Submitted:
02 February 2023
Posted:
06 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
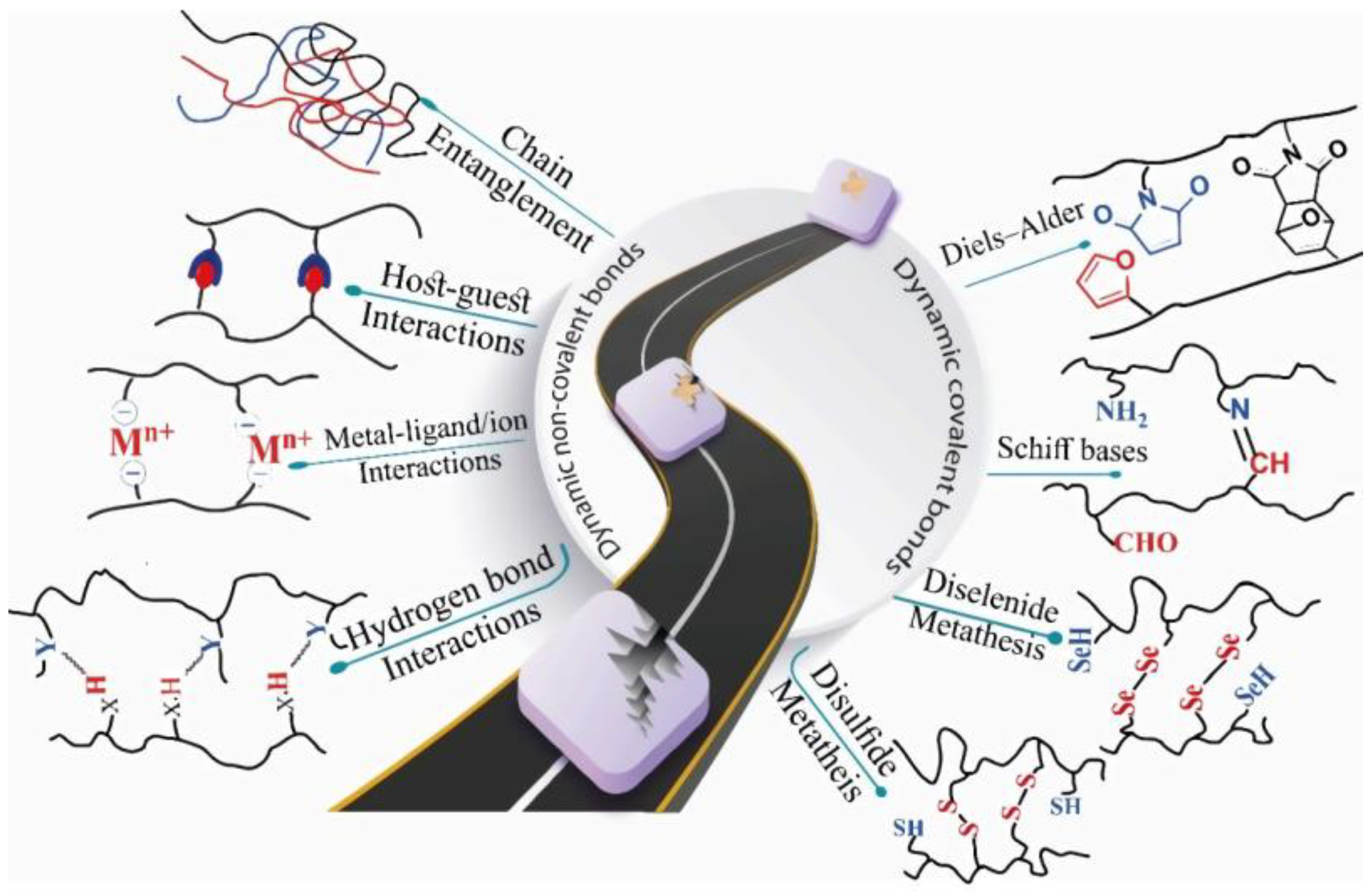
2. Hydrogels and their relationship with drug delivery systems (DDS)
3. D-printed hydrogels
4. Clays into hydrogels
5. Selected applications of hydrogels based on supramolecular strategies
5.1. Ophthalmic hydrogels
5.2. Adhesive hydrogels
5.3. Self-healing hydrogels systems
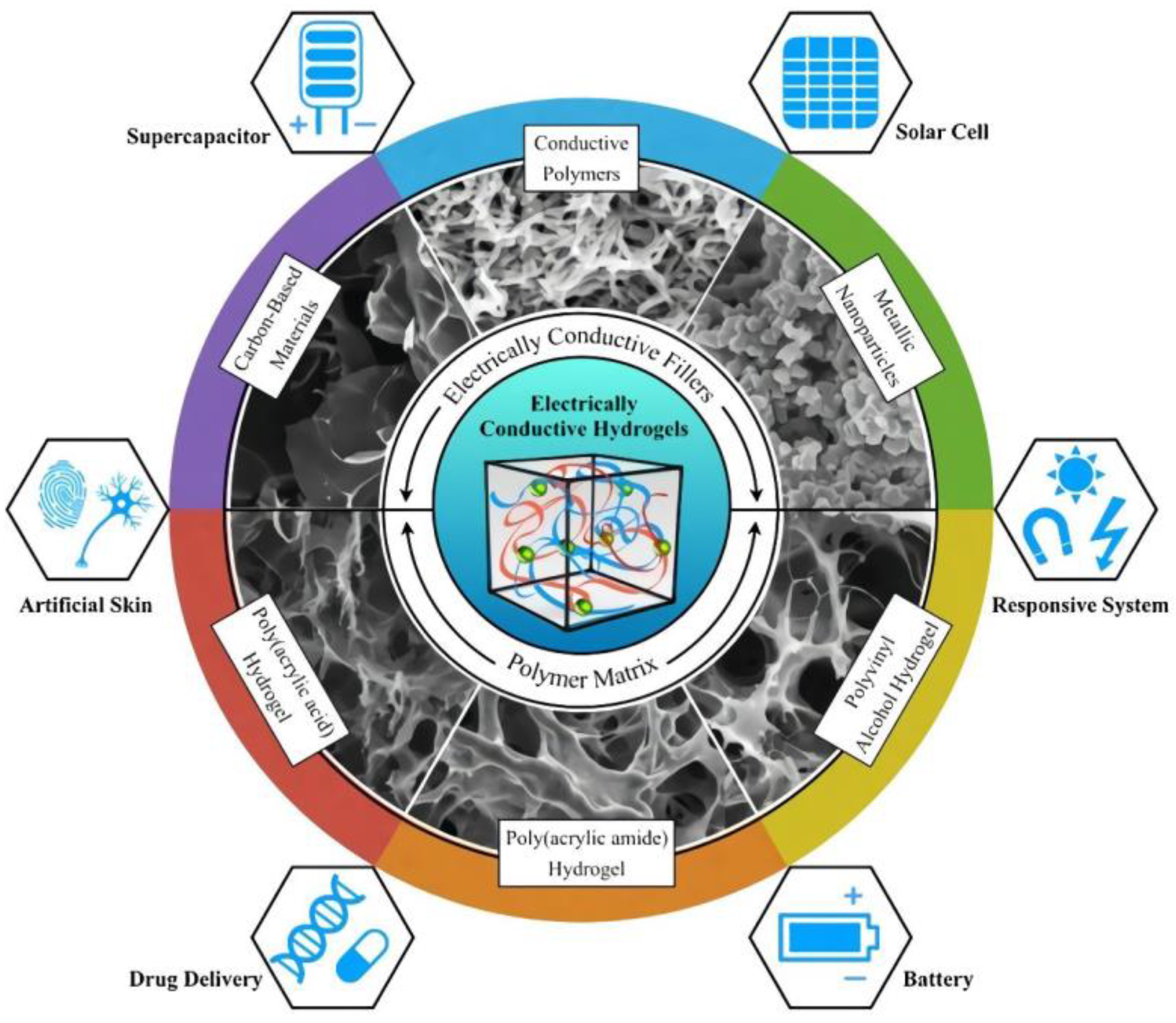
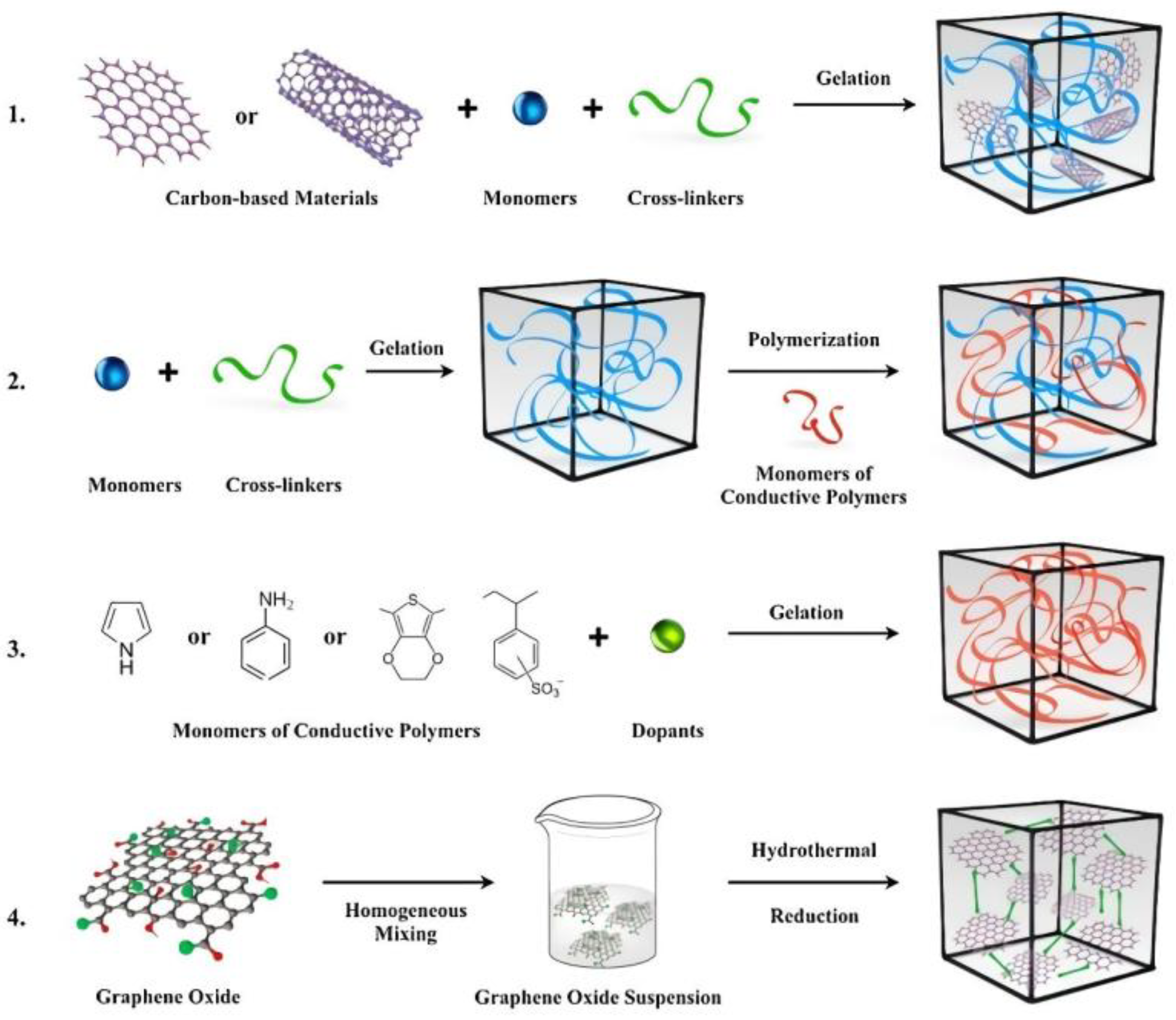
5.4. Electrically conducting hydrogels
5.5. Metallo-supramolecular hydrogels
6. Conclusions and perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Having, P.D.H.; Debertrand, L.; Zhao, J.; Creton, C. Metal – Ligand Coordination Bonds as Transient Crosslinks. Gels 2021, 7, 72. [Google Scholar] [CrossRef]
- Tan, Y.; Huang, H.; Ayers, D.C.; Song, J. Modulating Viscoelasticity, Stiffness, and Degradation of Synthetic Cellular Niches via Stoichiometric Tuning of Covalent versus Dynamic Noncovalent Cross-Linking. ACS Cent. Sci. 2018, 4, 971–981. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Wang, X.; Wang, Y.; Zhang, Q.; Cheng, Y. A Polydopamine Nanoparticle-Knotted Poly(Ethylene Glycol) Hydrogel for On-Demand Drug Delivery and Chemo-Photothermal Therapy. Chem. Mater. 2017, 29, 1370–1376. [Google Scholar] [CrossRef]
- Omar, J.; Ponsford, D.; Dreiss, C.A.; Lee, T.C.; Loh, X.J. Supramolecular Hydrogels: Design Strategies and Contemporary Biomedical Applications. Chem. Asian J. 2022, 17, e202200081. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Lin, Q.; Xue, K.; Loh, X.J. Recent Advances in Supramolecular Hydrogels for Biomedical Applications. Mater. Today Adv. 2019, 3, 100021. [Google Scholar] [CrossRef]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tibbitt, M.W. Engineering Hydrogel Adhesion for Biomedical Applications via Chemical Design of the Junction. ACS Biomater. Sci. Eng. 2021, 7, 4048–4076. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, Y.; Wang, W.; Sun, G.; Yan, K.; Wang, D. High Performance HKUST-1@PVA-Co-PE/PVA Hybrid Hydrogel with Enhanced Selective Adsorption. Compos. Commun. 2018, 10, 36–40. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Gao, J.; Yao, J.; Zhang, Q. Recent Progress in Metal-Organic Frameworks-Based Hydrogels and Aerogels and Their Applications. Coord. Chem. Rev. 2019, 398, 213016. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremião, M.P.D. Recent Advances in Smart Hydrogels for Biomedical Applications: From Self-Assembly to Functional Approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef]
- Gil, C.J.; Li, L.; Hwang, B.; Cadena, M.; Theus, A.S.; Finamore, T.A.; Bauser-Heaton, H.; Mahmoudi, M.; Roeder, R.K.; Serpooshan, V. Tissue Engineered Drug Delivery Vehicles: Methods to Monitor and Regulate the Release Behavior. J. Control. Release 2022, 349, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Svirskis, D.; Rees, S.W.P.; Barker, D.; Waterhouse, G.I.N.; Wu, Z. Photosensitive Drug Delivery Systems for Cancer Therapy: Mechanisms and Applications. J. Control. Release 2021, 338, 446–461. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, S.; Tibbitt, M.W. Supramolecular Engineering of Hydrogels for Drug Delivery. Adv. Drug Deliv. Rev. 2021, 171, 240–256. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1. [Google Scholar] [CrossRef]
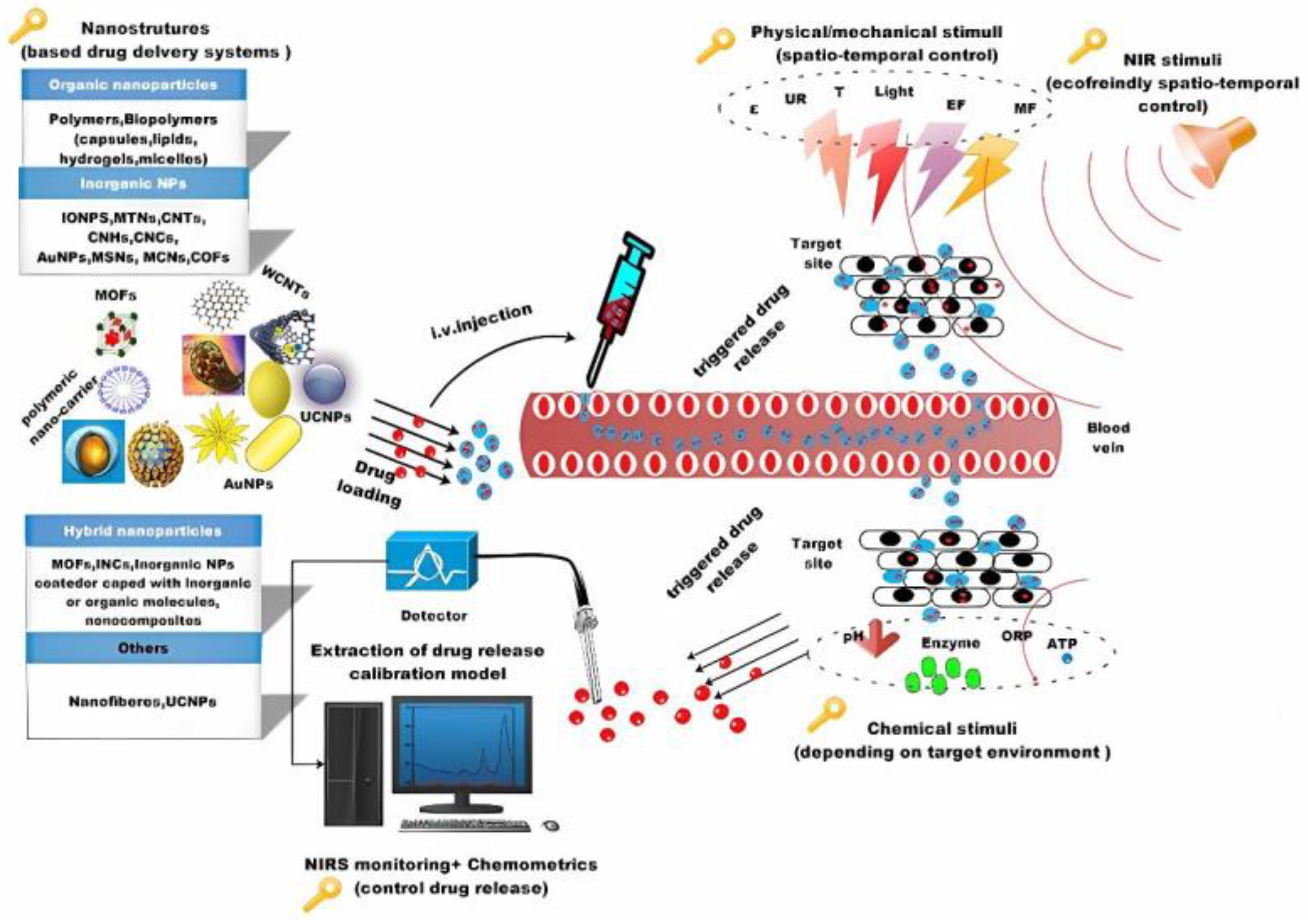
- Rahoui, N.; Jiang, B.; Taloub, N.; Huang, Y.D. Spatio-Temporal Control Strategy of Drug Delivery Systems Based Nano Structures. J. Control. Release 2017, 255, 176–201. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Fermin, L.; Ito, K.; Van Nostrum, C.F.; Vermonden, T. Hyaluronic Acid-Based Supramolecular Hydrogels for Biomedical Applications. Multifunct. Mater. 2021, 4, 032001. [Google Scholar] [CrossRef]
