Submitted:
06 February 2023
Posted:
08 February 2023
You are already at the latest version
Abstract
Keywords:
Introduction
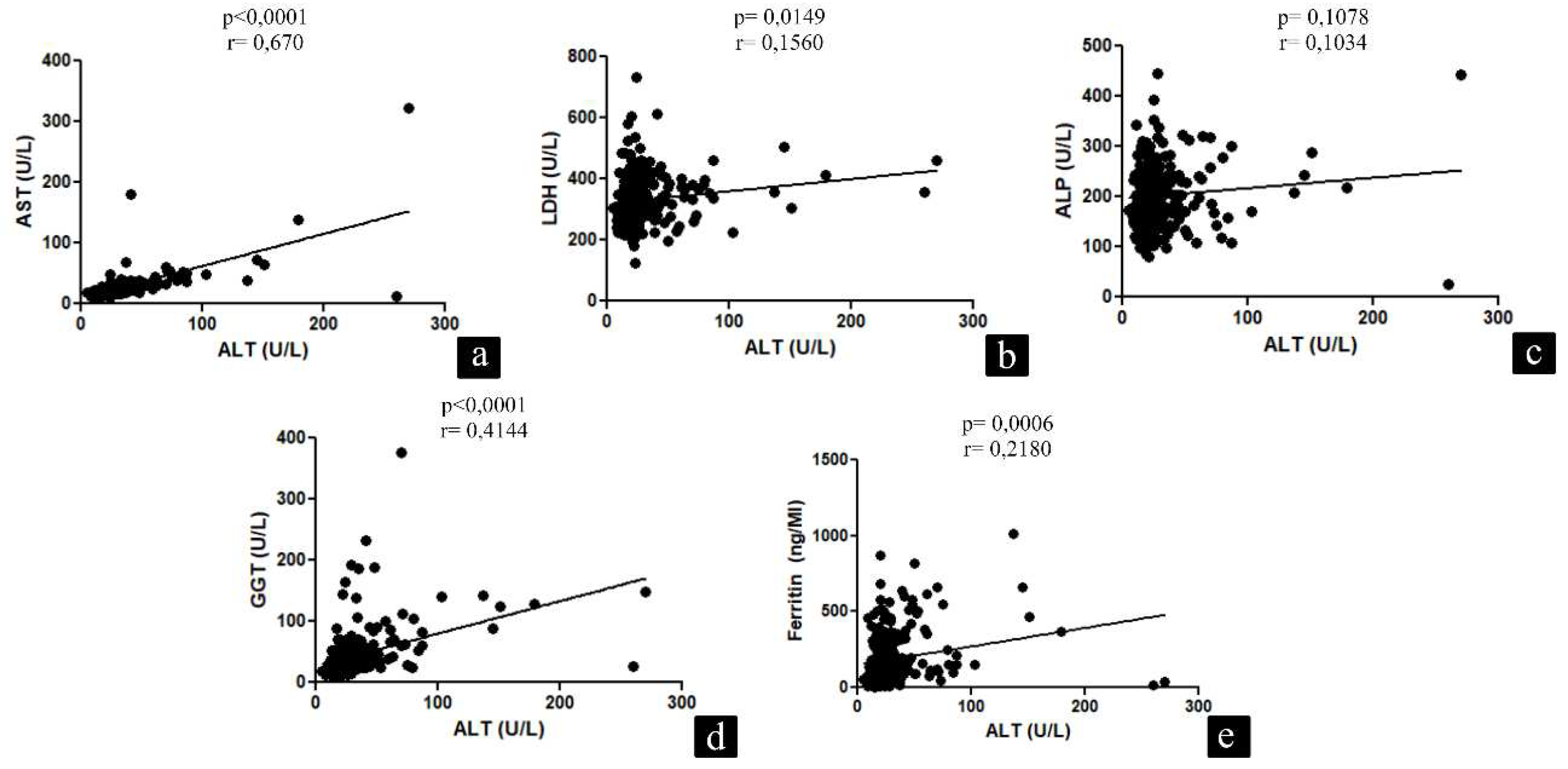
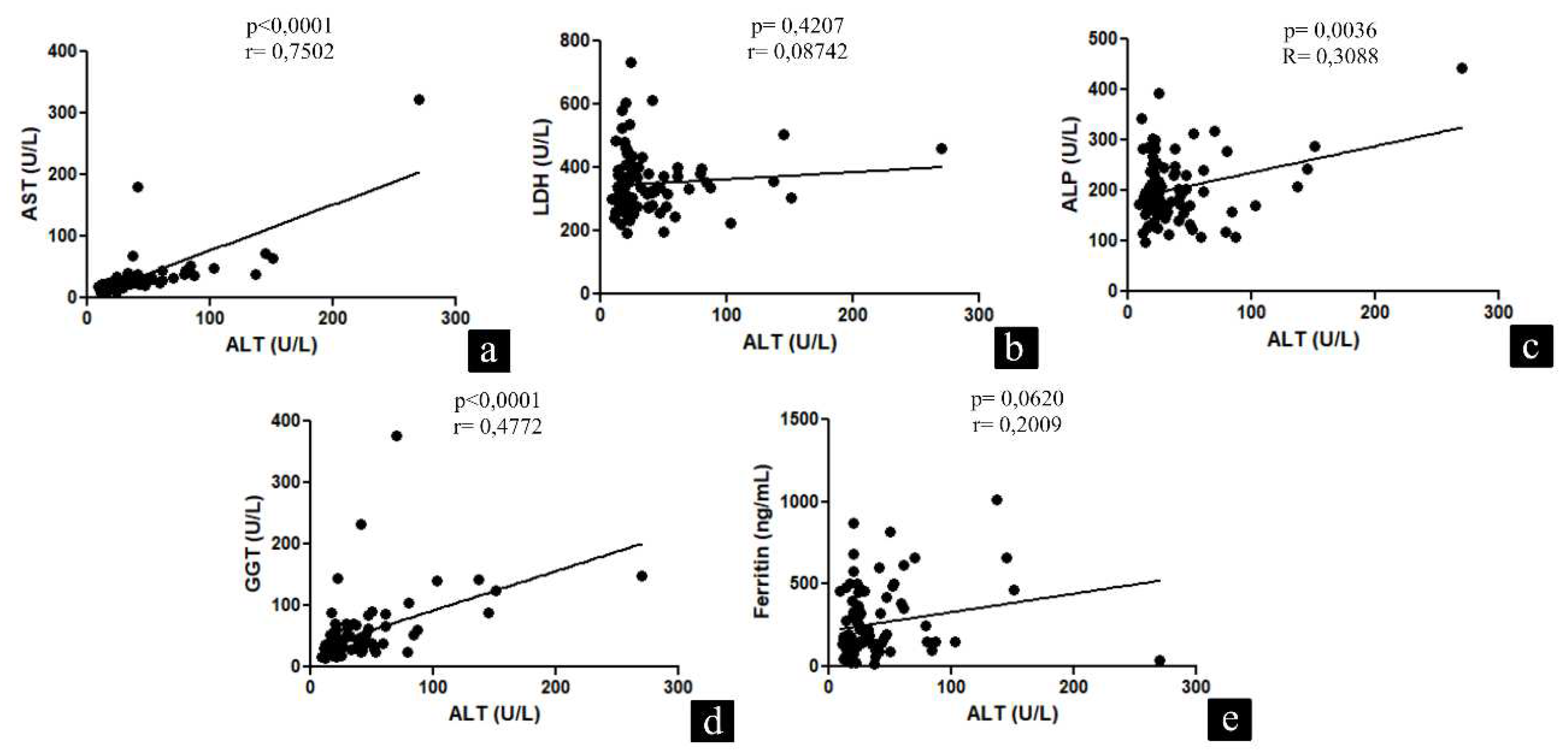
Results
Discussion
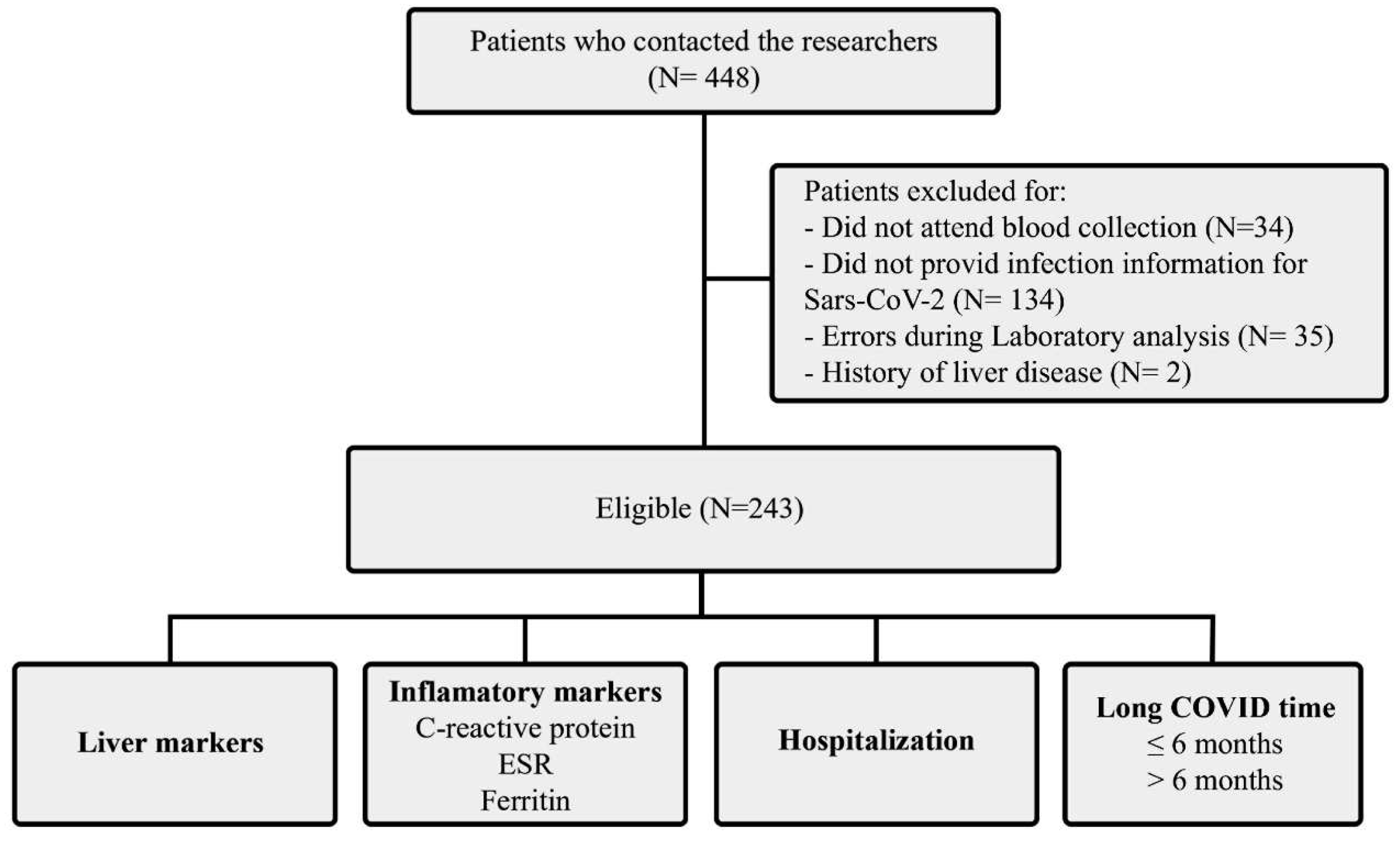
Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of post-acute COVID-19 in primary care. Brit. Med. J. 2020, 370, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; Molteni, E.; et al. Attributes and predictors of long COVID. Nat Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol. 2021, 93, 1013–1022. [Google Scholar] [CrossRef]
- Kamal, M.; Abo Omirah, M.; Hussein, A.; Saeed, H. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract. 2021, 75. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.A.K.; Arvind, A.; Bloom, P.P.; Chung, R.T. Interrelationship Between Coronavirus Infection and Liver Disease. Clin Liver Dis (Hoboken). 2020, 21, 175–180. [Google Scholar] [CrossRef]
- Mehandru, S.; Merad, M. Pathological sequelae of long-haul COVID. Nat Immunol. 2022, 23, 194–202. [Google Scholar] [CrossRef]
- Saviano, A.; Wrensch, F.; Ghany, M.G.; Baumert, T.F. Liver Disease and Coronavirus Disease 2019: From Pathogenesis to Clinical Care. Hepatology 2021, 74, 1088–1100. [Google Scholar] [CrossRef]
- Xu, L.; Liu, J.; Lu, M.; Yang, D.; Zheng, X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020, 40, 998–1004. [Google Scholar] [CrossRef]
- Roth, N. C.; Kim, A.; Vitkovski, T.; Xia, J.; Ramirez, G.; Bernstein, D.; Crawford, J.M. Post-COVID-19 Cholangiopathy: A Novel Entity. Am J Gastroenterol. 2021, 116, 1077–1082. [Google Scholar] [CrossRef]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Higgins, V.; Sohaei, D.; Diamandis, E.P.; Prassas, I. COVID-19: from an acute to chronic disease? Potential long-term health consequences. Crit Rev Clin Lab Sci. 2021, 58, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021; 11. [Google Scholar] [CrossRef]
