Submitted:
03 February 2023
Posted:
06 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- RQ: Are wearable technologies mature for EEG-based MI-BCI applications in uncontrolled environments?
- RQ1: Is there a significant amount of EEG-based MI-BCI studies using wearable technologies in the literature that implies a promising future development of this research field, especially in uncontrolled environments and outside the medical and clinical settings?
- RQ2: Are there common pipelines of processing that can be adopted from signal acquisition to feedback generation?
- RQ3: Are there consolidated experimental paradigms for wearable EEG-based MI-BCI applications?
- RQ4: Are there datasets available for the research community to properly compare classification models and data analysis?
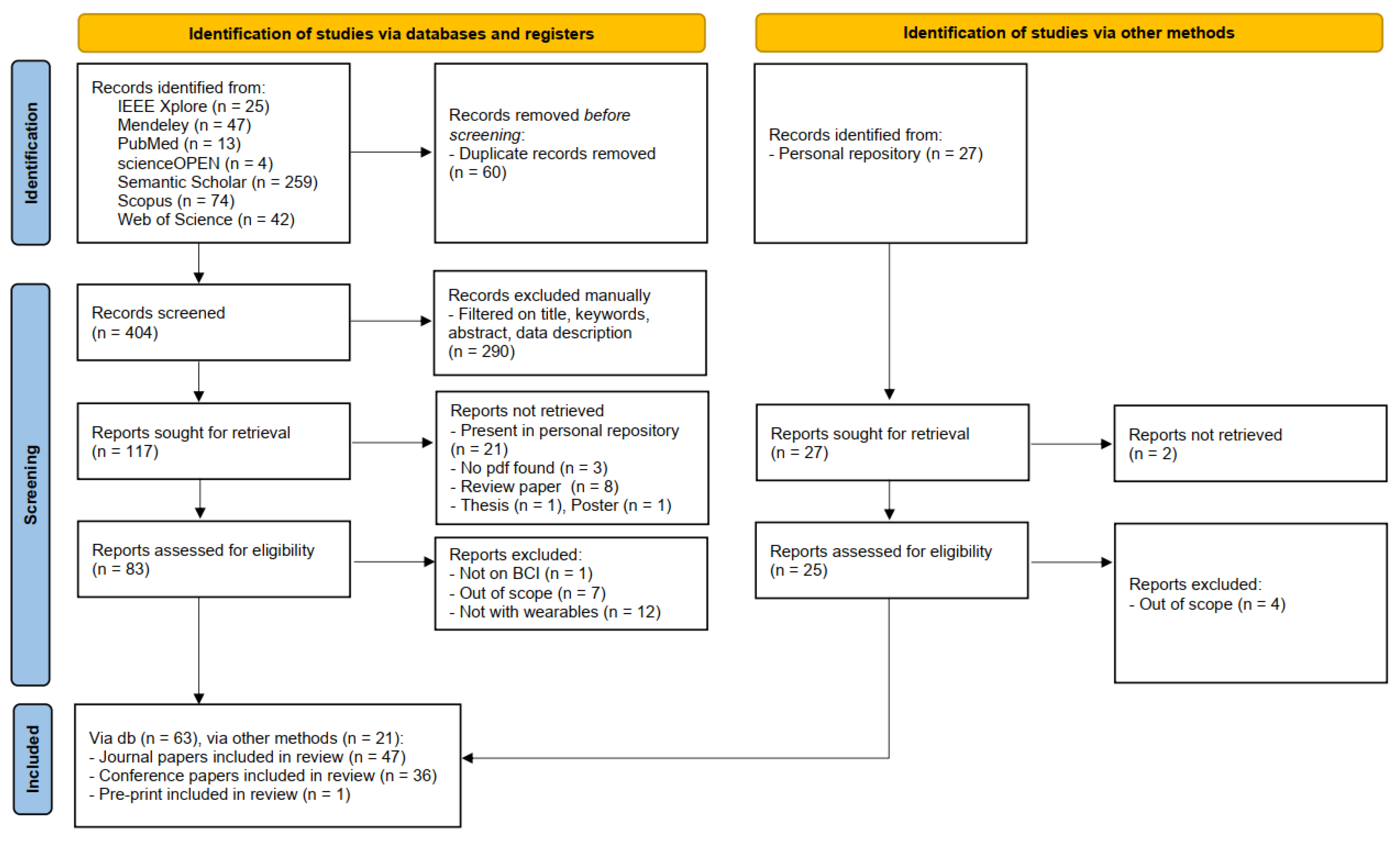
2. Systematic review search method
2.1. Eligibility criteria
- Studies published in the last 10 years (from January 01, 2012 to June 22, 2022).
- Studies published as journal articles, conference proceedings, and dataset reports.
- Non-English articles.
- Studies published as meeting abstracts, book chapters, posters, reviews, and Master and PhD dissertations.
2.2. Information sources
2.3. Search strategy
- a mean (std) of 4.76% (4.98%) works of the EEG & BCI search are present in the EEG & BCI & wearable field;
- a mean (std) of 26.01% (9.38%) works of the EEG & BCI search are present in the EEG & BCI & MI field;
- a mean (std) of 0.71% (0.65%) works of the EEG & BCI search are present in the EEG & BCI & wearable & MI field, target of the present survey.
2.4. Search outcome
3. Background Information
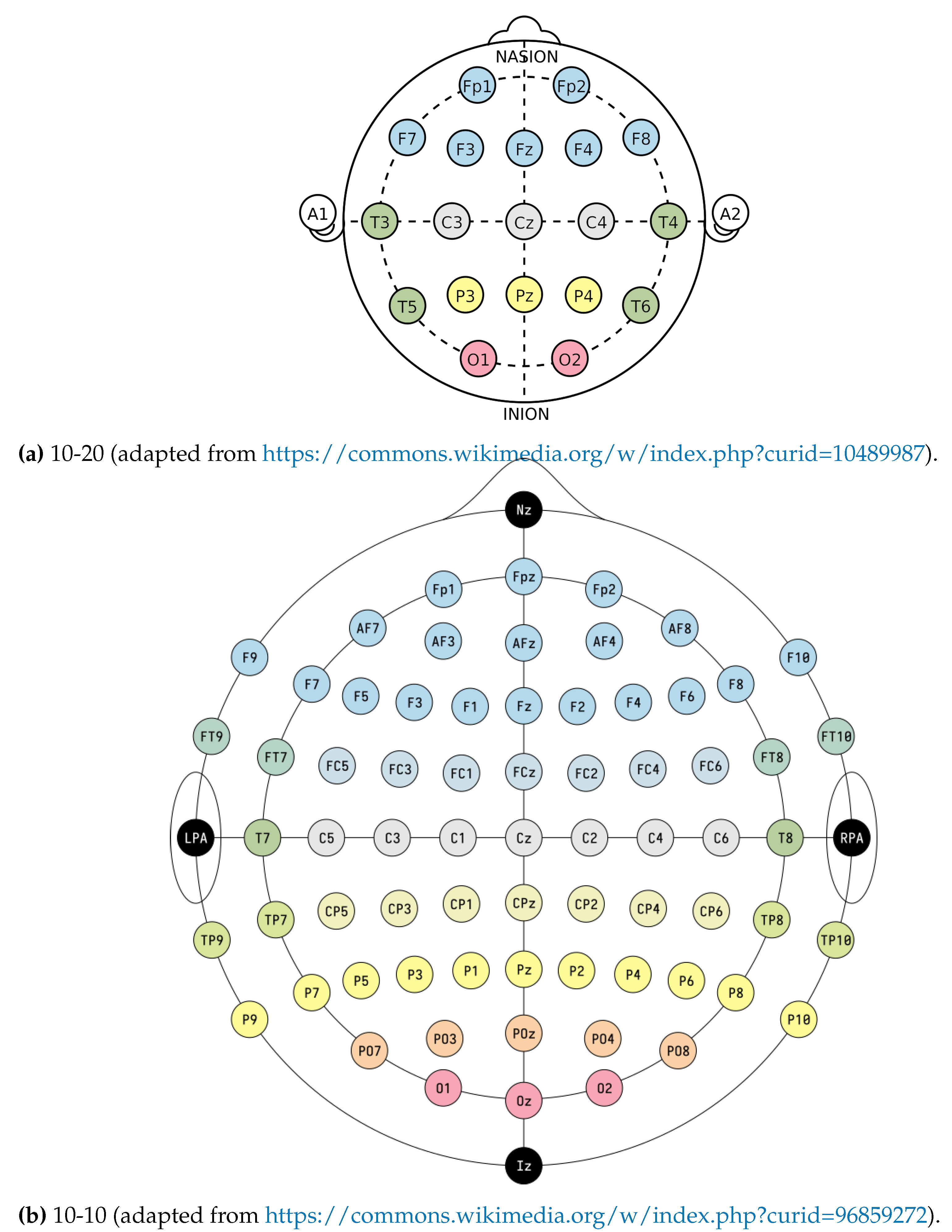
3.1. Electroencephalogram
- being non-stationary signals varying across time [18];
- being subject specific, due to the natural physiological differences between subjects [16];
- varying in a same subject depending on his/her physiological and psychological conditions, and changing from trial to trial [19];
3.1.1. EEG Rhythms
3.2. Motor Imagery
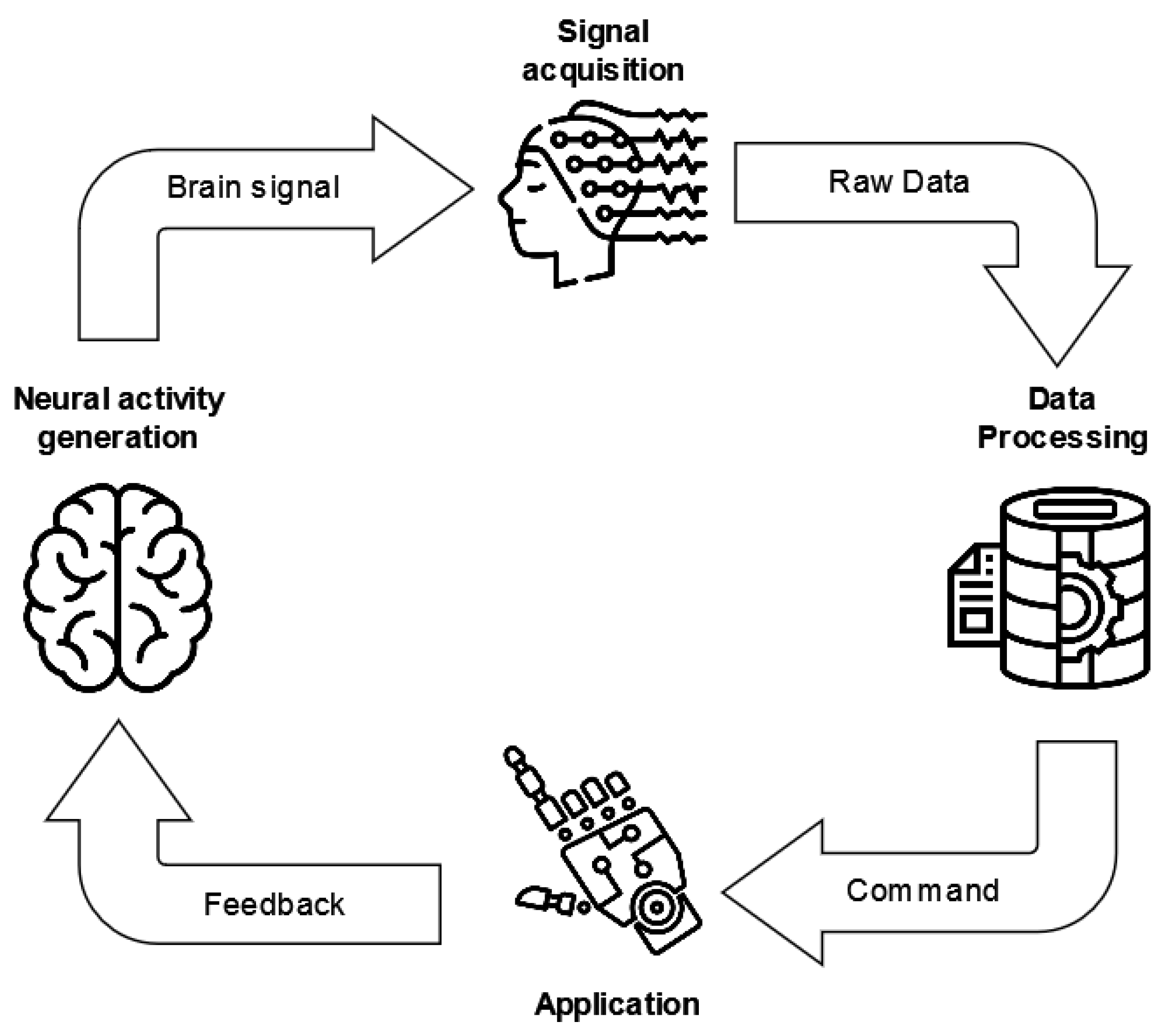
3.3. Brain Computer Interfaces
3.4. Wearable technologies
the evolution of ambulatory EEG units from the bulky, limited lifetime devices available today to small devices present only on the head that can record EEG for days, weeks, or months at a time.
