Submitted:
07 February 2023
Posted:
08 February 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
2.Mosquito Collection
2.Molecular Identification of Mosquitoes
2.Phylogenetic tree analyses based on COI sequences for MALDI-TOF comparison
2.Sample homogenization and MALDI-TOF MS analysis
2.MALDI-TOF MS parameters
2.MS spectra analysis
2.Database creation and blind tests
| Collection | ||||||
|---|---|---|---|---|---|---|
| Species | Site | Year | Number of specimens | BOLD# accession number (number of associated sequences) | COI gene sequence coverage (%) / identity (%) | Number of specimens included in the reference MS DB per body part (Thoraxes/Legs)§ |
| Cx. (Mel.) adamesi | Mac. | 2018 | 1 | FGMOS2220-20 (1) | 99% / 97% | 1 / 1 |
| Cx. (Cux.) declarator | Mac. | 2018, 2019 | 7 | FGMOS2272-20 (7) | 100% / 99‒100% | 2 / 1 |
| Cx. (Mel.) dunni | Mac. | 2018, 2019 | 30 | FGMOS2748-20 (20); FGMOS2750-20 (10) | 100% / 98‒100% | 4 / 3 |
| Cx. (Mel.) eastor | Mac. | 2018, 2019 | 3 | FGMOS2695-20 (1); FGMOS2743-20 (1); FGMOS2752-20 (1) | 100% / 99‒100% | 1 / 1 |
| Cx. (Mel.) idottus | Mac. | 2018 | 2 | FGMOS3098-23 (2) | 100% / 100% | 1 / 0 |
| Cx. (Cux.) nigripalpus | Mac. | 2018, 2019 | 12 | FGMOS225-16 (12) | 100% / 100% | 3 / 2 |
| Cx. (Mel.) pedroi | Mac. | 2018, 2019 | 15 | FGMOS2700-20 (12); FGMOS2758-20 (3) | 100% / 100% | 4 / 2 |
| Cx. (Mel.) phlogistus | Mac. | 2019 | 1 | FGMOS1542-20 (1) | 100% / 99% | 1 / 1 |
| Cx. (Mel.) portesi | Mac. | 2018 | 28 | FGMOS2416-20 (28) | 100% / 100% | 4 / 1 |
