Submitted:
02 February 2023
Posted:
07 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction

2. Materials and Methods
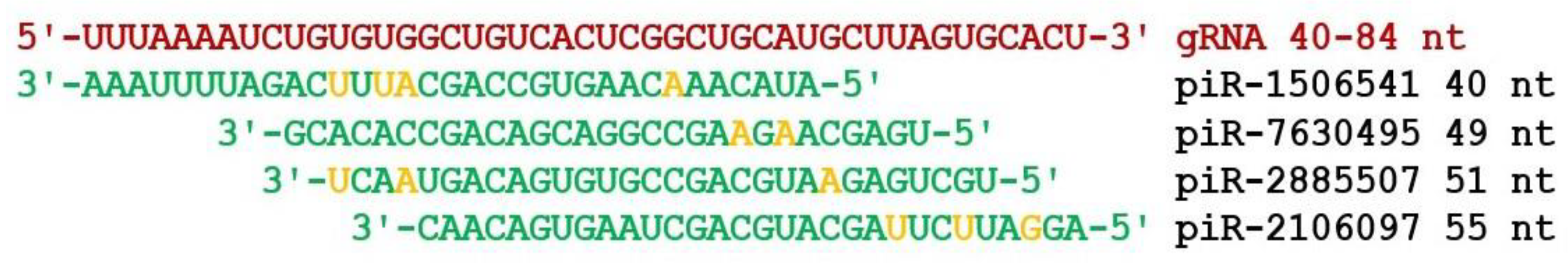
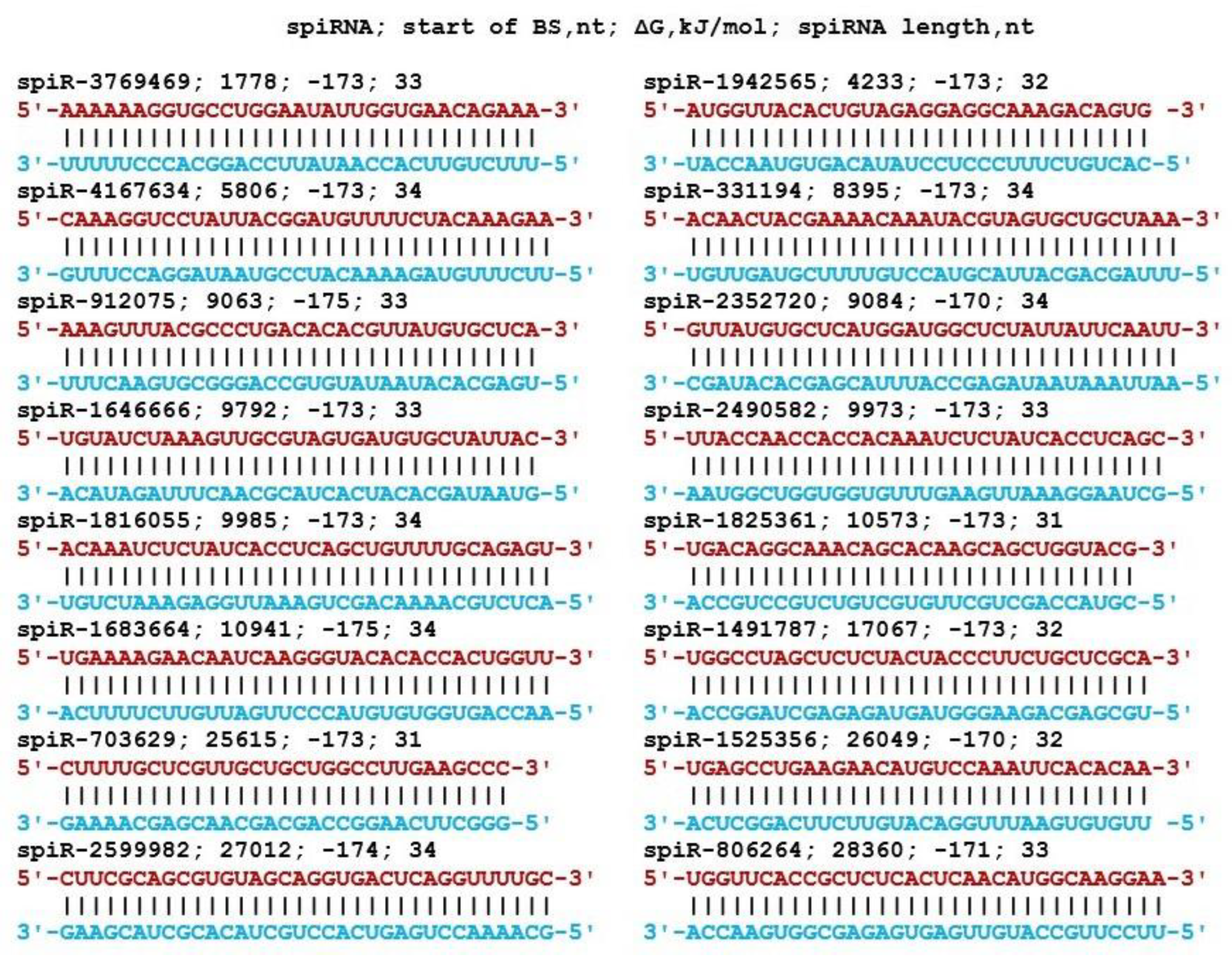
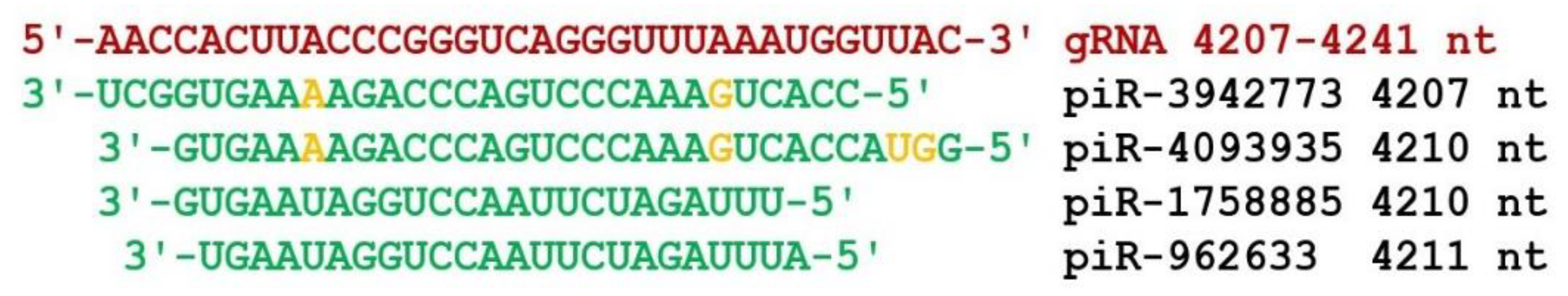
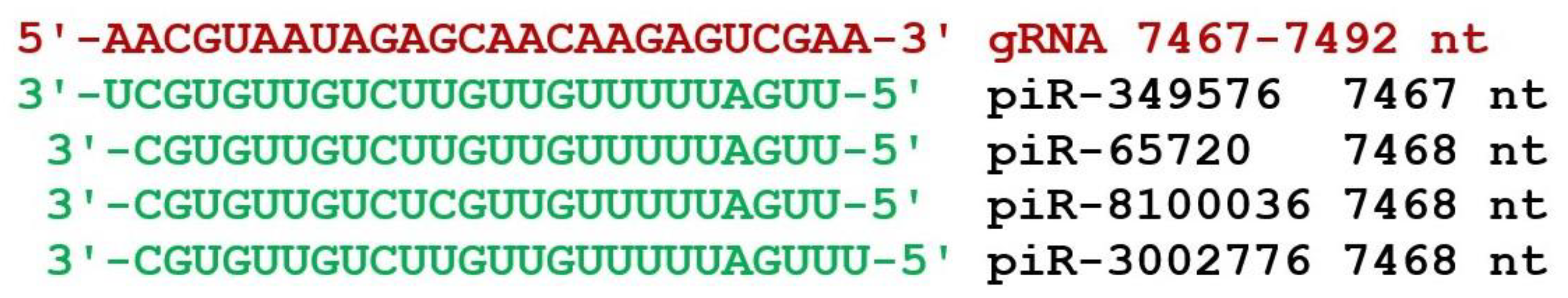
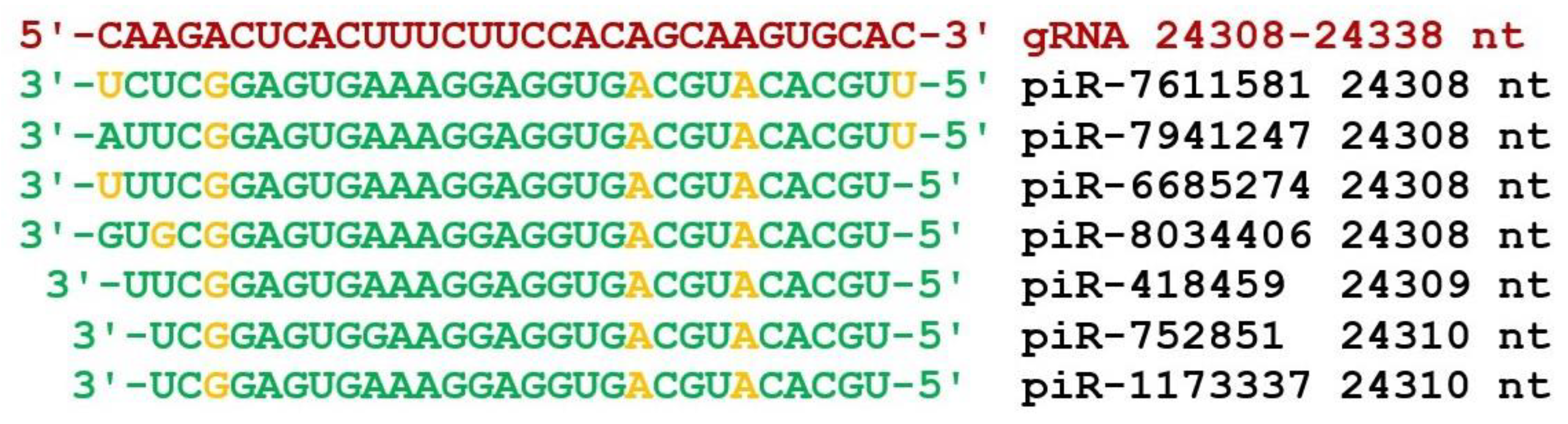
3. Results


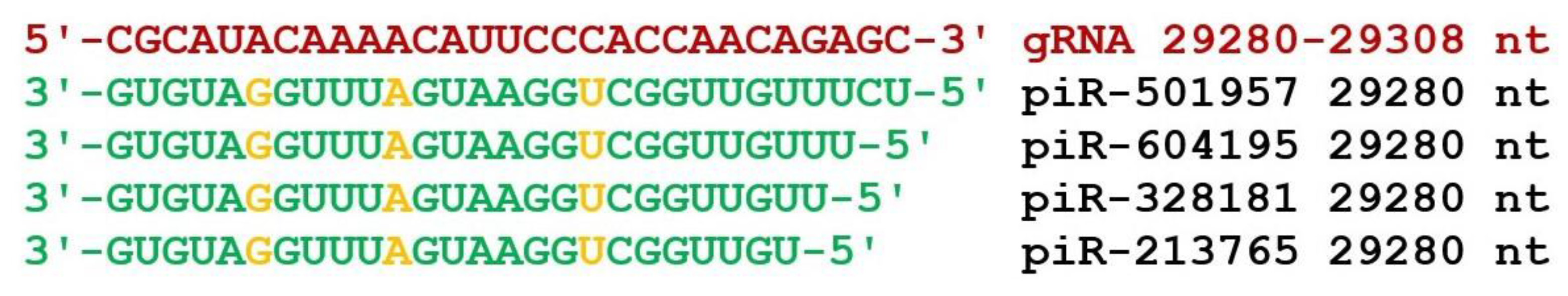
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, C.; Shan, K.J.; Wang, W.; Zhang, S.; Huan, Q.; Qian, W. Evidence for a mouse origin of the SARS-CoV-2 Omicron variant. J Genet Genomics. 2021, 48, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.W.; Xu, H.S.; Liu, S.J. COVID-19 and neurodegenerative diseases. Eur Rev Med Pharmacol Sci. 2022, 26, 4535–4544. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Bhushan, B.; Maurya, A.; Mishra, G.; Singh, S.K.; Awasthi, R. Novel coronavirus disease 2019 (COVID-19) and neurodegenerative disorders. Dermatol. Ther. 2020, 33, e13591. [Google Scholar] [CrossRef]
- Angelini, M.; Teglia, F.; Astolfi, L.; Casolari, G.; Boffetta, P. Decrease of cancer diagnosis during COVID-19 pandemic: a systematic review and meta-analysis. Eur. J. Epidemiol. 2023, 2023, 1–8. [Google Scholar] [CrossRef]
- Alrahawy, M.; Johnson, C.; Aker, M.; Eltyeb, H.A.; Green, S. Impact of COVID-19 on the Mode of Presentation and Stage at Diagnosis of Colorectal Cancer. Cureus. 2022, 14, e32037. [Google Scholar] [CrossRef]
- Ghosh, N.; Nandi, S.; Saha, I. A review on evolution of emerging SARS-CoV-2 variants based on spike glycoprotein. Int Immunopharmacol. 2022, 105, 108565. [Google Scholar] [CrossRef]
- Melidis, L.; Hill, H.J.; Coltman, N.J.; Davies, S.P.; Winczura, K.; Chauhan, T.; Craig, J.S.; Garai, A.; Hooper, C.A.J.; Egan, R.T.; McKeating, J.A.; Hodges, N.J.; Stamataki, Z.; Grzechnik, P.; Hannon, M.J. Supramolecular Cylinders Target Bulge Structures in the 5′ UTR of the RNA Genome of SARS-CoV-2 and Inhibit Viral Replication. Angew. Chem. Int. Ed. 2021, 60, 18144–18151. [Google Scholar] [CrossRef]
