1. Introduction
The airborne transmission of pathogen is a notable feature of the ongoing global pandemic of coronavirus disease 2019 (COVID-19) [
1]. Unlike droplet transmission, which has a quick deposition character and influences less than 1 to 2 m from the infected person, airborne transmission refers to the inhalation of respirable aerosols with particle sizes smaller than 5 μm and travel distances greater than 1 to 2 m from the infected person [
2,
3]. These aspects will result in a large number of opportunistic infections, posing a significant challenge to epidemic prevention and control. Aside from COVID-19 in 2019, airborne transmission played a role in the spreads of SARS pneumonia in 2003 and Influenza A (H1N1) in 2009 [
4].
The mechanism by which epidemic diseases are transmitted via aerosol is that viral particles and/or some attached to the environmental particulate matters (PMs) remain active and suspended in the air for long periods of time until being contacted by humans and deposited on mucous membranes, where they reproduce to form infections[
5,
6]. According to studies, the exhalatory actions of virus carriers, such as breathing, talking, singing, shouting, coughing, and sneezing, result in the production of a certain amount of viral aerosols [
7,
8]. The virus-related PMs have a wide range of particle sizes, from less than 100 nm to more than 1 mm [
9]. Large aerosol particles precipitate faster than small ones, but they are also more likely to impact and stick to other particles and form droplets due to adsorption [
3]. While factors such as UV light, humidity, and temperature inactivate viral particles, the droplet-forming viral particles reduce these effects to some extent and is thus more infectious than viral particles alone [
10,
11]. Accordingly, viral particles in ambient aerosols survive for up to 8 hours and remain infectious during the period, and if the air movement in indoor space is slow, they are infectious for a considerable period of time [
12]. The relationship between the length of stay of spatio-temporal concomitants in the space where the infected person has been and the probability of infection is an important issue. The investigation of this issue has significant implications for the development of prevention, isolation, and decontamination policies. However, more accurate qualitative or quantitative analyses rely on high-efficiency and sensitivity viral aerosol particle collection and detection device.
In the natural environment, the concentrations of viral particles are typically very low [
13]. The real-time monitoring and early detection of pathogenic PMs in bioaerosols can help meet the significant technical challenges posed by COVID-19. The qualitative or quantitative analysis of aerosol particles in the environment necessitates the use of bioaerosol samplers capable of separating and purifying particles from aerosols, which can then be combined with other biochemical analytical techniques. For example, quantitative real-time polymerase chain reaction (qRT-PCR), which is a common method for virus detection due to its high sensitivity and reliability. Certain comprehensive devices (CDs) are now available to enable real-time determination of parameters such as concentration, dispersion and composition of ambient aerosol particles [
14].
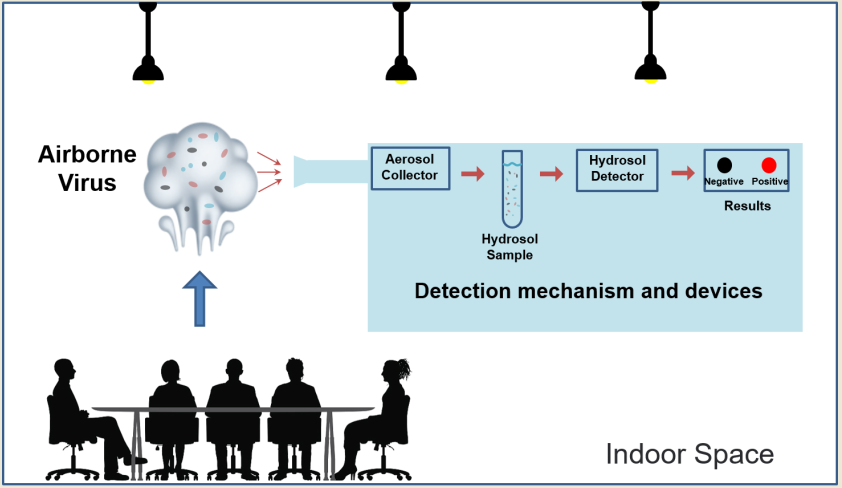
As shown in
Figure 1, the entire procedure for airborne virus detection is divided into three steps. The first step is to collect the particles from aerosol to hydrosol [
15,
16,
17,
18]. Up until now, there has been a lot of interest in the ability of aerosol to hydrosol (ATH) techniques to help with virus collection and enable downstream detection applications. The second step, called hydrosol to hydrosol (HTH), is to further concentrate and purify the detection target particles in hydrosol. The third step is to transport the concentrated liquid to a subsequent analytical device, which is a detection technique, such as direct or indirect detection of virus components like nucleic acids or proteins. The development and application of such device is obviously critical to ensuring the biosafety of environmental monitoring and the scientific nature of ventilation in indoor spaces. While combining bioaerosol samplers with other analytical detection techniques to achieve real-time monitoring of airborne viruses is challenging [
19].
Theoretical studies can aid in the development of devices that target viral particles in a certain particle size range, such as a well-designed high air flow-rate electrostatic sampler (HAFES) can target the H1N1 (peak diameter: 95 nm), as well as HCoV-229E (109 nm) [
14,
20]. However, the viral particles in the real environment are usually not alone in the air, but rather attach to other PMs to exist in the air with a wide particle size range. Chains of infection due to sequential presence in the same indoor environment are often frequently hard to track down and establish. Thus, broad-range collection, efficient purification, and sensitive analysis of viral particles in the environment are critical to resolving this issue. Furthermore, combining these three processes to create a CD that collects extremely low concentrations of viral aerosol particles and transfers them to a detection device with the appropriate limit of detection (LOD) in a small volume of liquid for subsequent detection would reduce the response time for outbreak prevention and control, the range of full nucleic acids, and the cost of controlling outbreaks. This will provide significant technical support for the prevention of diseases transmitted by aerosols, such as COVID-19. Therefore, this paper takes a review at recent advances in the collection and detection of airborne pathogen viruses from this perspective.
2. Methods
The reports reviewed in this study were sourced from ISI Web of Science, Pubmed and Google Scholar. Two categories of keywords were used: 1) aerosol, particle, sampler, collector, virus, and bioaerosol; 2) SARS-CoV-2, detection, nucleic acid detection, anti-gen/antibody detection, PCR, loop-mediated isothermal amplification (LAMP), clustered regularly interspaced short palindromic repeats (CRISPR), microfluid, and chip. At least one keyword from each category was used in each search. Results show more than 150 publications are relevant to aerosol particulate matter collecting, sampling and detection. Significant publications are summarized in
Table 1 and
Table 2. First, the primary mechanisms of aerosol PM collection are reviewed. Second, various techniques to enhance collection efficiency are presented and organized into enhanced collection principles. Third, current detection methods for SARS-CoV-2 are briefly described, with an emphasis on detection in the field of microfluidics. Finally, an indoor environment biosafety monitoring program is proposed based on various principles combined with ventilation of the indoor environment. For future research and development of aerosol collection-enrichment-detection devices, research and application directions are provided.