- Webber, M.J.; Pashuck, E.T. (Macro)Molecular Self-Assembly for Hydrogel Drug Delivery. Adv. Drug Deliv. Rev. 2021, 172, 275–295. [Google Scholar] [CrossRef]
- Vashahi, F.; Martinez, M.R.; Dashtimoghadam, E.; Fahimipour, F.; Keith, A.N.; Bersenev, E.A.; Ivanov, D.A.; Zhulina, E.B.; Popryadukhin, P.; Matyjaszewski, K.; et al. Injectable Bottlebrush Hydrogels with Tissue-Mimetic Mechanical Properties. Sci. Adv. 2022, 8, 1–13. [Google Scholar] [CrossRef]
- Ahsan, A.; Farooq, M.A.; Parveen, A. Thermosensitive Chitosan-Based Injectable Hydrogel as an Efficient Anticancer Drug Carrier. ACS Omega 2020, 5, 20450–20460. [Google Scholar] [CrossRef]
- Tang, Q.; Zhao, D.; Zhou, Q.; Yang, H.; Peng, K.; Zhang, X. Polyhistidine-Based Metal Coordination Hydrogels with Physiologically Relevant PH Responsiveness and Enhanced Stability through a Novel Synthesis. Macromol. Rapid Commun. 2018, 39, 1–6. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial Anti-Oxidant Electroactive Injectable Hydrogel as Self-Healing Wound Dressing with Hemostasis and Adhesiveness for Cutaneous Wound Healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, J.; Zhong, Y.; Li, K.; Zhang, L.; Cai, J. High-Strength and High-Toughness Double-Cross-Linked Cellulose Hydrogels: A New Strategy Using Sequential Chemical and Physical Cross-Linking. Adv. Funct. Mater. 2016, 26, 6279–6287. [Google Scholar] [CrossRef]
- Atta, S.; Khaliq, S.; Islam, A.; Javeria, I.; Jamil, T.; Athar, M.M.; Shafiq, M.I.; Ghaffar, A. Injectable Biopolymer Based Hydrogels for Drug Delivery Applications. Int. J. Biol. Macromol. 2015, 80, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, L.; Smith, A.; Kavanagh, K.; Devereux, M.; Colleran, J.; Breslin, C.; Richards, K.; McCann, M.; Rooney, A. Preparation and Antimicrobial Properties of Alginate and Serum Albumin/Glutaraldehyde Hydrogels Impregnated with Silver(I) Ions. Chemistry 2021, 3, 672–686. [Google Scholar] [CrossRef]
- Correa, S.; Grosskopf, A.K.; Lopez Hernandez, H.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef]
- Jiang, Y.; Krishnan, N.; Heo, J.; Fang, R.H.; Zhang, L. Nanoparticle–Hydrogel Superstructures for Biomedical Applications. J. Control. Release 2020, 324, 505–521. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan Hydrogels Incorporating Colloids for Sustained Drug Delivery. Carbohydr. Polym. 2022, 275, 118689. [Google Scholar] [CrossRef]
- Xu, L.; Wang, C.; Cui, Y.; Li, A.; Qiao, Y.; Qiu, D. Conjoined-Network Rendered Stiff and Tough Hydrogels from Biogenic Molecules. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef]
- Yu, A.C.; Lian, H.; Kong, X.; Lopez Hernandez, H.; Qin, J.; Appel, E.A. Physical Networks from Entropy-Driven Non-Covalent Interactions. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Zeng, L.; Song, M.; Gu, J.; Xu, Z.; Xue, B.; Li, Y.; Cao, Y. A Highly Stretchable, Tough, Fast Self-Healing Hydrogel Based on Peptide-Metal Ion Coordination. Biomimetics 2019, 4, 1–13. [Google Scholar] [CrossRef]
- Rahim, M.A.; Hata, Y.; Björnmalm, M.; Ju, Y.; Caruso, F. Supramolecular Metal–Phenolic Gels for the Crystallization of Active Pharmaceutical Ingredients. Small 2018, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted Drug Delivery Strategies for Precision Medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Jin, S.; Ye, K. Tissue and Organ 3D Bioprinting. SLAS Technol. 2018, 23, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Advincula, R.C.; Dizon, J.R.C.; Caldona, E.B.; Viers, R.A.; Siacor, F.D.C.; Maalihan, R.D.; Espera, A.H. On the Progress of 3D-Printed Hydrogels for Tissue Engineering. MRS Commun. 2021, 11, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Sather, N.A.; Sai, H.; Sasselli, I.R.; Sato, K.; Ji, W.; Synatschke, C.V.; Zambrotta, R.T.; Edelbrock, J.F.; Kohlmeyer, R.R.; Hardin, J.O.; et al. 3D Printing of Supramolecular Polymer Hydrogels with Hierarchical Structure. Small 2021, 17, 2005743. [Google Scholar] [CrossRef] [PubMed]