| Cx. (Cux.) quinquefasciatus | Cay. | 2019, 2020 | 34 | FGMOS2275-20 (34) | 100% / 100% | 4 / 4 |
| Cx. (Mel.) rabanicolus | Mac. | 2018 | 5 | FGMOS2744-20 (5) | 100% / 100% | 2 / 2 |
| Cx. (Mel.) spissipes | Mac. | 2018, 2019 | 9 | FGMOS2701-20 (9) | 100% / 97‒99% | 3 / 2 |
| Cx. (Cux.) usquatus | Mac., Rem. | 2018, 2019 | 22 | FGMOS046-16 (9); FGMOS049-16 (1); FGMOS2284-20 (12) | 100% / 99‒100% | 4 / 4 |
| Total | 169 | 34 / 24 | ||||
Results
3.Morphological and molecular identification of Culex specimens
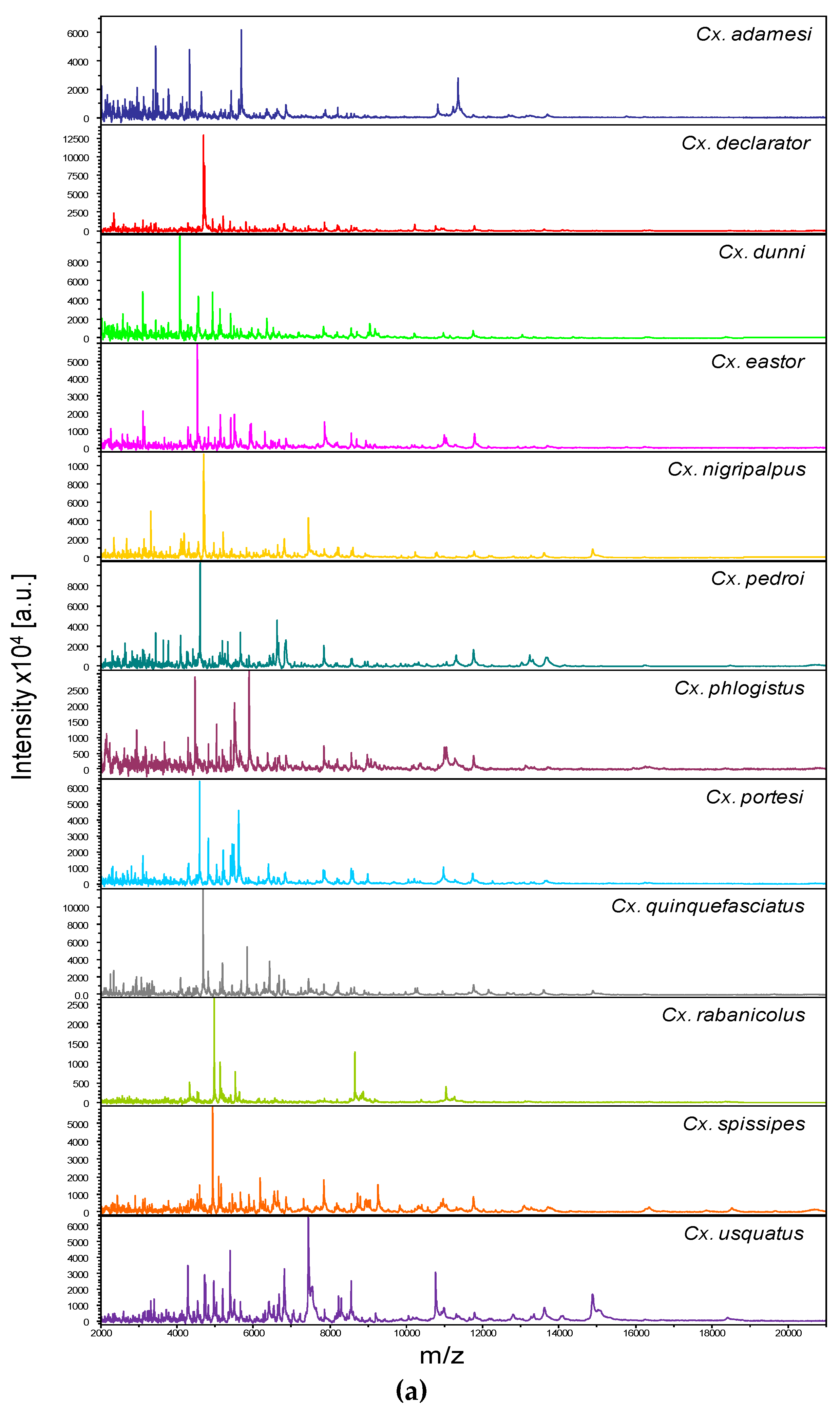
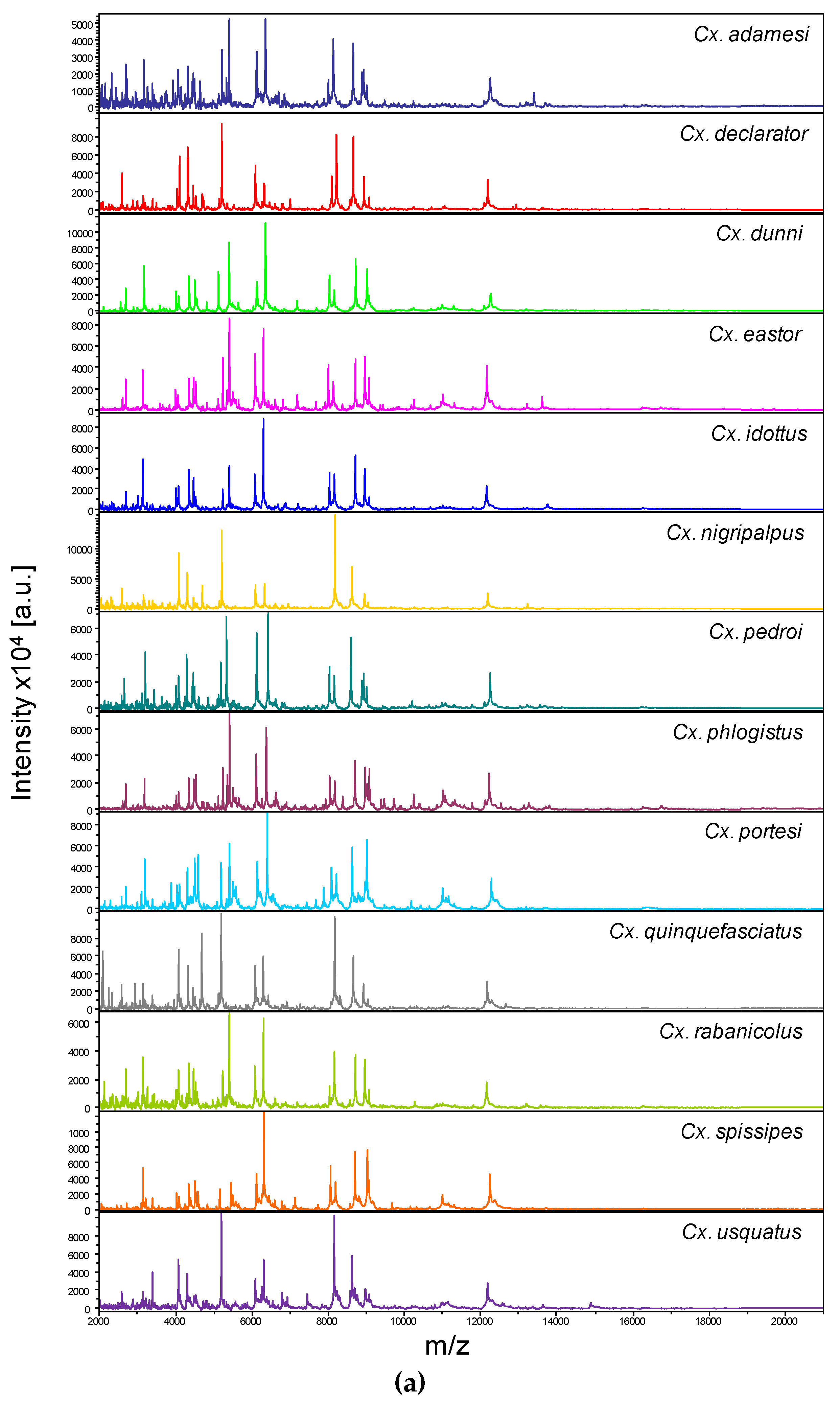
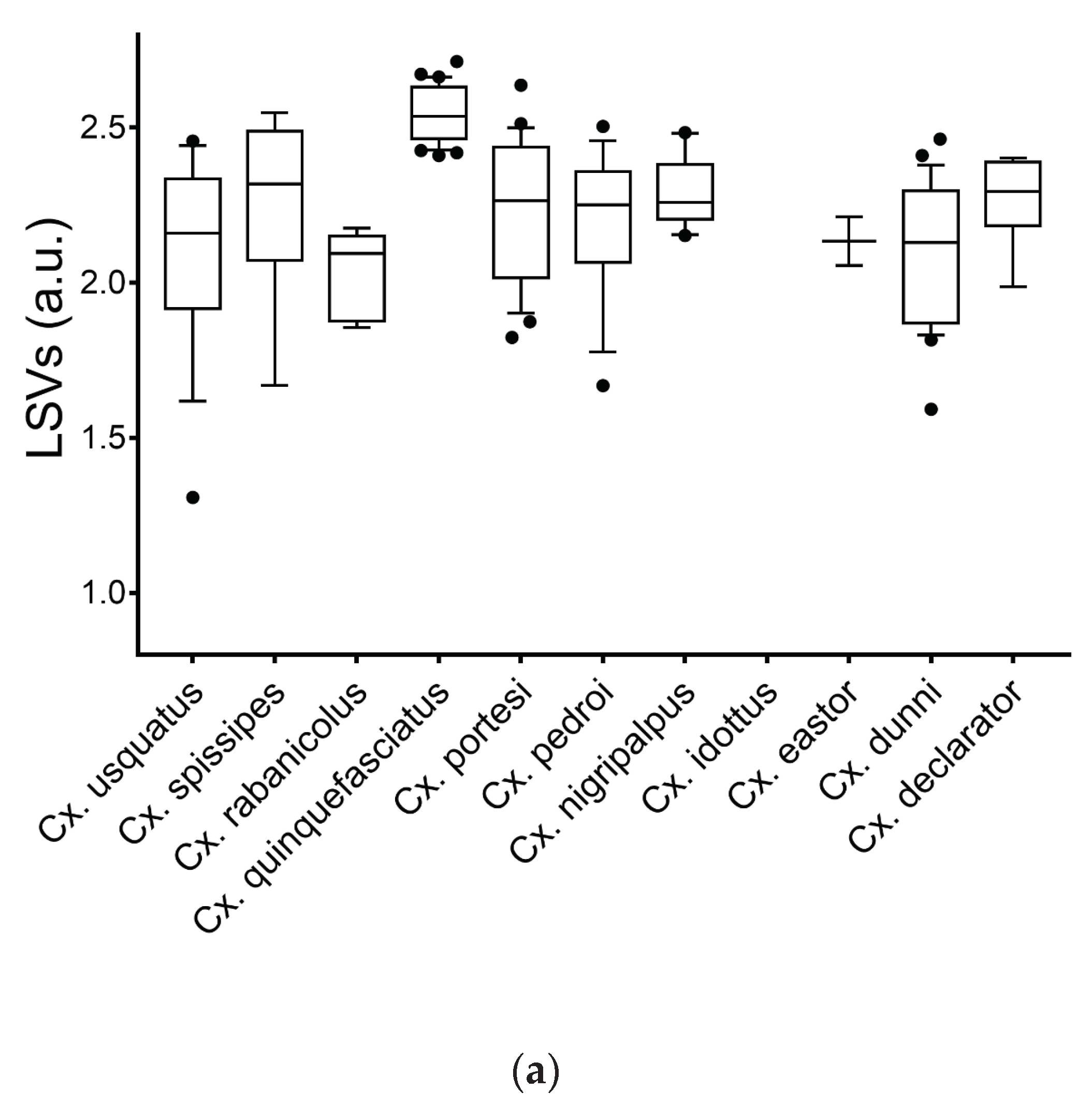
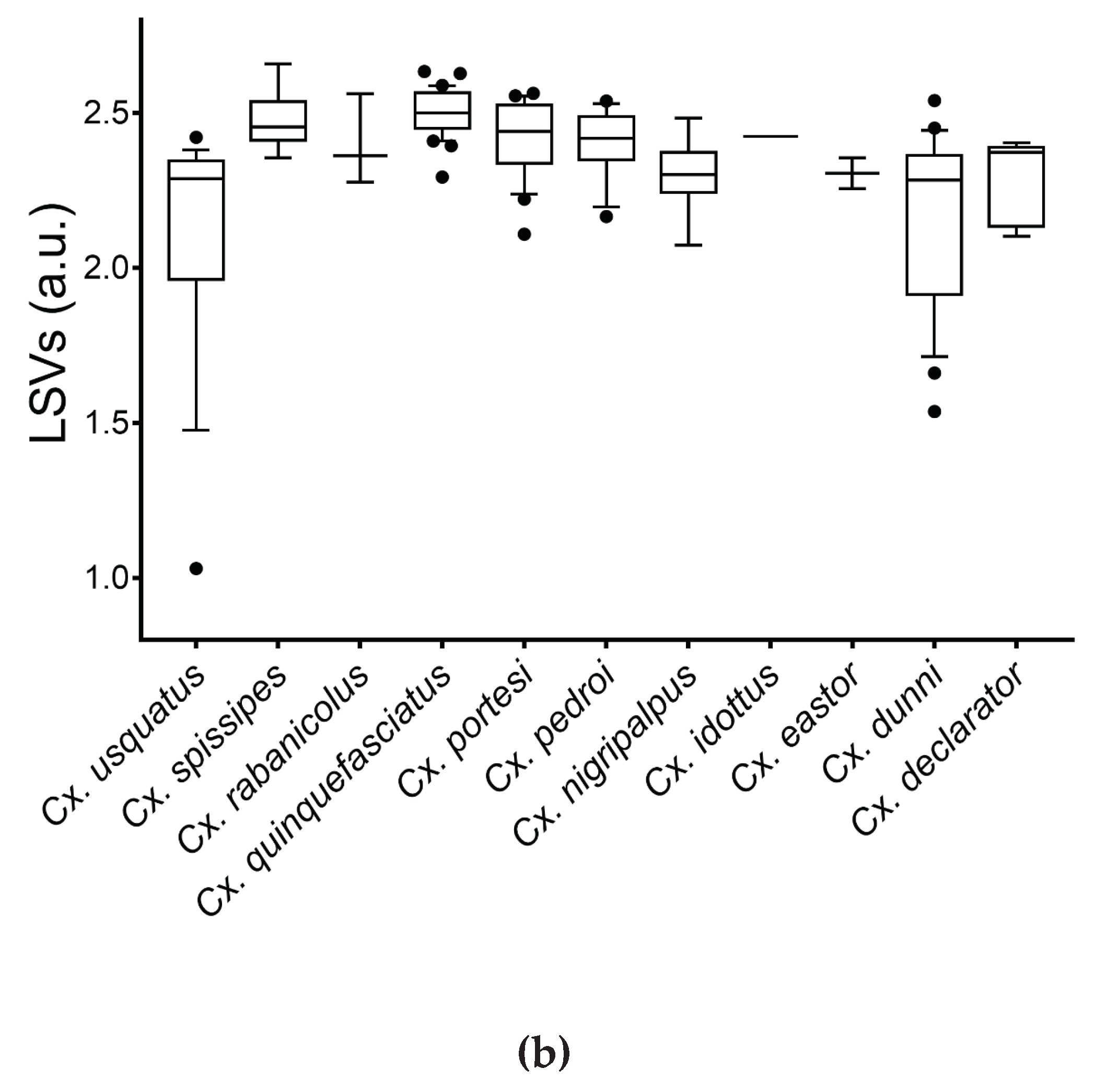
3.Reproducible and specific MS spectra from two Culex body parts
3.Leg and Thoraxe Biomarkers Distinguishing Culex Species
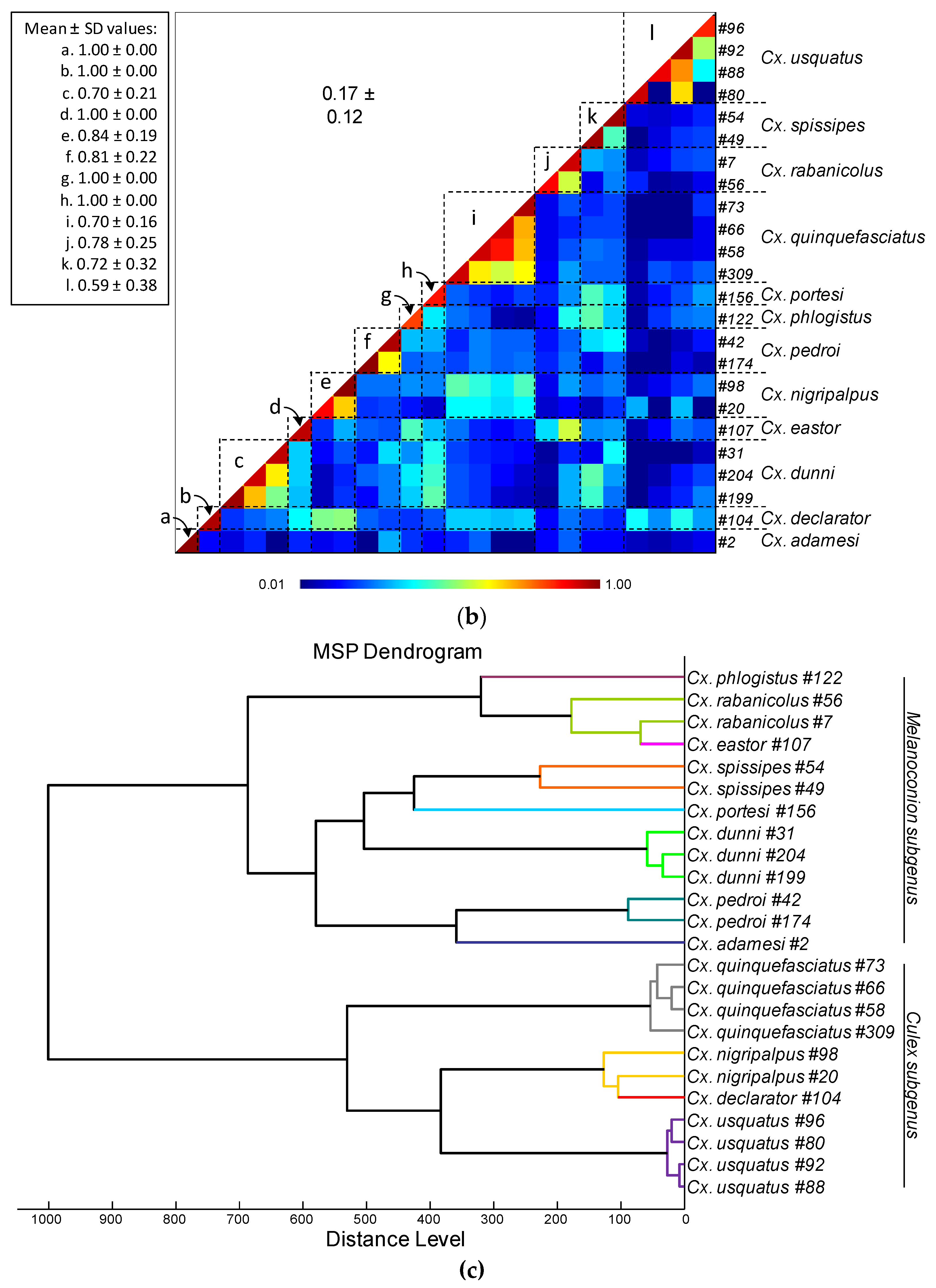
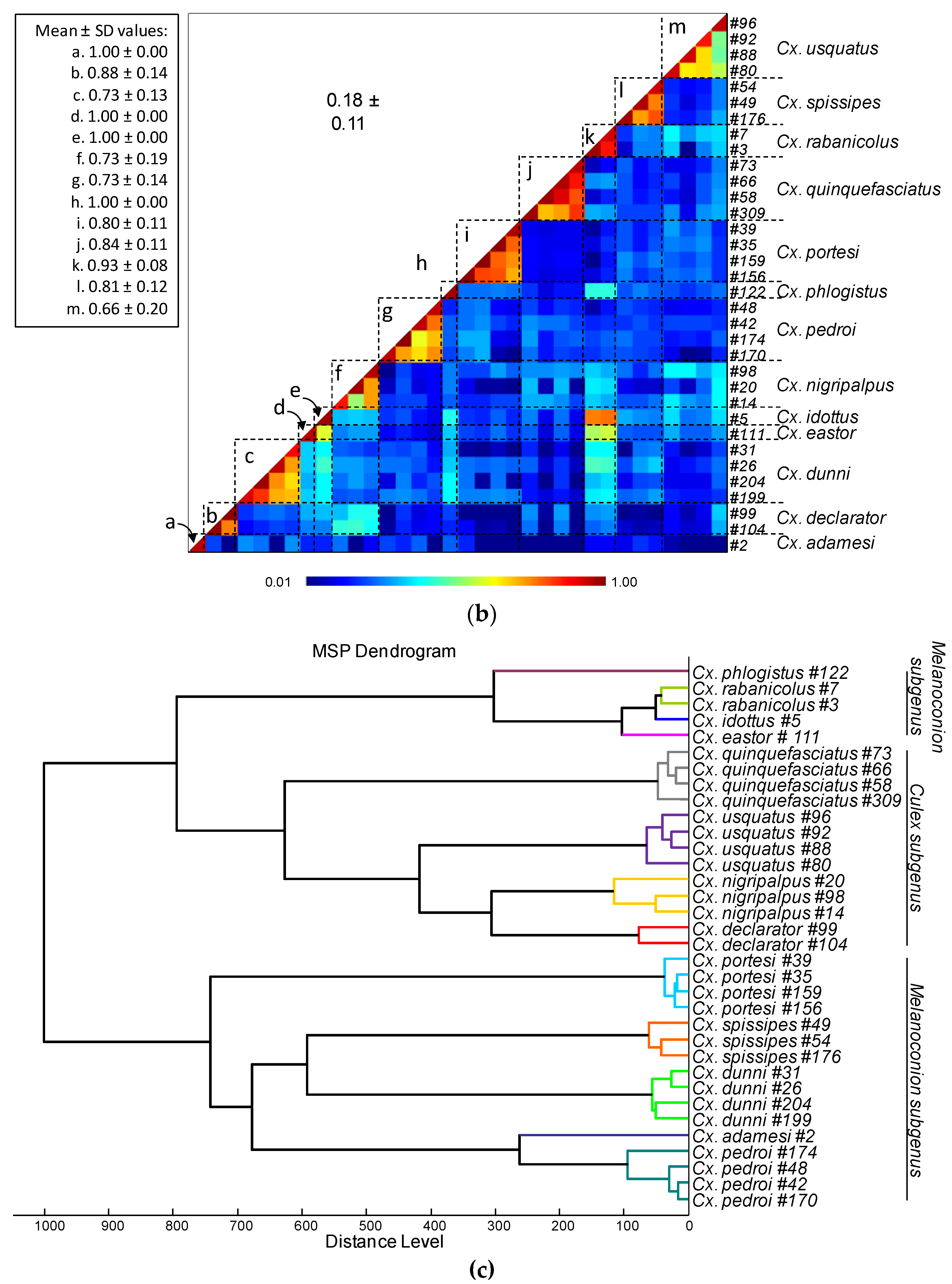
3.Creation of the MS reference spectra database and validation steps
Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foley, D.H.; Rueda, L.M.; Wilkerson, R.C. Insight into Global Mosquito Biogeography from Country Species Records. J. Med. Entomol. 2007, 44, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Talaga, S.; Dejean, A.; Carinci, R.; Gaborit, P.; Dusfour, I.; Girod, R. Updated Checklist of the Mosquitoes (Diptera: Culicidae) of French Guiana. J. Med. Entomol. 2015, 52, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Talaga, S.; Duchemin, J.-B.; Girod, R.; Dusfour, I. The Culex Mosquitoes (Diptera: Culicidae) of French Guiana: A Comprehensive Review With the Description of Three New Species. J. Med. Entomol. 2021, tjaa205. [Google Scholar] [CrossRef]
