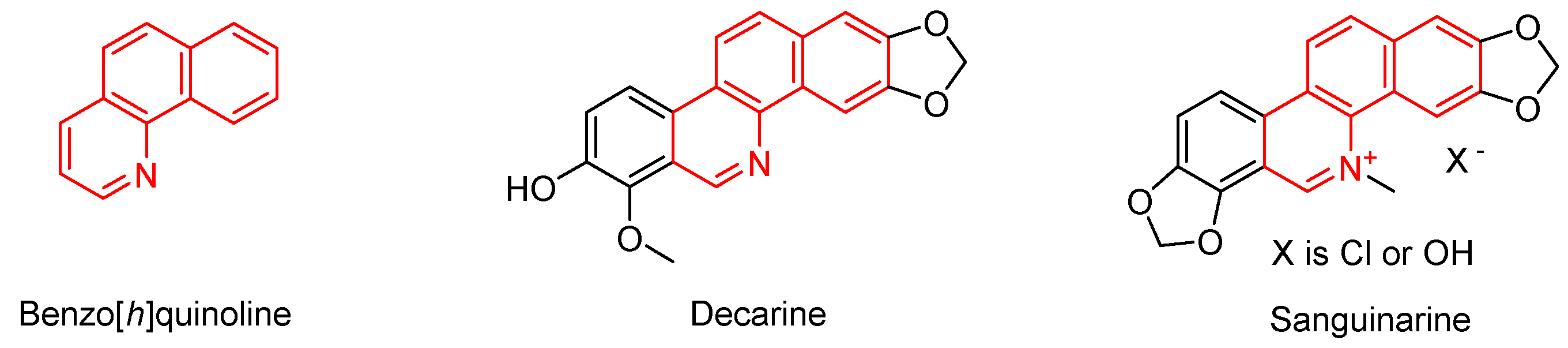
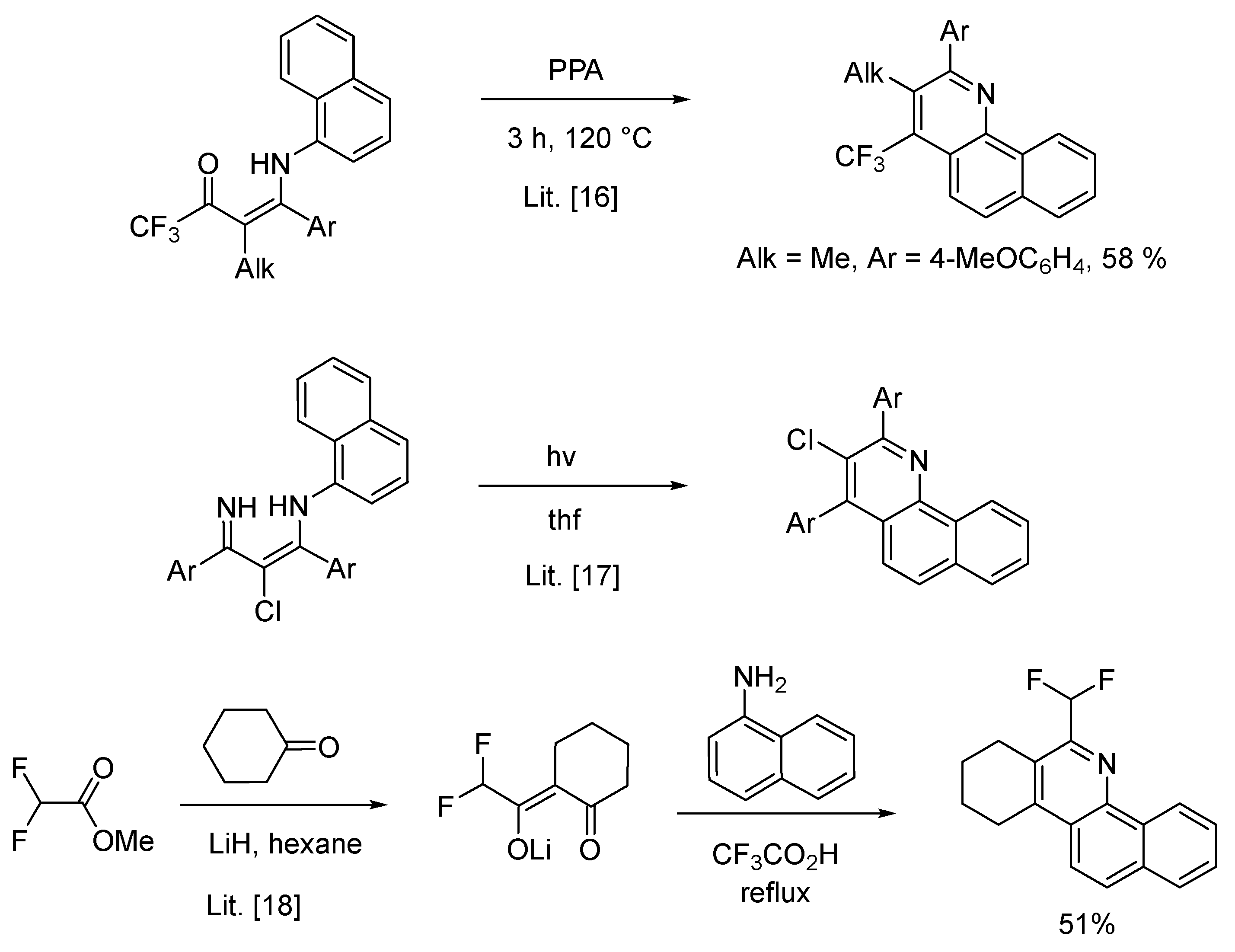
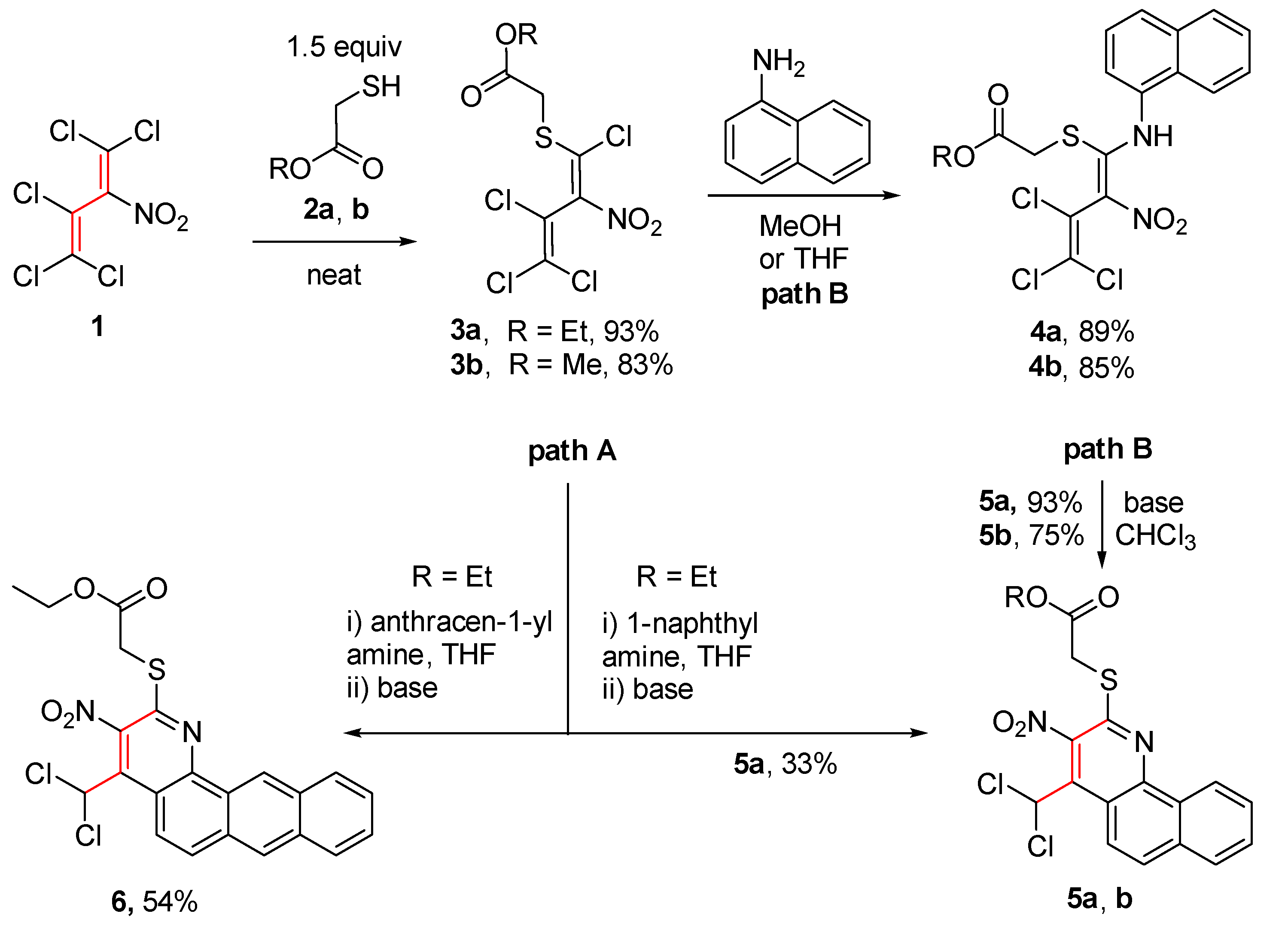
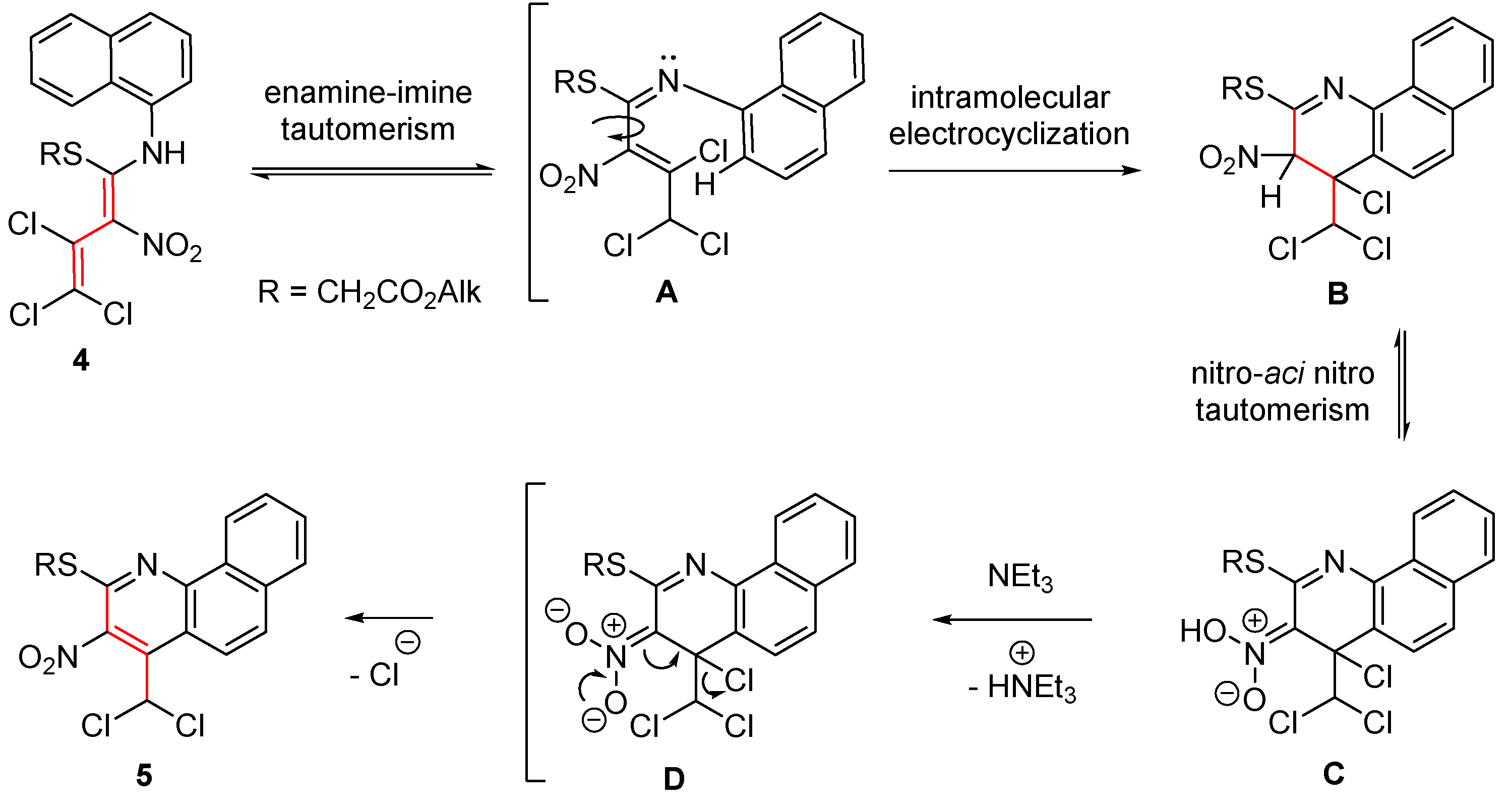
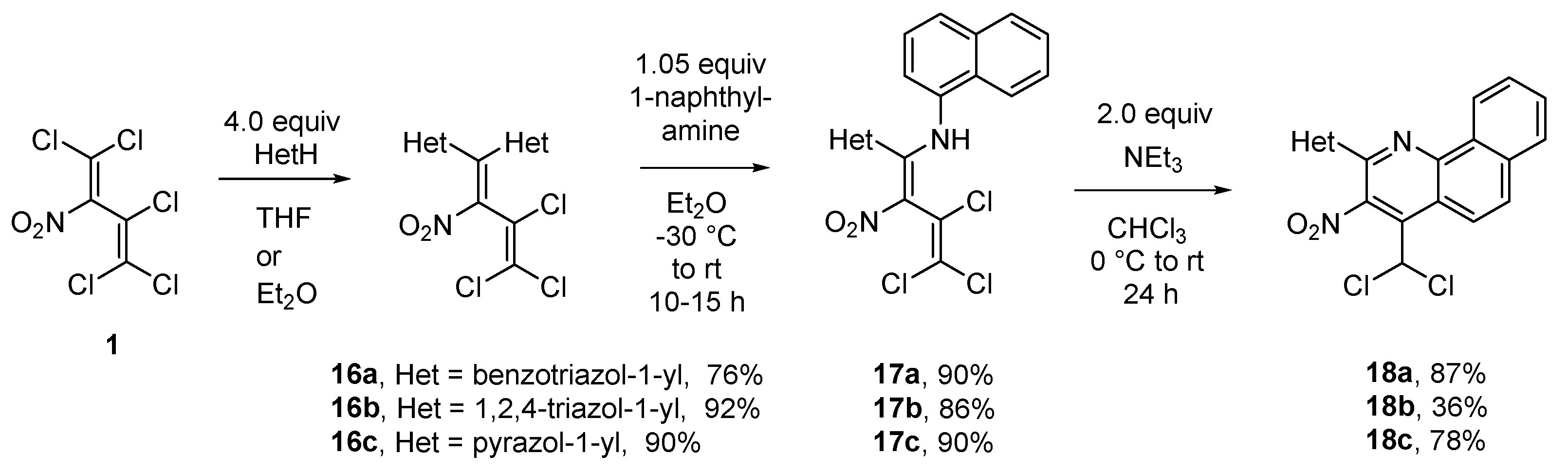3.1. General Remarks
Solvents and reagents were used as received from commercial sources without further purification. TLC was performed with Merck aluminum-backed TLC plates with silica gel 60, F254. Flash column chromatography was performed with Macherey–Nagel silica gel 60 M (0.040–0.063 mm) with appropriate mixtures of petroleum ether (PE, boiling range 60–70 °C) and ethyl acetate as eluents. Melting points were determined in capillary tubes with a Büchi B-520. FTIR spectra were recorded with a Bruker “Alpha-T” spectrometer with solid compounds measured as KBr pellets. ATR-IR spectra were measured on the same instrument with a Bruker “Alpha Platinum ATR” single reflection diamond ATR module.