- Mandal, S.; Barnett, J.; Brill, S.E.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; Hillman, T.E.; Jacob, J.; Jarvis, H.C.; et al. 'Long-COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021, 76, 396–398. [Google Scholar] [CrossRef]
- Bende, F.; Tudoran, C.; Sporea, I.; Fofiu, R.; Bâldea, V.; Cotrău, R.; Popescu, A.; Sirli, R.; Ungureanu, B.S.; Tudoran, M. A Multidisciplinary Approach to Evaluate the Presence of Hepatic and Cardiac Abnormalities in Patients with Post-Acute COVID-19 Syndrome-A Pilot Study. J Clin Med. 2021, 6. [Google Scholar] [CrossRef]
- An, Y.W.; Song, S.; Li, W.X.; Chen, Y.X.; Hu, X.P.; Zhao, J.; Li, Z.W.; Jiang, G.Y.; Wang, C.; Wang, J.C.; et al. Liver function recovery of COVID-19 patients after discharge, a follow-up study. Int J Med Sci. 2021, 18, 176–186. [Google Scholar] [CrossRef]
- Gameil, M.A.; Marzouk, R.E.; Elsebaie, A.H.; Rozaik, S.E. Long-term clinical and biochemical residue after COVID-19 recovery. Egypt Liver J. 2021, 11. [Google Scholar] [CrossRef]
- Patterson, B.K.; Francisco, E.B.; Yogendra, R.; Long, E.; Pise, A.; Rodrigues, H.; Hall, E.; Herrera, M.; Parikh, P.; Guevara-Coto, J.; et al. Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection. Front Immunol. 2022, 10, 746021. [Google Scholar] [CrossRef]
- Kumar-M, P.; Mishra, S.; Jha, D.K.; Shukla, J.; Choudhury, A.; Mohindra, R.; Mandavdhare, H.S.; Dutta, U.; Sharma, V. Coronavirus disease (COVID-19) and the liver: a comprehensive systematic review and meta-analysis. Hepatol Int. 2020, 14, 711–722. [Google Scholar] [CrossRef]
- Wu, Y.; Li, H.; Guo, X.; Yoshida, E.M.; Mendez-Sanchez, N.; Levi Sandri, G.B.; Teschke, R.; Romeiro, F.G.; Shukla, A.; Qi, X. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients: a systematic review and meta-analysis. Hepatol Int. 2020, 14, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Bloom, P.P.; Meyerowitz, E.A.; Reinus, Z.; Daidone, M.; Gustafson, J.; Kim, A.Y.; Schaefer, E.; Chung, R.T. Liver Biochemistries in Hospitalized Patients With COVID-19. Hepatology 2021, 73, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Moriyasu, F.; Oshiro, H.; Takeuchi, H.; Yoshimasu, Y.; Kasai, Y.; Furuichi, Y.; Itoi, T. Viscoelasticity Measurement in Rat Livers Using Shear-Wave US Elastography. Ultrasound Med Biol. 2018, 44, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Marjot, T.; Webb, G.J.; Barritt, A.S. 4th; Moon, A.M.; Stamataki, Z.; Wong, V.W.; Barnes, E. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021, 18, 348–364. [Google Scholar] [CrossRef]
- Fan, B.E.; Chong, V.C.L.; Chan, S.S.W.; Lim, G.H.; Lim, K.G.E.; Tan, G.B.; Mucheli, S.S.; Kuperan, P.; Ong, K.H. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020, 95, E131–E134. [Google Scholar] [CrossRef]
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020, 20, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020, 8, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Boeckmans, J.; Rodrigues, R.M.; Demuyser, T.; Piérard, D.; Vanhaecke, T.; Rogiers, V. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020, 94, 1367–1369. [Google Scholar] [CrossRef]
- Olry, A.; Meunier, L.; Délire, B.; Larrey, D.; Horsmans, Y.; Le Louët, H. Drug-Induced Liver Injury and COVID-19 Infection: The Rules Remain the Same. Drug Saf. 2020, 43, 615–617. [Google Scholar] [CrossRef]
| Variables | Patients |
|---|---|
| Women, n (%) | 182 (65,46) |
| Age (Mean ± SD, anos) | 49,55 ± 12,72 |
| Smoker/Ex-smoker | 82 (29,49) |
| Long COVID symptoms (n, %) | |
| Fatigue | 206 (74,10) |
| Dyspnoea | 201 (72,30) |
| Muscle weakness | 184 (66,18) |
| Muscle and joint pain | 177 (63,66) |
| loss of balance | 146 (52,51) |
| Insomnia | 135 (48,56) |
| Chest pain | 131 (47,12) |
| Cough | 110 (39,56) |
| Comorbidities (n, %) | |
| Comorbidities | 90 (32,37) |
| Respiratory disease | 42 (15,10) |
| DM | 22 (7,91) |
| Heart disease | 20 (7,19) |
| Kidney disease | 1 (0,35) |
| Hospital internment (n, %) | 101 (36,33) |
| Up to 30 days | 85 (30,57) |
| > 30 days | 16 (5,75) |
| Long COVID time (n, %) | |
| ≤ 6 months | 91 (32,73) |
| > 6 months | 187 (67,26) |