4. Overview of survey articles on EEG-based BCIs
5. EEG-based MI-BCIs through wearable systems
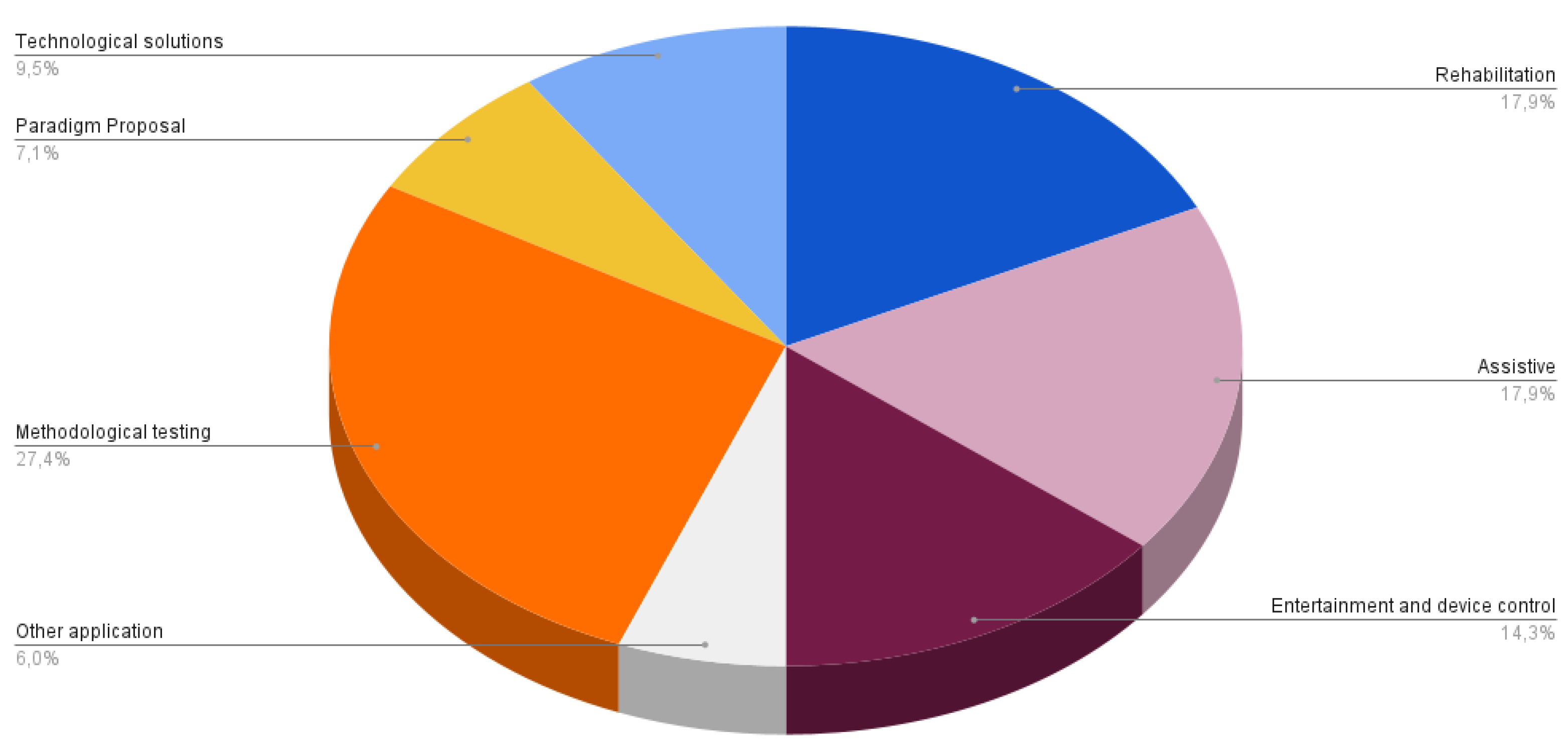
5.1. BCI application and feedback
5.2. Employed technologies
| Device | Producer | Electrodes | Price | Papers |
|---|---|---|---|---|
| B-Alert X-Series [164] | Advanced Brain Monitoring | up to 20 / W (gel) | ask to producer | [98] |
| BrainMaster Discovery 24E* [165] | bio-medical | 24 / W, D (compatible) | 5800.00$ | [110] |
| Cyton Biosensing Board* [166] | OpenBCI | 8 / W, D (compatible) | 999.00$ | [94,109,158] |
| eego rt [167] | ANT Neuro | 8-64 / W, D | ask to producer | [168] |
| Enobio 20 [169] | Neuroelectrics | 20 / W, D | ask to producer | [84,138] |
| Enobio 8 [170] | Neuroelectrics | 8 / W, D | ask to producer | [89,93,144,158,160,161] |
| EPOC+ [171] | Emotiv | 14 / W (saline) | discontinued | [91,106,111,117,122,123,125,128,129,131,132,135,152,163,172] |
| EPOC Flex [173] | Emotiv | up to 32 / W (gel, saline) | 1699.00-2099.00$ | [118,162] |
| EPOC X [174] | Emotiv | 14 / W (saline) | 849.00$ | [103] |
| g.USBamp* with(out) g.MOBIlab [175] | g.tec medical engineering | 16 / W, D | starting from 11900.00€ | [83,100,116,121] |
| Helmate [176] | abmedica | NA / NA | ask to producer | [107,153] |
| Insight [177] | Emotiv | 5 / S | 499.00$ | [102] |
| MindWave Mobile 2 [178] | Neurosky | 1 / NA | 109.99$ | [104,179] |
| Muse headband 2 [180] | InteraXon | 4 / NA | 269.99€ | [105,108,127,137,140] |
| g.Nautilus Multi-Purpose* [181] | g.tec medical engineering | 8-64 / W, D (compatible) | changing according to configuration, starting from 4990.00€ | [85,86,101,121,126,141,142,147,149,151] |
| g.Nautilus PRO [182] | g.tec medical engineering | 8-32 / W, D | changing according to configuration, starting from 5500.00€ | [85,86,101,121,126,141,142,147,149,151] |
| g.Nautilus RESEARCH [183] | g.tec medical engineering | 8-64 / W, D | changing according to configuration, starting from 4990.00€ | [85,86,101,121,126,141,142,147,149,151] |
| NuAmps* [184] | NeuroScan | 32 / NA | ask to producer | [88] |
| Quick-20 Dry EEG Headset [185] | Cognionics | 19 / D | ask to producer | [96,133] |
| Starstim [186] | neuroelectrics | 8-32 / tES-EEG | ask to producer | [99,136] |
| Synamps 2/RT* [187] | Neuroscan | 64 / NA | ask to producer | [114] |
5.3. Signal processing and analysis
5.3.1. EEG data preprocessing
- 9 works assumed that source signals are statistically independent from each other and instantaneously mixed, and apply Independent Component Analysis (ICA) to remove noise, mainly due to eye movements and eye blinks [89,91,99,137,143,146,148,154,160]. EEGLAB toolbox [191] is frequently employed by the authors to implement ICA.
- 11 works applied temporal filtering approaches like Butterworth of different orders and cutoffs: 3rd order filter in 0.5-30Hz [144] or in 4-33Hz [119], 4th order in 16-24Hz [84], 5th order in 8-30Hz [92,110,128,138,139] or in 1-40Hz [109], biquad tweaked Butterworth in 8-13Hz [134], 6th order in 8-30Hz [149].
5.3.2. Feature engineering
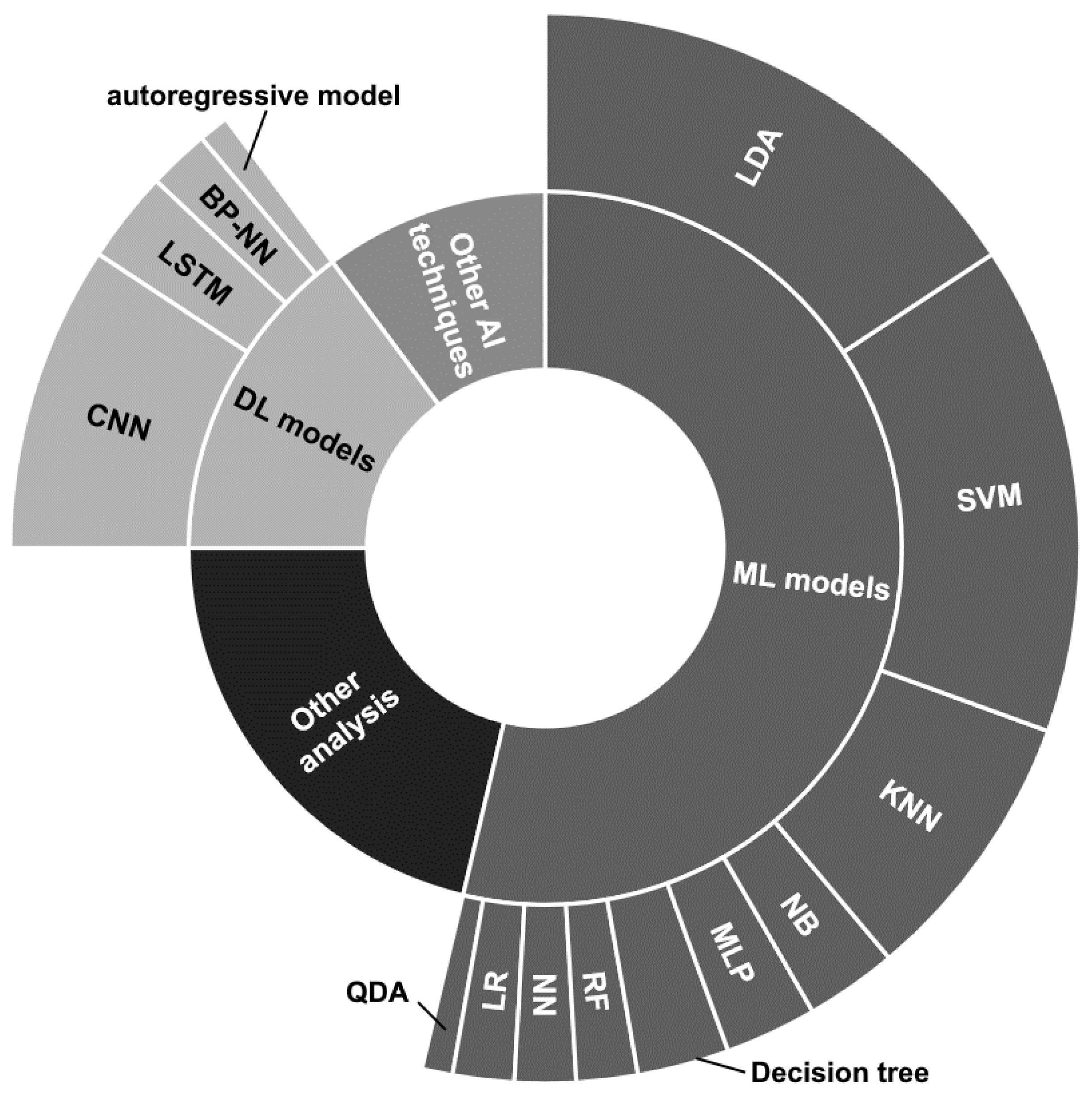
5.3.3. Classification and data analysis
- Questionnaire analyses have been also performed for quality assessment, by considering the opinions given by the subjects concerning a specific device [93] or employing Quebec User Evaluation of Satisfaction with Assistive Technology test to evaluate patients’ satisfaction [84], and for subject MI ability assessment [109].