1H NMR and
13C NMR spectra at 600 and 150 MHz, respectively, were recorded with an “Avance III” 600 MHz FTNMR spectrometer (Bruker, Rheinstetten, Germany).
1H NMR and
13C NMR spectra at 400 and 100 MHz, respectively, were recorded with an “Avance” 400 MHz FT-NMR spectrometer (also Bruker).
1H and
13C NMR spectra were referenced to the residual solvent peak: CDCl
3, δ = 7.26 (
1H) and 77.0 ppm (
13C); [D
6]DMSO, δ = 2.50 (
1H), and 39.7 ppm (
13C). The NMR-spectra of the newly synthesized compounds could be found in the
Supplementary Materials. Mass spectra were obtained with a Hewlett–Packard MS 5989B spectrometer, usually in direct mode with electron impact (70 eV). For chlorinated and brominated compounds, all peak values of molecular ions and fragments refer to the isotope
35Cl. High resolution mass spectra were recorded with a Waters mass spectrometer “VG Autospec” (EI), with a WATERS mass spectrometer “Q-Tof Premier” coupled with a Waters “Acquity UPLC” (ESI), or with a Micromass mass spectrometer “LCT” coupled with a Waters “Alliance 2965 HPLC” (ESI) at the Institute of Organic Chemistry, Leibniz University of Hannover.
Pentachloro-2-nitro-1,3-butadiene (
1). The product was prepared from 2H–penta chloro-1,3-butadiene in 53% yield (b.p. 69–71 °C / 1 mbar) according to the literature [
19].
Ethyl {[(1E)-1,3,4,4-tetrachloro-2-nitrobuta-1,3-dien-1-yl]sulfanyl}acetate (3a). A mixture of pentachloro-2-nitrobuta-1,3-diene (1) (75.30 g, 280 mmol) and ethyl 2-mercaptoacetate (50.60 g, 421 mmol) was stirred for 14 d at room temperature (rt). Then pentane was added (2 × 150 mL), each time the two-phase mixture was stirred for an additional 15 min and most of the solvent decanted after the product had solidified. It was then filtered with suction and dried in vacuo. Yellowish solid, yield 92.98 g (93%), m.p. 114–115 °C. IR (KBr): νmax = 2991, 1731 (C=O), 1528 (NO2), 1291 (NO2), 1205, 1006, 758 cm-1. 1H NMR (400 MHz, CDCl3): δ = 4.47 (q, 2 H, J = 7.1 Hz, OCH2), 3.91 (s, 2 H, SCH2), 1.28 (t, 3 H, J = 7.1 Hz, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ = 166.5 (C=O), 156.4 (SCCl), 138.9 (CNO2), 128.8 (CCl), 120.7 (CCl2), 62.4 (OCH2), 37.3 (SCH2), 13.8 (CH3) ppm. EIMS: m/z (%) = 355 (3) [M]+, 317 (10) [M - Cl]+, 272 (15) [M - Cl - NO2]+, 270 (55) [M - CCl2]+, 224 (30) [M - CCl2 - NO2]+, 120 (45) [SCH2COOEt]+. HRMS (EI): m/z calcd. for C8H8Cl4NO4S [M + H]+ 353.8923; found 353.8923.
Methyl {[(1E)-1,3,4,4-tetrachloro-2-nitrobuta-1,3-dien-1-yl]sulfanyl}acetate (3b). The product was prepared according to acetate 3a from buta-1,3-diene (1) (4.00 g, 14.7 mmol) and methyl 2-mercaptoacete (2.34 g, 22.1 mmol). Yellowish solid, yield 4.18 g (83%), m.p. 183 °C. IR (ATR): νmax = 3003, 1742 (C=O), 1538 (NO2), 1295 (NO2), 1160, 990, 760 cm-1. 1H NMR (400 MHz, CDCl3): δ = 3.94 (s, 2 H, SCH2), 3.81 (s, 3 H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ = 167.3 (C=O), 155.8 (SCCl), 139.6 (CNO2), 129.3 (CCl), 120.8 (CCl2), 53.4 (SCH2), 37.3 (CH3) ppm. EIMS: m/z (%) = 337 (65) [M]+, 233 (15) [M - SCH2COOCH3]+, 187 (45) [M - SCH2COOCH3 - NO2]+, 151 (50) [M - SCH2COOCH3 - CCl2]+, 105 (95) [SCH2COOCH3]+. HRMS (ESI): calcd. for C7H6Cl4NO4S [M + H]+: 339.87662; found 339.87687.
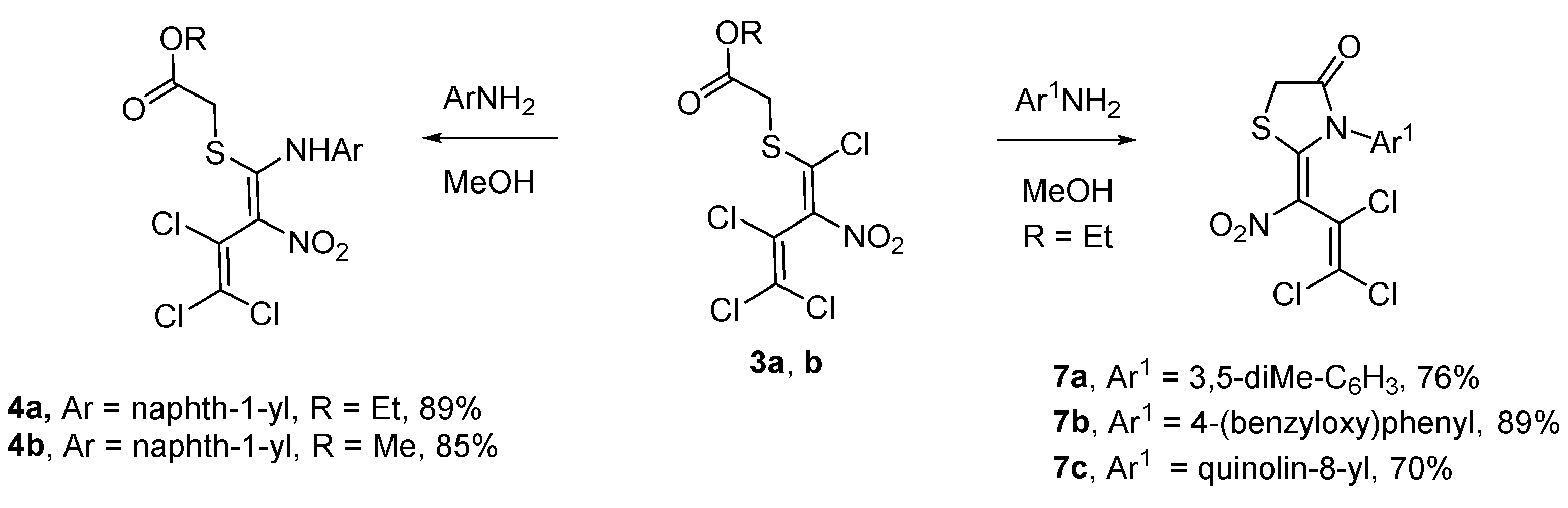
Ethyl {[(1E)-3,4,4-trichloro-1-(naphth-1-ylamino)-2-nitrobuta-1,3-dien-1-yl]sulfanyl}-acetate (4a). A solution of ethyl {[(1E)-1,3,4,4-tetrachloro-2-nitrobuta-1,3-dien-1-yl]sulfanyl}acetate (3a) (5.00 g, 14.0 mmol) and 1-naphthylamine (4.00 g, 28.0 mmol) in methanol (20 mL) was stirred for 3 h at rt, the precipitated product filtered off and washed with aqueous HCl (18%, 20 mL), water (20 mL) and cold methanol (20 mL). Yellowish solid, 5.73 g (89%), m.p.: 118–119 °C. IR (KBr): νmax = 2986, 1741, 1556, 1470, 1393, 1182, 829 cm-1. 1H NMR (400 MHz, CDCl3): δ = 12.16 (s, 1 H, NH), 8.03–7.93 (m, 3 H), 7.62–7.50 (m, 4 H), 4.98 (q, J = 7.1 Hz, 2 H), 2.95 (s, 2 H), 1.12 (t, J = 7.1 Hz, 3 H) ppm. 13C NMR (100 MHz, CDCl3): δ = 166.6 (C=O), 158.3 (NCS), 134.2 (Cq), 133.0 (Cq), 131.3 (Cq), 128.8 (CH), 128.7 (CH), 128.2 (CCl), 127.7 (CH), 127.3 (CH), 125.4 (CH), 123.6 (CCl2), 122.9 (CH), 122.2 (CNO2), 121.5 (CH), 62.1 (OCH2), 34.5 (SCH2), 13.9 (CH3) ppm. EIMS: m/z (%) = 462 (2) [M + H]+, 424 (2) [M - Cl]+, 414 (5) [M - NO2]+, 379 (5) [M - NO2 - Cl]+, 343 (10) [M - NO2 - Cl - Cl]+, 252 (10) [M - NO2 - Cl - naphthyl]+, 143 (100) [naphthylamine]+. HRMS (ESI): m/z calcd. for C18H15Cl3N2O4SNa [M + Na]+ 482.9710; found 482.9714.
Methyl {[(1E)-3,4,4-trichloro-1-(naphth-1-ylamino)-2-nitrobuta-1,3-dien-1-yl]sulfanyl}-acetate (4b). The product was prepared according to acetate 4a from acetate 3b (0.600 g, 1.759 mmol) and 1-naphthylamine (0.500 g, 3.519 mmol). Yellowish solid, yield: 0.668 g (85%), m.p. 110 °C. IR (ATR): νmax = 2905, 1745, 1553, 1527, 1390, 1170, 801 cm-1. 1H NMR (400 MHz, CDCl3): δ = 12.14 (s, 1 H, NH), 8.01 (d, J = 7.4 Hz, 1 H), 7.94 (d, J = 7.4 Hz, 1 H), 7.89 (d, J = 8.0 Hz, 1 H), 7.66–7.60 (m, 3 H), 7.52 (t, J = 8.0 Hz, 1 H), 3.53 (s, 1 H, CH3), 2.97 (s, 1 H, CH2) ppm. 13C-NMR (100 MHz, CDCl3): δ = 161.8 (C=O), 152.9 (NCS), 123.6 (2C, Cq, CH), 123.5 (CH), 123.2 (CNO2), 123.1 (CCl), 122.6 (CH), 122.1 (CH), 120.2 (CH), 118.2 (CCl2), 117.8 (CH), 116.3 (CH), 47.6 (CH3), 29.1 (CH2) ppm. EIMS: m/z (%) = 447 (25) [M + H]+, 351 (20) [M - Cl - COOCH3]+, 296 (20) [M - NO2 - SCH2COOCH3]+, 225 (40) [M - 2 Cl - NO2 - SCH2COOCH3]+ 143 (55) [naphthyl]+. HRMS (ESI): m/z calcd. for C17H13Cl3N2O4SNa [M + Na]+ 468.9554; found 468.9560.
