Submitted:
15 March 2023
Posted:
15 March 2023
You are already at the latest version
Abstract
Keywords:
Background
Material and Methods
Study periods and Setting
Tsetse Fly sampling
Animal Sample Collection and parasite detection
Method of statistical analysis
Ethics statement
Results
HAT case reports
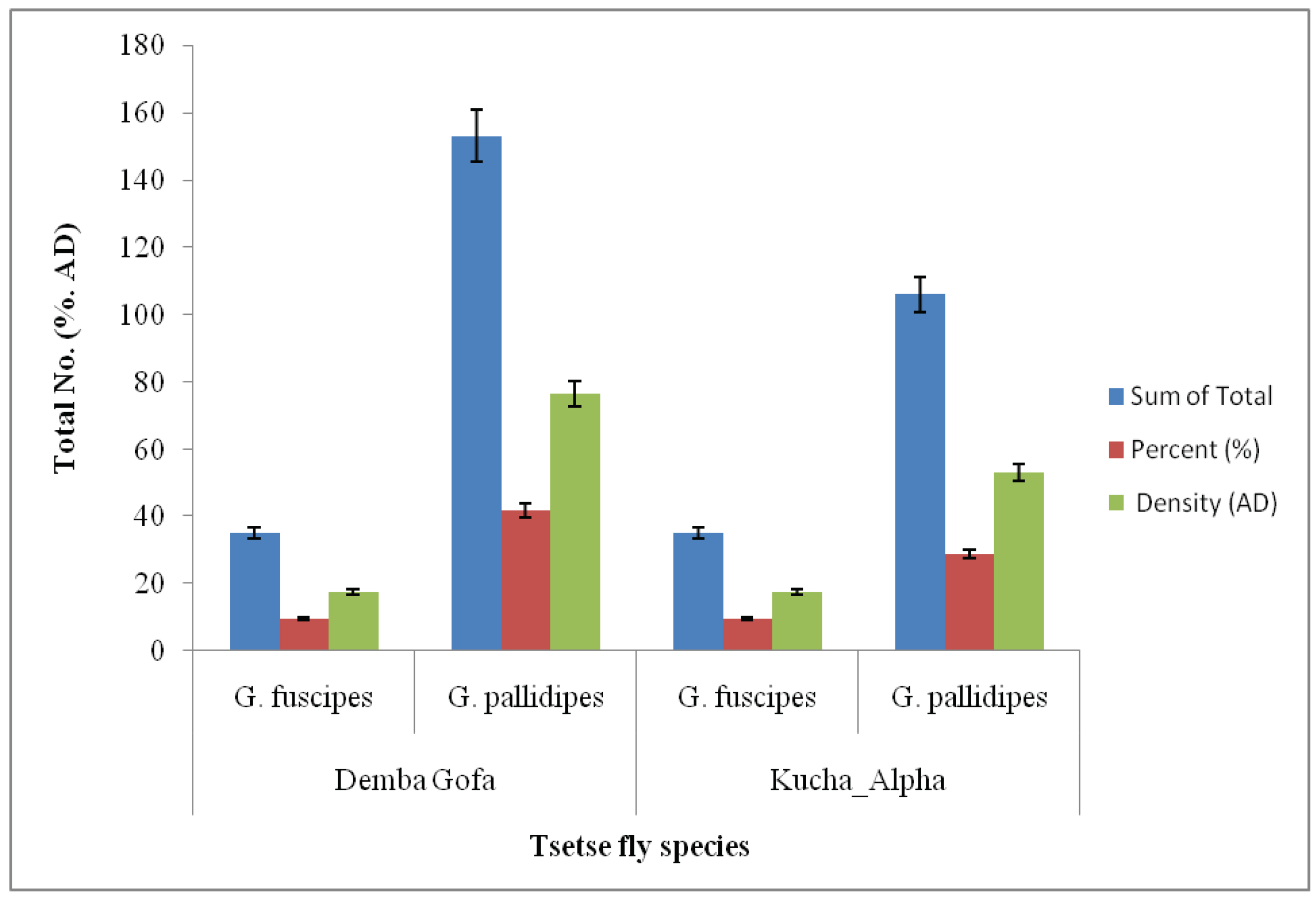
Fly composition and density
Abdominal status of tsetse flies
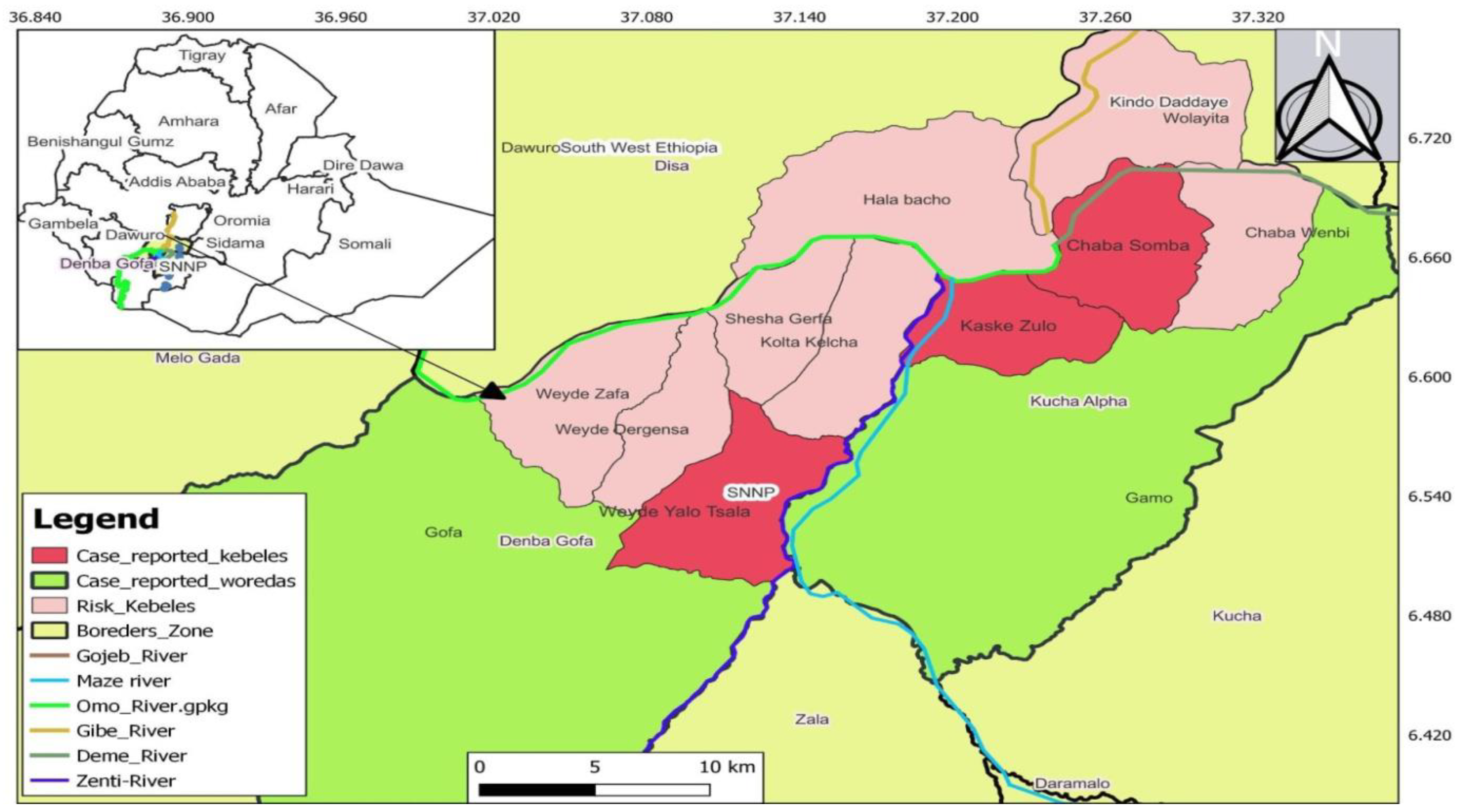
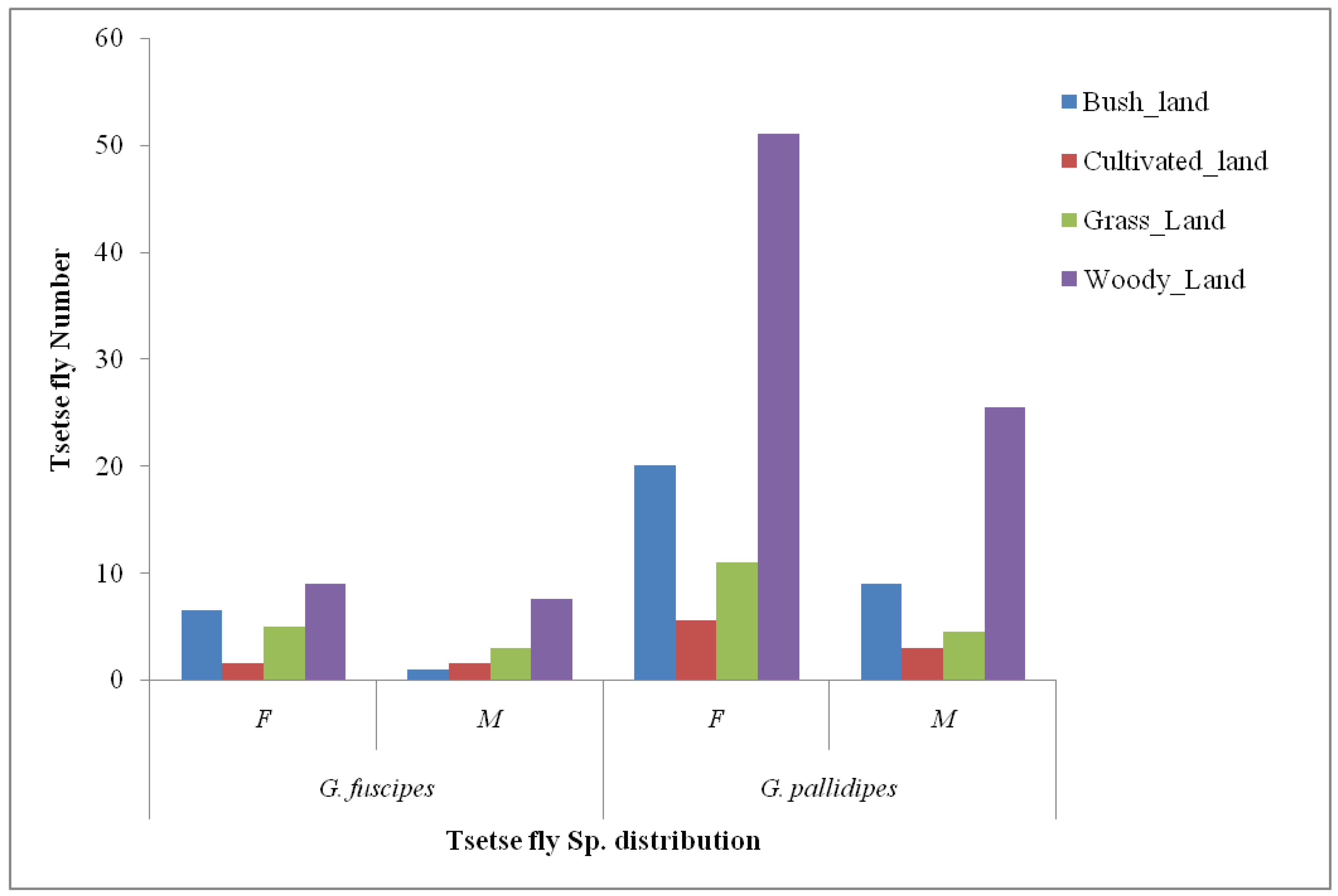
Ecological distribution of Tsetse fly
Animal investigation result
Discussion
Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Mitashi P, Hasker E, Lejon V, Kande V, Muyembe JJ, Lutumba P, et al. Human African Trypanosomiasis Diagnosis in First-Line Health Services of Endemic Countries, a Systematic Review. PLoS Negl Trop Dis. 2012;6(11).