- Pommier de Santi, V.P.; Girod, R.; Mura, M.; Dia, A.; Briolant, S.; Djossou, F.; Dusfour, I.; Mendibil, A.; Simon, F.; Deparis, X.; et al. Epidemiological and Entomological Studies of a Malaria Outbreak among French Armed Forces Deployed at Illegal Gold Mining Sites Reveal New Aspects of the Disease’s Transmission in French Guiana. Malar. J. 2016, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Dusfour, I.; Issaly, J.; Carinci, R.; Gaborit, P.; Girod, R. Incrimination of Anopheles (Anopheles) Intermedius Peryassú, An. (Nyssorhynchus) Nuneztovari Gabaldón, An. (Nys.) Oswaldoi Peryassú as Natural Vectors of Plasmodium Falciparum in French Guiana. Mem. Inst. Oswaldo Cruz 2012, 107, 429–432. [Google Scholar] [CrossRef]
- Pommier de Santi, V.; Dia, A.; Adde, A.; Hyvert, G.; Galant, J.; Mazevet, M.; Nguyen, C.; Vezenegho, S.B.; Dusfour, I.; Girod, R.; et al. Malaria in French Guiana Linked to Illegal Gold Mining. Emerg. Infect. Dis. 2016, 22, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Vezenegho, S.B.; Adde, A.; de Santi, V.P.; Issaly, J.; Carinci, R.; Gaborit, P.; Dusfour, I.; Girod, R.; Briolant, S. High Malaria Transmission in a Forested Malaria Focus in French Guiana: How Can Exophagic Anopheles Darlingi Thwart Vector Control and Prevention