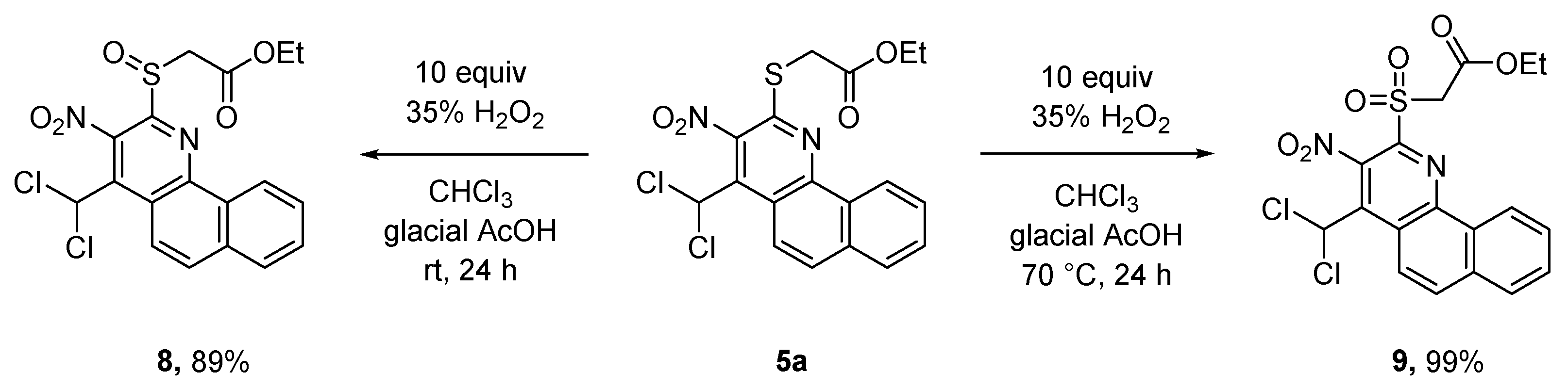
Ethyl {[4-dichloromethyl-3-nitrobenzo[h]quinolin-2-yl]sulfanyl}acetate (5a). To a solution of 4a (2.00 g, 4.3 mmol) in chloroform (20 mL) triethylamine (0.870 g, 0.60 mmol) was added at 0 °C and the reaction mixture stirred for 1 d at rt. The solvent was removed in vacuo and the residue dissolved in methanol (5 mL). The precipitated product was filtered off with suction and washed with aqueous HCl (18%, 20 mL), water (20 mL) and methanol (30 mL) and then dried in vacuo to give 5a, yellow solid. Yield 1.70 g (93%), m.p. 171–172 °C. IR (KBr): νmax = 3042, 2989, 1744, 1534, 1334, 831, 735 cm-1. 1H NMR (400 MHz, CDCl3): δ = 9.16 (d, J = 7.2 Hz, 1 H), 8.65 (d, J = 9.6 Hz, 1 H), 7.95 (d, J = 9.6 Hz, 1 H), 7.94 (d, J = 7.2 Hz, 1 H), 7.82–7.74 (m, 2 H), 7.20 (s, 1 H, CHCl2), 4.22 (q, J = 7.2 Hz, 2 H, OCH2), 4.20 (s, 2 H, SCH2), 1.28 (t, J = 7.2 Hz, 3 H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ = 168.6 (C=O), 149.0 (Cq), 147.9 (Cq), 139.1 (Cq), 135.7 (Cq), 134.3 (Cq), 130.4 (Cq), 130.3 (CH), 128.8 (CH), 128.0 (CH), 127.9 (CH), 125.7 (CH), 122.3 (CH), 119.6 (Cq), 63.4 (CHCl2), 62.1 (OCH2), 33.8 (SCH2), 14.2 (CH3) ppm. EIMS: m/z (%) = 424 (12) [M]+, 379 (5) [M - OEt]+, 355 (8) [M - Cl - Cl]+, 303 (25) [M- SCH2CO2Et]+, 192 (15) [M - HCl - HCl - SCH2CO2Et - NO2]+. HRMS (ESI): m/z calcd. for C18H14Cl2N2O4SNa [M + Na]+ 446.9944; found 446.9947.
Methyl {[4-(dichloromethyl)-3-nitrobenzo[h]quinolin-2-yl]sulfanyl}acetate (5b). The product was prepared according to benzo[h]quinoline 5a from acetate 4b (0.300 g, 0.67 mmol) and triethylamine (0.068 g, 0.126 mmol). Yellowish solid, yield 0.207 g (75%), m.p. 263 °C. IR (ATR): νmax = 2951, 1747, 1533, 1331, 827, 731 cm-1. 1H NMR (400 MHz, CDCl3): δ = 9.13 (d, J = 7.2 Hz, 1 H), 8.65 (d, J = 9.4 Hz, 1 H), 7.95 (d, J = 9.4 Hz, 1 H), 7.94 (d, J = 7.2 Hz, 1 H), 7.81–7.75 (m, 2 H), 7.02 (s, 1 H, CHCl2), 4.19 (s, 2 H, SCH2), 3.77 (s, 3 H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ = 169.1 (o, 1C, C=O), 148.9 (Cq), 148.0 (Cq), 139.1 (Cq 135.8 (Cq), 134.3 (Cq), 130.4 (Cq), 130.3 (CH), 128.9 (CH), 128.0 (2C, CH), 125.5 (CH), 122.3 (CH), 199.7 (Cq), 63.3 (CHCl2), 52.9 (CH3), 33.6 (SCH2) ppm. EIMS: m/z (%) = 410 (55) [M]+, 304 (40) [M - SCH2COOCH3]+, 260 (15) [M - SCH2COOCH3 - NO2]+, 252 (100) [M - SCH2COOCH3 - C4H4]+. HRMS (ESI): m/z calcd. for C17H12Cl2N2O4SNa [M + Na]+ 432.9787; found 432.9793.
Ethyl {[4-(dichloromethyl)-3-nitronaphtho[2,3-h]chinolin-2-yl]sulfanyl}acetate (6). A solution of ethyl {[(1E)-1,3,4,4-tetrachloro-2-nitrobuta-1,3-dien-1-yl]sulfanyl}acetate (3a) (0.500 g, 1.4 mmol) and 1-aminoanthracene (0.270 g, 1.40 mmol) in anhydrous THF (20 mL) was stirred at rt for 16 h. Subsequently, triethylamine (0.141 g, 1.4 mmol) was added, the mixture stirred at rt for 1 d and the solvent removed in vacuo. During addition of methanol (2 mL) a solid was precipitating and filtered off with suction, washed with aqueous HCl (18%, 20 mL), water (20 mL), and cold (methanol (25 mL). The yellowish solid was dried in vacuo. Yield 0.377 g (54%), m.p. 224 °C. IR (ATR): νmax = 1734, 1548, 1533, 1337, 1149, 987 cm-1. 1H NMR (400 MHz, CDCl3): δ = 9.56 (s, 1 H), 8.60 (s, 1 H), 8.38 (d, J = 9.5 Hz, 1 H), 8.20–8.16 (m, 3 H), 8.06 (s, 1 H, CHCl2), 7.72–7.69 (m, 2 H), 4.45 (s, 2 H, SCH2), 4.09 (q, J = 14.2, 7.1 Hz, 2 H, OCH2), 1.06 (t, J = 7.1 Hz, 3 H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ = 168.8 (C=O), 149.1 (Cq), 148.4 (Cq), 139.6 (Cq), 135.2 (Cq), 133.5 (Cq), 131.9 (Cq), 130.8 (Cq), 130.0 (CH), 129.0 (CH), 128.2 (CH), 127.9 (CH), 127.4 (Cq), 127.2 (2C, CH), 125.8 (CH), 120.6 (CH), 119.5 (Cq), 64.3 (CHCl2), 61.5 (OCH2), 33.6 (SCH2), 14.2 (CH3) ppm. EIMS: m/z (%) = 474 (77) [M]+, 429 (7) [M - OEt]+, 386 (10) [M - CH3COOEt]+, 355 (60) [M - SCH2COOEt]+, 302 (100) [M - CH3COOEt - CHCl2]+. HRMS (ESI): m/z calcd. for C22H16Cl2N2O4SNa [M + Na]+ 497.0100; found 497.0106.
(2Z)-3-(3,5-dimethylphenyl)-2-(2,3,3-trichloro-1-nitroprop-2-en-1-yliden)-1,3-thiazolidin-4-one (7a). A mixture of ethyl {[(1E) -1,3,4,4-tetrachloro-2-nitrobuta-1,3-dien-1-yl]sulfanyl}acetate (3a) (0.500 g, 1.4 mmol) and 4-(benzyloxy)aniline (0.350 g, 2.9 mmol) in methanol (2.5 mL) was stirred for 7 d at rt while the product was precipitating. It was filtered off with suction, washed with cold methanol (25 mL) and dried in vacuo. Beige solid, yield 0.415 g (76%), m.p. 240 °C. IR (ATR): νmax = 2983, 1756, 1595, 1369, 1287, 679 cm-1. 1H NMR (400 MHz, [D6]DMSO): δ = 7.14 (s, 1 H), 6.99 (s, 2 H), 4.16 (q, 2 H, J = 18.4 Hz, CH2), 2.31 (s, 6 H, CH3) ppm. 13C NMR (100 MHz, [D6]DMSO): δ = 173.7 (C=O), 165.7 (SCN), 138.7 (Cq), 138.4 (Cq), 134.0 (Cq), 131.7 (CH), 128.2 (CCl), 126.2 (CH), 124.6 (CH), 121.1 (CNO2), 120.9 (CCl2), 32.4 (CH2), 20.5 (2C, CH3) ppm. EIMS: m/z (%) = 391 (3) [M+], 356 (7) [M - Cl]+, 264 (30) [M - NO2 - CCl2]+, 247 (100) [M - NO2 - 2 CH3 - 2 Cl]+, 228 (25) [M - NO2 - CCl2 - Cl]+, 202 (70) [M - CCl2 - Ph(CH3)2]+, 104 (60) [Ph(CH3)2]+. HRMS (ESI): m/z calcd. for C14H11Cl3N2O3SNa [M + Na]+ 414.9448; found 414.9453.
(2Z)-3-[4-(benzyloxy)phenyl]-2-(2,3,3-trichloro-1-nitroprop-2-en-1-yliden)-1,3-thiazolidin-4-one (7b). The product was prepared according to thiazolidin-4-one 7a from acetate 3a (1.00 g, 2.80 mmol), 4-(benzyloxy)aniline hydrochloride (1.39 g, 7.02 mmol) and sodium hydroxide (0.28 g, 7.02 mmol) in methanol (10 mL) and stirred for 7 d at rt. Beige solid, yield 1.174 g (89%), m.p. 201 °C. IR (ATR): νmax = 2923, 1754, 1523, 1363, 1229, 743 cm-1. 1H NMR (400 MHz, [D6]DMSO): δ = 7.46 (d, J = 6.8 Hz, 2 H), 7.40 (d, J = 6.8 Hz, 1 H), 7.30 (d, J = 6.8 Hz, 1 H), 7.33 (t, J = 6.8 Hz, 1 H), 7.31 (d, J = 7.6 Hz, 2 H), 7.12–7.09 (m, 2 H), 5.17 (d, J = 8.2 Hz, 2 H, OCH2), 4.13 (q, J = 18.3 Hz, 2 H, SCH2) ppm. 13C NMR (100 MHz, [D6]DMSO): δ = 174.5 (C=O), 166.3 (SCN), 159.8 (Cq), 137.2 (Cq), 130.5 (CH), 129.1 (CH), 128.9 (2C, CH), 128.5 (Cq), 128.4 (CH), 128.1 (2C, CH), 127.4 (CCl), 121.7 (2C, CNO2, CCl2), 115.7 (CH), 115.6 (CH), 69.9 (OCH2), 32.8 (SCH2) ppm. EIMS: m/z (%) = 469 (10) [M+], 435 (20) [M - Cl]+, 378 (45) [M - Bz]+, 326 (50) [M - OBz - Cl]+, 313 (100) [M - OBz - NO2]+. HRMS (ESI): m/z calcd. for C19H13Cl3N2O4S [M+] 469.9656; found 469.9583.
(2Z)-3-(quinolin-8-yl)-2-(2,3,3-trichloro-1-nitroprop-2-en-1-yliden)-1,3-thiazolidin-4-one (7c). The product was synthesized according to thiazolidin-4-one 7a from acetate 3a (0.500 g, 1.40 mmol) and quinoline-8-amine (0.60 g, 4.20 mmol) in methanol (10 mL) by stirring for 6 h at rt. Orange solid, mixture of two rotamers, ratio (1: 0.9). Yield 0.404 g (70%), m.p. 197 °C. IR (ATR): νmax = 2978, 1747, 1519, 1374, 1285, 754 cm-1. Major Isomer: 1H NMR (600 MHz, [D6]DMSO): δ = 8.92 (dd, J = 4.2, 1.6 Hz, 1 H), 8.54 (ddd, J = 9.5, 8.2, 1.4 Hz, 1 H), 8.22 (dd, J = 8.2, 1.3 Hz, 1 H), 7.95 (dd, J = 7.3, 1.3 Hz, 1 H), 7.80 (dd, J = 7.4, 4.0 Hz, 1 H), 7.66 (dd, J = 8.3, 4.2 Hz, 1 H), 4.46 (q, J = 18.7 Hz, 2 H, CH2) ppm. 13C NMR (150 MHz, [D6]DMSO): δ = 174.0 (C=O), 166.2 (NCS), 151.6 (CH), 142.6 (Cq), 136.8 (CH), 131.2 (Cq), 131.1 (CH), 129.2 (Cq), 129.1 (CH), 127.8 (CCl), 126.2 (CH), 122.8 (CH), 121.3 (CNO2), 120.9 (CCl2), 32.1 (CH2) ppm. Minor Isomer: 1H NMR (600 MHz, [D6]DMSO): δ = 8.90 (dd, J = 8.2, 1.6 Hz, 1 H), 8.54 (ddd, J = 9.5, 8.2, 1.4 Hz, 1 H), 8.18 (dd, J = 8.2, 1.3 Hz, 1 H), 8.00 (dd, J = 7.3, 1.3 Hz, 1 H), 7.76 (dd, J = 7.4, 4.2 Hz, 1 H), 7.66 (dd, J = 8.3, 4.2 Hz, 1 H), 4.35 (q, J = 18.7 Hz, 2 H, CH2) ppm. 13C NMR (150 MHz, [D6]DMSO): δ = 174.0 (C=O), 166.8 (NCS), 151.6 (CH), 143.0 (Cq), 136.7 (CH), 131.3 (Cq), 131.2 (CH), 129.3 (Cq), 129.1 (CH), 128.7 (CCl), 126.2 (CH), 122.7 (CH), 121.6 (CNO2), 120.2 (CCl2), 32.3 (CH2) ppm. EIMS: m/z (%) = 414 (1) [M+], 369 (100) [M - NO2]+, 299 (15) [M - NO2 - Cl - Cl]+, 228 (15) [M - C3NO2Cl3]+, 128 (40) [quinoline]+. HRMS (ESI): m/z calcd. for C15H8Cl3N3O3S [M]+ 414.9346; found 414.9356.