3. Sampling Techniques and Devices of Airborne Viruses
3.1. Primary Mechanisms
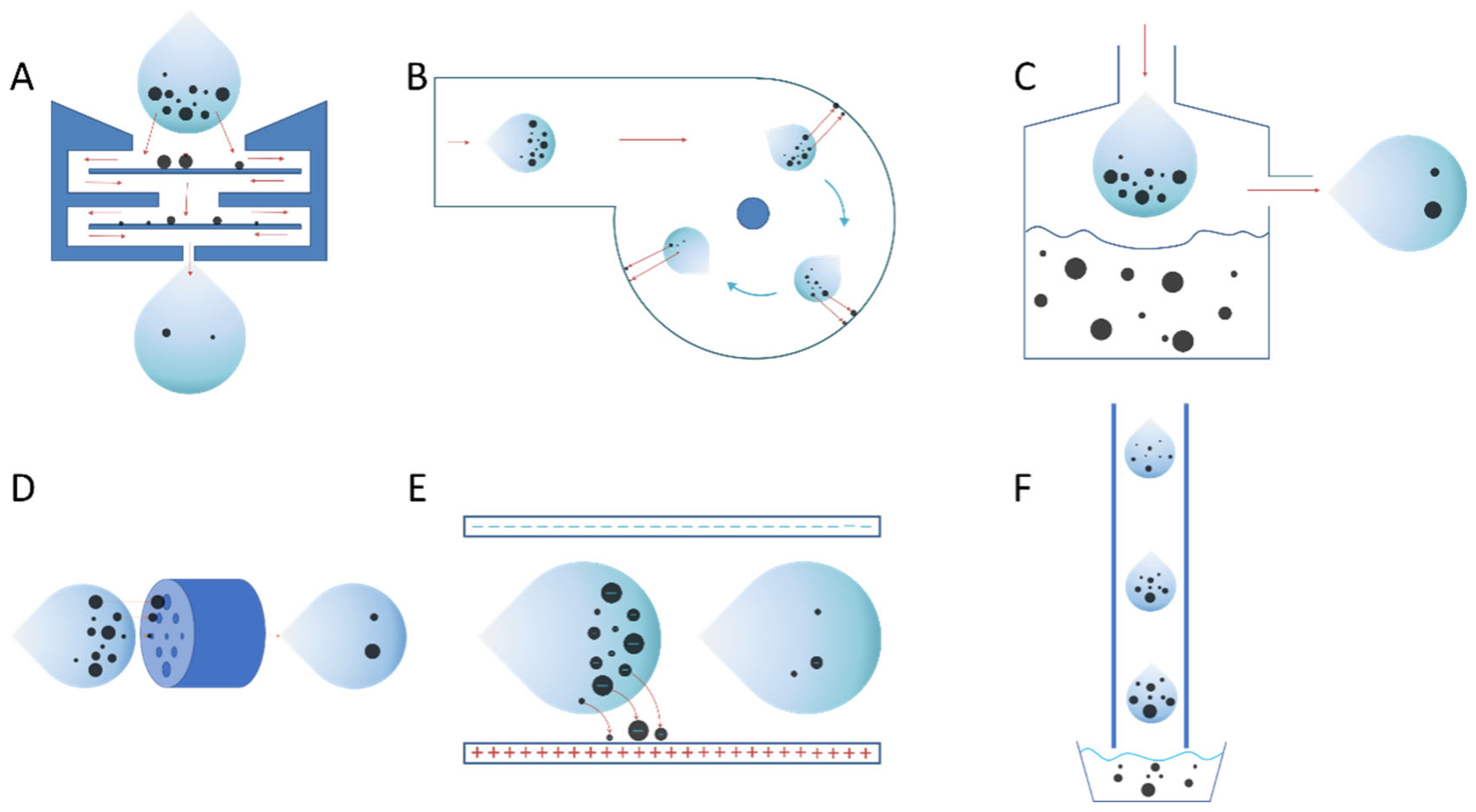
The fundamental mechanism of aerosol particle collection is that the particles are separated from the air by specific principles (inherent property of the PM or some force). These mechanisms or properties include but are not limited to size filtration, inertia, gravity, electrostatic force, centripetal force, etc (
Figure 2). Each of these collection principles has its applicable scenarios. The following is a brief description of each aerosol particle collection mechanism from the standpoint of aerosol flow within the device, as well as comments of each device.
3.1.1. Inertance
The samplers, such as impactor, impinger and cyclone are all use inertia to separate particles. The inertia of the particles in aerosol is greater than that of the rest of the aerosol components. When a high velocity aerosol rushes to the collection plate, the particles settle on the collection plate and the remaining aerosol is discharged from the outlet.
3.1.1.1. Impactor
As illustrated in
Figure 2A. Impactor fundamental principle: Under the action of a fan or pump, aerosols are sucked or sprayed into a channel with baffles (collection plates), particles are deposited on the collection plates by inertia, and the remaining aerosols are discharged from the outlet end with uncollected particles.
COMMENTS: This mechanism has been used successfully in a variety of contexts [
23,
50]. The flow rate, the pipe diameter of the channel, and the size of the baffles can all be modified to meet the required target particle size for collection. Additionally, depending on the requirement, it offers single-stage or multi-stage processing options. The single-stage can be directed to collect PM of a certain particle size section, whereas the multi-stage can collect a wide range of particles throughout a broad spectrum. The detachable multi-stage device has the capacity to collect particles within a certain target particle size range as needed. However, smaller particle sizes require extremely high velocities to collect the particles, which make high throughput collection impossible and increase the manufacturing precision, the cost, and the damages from clogging [
49]. The ambient aerosol PM, On the other hand, needs to be transported to the back-end analysis unit for following analysis after collection. The applicability of this principle to high-efficiency collection devices is limited, since the elution of PM from the collection plate is a challenging procedure.
3.1.1.2. Cyclone
As shown in
Figure 2B for Cyclone principle, aerosols are sucked or sprayed into the annular channel under the action of a fan. Centrifugal force deposits particles on the inner wall of the annular channel, and the remaining aerosol is discharged from the exit end with the uncollected particles.
COMMENTS: To achieve high aerosol throughput, the aerosol can be kept flowing at a high speed in the annular channel. However, experiments show that the decay of collection efficiency is more visible when the flow rate is high [
19]. An important research question is how to seek out the appropriate balance between flow rate and efficiency. In addition, the elution of particles from the inner wall is a challenging issue, making it difficult to transport samples to the detection device.
3.1.1.3. Impinger
As shown in
Figure 2C for Impinger’s mechanism, aerosol is rushed straight to a liquid surface under the action of a fan, particles are rushed into and dispersed or dissolved in the liquid by inertia, and the remaining aerosol and uncollected particles are discharged from the outlet end.
COMMENTS: Direct collection into the liquid greatly improves the efficiency of transporting the sample to the back-end detection module. Similar to the Cyclone, the balance of flow rate and collection efficiency is the focus. Additionally, using multiple collecting fluids to handle various types of particles with various properties, such as oily particles, non-oily particles, and improving the collection fluid’s affinity for certain types of particles improve the collection efficiency of the particles. However, re-aerosolize poses a serious concern due to the collection fluid is in a high flow rate aerosol environment, and the loss of collected particles during the process is a challenge [
51,
52].
3.1.2. Filtration
As shown in
Figure 2D. The aerosol is passed through a channel containing a filter plate or filter membrane under the action of a fan. The particles are trapped in the channel by the effects of the screen and the remaining aerosol is discharged from the outlet end with the uncollected particles. Filtration generally uses porous media like activated carbon, glass fiber nonwovens and medical stone as filter membranes [
53,
54,
55]. The particles in the aerosol are adsorbed in the pores inside the media when they pass through.
