Introduction
Optical Coherence Tomography Angiography (OCTA) is a non-invasive, rapid imaging modality that creates a cross sectional, in vivo image of the dynamic microvasculature of the choroid and retina [
1]. More than two decades ago, the first phase- resolved OCT-Optical Doppler Tomography (ODT) image of blood flow was demonstrated in blood vessels of human skin, with high velocity and sensitivity [
2]. This progressed to developments of a phase signal-based OCTA technique first seen in 2006, which included methods to minimise sample movement in the axial direction to improve image quality [
3]. Ophthalmic OCTA was initially in experimentation in 2006, before taking wider commercial effect in 2014. The software and scanning strategies are rapidly improving, and research in this is continuing to progress [
4]. Several OCTA devices are available internationally, widely in the context of clinical research. The emerging use of OCTA as a tool in the diagnosis of ocular pathology is of growing importance, as its potential clinical applications continue to be investigated through ongoing research [
5]. The technological advances in OCTA imaging since its inception have allowed the possibility of more detailed imaging of vascular structures and may herald an increase in its clinical utility in years to come [
6]. This paper aims to review the principles, limitations and benefits of OCTA, as well as its future potential for clinical use.
Principles of Coherence Tomography
OCT (Optical coherence tomography) utilises the optical principles of coherence and interferometry in producing a high-resolution cross-sectional image of anatomical structures. Based on the application of ‘wave-theory’ in physics where light energy behaves as a wave (as opposed to a particle) underpins the principle underlying coherence tomography. Coherence refers to the physical property of light waves to travel exactly in “phase”, where wave cycles run in parallel [
1].