Measures? Mem. Inst. Oswaldo Cruz 2016, 111, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Chouin-Carneiro, T.; Vega-Rua, A.; Vazeille, M.; Yebakima, A.; Girod, R.; Goindin, D.; Dupont-Rouzeyrol, M.; Lourenço-de-Oliveira, R.; Failloux, A.-B. Differential Susceptibilities of Aedes Aegypti and Aedes Albopictus from the Americas to Zika Virus. PLoS Negl. Trop. Dis. 2016, 10, e0004543. [Google Scholar] [CrossRef] [PubMed]
- Fouque, F.; Vazeille, M.; Mousson, L.; Gaborit, P.; Carinci, R.; Issaly, J.; Rodhain, F.; Failloux, A.-B. Aedes Aegypti in French Guiana: Susceptibility to a Dengue Virus. Trop. Med. Int. Health 2001, 6, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Girod, R.; Guidez, A.; Carinci, R.; Issaly, J.; Gaborit, P.; Ferrero, E.; Ardillon, V.; Fontaine, A.; Dusfour, I.; Briolant, S. Detection of Chikungunya Virus Circulation Using Sugar-Baited Traps during a Major Outbreak in French Guiana. PLoS Negl. Trop. Dis. 2016, 10, e0004876. [Google Scholar] [CrossRef] [PubMed]
- Harbach, R.E. Classification within the Cosmopolitan Genus Culex (Diptera: Culicidae): The Foundation for Molecular Systematics and Phylogenetic Research. Acta Trop. 2011, 120, 1–14. [Google Scholar] [CrossRef]
- Floch, H.; De Lajudie, P. Streptococci, Dick reaction and endemic lymphangitis in French Guiana. Bull. Soc. Pathol. Exot. Filiales 1945, 38, 127–132. [Google Scholar]