- Simo G, Mbida JAM, Eyenga VEO, Asonganyi T, Njiokou F, Grébaut P. Challenges towards the elimination of Human African Trypanosomiasis in the sleeping sickness focus of Campo in southern Cameroon. Parasites and Vectors. 2014;7(1):1–7.
- Malvy D, Chappuis F. Sleeping sickness. Clin Microbiol Infect. 2011;17(7):986–95. [CrossRef]
- Adungo F, Mokaya T, Makwaga O, Mwau M. Tsetse distribution, trypanosome infection rates, and small-holder livestock producers’ capacity enhancement for sustainable tsetse and trypanosomiasis control in Busia, Kenya. Trop Med Health. 2020;48(1).
- Franco JR, Cecchi G, Priotto G, Paone M, Diarra A, Grout L, et al. Monitoring the elimination of human African trypanosomiasis: Update to 2016. PLoS Negl Trop Dis. 2018;12(12):1–16.
- Odera JO. Population genetic studies of the tsetse fly, Glossina pallidipes (Diptera: Glossinidae). ProQuest Diss Theses. 2004;152. http://ezproxy.nottingham.ac.uk/login?url=https://search.proquest.com/docview/305169097?accountid=8018%5Cnhttp://sfx.nottingham.ac.uk/sfx_local/?url_ver=Z39.88-2004&rft_val_fmt=info:ofi/fmt:kev:mtx:dissertation&genre=dissertations+%26+theses&sid=ProQ:ProQ.
- Seck MT, Sall B, Ndiaye EY, Guerrini L, Vreysen MJB, Bouyer, J Seck, MT Sall, B Ndiaye, EY Guerrini, L Vreysen M. Stratified entomological sampling in preparation for an area-wide integrated pest management program : the example of Glossinapalpalisgambiensis ( Diptera : Glossinidae ) in the Niayes of. J Med Entomol. 2010;47(4):543–52.
- Geremew B, Zelalem A, Aster T, Ayantu H. Survey of Apparent Density of Tsetse and other Biting flies in Gimbi district, West Wollega, Western Ethiopia. SOJ Vet Sci. 2018.
- Longbottom J, Wamboga C, Bessell PR, Torr SJ, Stanton MC. Optimising passive surveillance of a neglected tropical disease in the era of elimination: A modelling study. PLoS Negl Trop Dis. 2021;15(3).
- World Health Organization. Control and surveillance of human African trypanosomiasis. World Health Organ Tech Rep Ser. 2013;(984):1–237.
- Serap A. Control of tsetse flies and trypanosomes using molecular genetics. Vet Parasitol. 2003;115(2003):125–45.
- Nagagi YP, Silayo RS, Kweka EJ. Advancements in bait technology to control Glossina swynnertoni Austen, the species of limited distribution in Kenya and Tanzania border : A review Advancements in bait technology to control Glossina swynnertoni Austen, the species of limited distributi. J Vector Borne Dis. 2017;54:16–24.
- Shereni W, Anderson NE, Nyakupinda L, Cecchi G. Spatial distribution and trypanosome infection of tsetse flies in the sleeping sickness focus of Zimbabwe in Hurungwe District. Parasit Vectors. 2016;1–9. [CrossRef]
- Solano, P. Need of entomological criteria to assess zero transmission of gambiense hat. PLoS Negl Trop Dis. 2021;15(3):1–8. [CrossRef]
- Pan African TsetseEradication Campaign Trypanosomosis (PATTEC). Pan African Tsetse and Trypanosomosis Eradication Campaign.
- Mihok S, Carlson DA, Krafsur ES, Foil LD, Walker C, Macquart H. Performance of the Nzi and other traps for biting flies in North America. Bull Entomol Res. 2006;96(2006):387–97.
- LSTM LS of TM. Use of Tiny Targets to control tsetse flies in Gambian HAT foci : standard operating procedures. 2016;(October):1–32.
- Vreysen MJB. Prospects for area-wide integrated control of tsetse flies ( Diptera : Glossinidae ) and trypanosomosis in sub-Saharan Africa. Rev Soc Entomol Argent 65. 2006;65(1–2):1–21.