5.4. Dataset and experimental paradigms
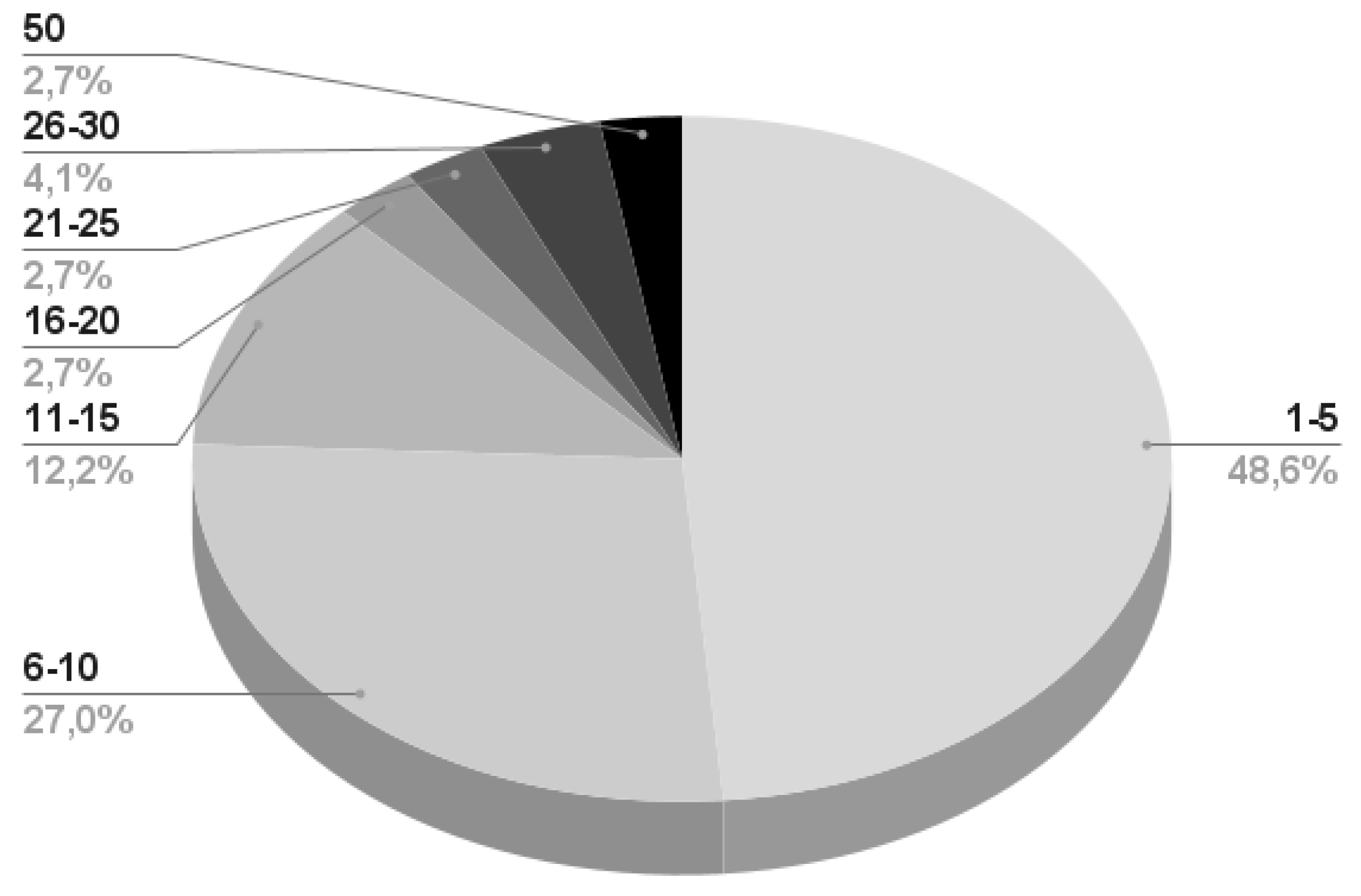
- 7/79 papers do not provide any information regarding the involved subjects.
- 36/79 papers specify the biological gender of the subjects and report most of the time the number of subjects divided per male and female.
- 50/79 papers recruited healthy subjects, while only 5 considered patients affected by specific patologies.
- 21/79 papers present information regarding the previous experience of the subjects with EEG, BCI, or MI based experiments.
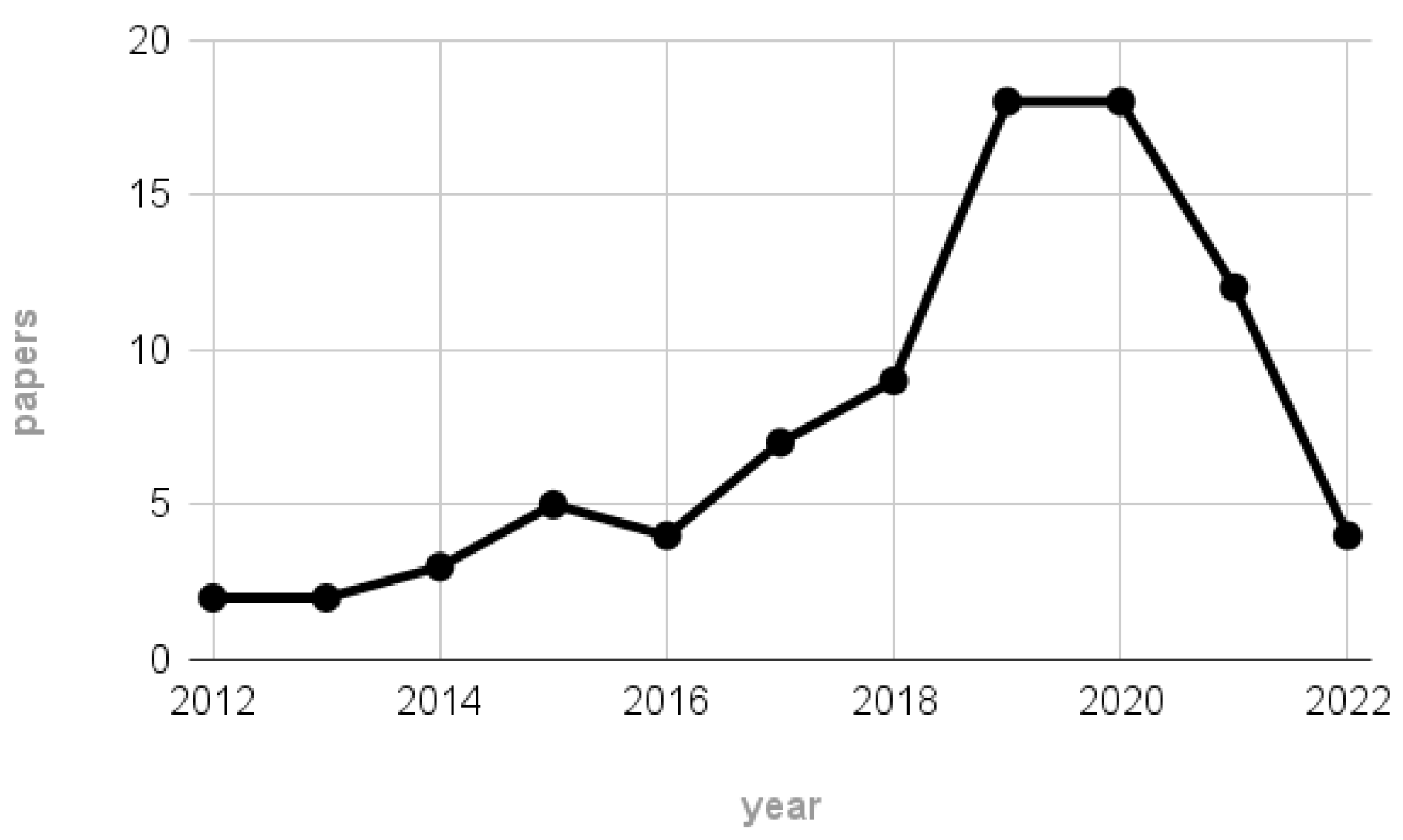
- Almost the 50% of the works reporting information on the subjects perform their experiment on a maximum of 5 participants, the 27% recruits a maximum of 10 subjects, and a very few number of works consider more than 20 participants. A detailed infographic is depicted in Figure 7.
5.4.1. BCI Competition III dataset IIIa
5.4.2. BCI Competition III dataset IVa
5.4.3. BCI Competition IV dataset 2a
5.4.4. BCI Competition IV dataset 2b
5.4.5. EEG Motor Movement/Imagery Dataset
5.4.6. MI-OpenBCI
5.4.7. EEG BCI dataset
6. Discussion
- Are wearable technologies mature for EEG-based MI-BCI applications in uncontrolled environments?
- RQ1: Is there a significant amount of EEG-based MI-BCI studies using wearable technologies in the literature that implies a promising future development of this research field, especially in uncontrolled environments and outside the medical and clinical settings?
- RQ2: Are there common pipelines of processing that can be adopted from signal acquisition to feedback generation?
- RQ3: Are there consolidated experimental paradigms for wearable EEG-based MI-BCI applications?
- RQ4: Are there datasets available for the research community to properly compare classification models and data analysis?