These principles can be applied to light in the infrared wavelength (780-10,000 nm) of the electromagnetic spectrum. Experiments showed that coherent infrared light targeted at different anatomical tissues can be utilised to produce images from the light reflected by those tissues of interest. For ophthalmic purposes the tissue of interest is the retina primarily. The OCT device comprises a low coherence infrared source which produces a beam of light, in addition to a beam splitter, a reference mirror, and a receiving source which can detect reflected light waves. Firstly, a generated infrared beam from the light source travels towards the beam splitter, which then acts to produce two beams, one which travels to a reference mirror and one which travels towards the eye. Light passes through the eye tissues at varying speeds and is reflected by the retina towards the detector. Light waves are also returned from the reference mirror and the analysis of the differences between returning waves in combination can be converted into computerised images to form a two-dimensional, tomographical map based on the reflective properties of each individual tissue layer.
As for standard OCT images, the reflection of emitted infrared coherent (in phase) light wave energy from eye tissues returning at varying speeds is what enables the computerised generation of OCTA images. Imaging of the retinal vasculature using OCTA is able to identify the movement pattern of erythrocytes within the retinal blood vessels, thus enabling real time depiction of blood flow, analogous to B scan ultrasonography. Indeed, doppler ultrasound scanning provided the initial foundation from which OCTA was developed. In order to quantify the volume of blood flow within vessels.
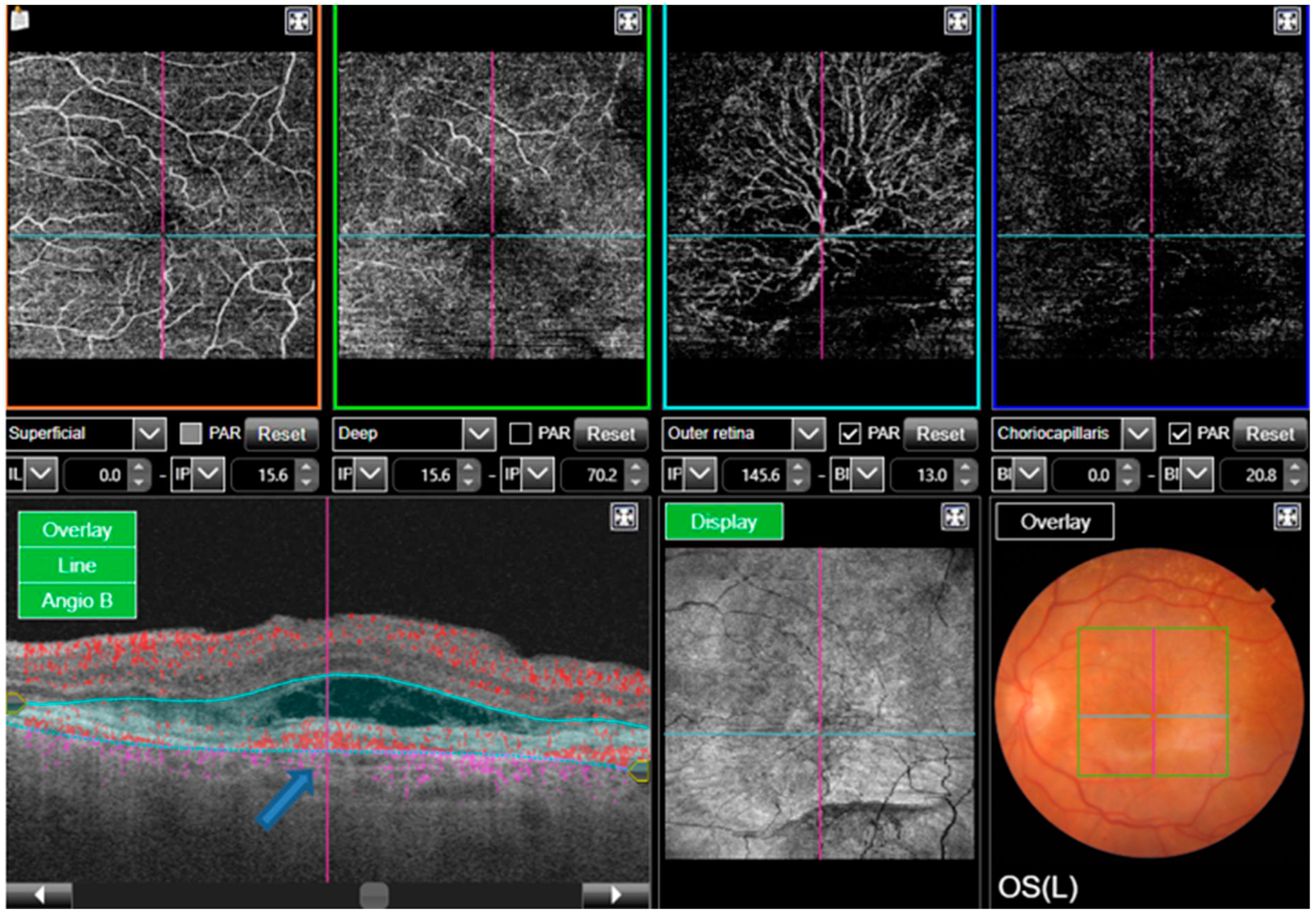
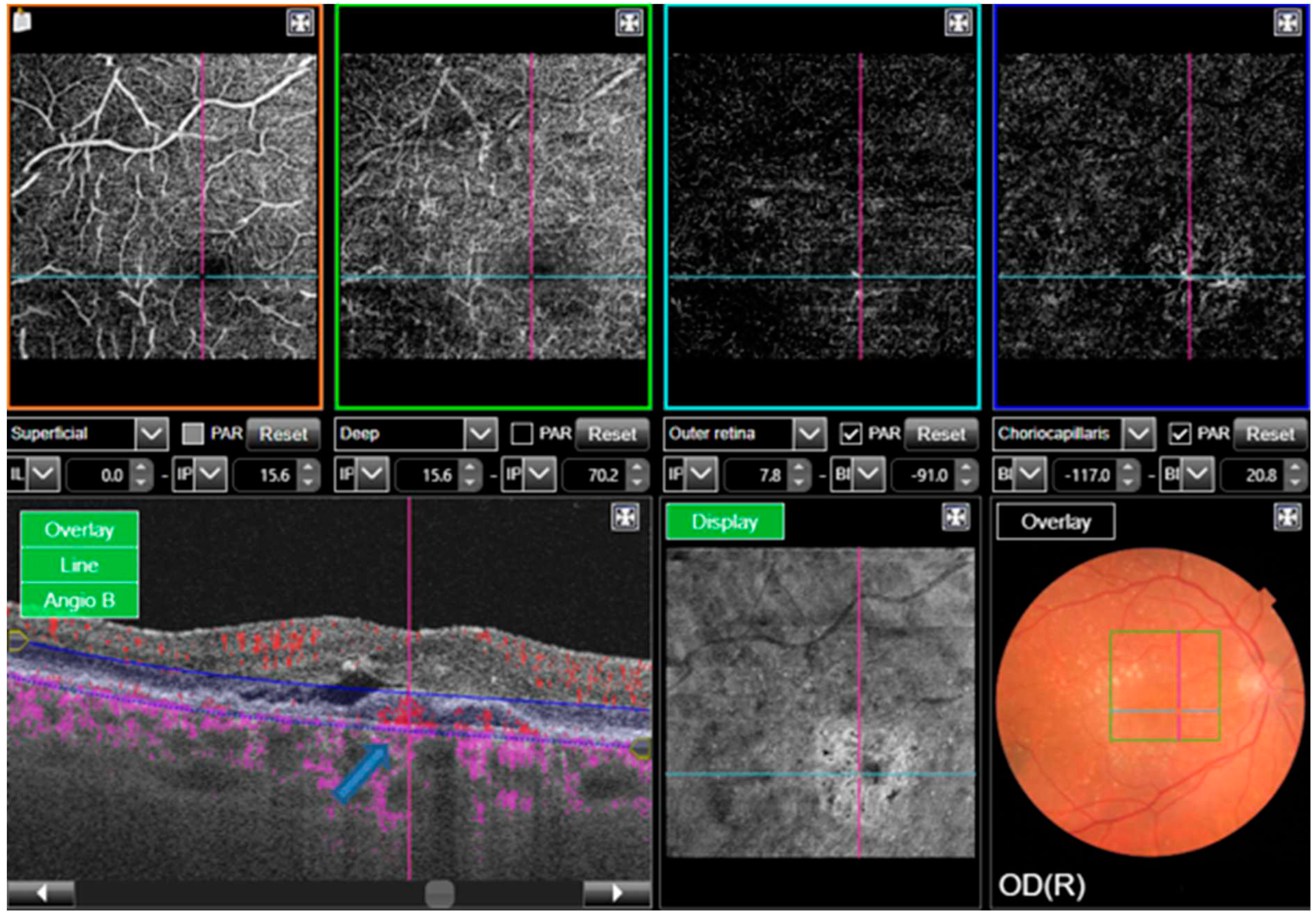
A series of successive retinal images are taken in close sequence which identifies the movement of erythrocytes from one point to another within blood vessels. This is compared to images of the static, non-moving retina for characterisation of areas of blood flow in different layers within the retina. These include the superficial, intermediate and deep plexus, the inner retinal plexus, the outer retina, the choriocapillaris and the choroid [