- Vale GA, Hargrove JW, Lehane MJ, Solano P, Torr SJ. Optimal Strategies for Controlling Riverine Tsetse Flies Using Targets : A Modelling Study. PLoS Negl Trop Dis. 2015;9(3):1–20. [CrossRef]
- Vincent D, Dramane K, Jean-baptiste R, Guiguigbaza-kossigan D, Bamoro C, Ernest S, et al. Detection and identification of pathogenic trypanosome species in tsetse flies along the Comoé River in Côte d ’ Ivoire. Parasite. 2015;22(18).
- Asmamaw Aki, Getachew D. Trypanosomosis in Cattle Population of Pawe District of Benishangul Gumuz Regional State, Western Ethiopia: Anemia, Vector Density and Associated Risks. Researcher. 2016;8(4):79–85.
- Zekarias T, Kapitano B, Mekonnen S, Zeleke G. The Dynamics of TseTse Fly in and Around Intensive Suppression Area of Southern Tsetse Eradication Project Site, Ethiopia. Ethiop J Agric science. 2014;24(2):59–67.
- Mesfin SM. Full Length Research Paper A cross-sectional study on the apparent density of tsetse flies and prevalence of bovine Trypanosomosis. Glob J Med Surg. 2016;4(10):151–5.
- Reid H, Kibona S, Rodney A, McPherson B, Sindato C, Malele I, et al. Assessment of the burden of human African trypanosomiasis by rapid participatory appraisal in three high-risk villages in Urambo District, northwest Tanzania. Afr Health Sci. 2012;12(2):104–13.
- Boundenga L, Mombo IM, Augustin MO, Barthélémy N, Nzassi PM, Moukodoum ND, et al. Molecular Identification of Trypanosome Diversity in Domestic Animals Reveals the Presence of Trypanosoma brucei gambiense in Historical Foci of Human African Trypanosomiasis in Gabon. Pathogens. 2022;11(9).
- Bemba I, Lenga A, Awono-Ambene HP, Antonio-Nkondjio C. Tsetse Flies Infected with Trypanosomes in Three Active Human African Trypanosomiasis Foci of the Republic of Congo. Pathogens. 2022;11(11):1275.
- A G, B Z, H K, A J. Characterization of Gofa Cattle Population, Production System, Production and Reproduction Performance in Southern Ethiopia. J Fish Livest Prod. 2017;05(03).
- Mutiso SM, Qureshi Z, Kinuthia J. Birth Preparedness Among Antenatal Clients in Rurar Health Centers in Kucha Woreda, Gamo-Gofa Zone, Southern Ethiopia. East Afr Med J. 2008;85(6):275–83.
- Senbeta Bedane A, Tanto TK, Asena TF. Malaria Distribution in Kucha District of Gamo Gofa Zone, Ethiopia: A Time Series Approach. Am J Theor Appl Stat. 2016;5(2):70.
- Zekarias B, Mesfin F, Mengiste B, Tesfaye A, Getacher L. Prevalence of goiter and associated factors among women of reproductive age group in Demba gofa woreda, Gamo Gofa zone, southwest Ethiopia: A community-based cross-sectional study. J Nutr Metab. 2020;2020.
- Rosemary B, Jingwen W, Yineng W, L WB, C WW, A MG, et al. Tsetse fly ( Glossina pallidipes ) midgut responses to Trypanosoma brucei challenge. Parasite and vectors. 2017;10(615):1–12.
- Ngomtcho SCH, Weber JS, Ngo Bum E, Gbem TT, Kelm S, Achukwi MD. Molecular screening of tsetse flies and cattle reveal different Trypanosoma species including T. grayi and T. theileri in northern Cameroon. Parasites and Vectors. 2017;10(1):1–16.
- Adugna T, Lamessa A, Hailu S, Habtamu T, Kebede B. A Cross-Sectional Study on the Prevalence of Bovine Trypanosomosis in Ankesha District of Awi Zone, Northwest Ethiopia. Austin J Vet Sci Anim Husb. 2017;4(2):1–4.
- Lemu M, Bekuma F, Abera D, Meharenet B. Prevalence of Bovine Trypanosomosis and Apparent Density of Tsetse Fly in Botor Tolay District, Jimma. Biomed J Sci Tech Res. 2019;13(3):9976–83.