- Sosnowski, P.; Tidu, A.; Eriani, G.; Westhof, E.; Martin, F. Correlated sequence signatures are present within the genomic 5’UTR RNA and NSP1 protein in coronaviruses. RNA. 2022, 28, 729–741. [Google Scholar] [CrossRef]
- Xu, T.; Li, L.X.; Jia, Y.; Wu, Q.; Zhu, W.; Xu, Z.; Zheng, B.; Lu, X. One microRNA has the potential to target whole viral mRNAs in a given human coronavirus. Front Microbiol. 2022, 13, 1035044. [Google Scholar] [CrossRef]
- Li, C.; Wang, R.; Wu, A.; Yuan, T.; Song, K.; Bai, Y.; Liu, X. SARS-COV-2 as potential microRNA sponge in COVID-19 patients. BMC Med Genomics. 2022, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.F.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.A.; Tian, Y.; Bruxvoort, K.J.; Tubert, J.E.; Florea, A.; Ku, J.H.; Lee, G.S.; Choi, S.K.; Takhar, H.S.; Aragones, M.; Qian, L. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med. 2022, 28, 1063–1071. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, H.; Chen, Y.; Tian, L.; Huang, D.; Liu, C.; Zhou, Y.; Wang, Y.; Wen, Z.; Yang, B.; Chen, X.; Pei, R. RBM24 inhibits the translation of SARS-CoV-2 polyproteins by targeting the 5’-untranslated region. Antiviral Res. 2022, 209, 105478. [Google Scholar] [CrossRef]
- Dhorne-Pollet, S.; Fitzpatrick, C.; Da Costa, B.; Bourgon, C.; Eléouët, J.F.; Meunier, N.; Burzio, V.A.; Delmas, B.; Barrey, E. Antisense oligonucleotides targeting ORF1b block replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Front Microbiol. 2022, 13, 915202. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.; Scott, G.; Calderon, M.; Haghighi, A.P. N6-Adenosine Methylation of SARS-CoV-2 5’-UTR Regulates Translation. bioRxiv. 2022, 2022.10.17.512569. [Google Scholar] [CrossRef]
- Condé, L.; Allatif, O.; Ohlmann, T.; de Breyne, S. Translation of SARS-CoV-2 gRNA Is Extremely Efficient and Competitive despite a High Degree of Secondary Structures and the Presence of an uORF. Viruses. 2022, 14, 1505. [Google Scholar] [CrossRef]