7].
The ability of OCTA to produce high-resolution images is dependent on the short interval between successive B scan images. Shorter interscan times will lead to higher sensitivity thresholds and minimise the fastest distinguishable flow rate [
8]. Advances in OCT technology have allowed the use of several different programme strategies, such as Swept-Source OCT (SS-OCT) and Spectral domain OCT (SD-OCT) which may be utilised to image retinal vasculature. These models have been developed to capture images at faster speeds and with larger fields of view. SS-OCT is of particular use in the setting of choroidal vascular anomalies as it enables visualisation of choroidal blood flow by emitting light of a longer wavelength for enhanced tissue penetration. Furthermore, SS-OCT generates images at a faster rate to improve image resolution. SD-OCT operates at increased image speed to directly compare the phase of A-scans. For example, the Carl Zeiss and Optovue modalities both use SD-OCT technology. Zeiss also has an SS-OCTA device which can be purchased in Europe and the United States, whereas the Topcon Medical System also using SS-OCTA is available in England, Brazil and Japan. It is expected that most manufacturers will develop OCTA platforms [
6].
Clinical Uses of OCTA in Current Ophthalmic Practice
Prior to the development of OCTA, imaging of the chorioretinal vasculature was predominately by using fundus fluorescein angiography (FA) and indocyanine green angiography (ICGA). These modalities continue to provide an essential tool for investigation of disorders of the fundus vasculature and remain the gold standard in current clinical practice. However, as a moderately invasive procedure involving the intravenous injection of sodium fluorescein dye which poses several important contraindications, for example dye allergy and pregnancy, the need for a less invasive imaging tool was a key impetus for the development of OCTA as an alternative imaging option.