- Nakamura Y, Hayashida K, Delesalle V, Qiu Y, Omori R, Simuunza M, et al. Genetic Diversity of African Trypanosomes in Tsetse Flies and Cattle From the Kafue Ecosystem. Front Vet Sci. 2021;8(January).
- Efrem D, Hagos A, Getachew T, Tesfu K, Nigatu K, Workineh S, et al. A Cross-sectional Study of Bovine Trypanosomosis in Sayo District, Oromia Regional State, Western Ethiopia. Int J Nutr Food Sci. 2018;7(2):56–64.
- Franco R, Paone M, Diarra A, Ruiz-postigo A, Fe EM. Estimating and Mapping the Population at Risk of Sleeping Sickness. 2012;6(10).
- Elenga VA, Lissom A, Ornelle D, Elion A, Vouvoungui JC, Djontu JC, et al. Risk factors and prevalence of human African trypanosomiasis in individuals living in remote areas of the republic of Congo. BMC Public Health. 2022;1–9. [CrossRef]
- Uba BV, Aliyu A, Abubakar A, Uba SA, Gidado S, Edukugho A, et al. Knowledge and prevalence of human african trypanosomiasis among residents of kachia grazing reserve, Kachia local government area, Kaduna state, Nigeria, 2012. Pan Afr Med J. 2016;23:1–8.
- Büscher P, Cecchi G, Jamonneau V, Priotto G. Human African trypanosomiasis. Lancet. 2017;390(10110):2397–409.
- Crump RE, Huang CI, Knock ES, Spencer SEF, Brown PE, Miaka EM, et al. Quantifying epidemiological drivers of gambiense human African Trypanosomiasis across the Democratic Republic of Congo. PLoS Comput Biol. 2021;17(1):1–23. [CrossRef]
- Gebre T, Kapitano B, Beyene D, Alemu D, Beshir A, Worku Z, et al. The national atlas of tsetse flies and African animal trypanosomosis in Ethiopia. Parasites and Vectors. 2022;15(1):1–15. [CrossRef]
- Mekonnen G, Mekonnen AN. Vector identification and prevalence of bovine trypanosomosis in Oda Buldigilu district of Benishangul Gumuze regional state , Western Ethiopia Vector identification and prevalence of bovine trypanosomosis in Oda Buldigilu district of Benishangul Gumuze r. J Entomol Zool Stud. 2017;5(5):1178-1183 E-ISSN:.
- Shumago N, Tekalign W. Distribution of Tsetse Fly in Selected Sites of Upper Omo Belt, Southern Ethiopia. Adv Life Sci Technol. 2016;44(1976):30–7.
- Opiro R, Opoke R, Angwech H, Nakafu E, Oloya FA, Openy G, et al. Apparent density, trypanosome infection rates and host preference of tsetse flies in the sleeping sickness endemic focus of northwestern Uganda. BMC Vet Res. 2021;17(1):1–12. [CrossRef]
- Aksoy E, Telleria EL, Echodu R, Wu Y, Okedi LM, Weiss BL, et al. Analysis of multiple tsetse fly populations in Uganda reveals limited diversity and species-specific gut microbiota. Appl Environ Microbiol. 2014;80(14):4301–12.
- Lord JS, Mthombothi Z, Lagat VK, Atuhaire F, Hargrove JW. Host-seeking efficiency can explain population dynamics of the tsetse fly Glossina morsitans morsitans in response to host density decline. PLoS Negl Trop Dis. 2017;11(7).
- Mekonnen G, Mekonnen AN, Golessa M, Asrese NM. Vector identification and prevalence of bovine trypanosomosis in Oda Buldigilu district of Benishangul Gumuze regional state, Western Ethiopia Vector identification and prevalence of bovine trypanosomosis in Oda Buldigilu district of Benishangul Gumuze r. J Entomol Zool Stud. 2017;5(5):1178–83.