- Bignon, E.; Miclot, T.; Terenzi, A.; Barone, G.; Monari, A. Structure of the 5’ untranslated region in SARS-CoV-2 genome and its specific recognition by innate immune system via the human oligoadenylate synthase 1. Chem Commun (Camb). 2022, 58, 2176–2179. [Google Scholar] [CrossRef]
- Vora, S.M.; Fontana, P.; Mao, T.; Leger, V.; Zhang, Y.; Fu, T.M.; Lieberman, J.; Gehrke, L.; Shi, M.; Wang, L.; Iwasaki, A.; Wu, H. Targeting stem-loop 1 of the SARS-CoV-2 5’ UTR to suppress viral translation and Nsp1 evasion. Proc Natl Acad Sci U S A. 2022, 119, e2117198119. [Google Scholar] [CrossRef]
- Garcia-Moran, E.; Hernández, M.; Abad, D.; Eiros, J.M. Putative Secondary Structure at 5’UTR as a Potential Antiviral Target against SARS-CoV-2. Rev Esp Quimioter. 2022, 35, 204–212. [Google Scholar] [CrossRef]
- Slobodin, B.; Sehrawat, U.; Lev, A.; Hayat, D.; Zuckerman, B.; Fraticelli, D.; Ogran, A.; Ben-Shmuel, A.; Bar-David, E.; Levy, H.; Ulitsky, I.; Dikstein, R. Cap-independent translation and a precisely located RNA sequence enable SARS-CoV-2 to control host translation and escape anti-viral response. Nucleic Acids Res. 2022, 50, 8080–8092. [Google Scholar] [CrossRef]
- Thakur, V.; Ratho, R.K. OMICRON (B.1.1.529): A new SARS-CoV-2 variant of concern mounting worldwide fear. J Med Virol. 2022, 94, 1821–1824. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G. Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein. J Med Virol. 2022, 94, 1641–1649. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Liang, Z.; Li, T.; Liu, S.; Cui, Q.; Nie, J.; Wu, Q.; Qu, X.; Huang, W.; Wang, Y. The significant immune escape of pseudotyped SARS-CoV-2 variant Omicron. Emerg Microbes Infect. 2022, 11, 1–5. [Google Scholar] [CrossRef]
- Saxena, S.K.; Kumar, S.; Ansari, S.; Paweska, J.T.; Maurya, V.K.; Tripathi, A.K.; Abdel-Moneim, A.S. Characterization of the novel SARS-CoV-2 Omicron (B.1.1.529) variant of concern and its global perspective. J Med Virol. 2022, 94, 1738–1744. [Google Scholar] [CrossRef]