COMMENTS: Particles larger than a specific particle size are collected using particular sieve membranes or screens (filtered). It is a good collection method if the target particle size is known. High flow rate collection is not possible when the particle size is at the nano or micron level since it only selects the lower limit, not the upper limit of the particle size. In addition, transporting of the particles from the filter membrane to the back-end detection device requires a more laborious process, and the filter membrane itself is prone to clogging that prevents it from being utilized for an extended period of time.
3.1.3. Electrostatic Precipitator
The electrostatic precipitator mechanism has been widely used in the field of dust removal [
56,
57]. Electrostatic precipitators are potential high-flow viral aerosol particulate collection devices because they can use Coulomb forces to all charged particles in the aerosol to deflect and deposit them on the pole plate. The aerosol enters a collection electric field under the action of a fan, and the particles are deflected and deposited on the collection plate by the Coulomb force (the particles themselves carry a small negative charge). The remaining aerosol, along with the uncollected particles, is discharged from the outlet end. As shown in
Figure 2E for electrostatic precipitator, the Coulombic force applied indiscriminately to the whole PM allows for high-efficiency collection, and, similar to the Cyclone and Impinger, the balance between flow and collection efficiency is critical. Simultaneously, the electric field may have some effects on the activity of the PM due to the ozone produced by the negative electrode, which may influence the sensitivity of the culture and the subsequent analytical techniques [
58,
59,
60]. However, certain techniques, such as targeting nuclear acid, may be unaffected.
3.1.4. Particle Amplifier
The particle amplifier is based on the principle of allowing particles to combine with other particles at high humidity. The particles are amplified and become easier to deposit before settling into the collection pool. As shown in
Figure 2F, the low temperature aerosol enters a warm and extremely humid pipe under the action of a fan. In the pipe, the droplets are continuously combined to form larger particles. The amplified particles are deposited in the collection pool at the back end, and the remaining aerosol along with the uncollected particles, is discharged from the outlet end.
COMMENTS: Particle amplifiers have the ability to achieve high collection efficiency while also meeting the needs of individual detection device. Multi-circulation of particles can further improve their collection efficiency [
61]. However, high flow rates are difficult to achieve due to the lengthy deposition process in the pipeline. Furthermore, the particle amplifier consumes quite a lot of energy.
Various devices have been developed based on the fundamental mechanisms stated above. The commercialized devices have previously been reviewed [
62,
63,
64,
65,
66,
67]. Particle collection using only primary mechanisms has two troubles. First, on the whole flow rate is low, and the collection efficiency decreases when the flow rate increases. Second, sampling particle size is limited, and the collection efficiency decreases when the particle size decreases. Even so, these primary mechanisms have been shown to be effective. Enhancement techniques, as well as the combination of several primary mechanisms may have an extra positive impact on the high-flow efficient particle sampling.
3.2. Enhancement Techniques
Researchers have used enhancement methods to increase the collection efficiency and developed comprehensive device to improve the sampler collection efficiency based on the primary methods. The collection of aerosol particles is divided into 3 steps as described above: Aerosol entry - aerosol collection via a specific principle - residual aerosols discharge. As an extension, the collection efficiency can be increased from various extra steps of enhancement techniques (
Figure 3).
3.2.1. Precharge
In nature, viral particles have relatively weak negative charges. The charge of the particles directly determines the magnitude of the Coulomb force on them when using electrostatic precipitation methods to collect particles. A series of theoretical and experimental studies have been conducted on the particle charging characteristics, and particle charging laws corresponding to various charging devices, particle size, and ionic density have been obtained. The precharge on aerosol particles increases the Coulomb force and shortens the length of the device’s collection section, enables particles to be deposited onto the collection plate faster (
Figure 3A) [
68,
69,
70,
71].
3.2.2. Aerosol Premixing
Aerosol premixing at the inlet involves mixing the target aerosol with aerosolized materials that has unique features (
Figure 3B). Premixing has two type’s results:
- 1)
The germicidal effect of the special materials on viral particles;
- 2)
The non-electrically charged aerosol is made by mixing the electrically charged particles.
Priyamvada et al. improved the collection efficiency of the subsequent precipitation fraction by using an aerosol mixing method in the device to mix charged aerosol particles with target aerosol particles in the inlet section to make them charged with a higher charge [
27]. Kalaiselvan et al. indicates that essential oil vapors of Melaleuca alternifolia act on aerosol coronaviruses, E. coli or fungal Aspergillus flavus spores to reduce the number of culturable microbial cells [
72]. This can help control aerosol-transmitted diseases like COVID-19. Miyaoka et al. has demonstrated that spraying on aerosols with acidic hypochlorite water (SAHW) can lower viral activity and hence prevent infections [
73]. Jung et al. developed a generation method for nanosilver particles [
74]. They disclosed that the nanosilver particles inhibited the activity of viral particles. Notably, the type of aerosol particles mixed in can be adjusted to change its affinity for specific particles. It is possible to collect a defined particle size interval while consuming less energy. Furthermore, this eliminates the waste caused by broad-spectrum collection.
3.2.3. Liquefied Collection Plate
The particles are collected by devices like impactor, cyclone, precipitation, etc. into a solid collection plate. These methods have a number of limitations:
- 1)
Particles have to be eluted into liquid before conducting further monitoring test;
- 2)
The activity of viral particles on a dry plate cannot be guaranteed;
- 3)
The high velocity of wind may cause particles to re-aerosolize.
Researchers have developed liquefied collection plates to address these issues (
Figure 3C/D). In order to improve the collection efficiency, it is possible to collect the viral particles directly in the liquid and delivered to the detection module at the back end by turning the collection plate of the above methods into a collection pool. It was also shown that liquefying and charging the collection plate for electrostatic aerosols can still generate an electric field with collection capability in verification experiments [
14,
19,
28]. The liquefied collection plate can be connected with the subsequent detection module using an automated device, which will shorten the overall process time and facilitate the automatic results display. Liquid samples can also be obtained using novel elution methods. The electrowetting-on-dielectric (EWOD) technique allows droplets on the substrate to be manipulated by applying an electrical signal to the flow of electro droplets, thereby increasing elution efficiency (
Figure 2D) [
75].
3.2.4. Cavities and Ribs on the Inner Wall of the Pipe
Well-designed cavities and ribs on the inner wall of an aerosol channel can improve the collection efficiency of particles by causing some of them to be deposited in cavities, and such studies are typically carried out using simulation methods. The mechanism of deposition enhancement and deposition efficiency ratio of particles with different particle sizes, rib spacing (p) and rib height (e) were investigated [
76] . The presence of ribs on the channel’s inner wall surface improves the particle deposition efficiency significantly, and the entrainment of turbulent eddies caused by ribs is better for the capture of particles with smaller particle sizes. Particle deposition efficiency improves as rib spacing decreases, especially for large particles, but rib height variation has no significant effect on deposition efficiency. The deposition enhancement was highest when the rib spacing p/e = 2. Lu et al. also simulated aerosol particle deposition in smooth and pipelines decorated with cavities and ribs [
76]. It was found that the addition of cavities and ribs to the inner wall of the duct increased the particle deposition rate by 10-4000 times either in the vertical or horizontal conditions. The maximum particle deposition enhancement occurred for particles of 0.2-3 μm in size, while the minimum enhancement occurred for particles of 20-50 μm in size. The design of cavities and ribs is an effective enhancement method for small particle sizes.