The ultimate goal toward smart wearable sensing with edge computing capabilities relies on a bespoke platform embedding sensors, front-end circuit interface, neuromorphic processor and memristive devices.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1DMSCNN | One-dimensional multi-scale convolutional neural network |
| AI | Artificial Intelligence |
| ANN | Artificial Neural Network |
| AUC | Area Under the Curve |
| BCI | Brain Computer Interface |
| CAR | Common Average Reference |
| CNN | Convolutional Neural Network |
| CSP | Common Spatial Pattern |
| DBN | Deep Belief Network |
| DL | Deep Learning |
| ECG | Electrocardiogram |
| ECoG | Electrocorticography |
| EEG | Electroencephalography |
| EMG | Electromyography |
| EOG | Electrooculography |
| ERD | Event-Related Desynchronization |
| ERP | Event Related Potentials |
| ErrP | Error Related Potential |
| ERS | Event-Related Synchronization |
| FFT | Fast Fourier Transform |
| FGMDRM | Framework with filter Geodesic Minimum & Distance to Riemannian Mean |
| FMRI | Functional Magnetic Resonance Imaging |
| ICA | Independent Component Analysis |
| KNN | K-Nearest Neighbor |
| LDA | Linear Discriminant Analysis |
| LPA | Left Pre-Auricolar point |
| LR | Logistic Regression |
| LSTM | Long-Short Term Memory |
| MI | Motor Imagery |
| ML | Machine Learning |
| MLP | Multi-Layer Perceptron |
| MSCNN | Multi-Scale Convolutional Neural Network |
| NB | Naive Bayes |
| NN | Neural Network |
| PSD | Power Spectral Density |
| PTFBCSP | Penalized Time-Frequency Band Common Spatial Patt |
| n QDA | Quadratic Discriminant Analysis |
| RF | Random forest |
| RNN | Recurrent Neural Network |
| RPA | Right Pre-Auricolar point |
| RQ | Research Question |
| SJGDA | Semisupervised Joint Mutual Information with Genera |
| Discriminate Analysis SNR | Signal to Noise Ratio |
| SSDT | Subject Specific Decision Tree |
| SSVEP | Steady-State Visual Evoked Potential |
| SVM | Support Vector Machine |
| TES | Transcranial Electrical Stimulation |
| VR | Virtual Reality |
| XGBoost | Extreme Gradient Boosting |
References
- Millett, D. Hans Berger: From psychic energy to the EEG. Perspectives in biology and medicine 2001, 44, 522–542. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.J.; Krusienski, D.J.; Wolpaw, J.R. Brain-computer interfaces in medicine. In Proceedings of the Mayo clinic proceedings. Elsevier, 2012, Vol. 87, pp. 268–279. [CrossRef]
- Kögel, J.; Schmid, J.R.; Jox, R.J.; Friedrich, O. Using brain-computer interfaces: a scoping review of studies employing social research methods. BMC medical ethics 2019, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Al-Saegh, A.; Dawwd, S.A.; Abdul-Jabbar, J.M. Deep learning for motor imagery EEG-based classification: A review. Biomedical Signal Processing and Control 2021, 63, 102172. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Systematic reviews 2021, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Nunez, P. Electroencephalography. In Encyclopedia of Human Behavior (Second Edition), Second Edition ed.; Ramachandran, V., Ed.; Academic Press: San Diego, 2012; pp. 15–23. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, Z.; Chen, P.; Nichele, S. Emotion recognition using multi-modal data and machine learning techniques: A tutorial and review. Information Fusion 2020, 59, 103–126. [Google Scholar] [CrossRef]
- Rojas, G.M.; Alvarez, C.; Montoya, C.E.; de la Iglesia-Vayá, M.; Cisternas, J.E.; Gálvez, M. Study of resting-state functional connectivity networks using EEG electrodes position as seed. Frontiers in neuroscience 2018, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Craik, A.; He, Y.; Contreras-Vidal, J.L. Deep learning for electroencephalogram (EEG) classification tasks: a review. Journal of neural engineering 2019, 16, 031001. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.P.; Hosseini, A.; Ahi, K. A Review on Machine Learning for EEG Signal Processing in Bioengineering. IEEE reviews in biomedical engineering 2020. [Google Scholar] [CrossRef]
- LaRocco, J.; Le, M.D.; Paeng, D.G. A systemic review of available low-cost EEG headsets used for drowsiness detection. Frontiers in neuroinformatics 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zhang, K.; Ramkumar, S.; Deny, J.; Emayavaramban, G.; Ramkumar, M.S.; Hussein, A.F. A review on electroencephalogram based brain computer interface for elderly disabled. IEEE Access 2019, 7, 36380–36387. [Google Scholar] [CrossRef]
- Oostenveld, R.; Praamstra, P. The five percent electrode system for high-resolution EEG and ERP measurements. Clinical neurophysiology 2001, 112, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Paranjape, R.; Mahovsky, J.; Benedicenti, L.; Koles, Z. The electroencephalogram as a biometric. In Proceedings of the Canadian Conference on Electrical and Computer Engineering 2001. Conference Proceedings (Cat. No. 01TH8555). IEEE, 2001, Vol. 2, pp. 1363–1366. [CrossRef]
- Xygonakis, I.; Athanasiou, A.; Pandria, N.; Kugiumtzis, D.; Bamidis, P.D. Decoding motor imagery through common spatial pattern filters at the EEG source space. Computational intelligence and neuroscience 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Roy, Y.; Banville, H.; Albuquerque, I.; Gramfort, A.; Falk, T.H.; Faubert, J. Deep learning-based electroencephalography analysis: a systematic review. Journal of neural engineering 2019, 16, 051001. [Google Scholar] [CrossRef] [PubMed]
- Bigdely-Shamlo, N.; Mullen, T.; Kothe, C.; Su, K.M.; Robbins, K.A. The PREP pipeline: standardized preprocessing for large-scale EEG analysis. Frontiers in neuroinformatics 2015, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Gramfort, A.; Strohmeier, D.; Haueisen, J.; Hämäläinen, M.S.; Kowalski, M. Time-frequency mixed-norm estimates: Sparse M/EEG imaging with non-stationary source activations. NeuroImage 2013, 70, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, S. Group nonnegative matrix factorization for EEG classification. In Proceedings of the Artificial Intelligence and Statistics. PMLR, 2009, pp. 320–327.
- Zhang, D.; Yao, L.; Chen, K.; Wang, S.; Chang, X.; Liu, Y. Making sense of spatio-temporal preserving representations for EEG-based human intention recognition. IEEE transactions on cybernetics 2019, 50, 3033–3044. [Google Scholar] [CrossRef] [PubMed]
- Vaid, S.; Singh, P.; Kaur, C. EEG signal analysis for BCI interface: A review. In Proceedings of the 2015 fifth international conference on advanced computing & communication technologies. IEEE, 2015, pp. 143–147. [CrossRef]
- Blinowska, K.; Durka, P. Electroencephalography (eeg). Wiley encyclopedia of biomedical engineering 2006. [Google Scholar]
- McFarland, D.J.; Miner, L.A.; Vaughan, T.M.; Wolpaw, J.R. Mu and beta rhythm topographies during motor imagery and actual movements. Brain topography 2000, 12, 177–186. [Google Scholar] [CrossRef]
- Decety, J. The neurophysiological basis of motor imagery. Behavioural brain research 1996, 77, 45–52. [Google Scholar] [CrossRef]
- Beisteiner, R.; Höllinger, P.; Lindinger, G.; Lang, W.; Berthoz, A. Mental representations of movements. Brain potentials associated with imagination of hand movements. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section 1995, 96, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Jeannerod, M. Mental imagery in the motor context. Neuropsychologia 1995, 33, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.; Halsband, U. Motor imagery. Journal of Physiology-paris 2006, 99, 386–395. [Google Scholar] [CrossRef] [PubMed]
- McAvinue, L.P.; Robertson, I.H. Measuring motor imagery ability: a review. European journal of cognitive psychology 2008, 20, 232–251. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Neuper, C. Motor imagery activates primary sensorimotor area in humans. Neuroscience letters 1997, 239, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Nam, C.S.; Kim, Y.J.; Whang, M.C. Event-related (De) synchronization (ERD/ERS) during motor imagery tasks: Implications for brain–computer interfaces. International Journal of Industrial Ergonomics 2011, 41, 428–436. [Google Scholar] [CrossRef]
- Munzert, J.; Lorey, B.; Zentgraf, K. Cognitive motor processes: the role of motor imagery in the study of motor representations. Brain research reviews 2009, 60, 306–326. [Google Scholar] [CrossRef] [PubMed]
- Dose, H.; Møller, J.S.; Iversen, H.K.; Puthusserypady, S. An end-to-end deep learning approach to MI-EEG signal classification for BCIs. Expert Systems with Applications 2018, 114, 532–542. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Da Silva, F.L. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clinical neurophysiology 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Brunner, C.; Schlögl, A.; Da Silva, F.L. Mu rhythm (de) synchronization and EEG single-trial classification of different motor imagery tasks. NeuroImage 2006, 31, 153–159. [Google Scholar] [CrossRef]
- Nam, C.S.; Jeon, Y.; Kim, Y.J.; Lee, I.; Park, K. Movement imagery-related lateralization of event-related (de) synchronization (ERD/ERS): motor-imagery duration effects. Clinical Neurophysiology 2011, 122, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Zheng, D.; Na, R.; Wang, S.; Zhang, S. EEG classification of motor imagery using a novel deep learning framework. Sensors 2019, 19, 551. [Google Scholar] [CrossRef] [PubMed]
- Wriessnegger, S.C.; Brunner, C.; Müller-Putz, G.R. Frequency specific cortical dynamics during motor imagery are influenced by prior physical activity. Frontiers in psychology 2018, 9, 1976. [Google Scholar] [CrossRef] [PubMed]
- Demarin, V.; Morović, S. Neuroplasticity. Periodicum biologorum 2014, 116, 209–211. [Google Scholar]
- Kaiser, V.; Bauernfeind, G.; Kreilinger, A.; Kaufmann, T.; Kübler, A.; Neuper, C.; Müller-Putz, G.R. Cortical effects of user training in a motor imagery based brain–computer interface measured by fNIRS and EEG. Neuroimage 2014, 85, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Reaves, J.; Flavin, T.; Mitra, B.; Mahantesh, K.; Nagaraju, V. Assessment And Application of EEG: A Literature Review. J Appl Bioinforma Comput Biol 10 2021, 7, 2. [Google Scholar]