- Papanikolaou, V.; Chrysovergis, A.; Ragos, V.; Tsiambas, E.; Katsinis, S.; Manoli, A.; Papouliakos, S.; Roukas, D.; Mastronikolis, S.; Peschos, D.; Batistatou, A.; Kyrodimos, E.; Mastronikolis, N. From delta to Omicron: S1-RBD/S2 mutation/deletion equilibrium in SARS-CoV-2 defined variants. Gene. 2022, 814, 146134. [Google Scholar] [CrossRef]
- Tian, D.; Sun, Y.; Xu, H.; Ye, Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J Med Virol. 2022, 94, 2376–2383. [Google Scholar] [CrossRef]
- Ma, W.; Yang, J.; Fu, H.; Su, C.; Yu, C.; Wang, Q.; de Vasconcelos, A.T.R.; Bazykin, G.A.; Bao, Y.; Li, M. Genomic Perspectives on the Emerging SARS-CoV-2 Omicron Variant. Genomics Proteomics Bioinformatics. 2022, 20, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Liu, B.; Li, F. Cryo-EM structure of a SARS-CoV-2 omicron spike protein ectodomain. Nat Commun. 2022, 13, 1214. [Google Scholar] [CrossRef] [PubMed]
- Rainville, C.; Sterner, D.E.; Suresh, K. A Method to Monitor Activity of SARS-CoV-2 Nsp3 from Cells. Methods Mol Biol. 2023, 2591, 269–282. [Google Scholar] [CrossRef]
- Gahbauer, S.; Correy, G.J.; Schuller, M.; Ferla, M.P.; Doruk, Y.U.; Rachman, M.; Wu, T.; Diolaiti, M.; Wang, S.; Neitz, R.J.; Fearon, D.; Radchenko, D.; Moroz, Y.; Irwin, J.J.; Renslo, A.R.; Taylor, J.C.; Gestwicki, J.E.; von Delft, F.; Ashworth, A.; Ahel, I.; Shoichet, B.K.; Fraser, J.S. Structure-based inhibitor optimization for the Nsp3 Macrodomain of SARS-CoV-2. bioRxiv 2022, 06.27.497816. [Google Scholar] [CrossRef]
- Diebold, O.; Gonzalez, V.; Venditti, L.; Sharp, C.; Blake, R.A.; Tan, W.S.; Stevens, J.; Caddy, S.; Digard, P.; Borodavka, A.; Gaunt, E. Using Species a Rotavirus Reverse Genetics to Engineer Chimeric Viruses Expressing SARS-CoV-2 Spike Epitopes. J Virol. 2022, 96, e0048822. [Google Scholar] [CrossRef]
- Akaishi, T.; Fujiwara, K.; Ishii, T. Insertion/deletion hotspots in the Nsp2, Nsp3, S1, and ORF8 genes of SARS-related coronaviruses. BMC Ecol Evol. 2022, 22, 123. [Google Scholar] [CrossRef]