- Fontes, G.; Leite, A.B.; Vasconcelos de Lima, A.R.; Freitas, H.; Ehrenberg, J.P.; da Rocha, E.M.M. Lymphatic Filariasis in Brazil: Epidemiological Situation and Outlook for Elimination. Parasit. Vectors 2012, 5, 272. [Google Scholar] [CrossRef] [PubMed]
- Gorris, M.E.; Bartlow, A.W.; Temple, S.D.; Romero-Alvarez, D.; Shutt, D.P.; Fair, J.M.; Kaufeld, K.A.; Del Valle, S.Y.; Manore, C.A. Updated Distribution Maps of Predominant Culex Mosquitoes across the Americas. Parasit. Vectors 2021, 14, 547. [Google Scholar] [CrossRef]
- Labarthe, N.; Guerrero, J. Epidemiology of Heartworm: What Is Happening in South America and Mexico? Vet. Parasitol. 2005, 133, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Rochlin, I.; Faraji, A.; Healy, K.; Andreadis, T.G. West Nile Virus Mosquito Vectors in North America. J. Med. Entomol. 2019, 56, 1475–1490. [Google Scholar] [CrossRef] [PubMed]
- Stenn, T.; Peck, K.J.; Rocha Pereira, G.; Burkett-Cadena, N.D. Vertebrate Hosts of Aedes Aegypti, Aedes Albopictus, and Culex Quinquefasciatus (Diptera: Culicidae) as Potential Vectors of Zika Virus in Florida. J. Med. Entomol. 2019, 56, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.J. da S.P.; Thies, S.F.; da Silva, D.J.F.; Kubiszeski, J.R.; Barreto, E.S.; Monteiro, H.A. de O.; Mondini, A.; São Bernardo, C.S.; Bronzoni, R.V. de M. Ecological Aspects of Potential Arbovirus Vectors (Diptera: Culicidae) in an Urban Landscape of Southern Amazon, Brazil. Acta Trop. 2020, 202, 105276. [Google Scholar] [CrossRef] [PubMed]
- Degallier, N. Les arbovirirus selvatiques en Guyane Francaise et leurs vecteurs, 1982.