Hemmati et al. used the Euler-Lagrange method to simulate granular gas flow for particle deposition in a turbulent incompressible gas flow in a horizontal channel with different two-dimensional artificial roughness shapes and heights [
77]. The shape of the ribs was discovered to influence the size of the gas flow field and recirculation zone, which in turn influence particle deposition efficiency. Triangular shaped ribs were found to have higher PM collection efficiency than other designs [
78,
79]. These studies have shown that cavities and ribs on the inner walls of sampler pipes improve the PM collection efficiency to some extent, as shown in
Figure 3E. The following factors have the greatest impact on the efficiency of cavities and ribs: 1) Ribs spacing, height ratio: m = p/h; 2) Ribs shape.
In addition to the studies mentioned above, several others have investigated the effect of cavities and ribs on particle deposition efficiency using computer simulations, which can be used to guide the design of rough surfaces as well as structural design of certain inner walls of particle collection devices [
80,
81,
82,
83].
3.2.5. Hydrosol to Hydrosol Enrichment
Generally, the concentration of hydrosol samples after collection by aerosol collector insufficient. Enhancing the concentration of viral particles in hydrosol samples with efficient enrichment can enhance the performance of CD and support downstream detection that requires small sample volume. Various HTH enrichment methods have been developed to meet this challenge. Yeh et al. developed a portable microfluidic platform using carbon nanotube arrays with differential filtration porosity for rapid identification and enrichment of viruses [
84]. Twist Bioscience has developed a variety of SARS-CoV-2 specific platforms that target fragments of viral DNA for enrichment [
85]. Chu et al. developed a nanoparticle-based sandwich immunosorbent assay as a sensing platform for effective detection of nonstructural protein 1 (NS1) of viruses such as enterovirus 71 (EV71) Japanese encephalitis virus (JEV) and Zika virus (ZIKV). It was illustrated that testing results for EV71 spiked human serum samples were in 95% agreement with PCR assays [
86]. Wylezich et al. developed a virionome based on the biotinylated RNA decoy principle for specific capture enrichment of endemic animal and zoonotic viruses (VirBaits) [
87]. Isaac A.M. Frias et al. used layered composite polyaniline-(electrospun nanofiber) hydrogel mats (ENM) for simultaneous enrichment and impedance sensing of ZIKV virus particles for specific identification of ZIKV in Vero cell cultures [
88]. Bai et al. developed a magnetic quantum dot nanobead (MQB)-based lateral flow assay for magnetic enrichment and fluorescence detection of influenza A virus (IAV) particles in clinical specimens. Their method enables quantitative detection of IAV viral particles in 35 min with a detection limit as low as 22 pfu/mL and shows good specificity between influenza B virus and two adenovirus strains [
89]. The aptamer used for synthesis and modification is capable of specific binding to the target, has demonstrated its functionality in bio-detectors, and is expected to be used for specific enrichment of aerosol detection systems [
90,
91,
92].
3.2.6. Other Issues
Enhancement from all CD steps is required to improve the performance of airborne virus collection devices. Other methods, in addition to the various enhancement techniques mentioned above, are listed below. As an example, the Ace glass impinger (AGI-30) has a very low collection efficiency (30%) for fine particulate collection, and the glass bead filling has increased the efficiency to 99% [
93].
The operators’ safety is another critical issue in the development of airborne detection CDs. Tea tree and Eucalyptus oils were used as a coating for filter fibers, which effectively improved the particulate collection efficiency while reducing the activity of viral PMs [
94,
95]. Damit et al. developed an ultra-high temperature (UHT) system to inactivate the virus and avoid secondary infection of the operator due to the operation of the device [
10].
3.3. Comparisons on Airborne Virus Sampling Devices
Devices for collecting aerosol PM using only one collection method already perform well, but them far from adequate for practical scenarios [
96]. Real-time surveillance is critical for airborne infectious diseases and the epidemics. Further advancements in performance may allow it to be applied to practical epidemic prevention. To achieve increased efficiency, new CDs have been developed that use a combination of multiple primary mechanisms or the method of enhancing techniques. The following are some advanced aerosol particulate collection devices. These devices are critical for the indoor airborne virus detection applications (
Table 1).
3.3.1. Combinations of Multiple Primary Mechanisms
Using cyclone and impactor, Sung et al. designed a CD in which aerosols enter from the outer wall in a cyclonic manner impacting on the collection plate and exit from the inner channel in the opposite direction. With a flow rate of 1000 L/min, the efficiency of the air sampler is about 50 % for a particle size of 1 µm and 78.3 % for a particle size of 1.5 µm. For particle size greater than 1.5 µm, collection efficiencies of approximately 100 % were observed [
21,
22]. A three-stage high-capacity bioaerosol sampler was designed [
23,
24]. Size-selective sampling of bioaerosols was performed by inhaling air at a high flow rate of 1000 L/min. In stage 1 and stage 2, PM >10 μm and PM between 2.5 and 10 μm were collected using the cyclone method, respectively. In stage 3, PMs < 2.5 μm were collected using the filtration method, and generic Phosphate buffered saline (PBS) elution was used to obtain a hydrosol sample. This device combines the primary mechanisms, cyclone and filtration, to expand the collection range of particle size spectrum and enhance the collection efficiency of each particle size spectrum under the condition of ultra-high flow rate. Hong et al. designed a personal electrostatic particle concentrator (EPC) using an impactor and an electrostatic precipitator. The collection efficiency was improved at a lower flow rate of the individual sampling device. At a flow rate of 1.2 L/min, the collection efficiency of polystyrene particles with particle sizes of 0.05 ‒ 2 μm was up to 99.3 ‒ 99.8% [
25]. McDevitt et al. designed and built a new sampler, Gesundheit II (G-II), using the amplifier and impactor principles to amplify PM and then secondarily collect the particulate matter to enhance the collection efficiency of small particles. This device provides a collection efficiency of >85% for particles larger than 50 nm for influenza viruses [
26].
3.3.2. Applications of Enhancing Techniques
The following describes the CDs using the enhancing techniques in combination with the primary mechanisms.