- Zhao, L.P.; Lybrand, T.P.; Gilbert, P.B.; Payne, T.H.; Pyo, C.W.; Geraghty, D.E.; Jerome, K.R. Rapidly identifying new coronavirus mutations of potential concern in the Omicron variant using an unsupervised learning strategy. Sci Rep. 2022, 12, 19089. [Google Scholar] [CrossRef]
- Peddireddy, S.P.; Rahman, S.A.; Cillo, A.R.; Vijay, G.M.; Somasundaram, A.; Workman, C.J.; Bain, W.; McVerry, B.J.; Methe, B.; Lee, J.S.; Ray, P.; Ray, A.; Bruno, T.C.; Vignali, D.A.A.; Kitsios, G.D.; Morris, A.; Singh, H.; Sarkar, A.; Das, J. Antibodies targeting conserved non-canonical antigens and endemic coronaviruses associate with favorable outcomes in severe COVID-19. Cell Rep. 2022, 39, 111020. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.S.; Yu, Y.; Li, Y.L.; Cui, L.; Zhao, Z.; Wang, M.; Wang, B.; Zhang, R.; Huang, Y.W. Expression Profile and Localization of SARS-CoV-2 Nonstructural Replicase Proteins in Infected Cells. Microbiol Spectr. 2022, 10, e0074422. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ouyang, M.; Yu, T.; Zhuang, J.; Wang, W.; Liu, X.; Duan, F.; Guo, D.; Peng, X.; Pan, J.A. Genome-Wide Analysis of the Indispensable Role of Non-structural Proteins in the Replication of SARS-CoV-2. Front Microbiol. 2022, 13, 907422. [Google Scholar] [CrossRef] [PubMed]
- Jupudi, S.; Rajagopal, K.; Murugesan, S.; Kumar, B.K.; Raman, K.; Byran, G.; Chennaiah, J.; Muthiah, V.P.; Dasan, P.B.; Sankaran, S.S. Identification of Papain-Like Protease inhibitors of SARS CoV-2 through HTVS, Molecular docking, MMGBSA and Molecular dynamics approach. Afr J Bot. 2021, 151, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, G.C.; Souza-Silva, F.; Sacramento, C.Q.; Trugilho, M.R.O.; Valente, R.H.; Napoleão-Pêgo, P.; Dias, S.S.G.; Fintelman-Rodrigues, N.; Temerozo, J.R.; Carels, N.; Alves, C.R.; Pereira, M.C.S.; Provance, D.W., Jr.; Souza, T.M.L.; De-Simone, S.G. SARS-CoV-2 Proteins Bind to Hemoglobin and Its Metabolites. Int J Mol Sci. 2021, 22, 9035. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.N. Small RNAs just got bigger: Piwi-interacting RNAs (piRNAs) in mammalian testes. Genes Dev. 2006, 20, 1993–1997. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Zhou, H.; Zhang, P.; Song, T.; Ying, Z.; Yu, H.; Li, Y.; Zhao, Y.; Zeng, X.; He, S.; Chen, R. piRBase: integrating piRNA annotation in all aspects. Nucleic Acids Res, 2021, 50, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Belkozhayev, A.; Niyazova, R.; Wilson, C.; Jainakbayev, N.; Pyrkova, A.; Ashirbekov, Y.; Akimniyazova, A.; Sharipov, K.; Ivashchenko, A. Bioinformatics Analysis of the Interaction of miRNAs and piRNAs with Human mRNA Genes. Having di- and Trinucleotide Repeats. Genes, 2022, 13, 800. [Google Scholar] [CrossRef]
- Akimniyazova, A.N.; Niyazova, T.K.; Yurikova, O.Y.; Pyrkova, A.Y.; Zhanuzakov, M.A.; Ivashchenko, A.T. piRNAs may regulate expression of candidate genes of esophageal adenocarcinoma. Front. Genet. 2022, 13, 1069637. [Google Scholar] [CrossRef]
- Akimniyazova, A.; Yurikova, O.; Pyrkova, A.; Rakhmetullina, A.; Niyazova, T.; Ryskulova, A.; Ivashchenko, A. In Silico Study of piRNA Interactions with the SARS-CoV-2 Genome. Int J Mol Sci. 2022, 23, 9919. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Lu, Y.; Li, Y.; Zheng, Y.; Kan, Y.; Chen, R.; He, S. piRBase: A comprehensive database of piRNA sequences. Nucleic Acids Res. 2018, 47, 175–180. [Google Scholar] [CrossRef]
- Ivashchenko, A.; Berillo, O.; Pyrkova, A.; Niyazova, R.; and Atambayeva, S. MiR-3960 binding sites with mRNA of human genes. Bioinformation 2014, 10, 423–427. [Google Scholar] [CrossRef]
- Friedman, R.A.; Honig, B.A. A free energy analysis of nucleic acid base stacking in aqueous solution. Biophys. J. 1995, 69, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Kool, E.T. Hydrogen Bonding, Base Stacking, and Steric Effects in DNA Replication. Annu. Rev. Biophys. Biomol. Struct. 2001, 30, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Heinemann, U.A. A novel form of RNA double helix based on G·U and C·A+ wobble base pairing. RNA 2018, 24, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Leontis, N.B.; Stombaugh, J.; Westhof, E. The non-watson-crick base pairs and their associated isostericity matrices. Nucleic Acids Res. 2002, 30, 3497–3531. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.D. A Brief History of the Discovery of RNA-Mediated Antiviral Immune Defenses in Vector Mosquitos. Microbiol Mol Biol Rev. 2022, e0019121. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, J.; Cao, J.; Liu, F.; Fu, M.; Xue, B.; Zhou, A.; Chen, S.; Liu, J.; Zhou, Y.; Shi, Y.; Peng, W.; Chen, X. SARS-CoV-2 infection activates CREB/CBP in cellular cyclic AMP-dependent pathways. J Med Virol. 2022. [Google Scholar] [CrossRef]
- Lin, M.H.; Li, D.; Tang, B.; Li, L.; Suhrbier, A.; Harrich, D. Defective Interfering Particles with Broad-Acting Antiviral Activity for Dengue, Zika, Yellow Fever, Respiratory Syncytial and SARS-CoV-2 Virus Infection. Microbiol Spectr. 2022, e0394922. [Google Scholar] [CrossRef]
- Casseb, S.M.M.; Khayat, A.S.; de Souza, J.E.S.; de Oliveira, E.H.C.; Dos Santos, S.E.B.; da Costa Vasconcelos, P.F.; de Assumpção, P.P. Anticipating the Next Chess Move: Blocking SARS-CoV-2 Replication and Simultaneously Disarming Viral Escape Mechanisms. Genes, 2022, 13, 2147. [Google Scholar] [CrossRef]
- Sarangi, M.K.; Padhi, S.; Rath, G.; Nanda, S.S.; Yi, D.K. Success of nano-vaccines against COVID-19: a transformation in nanomedicine. Expert Rev Vaccines 2022, 21, 1739–1761. [Google Scholar] [CrossRef]
- Lee, C.Y.; Lowen, A.C. Animal models for SARS-CoV-2. Curr Opin Virol 2021, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Cleary, S.J.; Pitchford, S.C.; Amison, R.T.; Carrington, R.; Robaina Cabrera, C.L.; Magnen, M.; Looney, M.R.; Gray, E.; Page, C.P. Animal models of mechanisms of SARS-CoV-2 infection and COVID-19 pathology. Br J Pharmacol. 2020, 177, 4851–4865. [Google Scholar] [CrossRef] [PubMed]
- Frazzini, S.; Amadori, M.; Turin, L.; Riva, F. SARS CoV-2 infections in animals, two years into the pandemic. Arch Virol. 2022, 167, 2503–2517. [Google Scholar] [CrossRef] [PubMed]