- Day, J.F.; Curtis, G.A. Annual Emergence Patterns of Culex Nigripalpus Females before, during and after a Widespread St. Louis Encephalitis Epidemic in South Florida. J. Am. Mosq. Control Assoc. 1993, 9, 249–255. [Google Scholar] [PubMed]
- Sardelis, M.R.; Turell, M.J.; Dohm, D.J.; O’Guinn, M.L. Vector Competence of Selected North American Culex and Coquillettidia Mosquitoes for West Nile Virus. Emerg. Infect. Dis. 2001, 7, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Alto, B.W.; Connelly, C.R.; O’Meara, G.F.; Hickman, D.; Karr, N. Reproductive Biology and Susceptibility of Florida Culex Coronator to Infection with West Nile Virus. Vector Borne Zoonotic Dis. 2014, 14, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Consoli, R.A.G.B.; Lourenço de Oliveira, R. Principais Mosquitos de Importância Sanitária No Brasil Available online:. Available online: https://www.arca.fiocruz.br/handle/icict/2708 (accessed on 20 September 2022).
- Forattini, O.P. Produto | Detalhes | Culicidologia Médica Vol. Available online: https://www.edusp.com.br/loja/produto/370/culicidologia-medica-vol--2--identificacao,-biologia,-epidemiologia (accessed on 20 September 2022).
- Digoutte, J.P. Ecologie des Arbovirus et Leur Rôle Pathogène chez L’Homme en Guyane Française; Institut Pasteur de la Guyane Française—Groupe I.N.S.E.R.M. U79, 1975.
- Fischer, C.; Pontier, D.; Filippi-Codaccioni, O.; Pons, J.-B.; Postigo-Hidalgo, I.; Duhayer, J.; Brünink, S.; Drexler, J.F. Venezuelan Equine Encephalitis Complex Alphavirus in Bats, French Guiana. Emerg. Infect. Dis. 2021, 27, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Mutricy, R.; Djossou, F.; Matheus, S.; Lorenzi-Martinez, E.; De Laval, F.; Demar, M.; Nacher, M.; Rousset, D.; Epelboin, L. Discriminating Tonate Virus from Dengue Virus Infection: A Matched Case–Control Study in French Guiana, 2003–Am. J. Trop. Med. Hyg. 2020, 102, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Auguste, A.J.; Volk, S.M.; Arrigo, N.C.; Martinez, R.; Ramkissoon, V.; Adams, A.P.; Thompson, N.N.; Adesiyun, A.A.; Chadee, D.D.; Foster, J.E.; et al. Isolation and Phylogenetic Analysis of Mucambo Virus (Venezuelan Equine Encephalitis Complex Subtype IIIA) in Trinidad. Virology 2009, 392, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Treangen, T.J.; Schoeler, G.; Phillippy, A.M.; Bergman, N.H.; Turell, M.J. Identification and Genomic Analysis of a Novel Group C Orthobunyavirus Isolated from a Mosquito Captured near Iquitos, Peru. PLoS Negl. Trop. Dis. 2016, 10, e0004440. [Google Scholar] [CrossRef]
- Ferro, C.; Boshell, J.; Moncayo, A.C.; Gonzalez, M.; Ahumada, M.L.; Kang, W.; Weaver, S.C. Natural Enzootic Vectors of Venezuelan Equine Encephalitis Virus in the Magdalena Valley, Colombia. Emerg. Infect. Dis. 2003, 9, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Padilla, S.L.; Prieto, K.; Dohm, D.J.; Turell, M.J.; Klein, T.A.; Fernández, R.; Watts, D.M.; Lowen, R.G.; Palacios, G.F.; Pitt, M.L.; et al. Complete Genomic Sequences of Venezuelan Equine Encephalitis Virus Subtype IIID Isolates from Mosquitoes. Arch. Virol. 2020, 165, 1715–1717. [Google Scholar] [CrossRef]
- Torres, R.; Samudio, R.; Carrera, J.-P.; Young, J.; Márquez, R.; Hurtado, L.; Weaver, S.; Chaves, L.F.; Tesh, R.; Cáceres, L. Enzootic Mosquito Vector Species at Equine Encephalitis Transmission Foci in the República de Panamá. PLoS ONE 2017, 12, e0185491. [Google Scholar] [CrossRef]
- Galindo, P. Los Arbovírus de Panama. Rev Med Panama 1978, 1–41. [Google Scholar]
- Viana, L.A.; Soares, P.; Paiva, F.; Lourenço-De-Oliveira, R. Caiman-Biting Mosquitoes and the Natural Vectors of Hepatozoon Caimani in Brazil. J. Med. Entomol. 2010, 47, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Sallum, M.A.; Forattini, O.P. Revision of the Spissipes Section of Culex (Melanoconion) (Diptera:Culicidae). J. Am. Mosq. Control Assoc. 1996, 12, 517–600. [Google Scholar] [PubMed]
- Laurito, M.; Oliveira, T.M. de; Almiron, W.R.; Sallum, M.A.M. COI Barcode versus Morphological Identification of Culex ( Culex ) (Diptera: Culicidae) Species: A Case Study Using Samples from Argentina and Brazil. Mem. Inst. Oswaldo Cruz 2013, 108, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Andrić, A.; Šikoparija, B.; Obreht, D.; Dan, M.; Preradović, J.; Radenković, S.; Pérez-Banon, C.; Vujić, A. DNA Barcoding Applied: Identifying the Larva of Merodon Avidus (Diptera: Syrphidae). Acta Entomol. Musei Natl. Pragae 2014, 54, 741–757. [Google Scholar]
- Hernández-Triana, M.; Brugman, A.; Nikolova, I.; Ruiz-Arrondo, I.; Barrero, E.; Thorne, L.; Fernández de Marco, M.; Krüger, A.; Lumley, S.; Johnson, N.; et al. DNA Barcoding of British Mosquitoes (Diptera, Culicidae) to Support Species Identification, Discovery of Cryptic Genetic Diversity and Monitoring Invasive Species. ZooKeys 2019, 832, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Bourke, B.P.; Oliveira, T.P.; Suesdek, L.; Bergo, E.S.; Sallum, M.A.M. A Multi-Locus Approach to Barcoding in the Anopheles Strodei Subgroup (Diptera: Culicidae). Parasit. Vectors 2013, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Raharimalala, F.N.; Andrianinarivomanana, T.M.; Rakotondrasoa, A.; Collard, J.M.; Boyer, S. Usefulness and Accuracy of MALDI-TOF Mass Spectrometry as a Supplementary Tool to Identify Mosquito Vector Species and to Invest in Development of International Database. Med. Vet. Entomol. 2017, 31, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Pflüger, V.; Wittwer, M.; Ziegler, D.; Chandre, F.; Simard, F.; Lengeler, C. Identification of Cryptic Anopheles Mosquito Species by Molecular Protein Profiling. PLoS ONE 2013, 8, e57486. [Google Scholar] [CrossRef] [PubMed]
- Yssouf, A.; Almeras, L.; Raoult, D.; Parola, P. Emerging Tools for Identification of Arthropod Vectors. Future Microbiol. 2016, 11, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Chavy, A.; Nabet, C.; Normand, A.C.; Kocher, A.; Ginouves, M.; Prévot, G.; Vasconcelos dos Santos, T.; Demar, M.; Piarroux, R.; de Thoisy, B. Identification of French Guiana Sand Flies Using MALDI-TOF Mass Spectrometry with a New Mass Spectra Library. PLoS Negl. Trop. Dis. 2019, 13, e0007031. [Google Scholar] [CrossRef]
- Bamou, R.; Costa, M.M.; Diarra, A.Z.; Martins, A.J.; Parola, P.; Almeras, L. Enhanced Procedures for Mosquito Identification by MALDI-TOF MS. Parasit. Vectors 2022, 15, 240. [Google Scholar] [CrossRef] [PubMed]
- Gaffigan, T.V.; Ward, R.A. Index to the Second Supplement to “A Catalog of the Mosquitoes of the World”, with Corrections and Additions (Diptera: Culicidae). Mosq. Syst. 1985, 52–63. [Google Scholar]
- Harbach, R.E. Mosquito Taxonomic Inventory Available online: http://mosquitotaxonomic- inventory.info/.