Tan et al. designed a new hemispherical electric field using Liquefied acquisition plate, pre-charging, electrostatic precipitator developed automated bioaerosol collection and output [
19]. After the aerosol is charged by the charging electrode, it enters the hemispherical electric field, where the travel path of the particles is shifted by the Coulomb force and falls into the central charged collection fluid, which is circulated by an external pipe and a peristaltic pump to achieve a continuous cycle and can be integrated with subsequent detection modules. Under the conditions of collection voltage 20 kV, charging voltage: 1.5V, and flow rate 1.2 L/min flow rate, the collection efficiency is 70% to 90% for particles of 0.3‒1.2μm, >90% for particles of 0.65‒0.8μm, and >90% for particles of 0.8‒2.0 μm; under the conditions of 6.2 L flow rate for each particle size segment The collection efficiency of PM is reduced to 20%‒60% [
19]. This device employs a hemispherical electric field and liquefied acquisition plates to efficiently collect PM into liquid samples. Kim et al. used a combination of Liquefied acquisition plate, electrostatic precipitator [
14]. A High air flow-rate electrostatic sampler (HAFES) was designed. The collection efficiencies were 88%, 79%, 82%, and 71% for HCoV-229E particles with peak particle size of 109 nm at a collection voltage of -10 kV and fluxes of 40 L/min, 60 L/min, 80 L/min, and 100 L/min, respectively. This device has a high collection efficiency and it has discovered that the collection efficiency decreases as the flow rate increases. Priyamvada et al. developed a new electrostatic precipitation-based portable low-cost sampler (TracB) using the principles of prefixing and electrostatic precipitator [
27]. The collection efficiency is above 50% for particles with particle size 0.01‒10μm at 8kV and flow rate: 10 L/min. The Liquefied acquisition plate, pre-charging and electrostatic precipitator principles were used by Ma et al [
28]. The integrated micropump automatically delivers the acquisition fluid through a half-open microchannel to the liquid outlet and flushes the deposited particles away into the collector, which can be integrated with the detection module at the back end. For particles <5 μm, the maximum sampling flow rate is 13.2 L/min at a charging voltage of -1.8 kV and a collection voltage of -7 kV, with a corresponding maximum effective collection efficiency of about 40%. This integrated microfluidic electrostatic sampler (IMES) is a bioaerosol sampling system ready for integration with subsequent automatic detection device. Lin et al. developed a high-flow water-based condenser by adding an aerosol premixing link to the front end of the cyclone to amplify the PM and enhance the collection efficiency [
29]. The results showed that when the aerosol flow rate was 19°C and the temperature of the mixed reservoir was 50°C, the physical capture efficiency of aerosol of 30‒100 nm was 70‒99%. This device makes better use of the amplifier to improve overall PM collection efficiency, but the amplified PM can result in inaccurate detection data at the back end. Novosselov et al. developed and tested a low-cost micro-channel collector (mCC) using a Micro-Channel cyclone [
30]. Particles are collected on the wall of the pipe due to centrifugal force. The typical collection efficiency is more than 50% for 0.5 μm particles and 90% for particles larger than 1 μm. This device has potential applications for front-end microfluidic chip acquisition.
3.4. Differences between Virus Sampling and Virus Detection
It is critical to differentiate between sampling and collection. Because sampling-based devices do not require real-time data, the samples are subsequently re-cultured, they require less efficiency. In less human-populated environments, such as farms and pastures, sampling equipment can be used for routine monitoring. Collection devices must be more efficient, as all particles passing through the device must be collected for subsequent testing of certain critical components. Devices for data collection can be used in densely populated environments such as conference rooms, subway stations, and large shopping malls. Based on the analysis and comparison in
Table 1, the following classification criteria are proposed.
- 1)
High flow rate: >1000 L/min; medium flow rate: 100-1000 L/min; low flow rate: <100 L/min.
- 2)
Particle size spectrum - collection efficiency: high collection efficiency ≥ 70%, medium collection efficiency: 40-70%, low collection efficiency: <30% low collection efficiency.
- 3)
Collection efficiency - particle size spectrum: full particle size collection device, partial particle size collection device, targeted collection device.
4. Detection Techniques of Airborne Virus
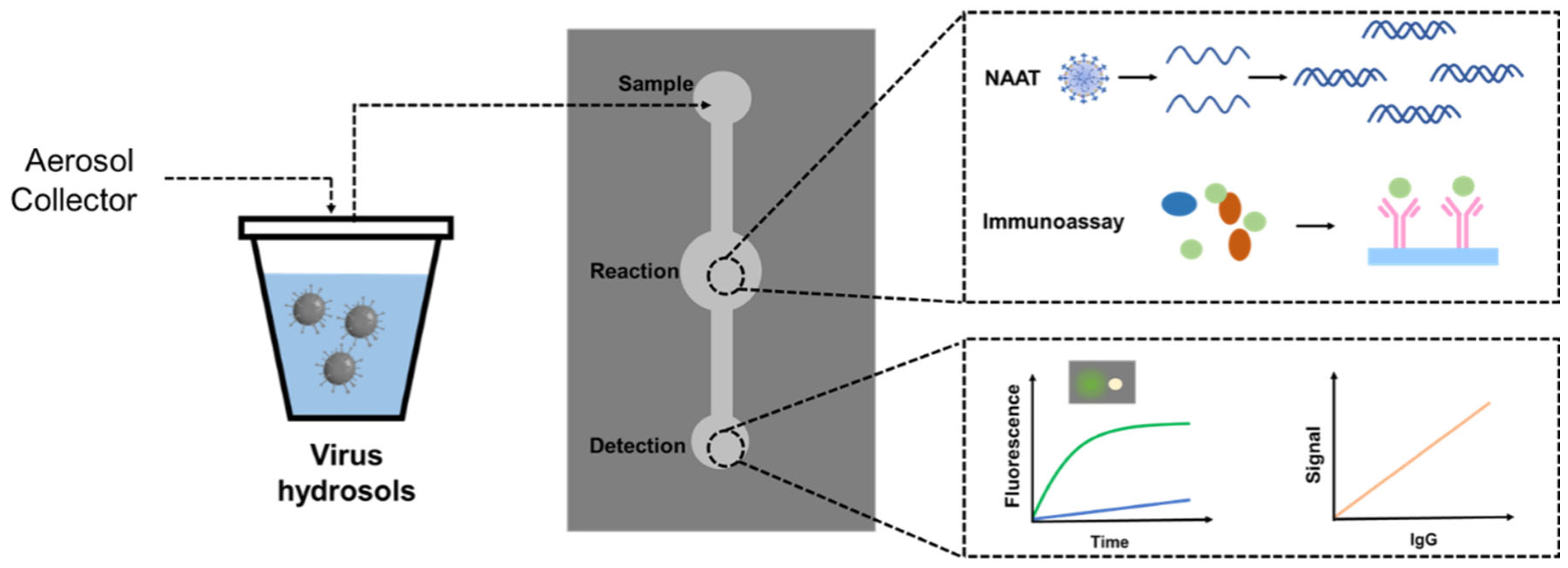
Detection techniques are important for compounding devices and determine the amount of hydrosol sample per unit time, sensitivity and response time. Virus assays are usually divided into two main categories, namely direct and indirect assays [
97]. Indirect detection methods involve virus isolation, where viruses are introduced into a suitable host cell line to propagate virus particles, which are later tested. This method usually requires a longer turnaround time. Direct detection methods include nucleic acid detection and immunoassay. Nucleic acid amplification test (NAAT) refers to the amplification and detection of genes in the virus and is characterized by early diagnosis, high sensitivity, and specificity, and the most widely used technique is RT-PCR. Immunoassay uses antibodies as the main means of detecting the virus in the sample, with the greatest advantage of convenience and short detection time. However, immunoassay detection of infection may be of limited use in the early stages due to the time required for the body to develop an immune response to the infection.
Table 2 summarized the typical detection techniques. Current conventional diagnostic techniques require expensive device and specialized operators and are inadequate to enable rapid, accurate, and on-site diagnosis during pandemics.
