Author Summary
Aedes mosquitoes are the vector of most arboviruses infecting humans and animals. In Ethiopia, yellow fever (YF) outbreak is frequently occurring and claiming the lives of several people. Therefore, understanding the cycle of transmission is crucial in designing the prevention and control strategies for YF outbreaks. We conducted an entomological sampling in two districts with recent YF outbreaks in southwestern Ethiopia to identify and characterize the behavior and ecology of the Aedes mosquito species playing role in transmission. The Ae. simpsoni complex was the predominant species identified in both study areas and tends to bite humans in the morning and afternoon when most people are active outdoors within the villages and forests. Although none of the tested Ae. simpsoni complex mosquitoes were positive for arboviruses, improving surveillance activities in reservoir hosts, including primates and vectors, could be a key to establishing prevention and control strategies.
1. Introduction
The emergence and re-emergence of arboviral diseases raise global concerns and threats to human health [
1]. The viruses usually circulate among wild animals, but spill over to humans sporadically, leading to epidemics [
2]. Arboviral diseases are increasingly causing morbidity in Ethiopia, with a significant increase in the number of outbreaks in the past years. Multiple outbreaks of yellow fever (YF) have occurred and affected the non-immune human population [
3]. The first YF outbreak occurred between 1960 and 1962 when 100,000 cases and 30,000 deaths were reported in the southwest of the country [
3]. The second YF outbreak took place between 2012 and 2013 in south Omo in southern Ethiopia [
4]. As a response, 550,000 people were vaccinated in the region [
5]. In 2016, 22 suspected YF cases and five deaths were reported in South Omo again [
6]. In October 2018, another YF outbreak has been confirmed in Wolaita in southwest Ethiopia resulting in the death of 10 people [
7]. Following confirmation of YF outbreaks, a vaccination campaign targeting 31,565 high-risk populations was conducted in October 2018 in six identified kebeles (villages; the lowest administrative unit). In addition, a ring vaccination campaign was carried out among 1,335,865 inhabitants in nine districts, seven in Wolaita zone and two in Gamo-Gofa zone [
8].
Recently, the YF outbreak occurred in the Gurage zone in southwestern Ethiopia [
9]. Although YF is endemic in the country, vaccination is not part of the childhood immunization program. Therefore, children and others living outside of vaccinated areas remain at risk for future YF outbreaks [
6].
In addition to YF, outbreaks of other arboviral diseases such as dengue (DEN) and chikungunya (CHIK) are also common [
10]. A previous study showed the presence of
Ae. simpsoni complex in South Omo in villages where the 2012-2014 YFV outbreaks occurred [
5].
Although arboviral disease outbreaks are common in Ethiopia, limited attention has been given to entomological monitoring and surveillance to identify appropriate vector control tools for outbreak prevention and control [
10]. The vector mediating the periodic outbreaks is unknown and there is so far no evidence of whether the outbreaks are initiated by spillover from forest catchments into urban areas or other reasons [
11]. Therefore, this study assessed the Aedes mosquito species composition, ecology, human biting activity and role in YFV transmission in sylvatic and human occupational spaces, in areas where YF outbreaks recently occurred in southern Ethiopia. This information is important to build on for further research eventually aiming to design appropriate vector control tools which might contribute to the prevention and control of future YF epidemics.
2. Materials and Methods
2.1. Ethical Consideration
Ethical clearance was obtained from Arba Minch University Institutional Review Board (Ref. No IRB/108/11). Local administrative bodies and district health offices were informed and involved in the study. Written and oral consent was obtained from the study participants (households and data collectors). For entomological collections, YF-vaccinated volunteers were included and provided with anti-malarial prophylaxis.
2.2. Study Areas Description
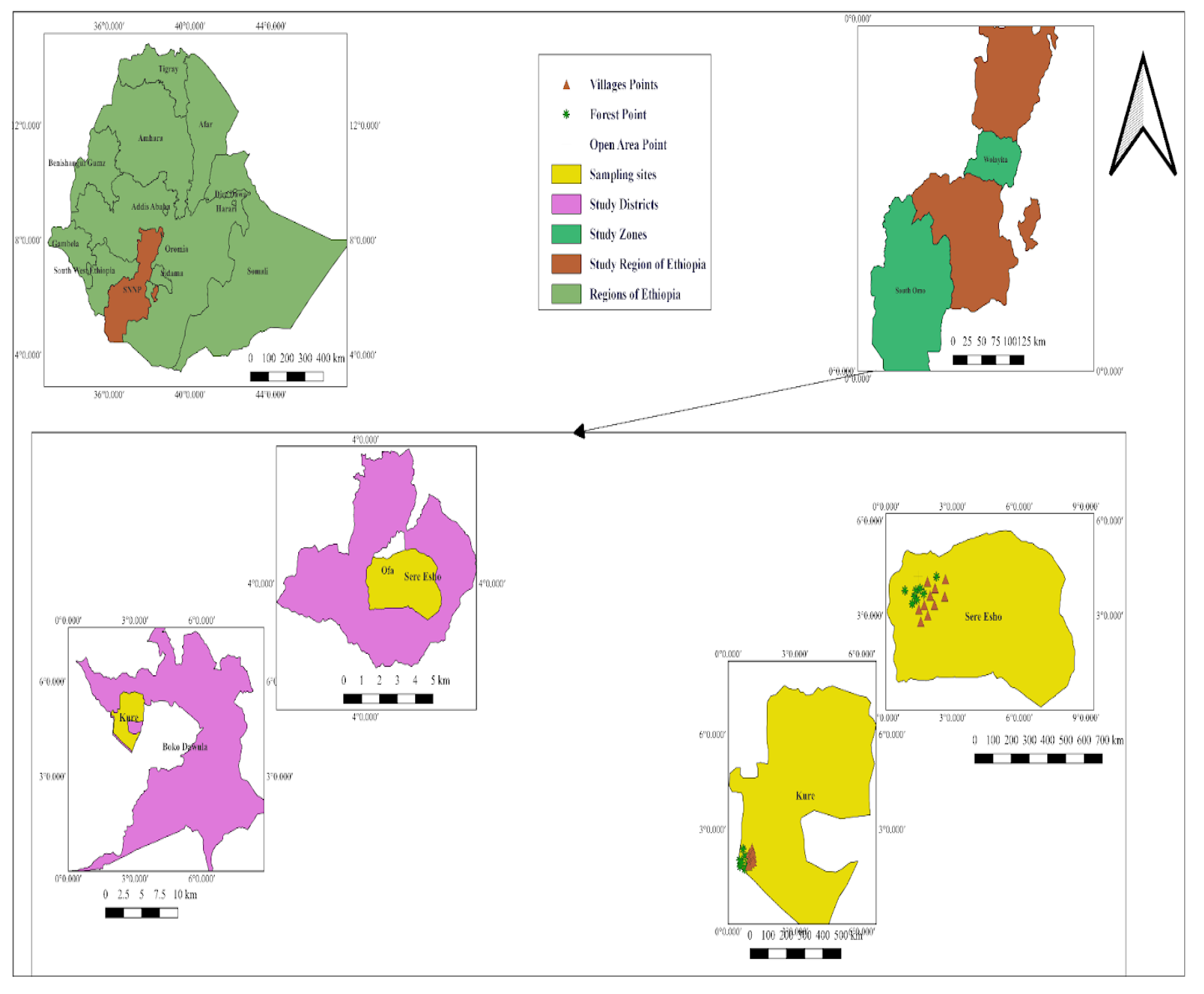
This study was conducted in Boko Dawula, former South Ari (South Omo zone) and Offa (Wolaita zone) districts in Southwestern Ethiopia (
Figure 1). The two study districts were affected by YF outbreaks in 2016 (South Omo) and 2018 (Wolaita). The selection of the study districts was made in consultation with the zonal health office, village administrations and health extension workers. Both study sites are located on the edge of a forest and in both districts, the village closest to the forest was selected for mosquito collection.