Ethyl {[4-(dichloromethyl)-3-nitrobenzo[h]quinolin-2-yl]sulfinyl}acetate (8). Ethyl {[4-dichloromethyl-3-nitrobenzo[h]quinolin-2-yl]sulfanyl}acetate (5a) (1.00 g, 2.350 mmol) was suspended in a mixture of chloroform (10 mL) and glacial acetic acid (10 mL). Aqueous hydrogen peroxide solution (35%, 1.5 mL) was added and the mixture stirred at rt for 2 d. Then ice (50 g) was added, the reaction solution stirred for an additional 5 min, followed by extraction with chloroform (3 × 60 mL). The organic phase was washed with water (2 × 50 mL) and dried (sodium sulfate). After evaporation of the solvent the obtained solid was purified by column chromatography (petroleum ether/ethyl acetate, 2:1) and finally dried in vacuo. Yellowish solid, yield 0.921 g (89%), m.p. 90 °C. IR (ATR): νmax = 2987, 1723, 1538, 1334, 1215, 775 cm-1. 1H NMR (400 MHz, [D6]DMSO): δ = 9.24 (d, J = 7.4 Hz, 1 H), 8.71 (d, J = 9.4 Hz, 1 H), 8.43 (d, J = 9.4 Hz, 1 H), 8.22 (d, J = 7.4 Hz, 1 H), 8.14 (s, 1 H, CHCl2), 8.00–7.92 (m, 2 H), 4.64 (d, J = 14.2 Hz, 1 H, SCH2), 4.41 (d, J = 14.2 Hz, 1 H, SCH2), 4.08 (q, J = 7.1 Hz, 2 H, OCH2), 1.06 (t, J = 7.1 Hz, 3 H, CH3) ppm. 13C NMR (100 MHz, [D6]DMSO): δ = 165.2 (C=O), 153.3 (Cq), 146.7 (Cq), 139.2 (Cq), 136.1 (Cq), 133.6 (Cq), 132.0 (CH), 131.1 (CH), 129.7 (Cq), 128.9 (CH), 128.5 (CH), 125.2 (CH), 122.9 (Cq), 121.5 (CH), 63.7 (CHCl2), 61.5 (OCH2), 57.9 (SCH2), 13.7 (CH3) ppm. EIMS: m/z (%) = 440 (12) [M+], 323 (75) [M - NO2 - Cl - Cl]+, 304 (20) [M - SOCH2COOEt]+, 226 (75) [M - NO2 - Cl - SOCH2COOEt]+, 191 (100) [M - NO2 - Cl - Cl - SOCH2COOEt]+. HRMS (ESI): m/z calcd. for C18H15Cl2N2O5S [M + H]+ 441.0073; found 441.0076.
Ethyl {[4-(dichloromethyl)-3-nitrobenzo[h]quinolin-2-yl]sulfonyl}acetate (9). Ethyl {[4-dichloromethyl-3-nitrobenzo[h]quinolin-2-yl]sulfanyl}acetate (5a) (0.200 g, 0.47 mmol) was suspended in a mixture of chloroform (4 mL) and glacial acetic acid (4 mL). Aqueous hydrogen peroxide solution (35%, 0.3 mL) was added and the mixture stirred at 70 °C for 6 h. Then ice (50 g) was added, the reaction mixture stirred for an additional 5 min, followed by extraction with chloroform (6 × 60 mL). The organic phase was washed with water (2 × 50 mL), dried with sodium sulfate, and the solvent removed in vacuo. Yellowish solid, yield 0.212 g (99%), m.p. 154 °C. IR (ATR): νmax = 3043, 1745, 1553, 1344, 1263, 749 cm-1. 1H NMR (400 MHz, [D6]DMSO): δ = 9.18 (d, J = 8.0 Hz, 1 H), 8.64 (d, J = 9.4 Hz, 1 H), 8.46 (d, J = 9.4 Hz, 1 H), 8.18 (d, J = 8.0 Hz, 1 H), 8.06 (s, 1 H, CHCl2), 7.97–7.88 (m, 2 H), 5.35 (s, 2 H, SCH2), 3.89 (q, J = 7.1 Hz, 2 H, OCH2), 0.80 (t, J = 7.1 Hz, 3 H, CH3) ppm. 13C NMR (100 MHz, [D6]DMSO): δ = 162.5 (C=O), 145.7 (Cq), 144.9 (Cq), 137.4 (Cq), 135.9 (Cq), 133.7 (CH), 133.6 (Cq), 131.4 (CH), 129.4 (Cq), 129.3 (CH), 128.6 (CH), 125.5 (CH), 124.1 (Cq), 121.5 (CH), 63.5 (CHCl2), 61.7 (OCH2), 56.8 (SCH2), 13.4 (CH3) ppm. EIMS: m/z (%) = 456 (20) [M+], 411 (10) [M - OEt]+, 363 (100) [M - OEt - NO2]+, 318 (45) [M - CH2COOEt - NO2]+, 177 (70) [benzo[h]quinoline]+. HRMS (ESI): m/z calcd. for C18H14Cl2N2O6SNa [M + Na]+ 478.9842; found 478.9847.
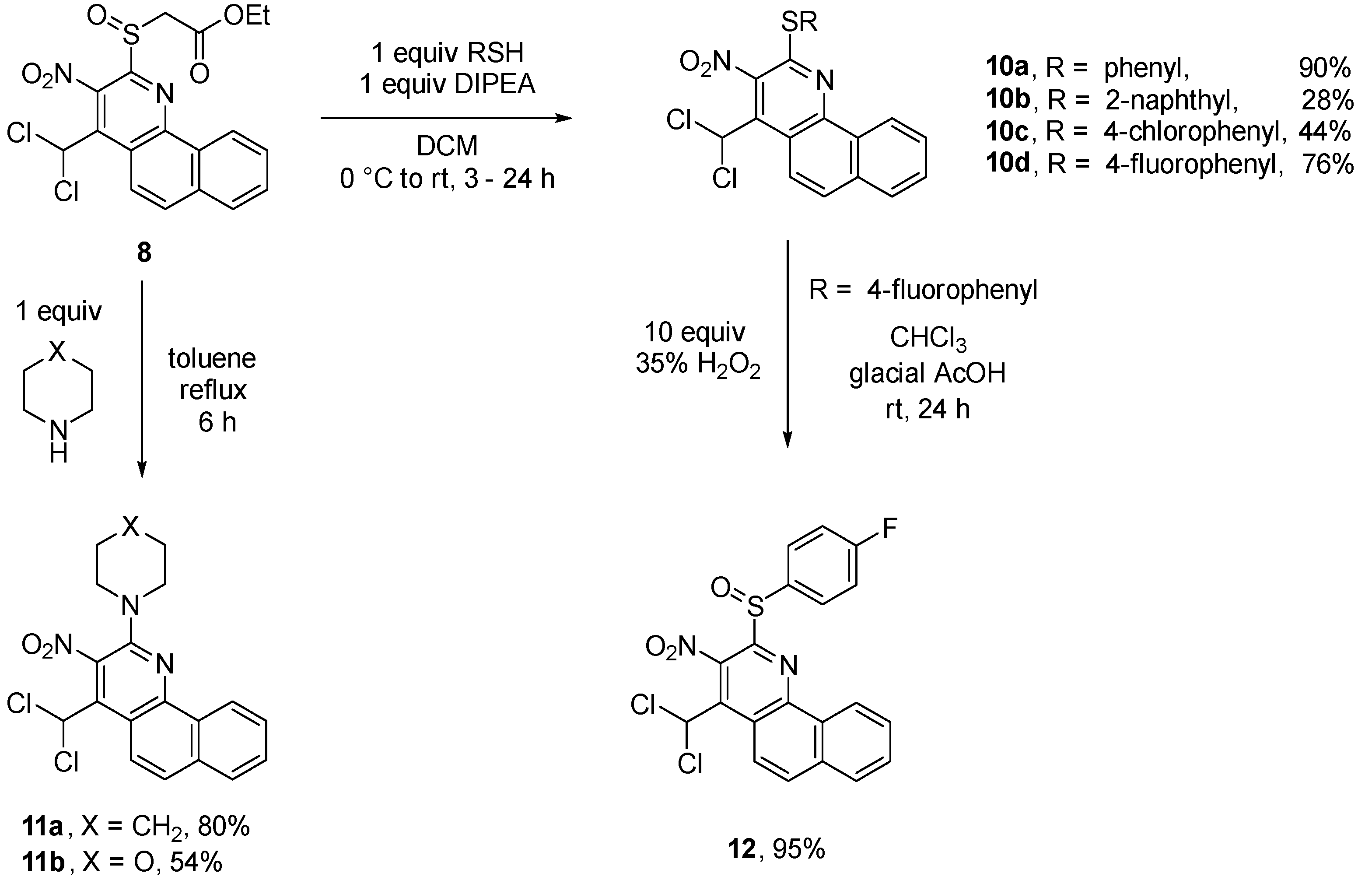
4-(Dichloromethyl)-3-nitro-2-(phenylsulfanyl)benzo[h]quinoline (10a). The product was synthesized according to benzo[h]quinoline 5a from quinoline 8 (0.200 g, 0.45 mmol), diisopropyl-N-ethylamine (0.058 g, 0.076 mmol) and benzenethiol (0.050 g, 0.45 mmol). Yellowish solid, yield 0.185 g (99%), m.p. 235 °C. IR (ATR): νmax = 3062, 1729, 1538, 1324, 1247, 714 cm-1. 1H NMR (400 MHz, CDCl3): δ = 8.59 (d, J = 9.6 Hz, 1 H), 8.28 (d, J = 8.0 Hz, 1 H), 7.89 (d, J = 9.6 Hz, 1 H), 7.85 (d, J = 8.0 Hz, 1 H), 7.71–7.68 (m, 3 H), 7.58–7.48 (m, 4 H), 7.17 (s, 1 H, CHCl2) ppm. 13C-NMR (100 MHz, CDCl3): δ = 150.5 (Cq), 147.8 (Cq), 136.3 (2C, CH), 136.2 (Cq), 135.2 (Cq), 134.0 (Cq), 130.5 (Cq), 130.0 (CH), 129.9 (CH 129.5 (2C, CH), 128.9 (CH), 128.6 (Cq), 127.8 (CH), 127.7 (CH), 125.5 (CH), 122.1 (CH), 199.6 (Cq), 63.5 (CHCl2) ppm. EIMS: m/z (%) = 414 (20) [M+], 304 (10) [M - SC6H5]+, 298 (15) [M - 2Cl - NO2]+, 186 (25) [M - SC6H5 - 2Cl - NO2]+. HRMS (MS): m/z calcd. for C20H12Cl2N2O2S [M+] 413.9991; found 413.9996.
4-(Dichloromethyl)-2-(naphth-1-ylsulfanyl)-3-nitrobenzo[h]quinoline (10b). The product was prepared according to benzo[h]quinoline 5a from quinoline 8 (0.200 g, 0.45 mmol), diisopropyl-N-ethylamine (0.058 g, 0. 076 mmol) and 2-naphthylthiol (0.072 g, 0.45 mmol). Yellowish solid, yield 0.059 g (28%), m.p. 254 °C. IR (ATR): νmax = 3053, 1538, 1323, 1220, 787, 712 cm-1. 1H-NMR (400 MHz, CDCl3): δ = 8.59 (d, J = 9.2 Hz, 1 H), 8.24 (s, 1 H), 8.15 (d, J = 8.4 Hz, 1 H), 7.97 (d, J = 8.4 Hz, 1 H), 7.96 (d, J = 8.0 Hz, 1 H), 7.89 (d, J = 9.2 Hz, 1 H), 7.87 (d, J = 9.0 Hz, 1 H), 7.81 (d, J = 8.0 Hz, 1 H), 7.69 (d, J = 9.0 Hz, 1 H), 7.64–7.56 (m, 3 H), 7.28 (t, J = 8.0 Hz, 1 H), 7.20 (s, 1 H, CHCl2) ppm. 13C-NMR (100 MHz, CDCl3): δ = 150.3 (Cq), 147.8 (Cq), 139.1 (Cq), 135.9 (CH), 135.2 (Cq), 134.0 (Cq), 133.8 (Cq), 133.6 (Cq), 132.3 (CH), 130.4 (Cq), 130.0 (CH), 128.9 (CH), 128.8 (CH), 128.1 (CH), 127.9 (CH), 127.8 (CH), 127.6 (CH), 127.4 (CH) 126.7 (CH), 126.0 (Cq), 125.5 (CH), 122.1 (CH), 199.7 (Cq), 63.5 (CHCl2) ppm. EIMS: m/z (%) = 464 (40) [M+], 365 (35) [M - NO2 - C4H4]+, 349 (15) [M - NO2 - 2Cl]+, 336 (10) [M - naphthyl]+, 191 (40) [M - NO2 - 2Cl - naphthyl]+, 127 (100) [naphthyl]+. HRMS (ESI): m/z calcd. for C24H14Cl2N2O2S [M]+ 464.0148; found 464.0153.
