1. Introduction
Thalassemia is the most prevalent genetic disorder in the world with WHO reporting around 60,000 infants afflicted with thalassemia major (TM-β), born every year [
1]. TM-β is described as the group of blood disorders with mutations in the β-globin gene that affects the globin chain synthesis to various degrees [
2]. TM-β patients exhibit three important factors which contribute to pathophysiology namely, ineffective erythropoiesis, chronic anemia and hypoxia, and iron overload [
3]. Gene drifts and founder effects are the reasons that thalassemia is increasingly prevalent in the sub-continent. It is interesting to note that consanguineous marriages are another reason cited for higher prevalence of thalassemia in North and central Africa, Mediterranean countries and the Middle east [
4]. Pakistan represents one of the highest burdened countries for thalassemia with an estimated 5000-9000 children affected with β-thalassemia born each year in Pakistan [
5]. Thalassemia Federation of Pakistan reports a prevalence rate of 6% for β-thalassemia (TM- β) in Pakistani population [
6]. The estimated carrier rate is reported to be 5-7%, with around 9.8million carriers in the total population [
7]. World Health Organisation (WHO) has warranted priority control for the blood disorders, specially βthalassemia, in the third world countries [
1].
β Thalassemia major (TM- β) exhibits distinctive oral and facial features along with its systemic manifestations. Protruded maxilla, severe crowding, open bite, protruded upper lip, flattened nose bridge, increased dental decay, and atrophic glossitis are some of the observed oral features in these patients [
8,
9,
10,
11]. Literature has consistently reported increased prevalence of periodontal problems in thalassemia patients compared to healthy controls [
9,
12,
13,
14]. Periodontal diseases are a group of infectious inflammatory diseases - affecting the supporting structures of the teeth - gingiva, alveolar bone, cementum [
15]. Periodontal diseases begin with gingivitis – localized inflammation of the gingiva initiated by bacterial plaque – a biofilm that forms on teeth and gingiva [
16]. Higher prevalence of gingivitis in these patients is explained by the following mechanisms. In addition to the systemic effects of TM- β, the condition also affects the local defense mechanism where neutrophils and B lymphocytes fail to respond effectively against gingival microbial attack in patients with gingivitis [
17]. The incidence of gingivitis in thalassemic patients is further favored by xerostomia due to patient’s inability to close the mouth over proclined teeth, resulting in inability of TM- β patients to benefit from the salivary local immune defense against gingivitis [
18]. Compromised oral hygiene, malocclusion and Chronic anoxemia in some cases also predisposes such patients to gingival disorders [
12].
Neutrophils represent the principal leukocyte (>95%), which are recruited as the first line defence against the bacterial biofilm [
19]. Their absence leads to periodontal tissue damage, whereas the excess also causes periodontal destruction. Hence, both the quantity as well as the distribution of the neutrophils is necessary for periodontal health [
15]. In diseases with neutrophil dysfunctions periodontal tissue is lost very rapidly [
20]. TM- β patients exhibit defective neutrophils and macrophages with compromised ability of phagocytosis [
21]. When there is supplementary gingival inflammation, that may in turn, alter the clinical signs of both the chronic diseases [
21]. This suggests that patients with TM- β and gingival inflammation have two chronic inflammatory conditions, each of which may affect the other.
The oral cavity is the most complex environment in the human body where interaction between the host and the microbes govern health and disease [
23]. The teeth are a connection from the inside to the outside environment, surrounded by a rich bacterial biofilm and hence provide a direct link from the outside world traversing through the bone and soft tissues, to the body systems. The literature on systemic conditions namely, chronic kidney disease (CKD) [
24], atherosclerotic cardiovascular disease [
25] diabetes [
26] and adverse pregnancy outcomes due to periodontal condition [
27] provide ample evidence to support the two-way effect of periodontal diseases on systemic diseases/conditions and vice versa. Non-surgical periodontal therapy (NSPT) is the gold standard in the treatment and management of periodontal inflammatory conditions [
28]. Evidence supports consistent improvement in clinical periodontal inflammatory markers in various patient population as well as improvement in systemic inflammatory markers following NSPT [
29].
In the light of these scientific evidences, it is assumed that there must be some association and correlation between the two chronic inflammatory conditions namely, gingivitis and thalassemia; each of which may exacerbate and/or effect the other condition. However, the bidirectional effect of periodontal diseases and thalassemia on each other lacks supporting literature and presents with the dearth of research in the domain. This study aims to explore the effect of the same and report the findings.