In comparison to Fluorescein Angiography (FA), OCTA offers a few key advantages. FA relies on the fluorescent properties of sodium fluorescein dye which be injected intravascularly to observe blood flow within the retina microvasculature. Due to the blocking effect of the retinal pigment epithelium (RPE), FA is only able to visualise more superficial layers of the retinal vasculature, thus limiting the potential diagnostic yield and necessitating the use of further invasive testing such as through ICG dye studies, to observe pathologies within deeper structures. OCTA is favourable because it is more time efficient taking approximately six seconds per image, and technically easier to perform – there is no need for a skilled photographer, and the patient does not require a dye injection [
9].
The retina receives more blood flow per gram of tissue than any other structure within the body [
1]. Thus, as a highly metabolically active structure, the health of retinal tissue is inextricably linked to the viability of its of blood supply for the provision of nutrients and oxygen, and the removal of metabolic waste products. Several retinal pathologies are characterised by an imbalance between blood supply and nutrient demand, which may lead to the growth of new vessels in response to tissue hypoxia, commonly mediated by upregulation of vascular endothelial growth factor (VEGF). This disease process may be seen in commonly encountered pathologies such as proliferative diabetic retinopathy (DR) and neovascular age-related macular degeneration (AMD) which are primarily associated with choroidal neovascularisation (CNV). Relative tissue hypoxia may also be seen in retinal vascular occlusions, which require prompt identification to prevent irreversible vision loss. OCTA provides a rapid, non-invasive tool in the diagnosis of retinal vascular pathologies as well as subsequent long-term monitoring in chronic disease.
Diabetic Retinopathy (DR)
Retinopathy secondary to diabetes mellitus is a well-characterised disease process implicating retinal vascular integrity through early loss of endothelial pericytes, microaneurysms and ultimately growth of abnormal “leaky” vessels leading to subsequent haemorrhage with severe implications for visual function. Diabetic macular oedema (DMO) is a complication of diabetic retinopathy from collection of tissue fluid leakage from abnormal vessels which may provoke profound visual impairment. OCTA can quantify characteristic features such as microaneurysms, intraretinal microvascular abnormalities and neovascularisations, and may even detect the extent of the microvascular damage prior to any clinical signs [
10]. The use of OCTA in monitoring the progression of DR may guide clinical management in offering therapeutics based on the stage of severity identified, in addition to assessment of treatment efficacy using anti-VEGF therapy [
4,
11]. In recent studies, it has been shown that OCTA has played a significant role in pinpointing regression, reactivation and resistance to treatment in DR [
12].
Retinal Vascular Occlusion
Retinal artery or vein occlusion typically involves obstruction of either the central retinal vessels or subsequent larger branches. Atheroembolic disease is by far the most common cause for both pathologies, with arterial occlusion commonly leading to secondary venous occlusion by compression at points of arteriovenous crossing [
15]. Relative retinal ischaemia following vessel obstruction leads to photoreceptor death and the upregulation of VEGF. OCTA can be utilised in this setting to detect non-perfusion within the superficial and deep choroidal plexuses because of vascular occlusion.
Areas of non-perfusion were evaluated in retinal vein occlusion by Wakabayashi et al, who used OCTA to assess for microvascular changes and disruption of the foveal avascular zone (FAZ). Best corrected visual acuity (BCVA) outcomes were measured and correlated to the OCTA findings on the vascular perfusion areas in the superficial and deep choroidal plexus within a defined area. Findings showed that better BCVA outcomes were significantly correlated to preservation of larger vascular perfusion areas [
16]. This tells us that preservation of the deep choroidal vasculature is significant in improving visual outcomes in branched retinal vein occlusion, and that OCTA is a useful imaging modality when evaluating and monitoring this, as well as predicting visual outcomes following treatment.