- Knight, K.L.; Stone, A. A Catalog of the Mosquitoes of the World (Diptera: Culicidae); 2nd ed.; Entomological Society of America: College Park, Maryland, 1977; Volume 6. [Google Scholar]
- Pecor, J.E.; Mallampalli, V.L.; Harbach, R.E.; Peyton, E.L. Catalog and Illustrated Review of the Subgenus Melanoconion of Culex (Diptera: Culicidae). Contrib. Am. Entomol. Inst. USA 1992. [Google Scholar]
- Sirivanakarn, S. A Review of the Systematics and a Proposed Scheme of Internal Classification of the New World Subgenus Melanoconion of Culex (Diptera, Culicidae). Mosq Syst 1982, 70. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Ratnasingham, S.; Hebert, P.D.N. Bold: The Barcode of Life Data System (Http://Www.Barcodinglife.Org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Ostell, J.; Pruitt, K.D.; Sayers, E.W. GenBank. Nucleic Acids Res. 2018, 46, D41–D47. [Google Scholar] [CrossRef] [PubMed]
- BLAST.
- Briolant, S.; Costa, M.M.; Nguyen, C.; Dusfour, I.; Pommier de Santi, V.; Girod, R.; Almeras, L. Identification of French Guiana Anopheline Mosquitoes by MALDI-TOF MS Profiling Using Protein Signatures from Two Body Parts. PLoS ONE 2020, 15, e0234098. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rúa, A.; Pagès, N.; Fontaine, A.; Nuccio, C.; Hery, L.; Goindin, D.; Gustave, J.; Almeras, L. Improvement of Mosquito Identification by MALDI-TOF MS Biotyping Using Protein Signatures from Two Body Parts. Parasit. Vectors 2018, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Nebbak, A.; Willcox, A.C.; Bitam, I.; Raoult, D.; Parola, P.; Almeras, L. Standardization of Sample Homogenization for Mosquito Identification Using an Innovative Proteomic Tool Based on Protein Profiling. PROTEOMICS 2016, 16, 3148–3160. [Google Scholar] [CrossRef] [PubMed]
- Lafri, I.; Almeras, L.; Bitam, I.; Caputo, A.; Yssouf, A.; Forestier, C.-L.; Izri, A.; Raoult, D.; Parola, P. Identification of Algerian Field-Caught Phlebotomine Sand Fly Vectors by MALDI-TOF MS. PLoS Negl. Trop. Dis. 2016, 10, e0004351. [Google Scholar] [CrossRef]
- Diarra, A.Z.; Almeras, L.; Laroche, M.; Berenger, J.-M.; Koné, A.K.; Bocoum, Z.; Dabo, A.; Doumbo, O.; Raoult, D.; Parola, P. Molecular and MALDI-TOF Identification of Ticks and Tick-Associated Bacteria in Mali. PLoS Negl. Trop. Dis. 2017, 11, e0005762. [Google Scholar] [CrossRef] [PubMed]
- Seng, P.; Drancourt, M.; Gouriet, F.; La Scola, B.; Fournier, P.-E.; Rolain, J.M.; Raoult, D. Ongoing Revolution in Bacteriology: Routine Identification of Bacteria by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2009, 49, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Karger, A. Current Developments to Use Linear MALDI-TOF Spectra for the Identification and Typing of Bacteria and the Characterization of Other Cells/Organisms Related to Infectious Diseases. PROTEOMICS – Clin. Appl. 2016, 10, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Sevestre, J.; Diarra, A.Z.; Laroche, M.; Almeras, L.; Parola, P. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry: An Emerging Tool for Studying the Vectors of Human Infectious Diseases. Future Microbiol. 2021, 16, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Yssouf, A.; Socolovschi, C.; Flaudrops, C.; Ndiath, M.O.; Sougoufara, S.; Dehecq, J.-S.; Lacour, G.; Berenger, J.-M.; Sokhna, C.S.; Raoult, D.; et al. Matrix-Assisted Laser Desorption Ionization - Time of Flight Mass Spectrometry: An Emerging Tool for the Rapid Identification of Mosquito Vectors. PLoS ONE 2013, 8, e72380. [Google Scholar] [CrossRef] [PubMed]
- Cupp, E.W.; Hassan, H.K.; Yue, X.; Oldland, W.K.; Lilley, B.M.; Unnasch, T.R. West Nile Virus Infection in Mosquitoes in the Mid-South USA, 2002–J. Med. Entomol. 2007, 44, 117–125. [Google Scholar] [CrossRef]
- Swei, A.; Couper, L.I.; Coffey, L.L.; Kapan, D.; Bennett, S. Patterns, Drivers, and Challenges of Vector-Borne Disease Emergence. Vector Borne Zoonotic Dis. 2020, 20, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; O’Guinn, M.L.; Jones, J.W.; Sardelis, M.R.; Dohm, D.J.; Watts, D.M.; Fernandez, R.; Travassos da Rosa, A.; Guzman, H.; Tesh, R.; et al. Isolation of Viruses from Mosquitoes (Diptera: Culicidae) Collected in the Amazon Basin Region of Peru. J. Med. Entomol. 2005, 42, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Torres-Gutierrez, C.; de Oliveira, T.M.P.; Emerson, K.J.; Sterlino Bergo, E.; Mureb Sallum, M.A. Molecular Phylogeny of Culex Subgenus Melanoconion (Diptera: Culicidae) Based on Nuclear and Mitochondrial Protein-Coding Genes. R. Soc. Open Sci. 2018, 5, 171900. [Google Scholar] [CrossRef] [PubMed]
- Wilai, P.; Namgay, R.; Made Ali, R.S.; Saingamsook, J.; Saeung, A.; Junkum, A.; Walton, C.; Harbach, R.E.; Somboon, P. A Multiplex PCR Based on Mitochondrial COI Sequences for Identification of Members of the Anopheles Barbirostris Complex (Diptera: Culicidae) in Thailand and Other Countries in the Region. Insects 2020, 11, 409. [Google Scholar] [CrossRef]