- Lopez Hernandez, H.; Souza, J.W.; Appel, E.A. A Quantitative Description for Designing the Extrudability of Shear-Thinning Physical Hydrogels. Macromol. Biosci. 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuk, H.; Lin, S.; Parada, G.A.; Tang, T.C.; Tham, E.; de la Fuente-Nunez, C.; Lu, T.K.; Zhao, X. 3D Printing of Living Responsive Materials and Devices. Adv. Mater. 2018, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, Y.J.; Kiran, R.; Tor, S.B.; Zhou, K. Submerged and Non-Submerged 3D Bioprinting Approaches for the Fabrication of Complex Structures with the Hydrogel Pair GelMA and Alginate/Methylcellulose. Addit. Manuf. 2021, 37, 101640. [Google Scholar] [CrossRef]
- Wang, R.; Yang, Z.; Luo, J.; Hsing, I.M.; Sun, F. B12-Dependent Photoresponsive Protein Hydrogels for Controlled Stem Cell/Protein Release. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 5912–5917. [Google Scholar] [CrossRef]
- Roh, H.H.; Kim, H.S.; Kim, C.; Lee, K.Y. 3D Printing of Polysaccharide-Based Self-Healing Hydrogel Reinforced With Alginate for Secondary Cross-Linking. Biomedicines 2021, 9, 1224. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, Y.W.; Jung, W.K.; Oh, J.; Nam, S.Y. Enhanced Rheological Behaviors of Alginate Hydrogels with Carrageenan for Extrusion-Based Bioprinting. J. Mech. Behav. Biomed. Mater. 2019, 98, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Rosales, A.M.; Anseth, K.S. The Design of Reversible Hydrogels to Capture Extracellular Matrix Dynamics. Nat. Rev. Mater. 2016, 1, 1–15. [Google Scholar] [CrossRef]
- Seliktar, D. Designing Cell-Compatible Hydrogels. 2012, 336, 1124–1128. [CrossRef]
- Shabbir, H.; Dellago, C.; Hartmann, M.A. A High Coordination of Cross-Links Is Beneficial for the Strength of Cross-Linked Fibers. Biomimetics 2019, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, M.; Roppolo, I.; Chiappone, A.; Larush, L.; Pirri, C.F.; Magdassi, S. 3D-Printed Self-Healing Hydrogels via Digital Light Processing. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hull, S.M.; Brunel, L.G.; Heilshorn, S.C. 3D Bioprinting of Cell-Laden Hydrogels for Improved Biological Functionality. Adv. Mater. 2022, 34, 2103691. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, S.; Fang, X.; Salmon, S. Advances in 3D Gel Printing for Enzyme Immobilization. Gels 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, S.R.; Sarabi, M.R.; Birtek, M.T.; Seyfi, S.; Sitti, M.; Tasoglu, S. 3D-Printed Microrobots from Design to Translation. Nat. Commun. 2022, 13, 5875. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.S.; Mostafavi, A.; Quint, J.P.; Panayi, A.C.; Baldino, K.; Williams, T.J.; Daubendiek, J.G.; Hugo Sánchez, V.; Bonick, Z.; Trujillo-Miranda, M.; et al. In Situ Printing of Adhesive Hydrogel Scaffolds for the Treatment of Skeletal Muscle Injuries. ACS Appl. Bio Mater. 2020, 3, 1568–1579. [Google Scholar] [CrossRef]
- Boyes, V.L.; Janani, R.; Partridge, S.; Fielding, L.A.; Breen, C.; Foulkes, J.; Le Maitre, C.L.; Sammon, C. One-Pot Precipitation Polymerisation Strategy for Tuneable Injectable Laponite®-PNIPAM Hydrogels: Polymerisation, Processability and Beyond. Polymer 2021, 233, 124201. [Google Scholar] [CrossRef]