Boko Dawula district is found at about 20 km south of Jinka town, the capital of the south Omo zone. The altitude ranges between 1000-1300 meters above sea level (masl) [
12]. The mean annual rainfall ranges between 601-1600 mm and its mean annual temperature lies between 10-30°C [
13]. Offa district is located 29 km southwest of the zonal capital, Wolaita Sodo. It is located at an altitude between 1000-2000 masl [
14]. The amount of rainfall is between 800 and 1400mm with a temperature ranging between 14-28°C [
15]. Due to high population pressure, agricultural expansion and deforestation are common. The edge of the forest is mainly used for agriculture. In forests and villages, there are natural and artificial breeding habitats for mosquitoes, such as Boko Dawula.
2.3. Study Design and Sampling Techniques
We collected Aedes mosquitoes in three different habitats: forests (sylvatic cycle), agricultural lands located between human settlements and forests, and rural human settlements (villages) for six months from February 2019 to July 2020 taking into account both the Ethiopian dry (February - April) and wet months (May - July). Human landing catches (HLC), pyrethrum space spray collection (PSC), and mechanical aspiration (MAC) were employed to collect host-seeking and resting mosquitoes.
2.3.1. Human Landing Collection (HLC)
Outdoor HLC was performed twice per month at three sampling habitats (villages, forest and agricultural lands) with a distance of at least 200m between sampling sites. In the forest and agricultural land, HLC was conducted at three sites in each district. In the villages of the two selected districts, HLC was conducted both indoors and outdoors in and around five selected houses. For each house, two collectors were assigned, one indoor and one outside with hourly rotation. The collection was performed between 6:00-10:00 and 15:00-20:00. Volunteers, wearing long-sleeve shirts were seated in chairs by exposing their legs below their knees to collect mosquitoes attempting to bite using an aspirator. Hourly collected mosquitoes were transferred into a paper cup and preserved as described below.
2.3.2. Pyrethrum Spray Collection (PSC)
Indoor resting mosquito collection was done twice per month in fifteen randomly selected houses (other than HLC) in both villages, following the WHO indoor residual spraying (IRS) operational guideline [
16]. The floor, beds and furniture were covered with white sheets, and food items were removed and properly closed before spraying an aerosol insecticide (Baygon, SC Johnson and Son Inc, Racine, Wisconsin, US) [
17]. Additionally, windows, doors and other openings were closed to prevent mosquitoes from escaping. The collection was done early in the morning before smoking the houses. About 10 minutes post-spraying, mosquitoes were collected and preserved as described below [
16].
2.3.3. Mechanical Aspirator Collection
The MAC collection was performed twice per month at each site using a mechanical aspirator. Twelve houses were randomly selected (other than HLC and PSC) to collect mosquitoes resting on interior and exterior walls. For the collection of outdoor resting mosquitoes, four additional sites including caves, rock holes, tree holes and leaves were selected. A collector spent 10 minutes at each site searching for resting mosquitoes, using a timer. The number of mosquitoes collected and the type of resting site was recorded. To minimize collector bias due to skill variation, collectors were alternated between collection sites.
2.3.4. Mosquito Species Identification
The collected mosquitoes were immediately identified at the genus level in the field using morphological keys [
18,
19].
Aedes mosquitoes were preserved individually in RNA later, transported in a coldchain and stored as soon as possible at -20°C for further analysis. From all collected
Aedes mosquitoes, 406 were selected for molecular species identification taking into account the habitat, collection method, time and month; to make certain the representativeness of the sample. Sampling sthis number for molecular species identification was due to limited laboratory resources (primers and probe, reagents shortage) and budget limits. The legs of each specimen were used for DNA extraction using the NucleoSpin Tissue Kit (Macherey Nagel, Düren, Germany) according to the manufacturer's instructions. Elution was done in 50 µL nuclease-free water. After that, the isolated DNA was subjected to a PCR targeting the mitochondrial
cytochrome oxidase 1 (COI) gene as described in [
20]
Amplicons were checked on a 1.5% gel and sent for Sanger sequencing using the same primers. The DNA sequences were cleaned, and consensus sequences were generated and subjected to alignment using the ClustalW tool [
21] implemented in MEGA X 10.1. Primers were trimmed to have sequences with equal lengths. Analyses of nucleotide composition and divergences among individuals were quantified using the Kimura 2-parameter (K2P) distance model [
22]. A neighborhood joining (NJ) tree based on the K2P distances was made to provide a graphic representation of the clustering pattern among different species [
23]. The presence or absence of a barcode gap was also determined for each species as a test of the reliability of its discrimination. This was to visualize and demonstrate the divergence in sequences obtained and to compare them to sequences held in GenBank. All sequences were compared with
Aedes species sequences in the GenBank, GenBank accessions: [
Ae. simpsoni (KT881398.1) and
Ae. aegypti (MK300226.1)].
2.3.5. Arboviral Screening
For arboviral screening, mosquito specimens (head, thorax and abdomen) were pooled from 8 to 12 according to the habitat and month in which they were collected. Then, a 500 µL DNA/RNA shield (Zymo Research – Baseclear, Leiden, the Netherlands) was added to each mosquito pool and specimens were homogenized with a sterile pestle. Homogenates were centrifuged and the supernatant was transferred to a nuclease-free tube. RNA extraction was done using the Quick-DNA/RNA Pathogen Miniprep kit (Zymo Research - Baseclear, Leiden, the Netherlands) according to the manufacturer’s instructions, but the columns were replaced by Zymo-Spin IICR columns (Zymo Research). The RNA isolates were tested with three different assays: (i) a multiplex DENV 1-4 RT qPCR [
20], (ii) a YFV RT-qPCR and, (iii) a CHIKV RT-qPCR [
24] as described before. Commercial positive and negative (nuclease-free water) PCR controls were included in each PCR to validate the run.