2. Materials and Methods
This study was a joint collaboration of Ziauddin College of Dentistry, Karachi and AfzaalMemorial Thalassemia Foundation (AMTF),Karachi. The ethical approval for the research was obtained from the Ethical Research Committee (ERC) at the Ziauddin University, bearing the reference code (0780119AHOM). A total of 137 patients, via consecutive sampling technique were screened, out of which 31 patients (16 males, 15 females) between the age range of 10y and 20y, fulfilled the selection criteria and were recruited in the study. All patients with any systemic comorbidities, history of antibiotic use during the past 3 months, history of dental prophylaxis during the past 6 months, history of active infection with HIV, HepB and HepC, history of splenectomy, history of cognitive challenges, were excluded. All patients diagnosed with TM- β, age >10years, patientswho received or are receiving iron chelation therapy with deferasirox, calcium, vitamin D, regular erythrocyte transfusion, regular physician’s follow-up, were selected.
Five milliliters (ml) of venous blood were obtained in tubes, with a silicone-coated interior, via standard venipuncture method (
Figure 1).
The collected blood samples were left at room temperature to allow clotting to occur and then centrifuged at 1,500 x g for 15 minutes at 4°C to remove the fibrin clot and cellular elements. The serum collection was done at the baseline, followed by the NSPT protocol (Sub and supragingival ultrasonic scaling and polishing, chemical plaque control using chlorhexidine 0.2% twice daily for 14 day and mechanical reinforcement of plaque control via fone’s brushing method) and re-collected 6 weeks after NSPT. All samples were collected by a trained