- Wolpaw, J.R.; Birbaumer, N.; McFarland, D.J.; Pfurtscheller, G.; Vaughan, T.M. Brain–computer interfaces for communication and control. Clinical neurophysiology 2002, 113, 767–791. [Google Scholar] [CrossRef] [PubMed]
- Schalk, G.; McFarland, D.J.; Hinterberger, T.; Birbaumer, N.; Wolpaw, J.R. BCI2000: a general-purpose brain-computer interface (BCI) system. IEEE Transactions on biomedical engineering 2004, 51, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Chugh, N. Signal processing techniques for motor imagery brain computer interface: A review. Array 2019, 1, 100003. [Google Scholar] [CrossRef]
- Abiri, R.; Borhani, S.; Sellers, E.W.; Jiang, Y.; Zhao, X. A comprehensive review of EEG-based brain–computer interface paradigms. Journal of Neural Engineering 2019, 16, 011001. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, F.; Cazzaniga, E.; Saibene, A. Inner speech recognition through electroencephalographic signals. arXiv 2022, arXiv:2210.06472. [Google Scholar]
- Ramele, R.; Villar, A.J.; Santos, J.M. EEG Waveform Analysis of P300 ERP with Applications to Brain Computer Interfaces. Brain Sciences 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Friman, O.; Volosyak, I.; Graser, A. Multiple Channel Detection of Steady-State Visual Evoked Potentials for Brain-Computer Interfaces. IEEE Transactions on Biomedical Engineering 2007, 54, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Konar, A.; Tibarewala, D.N. Motor imagery and error related potential induced position control of a robotic arm. IEEE/CAA Journal of Automatica Sinica 2017, 4, 639–650. [Google Scholar] [CrossRef]
- Kawala-Sterniuk, A.; Browarska, N.; Al-Bakri, A.; Pelc, M.; Zygarlicki, J.; Sidikova, M.; Martinek, R.; Gorzelanczyk, E.J. Summary of over fifty years with brain-computer interfaces—A review. Brain Sciences 2021, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Han, J.; Wang, Y.; Jung, T.P.; Ming, D. Implementing over 100 command codes for a high-speed hybrid brain-computer interface using concurrent P300 and SSVEP features. IEEE Transactions on Biomedical Engineering 2020, 67, 3073–3082. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Li, H.; Deng, L.; Yang, H.; Lv, X.; Li, P.; Li, F.; Zhang, R.; Liu, T.; Yao, D.; et al. The hybrid BCI system for movement control by combining motor imagery and moving onset visual evoked potential. Journal of neural engineering 2017, 14, 026015. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Lin, D.; Li, W.; Zhang, Z. Design of a multimodal EEG-based hybrid BCI system with visual servo module. IEEE Transactions on Autonomous Mental Development 2015, 7, 332–341. [Google Scholar] [CrossRef]
- Khan, M.A.; Das, R.; Iversen, H.K.; Puthusserypady, S. Review on motor imagery based BCI systems for upper limb post-stroke neurorehabilitation: From designing to application. Computers in Biology and Medicine 2020, 123, 103843. [Google Scholar] [CrossRef] [PubMed]
- Abdulkader, S.N.; Atia, A.; Mostafa, M.S.M. Brain computer interfacing: Applications and challenges. Egyptian Informatics Journal 2015, 16, 213–230. [Google Scholar] [CrossRef]
- Kerous, B.; Skola, F.; Liarokapis, F. EEG-based BCI and video games: a progress report. Virtual Reality 2018, 22, 119–135. [Google Scholar] [CrossRef]
- Sanchez-Fraire, U.; Parra-Vega, V.; Martinez-Peon, D.; Sepúlveda-Cervantes, G.; Sánchez-Orta, A.; Muñoz-Vázquez, A.J. On the brain computer robot interface (bcri) to control robots. IFAC-PapersOnLine 2015, 48, 154–159. [Google Scholar] [CrossRef]
- Perrin, X.; Chavarriaga, R.; Colas, F.; Siegwart, R.; Millán, J.d.R. Brain-coupled interaction for semi-autonomous navigation of an assistive robot. Robotics and Autonomous Systems 2010, 58, 1246–1255. [Google Scholar] [CrossRef]
- Balderas, D.; Ponce, P.; Lopez-Bernal, D.; Molina, A. Education 4.0: Teaching the Basis of Motor Imagery Classification Algorithms for Brain-Computer Interfaces. Future Internet 2021, 13, 202. [Google Scholar] [CrossRef]
- Myrden, A.; Chau, T. A passive EEG-BCI for single-trial detection of changes in mental state. IEEE Transactions on neural systems and rehabilitation Engineering 2017, 25, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, J.R. Brain–computer interfaces. In Handbook of Clinical Neurology; Elsevier, 2013; Vol. 110, pp. 67–74. [CrossRef]
- Fiedler, P.; Fonseca, C.; Supriyanto, E.; Zanow, F.; Haueisen, J. A high-density 256-channel cap for dry electroencephalography. Human Brain Mapping 2022, 43, 1295–1308. [Google Scholar] [CrossRef]
- Casson, A.J.; Yates, D.C.; Smith, S.J.; Duncan, J.S.; Rodriguez-Villegas, E. Wearable electroencephalography. IEEE engineering in medicine and biology magazine 2010, 29, 44–56. [Google Scholar] [CrossRef]
- Soufineyestani, M.; Dowling, D.; Khan, A. Electroencephalography (EEG) technology applications and available devices. Applied Sciences 2020, 10, 7453. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Z. EEG signal processing and feature extraction; Springer, 2019. [CrossRef]
- Li, G.L.; Wu, J.T.; Xia, Y.H.; He, Q.G.; Jin, H.G. Review of semi-dry electrodes for EEG recording. Journal of Neural Engineering 2020, 17, 051004. [Google Scholar] [CrossRef]
- Casson, A.J. Wearable EEG and beyond. Biomedical engineering letters 2019, 9, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Mihajlović, V.; Grundlehner, B.; Vullers, R.; Penders, J. Wearable, wireless EEG solutions in daily life applications: what are we missing? IEEE journal of biomedical and health informatics 2014, 19, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Blum, S.; Emkes, R.; Minow, F.; Anlauff, J.; Finke, A.; Debener, S. Flex-printed forehead EEG sensors (fEEGrid) for long-term EEG acquisition. Journal of neural engineering 2020, 17, 034003. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Cho, B.H.; Yook, S.; Kim, J.Y.; Shon, Y.M.; Seo, D.W.; Kim, I.Y. Unsupervised automatic seizure detection for focal-onset seizures recorded with behind-the-ear EEG using an anomaly-detecting generative adversarial network. Computer Methods and Programs in Biomedicine 2020, 193, 105472. [Google Scholar] [CrossRef] [PubMed]
- Do Valle, B.G.; Cash, S.S.; Sodini, C.G. Wireless behind-the-ear EEG recording device with wireless interface to a mobile device (iPhone/iPod touch). In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE, 2014, pp. 5952–5955. [CrossRef]
- Wang, Y.; Yin, L.; Bai, Y.; Liu, S.; Wang, L.; Zhou, Y.; Hou, C.; Yang, Z.; Wu, H.; Ma, J.; et al. Electrically compensated, tattoo-like electrodes for epidermal electrophysiology at scale. Science advances 2020, 6, eabd0996. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Chen, D.; Ge, S.; Liu, Y.; Wang, Z.; Zhang, X.; Guo, Q.; Yang, J. Robust tattoo electrode prepared by paper-assisted water transfer printing for wearable health monitoring. IEEE Sensors Journal 2022. [Google Scholar] [CrossRef]
- Casson, A.J.; Abdulaal, M.; Dulabh, M.; Kohli, S.; Krachunov, S.; Trimble, E. Electroencephalogram. In Seamless healthcare monitoring; Springer, 2018; pp. 45–81. [CrossRef]
- Palumbo, A.; Gramigna, V.; Calabrese, B.; Ielpo, N. Motor-imagery EEG-based BCIs in wheelchair movement and control: A systematic literature review. Sensors 2021, 21, 6285. [Google Scholar] [CrossRef]
- Abiri, R.; Borhani, S.; Sellers, E.W.; Jiang, Y.; Zhao, X. A comprehensive review of EEG-based brain–computer interface paradigms. Journal of neural engineering 2019, 16, 011001. [Google Scholar] [CrossRef] [PubMed]
- Udovičić, G.; Topić, A.; Russo, M. Wearable technologies for smart environments: a review with emphasis on BCI. In Proceedings of the 2016 24th International Conference on Software, Telecommunications and Computer Networks (SoftCOM). IEEE, 2016, pp. 1–9. [CrossRef]
- TajDini, M.; Sokolov, V.; Kuzminykh, I.; Shiaeles, S.; Ghita, B. Wireless sensors for brain activity—a survey. Electronics 2020, 9, 2092. [Google Scholar] [CrossRef]
- Portillo-Lara, R.; Tahirbegi, B.; Chapman, C.A.; Goding, J.A.; Green, R.A. Mind the gap: State-of-the-art technologies and applications for EEG-based brain–computer interfaces. APL bioengineering 2021, 5, 031507. [Google Scholar] [CrossRef] [PubMed]
- Jamil, N.; Belkacem, A.N.; Ouhbi, S.; Lakas, A. Noninvasive Electroencephalography Equipment for Assistive, Adaptive, and Rehabilitative Brain–Computer Interfaces: A Systematic Literature Review. Sensors 2021, 21, 4754. [Google Scholar] [CrossRef]
- Gu, X.; Cao, Z.; Jolfaei, A.; Xu, P.; Wu, D.; Jung, T.P.; Lin, C.T. EEG-based brain-computer interfaces (BCIs): A survey of recent studies on signal sensing technologies and computational intelligence approaches and their applications. IEEE/ACM transactions on computational biology and bioinformatics 2021, 18, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- Neuper, C.; Pfurtscheller, G. Neurofeedback training for BCI control. Brain-computer interfaces, 2009, pp. 65–78. [CrossRef]
- Jiang, Y.; Hau, N.T.; Chung, W.Y. Semiasynchronous BCI using wearable two-channel EEG. IEEE Transactions on Cognitive and Developmental Systems 2017, 10, 681–686. [Google Scholar] [CrossRef]
- Mattia, D.; Pichiorri, F.; Colamarino, E.; Masciullo, M.; Morone, G.; Toppi, J.; Pisotta, I.; Tamburella, F.; Lorusso, M.; Paolucci, S.; et al. The Promotoer, a brain-computer interface-assisted intervention to promote upper limb functional motor recovery after stroke: a study protocol for a randomized controlled trial to test early and long-term efficacy and to identify determinants of response. BMC neurology 2020, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Barria, P.; Pino, A.; Tovar, N.; Gomez-Vargas, D.; Baleta, K.; Díaz, C.A.; Múnera, M.; Cifuentes, C.A. BCI-Based Control for Ankle Exoskeleton T-FLEX: Comparison of Visual and Haptic Stimuli with Stroke Survivors. Sensors 2021, 21, 6431. [Google Scholar] [CrossRef] [PubMed]
- Karakullukcu, N.; Yilmaz, B. Detection of Movement Intention in EEG-Based Brain-Computer Interfaces Using Fourier-Based Synchrosqueezing Transform. International Journal of Neural Systems 2022, 32, 2150059. [Google Scholar] [CrossRef]
- Du Bois, N.; Bigirimana, A.D.; Korik, A.; Kéthina, L.G.; Rutembesa, E.; Mutabaruka, J.; Mutesa, L.; Prasad, G.; Jansen, S.; Coyle, D. Neurofeedback with low-cost, wearable electroencephalography (EEG) reduces symptoms in chronic Post-Traumatic Stress Disorder. Journal of affective disorders 2021, 295, 1319–1334. [Google Scholar] [CrossRef] [PubMed]