2.4. Data Analysis
Data analysis was done in SPSS (version 21) software. Descriptive statistics (percentages, mean and standard deviations), were used to summarize the data. Generalized linear, model (ANOVA), chi-square, and t-test were used to compare vector density and behaviors of the vectors between villages/land cover types. Human biting rates (HBR) were measured directly from human-landing collections. In all analyses (p < 0.05) was considered significant. The hourly mean of the biting number of adult female mosquitoes was calculated using Pearson’s correlation.
3. Results
3.1. Mosquito Species Composition and Ecology
A total of 1689 mosquitoes 93.7% (1582/1689) of
Aedes and 6.3% (107/1689)
of Culex were collected. Of the total collected mosquitoes, 58.7% (991/1689) were from Ofa study sites whereas the left 41.3% (698/1689) were from Boko Dawula. Most of the mosquitoes were collected during the wet season 97.9% (1653/1689). 93.5% (1579/1689) of
Aedes complex were collected during wet season. Of the 1582
Aedes simpsoni complex, 57.7% (913/1582) were from Ofa district and the remaining 42.3% (669/1582) were from Boko Dawula district (
Table 1).
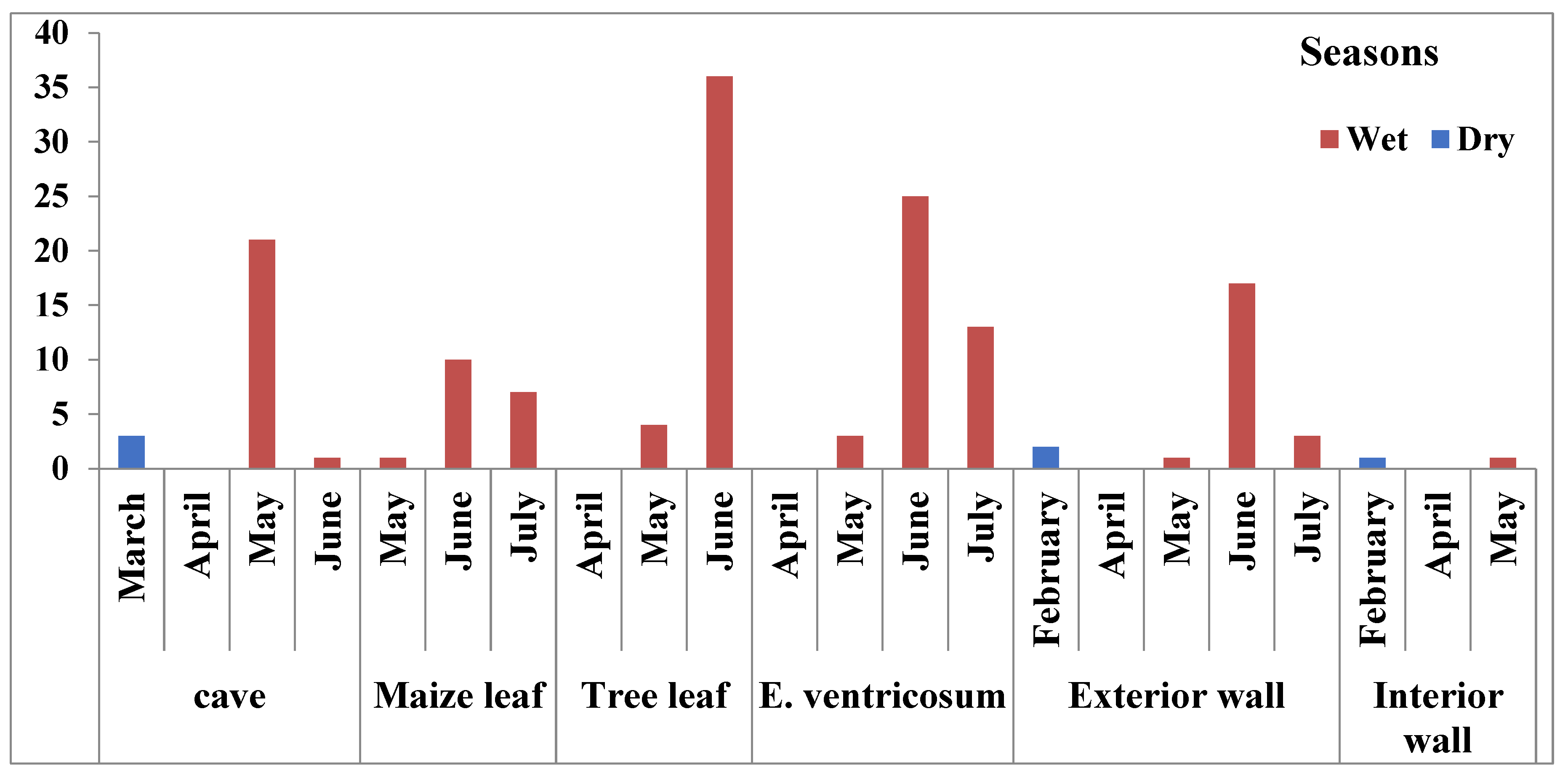
A high number of
Aedes mosquitoes was collected from tree leaves, E
. ventricosum Caves and interior walls during wet seasons (June to July) (
Figure 2).
The majority of
Ae. simpsoni complex were collected by HLC (91.2%, n = 1442, mean = 15.02) from all land covers. Of these, 1475 (93.2%) were unfed whereas only 107 (6.8%) fed Aedes mosquitoes (
Table 2).