| Variables | Hospitalization | Long COVID time | ALT >29 U/L | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | P value | ≤6 months | >6 months | P value | Yes | No | P value | |
| ALT, M ± SD | 37,68±37,84 | 27,73±26,42 | 0,0182& | 31,74±26,86 | 31,07±33,31 | 0,5740 | - | - | - |
| ALT >29 U/L, n. (%) | 33 (13,58) | 41 (16,87) | 0,0808 | 25 (10,28) | 49 (20,16) | 0,9674 | - | - | - |
| AST, M ± SD | 30,85±37,55 | 22,79±12,34 | 0,0042& | 23,37±9,79 | 26,80±29,43 | 0,7790 | 39,43 ±41,30 | 19,65±4,76 | < 0,0001& |
| AST >25 U/L, n. (%) | 32 (13,16) | 31 (12,75) | 0,0063’ | 21 (8,64) | 42 (17,28) | 0,9402 | 49 (20,16) | 14 (5,76) | < 0,0001’ |
| LDH, M ± SD | 348,01±97,94 | 322,23±66,78 | 0,1474 | 345,01±92,30 | 324,81±72,79 | 0,0873 | 343,11±70,56 | 326,36±83,64 | 0,0269& |
| LDH >460 U/L, n. (%) | 10 (4,11) | 3 (1,23) | 0,0024’’ | 7 (2,88) | 6 (2,47) | 0,1289 | 3 (1,23) | 10 (4,11) | 0,7594 |
| ALP, M ± SD | 203,10±64,25 | 201,74±62,18 | 0,8535 | 201,81±54,45 | 202,43±66,67 | 0,7035 | 206,32±67,17 | 200,44±60,91 | 0,4821 |
| ALP >190 µg/L, n. (%) | 46 (18,93) | 84 (34,56) | 0,9908 | 47 (19,34) | 83 (34,15) | 0,3110 | 42 (17,28) | 88 (36,21) | 0,5932 |
| GGT*, M ± SD | 60,15±61,14 | 37,07±21,46 | 0,0024& | 64,02±64,78 | 35,68±15,97 | 0,0013& | 69,60±68,45 | 35,04±14,03 | 0,0004& |
| GGT >50 µg/L, n. (%) | 17 (6,99) | 8 (3,29) | 0,1371 | 17 (6,99) | 8 (3,29) | 0,0192 | 18 (7,40) | 7 (2,88) | 0,0006’ |
| GGT**, M ± SD | 44,47±34,94 | 39,09±34,82 | 0,3912 | 37,02±26,60 | 44,30±36,46 | 0,5645 | 64,97±45,70 | 32,56±26,14 | < 0,0001& |
| GGT >32 µg/L, n. (%) | 19 (7,81) | 42 (17,28) | 0,3773 | 16(6,58) | 45 (18,51) | 0,9318 | 26 (10,70) | 35 (14,40) | < 0,0001’ |
| PT, M ± SD | 12,29±1,01 | 12,87±5,63 | 0,2142 | 12,35±1,18 | 12,82±5,50 | 0,4390 | 13,20±8,08 | 12,43±1,17 | 0,3034 |
| PT > 15 segundos, n. (%) | 1 (0,41) | 2 (0,82) | 1,0000 | 2 (0,82) | 1 (0,41) | 0,2528 | 1 (0,41) | 1 (0,41) | 0,2937 |