- Thorpe, A.A.; Boyes, V.L.; Sammon, C.; Le Maitre, C.L. Thermally Triggered Injectable Hydrogel, Which Induces Mesenchymal Stem Cell Differentiation to Nucleus Pulposus Cells: Potential for Regeneration of the Intervertebral Disc. Acta Biomater. 2016, 36, 99–111. [Google Scholar] [CrossRef]
- Chakraborty, A.; Sutar, P.; Yadav, P.; Eswaramoorthy, M.; Maji, T.K. Charge-Assisted Self-Assembly of ZIF-8 and Laponite Clay toward a Functional Hydrogel Nanocomposite. Inorg. Chem. 2018, 57, 14480–14483. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Yang, L.; Hong, L.; Hu, Q. A Chitosan Hydrogel Sealant with Self-Contractile Characteristic: From Rapid and Long-Term Hemorrhage Control to Wound Closure and Repair. Carbohydr. Polym. 2021, 271, 118428. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhuang, Y.; Rampal, N.; Hewitt, R.; Divitini, G.; O’Keefe, C.A.; Liu, X.; Whitaker, D.J.; Wills, J.W.; Jugdaohsingh, R.; et al. Formulation of Metal-Organic Framework-Based Drug Carriers by Controlled Coordination of Methoxy PEG Phosphate: Boosting Colloidal Stability and Redispersibility. J. Am. Chem. Soc. 2021, 143, 13557–13572. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Liu, Z.; Yang, C.; Wang, W.; Ju, X.J.; Xie, R.; Chu, L.Y. Smart Hydrogels with Inhomogeneous Structures Assembled Using Nanoclay-Cross-Linked Hydrogel Subunits as Building Blocks. ACS Appl. Mater. Interfaces 2016, 8, 21721–21730. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Tsang, W.P.; Wan, C.; Wu, C. Fabrication of Injectable High Strength Hydrogel Based on 4-Arm Star PEG for Cartilage Tissue Engineering. Biomaterials 2017, 120, 11–21. [Google Scholar] [CrossRef]
- Rezanejad Gatabi, Z.; Heshmati, N.; Mirhoseini, M.; Dabbaghianamiri, M. The Application of Clay-Based Nanocomposite Hydrogels in Wound Healing. Arab. J. Sci. Eng. 2022, 1–14. [Google Scholar] [CrossRef]
- An, H.; Gu, Z.; Zhou, L.; Liu, S.; Li, C.; Zhang, M.; Xu, Y.; Zhang, P.; Wen, Y. Janus Mucosal Dressing with a Tough and Adhesive Hydrogel Based on Synergistic Effects of Gelatin, Polydopamine, and Nano-Clay. Acta Biomater. 2022, 149, 126–138. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Nikolakis, S.P.; Pamvouxoglou, A.; Koutsopoulou, E. Physicochemical Properties of Electrostatically Crosslinked Carrageenan/Chitosan Hydrogels and Carrageenan/Chitosan/Laponite Nanocomposite Hydrogels. Int. J. Biol. Macromol. 2023, 225, 565–573. [Google Scholar] [CrossRef]
- Gokaltun, A.A.; Fan, L.; Mazzaferro, L.; Byrne, D.; Yarmush, M.L.; Dai, T.; Asatekin, A.; Usta, O.B. Supramolecular Hybrid Hydrogels as Rapidly On-Demand Dissoluble, Self-Healing, and Biocompatible Burn Dressings. Bioact. Mater. 2022, No. May. [CrossRef]
- Zhai, X.; Ma, Y.; Hou, C.; Gao, F.; Zhang, Y.; Ruan, C.; Pan, H.; Lu, W.W.; Liu, W. 3D-Printed High Strength Bioactive Supramolecular Polymer/Clay Nanocomposite Hydrogel Scaffold for Bone Regeneration. ACS Biomater. Sci. Eng. 2017, 3, 1109–1118. [Google Scholar] [CrossRef]
- Li, P.; Zhong, Y.; Wang, X.; Hao, J. Enzyme-Regulated Healable Polymeric Hydrogels. ACS Cent. Sci. 2020, 6, 1507–1522. [Google Scholar] [CrossRef]
- Fang, G.; Yang, X.; Wang, Q.; Zhang, A.; Tang, B. Hydrogels-Based Ophthalmic Drug Delivery Systems for Treatment of Ocular Diseases. Mater. Sci. Eng. C 2021, 127, 112212. [Google Scholar] [CrossRef]
- Fang, G.; Wang, Q.; Yang, X.; Qian, Y.; Zhang, G.; Tang, B. γ-Cyclodextrin-Based Polypseudorotaxane Hydrogels for Ophthalmic Delivery of Flurbiprofen to Treat Anterior Uveitis. Carbohydr. Polym. 2022, 277, 118889. [Google Scholar] [CrossRef] [PubMed]