4.1. Nucleic Acid Detection Techniques
Nucleic acid testing has a relatively high sensitivity. In general, nucleic acid testing is used to confirm a viral infection diagnosis. PCR, LAMP, CRISPR, and other nucleic acid detection methods are commonly used. Current conventional diagnostic techniques require expensive devices and specialized operators and are insufficient for rapid, accurate, and on-site diagnosis during pandemics. Microfluidic devices provide a favorable platform for rapid testing because they offer highly integrated disposable chips that enable potential automation from sample preparation to result output (
Figure 4). Microfluidic technology has recently provided a breakthrough in viral detection. Several microfluidic-based nucleic acid detection platforms have been introduced.
4.1.1. Polymerase Chain Reaction
Many studies on microfluidic systems related to PCR have been conducted as a hot topic in nucleic acid diagnostics. PCR is a thermal cycle nucleic acid temperature amplification technology. Lee et al. described a disposable, polymer-based RT-PCR detection chip for human immunodeficiency virus (HIV) testing [
98]. The chip incorporates embedded micro pinch valves and RT-PCR to produce a portable analyzer with an infrared lamp temperature control system and an optical detection system for analytical chemiluminescence assays. The detection takes only 35 minutes, reducing contamination and sample loss. Oshiki et al. used microfluidic nested PCR followed by MiSeq amplicon sequencing (MFnPCR–MiSeq) to create a high-throughput tool for detecting and genotyping 11 human pathogenic RNA viruses [
99]. This is the first study to use microfluidic PCR and next-generation sequencing technologies in a genotyped environment to detect and genotype multiple human RNA viruses. Li et al developed an automated sample-to-answer disk based on a dual rotary axis centrifugal microfluidic platform to detect hepatitis B virus (HBV) in whole blood [
100]. The disc can separate serum from whole blood, extract DNA using magnetic beads, aliquot nucleic acids, and perform real-time polymerization chain reaction. For HBV DNA detection, the sample-to-answer time is about 48 minutes, the detection limit is 100 copies/mL, and the only manual processing step is supplying the sample to the disc.
4.1.2. Loop-mediated Isothermal Amplification
Isothermal amplification has requires few devices, can be done in a thermostatic water bath, and has a quick reaction time. It can be highly integrated with microfluidic devices to achieve device miniaturization, high sensitivity and precision of nucleic acid detection. Ma et al developed a self-driven microfluidic device that detects H1N1 influenza virus using reverse transcription loop-mediated isothermal amplification (RT-LAMP) [
101]. A hydrophobic soft valve, novel polydimethylsiloxane surface treatment, and capillary force were used to build the device, which performs virus isolation and lysis, isothermal nucleic acid amplification, and virus colorimetric detection. The entire procedure only takes 40 minutes and has a detection limit of 0.8 ng/mL. It is adequate for use in an environmental detection setting. LAMP reaction can also be used for factual monitoring. The use of fluorescent dyes causes false positives due to non-specific amplification. It can be used to distinguish nonspecific amplification by labeled primers [
102,
103], binding quenching probes [
104], hybeacons probes [
105], and molecular beacons [
106]. The future development trend is a fully integrated inspection system. There have been numerous reports on materials suitable for nucleic acid extraction, including modified magnetic beads, silica beads/sol gel solid-phase extraction (SPE) beds, and silica/glass columns. Wang et al., for example, proposed a microfluidic system that integrates RNA extraction and LAMP detection processes for detecting nervous necrosis virus (NNV) in aquacultured grouper [
107].
4.1.3. Clustered Regularly Interspaced Short Palindromic Repeats
Due to their high sensitivity and specificity, mobility, quickness, and low cost, CRISPR have been created as effective instruments for nucleic acid detection [
108,
109,
110,
111,
112]. The CRISPR approach is also suitable for bedside testing because it can be carried out at physiological temperatures. The creation of CRISPR-based biosensors as point-of-care (POC) devices is made possible by microfluidic technologies. The SARS-CoV-2 RNA can be concentrated and purified from a significant amount of swab eluate using a single-step CRISPR-Cas-assisted assay (POC-CRISPR), which uses magnetic-based capture and transport of nucleic acid-binding magnetic beads to transport the RNA to downstream amplification and detection [
113]. POC-CRISPR can detect SARS-CoV-2 RNA - 1 genome equivalent/μL from a sample volume of 100 μL less than 30 min. In an effort to speed up the CRISPR-Cas12 reaction, Ramachandran et al. used isoelectrophoresis (ITP) on a microfluidic chip to harvest RNA from nasopharyngeal swabs. The procedure includes isothermal reverse transcription and amplification, ITP-based nucleic acid extraction from the original sample, and ITP-enhanced CRISPR testing [
114]. With an average LOD of about 10 copies/μL and a sample to result time of only 40 minutes, this approach uses approximately 100 times less volume of all reagents than the average quantity of only 0.2 μL.
4.2. Antigen Detection Techniques
Antigen detection is to determine the presence or absence of a virus by detecting proteins on the surface or inside the virus. Here we focus on several virus sensing platforms.
4.2.1. Surface Plasmon Resonances
Surface plasmon resonances (SPRs) have been used to identify biomolecular interactions for therapeutic or scientific purposes. They can detect very low concentrations of chemical and biological substances near the measured surface by monitoring the region's refractive index value in real time [
115]. For example, Yoo et al. reported a reusable magnetic SPR sensor chip in which they used ferromagnetic patterns fabricated on the SPR sensor chip to trap a layer of magnetic particles, and those patterns with trapped magnetic particles [
116]. This SPR sensor chip can be reused in a conventional SPR system without any chemical process for renewal. The use of SPR technology to create new biosensors for virus samples shows promise. Such biosensors are being used to identify RNA viruses such as influenza A/B, SARS-Corona, Ebola virus, Middle East Respiratory Syndrome (MERS), Zika virus, and Dengue virus.
4.2.2. Surface-enhanced Raman Spectroscopy
Surface-enhanced Raman spectroscopy (SERS) is an attractive, highly sensitive and multiplexed assay. Raman reporter molecules modified aptamers are usually immobilized on a nanostructure metal-dielectric substrate to recognize and capture viruses. Negri et al. first proposed a label-free SERS-based platform for detecting influenza virus nucleoproteins [
117]. Wang et al. proposed a SERS-based immunoassay with digital microfluidics (DMF) for disease biomarker detection [
118]. Magnetic beads that have been coated with antibodies are used as solid supports to capture antigen from the sample, resulting in the formation of a bead-antibody-antigen immunocomplex. Antigens can be detected sensitively by strong SERS signals by labeling immune complexes with SERS tags functionalized with detection antibodies. The quantitative detection of avian influenza virus H5N1 was used to demonstrate the utility of the DMF-SERS method, which has a sensitivity of 74 pg/mL, a detection time of less than 1 h, and significantly less reagent consumption (30 μL) than standard ELISA.
4.2.3. Electrochemical Detection Electrochemical Aptamer-based Techniques
Electrochemistry measures the current response or resistivity change to determine the concentration of a detection target. Idili et al. created an electrochemical aptamer (EAB)-based sensor for measuring SARS-CoV-2 stinger (S) protein in a reagent-free and quantitative manner [
119]. The technique is based on signals generated by the binding-induced conformational changes of redox reporter aptamers on the surface of gold electrodes. S protein in buffer, serum, and 50% artificial saliva were measured at the picomolar level. The assay results were also shown to be specific.