| Ferritin*, M ± SD | 338,11±229,71 | 226,72±155,61 | 0,0235& | 368,02±238,19 | 208,27±146,69 | 0,0056& | 352,28±236,85 | 239,32±166,66 | 0,0239 |
| Ferritin >300 ng/mL, n. (%) | 24 (9,87) | 11 (4,52) | 0,0350’ | 23 (9,46) | 13 (5,35) | 0,0084’ | 18 (7,40) | 17 (6,99) | 0,1904 |
| Ferritin **, M ± SD | 177,09±154,13 | 119,38±116,52 | 0,0048& | 122,92±110,85 | 175,07±167,44 | 0,2090 | 161,74±133,42 | 125,81±127,60 | 0,0148& |
| Ferritin >300 ng/mL, n. (%) | 6 (2,47) | 11 (4,52) | 0,3907 | 3 (1,23) | 14 (5,76) | 0,5628 | 6 (2,47) | 11 (4,52) | 0,3691 |
| CRP-positiva***, n. (%) | 10 (4,11) | 23 (9,46) | 0,6076 | 9 (3,70) | 24 (9,87) | 0,5867 | 11 (4,52) | 22 (9,05) | 0,8545 |
| ESR*, M ± SD | 35,22±23,63 | 33,10±28,35 | 0,3698 | 33,95±23,81 | 34,31±27,72 | 0,8629 | 30,71±21,07 | 36,75±28,64 | 0,5343 |
| ESR >20 mm, n. (%) | 31 (12,75) | 22 (9,05) | 0,3394 | 26 (10,69) | 27 (11,11) | 0,6856 | 22 (9,05) | 31 (12,75) | 0,8484 |
| ESR**, M ± SD | 41,16±28,33 | 43,93±25,24 | 0,4544 | 51,24±32,88 | 41,72±26,49 | 0,1343 | 48,66±33,94 | 41,42±22,77 | 0,4176 |
| ESR >30 mm, n. (%) | 25 (10,29) | 77 (31,68) | 0,5882 | 27 (11,11) | 75 (30,86) | 0,9403 | 28 (11,52) | 74 (30,45) | 0,3403 |
| TB, M ± SD | 0,46±0,23 | 0,47±0,21 | 0,7087 | 0,48±0,28 | 0,46±0,18 | 0,7587 | 0,47±0,25 | 0,47±0,21 | 0,7099 |
| TB >1.0 mg/dL | 2 (0,82) | 1 (0,41) | 0,5634 | 3 (1,23) | 0 | 0,0370’’ | 1 (0,41) | 2 (0,82) | 0,9756 |
| DB, M ± SD | 0,17±0,05 | 0,17±0,07 | 0,7317 | 0,17±0,07 | 0,17±0,06 | 0,8638 | 0,17±0,07 | 0,17±0,06 | 0,9341 |
| DB >0,2 mg/dL, n. (%) | 1 (0,41) | 7 (2,88) | 0,1369 | 3 (1,23) | 5 (2,05) | 0,9980 | 4 (1,64) | 4 (1,64) | 0,2326 |
| IB, M ± SD | 0,31±0,21 | 0,31±0,18 | 0,5453 | 0,3094±0,23 | 0,3145±0,17 | 0,3736 | 0,30±0,18 | 0,31±0,19 | 0,6345 |
| IB >0,8 mg/dL, n. (%) | 1 (0,41) | 1 (0,41) | 1,0000 | 2 (0,82) | 0 | 0,1135 | 0 | 2 (0,82) | 0,5772 |
| Albumin, M ± SD | 4,09±0,34 | 4,12±0,38 | 0,2321 | 3,9969±0,37 | 4,1677±0,35 | 0,6949 | 4,13±0,34 | 4,10±0,38 | 0,6542 |
| Albumin>4,8 g/dL, n. (%) | 0 | 0 | 1,0000 | 0 | 0 | 1,0000 | 0 | 0 | 1,0000 |