- Cassano, R.; Di Gioia, M.L.; Trombino, S. Gel-Based Materials for Ophthalmic Drug Delivery. Gels 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Kianersi, S.; Solouk, A.; Saber-Samandari, S.; Keshel, S.H.; Pasbakhsh, P. Alginate Nanoparticles as Ocular Drug Delivery Carriers. J. Drug Deliv. Sci. Technol. 2021, 66, 102889. [Google Scholar] [CrossRef]
- Sani, E.S.; Kheirkhah, A.; Rana, D.; Sun, Z.; Foulsham, W.; Sheikhi, A.; Khademhosseini, A.; Dana, R.; Annabi, N. Sutureless Repair of Corneal Injuries Using Naturally Derived Bioadhesive Hydrogels. Sci. Adv. 2019, 5, eaav1281. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Chen, H.; Yang, F.; Hong, J.; Cheng, Y.; Hu, J. All-Small-Molecule Supramolecular Hydrogels Assembled from Guanosine 5′-Monophosphate Disodium Salt and Tobramycin for the Treatment of Bacterial Keratitis. Bioact. Mater. 2022, 16, 293–300. [Google Scholar] [CrossRef]
- Fang, G.; Yang, X.; Chen, S.; Wang, Q.; Zhang, A.; Tang, B. Cyclodextrin-Based Host–Guest Supramolecular Hydrogels for Local Drug Delivery. Coord. Chem. Rev. 2022, 454, 214352. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.D.; Das, G.; Ajayaghosh, A. Stepwise Control of Host-Guest Interaction Using a Coordination Polymer Gel. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Fernandes-Cunha, G.M.; Jeong, S.H.; Logan, C.M.; Le, P.; Mundy, D.; Chen, F.; Chen, K.M.; Kim, M.; Lee, G.H.; Na, K.S.; et al. Supramolecular Host-Guest Hyaluronic Acid Hydrogels Enhance Corneal Wound Healing through Dynamic Spatiotemporal Effects. Ocul. Surf. 2022, 23, 148–161. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Song, S.; Ding, Y.; Dong, Y.; Lu, Y.; Liu, D.; Zhang, C. DNA Supramolecular Hydrogel as a Biocompatible Artificial Vitreous Substitute. Adv. Mater. Interfaces 2022, 9, 1–9. [Google Scholar] [CrossRef]
- Cheng, M.; Chang, W.H.; Yang, S.T.; Huang, H.Y.; Tsui, K.H.; Chang, C.P.; Lee, W.L.; Wang, P.H. Efficacy of Applying Hyaluronic Acid Gels in the Primary Prevention of Intrauterine Adhesion after Hysteroscopic Myomectomy: A Meta-Analysis of Randomized Controlled Trials. Life 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, C.; Yang, M.; Pan, Y.; Yin, Q.; Tang, J.; Qi, H.J.; Suo, Z. Printing Hydrogels and Elastomers in Arbitrary Sequence with Strong Adhesion. Adv. Funct. Mater. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Jung, H.; Kim, M.K.; Lee, J.Y.; Choi, S.W.; Kim, J. Adhesive Hydrogel Patch with Enhanced Strength and Adhesiveness to Skin for Transdermal Drug Delivery. Adv. Funct. Mater. 2020, 30, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel Machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Wirthl, D.; Pichler, R.; Drack, M.; Kettlguber, G.; Moser, R.; Gerstmayr, R.; Hartmann, F.; Bradt, E.; Kaltseis, R.; Siket, C.M.; et al. Instant Tough Bonding of Hydrogels for Soft Machines and Electronics. Sci. Adv. 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Celiz, A.D.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B.R.; Vasilyev, N.V.; Vlassak, J.J.; Suo, Z.; et al. Tough Adhesives for Diverse Wet Surfaces. Science 2017, 357, 378–381. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, S.; Ren, X.; Zhang, J.; Lin, Q.; Zhao, Y. Supramolecular Adhesive Hydrogels for Tissue Engineering Applications. Chem. Rev. 2022, 122, 5604–5640. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, J.S.; Lee, C.; Park, H.J.; Yang, K.; Jin, Y.; Ryu, J.H.; Hong, K.S.; Moon, S.H.; Chung, H.M.; et al. Tissue Adhesive Catechol-Modified Hyaluronic Acid Hydrogel for Effective, Minimally Invasive Cell Therapy. Adv. Funct. Mater. 2015, 25, 3814–3824. [Google Scholar] [CrossRef]