phlebotomist at AMTF who was double blinded to the study results, in the presence of the principal investigator (AH). All of the serum samples were immediately stored at -40oC at AMTF facility and periodically transferred, carefully wrapped in icebox and stored at -40oC the MDRL lab at Ziauddin University, Clifton campus. All transferred serum samples were monitored by the biochemical expert (MA) prior to storing them at the MDRL Ziauddin University. Serum IL-6 and IL-8 were determined by Enzyme linked Immunosorbent assay (IL-6 human ELISA kit – Invitrogen – Thermo Fisher Scientific (life tec) and IL-8 Human ELISA kit – Invitrogen – Thermo Fisher Scientific (life tec) according to the guidelines of the manufacturer. The tests were conducted by the principal investigator (AH) in the presence of an expert laboratory personnel (MA) who was blinded of the clinical details.
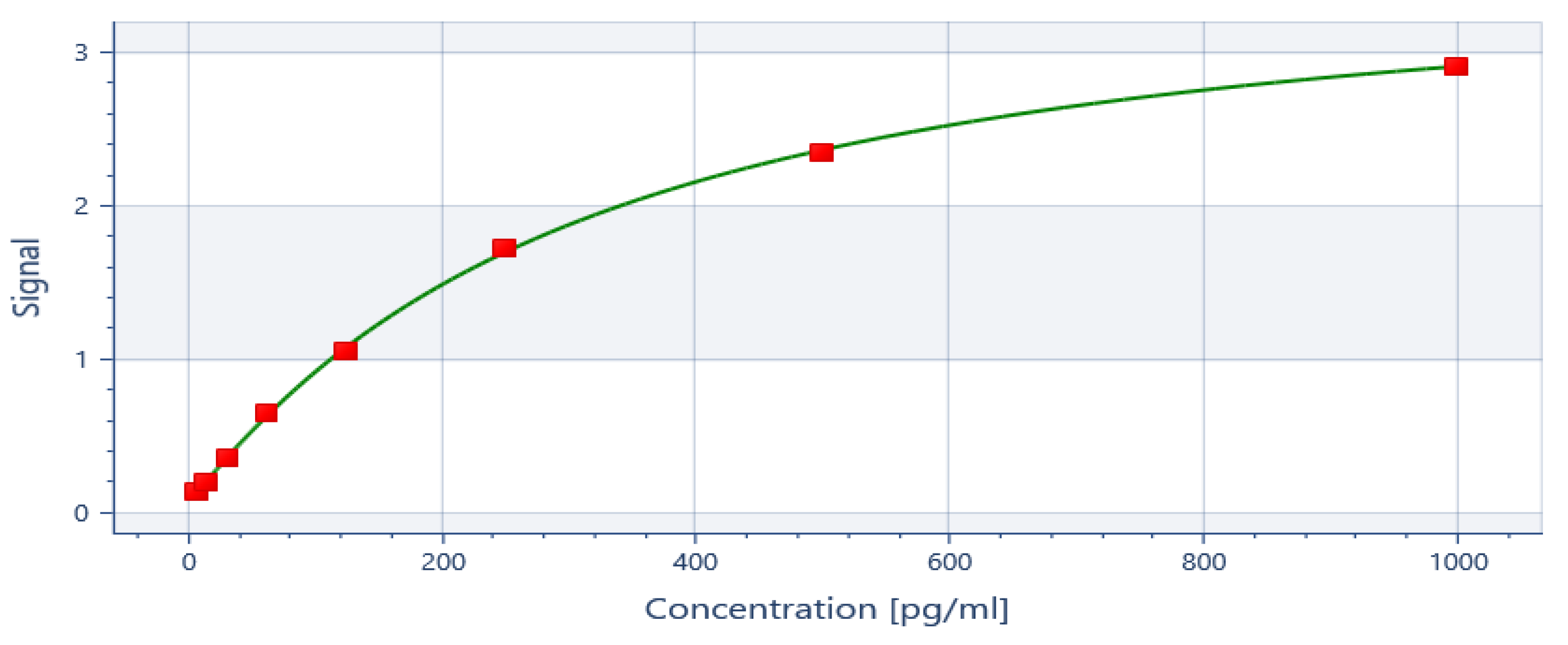
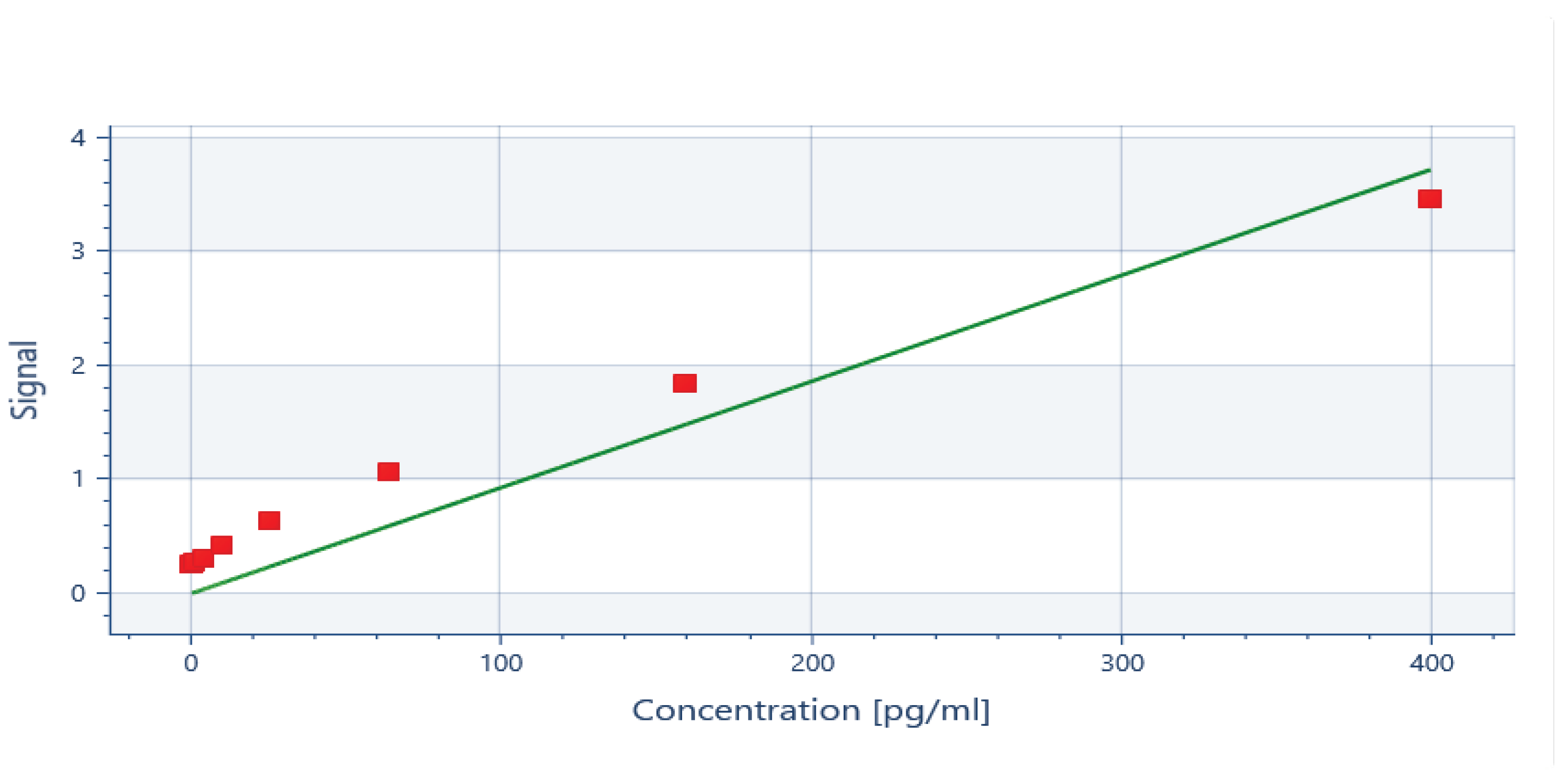
ELISA generated a standard curve and the readings for IL-6 and IL-8 were inferred at the spectrum of 450nm (
Appendix A). The detection limit of the assay for IL-6 were 0.655 pg/ml and 7.8 pg/ml for IL-8. The standard curves of correlation showed R2 : 1 for IL-8 and R2 : 0.98 for IL-6. The data was transferred to SPSS and statistical analysis was then performed on SPSS version 23 for windows. Shapiro-wilk test of normality shows significant p-value which confirms that the data for IL-6 baseline and 6weeks and IL-8 at baseline and 6 weeks is not normally distributed. The comparison from the baseline to 6-weeks follow-up for both IL-6 and IL-8 was done using Wilcoxon Signed rank test. The descriptive statistics are represented as median and inter quartile range (IQR).