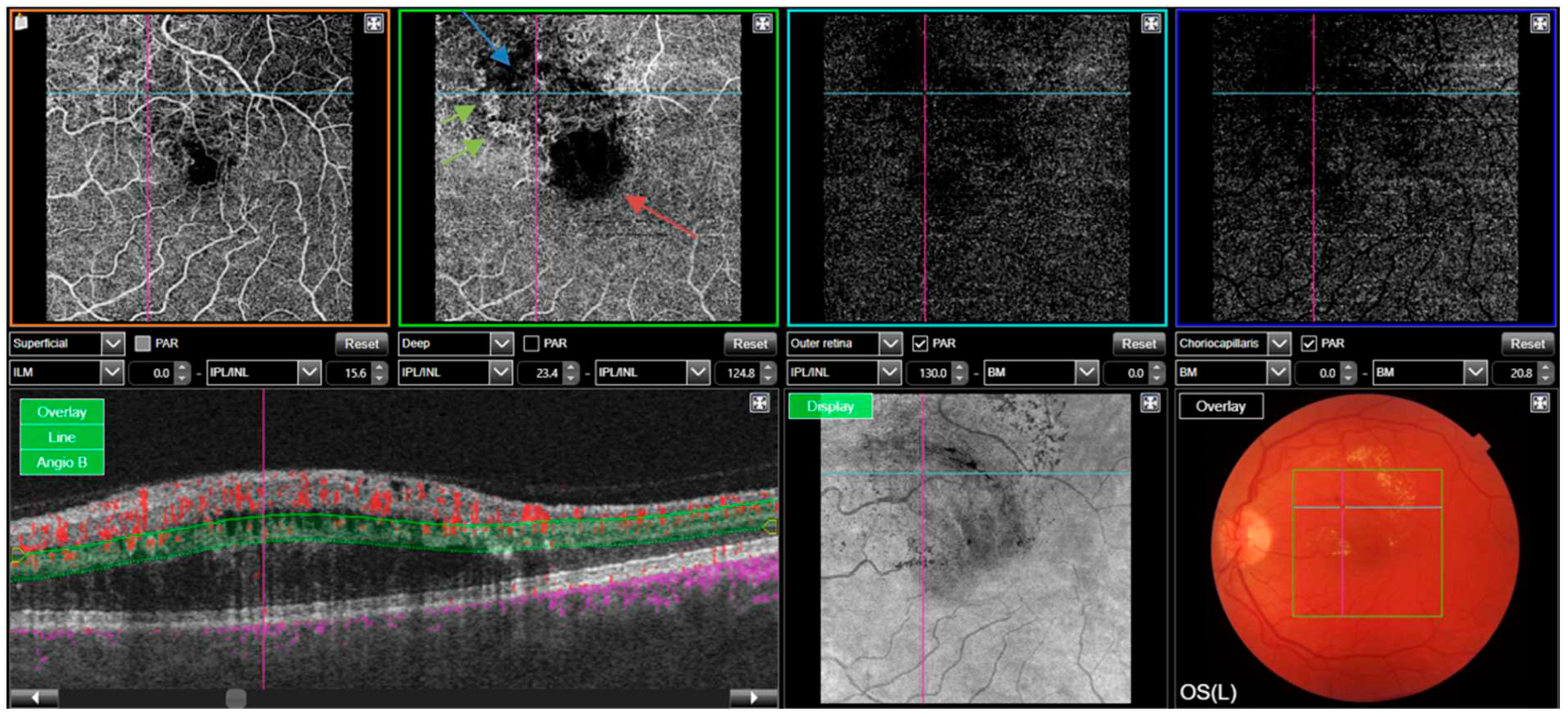
In retinal vein occlusion, OCTA can detect characteristic findings such as venous dilation, cotton wool spots and retinal haemorrhages. Kashani et al’s review on the topic reports that OCTA is useful for diagnosis and management of retinal artery occlusion (RAO) and vascular complications of retinal vein occlusions (RVO) in the macula, with evidence suggesting that OCTA is as effective for the management of RVO, as FA. Interestingly, they also found that a patient with branched retinal artery occlusion with insignificant symptoms and normal visual acuity and unremarkable clinical examination was showed to have capillary and neurosensory loss of the superior macula on OCTA [
6]. OCTA has been shown to precisely characterise macular ischaemia by demonstrate vascular remodelling of the capillary layer in retinal artery occlusion over time, leading it to be considered for monitoring these vascular flow changes during the management of this disease [
17].
A representative case of OCT-A in RVO is depicted in
Figure 3.
Glaucoma
An emerging use for OCTA is within optic nerve disorders. Glaucoma refers to a spectrum of diseases of progressive optic neuropathy where raised intraocular pressure is a major risk factor. OCTA can measure optic disc perfusion which may be decreased in glaucoma and therefore can contribute to evaluation of disease progression [
18]. It has been noted that OCTA has been able to detect a reduction in the peripapillary vessel density, where there has not yet been evidence of visual field loss. This could suggest earlier detection and therefore diagnosis for glaucoma, leading to improved visual outcomes in this condition [
19,
20].