- Kamenova, S.; Sharapkhanova, A.; Akimniyazova, A.; Kuzhybayeva, K.; Kondybayeva, A.; Rakhmetullina, A.; Pyrkova, A.; Ivashchenko, A. piRNA and miRNA can suppress the expression of multiple sclerosis candidate genes. Nanomaterials 2022, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Kondybayeva, A.; Akimniyazova, A.N.; Kamenova, S.U.; Ivashchenko, A. The characteristics of miRNA binding sites in mRNA of ZFHX3 gene And its orthologs. Vavilov J. Genet. Breed. 2018, 22, 438–444. [Google Scholar] [CrossRef]
- Aisina, D.; Niyazova, R.; Atambayeva, S.; Ivashchenko, A. Prediction of clusters of miRNA binding sites in mRNA candidate genes of breast cancer subtypes. PeerJ 2019, 7, e8049. [Google Scholar] [CrossRef] [PubMed]
- Kondybayeva, A.; Akimniyazova, A.; Kamenova, S.; Duchshanova, G.; Aisina, D.; Goncharova, A.; Ivashchenko, A. Prediction of miRNA interaction with mRNA of stroke candidate genes. Neurol. Sci. 2019, 41, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Mukushkina, D.; Aisina, D.; Pyrkova, A.; Ryskulova, A.; Labeit, S.; Ivashchenko, A. In silico Prediction of miRNA Interactions With Candidate Atherosclerosis Gene mRNAs. Front. Genet. 2020, 11, 605054. [Google Scholar] [CrossRef] [PubMed]
- Kamenova, S.; Aralbayeva, A.; Kondybayeva, A.; Akimniyazova, A.; Pyrkova, A.; Ivashchenko, A. Evolutionary Changes in the Interaction of miRNA With mRNA of Candidate Genes for Parkinson’s Disease. Front. Genet. 2021, 12, 647288. [Google Scholar] [CrossRef] [PubMed]
- Akimniyazova, A.; Pyrkova, A.; Uversky, V.; Ivashchenko, A. Predicting Associations of miRNAs and Candidate Gastric Cancer Genes for Nanomedicine. Nanomaterials 2021, 11, 691. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory Mechanisms and Intercellular Transfer of MicroRNAs in Living Cells. JBC. 2010, 285, 17442–17452. [Google Scholar] [CrossRef]
- Urbanelli, L.; Magini, A.; Buratta, S.; Brozzi, A.; Sagini, K.; Polchi, A.; Tancini, B.; Emiliani, C. Signaling Pathways in Exosomes Biogenesis, Secretion and Fate. Genes 2013, 4, 152–170. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