- Conejo-Cuevas, G.; Ruiz-Rubio, L.; Sáez-Martínez, V.; Pérez-González, R.; Gartziandia, O.; Huguet-Casquero, A.; Pérez-Álvarez, L. Spontaneous Gelation of Adhesive Catechol Modified Hyaluronic Acid and Chitosan. Polymers 2022, 14, 1209. [Google Scholar] [CrossRef]
- Zheng, K.; Gu, Q.; Zhou, D.; Zhou, M.; Zhang, L. Recent Progress in Surgical Adhesives for Biomedical Applications. Smart Mater. Med. 2022, 3, 41–65. [Google Scholar] [CrossRef]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tibbitt, M.W. Engineering Hydrogel Adhesion for Biomedical Applications via Chemical Design of the Junction. ACS Biomater. Sci. Eng. 2021, 7, 4048–4076. [Google Scholar] [CrossRef]
- Annabi, N.; Yue, K.; Tamayol, A.; Khademhosseini, A. Elastic Sealants for Surgical Applications. Eur. J. Pharm. Biopharm. 2015, 95, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Dhibar, S.; Dey, A.; Majumdar, S.; Ghosh, D.; Mandal, A.; Ray, P.P.; Dey, B. A Supramolecular Cd(Ii)-Metallogel: An Efficient Semiconductive Electronic Device. Dalt. Trans. 2018, 47, 17412–17420. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Suo, Z. Hydrogel Ionotronics. Nat. Rev. Mater. 2018, 3, 125–142. [Google Scholar] [CrossRef]
- Nam, S.; Mooney, D. Polymeric Tissue Adhesives. Chem. Rev. 2021, 121, 11336–11384. [Google Scholar] [CrossRef] [PubMed]
- 88 Taboada, G.M.; Yang, K.; Pereira, M.J.N.; Liu, S.S.; Hu, Y.; Karp, J.M.; Artzi, N.; Lee, Y. Overcoming the Translational Barriers of Tissue Adhesives. Nat. Rev. Mater. 2020, 5, 310–329. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The Stiffness of Living Tissues and Its Implications for Tissue Engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Feng, B.; Wang, S.; Hu, D.; Fu, W.; Wu, J.; Hong, H.; Domian, I.J.; Li, F.; Liu, J. Bioresorbable Electrospun Gelatin/Polycaprolactone Nanofibrous Membrane as a Barrier to Prevent Cardiac Postoperative Adhesion. Acta Biomater. 2019, 83, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Hu, S.; Qin, W.; Tang, Y.; Guo, R.; Han, L. Bioprinting of a Blue Light-Cross-Linked Biodegradable Hydrogel Encapsulating Amniotic Mesenchymal Stem Cells for Intrauterine Adhesion Prevention. ACS Omega 2021. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Yu, J.; Chen, X.; Chen, X.; Cui, C.; Zhang, J.; Zhang, Q.; Zhang, Y.; Wang, S.; et al. H-Bonding Supramolecular Hydrogels with Promising Mechanical Strength and Shape Memory Properties for Postoperative Antiadhesion Application. ACS Appl. Mater. Interfaces 2020, 12, 34161–34169. [Google Scholar] [CrossRef]
- Du, R.; Wu, J.; Chen, L.; Huang, H.; Zhang, X.; Zhang, J. Hierarchical Hydrogen Bonds Directed Multi-Functional Carbon Nanotube-Based Supramolecular Hydrogels. Small 2014, 10, 1387–1393. [Google Scholar] [CrossRef]
- Burattini, S.; Greenland, B.W.; Merino, D.H.; Weng, W.; Seppala, J.; Colquhoun, H.M.; Hayes, W.; MacKay, M.E.; Hamley, I.W.; Rowan, S.J. A Healable Supramolecular Polymer Blend Based on Aromatic π-π Stacking and Hydrogen-Bonding Interactions. J. Am. Chem. Soc. 2010, 132, 12051–12058. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Y.; Sun, Y.; Qin, Y.; Zhang, H.; Yu, X.; Liu, Y. Stretchable Slide-Ring Supramolecular Hydrogel for Flexible Electronic Devices. Commun. Mater. 2022, 3, 1–9. [Google Scholar] [CrossRef]
- Freeman, R.; Han, M.; Álvarez, Z.; Lewis, J.A.; Wester, J.R.; Stephanopoulos, N.; McClendon, M.T.; Lynsky, C.; Godbe, J.M.; Sangji, H.; et al. Reversible Self-Assembly of Superstructured Networks. Science 2018, 362, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Gharakhloo, M.; Karbarz, M. Autonomous Self-Healing Hydrogels: Recent Development in Fabrication Strategies. Eur. Polym. J. 2022, 165, 111004. [Google Scholar] [CrossRef]