3.2. Hourly Biting Activity
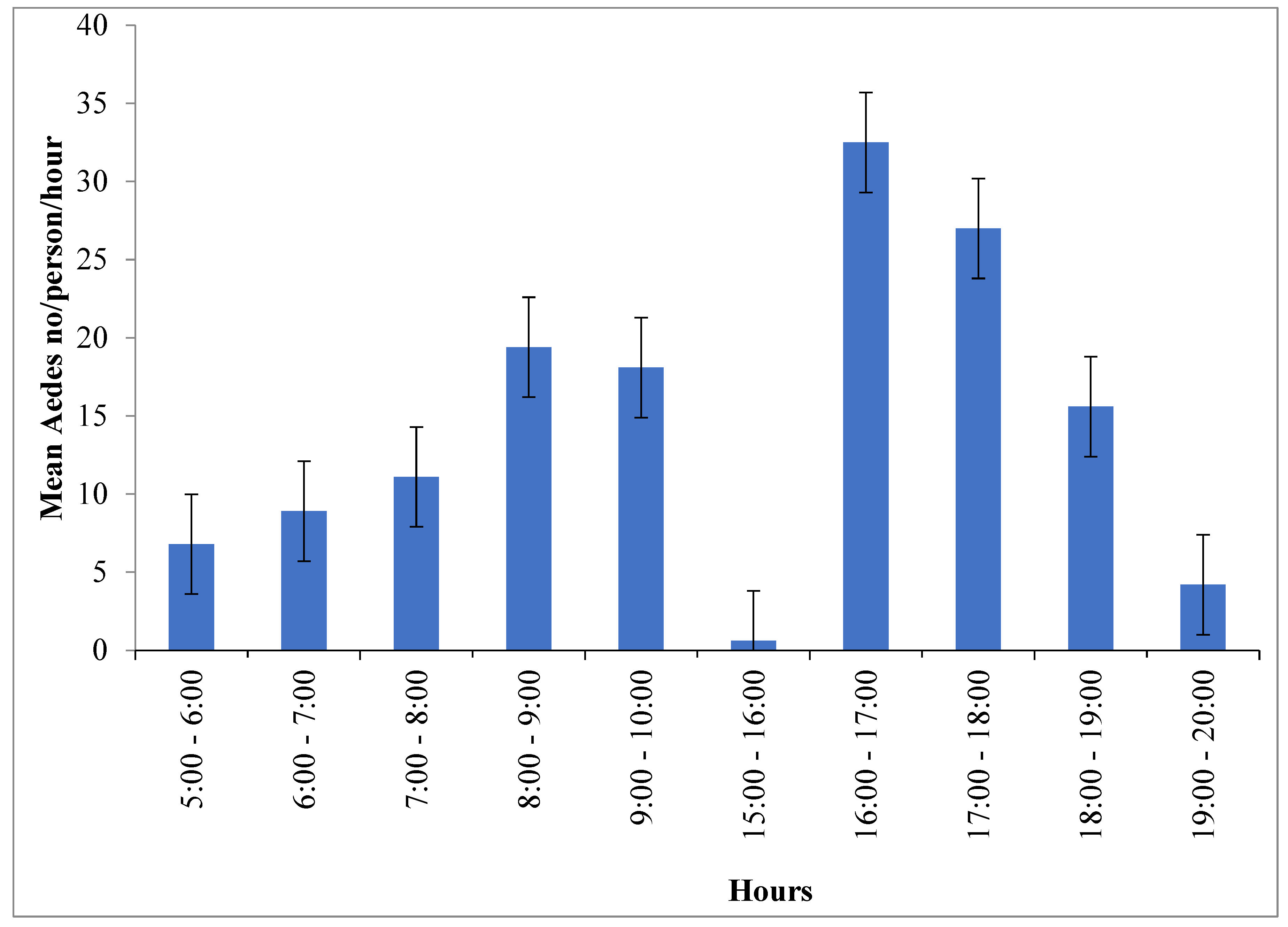
Of the total collected
Aedes mosquitoes with HLC techniques (n = 1442) (
Table 2), the hourly biting number of
Ae. simpsoni complex significantly varied across collection times (F= 19.2; DF = 9; P ≤ 0.001). The highest peak biting time of
Ae. simpsoni complex was observed between 16:00 and 17:00hour (32.5/hour/person). In the morning, the relative peak biting activity was reported between 8:00 and 9:00 hours (19.4/hour/person). The peak biting activity of
Ae. simpsoni complex sharply declined after 10h in the morning and 18h in the afternoon (
Figure 3).
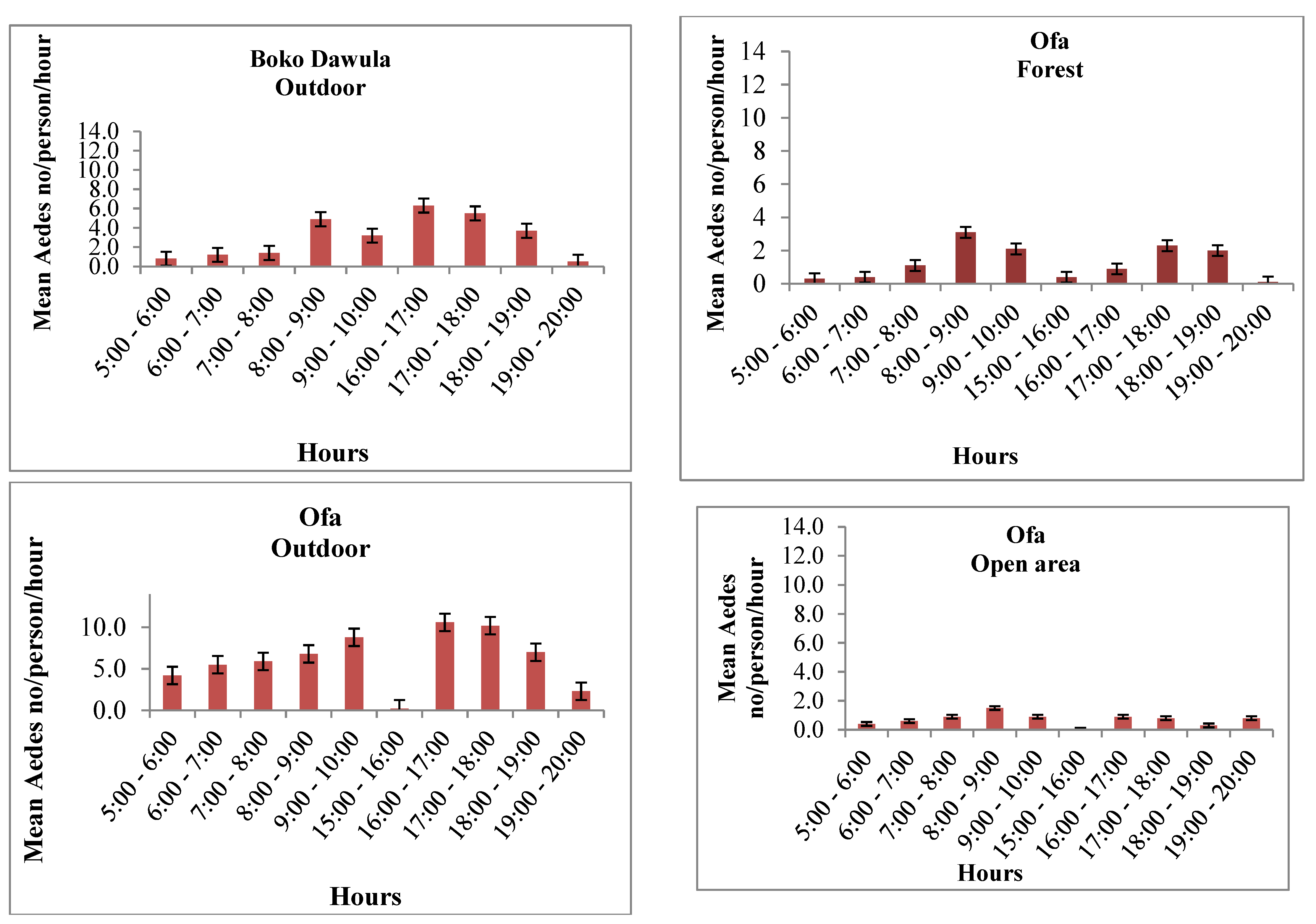
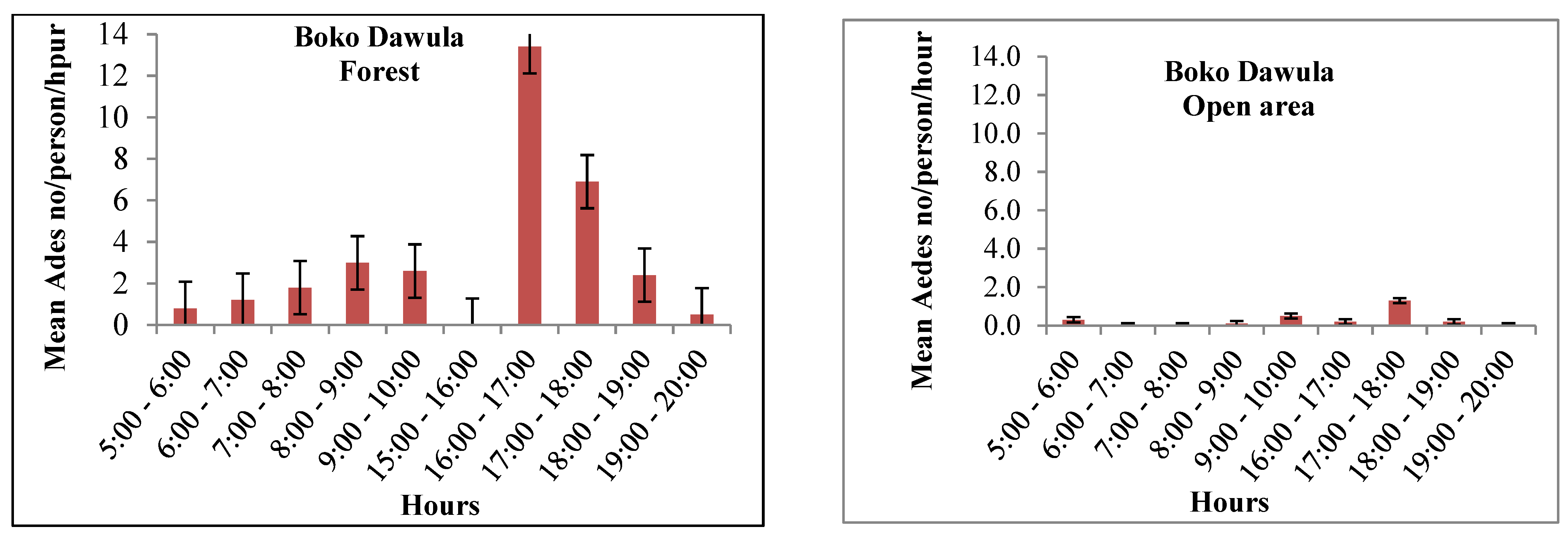
Ae. simpsoni complex was consistently active with a similar pattern outdoors within the villages and inside the forests. The peak human biting activity of
Ae. simpsoni observed outdoors averaging 10.6 bites/person/hour in Offa and 6.3 bites/person/hour in Boko Dawula from 16:00-17:00hours. Morning peak human biting activity in the forest was observed from 8 to 10 am in both Ofa and Boko Dawula. The highest biting peak of
Ae. simpsoni complex in the forest was observed between 16:00 and 17:00 hour (13.4/person/hour) in Boko Dawula. The peak biting times of Aedes was also relatively observed in Open land (
Figure 4).
3.3. Resting Sites
To assess resting behavior,
Ae. simpsoni complex collected by MAC was considered, by which 137 Aedes mosquitoes were collected overall. The density of the
Ae. simpsoni complex varied by resting sites (
P< 0.001, Df = 11). Relatively larger numbers of
Ae. simpsoni were collected from
E. ventricosum followed by tree leaves (
Table 3). A single
Ae. simpsoni complex was collected on the interior wall. Overall, most
Ae. simpsoni were collected during the rainy months of May, June and July.
3.4. Molecular Speciation of Aedes Mosquitoes
Of the 406
Aedes (198; 48. 8% from South Ari and 208; 51.2% from Ofa) mosquitoes screened for species identification, 404 (99.5%) were
Ae. simpsoni complex. The other 404
Aedes mosquitoes had more than 84% closeness to the
Ae. simpsoni (
Table 4).