2-[(4-Chlorophenyl)sulfanyl]-4-(dichloromethyl)-3-nitrobenzo[h]quinoline (10c). The product was prepared according to benzo[h]quinoline 5a from quinoline 8 (0.200 g, 0.45 mmol), diisopropyl-N-ethylamine (0.058 g, 0. 076 mmol) and 4-chlorobenzenethiol (0.065 g, 0.45 mmol). Yellowish solid, yield 0.089 g (44%), m.p. 265 °C. IR (ATR): νmax = 1520, 1475, 1326, 1088, 822, 734 cm-1. 1H NMR (400 MHz, [D6]DMSO): δ = 8.52 (d, J = 9.2 Hz, 1 H), 8.18 (d, J = 7.8 Hz, 1 H), 8.16 (d, J = 9.2 Hz, 1 H), 8.10 (s, 1 H, CHCl2), 8.05 (d, J = 7.8 Hz, 1 H), 7.79 (t, J = 7.8 Hz, 1 H), 7.76 (d, J = 8.6 Hz, 2 H), 7.67 (d, J = 8.6 Hz, 2 H), 7.60 (t, J = 7.8 Hz, 1 H) ppm. 13C-NMR (100 MHz, [D6]DMSO): δ = 149.2 (Cq), 146.6 (Cq), 139.2 (Cq), 137.5 (2C, CH), 135.3 (Cq), 135.2 (Cq), 133.6 (Cq), 130.5 (CH), 129.6 (2C, CH), 129.5 (CH), 128.1 (2C, CH), 127.1 (Cq), 124.3 (CH), 121.3 (CH), 119.4 (Cq), 64.1 (CHCl2) ppm. EIMS: m/z (%) = 447 (45) [M+], 348 (45) [M - C5H4Cl]+, 304 (10) [M - SC6H4Cl]+, 235 (60) [M - SC6H4Cl - 2Cl]+, 191 (25) [M - SC6H4Cl - 2Cl - NO2]+, 177 (100) [benzo[h]quinoline]+. HRMS (ESI): m/z calcd. for C20H11Cl3N2O2S [M+] 447.9601; found 447.9607.
4-(Dichloromethyl)-2-[(4-fluorophenyl)sulfanyl]-3-nitrobenzo[h]quinoline (10d). To a solution of ethyl {[4-(dichloromethyl)-3-nitrobenzo[h]quinolin-2-yl]sulfinyl}acetate (8) (0.400 g, 0.90 mmol) in dichloromethane (10 mL) diisopropyl-N-ethylamine (0.116 g, 0.90 mmol) was added under stirring. The solution was cooled to 0 °C and treated with 4-fluorobenzenethiol (0.115 g, 0.90 mmol). The reaction mixture was stirred for 3.5 h at rt. Subsequently, the reaction mixture was acidified with aqueous HCl (18%, 1 mL), extracted with chloroform (3 × 30 mL), washed with water (1 × 50 mL) and dried with calcium chloride. After evaporation of the solvent the obtained solid was purified by column chromatography (petroleum ether/ethyl acetate, 10:1). The solvents were removed in vacuo to receive a yellowish solid, yield 0.296 g (74%), m.p. 267 °C. IR (ATR): νmax = 1522, 1488, 1328, 1220, 827, 744 cm-1. 1H NMR (600 MHz, CDCl3): δ = 8.61 (d, J = 9.3 Hz, 1 H), 8.30 (d, J = 7.9 Hz, 1 H), 7.89 (d, J = 7.9 Hz, 1 H), 7.85 (d, J = 7.9 Hz, 1 H), 7.69 (t, J = 7.9 Hz, 1 H), 7.69–7.65 (m, 2 H), 7.55 (t, J = 7.9 Hz, 1 H), 7.26–7.22 (m, 2 H), 7.17 (s, 1 H, CHCl2) ppm. 13C NMR (150 MHz, CDCl3): δ = 164.0 (CF, 1JC,F = 250.9 Hz), 150.3 (Cq), 147.8 (Cq), 139.0 (Cq), 138.5 (CH, 3JC,F = 8.3 Hz), 135.3 (Cq), 134.1 (Cq), 130.4 (Cq), 130.1 (CH), 129.0 (CH), 127.9 (CH), 127.8 (CH), 125.3 (CH), 123.9 (Cq, 4JC,F = 3.4 Hz), 122.1 (CH), 119.8 (Cq), 116.7 (CH, 2JC,F = 22.0 Hz), 63.4 (CHCl2) ppm. EIMS: m/z (%) = 432 (30) [M+], 397 (8) [M - Cl]+, 333 (30) [M - Cl - F - NO2]+, 285 (20) [M - CCl2 - F - NO2]+. HRMS (EI): m/z calcd. for C20H11Cl2FN2O2S [M+] 431.9897; found 431.9902.
4-(Dichloromethyl)-3-nitro-2-(piperidin-1-yl)benzo[h]quinoline (11a). To a solution of ethyl {[4-(dichloromethyl)-3-nitrobenzo[h]quinolin-2-yl]sulfinyl}acetate (8) (0.127 g, 0.29 mmol) in dry toluene (10 mL) piperidine (0.025 g, 0.29 mmol) was added under stirring, followed by refluxing for an additional 6 h. Subsequently, the reaction mixture was cooled to rt, acidified with aqueous HCl (18%, 1 mL), extracted with chloroform (3 × 30 mL), washed with water (1 × 50 mL) and dried with calcium chloride. After evaporation of the solvent the obtained solid was purified by column chromatography (petroleum ether/ethyl acetate, 10:1). Red solid, yield 0.091 g (80%), m.p. 236 °C. IR (ATR): νmax = 2930, 2832, 1582, 1527, 1332, 1234, 727 cm-1. 1H NMR (400 MHz, CDCl3): δ = 9.07 (d, J = 7.6 Hz, 1 H), 8.54 (d, J = 9.2 Hz, 1 H), 7.87 (d, J = 7.6 Hz, 1 H), 7.76 (d, J = 9.2 Hz, 1 H), 7.73–7.65 (m, 2 H), 7.08 (s, 1 H, CHCl2), 3.53–3.50 (m, 4 H) 1.77–1.67 (m, 6 H) ppm. 13C NMR (100 MHz, CDCl3): δ = 149.5 (Cq), 146.9 (Cq), 135.9 (Cq), 134.3 (Cq), 134.0 (Cq), 130.5 (Cq), 129.4 (CH), 127.8 (CH), 127.0 (CH), 125.7 (CH), 125.4 (CH), 122.6 (CH), 116.0 (Cq), 63.8 (CHCl2), 49.9 (2C, CH2), 25.7 (2C, CH2), 24.4 (CH2) ppm. EIMS: m/z (%) = 389 (100) [M+], 318 (60) [M - 2Cl]+, 304 (40) [M - piperidine]+. HRMS (MS): m/z calcd. for C19H17Cl2N3O2 [M]+ 389.0692; found 389.0699.
4-(Dichloromethyl)-2-(morpholin-4-yl)-3-nitrobenzo[h]quinoline (11b). The product was prepared according to benzo[h]quinoline 11a from quinoline 8 (0.200 g, 0.45 mmol) and morpholine (0.039 g, 0.45 mmol). The reaction mixture was stirred for 8 h at 70 °C. Purification by column chromatography (petroleum ether/ethyl acetate, 10:1) led to a red solid, yield 0.095 g (54%), m.p. 233 °C. IR (ATR): νmax = 2960, 2857, 1587, 1525, 1352, 1227, 734 cm-1. 1H NMR (400 MHz, [D6]DMSO): δ = 9.02 (d, J = 7.8 Hz, 1 H), 8.47 (d, J = 9.3 Hz, 1 H), 8.05 (d, J = 7.8 Hz, 1 H), 8.02 (d, J = 9.3 Hz, 1 H), 7.84–7.59 (m, 2 H), 7.83 (s, 1 H, CHCl2), 3.77 (t, J = 4.5 Hz, 4 H, CH2), 3.47 (t, J = 4.5 Hz, 4 H, CH2) ppm. 13C-NMR (100 MHz, [D6]DMSO): δ = 148.6 (Cq), 145.6 (Cq), 135.8 (Cq), 133.9 (Cq), 133.7 (Cq), 129.9 (CH), 129.4 (Cq), 128.0 (CH), 127.6 (CH), 126.5 (CH), 124.9 (CH), 121.7 (CH), 116.0 (Cq), 65.8 (2C, CH2), 64.4 (CHCl2), 48.7 (2C, CH2) ppm. EIMS: m/z (%) = 391 (100) [M+], 357 (10) [M - Cl]+, 321 (20) [M - 2Cl]+, 305 (40) [M - morpholine]+, 177 (65) [benzo[h]quinoline]+. HRMS (ESI): m/z calcd. for C18H15Cl2N3O3 [M]+ 391.0485; found 391.0490.
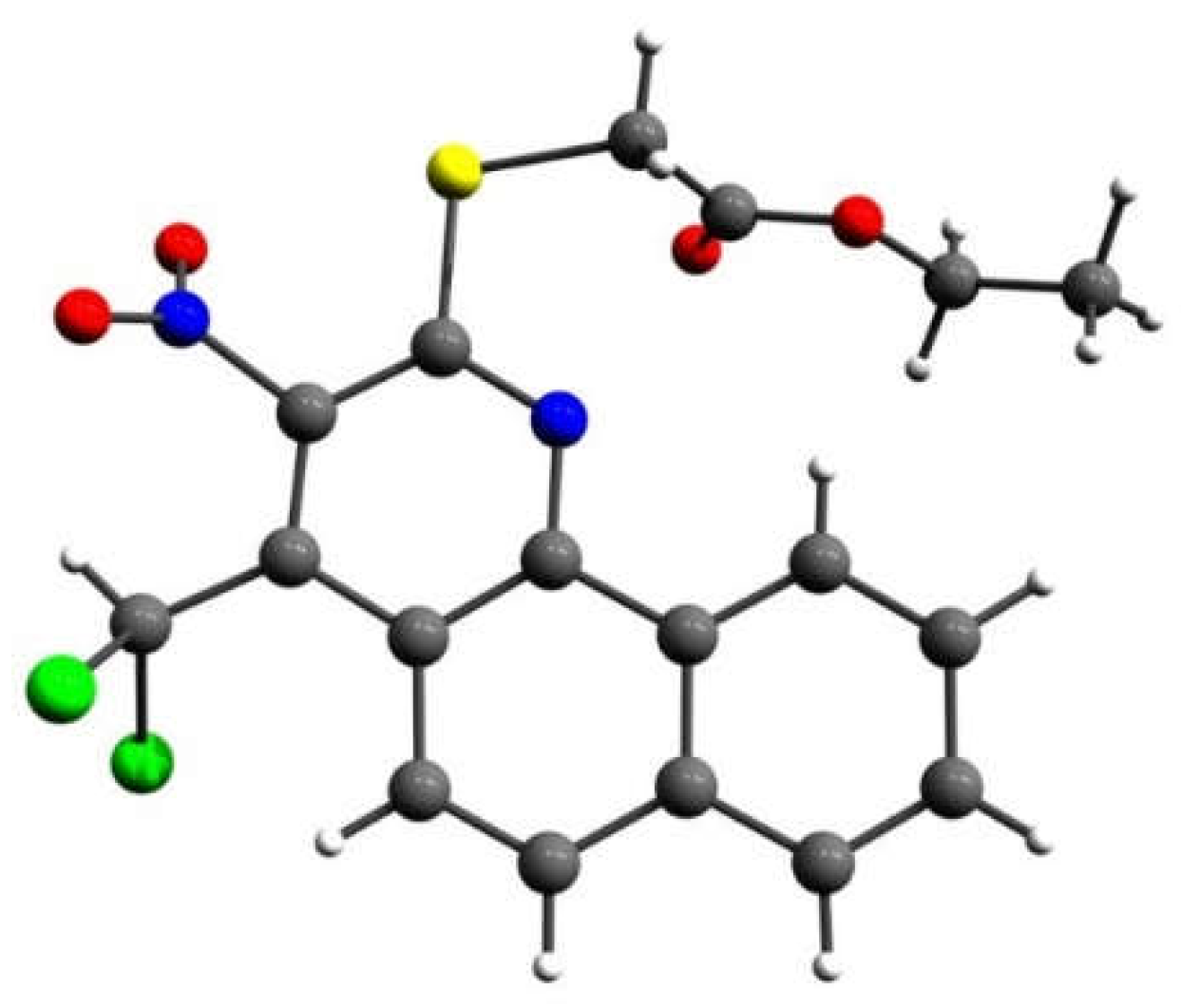
4-(Dichloromethyl)-2-[(4-fluorophenyl)sulfinyl]-3-nitrobenzo[h]quinoline (12). 4-(Dichloromethyl)-2-[(4-fluorophenyl)sulfanyl]-3-nitrobenzo[h]quinoline (10d) (0.200 g, 0.45 mmol) was dissolved in a mixture of chloroform (5 mL) and glacial acetic acid (5 mL). Under ice cooling aqueous hydrogen peroxide solution (35%, 0.3 mL) was added, followed by stirring for an additional 2 d at rt. Subsequently, ice (50 g) was added, the reaction mixture stirred for 5 min, extracted with chloroform (5 × 60 mL), the organic phase washed with water (2 × 50 mL) and dried (sodium sulfate). After evaporation of the solvent the obtained solid was purified by column chromatography (petroleum ether/ethyl acetate, 2:1). Yellowish solid, yield 0.196 g (95%), m.p. 187 °C. IR (ATR): νmax = 1585, 1542, 1353, 1223, 1090, 754 cm-1. 1H NMR (400 MHz, CDCl3): δ = 9.29 (d, J = 9.2 Hz, 1 H), 8.71 (d, J = 9.2 Hz, 1 H), 8.11 (d, J = 9.2 Hz, 1 H), 8.05 (d, J = 8.8 Hz, 1 H), 8.04 (d, J = 8.8 Hz, 1 H), 7.99–7.96 (m, 1 H), 7.87–7.85 (m, 2 H), 7.24–7.20 (m, 3 H) ppm. 13C NMR (100 MHz, CDCl3): δ = 164.9 (CF, 1JC,F = 253.3 Hz), 153.7 (Cq), 148.2 (Cq), 138.2 (Cq, 3JC,F = 8.2 Hz), 138.1 (Cq), 136.0 (Cq), 134.0 (Cq), 131.9 (CHTh), 131.0 (CHTh), 130.6 (Cq), 128.8 (CH), 128.3 (CH), 128.2 (2 C, CH), 125.8 (CH), 123.3 (Cq, 4JC,F = 3.0 Hz), 122.0 (CH), 116.8 (CH, 2JC,F = 22.6 Hz), 62.5 (CHCl2) ppm. EIMS: m/z (%) = 446 (3) [M+], 403 (10) [M - NO2]+, 368 (20) [M - NO2 - Cl]+, 333 (8) [M - NO2 - 2Cl]+, 143 (100) [SOPhF]+. HRMS (ESI): m/z calcd. for C20H11Cl2FN2O3SNa [M + Na]+ 470.9744; found 470.9749.