- Shin, M.; Lee, H. Gallol-Rich Hyaluronic Acid Hydrogels: Shear-Thinning, Protein Accumulation against Concentration Gradients, and Degradation-Resistant Properties. Chem. Mater. 2017, 29, 8211–8220. [Google Scholar] [CrossRef]
- Hardman, D.; George Thuruthel, T.; Iida, F. Self-Healing Ionic Gelatin/Glycerol Hydrogels for Strain Sensing Applications. NPG Asia Mater. 2022, 14, 11. [Google Scholar] [CrossRef]
- Tamate, R.; Watanabe, M. Recent Progress in Self-Healable Ion Gels. Sci. Technol. Adv. Mater. 2020, 21, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Cunha, I.; Martins, J.; Gaspar, D.; Bahubalindruni, P.G.; Fortunato, E.; Martins, R.; Pereira, L. Healable Cellulose Iontronic Hydrogel Stickers for Sustainable Electronics on Paper. Adv. Electron. Mater. 2021, 7, 2001166. [Google Scholar] [CrossRef]
- Majumdar, S.; Ray, P.P.; Sahu, R.; Dey, A.; Dey, B. Strategic Fabrication of Efficient Photo-Responsive Semiconductor Electronic Diode-Devices by Bovine Serum Albumin Protein-Based Cu(II)-Metallohydrogel Scaffolds. Int. J. Biol. Macromol. 2022, 195, 287–293. [Google Scholar] [CrossRef]
- Li, C.H.; Zuo, J.L. Self-Healing Polymers Based on Coordination Bonds. Adv. Mater. 2020, 32, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Breul, K.; Kissel, S.; Seiffert, S. Sticker Multivalency in Metallo-Supramolecular Polymer Networks. Macromolecules 2021, 54, 8407–8422. [Google Scholar] [CrossRef]
- Xu, X.; Jerca, V.V.; Hoogenboom, R. Self-Healing Metallo-Supramolecular Hydrogel Based on Specific Ni2+ Coordination Interactions of Poly(Ethylene Glycol) with Bistriazole Pyridine Ligands in the Main Chain. Macromol. Rapid Commun. 2020, 41, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Choi, W.; Kim, J.; Choi, J.; Choi, N.; Hwang, K.S. Highly Sensitive Three-Dimensional Interdigitated Microelectrode Biosensors Embedded with Porosity Tunable Hydrogel for Detecting Proteins. Sensors Actuators, B Chem. 2020, 302, 127190. [Google Scholar] [CrossRef]
- Volpi, M.; Paradiso, A.; Costantini, M.; Świȩszkowski, W. Hydrogel-Based Fiber Biofabrication Techniques for Skeletal Muscle Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 379–405. [Google Scholar] [CrossRef] [PubMed]
- Farr, A.C.; Hogan, K.J.; Mikos, A.G. Nanomaterial Additives for Fabrication of Stimuli-Responsive Skeletal Muscle Tissue Engineering Constructs. Adv. Healthc. Mater. 2020, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; Boccaccini, A.R. 3D Printing of Electrically Conductive Hydrogels for Tissue Engineering and Biosensors – A Review. Acta Biomater. 2020, 101, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rong, Q.; Lei, W.; Liu, M. Conductive Hydrogels as Smart Materials for Flexible Electronic Devices. Chem. - A Eur. J. 2018, 24, 16930–16943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Feng, P.; Chen, J.; Sun, Z.; Zhao, B. Electrically Conductive Hydrogels for Flexible Energy Storage Systems. Prog. Polym. Sci. 2019, 88, 220–240. [Google Scholar] [CrossRef]
- Guan, X.; Yang, Z.; Zhou, M.; Yang, L.; Peymanfar, R.; Aslibeiki, B.; Ji, G. 2D MXene Nanomaterials: Synthesis, Mechanism, and Multifunctional Applications in Microwave Absorption. Small Struct. 2022, 3, 2200102. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, H.; Zhu, W.; Guan, L.; Yang, X.; Zvyagin, A.V.; Zhao, Y.; Shen, C.; Yang, B.; Lin, Q. Muscle-Inspired MXene Conductive Hydrogels with Anisotropy and Low-Temperature Tolerance for Wearable Flexible Sensors and Arrays. Adv. Funct. Mater. 2021, 31, 2105264. [Google Scholar] [CrossRef]
- Mariani, F.; Gualandi, I.; Schuhmann, W.; Scavetta, E. Micro - and Nano - Devices for Electrochemical Sensing. Microchim. Acta 2022, 189, 459. [Google Scholar] [CrossRef]