4.3. Serological Tests
Antibodies produced by the body in response to antigenic stimulation are detected by serological tests. Methods that are commonly used include enzyme-linked immunosorbent assay, chemiluminescence assay (CLIA), and LFIA. Many efforts have been made in recent years to improve diagnostic sensitivity, such as Wang's development of a selenium nanoparticle-modified SARS-CoV-2 nucleoprotein-based lateral flow immunoassay kit, and Devora's development of a device for simultaneous detection of SARS-CoV-2 RNA and SARS-CoV-2 antibody [
120,
121].
4.4. Other Methods
For large and heterogeneous samples, conventional mass spectrometry (MS) cannot provide accurate molecular weight measurements. MS has driven the development of the histological revolution, including proteomics, glycomics, and metabolomics, with the development of Matrix Assisted Laser Desorption and Ionization (MALDI), particularly electrospray ionization [
122]. As a result, mass spectrometry can accurately detect the molecular weight and purity of biological macromolecules such as polypeptides, proteins, nucleic acids and polysaccharides with high accuracy. Virus detection is now being done using liquid chromatography mass spectrometry, electrospray mass spectrometry, and Matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) [
123,
124,
125,
126,
127]. Many assays have been developed on a chip as modern detection techniques become more miniaturized and portable. Mass spectrometry can also be combined with nanoelectromechanical systems to allow for miniaturization, as this technique allows for direct mass measurement without relying on the charge state of the sample [
128].
4.5. Advantages and Limitations of Detection Techniques
In general, nucleic acid assays are sensitive, specific, rapid and accurate, and can generally be used as a detection basis. RT-PCR is a mature technology, but it requires strict process control to avoid false negatives or false positives. The LAMP assay is currently thought to be inferior to RT-qPCR in terms of assay sensitivity and specificity, but it has the advantage of a quick turnaround time and ease of setup [
129]. For the new crown assay, the commercially available RT-LAMP kit results are consistent with RT-PCR results with a clinical sensitivity of 76.3% [
130]. The CRISPR assay's performance varies, with sensitivity ranging from 80% to 100% and specificity of 100%, and it has great potential for use with POCT devices or high-throughput testing platforms. Although the antigen assay is less sensitive than the nucleic acid assay, it has the advantages of convenience and speed, and it can be used as an individual assay in most cases. The sensitivity of serological methods is related to the duration of onset, with little difference in specificity, which is greater than 95%. It is a powerful diagnostic tool, but it is susceptible to false positive results due to interference from other substances in the blood sample [
131].
The first limiting factor is that most current detection systems rely on fluorescence detection, which has high sensitivity, high specificity, and real-time monitoring. However, this means that it requires the integration of a number of optical components, excitation sources, photodetectors, and filters outside of the device [
132]. Another limitation is the requirement for an additional sample amplification step for diseases with low viral load, which requires a thermal module to achieve PCR or isothermal amplification [
133,
134]. Furthermore, NAAT technologies based on microfluidics lack multiplex capabilities [
135,
136]. Rigorous validation is still required to translate these technologies from research to practical usage. Therefore, microfluidic-based integrated diagnostic devices provide novel approaches to airborne virus detection.
To summarize, responding to viral disease outbreaks necessitates the use of rapid, accurate, and sensitive detection technologies to expedite diagnosis. Microfluidic chips have become a powerful tool for virus molecular analysis over the last 20 years due to inherent advantages such as high integration, accuracy, low reagent consumption, and fast response time [
137,
138]. The ideal microfluidic assay chip should be capable of automating its operation and meeting the goal of "sample input and result output" [
139,
140,
141]. Therefore, the three fundamental steps for conducting experiments on the chip are sample preparation, target amplification, and product detection. Despite the large number of papers on nucleic acid amplification and detection that have been published, truly miniaturized chip-scale devices remain elusive in the laboratory and industry, with few examples of fully integrated "sample-to-result" microfluidic devices.
5. Application Scenario-Dependent Devices for Airborne Virus Detection
The combination of an aerosol collector and a detector into a single CD reduces the detection time of airborne viruses and speeds up emergency response. The performances of CDs are crucial for the various application scenarios [
4,
62,
63]. As illustrated in
Figure 5, the front-end aerosol collector determines the CD's flow rate and collection efficiency, while the back-end hydrosol detector determines the CD's sensitivity and other crucial factors, such as response time.
Various viral aerosol particulate samplers, collectors, and detectors have been developed. The primary performance parameters of these devices are collection efficiency, flow rate, sensitivity and response time [
142,
143]. It is technically difficult to create a device with extreme high performance across the board. As a result, different combinations of principles can be used to improve some of the performance to meet the needs of the application scenarios. The performance indicators should meet varied requirements of the application scenarios. To address this issue, different primary mechanisms and enhancement techniques should be used in the development of aerosol collector. Meanwhile, the back-end hydrosol detector should employ appropriate detection techniques. As shown in
Figure 6, The CDs are categorized into environmental detection device and individual detection device in accordance with various application scenarios. Environmental detection device is further subdivided into environmental monitoring device and environmental detectors.
For the actual circumstances of various scenarios, there are various options. Thus, it is feasible to combine environmental monitoring device, environmental detectors, and individual detection device in various forms for a high efficiency detection of airborne virus. This chapter will provide a building ventilation and airborne virus detection strategy based on the above-mentioned device categories. Aside from the scenarios mentioned above, the main ventilation pipe and sewer pipe in a building both contribute to high levels of indoor air pollution. It is critical to monitor these circumstances using environmental monitoring devices.
5.1. Device Classification
5.1.1. Environmental Monitoring Device
The outdoor environment is multi-factorial and complex, and outdoor aerosol monitoring necessitates collaboration with related fields such as environmental monitoring, air pollution control and weather forecasting, as well as the establishment of large monitoring stations for multi-indicator environmental aerosol monitoring and prediction [
144]. The CDs mentioned in this review are for monitoring, detection, and early warning of viruses in the indoor environment, and their proper application is critical for developing solutions for fighting airborne diseases (
Table 1/2) [
145]. Here, we classify indoor environmental monitoring equipment into two groups: high-flow environment aerosol monitoring devices and portable environmental sensor.
5.1.1.1. High-Flow Environment Aerosol Monitoring Device
The volume of aerosols in the living environment is large for indoor environments that require constant ventilation [
146]. For example, an office with a floor area of 20 m
2 and a floor height of 2.5 m contains nearly 50 m
3 of aerosols. In the environment, viral aerosol particles have a low concentration and a wide particle size distribution (attached to other particles) [
13,
19]. Therefore, aerosol particle collection devices for environmental monitoring are expected to possess the following characteristics in order to meet the requirements:
- 1)
Aerosol collector: high flow rate, high collection efficiency for the full particle size range.
- 2)
Hydrosol collector: high hydrosol flow rate, high sensitivity, short response time.
- 3)
Many researchers have developed such CDs, and environmental monitoring devices should be able to maintain a high level of particle collection efficiency at high flow rate while also delivering hydrosol samples to the back-end high-sensitivity detection module in real time.
5.1.1.2. Portable Environment Sensor
For indoor environments with complex ventilation, it is difficult to monitor inlets and outlets uniformly or its monitoring cannot meet the actual prevention and control requirements. The development of portable environmental sensor to patrol or deployment of indoor environmental aerosol detection can help monitor the indoor environment of the virus aerosol. Such portable environmental sensors should possess the following characteristics:
- 1)
Aerosol collector: high flow rate, high collection efficiency for particle size range of human-generated PM.