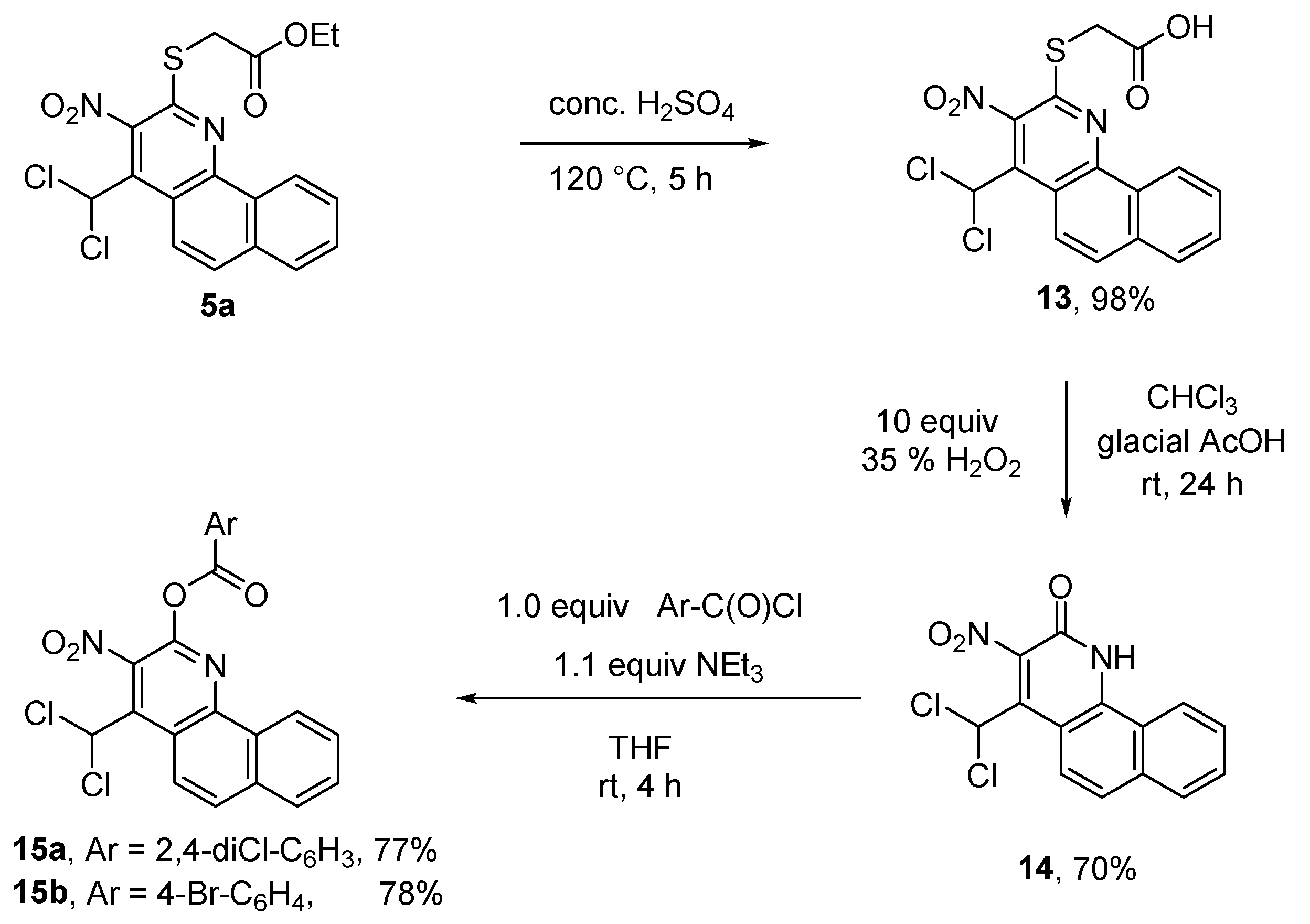
{[4-(Dichloromethyl)-3-nitrobenzo[h]quinolin-2-yl]sulfanyl}acetic acid (13). Ethyl {[4-(dichloromethyl)-3-nitrobenzo[h]quinolin-2-yl]sulfanyl}acetate (5a) (1.500 g, 3.527 mmol) was suspended in conc. sulfuric acid (25 mL) and stirred at 90 ° C for 5 h. Subsequently, ice-water (100 mL) was added under stirring. The reaction mixture was extracted with chloroform (4 × 50 mL) and washed with water (3 × 70 mL). The organic phase was dried with sodium sulfate and the solvent removed in vacuo. Yellowish solid, yield 1.379 g (99%), m.p. 204 °C. IR (ATR): νmax = 2119, 1705, 1524, 1359, 1221, 968, 751 cm-1. 1H NMR (600 MHz, [D6]DMSO): δ = 13.05 (bs, 1 H, OH), 9.13 (d, J = 8.0 Hz, 1 H), 8.52 (d, J = 9.6 Hz, 1 H), 8.17 (d, J = 8.0 Hz, 1 H), 8.09 (d, J = 8.0 Hz, 1 H), 8.07 (s, 1 H, CHCl2), 7.87 (d, J = 8.0 Hz, 1 H), 7.80 (d, J = 8.0 Hz, 1 H), 4.29 (s, 2 H, SCH2) ppm. 13C NMR (150 MHz, [D6]DMSO): δ = 170.1 (C=O), 149.6 (Cq), 147.2 (Cq), 139.8 (Cq), 135.3 (Cq) 134.2 (Cq), 130.9 (CH), 129.9 (Cq), 129.6 (CH), 128.7 (CH), 128.6 (CH), 125.8 (CH), 121.9 (CH), 119.2 (Cq), 64.4 (CHCl2), 34.0 (SCH2) ppm. EIMS: m/z (%) = 396 (20) [M+], 303 (25) [M - SCH2COOH]+, 252 (100) [M - SCH2COOH - C4H4]+, 177 (30) [benzo[h]quinoline]+. HRMS (ESI): m/z calcd. for C16H11Cl2N2O4S [M + H]+ 396.9811; found 396.9813.
4-(Dichloromethyl)-3-nitrobenzo[h]quinolin-2(1H)-one (14). To a suspension of {[4-(dichloromethyl)-3-nitrobenzo[h]quinolin-2-yl]sulfanyl}acetic acid (13) (0.840 g, 2.120 mmol) in a mixture of chloroform (15 mL) and glacial acetic acid (15 mL) aqueous hydrogen peroxide solution (35%, 1.2 mL) was added and stirred at rt for 2 d. Subsequently, water (50 mL) was added and the reaction mixture stirred for an additional 30 min. After extraction with chloroform (4 × 50 mL) the organic phase was dried with sodium sulfate and the solvent removed in vacuo. Yellowish solid, yield 0.475 g (70%), m.p. 286 °C. IR (ATR): νmax = 2923, 1654, 1537, 1350, 1216, 736 cm-1. 1H NMR (400 MHz, [D6]DMSO): δ = 8.90 (d, J = 8.0 Hz, 1 H), 8.33 (d, J = 9.2 Hz, 1 H), 8.01 (d, J = 8.0 Hz, 1 H), 7.88 (s, 1 H, CHCl2), 7.86 (d, J = 8.0 Hz, 1 H), 7.76 (t, J = 8.0 Hz, 1 H), 7.69 (t, J = 8.0 Hz, 1 H) ppm. 13C-NMR (100 MHz, [D6]DMSO): δ = 153.9 (Cq), 136.5 (Cq), 134.0 (Cq), 131.3 (Cq), 130.0 (Cq), 129.7 (CH), 128.4 (CH), 127.4 (CH), 123.9 (Cq), 123.8 (CH), 123.2 (CH), 121.9 (CH), 119.0 (Cq), 64.0 (CHCl2) ppm. EIMS: m/z (%) = 322 (100) [M+], 252 (25) [M - 2Cl]+, 229 (75) [M - Cl - NO2 - O]+, 207 (20) [M - 2Cl - NO2]+, 177 (60) [benzo[h]quinoline]+. HRMS (ESI): m/z calcd. for C14H8Cl2N2O3Na [M + Na]+ 344.9804; found 344.9816.
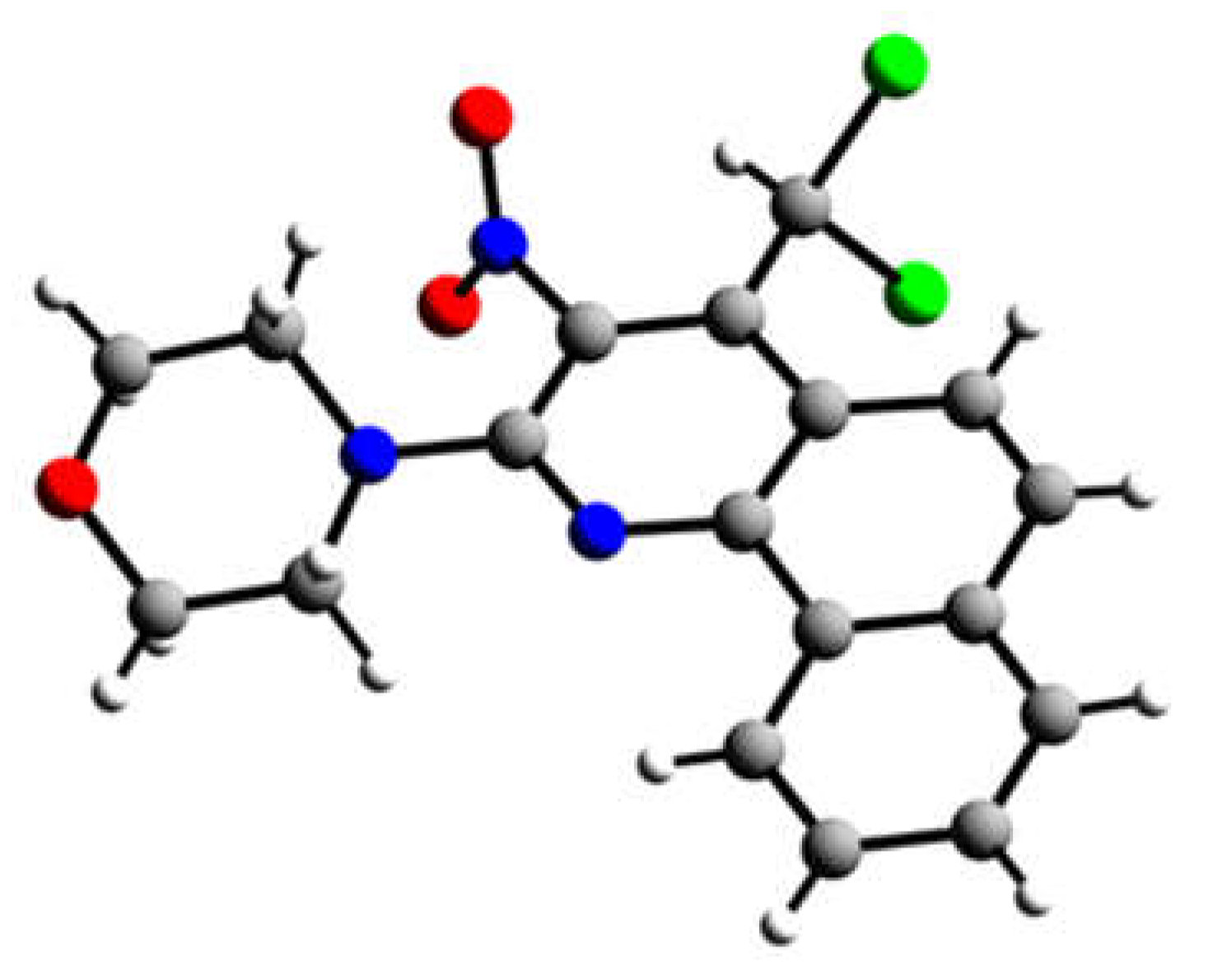
4-(Dichloromethyl)-3-nitrobenzo[h]quinolin-2-yl 2,4-dichlorobenzoate (15a). To a solution of 4-(dichloromethyl)-3-nitrobenzo[h]quinolin-2(1H)-one (14) (0.086 g, 0.269 mmol) and 2,4-dichlorobenzoyl chloride (0.056 g, 0.269 mmol) in anhydrous THF (5 mL) under nitrogen atmosphere triethylamine (0.029 g, 0.290 mmol) was added at rt and stirred for 1 d. Subsequently, the reaction mixture was diluted with ice-water (30 mL) at stirring, extracted with chloroform (3 × 30 mL), washed with water (1 × 50 mL), saturated aqueous NaHCO3 solution (10 mL) and water (30 mL). The organic phase was dried with sodium sulfate, the solvent removed in vacuo, and the residue purified by column chromatography (petroleum ether/ethyl acetate, 3:1). Yellowish solid, yield 0.100 g (77%), m.p. 177 °C. IR (ATR): νmax = 2956, 1757, 1535, 1335, 1214, 1150, 732 cm-1. 1H NMR (400 MHz, CDCl3): δ = 9.09 (d, J = 7.8 Hz, 1 H), 8.64 (d, J = 9.4 Hz, 1 H), 8.05 (d, J = 8.5 Hz, 1 H), 8.02 (d, J = 9.4 Hz, 1 H), 7.92 (d, J = 7.8 Hz, 1 H), 7.76 (t, J = 7.8 Hz, 1 H), 7.70 (t, J = 7.8 Hz, 1 H), 7.54 (s, 1 H), 7.36 (d, J = 8.5 Hz, 1 H), 7.13 (s, 1 H, CHCl2) ppm. 13C NMR (100 MHz, CDCl3): δ = 161.0 (C=O), 147.1 (Cq), 145.4 (Cq), 140.5 (Cq), 137.7 (Cq), 136.8 (Cq), 134.1 (Cq), 133.7 (CH), 131.8 (CH), 130.6 (Cq), 130.6 (CH), 130.5 (Cq), 130.2 (CH), 128.2 (CH), 128.0 (CH), 127.5 (CH), 126.0 (CH), 125.3 (Cq), 121.9 (CH), 121.8 (Cq), 62.8 (CHCl2) ppm. EIMS: m/z (%) =493 (3) [M+], 172 (100) [COC6H3Cl2]+. HRMS (ESI): C21H10Cl4N2O4: m/z calcd. for [M+] 493.9389; found 493.9397.