3. Results
3.1. Paired Comparison of IL-6 and IL-8 from Baseline to 6 Weeks after Intervention
The results show highly significant (p-value <0.000) improvement in the systemic burden of the pro-inflammatory cytokines from baseline to 6 weeks after intervention for both IL-6 and IL-8 [
Table 1].
3.2. Median and Inter-Quartile Range (IQR) for IL-6 and IL-8
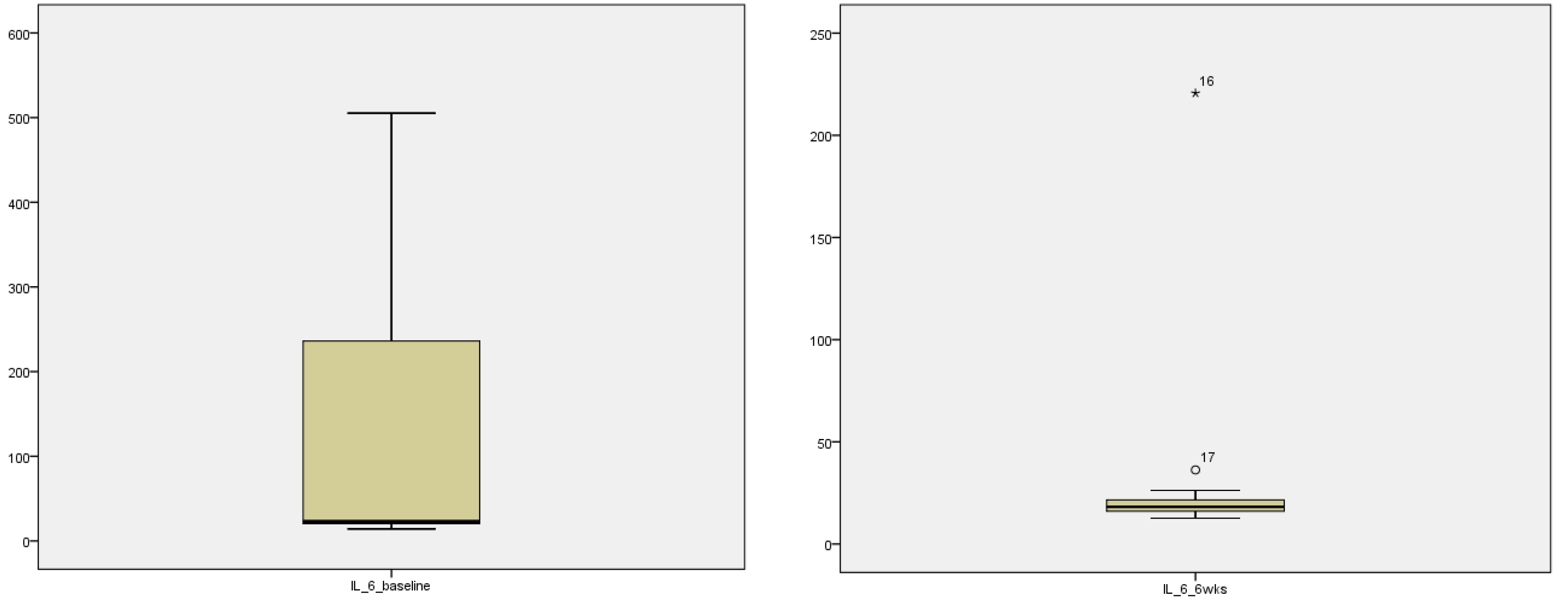
The descriptive statistics for the interleukin 6 and 8 are explained in table 4. Marked improvement in median and inter-quartile range (IQR) from the baseline data to 6 weeks post intervention is seen for both IL-6 and IL-8 [
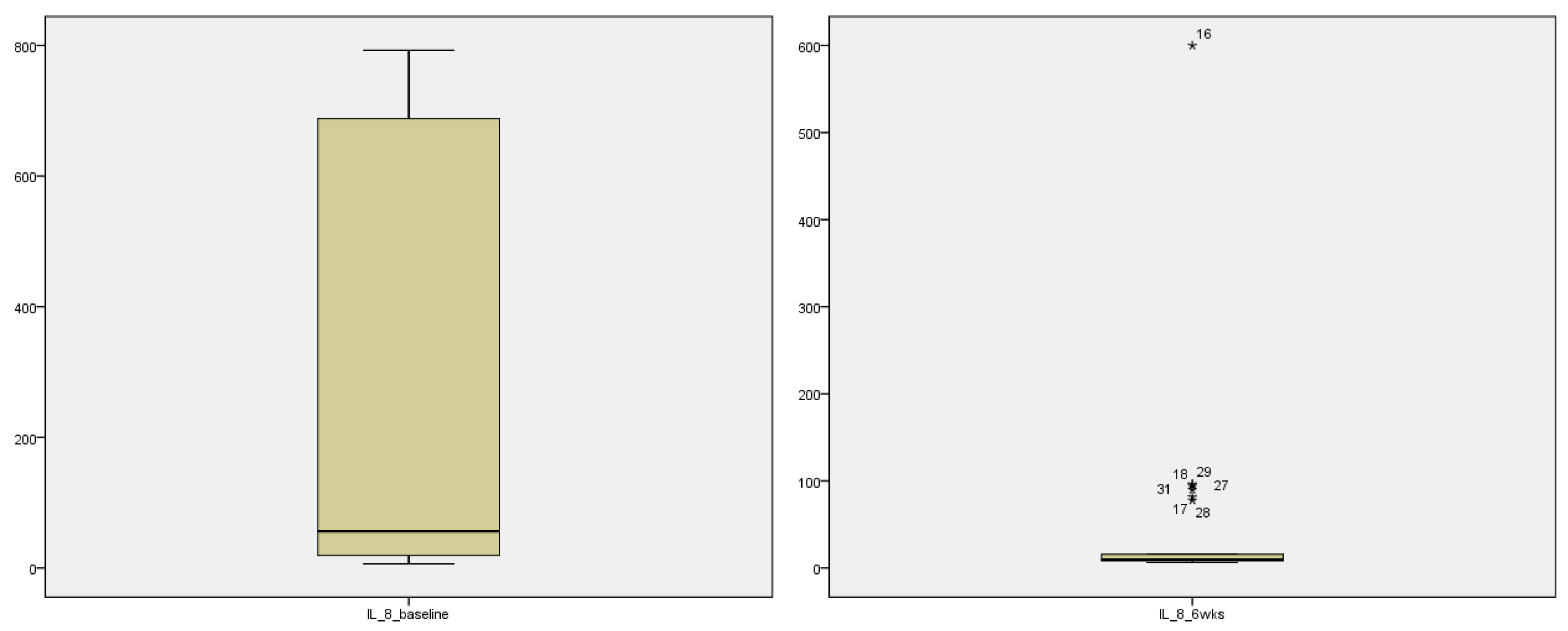
Table 2]. Stem and leaf plot further exhibit marked improvement in the systemic pro-inflammatory markers as seen graphically for IL-6 (
Figure 2) and IL-8 (
Figure 3)
3.3. Stem and leaf plot showing pre and post intervention levels of IL-6 and IL-8
4. Discussion
Despite extensive study on the pathophysiology of the thalassemia, there is little information about the association between periodontal diseases and thalassemia. It is interesting to note that although there are various studies internationally, which report the higher prevalence of periodontal diseases and systemic pro-inflammatory markers in these individuals, no study has reported clinical or dental intervention in this cohort. To the best of the author knowledge, no study has evaluated the effect of NSPT of the systemic burden of pro-inflammatory markers in TM-β patients and this is the first study to evaluate the effect of NSPT on the clinical parameters of periodontal inflammation as well as serum levels of IL-6 and IL-8 and then correlate the two in TM-β patients with gingivitis.