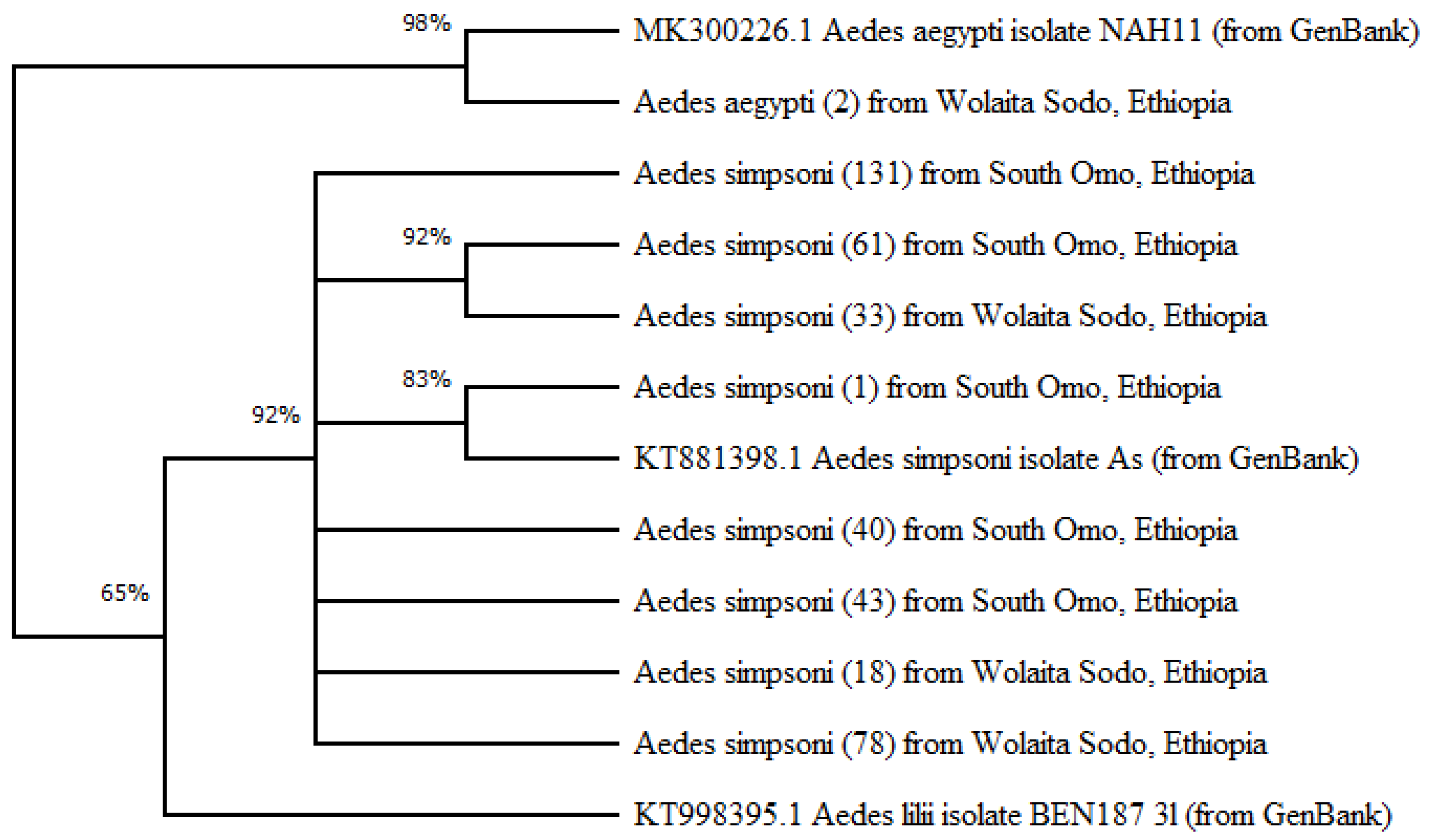
Of the species identified through amplification of mitochondrial gene COI, two
Ae. aegypti had 98% close proximity to
Ae. aegypti in the GenBank. The other 404
Ae. simpsoni complex had more than 84% closeness to the
Ae. simpsoni. All coded and identified as being very close to the mosquitoes in the GenBank and phylogenetic tree was made. Of the species identified through amplification of mitochondrial gene COI, two
Ae. aegypti had 98% proximity to
Ae. aegypti in the GenBank. The other 404
Ae. simpsoni complex had more than 84% closeness to the
Ae. simpsoni from GenBank. All were coded and identified as being very close to the mosquitoes in the GenBank and the phylogenetic tree was made (
Figure 5). Of the 934
Ae. simpsoni complex screened for viral infection by RT-qPCR, none of the pools was positive for YFV, DENV, serotype 1-4, or CHKV.
Evolutionary relationships of taxa: The evolutionary history was inferred using the Neighbor-Joining method [
25]. The optimal tree with the sum of branch length = 0.17579417 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) is shown next to the branches. The evolutionary distances were computed using the Maximum Composite Likelihood method [
20] and are in the units of the number of base substitutions per site. The differences in the composition bias among sequences were considered in evolutionary comparisons. The proportion of sites where at least 1 unambiguous base is present in at least 1 sequence for each descendent clade is shown next to each internal node in the tree. This analysis involved 12 nucleotide sequences. All ambiguous positions were removed for each sequence pair (pairwise deletion option). There were a total of 724 positions in the final dataset. Evolutionary analyses were conducted in MEGA X [
23].
4. Discussion
The current study was conducted to assess the
Aedes mosquito species composition, ecology, human biting activity and their role in viral transmission in selected districts with a previous history of YF outbreaks in southwestern Ethiopia.
Ae. simpsoni complex was the dominant
Aedes mosquito species in the two districts. In the current study sites, there is no previously conducted biting behavioral studies on
Aedes mosquitoes. The species was predominantly captured outdoors, particularly for human biting activity. Majority of the
Aedes mosquito were collected outdoors and within the forest.Very rarely, Aedes mosquitoes were collected indoors.This indicated that
Aedes mosquitoes are commonly biting human outdoors and within the forests. The current observation supports the finding of Captain-esoah et al. (2020) who reported larger numbers of
Aedes biting outdoors than indoors [
26]. In consistent to the study conducted in southeastern Senegal by [
27], the outdoor biting behavior of
Aedes might lead to an increase in risk of exposure toYF transmission outdoors than indoors.
Aedes simpsoni complex specifically
Ae. bromeliae and
Ae. aegypti have both previously been documented as the responsible vectors for the reemergence of yellow fever outbreak in South Ari, southwest Ethiopia [
3]. Adult mosquitoes have been collected both indoors and outdoors in south Omo (South Ari) and were identified as
Ae. simpsoni complex [
5].
Ae. simpsoni complex was the predominant species in these previous studies; which may imply that the species is adapted to the sylvatic environment. in contrast to our study, in the previous tudy of [
5], adult mosquitoes collected from south Omo were most likely
Ae. lilii. In contrast,
Ae. aegypti was dominant in Dire Dawa town, an urban setting in the eastern part of the country where YF outbreaks previously occurred [
11]. This might be due to differences in the sylvatic and urban environments could be different and the species distribution could vary due to the adaptabilityor the species’ ability to adapt to particular environments. The same study screened
Ae. simpsoni complex for arboviral infection and none of them were positive for YFV and other arboviruses.
Majority of the host seeking Ae. simpsoni complex mosquitoes were collected outdoors within the village and forest. The tendency of Ae. simpsoni complex to bite humans inside the forest and outdoors within the village might increase human exposure to YFV infection when they go into the forests to cut trees for agricultural expansion and other activities. Moreover, this behavior of Ae. simpsoni complex might make them suitable as a vector for the transmission of YFV in the sylvatic cycle.
The human biting activity of
Ae. simpsoni was found to be high in the afternoon, with peak biting time at 16:00-17:00h both within the villages and forests. Peak biting times are usually altered to times when their potential hosts are less protected [
26]. This indicates the highest risk to get into contact with
Ae. Simpsoni complex is during outdoor activities in the afternoon. However, the mosquitoes are also quite active in the morning, when lots of human activities take place in the forests as it is generally colder then. This might increase the risk of infection due to high-human vector contacts [
28,
29].