4-(Dichloromethyl)-3-nitrobenzo[h]quinolin-2-yl 4-bromobenzoate (15b). The product was prepared according to benzo[h]quinoline 15a from quinoline 14 (0.100 g, 0.31 mmol), 4-bromobenzoyl chloride (0.068 g, 0.31 mmol) and triethylamine (0.034 g, 0.34 mmol). The reaction mixture was stirred for 4 h. Yellow solid, yield 0.122 g (78%), m.p. 239 °C. IR (ATR): νmax = 2924, 1754, 1558, 1508, 1398, 1227, 1056, 752 cm-1. 1H NMR (400 MHz, CDCl3): δ = 9.14 (d, J = 8.0 Hz, 1 H), 8.71 (d, J = 9.3 Hz, 1 H), 8.08 (d, J = 9.3 Hz, 1 H), 8.07 (d, J = 8.5 Hz, 2 H), 7.98 (d, J = 8.0 Hz, 1 H), 7.82 (t, J = 8.0 Hz, 1 H), 7.76 (t, J = 8.0 Hz, 1 H), 7.71 (d, J = 8.5 Hz, 2 H), 7.20 (s, 1 H, CHCl2) ppm. 13C NMR (100 MHz, CDCl3): δ = 163.0 (C=O), 147.1 (Cq), 145.7 (Cq), 137.6 (Cq), 134.1 (Cq), 132.4 (Cq), 132.3 (2C, CH), 132.2 (2C, CH), 130.6 (CH), 130.5 (Cq), 130.2 (Cq), 130.1 (CH), 128.2 (CH), 128.0 (CH), 126.7 (Cq , 126.0 (CH), 121.9 (CH), 121.7 (Cq), 62.8 (CHCl2) ppm. EIMS: m/z (%) = 505 (75) [M+], 392 (100) [M - Cl - Br]+. HRMS (MS): m/z calcd. for C21H11Cl2N2O4Br [M+] 503.9274; found 503.9280.
1,1-Bis(benzotriazol-1-yl)-2-nitro-3,4,4-trichlorobuta-1,3-diene (
16a). The product was prepared according to the published literature [
20] from the nitrodiene
1 and 1
H-benzotriazole. Yield: 76%. All spectral data were in accordance with the literature.
1,1-Bis(1H-1,2,4-triazol-1-yl)-2-nitro-3,4,4-trichlorobuta-1,3-diene (
16b). The product was synthesized according to a previously published procedure [
21] from nitrodiene
1 and 1
H-1,2,4-triazole. Yield: 92%. All spectral data were in accordance with the literature.
1,1-Bis(1H-pyrazol-1-yl)-2-nitro-3,4,4-trichlorobuta-1,3-diene (16c). To a solution of 2.72 g (40.00 mmol) of 1H-pyrazole in diethyl ether (50 mL) at 0 °C was added a solution of nitrodiene 1 (2.71 g, 10.00 mmol) in ether (5 mL). The resulting mixture was stirred at 0 °C for 1 h and at rt for an additional 20 h. The solvent was removed and then cold water (50 mL) was added with stirring. The resulting precipitate was filtered off with suction, washed with water (2 × 20 mL) and cold methanol (5 mL) and dried in vacuo to give 16c. Yellow solid, yield 3.01 g (90%), m.p. 133–134 °C. IR (KBr): νmax = 3102, 1653, 1532 (NO2), 1394, 1314 (NO2), 957, 772 cm-1. 1H NMR (400 MHz, CDCl3): δ = 7.93 (d, J = 1.3 Hz, 1 H), 7.87 (d, J = 1.3 Hz, 1 H), 7.56 (d, J = 3.0 Hz, 1 H), 7.51 (d, J = 2.8 Hz, 1 H), 6.63–6.58 (m, 2 H) ppm. 13C NMR (100 MHz, CDCl3): δ = 146.0 (CH), 145.4 (CH), 138.3 (C1), 132.1 (CH), 131.8 (CH), 129.7 (C-NO2), 129.0 and 121.1 (C2Cl3), 111.0 (2 CH) ppm. EIMS: m/z (%) = 333 (2) [M+], 298 (100) [M - Cl]+, 263 (10) [M - 2Cl], 252 (37) [M - Cl - NO2]+, 217 (55) [M - 2Cl - NO2]+. HRMS (ESI): m/z calcd. for C10H6Cl3N5O2Na [M + Na]+ 355.9479; found 355.9485.
(E)-1-(1H-Benzotriazol-1-yl)-1-(naphth-1-ylamino)-3,4,4-trichloro-2-nitrobuta-1,3-diene (
17a). The product was obtained according to a previously published procedure [
22] from bisazole
16a and 1-naphthylamine. Yield: 90%. All spectral data were in accordance with the literature.
(E)-1-(1H-1,2,4-Triazol-1-yl)-1-(naphth-1-ylamino)-3,4,4-trichloro-2-nitrobuta-1,3-diene (17b). 1-Naphthylamine (1.50 g, 10.5 mmol) was added to a suspension of the bisazole 16b (3.37 g, 10.0 mmol) in MeOH (40 mL) at 0 °C within 2 min. The resulting mixture was stirred at 0 °C for 1 h, and was then kept at rt overnight. Subsequently, the supernatant liquid was concentrated to a volume of about 15 mL, cooled to 10 °C and then treated with aqueous HCl (5%, 80 mL). The mixture was stirred for an additional 20 min. The formed precipitate was collected on a suction filter, washed with water (2 × 20 mL) and cold MeOH (1 × 10 mL), and then finally dried under reduced pressure. Yellow solid, yield: 3.53 g (86%), m.p. 114–115 °C. IR (KBr): νmax = 3129, 1620, 1575 (NO2), 1334 (NO2), 1262, 1179, 773 cm-1. 1H NMR (400 MHz, CDCl3): δ = 11.73 (br s, 1 H, NH), 8.04 (d, J = 8.3 Hz, 1 H), 8.00 (s, 1 H, CHN), 7.89 (d, J = 7.3 Hz, 1 H), 7.87 (s, 1 H, CHN), 7.78 (d, J = 8.3 Hz, 1 H), 7.72–7.55 (m, 2 H), 7.26 (t, J = 7.7 Hz, 1 H), 6.91 (d, J = 7.3 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 153.0 (CHN), 147.8 (C1), 144.6 (CHN), 134.0 (NH-Cq), 130.6 (Cq), 129.2 (CH), 128.9 (Cq), 128.8 (CH), 128.5 (Cq), 128.1 (CH), 127.3 (CH), 125.1 (CH), 122.5 (CH), 121.0 (CH), 120.6 (Cq), 118.8 (C-NO2) ppm. EIMS: m/z (%) = 409 (35) [M+], 374 (1) [M - Cl]+, 327 (6) [M - Cl - HNO2]+, 214 (24), 143 (74), 133 (100). HRMS (ESI): m/z calcd. for C16H11Cl3N5O2 [M + H]+ 409.9973; found 409.9978.
(E)-1-(1H-Pyrazol-1-yl)-1-(naphthalen-1-ylamino)-3,4,4-trichloro-2-nitrobuta-1,3-diene (17c). To a suspension of the bisazole 16c (3.35 g, 10.0 mmol) in diethyl ether (40 mL) was added 1-aminonaphthalene (1.50 g, 10.5 mmol) at - 30 °C within 5 min. The resulting mixture was stirred at - 30 °C for 4 h, then kept at rt overnight. The mixture was concentrated to a volume of about 10 mL by means of a rotary evaporator. Subsequently, after addition of cold water (200 mL), aqueous HCl (37%, 5 mL) was added dropwise. After 20 min stirring, the mixture was extracted with chloroform (3 × 70 mL). The combined organic layers were washed with brine (150 mL) and dried with calcium chloride. After evaporation of the solvent the crude product was purified by means of column chromatography (petroleum ether/ethyl acetate, 3:1). Evaporation of all solvents gave nitrodiene 17c as a light-brown solid. Yield 3.69 g (90%), m.p. 177–179 °C. IR (KBr): νmax = 3222, 1617, 1571 (NO2), 1485, 1360 (NO2), 1084, 757 cm-1. 1H NMR (400 MHz, CDCl3): δ = 11.91 (br s, 1 H, NH), 8.11 (d, J = 8.2 Hz, 1 H), 7.89 (d, J = 8.1 Hz, 1 H), 7.75 (d, J = 8.3 Hz, 1 H), 7.67 (ddd, J = 8.2, 7.2, 1.1 Hz, 1 H), 7.59 (ddd, J = 8.2, 7.0, 1.1 Hz, 1 H), 7.58 (d, J = 2.0 Hz 1 H, CH-N), 7.37 (d, J = 2.4 Hz, 1 H, CHN), 7.24 (t, J = 7.8 Hz, 1 H), 6.78 (d, J = 7.3 Hz, 1 H), 6.23 (dd, J = 2.4, 2.0 Hz, 1 H) ppm. 13C NMR (100 MHz, CDCl3): δ = 150.6 (C1), 143.3 (CHN), 134.0 (NH-Cq), 131.6 (Cq), 131.4 (CH), 128.7 (CH), 128.3 (CH), 128.2 (Cq), 127.9 (CH), 127.7 (Cq), 127.1 (CH), 125.2 (CH), 122.1 (Cq), 121.44 (CH), 121.2 (CH), 118.1 (C-NO2), 109.1 (CH) ppm. EIMS: m/z (%) = 408 (2) [M+], 372 (4) [M - HCl]+, 362 (5) [M - NO2]+, 327 (3) [M - NO2 - HCl]+, (7), 292 (4) [M - 2 Cl - NO2]+, 169 (100). HRMS (ESI): m/z calcd. for C17H11Cl3N4O2Na [M + Na]+ 430.9840; found 430.9845.
2-(1H-Benzotriazol-1-yl)-4-(dichloromethyl)-3-nitrobenzo[h]quinoline (18a). Triethylamine (202 mg, 2.00 mmol) was added to a solution of nitrodiene 17a (461 mg, 1.00 mmol) in anhydrous chloroform (20 mL) at 0 °C within 2 min. The resulting mixture was stirred at 0 °C for 1 h, then kept at rt overnight. Subsequently, the supernatant liquid was evaporated in vacuo, and MeOH (10 mL) was added to the residue. The mixture was stirred for 10 min, the formed precipitate filtered off with suction, washed successively with aqueous HCl (5%, 1 × 10 mL), water (2 × 10 mL) and MeOH (1 × 5 mL), and then dried under reduced pressure. Yellowish solid, yield: 369 mg (87%), m.p. 260–262 °C. IR (KBr): νmax = 3007, 1578, 1542 (NO2), 1459, 1359 (NO2), 1205, 741 cm-1. 1H NMR (600 MHz, [D6)DMSO): δ = 9.08 (d, J = 7.9 Hz, 1 H), 8.72 (d, J = 9.3 Hz, 1 H), 8.53 (d, J = 8.3 Hz, 1 H), 8.39 (d, J = 9.4 Hz, 1 H), 8.33 (ddd, J = 8.3, 0.8, 0.8 Hz, 1 H), 8.22 (dd, J = 7.3, 1.2 Hz, 1 H), 8.17 (br s, 1 H, CHCl2), 7.96 (ddd, J = 7.3, 7.3, 1.4 Hz, 1 H), 7.95–7.91 (m, 2H), 7.69 (ddd, J = 8.3, 7.1, 1.0 Hz, 1 H) ppm. 13C NMR (150 MHz, [D6]DMSO): δ = 146.0 (Cq), 145.5 (Cq), 137.7 (Cq), 137.5 (Cq), 134.6 (C-NO2), 134.0 (Cq), 131.8 (Cq), 131.2 (CH), 131.1 (CH), 130.7 (CH), 129.9 (Cq), 129.1 (CH), 128.7 (CH), 126.4 (CH), 125.1 (CH), 121.6 (CH), 121.5 (Cq), 120.3 (CH), 113.4 (CH), 64.1 (CHCl2) ppm. EIMS: m/z (%) = 423 (12) [M+], 395 (4) [M - N2], 349 (7) [M - N2 - NO2], 302 (20), 279 (10), 266 (10), 240 (12), 92 (100). HRMS (ESI): m/z calcd. for C20H11Cl2N5O2Na [M + Na]+ 446.0182; found 446.0187.