The present study confirms elevated serum IL-6 and IL-8 in TM-β patients with gingivitis as is seen in the literature discussed above. However, the levels of IL-6 and IL-8 reported by Öztürk et al., [
30] in thalassemia cohort of their study are significantly lower than the baseline levels of IL-6 and IL-8 in this study. Öztürk et al., report baseline IL-8 levels of 9.34±1.3 pg/ml and 3.87±0.46 pg/ml for IL-6. In contrast, this study reports baseline levels of 133.51 ± 186.94 pg/ml and 265.55 ± 318.80 pg/ml for IL-6 and IL-8 respectively. However, the present findings are in sync with the finding of Öztürk et al., [
30] where the levels of IL-8 are higher than the levels of IL-6 at baseline as are the levels of IL-8 higher than the levels of IL-6 in the present study. This prompts further investigation on the factors leading to significantly elevated levels of pro-inflammatory markers in the TM-β in the local population. It is reported that iron accumulation due to multiple transfusions in these patients causes elevated systemic levels of inflammatory mediators [
31]. To avoid the effect of this variable, all recruited patients who were on iron chelation therapy with either DFX (n=28) or DFP (n=3) were selected.
Following NSPT, the systemic levels of IL-6 and IL-8 were markedly reduced and showed highly significant result (p-value <0.000) for both the pro-inflammatory cytokines. The average levels of IL-6 at baseline were 133.51 ± 186.94 pg/ml which were reduced to 25.75 ± 37.08 pg/ml after 6 weeks of intervention. The average levels of IL-8 at baseline were 265.55 ± 318.80 pg/ml which were reduced to 45.54 ± 109.57 pg/ml after 6 weeks of intervention [table 1]. These findings validate evidence based informed propositions by the author. NSPT reduces the systemic burden of inflammation by reducing the levels of inflammatory cytokines. In a study by Konopka, Pietrzak and Brzezińska-Błaszczyk, [
32], the levels of GCF IL-8 in patients with periodontal inflammatory diseases, reduced from the range of 7.4 to 96.1pg/ml to 2.7 ± 20.3 pg/ml at 4 weeks of intervention and was significantly lower than at baseline (p-value <0.001). Escobar Arregocés et al., [
33] evaluated the systemic pro-inflammatory markers levels in hypertensive patients with periodontal conditions. Although the result of their study shows reduced levels of IL-6 (from 0.31 ± 0.18pm/ml to 0.24 ± 0.13pg/ml) and IL-8 (from 9.17 ± 1.05pg/ml to 7.79 ± 0.62pg/ml) but not statistically significant. Reduction of serum levels of IL-6 and IL-8, following NSPT in the present study, strengthens the positive effect of periodontal therapy on the systemic inflammatory burden in TM-β patients with gingivitis and provides a new dimension to explore the association of periodontal diseases and thalassemia. To further validate the finding, the study should be tested on a larger cohort.
In blood disorders, as with any other diseases, achieving and maintaining inflammatory homeostasis is the key goal of any treatment modality to ensure survival. Within the limits of the present study, the results not only show biologic and mechanistic association of periodontal diseases and thalassemia, but also provide evidence at the reduction of systemic pro-inflammatory markers following a simple yet effective local measure.
Although there are thalassemia prevention legislations in Sindh and Balochistan [
34,
35] there exists regulatory oversight in their planning since the legislations did not take into account any public and professional dialogue with the thalassemia community. Management of thalassemia in countries like Pakistan pose a major challenge. A disease that is easily preventable but keeps increasing the burden because of lack of preventive measures and protocols. There are more than 40 thalassemia centers currently operating across the country [
36] yet majority of them are just focusing on the transfusional support. These centers lack the multi-specialty treatment facilities mostly because of the lack of government support and unawareness about other systemic health challenges, including oral health
5. Conclusions
This pioneer study demonstrates that thalassemia and periodontal diseases are synergistically inclined towards each other. The effect of locally administered measure of non-surgical periodontal treatment to alleviate/reduce systemic burden of the disease has long been studied and implemented. Literature supports that the periodontal conditions and systemic conditions/diseases have a bi-directional effect on each other and prompted the rationale of the present study. The locally administered oral hygiene care and regime altering the systemic pro-inflammatory markers in the TM-β patients proposes and supports the synergistic effect of the two chronic inflammatory conditions. The results prove the association of periodontal disease with thalassemia and provide a promising new horizon of limiting the systemic burden of the disease while requiring no expensive or fancy treatment modality and also contributing towards patient’s quality of life.