Aedes asimpsoni complex preferred to rest outdoors under the leaves of
E. vetricosum and other plants.
E. vetricosum was previously reported as the main breeding habitat for the same species in Southern Ethiopia [
3,
5]. This plant has a stem resistant to evaporation and stores water for a long time which makes it ideal for mosquito breeding and resting [
30]. This implies that the
E. vetricosum could be a potential site for monitoring and surveillance of both immature and adult stages.
None of the
Ae. simpsoni complex screened for arboviral diseases was found to be positive. A few specimens of the same species from south Omo (South Ari) have been screened for different arboviruses previously and none of the were also positive [
5]. The absence of virus circulation in this species might be due to the small number of mosquitoes screened or the absence of the movement of viremic vertebrates. Moreover, the YF virus transmission depends on the density of mosquitoes and wild primates [
31]. During the outbreak of YF in the two districts, the majority of the community was vaccinated, which might also contribute to the absence of the virus in the vector population [
32].
Additional to
Ae. Simpsoni complex, our study also found 2
Ae. Aegypti mosquitoes in village habitats during afternoon times with outdoor HLC method. In contrast to the current study, high abundance of
Ae.aegypti was reported in Dire Dawa, Eastern Ethiopia by [
10] from immature stage collection. This indicates that
Ae. aegypti complex is exophagic in parallel to the study conducted in Northern Ghana, that confirmed the
Ae. aegypti outdoor bitinag tendency [
26]. Similarly, the study from southeastern Senegal documented the outdoor biting tendency of
Ae. aegypti [
27]. Similar reports in Northern Ghana was shown that adult
Aedes spp. are diurnal feeders, with exophilic feeding behavior during the early or late hours of the day, usually bite and rest outdoors before and after feeding [
26].
Despite regular outbreaks of YF in Ethiopia, very little is known about the vectors and their behavior. However, this information is pivotal to designing vector control tools to prevent and control future YF epidemics. Our research findings give new insight into the temporal and spatial dynamics of the unusually Aedes mosquito species as a vecors of arboviral diseases transmission and its amplifications in their animal reservoirs that result in spillover infection of humans in an area. While many vectors may participate in maintenance of sylvatic Arboviral diseasas, Ae. simpsoni complex is most likely to be responsible for spillover into humans due to its broad land cover preferences and rates of human contact within village perimeters. This information can be used to inform the local population of the places and times of greatest risk for exposure so that mosquito avoidance or protective measures can be implemented.
There are several weaknesses in the reported study design, which need to be considered when interpreting the result of the current study data. Moreover, the current study has not covered large geographical areas to diversify the species and get positive arboviruses. As this study was limited to limited study sites, it would not have been appropriate to perform a study on non-human primates and mosquito species diversity for the detection of arboviruses. Regarding entomological indices, Immature stage collection for entomological risk analysis was not conducted, and it was not possible to assess the relative importance of each breeding site over time about Aedes mosquito’s species distribution factors (temperature, Humidity, and rainfall) analysis. As this study was employed to use only three collection techniques, it would not have been appropriate to collect a high number of blood-fed mosquitoes for blood meal origin analysis.
5. Conclusions
Aedes simpsoni complex was the most dominant species in the region and could be a potential vector responsible for the transmission of YFV. The human biting activity of Ae. simpsoni complex was predominantly in the morning (8:00 – 9:00hr) and afternoon (16:00 – 17:00hr) both outdoors within the villages and inside the forests, where people are active. Screening more mosquito samples are recommended to identify the most important vectors mediating transmissions. Future research should focus on sylvatic vector distribution at geographic large areas, blood meal sources, including reservoirs, using diversified traps types to collect Aedes before, during the outbreak and after the outbreak. Assessing the blood meal sources for a comprehensive assessment of arbovirus risk and estimating the reservoir hosts of YFV should be required.
Supplementary Materials
Supplemental information for this article can be found online at https://doi.org/10.6084/m9.figshare.19187822;https://doi.org/10.6084/m9.figshare.19187843; https://doi.org/10.6084/m9.figshare.19187849;https://doi.org/10.6084/m9.figshare.21960107; https://doi.org/10.6084/m9.figshare.19187660;https://doi.org/10.6084/m9.figshare.19187657; https://doi.org/10.6084/m9.figshare.19187654; https://doi.org/10.6084/m9.figshare.19187642.
Author Contributions
Abate Waldetensai: Conceptualization, Methodology, Resources, Investigation, Visualization, Validation, Data curation, Formal analysis, Writing-original draft, and Writing-review and editing, Fekadu Massebo: Conceptualization, Methodology, Resources, Supervision, Project administration, Visualization, Validation, Formal analysis and Writing-review and editing, Myrthe Pareyn: Methodology, Supervision, Visualization, Validation, Formal analysis, Writing-original draft and Writing-review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The Norwegian Programme for Capacity Development in Higher Education and Research for Development-Arba Minch University (ETH-13/0025) funded this work. The funders played no role in the study design, data collection and analysis, and preparation of the manuscript.
Data Availability Statement
All relevant data are within the manuscript are available at https://doi.org/10.6084/m9.figshare.19188638;https://doi.org/10.6084/m9.figshare.19188641; https://doi.org/10.6084/m9.figshare.19188644;https://doi.org/10.6084/m9.figshare.19188647.
Acknowledgments
We are very grateful to South Omo and Wolaita zone Health department office leaders, the village head, Health extension workers, and volunteers for their cooperation during data collection. We are so proud to express our deep gratitude to the data collectors for their strong commitment to collecting mosquitoes. Our special thanks go out to Girum Tamiru (Arba Minch University) and Natalie van Houtte of the Evolutionary ecology group (University of Antwerp, Belgium) and Joachim Mariën from the Outbreak Research Team (Institute of Tropical Medicine Antwerp, Belgium) for their technical assistance in the laboratory process and activities.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Qasim M, Naeem M, Bodlah I, Qasim M. Mosquito ( Diptera : Culicidae ) of Murree Hills, Punjab, Pakistan. Pak J Zool. 2014, 46, 523–529. [Google Scholar]
- Kuna A, Gajewski M, Biernat B. Selected arboviral diseases imported to Poland – current state of knowledge and perspectives for research. Ann Agric Environ Med. 2019, 26, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Lilay A, Asamene N, Bekele A, Mengesha M, Wendabeku M, Tareke I, et al. Reemergence of yellow fever in Ethiopia after 50 years , 2013 : Epidemiological and entomological investigations. BMC Infect Dis. 2017; 2–7.
- Legesse M, Endale A, Erku W, Tilahun G, Medhin G. Community knowledge , attitudes and practices on Yellow fever in South Omo area , Southern Ethiopia. PLoS Negl Trop Dis. 2018; 1–13.