| Variables | CRP*** | ESR (mm/h) * | ESR (mm/h) ** | Ferritin* (ng/mL) | Ferritin** (ng/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | - | P value | > 20 | ≤20 | P value | >30 | ≤30 | P value | > 300 | ≤300 | P value | > 300 | ≤300 | P value | |
| ALT, M ± SD | 43,79±59,36 | 29,33±23,77 | 0,1145 | 35,04±23,19 | 42,58±41,21 | 0,7073 | 27,92±28,78 | 27,70±34,76 | 0,4037 | 49,48±41,58 | 29,49±16,52 | 0,0104& | 25,88±10,55 | 28,07±32,56 | 0,2520 |
| ALT >29 U/L n. (%) | 11 (4,52) | 63 (25,92) | 0,8545 | 22 (9,05) | 13 (5,35) | 0,0370’ | 28 (11,52) | 11 (4,52) | 0,3403 | 18 (7,40) | 17 (6,99) | 0,1904 | 6 (24,69) | 33 (13,58) | 0,3691 |
| AST, M ± SD | 32,33±52,94 | 24,63±16,53 | 0,9236 | 24,37±7,73 | 34,87±36,62 | 0,6597 | 26,00±31,27 | 21,30±6,77 | 0,3626 | 31,83±22,67 | 25,69±23,82 | 0,0064& | 24,06±6,25 | 24,34±26,83 | 0,0491& |
| AST >25 U/L n. (%) | 9 (3,70) | 54 (22,22) | 0,9811 | 18 (7,40) | 10 (4,11) | 0,9363 | 25 (10,29) | 10 (41,15) | 0,4139 | 17 (6,99) | 11 (4,52) | 0,0233’ | 4 (1,64) | 31 (12,75) | 0,9970 |
| LDH M ± SD | 332,06±81,41 | 331,37±80,10 | 0,8762 | 334,15±100,17 | 331,13±95,05 | 0,9078 | 333,13±70,74 | 326,16±67,18 | 0,4893 | 348,03±107,76 | 322,32±89,52 | 0,2968 | 343,76±72,56 | 329,06±69,06 | 0,4222 |
| LDH >460 U/L n. (%) | 2 (0,82) | 11 (4,52) | 0,9803 | 3 (1,23) | 4 (1,64) | 0,4148 | 3 (1,23) | 3 (1,23) | 4 (1,64) | 3 (1,23) | 1 (0,42) | 5 (2,06) | 0,4817 | ||
| ALP M ± SD | 232,97±84,13 | 197,40±57,53 | 0,0092& | 199,13±63,82 | 193,77±48,21 | 0,2808 | 210,38±68,42 | 195,12±57,88 | 0,3712 | 209,02±53,64 | 188,67±60,53 | 0,5312 | 225,41±59,24 | 202,46±65,49 | 0,1520 |
| ALP >190 µg/L | 23 (9,46) | 107 (44,03) | 0,0689 | 30 (12,34) | 16 (6,58) | 0,8287 | 54 (22,22) | 30 (12,34) | 0,8980 | 23 (9,46) | 23 (9,46) | 0,1383 | 11 (4,52) | 73 (30,04) | 0,4349 |
| GGT* M ± SD | 49,80±31,46 | 49,42±49,24 | 0,9774 | 52,24±57,38 | 44,64±26,30 | 0,8457 | - | - | - | 66,31±69,34 | 37,39±16,17 | 0,0164& | - | - | - |