- Looned, R.; Webb, J.; Xiao, Z.G.; Menon, C. Assisting drinking with an affordable BCI-controlled wearable robot and electrical stimulation: a preliminary investigation. Journal of neuroengineering and rehabilitation 2014, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yuan, Y.; Luo, L.; Su, W.; Zhao, K.; Xu, C.; Huang, J.; Pi, M. Hybrid brain/muscle signals powered wearable walking exoskeleton enhancing motor ability in climbing stairs activity. IEEE Transactions on Medical Robotics and Bionics 2019, 1, 218–227. [Google Scholar] [CrossRef]
- Vourvopoulos, A.; Jorge, C.; Abreu, R.; Figueiredo, P.; Fernandes, J.C.; Bermudez i Badia, S. Efficacy and brain imaging correlates of an immersive motor imagery BCI-driven VR system for upper limb motor rehabilitation: A clinical case report. Frontiers in human neuroscience 2019, 13, 244. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Fu, S.; Deng, B.; Zeng, H.; Zhang, J.; Guo, S. Embedded BCI Rehabilitation System for Stroke. Journal of Beijing Institute of Technology 2019, 28, 35–41. [Google Scholar]
- Athanasiou, A.; Arfaras, G.; Xygonakis, I.; Kartsidis, P.; Pandria, N.; Kavazidi, K.R.; Astaras, A.; Foroglou, N.; Polyzoidis, K.; Bamidis, P.D. Commercial BCI Control and functional brain networks in spinal cord injury: a proof-of-concept. In Proceedings of the 2017 IEEE 30th International Symposium on Computer-Based Medical Systems (CBMS). IEEE, 2017, pp. 262–267. [CrossRef]
- Simon, C.; Ruddy, K.L. A wireless, wearable Brain-Computer Interface for neurorehabilitation at home; A feasibility study. In Proceedings of the 2022 10th International Winter Conference on Brain-Computer Interface (BCI). IEEE, 2022, pp. 1–6. [CrossRef]
- Quiles, E.; Suay, F.; Candela, G.; Chio, N.; Jiménez, M.; Álvarez-Kurogi, L. Low-cost robotic guide based on a motor imagery brain–computer interface for arm assisted rehabilitation. International journal of environmental research and public health 2020, 17, 699. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Delisle-Rodriguez, D.; Romero-Laiseca, M.A.; Loterio, F.A.; Gurve, D.; Floriano, A.; Valadão, C.; Silva, L.; Krishnan, S.; Frizera-Neto, A.; et al. Effect of a Brain–Computer Interface Based on Pedaling Motor Imagery on Cortical Excitability and Connectivity. Sensors 2021, 21, 2020. [Google Scholar] [CrossRef]
- Wang, H.; Bezerianos, A. Brain-controlled wheelchair controlled by sustained and brief motor imagery BCIs. Electronics Letters 2017, 53, 1178–1180. [Google Scholar] [CrossRef]
- Lisi, G.; Hamaya, M.; Noda, T.; Morimoto, J. Dry-wireless EEG and asynchronous adaptive feature extraction towards a plug-and-play co-adaptive brain robot interface. In Proceedings of the 2016 IEEE International Conference on Robotics and Automation (ICRA). IEEE, 2016, pp. 959–966. [CrossRef]
- Carrino, F.; Dumoulin, J.; Mugellini, E.; Abou Khaled, O.; Ingold, R. A self-paced BCI system to control an electric wheelchair: Evaluation of a commercial, low-cost EEG device. In Proceedings of the 2012 ISSNIP biosignals and biorobotics conference: biosignals and robotics for better and safer living (BRC). IEEE, 2012, pp. 1–6. [CrossRef]
- Gant, K.; Guerra, S.; Zimmerman, L.; Parks, B.A.; Prins, N.W.; Prasad, A. EEG-controlled functional electrical stimulation for hand opening and closing in chronic complete cervical spinal cord injury. Biomedical physics & engineering express 2018, 4, 065005. [Google Scholar]
- Gaxiola-Tirado, J.A.; Iáñez, E.; Ortíz, M.; Gutiérrez, D.; Azorín, J.M. Effects of an exoskeleton-assisted gait motor imagery training in functional brain connectivity. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE, 2019, pp. 429–432. [CrossRef]
- Khan, M.J.; Hong, K.S.; Naseer, N.; Bhutta, M.R. Motor imagery performance evaluation using hybrid EEG-NIRS for BCI. In Proceedings of the 2015 54th Annual Conference of the Society of Instrument and Control Engineers of Japan (SICE). IEEE, 2015, pp. 1150–1155. [CrossRef]
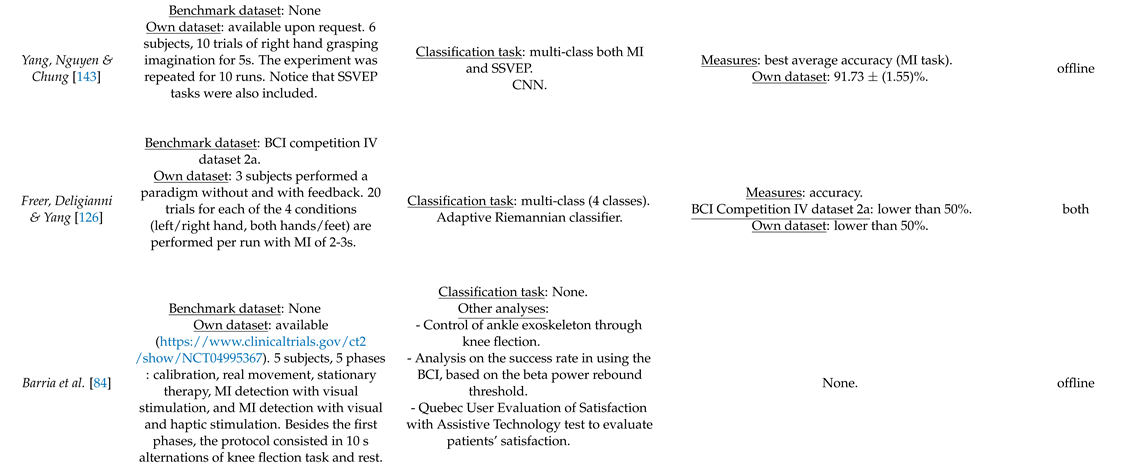
- Freer, D.; Yang, G.Z. MIndGrasp: A New Training and Testing Framework for Motor Imagery Based 3-Dimensional Assistive Robotic Control. arXiv 2020, arXiv:2003.00369. [Google Scholar]
- Jameel, H.F.; Mohammed, S.L.; Gharghan, S.K. Electroencephalograph-based wheelchair controlling system for the people with motor disability using advanced brainwear. In Proceedings of the 2019 12th International Conference on Developments in eSystems Engineering (DeSE). IEEE, 2019, pp. 843–848. [CrossRef]
- Ketola, E.; Lloyd, C.; Shuhart, D.; Schmidt, J.; Morenz, R.; Khondker, A.; Imtiaz, M. Lessons Learned from the Initial Development of a Brain Controlled Assistive Device. In Proceedings of the 2022 IEEE 12th Annual Computing and Communication Workshop and Conference (CCWC). IEEE, 2022, pp. 0580–0585. [CrossRef]
- Permana, K.; Wijaya, S.; Prajitno, P. Controlled wheelchair based on brain computer interface using Neurosky Mindwave Mobile 2. In Proceedings of the AIP Conference Proceedings. AIP Publishing LLC, 2019, Vol. 2168, p. 020022. [CrossRef]
- Priyatno, S.B.; Prakoso, T.; Riyadi, M.A. Classification of motor imagery brain wave for bionic hand movement using multilayer perceptron. Sinergi 2022, 26, 57–64. [Google Scholar] [CrossRef]
- Tang, X.; Li, W.; Li, X.; Ma, W.; Dang, X. Motor imagery EEG recognition based on conditional optimization empirical mode decomposition and multi-scale convolutional neural network. Expert Systems with Applications 2020, 149, 113285. [Google Scholar] [CrossRef]
- Apicella, A.; Arpaia, P.; Frosolone, M.; Moccaldi, N. High-wearable EEG-based distraction detection in motor rehabilitation. Scientific Reports 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Moreno, F.M.; Bermudez-Edo, M.; Rodríguez-Fórtiz, M.J.; Garrido, J.L. A CNN-LSTM deep Learning classifier for motor imagery EEG detection using a low-invasive and low-Cost BCI headband. In Proceedings of the 2020 16th International Conference on Intelligent Environments (IE). IEEE, 2020, pp. 84–91. [CrossRef]
- Peterson, V.; Galván, C.; Hernández, H.; Spies, R. A feasibility study of a complete low-cost consumer-grade brain-computer interface system. Heliyon 2020, 6, e03425. [Google Scholar] [CrossRef]
- Tariq, M.; Trivailo, P.M.; Simic, M. Motor imagery based EEG features visualization for BCI applications. Procedia computer science 2018, 126, 1936–1944. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, L.; Sheng, Q.Z.; Kanhere, S.S.; Gu, T.; Zhang, D. Converting your thoughts to texts: Enabling brain typing via deep feature learning of eeg signals. In Proceedings of the 2018 IEEE international conference on pervasive computing and communications (PerCom). IEEE, 2018, pp. 1–10. [CrossRef]
- Chowdhury, A.; Raza, H.; Meena, Y.K.; Dutta, A.; Prasad, G. Online covariate shift detection-based adaptive brain–computer interface to trigger hand exoskeleton feedback for neuro-rehabilitation. IEEE Transactions on Cognitive and Developmental Systems 2017, 10, 1070–1080. [Google Scholar] [CrossRef]
- Daeglau, M.; Wallhoff, F.; Debener, S.; Condro, I.S.; Kranczioch, C.; Zich, C. Challenge accepted? Individual performance gains for motor imagery practice with humanoid robotic EEG neurofeedback. Sensors 2020, 20, 1620. [Google Scholar] [CrossRef]
- LaFleur, K.; Cassady, K.; Doud, A.; Shades, K.; Rogin, E.; He, B. Quadcopter control in three-dimensional space using a noninvasive motor imagery-based brain–computer interface. Journal of neural engineering 2013, 10, 046003. [Google Scholar] [CrossRef] [PubMed]
- Alanis-Espinosa, M.; Gutiérrez, D. On the assessment of functional connectivity in an immersive brain-computer interface during motor imagery. Frontiers in Psychology 2020, 11, 1301. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, W.; He, X.; Wei, Z.; Zhang, D.; Wu, C.; Song, A. Motor imagery based continuous teleoperation robot control with tactile feedback. Electronics 2020, 9, 174. [Google Scholar] [CrossRef]
- Zhuang, J.; Geng, K.; Yin, G. Ensemble learning based brain–computer interface system for ground vehicle control. IEEE Transactions on Systems, Man, and Cybernetics: Systems 2019, 51, 5392–5404. [Google Scholar] [CrossRef]
- Liu, Y.; Habibnezhad, M.; Jebelli, H. Brain-computer interface for hands-free teleoperation of construction robots. Automation in Construction 2021, 123, 103523. [Google Scholar] [CrossRef]
- Mahmood, M.; Kwon, S.; Kim, H.; Kim, Y.S.; Siriaraya, P.; Choi, J.; Otkhmezuri, B.; Kang, K.; Yu, K.J.; Jang, Y.C.; et al. Wireless Soft Scalp Electronics and Virtual Reality System for Motor Imagery-Based Brain–Machine Interfaces. Advanced Science 2021, 8, 2101129. [Google Scholar] [CrossRef]
- Djamal, E.C.; Abdullah, M.Y.; Renaldi, F. Brain computer interface game controlling using fast fourier transform and learning vector quantization. Journal of Telecommunication, Electronic and Computer Engineering (JTEC) 2017, 9, 71–74. [Google Scholar]
- Mitocaru, A.; Poboroniuc, M.S.; Irimia, D.; Baciu, A. Comparison Between Two Brain Computer Interface Systems Aiming to Control a Mobile Robot. In Proceedings of the 2021 International Conference on Electromechanical and Energy Systems (SIELMEN). IEEE, 2021, pp. 1–5. [CrossRef]
- Mwata-Velu, T.; Ruiz-Pinales, J.; Rostro-Gonzalez, H.; Ibarra-Manzano, M.A.; Cruz-Duarte, J.M.; Avina-Cervantes, J.G. Motor imagery classification based on a recurrent-convolutional architecture to control a hexapod robot. Mathematics 2021, 9, 606. [Google Scholar] [CrossRef]
- Parikh, D.; George, K. Quadcopter Control in Three-Dimensional Space Using SSVEP and Motor Imagery-Based Brain-Computer Interface. In Proceedings of the 2020 11th IEEE Annual Information Technology, Electronics and Mobile Communication Conference (IEMCON). IEEE, 2020, pp. 0782–0785. [CrossRef]
- Wu, S.L.; Liu, Y.T.; Chou, K.P.; Lin, Y.Y.; Lu, J.; Zhang, G.; Chuang, C.H.; Lin, W.C.; Lin, C.T. A motor imagery based brain-computer interface system via swarm-optimized fuzzy integral and its application. In Proceedings of the 2016 IEEE International Conference on Fuzzy Systems (FUZZ-IEEE). IEEE, 2016, pp. 2495–2500. [CrossRef]