Author Contributions
Data curation, Ayesha Hanif and Komal Khan; Formal analysis, Ayesha Hanif and Moazzam Shahid; Investigation, Ayesha Hanif; Methodology, Ayesha Hanif; Project administration, Asim Qidwai; Software, Ayesha Hanif and Moazzam Shahid; Supervision, Moazzam Shahid and Asim Qidwai; Visualization, Ayesha Hanif; Writing – original draft, Ayesha Hanif; Writing – review & editing, Komal Khan and Asim Qidwai. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to acknowledge the whole team at the AMTF who were extremely accommodating and made this research a smooth process. Ma’am Syeda who was always there for everything.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Figure A1.
Standard curve for IL-6 at 450n.
Figure A1.
Standard curve for IL-6 at 450n.
Figure A2.
Standard curve for IL-8 at 450nm.
Figure A2.
Standard curve for IL-8 at 450nm.
References
- Modell B, Darlison M., 2008. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ, 86, pp. 480-487.
- Cooley TB, Lee P., 1925. A series of cases of splenomegaly in children with anemia and peculiar bone changes. Trans Amer Pediatr Soc, 37, pp. 29-30.
- Sanchez-Villalobos, M. et al., 2022. New Insights Into Pathophysiology of β-Thalassemia. Frontiers in Medicine, 9. [CrossRef]
- Weatherall, D., Williams, T., Allen, S. and ODonnell, A., 2010. The Population Genetics and Dynamics of the Thalassemias. Hematology/Oncology Clinics of North America, 24(6), pp.1021-1031. [CrossRef]
- Zaheer, H., Waheed, U., Abdella, Y. and Konings, F., 2020. Thalassemia in Pakistan: A forward-looking solution to a serious health issue. Global Journal of Transfusion Medicine, 5(1), p.108. [CrossRef]
- Tfp.org.pk. 2021. What is Thalasseamia – Thalassaemia. [online] Available at: <http://tfp.org.pk/what-is-thalasseamia/> [Accessed 3 December 2021].
- Ahmed S, Saleem M, Modell B, Petrou M., 2002. Screening extended families for genetic hemoglobin disorders in Pakistan. The New England Journal of Medicine, 347, pp. 1162–1168. [CrossRef]
- Hattab FN. Periodontal condition and orofacial changes in patients with thalassemia major: a clinical and radiographic overview. J Clin Pediatr Dent 2012; 36: 301-307. [CrossRef]
- Abu Alhaija ESJ, Hattab FN, Al-Omari MAO. Cephalometric measurements and facial deformities in subjects with B-thalassaemia major. Eur J Orthod 2002; 24: 9-19. [CrossRef]
- Wang Y, Yu-Fong Chang J, Wu Y, Cheng S, Chen H, Sun A. Oral manifestations and blood profile in patients with thalassemia trait. J Formos Med Assoc 2013; 112(12): 761-765. [CrossRef]
- Helmi, N., Bashir, M., Shireen, A. and Mirza Ahmed, I., 2017. Thalassemia review: features, dental considerations and management. Electronic physician, 9(3), pp.4003-4008. [CrossRef]
- Singh J, Singh N, Kumar A, Kedia NB, Agarwal A., 2013. Dental and periodontal health status of beta thalassemia major and sickle cell anemic patients: A comparative study. J Int Oral Health, 5, pp. 53-58.
- Tamaddoni A, fereidooni M, khafri S, faghani M., 2014. The relationship between gingivitis and periodontitis with β-thalassemia disease. J Babol Univ Med Sci, 16 (11), pp. 22-27.
- Eugenio, P., 2015. Dental and Periodontal Condition in Patients affected by β-Thalassemia Major and β-Thalassemia Intermedia: A Study among Adults in Sicily, Italy. J Dent Health Oral Disord Ther, 3(1). [CrossRef]
- Cortés-Vieyra, R., Rosales, C. and Uribe-Querol, E., 2016. Neutrophil Functions in Periodontal Homeostasis. J Immunol Res, 2016, pp.1-9. [CrossRef]
- Kinane DF, Stathopoulou PG, Papapanou PN., 2017. Periodontal diseases. Nature reviews. Disease primers, 22, pp. 17-38. https://doi.org/10.1038/nrdp.2017.38. [CrossRef]
- Arabsolghar M, Mohammadi M, Kaheh A, Norouzifard A, Ahmadzade S. Different type of periodontitis and gingivitis in patients with major thalassemia comparing to healthy people. J Oral Health Oral Epidemiol 2015; 4(1): 24-9.