2-(1H-1,2,4-Triazol-1-yl)-4-(dichloromethyl)-3-nitro-benzo[h]quinoline (18b). The product was prepared according to quinoline 18a from nitrodiene 17b (411 mg, 1.00 mmol) and triethylamine (202 mg, 2.00 mmol). Beige solid, yield: 135 mg (36%), m.p. 247–249 °C. IR (KBr): νmax = 3008, 1547 (NO2), 1501, 1364 (NO2), 1198, 1009, 732 cm-1. 1H NMR (600 MHz, [D6]DMSO): δ = 9.89 (s, 1 H, 1JC,H = 222 Hz, CHN), 9.27 (d, J = 8.1 Hz, 1 H), 8.63 (d, J = 9.3 Hz, 1 H), 8.44 (s, 1 H, 1JC,H = 209 Hz, CHN), 8.32 (d, J = 9.3 Hz, 1 H), 8.15 (d, J = 8.3 Hz, 1 H), 8.05 (br s, 1 H, CHCl2), 7.92 (ddd, J = 7.8, 7.1, 1.0 Hz, 1 H), 7.87 (ddd, J = 8.1, 7.1, 1.1 Hz, 1 H) ppm. 13C NMR (150 MHz, [D6]DMSO): δ = 153.9 (CHN), 145.6 (Cq), 145.5 (CHN), 137.2 (Cq), 136.5 (Cq), 133.9 (Cq), 132.9 (C-NO2), 131.04 (CH), 130.98 (CH), 129.6 (Cq), 128.7 (CH), 128.5 (CH), 125.8 (CH), 121.6 (Cq), 121.5 (CH), 64.0 (CHCl2) ppm. EIMS: m/z (%) = 373 (100) [M+], 338 (2) [M - Cl]+, 327 (7) [M - NO2]+, 303 (20), 292 (10) [M - NO2 - Cl]+, 280 (45), 269 (15), 253 (25). HRMS (ESI): m/z calcd. for C16H10Cl2N5O2 [M + H]+ 374.0206; found 374.0211.
2-(1H-Pyrazol-1-yl)-4-(dichloromethyl)-3-nitro-benzo[h]quinoline (18c). The product was prepared according to quinoline 18a from nitrodiene 17c (410 mg, 1.00 mmol) and triethylamine (202 mg, 2.00 mmol). Light brown solid, yield 291mg (78%), m.p. 197–199 °C. IR (KBr): νmax = 3006, 1545 (NO2), 1502, 1395, 1359 (NO2), 1260, 739 cm-1. 1H NMR (400 MHz, CDCl3): δ = 9.14–9.09 (m, 1 H), 8.77 (dd, J = 2.7, 0.6 Hz, 1 H, 1JC,H = 194 Hz, CH-N), 8.69 (d, J = 9.3 Hz, 1 H), 7.99 (d, J = 9.3 Hz, 1 H), 7.96–7.92 (m, 1 H), 7.81 (dd, J = 1.6, 0.6 Hz, 1 H, CH=N), 7.79 (ddd, J = 7.2, 7.1, 1.7 Hz, 1 H), 7.79 (ddd, J = 7.3, 7.3, 1.6 Hz, 1 H), 7.11 (s, 1JC,H = 179 Hz, 1 H, CHCl2), 6.60 (dd, J = 2.7, 1.6 Hz, 1JC,H = 179 Hz, 1 H) ppm. 13C NMR (100 MHz, CDCl3): δ = 146.1 (Cq), 143.7 (CH=N), 138.3 (Cq), 136.8 (Cq), 134.0 (Cq), 132.8 (C-NO2), 130.3 (Cq), 130.2 (CH), 129.3 (CH), 129.0 (CH), 128.1 (CH), 128.0 (CH), 125.2 (CH), 122.2 (CH), 120.7 (Cq), 108.8 (CH), 63.2 (CHCl2) ppm. EIMS: m/z (%) = 372 (100) [M+], 355 (2) [M - OH]+, 326 (10) [M - NO2]+, 291 (24) [M - NO2 - Cl]+, 279 (23), 256 (25), 243 (12) [M - NO2 - CHCl2]+. HRMS (ESI): m/z calcd. for C16H10Cl2N4O2Na [M + Na]+ 395.0073; found 395.0079.
Crystal Data
X-Ray structure analysis for ethyl {[(1E)-1,3,4,4-tetrachloro-2-nitrobuta-1,3-dien-1-yl]sulfanyl}acetate C8H7Cl4NO4S (3a), M = 355.01 g mol–1: A suitable single crystal of the title compound was selected under a polarization microscope and mounted in a glass capillary (d = 0.3 mm). The crystal structure was determined by X-ray diffraction analysis using graphite monochromated Mo-K
α radiation (0.71073 Å) [T = 223(2) K], whereas the scattering intensities were collected with a single crystal diffractometer (STOE IPDS II). The crystal structure was solved by Direct Methods using SHELXS and refined using alternating cycles of least squares refinements against F
2 (SHELXL). All non-H atoms were located in Difference Fourier maps and were refined with anisotropic displacement parameters. The H positions were determined by a final Difference Fourier Synthesis [
23].
C8H7Cl4NO4S (3a) crystallized in the triclinic space group P1 (no. 2), lattice parameters a = 7.685(1) Å, b = 8.189(2) Å, c = 12.236(2) Å, α = 77.66(1) °, β = 76.20(1) °, γ = 68.33(1) °, V = 688.2(2) Å3, Z = 2, dcalc. = 1.713 g cm–3, F(000) = 356 using 2558 independent reflections and 191 parameters. R1 = 0.0568 [I > 2σ(I)], wR2 = 0.1527 [I > 2σ(I)], goodness of fit on F2 = 1.043, residual electron density 1.145 and –1.098 e Å–3. Further details of the crystal structure investigations have been deposited with the Cambridge Crystallographic Data Center, CCDC 1583680. Copies of this information may be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK (Fax: +44(1223)-336033; e-mail: fileserv@ccdc.ac.uk or http://www.ccdc.cam.ac.uk).
X-Ray structure analysis for ethyl {[4-dichloromethyl-3-nitrobenzo[h]quinolin-2-yl]sulfanyl}acetate C18H14Cl2N2O4S (5a), M = 425.27 g mol
–1: A suitable single crystal of the title compound was selected under a polarization microscope and mounted in a glass capillary (d = 0.3 mm). The crystal structure was determined by X-ray diffraction analysis using graphite monochromated Mo-K
α radiation (0.71073 Å) [T = 223(2) K], whereas the scattering intensities were collected with a single crystal diffractometer (STOE IPDS II). The crystal structure was solved by Direct Methods using SHELXS and refined using alternating cycles of least squares refinements against F
2 (SHELXL). All non-H atoms were located in Difference Fourier maps and were refined with anisotropic displacement parameters. The H positions were determined by a final Difference Fourier Synthesis [
23].
C
18H
14Cl
2N
2O
4S (
5a) crystallized in the monoclinc space group P2
1/n (no. 14), lattice parameters a = 11.504(2) Å, b = 11.520(2) Å, c = 14.139(2) Å, β = 101.17(1) °, V = 1838.3(5) Å
3, Z = 4, d
calc. = 1.537 g cm
–3, F(000) = 872 using 3257 independent reflections and 295 parameters. R1 = 0.0642 [I > 2σ(I)], wR2 = 0.1298 [I > 2σ(I)], goodness of fit on F
2 = 1.032, residual electron density 1.123 and –1.042 e Å
–3. Further details of the crystal structure investigations have been deposited with the Cambridge Crystallographic Data Center, CCDC 1583675. Copies of this information may be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK (Fax: +44(1223)-336033; e-mail: fileserv@ccdc.ac.uk or
http://www.ccdc.cam.ac.uk).
X-Ray structure analysis for 4-(dichloromethyl)-2-(morpholin-4-yl)-3-nitrobenzo[h]quinoline C18H15Cl2N3O3 (11b), M = 392.23 g mol
–1: A suitable single crystal of the title compound was selected under a polarization microscope and mounted in a glass capillary (d = 0.3 mm). The crystal structure was determined by X-ray diffraction analysis using graphite monochromated Mo-K
α radiation (0.71073 Å) [T = 223(2) K], whereas the scattering intensities were collected with a single crystal diffractometer (STOE IPDS II). The crystal structure was solved by Direct Methods using SHELXS and refined using alternating cycles of least squares refinements against F
2 (SHELXL). All non-H atoms were located in Difference Fourier maps and were refined with anisotropic displacement parameters. The H positions were determined by a final Difference Fourier Synthesis [
23].
C
18H
15Cl
2N
3O
3 (
11b) crystallized in the monoclinc space group P2
1/c (no. 14), lattice parameters a = 7.1031(9) Å, b = 22.053(2) Å, c = 11.362(1) Å, β = 99.70(1) °, V = 1754.3(4) Å
3, Z = 4, d
calc. = 1.485 g cm
–3, F(000) = 808 using 3328 independent reflections and 295 parameters. R1 = 0.0528 [I > 2σ(I)], wR2 = 0.1378 [I > 2σ(I)], goodness of fit on F
2 = 1.075, residual electron density 0.335 and –0.577 e Å
–3. Further details of the crystal structure investigations have been deposited with the Cambridge Crystallographic Data Center, CCDC 1583679. Copies of this information may be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK (Fax: +44(1223)-336033; e-mail: fileserv@ccdc.ac.uk or
http://www.ccdc.cam.ac.uk).
Antibacterial assays
Overnight cultures of the bacteria were grown aerobically at 37 °C in Müller Hinton broth with added 1% glucose and pH 7.2 for Gram-negative strains, or with Trypticase soy yeast extract medium (TSY – 30 g/l Trypticase soy broth, 3 g/L yeast extract, pH 7.2) for Gram-positive strains. The cultures were adjusted to an OD600 nm of 0.001, which resulted in a final start OD600 nm of 0.0005 in the test. 25 μL of test culture was added to 25 μL of a serial dilution of the test compounds in the appropriate medium for the different strains in accordance with standardized procedures in 384 well plates. For screening purposes, the residual absorbance in % was tested at compound concentrations of 5 and 50 µM. For selected compounds, concentration-dependent growth inhibition curves were recorded from stock solutions in DMSO at final concentrations of 100, 50, 25, 12.5, 6,25, 3.125, 1.56, 0.78, 0.39, 0.2 µM. As positive control compounds, Linezolid (both MRSA strains) Ciprofloxacin (E. faecium, E. coli), and Amikacin (P. aeruginosa) were applied. The highest DMSO concentration in the assay was 1%, which had no apparent effect on the growth of the bacteria. After an incubation time of 18 h at 37 °C under moist conditions, the optical density at 600 nm was measured with a Fusion Universal Microplate Analyzer (Perkin–Elmer, Waltham, USA). The lowest concentration that completely suppressed growth defined the MIC values. The following bacterial strains were used: Gram-negative: Escherichia coli (DSM 1116) and Pseudomonas aeruginosa PA7 (DSM 24068). Gram-positive: Staphylococcus aureus MRSA (clinical isolate, RKI 11-02670) and Staphylococcus aureus MRSA (DSM 11822). The MIC values were determined by curve fitting with Sigma Plot.
Antiproliferative assays
The effect of compounds on cell viability was probed with a WST-1 test using the procedure of Ishiyama et al. [
24] as modified by Sasse et al [
25]. The following cell lines were used: mouse fibroblast cell line L929 (DSM ACC 2), human cervix carcinoma cell line KB-3-1 (DSM ACC 158) and human breast cancer cell line MCF-7 (DSM ACC 115). In addition, the conditional immortalized human fibroblast cell line FS4-LTM (InScreenex, Braunschweig, Germany) was used without doxycyclin to induce primary cell-like behavior (Pub. No.: US2011/0189142 A2). Briefly, the subconfluent cells were washed with Earle’s Balanced Salt Solution (Gibco) without Ca and Mg, trypsinized and re-suspended in Dulbecco’s modified eagle’s medium that contained 5% fetal bovine serum (FBS; L929, KB-3-1, FS4-LTM) or Roswell Park Memorial Institute medium that contained 5% FBS, 0.5% Minimum Essential Medium Non-Essential Amino Acids, Gibco (MEM NEAA), 0.5% GlutaMAX (Gibco) and insulin at 5 μg/mL (MCF-7). 25 µl of serial dilutions of the test compounds (100-0.2 µM), that were made with a pipetting robot (epMotion, Eppendorf, Hamburg, Germany), were added to 25 µl aliquots of a cell suspension (1500 cells for KB3-1 and L929, 3000 cells for MCF-7 and 7500 cells for FS4-LTM) in 384 well microtiter plates. Blank and solvent controls were incubated under identical conditions. After an incubation period of 5 days (for L929, KB-3-1, and MCF-7) or 24 h (for FS4-LTM), 3 μL WST-1 (ready to use solution by Roche) was added. The incubation time of the plates at 37 °C varied between the cell lines from 20 min for KB-3-1, 30 min for L929, 1 h for FS4-LTM to 2 h for MCF-7, before measuring absorbance at 450 nm (reference 600 nm) with an Infinite 200 PRO plate reader (Tecan, Männedorf, Switzerland). As positive control compounds, Auranofin and Staurosporin were applied. The absorbance of the solvent control was set to 100%. The EC
50 values were determined with Sigma Plot.