- Abdulwahab, S.S.; Khleaf, H.K.; Jassim, M.H. EEG Motor-Imagery BCI System Based on Maximum Overlap Discrete Wavelet Transform (MODWT) and cubic SVM. Journal of Physics: Conference Series. IOP Publishing, 2021, Vol. 1973, p. 012056. [CrossRef]
- Freer, D.; Deligianni, F.; Yang, G.Z. Adaptive Riemannian BCI for enhanced motor imagery training protocols. In Proceedings of the 2019 IEEE 16th International Conference on Wearable and Implantable Body Sensor Networks (BSN). IEEE, 2019, pp. 1–4. [CrossRef]
- Garcia-Moreno, F.M.; Bermudez-Edo, M.; Garrido, J.L.; Rodríguez-Fórtiz, M.J. Reducing response time in motor imagery using a headband and deep learning. Sensors 2020, 20, 6730. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Li, J.; Wang, F.; Yuan, Z.; Kang, X.; Lu, B. Discriminating three motor imagery states of the same joint for brain-computer interface. PeerJ 2021, 9, e12027. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Zhao, K.; Yang, S. Motor imagery EEG classification based on decision tree framework and Riemannian geometry. Computational intelligence and neuroscience 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Jawanjalkar, A.R.; Padole, D.V. Development of soft computing technique for classification of EEG signal. In Proceedings of the 2017 International Conference on Innovations in Information, Embedded and Communication Systems (ICIIECS). IEEE, 2017, pp. 1–6. [CrossRef]
- Khan, J.; Bhatti, M.H.; Khan, U.G.; Iqbal, R. Multiclass EEG motor-imagery classification with sub-band common spatial patterns. EURASIP Journal on Wireless Communications and Networking 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Kim, H.H.; Jeong, J. Decoding electroencephalographic signals for direction in brain-computer interface using echo state network and Gaussian readouts. Computers in biology and medicine 2019, 110, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Lisi, G.; Rivela, D.; Takai, A.; Morimoto, J. Markov switching model for quick detection of event related desynchronization in EEG. Frontiers in neuroscience 2018, 12, 24. [Google Scholar] [CrossRef]
- Lo, C.C.; Chien, T.Y.; Chen, Y.C.; Tsai, S.H.; Fang, W.C.; Lin, B.S. A wearable channel selection-based brain-computer interface for motor imagery detection. Sensors 2016, 16, 213. [Google Scholar] [CrossRef]
- Mladenov, T.; Kim, K.; Nooshabadi, S. Accurate motor imagery based dry electrode brain-computer interface system for consumer applications. In Proceedings of the 2012 IEEE 16th International Symposium on Consumer Electronics. IEEE, 2012, pp. 1–4. [CrossRef]
- Rodriguez-Ugarte, M.D.l.S.; Iáñez, E.; Ortiz-Garcia, M.; Azorín, J.M. Effects of tDCS on real-time BCI detection of pedaling motor imagery. Sensors 2018, 18, 1136. [Google Scholar] [CrossRef]
- Riyadi, M.A.; Setiawan, I.; Amir, A. EEG Multiclass Signal Classification Based on Subtractive Clustering-ANFIS and Wavelet Packet Decomposition. In Proceedings of the 2021 International Conference on Electrical and Information Technology (IEIT). IEEE, 2021, pp. 81–86. [CrossRef]
- Shajil, N.; Mohan, S.; Srinivasan, P.; Arivudaiyanambi, J.; Arasappan Murrugesan, A. Multiclass classification of spatially filtered motor imagery EEG signals using convolutional neural network for BCI based applications. Journal of Medical and Biological Engineering 2020, 40, 663–672. [Google Scholar] [CrossRef]
- Shajil, N.; Sasikala, M.; Arunnagiri, A. Deep learning classification of two-class motor imagery EEG signals using transfer learning. In Proceedings of the 2020 International Conference on e-Health and Bioengineering (EHB). IEEE, 2020, pp. 1–4. [CrossRef]
- Tiwari, S.; Goel, S.; Bhardwaj, A. Machine learning approach for the classification of EEG signals of multiple imagery tasks. In Proceedings of the 2020 11th International Conference on Computing, Communication and Networking Technologies (ICCCNT). IEEE, 2020, pp. 1–7. [CrossRef]
- Triana Guzmán, N.; Orjuela-Cañón, Á.D.; Jutinico Alarcon, A.L. Incremental training of neural network for motor tasks recognition based on brain-computer interface. In Proceedings of the Iberoamerican Congress on Pattern Recognition. Springer, 2019, pp. 610–619. [CrossRef]
- Yang, B.; Tang, J.; Guan, C.; Li, B. Motor imagery EEG recognition based on FBCSP and PCA. In Proceedings of the International Conference on Brain Inspired Cognitive Systems. Springer, 2018, pp. 195–205. [CrossRef]
- Yang, D.; Nguyen, T.H.; Chung, W.Y. A Synchronized Hybrid Brain-Computer Interface System for Simultaneous Detection and Classification of Fusion EEG Signals. Complexity 2020, 2020. [Google Scholar] [CrossRef]
- Yusoff, M.Z.; Mahmoud, D.; Malik, A.S.; Bahloul, M.R.; et al. Discrimination of four class simple limb motor imagery movements for brain–computer interface. Biomedical Signal Processing and Control 2018, 44, 181–190. [Google Scholar]
- Zhou, B.; Wu, X.; Lv, Z.; Zhang, L.; Zhang, C. Independent component analysis combined with compressed sensing for EEG compression in BCI. In Proceedings of the 2015 10th International Conference on Information, Communications and Signal Processing (ICICS). IEEE, 2015, pp. 1–4. [CrossRef]
- Zich, C.; Schweinitz, C.; Debener, S.; Kranczioch, C. Multimodal evaluation of motor imagery training supported by mobile EEG at home: A case report. In Proceedings of the 2015 IEEE International Conference on Systems, Man, and Cybernetics. IEEE, 2015, pp. 3181–3186. [CrossRef]
- Verma, P.; Heilinger, A.; Reitner, P.; Grünwald, J.; Guger, C.; Franklin, D. Performance investigation of brain-computer interfaces that combine EEG and fNIRS for motor imagery tasks. In Proceedings of the 2019 IEEE International Conference on Systems, Man and Cybernetics (SMC). IEEE, 2019, pp. 259–263. [CrossRef]
- Rodríguez-Ugarte, M.; Angulo-Sherman, I.; Iáñez, E.; Ortiz, M.; Azorín, J. Preliminary study of pedaling motor imagery classification based on EEG signals. In Proceedings of the 2017 International Symposium on Wearable Robotics and Rehabilitation (WeRob). IEEE, 2017, pp. 1–2. [CrossRef]
- Hirsch, G.; Dirodi, M.; Xu, R.; Reitner, P.; Guger, C. Online classification of motor imagery using EEG and fNIRS: a hybrid approach with real time human-computer interaction. In Proceedings of the International Conference on Human-Computer Interaction. Springer, 2020, pp. 231–238. [CrossRef]
- Dehzangi, O.; Zou, Y.; Jafari, R. Simultaneous classification of motor imagery and SSVEP EEG signals. 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER). IEEE, 2013, pp. 1303–1306. [CrossRef]
- Cha, K.; Lee, J.; Kim, H.; Kim, C.; Lee, S. Steady-State Somatosensory Evoked Potential based Brain-Computer Interface for Sit-to-Stand Movement Intention. In Proceedings of the 2019 7th International Winter Conference on Brain-Computer Interface (BCI). IEEE, 2019, pp. 1–3. [CrossRef]
- Arfaras, G.; Athanasiou, A.; Pandria, N.; Kavazidi, K.R.; Kartsidis, P.; Astaras, A.; Bamidis, P.D. Visual versus kinesthetic motor imagery for BCI control of robotic arms (Mercury 2.0). In Proceedings of the 2017 IEEE 30th International Symposium on Computer-Based Medical Systems (CBMS). IEEE, 2017, pp. 440–445. [CrossRef]
- Angrisani, L.; Arpaia, P.; Donnarumma, F.; Esposito, A.; Frosolone, M.; Improta, G.; Moccaldi, N.; Natalizio, A.; Parvis, M. Instrumentation for Motor Imagery-based Brain Computer Interfaces relying on dry electrodes: a functional analysis. In Proceedings of the 2020 IEEE International Instrumentation and Measurement Technology Conference (I2MTC). IEEE, 2020, pp. 1–6. [CrossRef]
- Bista, S.; BikramAdhikari, N. Performance Analysis of Tri-channel Active Electrode EEG Device Designed for Classification of Motor Imagery Brainwaves for Brain ComputerInterface. In Proceedings of the 2018 International Conference on Advances in Computing, Communication Control and Networking (ICACCCN). IEEE, 2018, pp. 662–667.
- Liao, L.D.; Wu, S.L.; Liou, C.H.; Lu, S.W.; Chen, S.A.; Chen, S.F.; Ko, L.W.; Lin, C.T. A novel 16-channel wireless system for electroencephalography measurements with dry spring-loaded sensors. IEEE Transactions on Instrumentation and Measurement 2014, 63, 1545–1555. [Google Scholar] [CrossRef]
- Lin, C.L.; Chu, T.Y.; Wu, P.J.; Wang, C.A.; Lin, B.S. Design of wearable brain computer interface based on motor imagery. In Proceedings of the 2014 Tenth International Conference on Intelligent Information Hiding and Multimedia Signal Processing. IEEE, 2014, pp. 33–36. [CrossRef]
- Lin, B.S.; Pan, J.S.; Chu, T.Y.; Lin, B.S. Development of a wearable motor-imagery-based brain–computer interface. Journal of medical systems 2016, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vourvopoulos, A.; Niforatos, E.; Giannakos, M. EEGlass: An EEG-eyeware prototype for ubiquitous brain-computer interaction. Adjunct proceedings of the 2019 ACM international joint conference on pervasive and ubiquitous computing and proceedings of the 2019 ACM international symposium on wearable computers, 2019, pp. 647–652. [CrossRef]
- Zhang, Y.; Zhang, X.; Sun, H.; Fan, Z.; Zhong, X. Portable brain-computer interface based on novel convolutional neural network. Computers in biology and medicine 2019, 107, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Venot, T.; Corsi, M.C.; Saint-Bauzel, L.; de Vico Fallani, F. Towards multimodal BCIs: the impact of peripheral control on motor cortex activity and sense of agency. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). IEEE, 2021, pp. 5876–5879. [CrossRef]
- Abdalsalam, E.; Yusoff, M.Z.; Malik, A.; Kamel, N.S.; Mahmoud, D. Modulation of sensorimotor rhythms for brain-computer interface using motor imagery with online feedback. Signal, Image and Video Processing 2018, 12, 557–564. [Google Scholar] [CrossRef]
- Paszkiel, S.; Dobrakowski, P. Brain–computer technology-based training system in the field of motor imagery. IET Science, Measurement & Technology 2020, 14, 1014–1018. [Google Scholar]
- Zich, C.; De Vos, M.; Kranczioch, C.; Debener, S. Wireless EEG with individualized channel layout enables efficient motor imagery training. Clinical Neurophysiology 2015, 126, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Advanced Brain Monitoring, I. B-Alert X-Series Wireless & Mobile EEG System. Available online: https://www.advancedbrainmonitoring.com/products/b-alert-x-series (accessed on 03 October 2022).