Chemical Eye Injuries
Acute chemical injury to the eye is a sight-threatening emergency, characterised by diffuse corneal epithelial erosion, limbal stem cell disruption, conjunctival vessel infiltration, and may progress to damage of deeper ocular structures. The primary function of the limbal stem cells is to maintain the integrity of the corneal epithelium and prevent conjunctivalisation. Injury to this region is often catastrophic for visual outcomes. Ischaemia of limbal stem cells following acute chemical injury may be detected and monitored using OCTA of the Anterior Segment (AS-OCTA). This is done by capturing blood flow in the corneal and limbal vessels. The degree of ischaemia can be quantified using different vessel-related parameters, of which the density of the vessels correlates with the severity of the injury. Clinical examination using slit-lamp biomicroscopy is accurate in the diagnosis of limbal stem cell injury but is unable to formally quantify the extent of the ischaemia to the region. Although this is a new use of this technology and will need further evidence for utility, it holds potential to be incorporated into the clinical setting for the management chemical injuries. Potential opportunity lies in the ability to identify patients who would benefit from revascularisation therapies such as stem cell transplants [
21].
Uveitis
OCTA is a useful tool for identifying inflammation, vascular changes, and structural changes in the retina and choroid and for monitoring disease activity and treatment response in uveitis [
7]. OCTA (together with Wide-field OCT) allows for the identification of inflammation and vascular changes in the peripheral retina and choroid, which may not be visible on traditional imaging modalities. This is particularly important in uveitis, as inflammation and vascular changes in the peripheral retina and choroid are often associated with poor visual outcomes [
22].
In a study by Vita et al., OCTA was found to be a valuable diagnostic tool in uveitis, as it was able to detect inflammation and vascular changes in the retina and choroid that were not visible on conventional imaging techniques. The study also found that OCTA was able to detect inflammation in areas that were not visible on clinical examination, suggesting that it may be able to identify early stages of inflammation [
23].
However, a study by Herbort et al. highlights the limitations of OCTA in the diagnosis and follow-up of posterior intraocular inflammation, and suggests that it should be used in combination with other imaging modalities and clinical examination. The study also suggests that the interpretation of OCTA images requires a high level of expertise, and that false positive and false negative results can occur if the images are not properly interpreted [
24].
Clinical Limitations of OCTA Imaging
The primary difference in OCTA versus other imaging modalities of the retinal circulation is the method used to detect vascular leakage. Following intravenous administration, fluorescein dye may be shed into retinal tissues and captured using a blue excitation filter. Unlike Fluorescein OCTA is unable to image direct leakage pathological blood vessels because it can only detect motion of erythrocytes within vessels. In such cases where possible vascular leakage should be assessed, FA may be preferable in identifying leakage sites [
9].
Artefacts may hinder the image quality produced by OCTA and can arise from several sources. As OCTA works by detecting motion, the quality of the images produced by OCTA is therefore impacted by the patient’s loss of fixation and eye movements. Adequate fixation may not be achieved in non-compliant patients and may contribute to a degree of motion artefact. Methods such as eye tracking and motion correction techniques have been incorporated into some OCTA protocols to reduce the impact of patient-related movement artefact [
25].
Light scatter caused by superficial structures including moving erythrocytes can mean that the deeper retinal vascular layers are more prone to projection artefact [
26]. Software editing targets artefact projection removal, however, these developments have not yet matured and often can remain.
Anterior segment opacities can create shadow artefacts often from corneal scarring, cataracts or vitreous floaters leading to signal attenuation. Anterior opacities may hide portions of the OCTA and lead to diffuse reduction in image quality [
27]. If a patient has a cataract or if the pupils are poorly dilated the image produced may show a vignetting. This is an artefact where the centre of the image seems brighter than the area surrounding it, giving the appearance of irregular illumination. This poses difficulty when differentiating between the peripheral areas of the vignette artefact and areas of true low contrast from pathological vessels. There is ongoing research to develop algorithms to minimise artefact to improve the use of OCTA [
28].
Other patient factors may also affect image quality, for example those who are myopic may have extra features which disturb the image formed, such as RPE atrophy, retinoschisis and lacquer cracks (a feature of pathologic myopia) [
26].
OCTA is limited in its field of view because the sequential B scans are taken over a small area. This can be advantageous if the desired scan position is known, however it may result in a compromise of the detection of peripheral vascular changes. To increase the field of view of OCTA, multiple images can be put together to give a more inclusive image [
1].