| GGT >50 µg/L | 2 (0,82) | 23 (9,46) | 0,6297 | 16 (6,58) | 9 (3,70) | 0,8923 | - | - | - | 14 (5,76) | 11 (4,52) | 0,1356 | - | - | - |
| GGT** M ± SD | 46,25±33,47 | 39,29±35,11 | 0,0345& | - | - | - | 40,65±33,79 | 40,26±36,91 | 0,3522 | - | - | - | 56,70±53,14 | 38,57±31,67 | 0,1226 |
| GGT >32 µg/L | 15 (6,17) | 46 (18,93) | 0,1076 | - | - | - | 39 (16,05) | 22 (9,05) | 0,8980 | - | - | - | 9 (3,70) | 52 (21,40) | 0,2965 |
| PT M ± SD | 14,57±11,98 | 12,36±1,18 | 0,2444 | 12,33±1,27 | 12,66±0,99 | 0,3124 | 12,37±1,26 | 13,50±9,15 | 0,8224 | 12,42±1,35 | 12,47±1,06 | 0,6394 | 12,77±1,20 | 12,78±5,88 | 0,1465 |
| PT > 15 segundos | 1 (0,42) | 2 (0,82) | 0,3535 | 1 (0,42) | 0 | 1,0000 | 1 (0,42) | 1 (0,42) | 1,0000 | 1 (0,42) | 3 (1,23) | 0,6374 | 1 (0,42) | 2 (0,82) | 0,2883 |
| TB M ± SD | 0,45±0,21 | 0,47±0,22 | 0,8213 | 0,49±0,26 | 0,50±0,22 | 0,9049 | 0,42±0,19 | 0,52±0,22 | 0,0755 | 0,48±0,27 | 0,50±0,24 | 0,7357 | 0,41±0,19 | 0,46±0,21 | 0,4735 |
| TB >1.0 mg/dL | 0 | 3 (1,23) | 1,0000 | 1 (0,42) | 1 (0,42) | 1,0000 | 0 | 1 (0,42) | 0,3390 | 1 (0,42) | 1 (0,42) | 1,0000 | 0 | 1 (0,42) | 1,0000 |
| DB M ± SD | 0,16±0,06 | 0,17±0,06 | 0,4465 | 0,17±0,06 | 0,17±0,06 | 1,0000 | 0,16±0,06 | 0,18±0,07 | 0,4565 | 0,16±0,05 | 0,18±0,06 | 0,4773 | 0,14±0,05 | 0,17±0,07 | 0,3428 |
| DB >0,2 mg/dL | 1 (0,42) | 7 (2,88) | 1,0000 | 2 (0,82) | 1 (0,42) | 0,5508 | 3 (1,23) | 2 (0,82) | 0,9969 | 0 | 3 (1,23) | 0,5361 | 0 | 5 (2,06) | 0,5860 |
| IB M ± SD | 0,33±0,20 | 0,31±0,19 | 0,6692 | 0,31±0,21 | 0,32±0,19 | 0,7328 | 0,27±0,17 | 0,37±0,20 | 0,0537 | 0,32±0,25 | 0,31±0,19 | 0,7899 | 0,26±0,15 | 0,33±0,19 | 0,5066 |
| IB >0,8 mg/dL | 0 | 2 (0,82) | 1,0000 | 1 (0,42) | 1 (0,42) | 1,0000 | 0 | 0 | 1,0000 | 1 (0,42) | 1 (0,42) | 1,0000 | 0 | 0 | 1,0000 |
| Albumin M ± SD | 4,17±0,40 | 4,09±0,36 | 0,4643 | 4,01±0,34 | 4,13±0,37 | 0,2978 | 4,17±0,36 | 4,07±0,39 | 0,2630 | 4,03±0,41 | 4,06±0,34 | 0,6314 | 4,08±0,36 | 4,15±0,37 | 0,5909 |
| Albumin >4,8 g/dL | 0 | 0 | 1,0000 | 0 | 0 | 1,0000 | 0 | 0 | 1,0000 | 0 | 0 | 1,0000 | 0 | 0 | 1,0000 |