- Mulchandani R, Massebo F, Bocho F, Jeffries CL, Walker T, Messenge LA; et al. A community-level investigation following a yellow fever virus outbreak in South Omo. Peer J. 2019, 7, e6466–e6466. [Google Scholar] [CrossRef] [PubMed]
- African Union Permanent Representatives Committe (PRC). Briefing on the outbreaks of communicable diseases of 2016 in different parts of Arica. Addis Ababa; 2016.
- Ministry of Health, M. Yellow fever Outbreak In Wolaita, SNNPRs, Ethiopia. Addis Ababa; 2018.
- UNICEF. Humanitarian Situation Report. 2018.
- Waldetensai A, Nigatu W, Asrat Y, Sisay C, Gunta M, Belay D; et al. Aedes Mosquitoes distribution and risk of Yellow Fever transmission in Gurage Zone, Southwest Ethiopia. Ethiop j public Heal nutr. 2019, 4, 19–23. [Google Scholar]
- Getachew D, Tekie H, Gebre-michael T, Balkew M, Mesfin A. Breeding Sites of Aedes aegypti : Potential Dengue Vectors in Dire Dawa, East Ethiopia. Hindawi. 2015, 2015, 8. [Google Scholar]
- Burkett-cadena ND, Eubanks MD, Unnasch TR. Preference of Female Mosquitoes For Natural and Artificial Resting sites. J Am Mosq Control Assoc. 2008, 24, 228–235. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Migration (IOM). Ethiopia National Displacement Report 7: Site Assessment Round 28 & Village Assessment Survey Round 11. 2021.
- Melese T, Belay T. Groundwater Potential Zone Mapping Using Analytical Hierarchy Process and GIS in Muga Watershed, Abay Basin, Ethiopia. Glob Challenges. 2022, 6, 2100068. [Google Scholar] [CrossRef] [PubMed]
- Shumbulo A, Gecho Y, Tora M. Diversity , Challenges and Potentials of Enset ( Ensete ventricosum ) Production : In Case of Offa Woreda ,. 2012, 7, 24–32.
- Menagemeso, M. Factors Affecting Diffusion of Cassava Root : The Case of Ofa Woreda, Wolaita Zone, Southern Ethiopia The Thesis Submitted to Addis Ababa University School of Commerce in Partial Fulfillment of the Requirements for the Master of Business Administration. Addis Ababa; 2018.
- WHO. An operational mannual for indoor residual spraying (IRS) for malaria transmission control and ellimination, 2nd eddition. 2015; www.who.int.
- PMI. President’s malaria initiative. Africa indoor residual spraying project. Ethiopia PMI country profile 2015. 2015;
- G. H. E. Hopkins. Mosquitoes of the Ethiopian region. 1952, 1 362.
- Kamgang B, Kusimo MO, Wilson-bahun TA, Irving H, Lenga A, Wondji CS. Geographical distribution of Aedes aegypti and Aedes albopictus ( Diptera : Culicidae ) and genetic diversity of invading population of Ae . albopictus in the Republic of the Congo [ version 1 ; referees : 1 approved ] Referee Status : Wellcome Open Res 2018. 2018, 3, 1–11.
- Anoopkumar AN, Puthur S, Rebello S, Aneesh EM. Molecular characterization of Aedes, Culex, Anopheles, and Armigeres vector mosquitoes inferred by mitochondrial cytochrome oxidase I gene sequence analysis. Biologia (Bratisl). 2019, 74, 1125–1138. [Google Scholar] [CrossRef]
- Behuraa MsK, Loboa NF, Haas B, DeBruyna B, Lovin DD, Shumway MF; et al. Complete Sequences of Mitochondria Genomes of Aedes aegypti and Culex quinquefasciatus and Comparative Analysis of Mitochondrial DNA Fragments Inserted in the Nuclear Genomes. Insect Bichemistry Mol Biol. 2012, 41, 770–777. [Google Scholar]
- Vedururu R, Neave MJ, Tachedjian M, Klein MJ, Gorry PR, Duchemin J; et al. RNASeq Analysis of Aedes albopictus Mosquito Midguts after Chikungunya Virus Infection. Viruses. 2019, 11, 1–12. [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Vadivalagan C, Karthika P, Murugan K, Wei H, Aziz AT, Alsalhi MS; et al. Genetic deviation in geographically close populations of the dengue vector Aedes aegypti ( Diptera : Culicidae ): Influence of environmental barriers in South India. Parasite Res. 2015;
- Wilkerson RC, Linton Y, Fonseca DM, Schultz TR. Making Mosquito Taxonomy Useful : A Stable Classification of Tribe Aedini that Balances Utility with Current Knowledge of Evolutionary Relationships. PLoS Negl Trop Dis. 2015, 10, 1–26. [Google Scholar]
- Captain-esoah M, Baidoo PK, Frempong KK, Adabie-gomez D, Chabi J, Obuobi D; et al. Biting Behavior and Molecular Identification of Aedes aegypti ( Diptera : Culicidae ) Subspecies in Some Selected Recent Yellow Fever Outbreak Communities in Northern Ghana. J Med Entomol. 2020, xx(X):1–7.
- Diallo D, Sall AA, Diagne CT, Faye OO, Hanley KA, Buenemann M; et al. Patterns of a Sylvatic Yellow Fever Virus Amplification in Southeastern Senegal, 2010. Am J Trapocal Med Hyg. 2014, 90, 1003–1013. [Google Scholar] [CrossRef]
- Joseph AO, Adepeju SI, Omosalewa OB, Joseph AO. Distribution, abundance and diversity of mosquitoes in Akure, Ondo State, Nigeria. J Parasitol Vector Biol. 2013, 5, 132–136. [Google Scholar]
- Lutomiah J, Bast J, Clark J, Richardson J, Yalwala S, Oullo D; et al. Abundance, diversity, and distribution of mosquito vectors in selected ecological regions of Kenya : Public health implications. J Vector Ecol. 2013, 38, 134–142. [Google Scholar] [CrossRef]
- Waldetensai A, Nigatu W, Asrat Y, Sisay C, Gunta M, Belay D; et al. Aedes Mosquitoes distribution and risk of Yellow Fever transmission in Gurage Zone, Southwest Ethiopia. Ethiop J Public Heal Nutr. 2020, 4, 21–31. [Google Scholar]
- Hanley KA, Monath TP, Weaver SC, Rossi SL, Richman RL, Vasilakis N. Infection, Genetics and Evolution Fever versus fever : The role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. IInfection, Genet Evol. 2013, xxx(2013):xxx–xxx. [CrossRef]
- Diallo M, Sall AA, Moncayo AC, Ba Y, Fernandez Z. Potential Role of Sylvatic and Domestic African mosquito species in dengue emergence. Am J Trop Med Hyg,. 2005, 73, 445–449. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).