As OCTA relies on the flow of erythrocytes through a vessel for imaging a low-flow or diseased vessel may have a flow rate below the slowest detectable rate. This can occur in fibrotic neovascularization. Where the pathological blood vessels have become fibrosed and only leak slowly. In some systems the slowest detectable flow can be customized to minimise this limitation and allow for better investigation of the vessel flow [
28]. Increasing the time between consecutive OCT B scans could allow for increased flow detection but would the increase the risk of movement artefact [
7].
All these factors impact the quality of the OCTA and can decrease the accuracy of detecting blood vessel abnormalities, potentially leading to misinterpretation of the image.
Conclusions
OCTA holds great promise in contributing to the clinical investigation and management of retinal diseases [
9]. The use of OCTA has improved our understanding of retinal and macular diseases in recent years [
4]. Although the technology may not currently be easily accessible and of higher cost in comparison to FA or ICGA, it can provide clinical benefit to patients with poor vascular access, or in those cases where dye containing investigation are contraindicated, such as pregnancy, breast feeding, renal impairment, or allergy to fluorescein sodium or indocyanine green dye.
OCTA is an advancing technology, which provides a potential use in clinical practice and research. It has the potential to involve artificial intelligence (AI) and deep learning to potentially enhance diagnosis and management of retinal diseases such as DR in the upcoming years [
29]. A recent study demonstrated the potential clinical applications of deep learning for early detection and progression assessment of DR. Deep-learning analysis has already demonstrated parity with human grading in several tasks on mostly small datasets. OCTA was used with deep learning principles, where avascular areas were segmented in diabetic eyes. This was successful in classifying the image findings and showed accuracy in decision making [
30]. With the growing implementation of OCTA in clinical practice and corresponding increase in the amount of available OCTA data, AI-based analysis can only expand, and work to aid clinical decisions [
31].
References
- Choi W, Mohler KJ, Potsaid B, Lu CD, Liu JJ, Jayaraman V, et al. Choriocapillaris and choroidal microvasculature imaging with ultrahigh speed OCT angiography. PLoS One. 2013, 8(12), e81499.
- Zhao Y, Chen Z, Saxer C, Xiang S, de Boer JF, Nelson JS. Phase-resolved optical coherence tomography and optical Doppler tomography for imaging blood flow in human skin with fast scanning speed and high velocity sensitivity. Opt Lett. 2000, 25(2), 114–116. [CrossRef] [PubMed]
- Makita S, Hong Y, Yamanari M, Yatagai T, Yasuno Y. Optical coherence angiography. Opt Express. 2006, 14(17), 7821–7840.
- Spaide RF, Fujimoto JG, Waheed NK. Optical Coherence Tomography Angiography. Retina. 2015, 35(11), 2161–2162. [CrossRef] [PubMed]
- Wylegala A, Teper S, Dobrowolski D, Wylegala E. Optical coherence angiography: A review. Medicine (Baltimore), 2016; 95, 41, e4907.
- Kashani AH, Chen CL, Gahm JK, Zheng F, Richter GM, Rosenfeld PJ, et al. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017, 60, 66–100. [CrossRef] [PubMed]
- de Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous. 2015, 1, 5. [CrossRef] [PubMed]
- Waheed NK, Moult EM, Fujimoto JG, Rosenfeld PJ. Optical Coherence Tomography Angiography of Dry Age-Related Macular Degeneration. Dev Ophthalmol. 2016, 56, 91–100.
- Schwartz DM, Fingler J, Kim DY, Zawadzki RJ, Morse LS, Park SS, et al. Phase-variance optical coherence tomography: a technique for noninvasive angiography. Ophthalmology 2014, 121(1), 180–187.
- Sun Z, Yang D, Tang Z, Ng DS, Cheung CY. Optical coherence tomography angiography in diabetic retinopathy: an updated review. Eye (Lond) 2021, 35(1), 149–161. [CrossRef] [PubMed]
- Pan J, Chen D, Yang X, Zou R, Zhao K, Cheng D, et al. Characteristics of Neovascularization in Early Stages of Proliferative Diabetic Retinopathy by Optical Coherence Tomography Angiography. Am J Ophthalmol. 2018, 192, 146–156. [CrossRef] [PubMed]
- Vaz-Pereira S, Morais-Sarmento T, Engelbert M. Update on Optical Coherence Tomography and Optical Coherence Tomography Angiography Imaging in Proliferative Diabetic Retinopathy. Diagnostics (Basel). 2021, 11(10).