- Kweka, E.J.; Kamau, L.; Munga, S.; Lee, M.-C.; Githeko, A.K.; Yan, G. A First Report of Anopheles Funestus Sibling Species in Western Kenya Highlands. Acta Trop. 2013, 128, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Vezenegho, S.B.; Bass, C.; Puinean, M.; Williamson, M.S.; Field, L.M.; Coetzee, M.; Koekemoer, L.L. Development of Multiplex Real-Time PCR Assays for Identification of Members of the Anopheles Funestus Species Group. Malar. J. 2009, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, F.; Picard, M.; Sulesco, T.; Haddad, N.; Harrat, Z.; Sawalha, S.S.; Günay, F.; Kanani, K.; Shaibi, T.; Akhramenko, D.; et al. Identification of Mosquitoes (Diptera: Culicidae): An External Quality Assessment of Medical Entomology Laboratories in the MediLabSecure Network. Parasit. Vectors 2018, 11, 553. [Google Scholar] [CrossRef]
- Jinbo, U.; Kato, T.; Ito, M. Current Progress in DNA Barcoding and Future Implications for Entomology. Entomol. Sci. 2011, 14, 107–124. [Google Scholar] [CrossRef]
- Paskewitz, S.M.; Wesson, D.M.; Collins, F.H. The Internal Transcribed Spacers of Ribosomal DNA in Five Members of the Anopheles Gambiae Species Complex. Insect Mol. Biol. 1993, 2, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Huynh, L.N.; Diarra, A.Z.; Nguyen, H.S.; Tran, L.B.; Do, V.N.; Ly, T.D.A.; Ho, V.H.; Nguyen, X.Q.; Parola, P. MALDI-TOF Mass Spectrometry Identification of Mosquitoes Collected in Vietnam. Parasit. Vectors 2022, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Bamou, R.; Costa, M.M.; Diarra, A.Z.; Martins, A.J.; Parola, P.; Almeras, L. Enhanced Procedures for Mosquito Identification by MALDI-TOF MS. Parasit. Vectors 2022, 15, 240. [Google Scholar] [CrossRef] [PubMed]
- Nabet, C.; Kone, A.K.; Dia, A.K.; Sylla, M.; Gautier, M.; Yattara, M.; Thera, M.A.; Faye, O.; Braack, L.; Manguin, S.; et al. New Assessment of Anopheles Vector Species Identification Using MALDI-TOF MS. Malar. J. 2021, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.V.; Bogale, H.N.; Bhalerao, D.; Keita, K.; Camara, D.; Barry, Y.; Keita, M.; Coulibaly, D.; Kone, A.K.; Doumbo, O.K.; et al. High-Throughput Detection of Eukaryotic Parasites and Arboviruses in Mosquitoes. Biol. Open 2021, 10, bio058855. [Google Scholar] [CrossRef] [PubMed]
- Kent, R.J. Molecular Methods for Arthropod Bloodmeal Identification and Applications to Ecological and Vector-Borne Disease Studies. Mol. Ecol. Resour. 2009, 9, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Niare, S.; Berenger, J.-M.; Dieme, C.; Doumbo, O.; Raoult, D.; Parola, P.; Almeras, L. Identification of Blood Meal Sources in the Main African Malaria Mosquito Vector by MALDI-TOF MS. Malar. J. 2016, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Tahir, D.; Almeras, L.; Varloud, M.; Raoult, D.; Davoust, B.; Parola, P. Assessment of MALDI-TOF Mass Spectrometry for Filariae Detection in Aedes Aegypti Mosquitoes. PLoS Negl. Trop. Dis. 2017, 11, e0006093. [Google Scholar] [CrossRef]
- Rakotonirina, A.; Pol, M.; Kainiu, M.; Barsac, E.; Tutagata, J.; Kilama, S.; O’Connor, O.; Tarantola, A.; Colot, J.; Dupont-Rouzeyrol, M.; et al. MALDI-TOF MS: Optimization for Future Uses in Entomological Surveillance and Identification of Mosquitoes from New Caledonia. Parasit. Vectors 2020, 13, 359. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A.L.; Batovska, J.; Webb, C.E.; Lynch, S.E.; Blacket, M.J.; Šlapeta, J.; Parola, P.; Laroche, M. Accurate identification of Australian mosquitoes using protein profiling. Parasitology 2019, 146, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Tandina, F.; Laroche, M.; Davoust, B.; K Doumbo, O.; Parola, P. Blood Meal Identification in the Cryptic Species Anopheles Gambiae and Anopheles Coluzzii Using MALDI-TOF MS. Parasite 2022, 25, 40. [Google Scholar] [CrossRef] [PubMed]
- Dieme, C.; Yssouf, A.; Vega-Rúa, A.; Berenger, J.-M.; Failloux, A.-B.; Raoult, D.; Parola, P.; Almeras, L. Accurate Identification of Culicidae at Aquatic Developmental Stages by MALDI-TOF MS Profiling. Parasit. Vectors 2014, 7, 544. [Google Scholar] [CrossRef] [PubMed]
- Nebbak, A.; Koumare, S.; Willcox, A.C.; Berenger, J.-M.; Raoult, D.; Almeras, L.; Parola, P. Field application of MALDI-TOF MS on mosquito larvae identification. Parasitology 2018, 145, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Nebbak, A.; Willcox, A.C.; Koumare, S.; Berenger, J.-M.; Raoult, D.; Parola, P.; Fontaine, A.; Briolant, S.; Almeras, L. Longitudinal Monitoring of Environmental Factors at Culicidae Larval Habitats in Urban Areas and Their Association with Various Mosquito Species Using an Innovative Strategy. Pest Manag. Sci. 2019, 75, 923–934. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