- Siamopoulou-Mavridou A, Mavridis A, Galanakis E, Vasakos S, Fatourou H, Lapatsanis P., 1992. Flow rate and chemistry of parotid saliva related to dental caries and gingivitis in patients with thalassemia major. Int J Paediatr Dent, 2, pp. 93–7. [CrossRef]
- Hajishengallis E., Hajishengallis G., 2014. Neutrophil homeostasis and periodontal health in children and adults. Journal of Dental Research, 93(3) pp. 231–237. [CrossRef]
- N. M. Moutsopoulos, J. Konkel, M. Sarmadi et al., 2014. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Science Translational Medicine, 6(229). [CrossRef]
- Consolini R, Calleri A, Legitimo A, Massei F., 2001. Immunological evaluation of patients with beta-thalassemia major. Acta Haematology, 105, pp. 7-12. [CrossRef]
- Akcalı, Aliye et al., 2015. The association between thalassemia major and periodontal health. Journal of Periodontology, pp. 1047-1057. [CrossRef]
- Sedghi, L., DiMassa, V., Harrington, A., Lynch, S. and Kapila, Y., 2021. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontology 2000, 87(1), pp.107-131. [CrossRef]
- Li, L., Zhang, Y.-L., Liu, X.-Y., Meng, X., Zhao, R.-Q., Ou, L.-L., Li, B.-Z. and Xing, T., 2021. Periodontitis Exacerbates and Promotes the Progression of Chronic Kidney Disease Through Oral Flora, Cytokines, and Oxidative Stress. Frontiers in Microbiology, 12. [CrossRef]
- Tonetti, M.S. and Van Dyke, T.E., 2013. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAPWorkshop on Periodontitis and Systemic Diseases. Journal of Periodontology, 84(4-s), pp.S24–S29. [CrossRef]
- Chapple, I.L.C. and Genco, R. (2013). Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. Journal of Periodontology, 84(4-s), pp. S106– S112. [CrossRef]
- Sanz, M., Ceriello, A., Buysschaert, M., Chapple, I., Demmer, R., Graziani, F., Herrera, D., Jepsen, S., Lione, L., Madianos, P., Mathur, M., Montanya, E., Shapira, L., Tonetti, M. and Vegh, D., 2018. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International diabetes Federation and the European Federation of Periodontology. Diabetes Research and Clinical Practice, 137, pp.231-241. [CrossRef]
- Md Tahir, K., Ab Malek, A., Vaithilingam, R., Saub, R., Safii, S., Rahman, M., Abdul Razak, F., Alabsi, A. and Baharuddin, N., 2020. Impact of non-surgical periodontal therapy on serum Resistin and periodontal pathogen in periodontitis patients with obesity. BMC Oral Health, 20(1). [CrossRef]
- Leite SA, Casanovas RC, Rodrigues VP, Pereira ade, ferreira tc, nascimento fr, et al. The effect of nonsurgical periodontal therapy on hepcidin and on inflammatory and iron marker levels. Brazilian Oral Research. 2019;33. [CrossRef]
- Öztürk, Oguz et al., 2001. Increased plasma levels of interleukin-6 and interleukin-8 in β-Thalassaemia major. Haematologia, pp. 237-244. [CrossRef]
- Calisxkan U, Tongucx MO, Cirisx M, et al., 2011. The investigation of gingival iron accumulation in thalassemia major patients. Journal of Pediatric Hematology and Oncology, 33, pp. 98-102. [CrossRef]
- Konopka Ł, Pietrzak A, Brzezińska-Błaszczyk E. Effect of scaling and root planing on interleukin-1β, interleukin-8 and MMP-8 levels in gingival crevicular fluid from chronic periodontitis patients. Journal of Periodontal Research. 2012;47(6):681–8. [CrossRef]
- Escobar Arregocés, F.M., Del Hierro Rada, M., Sáenz Martinez, M.J., Hernández Meza, F.J., Roa, N.S., Velosa-Porras, J. and Latorre Uriza, C. (2021). Systemic inflammatory response to non-surgical treatment in hypertensive patients with periodontal infection. Medicine, 100(13), p.e24951. [CrossRef]
- The Balochistan Gazette. The Balochistan Prevention and Control of Thalassemia Bill, 2015, Act No. XIV of 2015. Balochistan Provincial Assembly Secretariat. Available from: http://pabalochistan.gov.pk/pab/pab/tables/alldocuments/actdocx/2018-10-23%2012:23:49act-14-2015.pdf. [Last accessed on 2021 Aug 29].
- The Sindh Government Gazette. The Sindh Prevention and Control of Thalassemia Act, 2013, Act No. I of 2013.Provincial Assembly of Sindh. Available from: http://www.pas.gov.pk/uploads/acts/Sindh%20Act%20No.I%20of%202014.pdf. [Last accessed on 2021 Aug 29].
- Naghmi A, Khalid H., 2016. Management of Thalassemia in Pakistan. Journal of Islamabad Medical and Dental College, 5(4) pp.152-153.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).