- bio medical.com. BrainMaster Discovery 24E - 24 Channel qEEG. Available online: https://bio-medical.com/brainmaster-discovery-24e-24-channel-qeeg.html (accessed on 03 October 2022).
- by Shopify, O.O.S.P. Cyton Biosensing Board (8-channels) – OpenBCI Online Store. Available online: https://shop.openbci.com/products/cyton-biosensing-board-8-channel?variant=38958638542 (accessed on 03 October 2022).
- Neuro, A. eego™rt | ANT Neuro. Available online: https://www.ant-neuro.com/products/eego_rt (accessed on 03 October 2022).
- Peterson, V.; Wyser, D.; Lambercy, O.; Spies, R.; Gassert, R. A penalized time-frequency band feature selection and classification procedure for improved motor intention decoding in multichannel EEG. Journal of neural engineering 2019, 16, 016019. [Google Scholar] [CrossRef] [PubMed]
- Neuroelectrics. Enobio 20 | Solutions | Neuroelectrics. Available online: https://www.neuroelectrics.com/solutions/enobio/20 (accessed on 03 October 2022).
- Neuroelectrics. Enobio 8 | Solutions | Neuroelectrics. Available online: https://www.neuroelectrics.com/solutions/enobio/8 (accessed on 03 October 2022).
- EMOTIV. EMOTIV EPOC+ 14-Channel Wireless EEG Headset - EMOTIV. Available online: https://www.emotiv.com/epoc/ (accessed on 03 October 2022).
- Zhang, S.; Yuan, S.; Huang, L.; Zheng, X.; Wu, Z.; Xu, K.; Pan, G. Human mind control of rat cyborg’s continuous locomotion with wireless brain-to-brain interface. Scientific reports 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- EMOTIV. EPOC Flex - 32-Channel Wireless EEG Device - EMOTIV. Available online: https://www.emotiv.com/epoc-flex/ (accessed on 03 October 2022).
- EMOTIV. EMOTIV EPOC X - 14 Channel Wireless EEG Headset - EMOTIV. Available online: https://www.emotiv.com/epoc-x/ (accessed on 03 October 2022).
- g.tec medical engineering GmbH Austria. g.USBAMP RESEARCH | EEG/Biosignal Amplifier | g.tec medical engineering GmbH medical engineering. Available online: https://www.gtec.at/product/gusbamp-research/ (accessed on 03 October 2022).
- ab medica s.p.a.. Dispositivi medici innovativi - Robotica - Telemedicina - ab medica. Available online: https://www.abmedica.it/ (accessed on 03 October 2022).
- EMOTIV. Insight Brainwear® 5 Channel Wireless EEG Headset - EMOTIV. Available online: https://www.emotiv.com/insight/ (accessed on 03 October 2022).
- NeuroSky. MindWave. Available online: https://store.neurosky.com/pages/mindwave (accessed on 03 October 2022).
- Kevric, J.; Subasi, A. The impact of Mspca signal de-noising in real-time wireless brain computer interface system. Southeast Europe Journal of Soft Computing 2015, 4. [Google Scholar] [CrossRef]
- Muse. Muse 2: Brain Sensing Headband - Technology Enhanced Meditation. Available online: https://choosemuse.com/muse-2/ (accessed on 03 October 2022).
- g.tec medical engineering GmbH Austria. g.Nautilus Multiple Biosignal Recording | g.tec medical engineering GmbH. Available online: https://www.gtec.at/product/gnautilus-multipurpose/ (accessed on 03 October 2022).
- g.tec medical engineering GmbH Austria. g.Nautilus PRO Wearable EEG | g.tec medical engineering GmbH. Available online: https://www.gtec.at/product/gnautilus-pro/ (accessed on 03 October 2022).
- g.tec medical engineering GmbH Austria. g.NAUTILUS RESEARCH | Wearable EEG Headset | g.tec medical engineering GmbH. Available online: https://www.gtec.at/product/gnautilus-research/ (accessed on 03 October 2022).
- Neuroscan, C. nuamps – Compumedics Neuroscan. Available online: https://compumedicsneuroscan.com/nuamps-4/ (accessed on 03 October 2022).
- COGNIONICS, I. Quick-20 Dry EEG Headset (2). Available online: http://www.cognionics.com/index.php/32-uncategorised/94-quick-20-dry-headset-2 (accessed on 03 October 2022).
- Neuroelectrics. Starstim® tES-EEG systems | Neuroelectrics. Available online: https://www.neuroelectrics.com/solutions/starstim (accessed on 03 October 2022).
- AG, N. Synamps 2/RT | NEUROSPEC AG Research Neurosciences. Available online: https://www.neurospec.com/Products/Details/1017/synamps-2rt (accessed on 03 October 2022).
- Rashmi, C.; Shantala, C. EEG artifacts detection and removal techniques for brain computer interface applications: a systematic review. International Journal of Advanced Technology and Engineering Exploration 2022, 9, 354. [Google Scholar]
- Fatourechi, M.; Bashashati, A.; Ward, R.K.; Birch, G.E. EMG and EOG artifacts in brain computer interface systems: A survey. Clinical Neurophysiology 2007, 118, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Seok, D.; Lee, S.; Kim, M.; Cho, J.; Kim, C. Motion artifact removal techniques for wearable EEG and PPG sensor systems. Frontiers in Electronics 2021, 2, 685513. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of neuroscience methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Blankertz, B.; Muller, K.R.; Krusienski, D.J.; Schalk, G.; Wolpaw, J.R.; Schlogl, A.; Pfurtscheller, G.; Millan, J.R.; Schroder, M.; Birbaumer, N. The BCI competition III: Validating alternative approaches to actual BCI problems. IEEE transactions on neural systems and rehabilitation engineering 2006, 14, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Tangermann, M.; Müller, K.R.; Aertsen, A.; Birbaumer, N.; Braun, C.; Brunner, C.; Leeb, R.; Mehring, C.; Miller, K.J.; Mueller-Putz, G. ; others. Review of the BCI competition IV. Frontiers in neuroscience, 2012, p. 55. [CrossRef]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Binli, M.K.; Ozbay, E.; Yanar, H.; Mishchenko, Y. A large electroencephalographic motor imagery dataset for electroencephalographic brain computer interfaces. Scientific data 2018, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Leeb, R.; Lee, F.; Keinrath, C.; Scherer, R.; Bischof, H.; Pfurtscheller, G. Brain–computer communication: motivation, aim, and impact of exploring a virtual apartment. IEEE Transactions on Neural Systems and Rehabilitation Engineering 2007, 15, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Covi, E.; Donati, E.; Liang, X.; Kappel, D.; Heidari, H.; Payvand, M.; Wang, W. Adaptive extreme edge computing for wearable devices. Frontiers in Neuroscience, 2021, p. 429. [CrossRef]
- Jin, X.; Li, L.; Dang, F.; Chen, X.; Liu, Y. A survey on edge computing for wearable technology. Digital Signal Processing 2022, 125, 103146. [Google Scholar] [CrossRef]
- Huang, D.; Wang, M.; Wang, J.; Yan, J. A Survey of Quantum Computing Hybrid Applications with Brain-Computer Interface. Cognitive Robotics 2022. [Google Scholar] [CrossRef]
- Miranda, E.R.; Martín-Guerrero, J.D.; Venkatesh, S.; Hernani-Morales, C.; Lamata, L.; Solano, E. Quantum Brain Networks: A Perspective. Electronics 2022, 11, 1528. [Google Scholar] [CrossRef]
- Miranda, E.R.; Venkatesh, S.; Martın-Guerrero, J.D.; Hernani-Morales, C.; Lamata, L.; Solano, E. An approach to interfacing the brain with quantum computers: practical steps and caveats. arXiv 2022, arXiv:2201.00817. [Google Scholar]
| 1 | The montage is depicted in the official dataset description available at https://www.bbci.de/competition/iii/desc_IIIa.pdf
|
| 2 | More details on the official dataset description available at https://www.bbci.de/competition/iii/desc_IVa.html. |
| 3 |
| Search engine | Author | Last consultation date |
|---|---|---|
| IEEE Xplore | F.G. | 2022/06/22 |
| Mendeley | F.G. | 2022/06/22 |
| PubMed | S.C. | 2022/06/22 |
| ScienceOPEN | A.S. | 2022/06/21 |
| Semantic Scholar | A.S. | 2022/06/21 |
| Scopus | A.S. | 2022/06/22 |
| Web of Science | S.C. | 2022/06/22 |
| Google Scholar | M.C. & A.S. | 2022/06/20 |
| Search engine | EEG & BCI | EEG & BCI & wearable | EEG & BCI & MI | EEG & BCI & wearable & MI |
|---|---|---|---|---|
| IEEE Xplore | 3584 | 121 | 546 | 13 |
| Mendeley | 10723 | 286 | 3118 | 60 |
| PubMed | 2777 | 57 | 877 | 14 |
| ScienceOPEN | 2659 | 22 | 303 | 4 |
| Semantic Scholar | 12200 | 1879 | 3630 | 259 |
| Scopus | 10733 | 670 | 2909 | 74 |
| Web of Science | 6564 | 180 | 2491 | 40 |
| Rhythm | Frequency range (Hz) | Occurrence |
|---|---|---|
| ≤ 4 | infants, deep sleep | |
| 4 - 8 | emotional stress, drowsiness | |
| 8 - 13 | relaxed awake state | |
| 8 - 13 | motor cortex functionalities | |
| 13 - 30 | alert state, active thinking/attention, anxiety | |
| ≥ 31 | intensive brain activity |
 |
 |
 |
 |
| Dataset | Link | Device | Experimental paradigm |
|---|---|---|---|
| BCI Competition III dataset IIIa [192] | https://www.bbci.de/competition/iii/ | Neuroscan, 64 channel EEG amplifier (wired) | cue-based left/right hand, foot, tongue MI |
| BCI Competition III dataset IVa [192] | https://www.bbci.de/competition/iii/ | BrainAmps and 128 channel ECI cap (wired) | cue-based left/right hand, right foot MI |
| BCI Competition IV dataset 2a [193] | https://www.bbci.de/competition/iv/ | 22 electrodes (wired) | cue-based MI-BCI left/right hand, both feet, tongue MI |
| BCI Competition IV dataset 2b [193] | https://www.bbci.de/competition/iv/ | 3 electrodes (wired) | cue-based MI-BCI left/right hand MI |
| EEG Motor Movement/Imagery Dataset [42,194] | https://physionet.org/content/eegmmidb/1.0.0/ | 64 electrodes (wired) | cue-based motor execution/imagination left/right fist and both feet/fists opening/closing |
| MI-OpenBCI [109] | https://github.com/vpeterson/MI-OpenBCI | OpenBCI Cyton and Daisy Module, Electrocap System II, 15 electrodes (wearable) | cue-based dominant hand grasping MI |
| EEG BCI dataset [195] | https://figshare.com/collections/A_large_electroencephalographic_motor_imagery_dataset_for_electroencephalographic_brain_computer_interfaces/3917698 | EEG-1200 JE-921A EEG system, 19 electrodes (wired) | left/right hand, left/right leg, tongue and finger MI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