- Zahed, M.A.; Sharifuzzaman, M.; Yoon, H.; Asaduzzaman, M.; Kim, D.K.; Jeong, S.; Pradhan, G.B.; Shin, Y. Do; Yoon, S.H.; Sharma, S.; et al. A Nanoporous Carbon-MXene Heterostructured Nanocomposite-Based Epidermal Patch for Real-Time Biopotentials and Sweat Glucose Monitoring. Adv. Funct. Mater. 2022, 2208344, 1–17. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, Y.; Cuthbert, T.J.; Shokurov, A.V.; Chu, P.K.; Feng, S.P.; Menon, C. Hydrogels as Soft Ionic Conductors in Flexible and Wearable Triboelectric Nanogenerators. Adv. Sci. 2022, 9, 1–24. [Google Scholar] [CrossRef]
- Carvalho, J.T.; Cunha, I.; Coelho, J.; Fortunato, E.; Martins, R.; Pereira, L. Carbon-Yarn-Based Supercapacitors with in Situ Regenerated Cellulose Hydrogel for Sustainable Wearable Electronics. ACS Appl. Energy Mater. 2022, 5, 11987–11996. [Google Scholar] [CrossRef]
- Yuk, H.; Wu, J.; Zhao, X. Hydrogel Interfaces for Merging Humans and Machines. Nat. Rev. Mater. 2022, 7, 25–29. [Google Scholar] [CrossRef]
- Mahendar, C.; Kumar, Y.; Dixit, M.K.; Mukherjee, M.; Kalam, A.; Dubey, M. Conductive Zn(Ii)-Metallohydrogels: The Role of Alkali Metal Cation Size in Gelation, Rheology and Conductance. Mol. Syst. Des. Eng. 2021, 6, 654–661. [Google Scholar] [CrossRef]
- Kawamoto, K.; Grindy, S.C.; Liu, J.; Holten-Andersen, N.; Johnson, J.A. Dual Role for 1,2,4,5-Tetrazines in Polymer Networks: Combining Diels-Alder Reactions and Metal Coordination to Generate Functional Supramolecular Gels. ACS Macro Lett. 2015, 4, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Falcone, N.; Kraatz, H.B. Supramolecular Peptide Gels: Influencing Properties by Metal Ion Coordination and Their Wide-Ranging Applications. ACS Omega 2020, 5, 1312–1317. [Google Scholar] [CrossRef]
- Das, P.; Pan, I.; Cohen, E.; Reches, M. Self-Assembly of a Metallo-Peptide into a Drug Delivery System Using a “Switch on” Displacement Strategy. J. Mater. Chem. B 2018, 6, 8228–8237. [Google Scholar] [CrossRef]
- Song, W.J.; Tezcan, F.A. A Designed Supramolecular Protein Assembly with in Vivo Enzymatic Activity. Science 2014, 346, 1525–1528. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Panahi, F.; Bahri-Laleh, N.; Sabzi, M.; Pareras, G.; Falcone, B.N.; Poater, A. PH-Responsive Gelation in Metallo-Supramolecular Polymers Based on the Protic Pyridinedicarboxamide Ligand. Chem. Mater. 2022, 34, 6155–6169. [Google Scholar] [CrossRef]
- Ahmadi, M.; Seiffert, S. Coordination Geometry Preference Regulates the Structure and Dynamics of Metallo-Supramolecular Polymer Networks. Macromolecules 2021, 54, 1388–1400. [Google Scholar] [CrossRef]
- Nicolella, P.; Koziol, M.F.; Löser, L.; Saalwächter, K.; Ahmadi, M.; Seiffert, S. Defect-Controlled Softness, Diffusive Permeability, and Mesh-Topology of Metallo-Supramolecular Hydrogels. Soft Matter 2022, 18, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Seiffert, S. Thermodynamic Control over Energy Dissipation Modes in Dual-Network Hydrogels Based on Metal-Ligand Coordination. Soft Matter 2020, 16, 2332–2341. [Google Scholar] [CrossRef]
- Kurbah, S.D.; Lal, R.A. Vanadium(V) Complex Based Supramolecular Metallogel: Self-Assembly and (Metallo)Gelation Triggered by Non-Covalent and N+[Sbnd]H…O Hydrogen Bonding Interactions. Inorg. Chem. Commun. 2020, 111, 107642. [Google Scholar] [CrossRef]
- Dasgupta, A.; Das, D. Designer Peptide Amphiphiles: Self-Assembly to Applications. Langmuir 2019, 35, 10704–10724. [Google Scholar] [CrossRef]
- Kerns, S.A.; Biswas, A.; Minnetian, N.M.; Borovik, A.S. Artificial Metalloproteins: At the Interface between Biology and Chemistry. JACS Au 2022, 2, 1252–1265. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