| Risk variables | ALT >29 U/L | AST >25 U/L | Ferritin >300 ng/mL | Long COVID time ≤6 | >5 long COVID symptoms | Hospitalization | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | P value | Odds ratio | P value | Odds ratio | P value | Odds ratio | P value | Odds ratio | P value | Odds ratio | P value | |
| Hospitalization, Yes | 0,8948 | 0,8025 | 2,1662 | 0,0807 | 1,7693 | 0,1495 | 2,4271 | 0,0059 | 1,9802 | 0,0542 | - | - |
| Long COVID time ≤6 | 0,7877 | 0,5933 | 0,6341 | 0,3236 | 1,5344 | 0,2711 | - | - | 2,8343 | 0,0042 | 2,4542 | 0,0052 |
| Age ≥60 years | 0,2855 | 0,0214 | 1,1367 | 0,8038 | 2,4342 | 0,0331 | 1,5585 | 0,2126 | 0,9407 | 0,8678 | 1,4307 | 0,3218 |
| Male | 2,6959 | 0,0274 | 0,9369 | 0,8878 | 4,8173 | < 0,0001 | 2,0594 | 0,0351 | 0,6988 | 0,3046 | 2,4365 | 0,0087 |
| >5 long COVID symptoms | 1,1528 | 0,7536 | 0,6118 | 0,2554 | 0,6997 | 0,3829 | 2,8768 | 0,0041 | - | - | 1,9906 | 0,0539 |
| ALT >29 U/L | - | - | 21,3046 | < 0,0001 | 1,7447 | 0,2720 | 0,8798 | 0,7707 | 1,0923 | 0,8321 | 0,8981 | 0,8054 |
| AST >25 U/L | 21,5317 | < 0,0001 | - | - | 1,2285 | 0,6807 | 0,7166 | 0,4531 | 0,6006 | 0,2344 | 2,2260 | 0,0670 |
| LDH ≥460 U/L | 0,2712 | 0,1577 | 5,4802 | 0,0266 | 1,3118 | 0,7063 | 1,2126 | 0,7673 | 2,0278 | 0,3957 | 4,6938 | 0,0370 |
| ALP ≥ 190 U/L | 0,7481 | 0,4745 | 1,0868 | 0,8380 | 1,9531 | 0,0852 | 1,3307 | 0,3638 | 0,7894 | 0,4398 | 0,7015 | 0,2669 |
| GGT ≥ 50 U/L* ou 32 U/L** | 3,5989 | 0,0019 | 1,9687 | 0,1094 | 1,5007 | 0,3431 | 1,3967 | 0,3401 | 1,7849 | 0,1055 | 1,2841 | 0,4755 |
| Ferritin ≥ 300 ng/mL | 1,6449 | 0,3357 | 1,3499 | 0,5338 | - | - | 1,5394 | 0,2654 | 0,6965 | 0,3639 | 1,9245 | 0,0966 |
| ESR ≥ 20 mm/h* ou 30 mm/h ** | 1,1198 | 0,7887 | 1,2075 | 0,6576 | 1,9377 | 0,1069 | 1,3981 | 0,3084 | 0,6022 | 0,1178 | 1,0869 | 0,8008 |
| Positive C-reactive protein *** | 1,2672 | 0,6725 | 0,8917 | 0,8434 | 0,4243 | 0,1865 | 0,7255 | 0,5023 | 1,1572 | 0,7462 | 0,9796 | 0,9651 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