- Regasa T, Bedada M, Workine M. Study on Spatial Distribution of Tsetse Fly and Prevalence of Bovine Trypanosomosis and Other Risk Factors : Case Study in Bedele Woreda, Ilu Aba Bora Zone, South Western Ethiopia. Acta Parasitol Glob 6. 2018;6(3):174–81.
| Sex | Age-group | Number of confirmed Cases |
|---|---|---|
| Female | 0 - 4 years | 1 |
| Male | 5 - 14 years | 2 |
| Male | 15 - 44 years | 2 |
| Total | 5 |
| Zone | Districts | AR/100,000 pop |
|---|---|---|
| Gamo | Kucha Alpha | 38 |
| Gofa | DembaGofa | 37 |
| Fly species | Number (%) | Apparent Density (AD) |
| G. Fuscipes | 70 (16.3) | 35 |
| G. pallidipes | 259 (60.4) | 129.5 |
| Total | 329 (76.7) | 164.5 |
| Stomoxys | 73 (17) | 34.5 |
| Tabanids | 27 (6.3) | 13.5 |
| Total | 100 (23.3) | 50 |
| Grand Total | 429 (100) | 214.5 |
| Tsetse species | Fed (%, AD) | Unfed (%, AD) | Total (%, AD) | |
| Bi-conical | 39 (11.8, 19.5) | 255 (77.5, 127.5) | 294 (89.4, 147) | |
| G. Fuscipes | 7 (2.1, 3.5)) | 55 (16.7, 27.5) | 62 (18.8, 31) | |
| G. pallidipes | 32 (9.7, 16) | 200 (60.8, 100) | 232 (70.5, 116) | |
| NGU | 7 (2.1, 3.5) | 28 (8.5, 14) | 35 (10.6, 17.5) | |
| G. Fuscipes | 2 (0.6, 1) | 6 (1.8, 3) | 8 (2.4, 4) | |
| G. pallidipes | 5 (1.5, 2.5) | 22 (6.7, 11) | 27 (8.2, 13.5) | |
| Grand Total | 46 (14, 23) | 283 (86.0, 141.5) | 329 (100, 164.5) | |
|
Factors |
Level |
Total tested |
-ve |
Positive (%) |
Total +ve |
||
| Brucei_suspected | T_congo | T_vivax | |||||
| Sex | Female | 139 | 134 | 1(0.7) | 4 (2.9) | 0 (0) | 5 (3.6) |
| Male | 162 | 158 | 1(0.6) | 2 (1.2) | 1(0.6) | 4 (2.5) | |
| Bcs | Good | 55 | 54 | 0 (0) | 1(1.8) | 0 (0) | 1(1.8) |
| Medium | 159 | 155 | 1 (0.6) | 2 (1.3) | 1(0.6) | 4 (2.5) | |
| Poor | 87 | 83 | 1((1.1) | 3 (3.4) | 0 (0) | 4 (4.6) | |
| Age | 1 - 4 yrs | 160 | 158 | 1 ((0.6) | 0 (0) | 1(0.6) | 2 (1.3) |
| 5 - 9 yrs | 132 | 125 | 1(0.8) | 6 (4.5) | 0 (0) | 7 (5.3) | |
| 10 - 15 yrs | 9 | 9 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Grand Total | 301 | 292 | 2 (0.7) | 6 (2) | 1(0.3) | 9 (3) | |
|
Animal color |
Total tested |
-ve |
Positive (+ve) |
Total +ve |
IR (%) |
|||
| Brucei | T_congo | T_vivax | ||||||
| Dambagofa | 96 | 94 | 0 | 2 | 0 | 2 | 2.1 | |
| Black | 15 | 14 | 0 | 1 | 0 | 1 | 6.7 | |
| Red | 44 | 43 | 0 | 1 | 0 | 1 | 2.3 | |
| White | 37 | 37 | 0 | 0 | 0 | 0 | 0.0 | |
| Kucha Alpha | 205 | 198 | 2 | 4 | 1 | 7 | 3.4 | |
| Black | 31 | 29 | 1 | 1 | 0 | 0 | 0.0 | |
| Red | 114 | 111 | 1 | 2 | 0 | 0 | 0.0 | |
| White | 60 | 58 | 0 | 1 | 1 | 2 | 3.3 | |
| Grand Total | 301 | 292 | 2 | 6 | 1 | 9 | 3.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