- McClintic SM, Jia Y, Huang D, Bailey ST. Optical coherence tomographic angiography of choroidal neovascularization associated with central serous chorioretinopathy. JAMA Ophthalmol. 2015, 133(10), 1212–1214. [CrossRef] [PubMed]
- Huang D, Jia Y, Rispoli M, Tan O, Lumbroso B. Optical Coherence Tomography Angiography of Time Course of Choroidal Neovascularization in Response to Anti-Angiogenic Treatment. Retina. 2015, 35(11), 2260-2264.
- Fraser-Bell S, Symes R, Vaze A. Hypertensive eye disease: a review. Clin Exp Ophthalmol. 2017, 45(1), 45-53.
- Wakabayashi T, Sato T, Hara-Ueno C, Fukushima Y, Sayanagi K, Shiraki N, et al. Retinal Microvasculature and Visual Acuity in Eyes With Branch Retinal Vein Occlusion: Imaging Analysis by Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2017, 58(4), 2087-2094.
- Bonini Filho MA, Adhi M, de Carlo TE, Ferrara D, Baumal CR, Witkin AJ, et al. Optical Coherence Tomography Angiography in Retinal Artery Occlusion. Retina. 2015, 35(11), 2339–2346. [CrossRef] [PubMed]
- Jia Y, Wei E, Wang X, Zhang X, Morrison JC, Parikh M, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014, 121(7), 1322–1332. [CrossRef] [PubMed]
- Yarmohammadi A, Zangwill LM, Diniz-Filho A, Saunders LJ, Suh MH, Wu Z, et al. Peripapillary and Macular Vessel Density in Patients with Glaucoma and Single-Hemifield Visual Field Defect. Ophthalmology. 2017, 124(5), 709-719.
- Koutropoulou N, Panos GD. The Diagnostic Value of Optical Coherence Tomography Angiography in Glaucoma. Curr Med Imaging. 2021, 17(10), 1179–1182. [CrossRef] [PubMed]
- Kate A, Basu S. Role of Anterior Segment-Optical Coherence Tomography Angiography in Acute Ocular Burns. Diagnostics (Basel). 2022, 12(3).
- Grewal DS, Agarwal M, Munk MR. Wide Field Optical Coherence Tomography and Optical Coherence Tomography Angiography in Uveitis. Ocul Immunol Inflamm. 2022, 1–11.
- Dingerkus VLS, Munk MR, Brinkmann MP, Freiberg FJ, Heussen FMA, Kinzl S, et al. Optical coherence tomography angiography (OCTA) as a new diagnostic tool in uveitis. J Ophthalmic Inflamm Infect. 2019, 9(1), 10.
- Herbort CP, Jr., Papasavvas I, Tugal-Tutkun I. Benefits and Limitations of OCT-A in the Diagnosis and Follow-Up of Posterior Intraocular Inflammation in Current Clinical Practice: A Valuable Tool or a Deceiver? Diagnostics (Basel). 2022, 12(10).
- Camino A, Zhang M, Gao SS, Hwang TS, Sharma U, Wilson DJ, et al. Evaluation of artifact reduction in optical coherence tomography angiography with real-time tracking and motion correction technology. Biomed Opt Express. 2016, 7(10), 3905-3915.
- Chen FK, Viljoen RD, Bukowska DM. Classification of image artefacts in optical coherence tomography angiography of the choroid in macular diseases. Clin Exp Ophthalmol. 2016, 44(5), 388–399. [CrossRef] [PubMed]
- Spaide RF, Klancnik JM, Jr., Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015, 133(1), 45-50.
- Chua J, Sim R, Tan B, Wong D, Yao X, Liu X, et al. Optical Coherence Tomography Angiography in Diabetes and Diabetic Retinopathy. J Clin Med. 2020, 9(6).
- Ting DSW, Pasquale LR, Peng L, Campbell JP, Lee AY, Raman R, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019, 103(2), 167–175.
- Guo Y, Camino A, Wang J, Huang D, Hwang TS, Jia Y. MEDnet, a neural network for automated detection of avascular area in OCT angiography. Biomed Opt Express. 2018, 9(11), 5147-5158.
- Hormel TT, Hwang TS, Bailey ST, Wilson DJ, Huang D, Jia Y. Artificial intelligence in OCT angiography. Prog Retin Eye Res. 2021, 85, 100965.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).