- 2)
Hydrosol collector: high sensitivity, short response time.
In addition, this device is expected to be portable and have low energy consumption. Due to energy consumption, portability and other needs, the flow rate of portable environment sensor can be appropriately reduced compared to high-flow environment aerosol monitoring device.
5.1.2. Individual Detection Device
Individual detection device is a device that collects air directly from a person's exhalation [
147]. The amount of air exhaled by a person in a single breath is about 10 ml/kg, and with an average weight of 70 kg, the amount of aerosol exhaled by a person in a single breath is about 70 ml [
148]. PMs contained in human exhaled aerosols ranges from 0.3 to 10 μm, which requires individual detection devices to have ultra-high sensitivity with very small sample sizes [
149]. This requirement brings an extremely high challenge to both the front-end collection technology and the back-end detection technology. In addition, people directly to the device and exhale, without settling, the exhaled PM particle size segment is more stable [
150,
151]. Therefore, the individual CD should possess the following characteristics:
- 1)
Aerosol collector: low flow rate, high collection efficiency for particle size range of human-generated PM.
- 2)
Hydrosol collector: ultra-high sensitivity, short response time.
The individual detection device should achieve the collection of approximately all the particles in a limited sample volume and deliver them to the detection module. Ensuring that individuals entering important sites do not produce viral PM has significant implications for localized outbreak prevention and control.
5.2. Building Ventilation and Airborne Virus Detection
Airborne virus collection and detection for various indoor environments are achievable using a combination of the various devices mentioned above (
Table 1). Common indoor environments ventilation is categorized into three types: mechanical (full ventilation), hybrid ventilation (partial ventilation), and natural (natural ventilation) [
152]. Full ventilation means air exchange through ventilation equipment, no air leakage or air leakage can be ignored. Partial ventilation means, on the basis of ventilation equipment, also accompanied by windows, doors and other air leaks [
153]. Natural ventilation refers to ventilation only through windows, doors and other vents. As illustrated in
Figure 6, various types of devices correspond to their appropriate application scenarios.
5.2.1. Critical Sites
The use of nucleic acid testing on all personnel entering a critical site is inefficient, impractical, and expensive. As shown in
Figure 6A, it is critical to screen people entering a critical site with an individual detection device at the entrance. The strategy will ensure that people entering a critical site do not expel viral PMs. Following the subject's exhalation into the mouthpiece, the individual detection device detects whether the exhaled breath contains virus particles in a relatively short period of time.
Individual detection device needs to possess ultra-high collection efficiency, and the water-based condenser of PM is particularly suitable for the development of such device. However, a problem is that the energy consumption of such devices is generally high [
154]. The miniaturized material of the valley power storage technology for the heating section of the water-based condenser is expected to solve the high energy consumption problem of such devices [
155].
5.2.2. Full Ventilation Environment
A full ventilation system: with an inlet and an outlet, and two ventilation devices, as shown in
Figure 6B. Because the inlet air volume is slightly greater than the outlet air volume, the system is in a positive pressure state to prevent outdoor air from entering. The inlet and outlet of all ventilation systems require a high flow rate and an efficient sampling device for the indoor environment. Several situations will occur when the CD of the inlet and outlet produce positive or negative results. Positive inlet, negative outlet: virus aerosol is imported from the outside. Negative inlet, positive outlet: infected person indoors, not imported from outside. Both indoor and outdoor viral aerosols have a positive inlet and outlet. Inlet and outlet both positive: both indoor and outdoor viral aerosols. The staff of Centers for Disease Control (CDC) can respond quickly to the above situations and target different means of prevention and control.
5.2.3. Partial Ventilation Environment
5.2.3.1. Partial Ventilation -with Return Air
As shown in
Figure 6C, local ventilation system with return air: with an inlet and an air returning duct. Inlet ventilators deliver fresh air, air returning ventilators circulate air, and the system is under positive pressure.
For the indoor environment of partial ventilation - with return air system, which is the same as the full fresh air system, a high flow rate and high efficiency collection device is used in the inlet and air returning, which can produce a variety of positive/negative results. When the return system's air volume does not differ significantly from the inlet air volume, or when the natural fugitive air volume is low, these two devices work together to achieve better disease control. Inadequate return air volume can cause some of the air to naturally escape. The air returning to the CD does not fully reflect the positive situation of people inside the environment, and sampling every air escaping is impractical. Using the portable environmental sensors with patrolled or distributed detection can compensate for these deficiencies.
5.2.3.2. Partial Ventilation-without Return Air
The local fresh air-no-return air system, as depicted in the figure, has one or more inlets, gas fugitives occur in gaps such as windows and doors, and the system is under positive pressure. The CD of inlets can still monitor the pollution of the indoor environment by external aerosols for the partial ventilation - no return air system, but the airflow of the indoor environment is more complex and cannot be monitored for each fugitive port. Portable environmental sensors for patrol or distributed detection can improve indoor viral aerosol monitoring.
5.2.3.3. Natural Ventilation
Natural ventilation, as shown in
Figure 6D, relies on the flow of gas through windows and doors to achieve air exchange. Detecting all air leaks is inefficient and impractical due to the uncertainty of wind direction. The use of a portable environment sensor has the potential to improve building ventilation and airborne virus detection strategies.
In summary, full ventilation with its well-defined outlets and inlets enables clear monitoring using High-flow environment aerosol monitoring device. Partial ventilation with well-defined inlets, but with more outlets and air leaks, can be monitored by placing high flow monitoring equipment in the inlet section, combined with Portable environment sensor. Natural ventilation does not have clear entrances and exits, and can only be monitored using Portable environment sensor for distributed or patrol monitoring.
5.3. Forward to an Intelligent Detection Strategy of Airborne Virus
Numerous studies show that improving the quality of the indoor environment can reduce viral transmission [
156,
157,
158,
159]. To improve indoor air quality, the airborne virus sampling devices can be combined with air purification/filtration techniques and other processes. Airborne virus sampling devices, particularly those with high throughput, enable virus detection to be combined with indoor space ventilation in future building design. Indoor aerosol environmental monitoring requires three types of devices: conventional monitoring devices with certain collection efficiency at high flow rates, high efficiency collection devices with a portable premise at high flow rates, and individual monitoring devices with nearly 100% collection efficiency at ultra-low flow rates. The bullets in this section are a comment on how to develop an intelligent strategy for airborne virus detection.
- 1)
Individual detection devices with ultra-high sensitivity and low flow rates are used to ensure that no viral aerosol particles are produced by people entering a critical site.
- 2)
A high-flow environment aerosol monitoring device with an ultra-high flow rate is used to collect and monitor viral particles in the full ventilation environment's inlet and outlet, as well as the partial ventilation environment's inlet and return pipe.
- 3)
A portable environmental sensor is used as a supplement because the air leakage from partial and natural ventilation is insufficient for regular monitoring.
- 4)
The collection device at the front end determines CD collection efficiency, but the detection module at the back end determines the specific length of response time, and the detection limit is influenced by both the collection efficiency and the detection method.
- 5)
The enhancement technique improves indoor air quality and contributes to the prevention and control of airborne diseases in indoor environments by lowering PM concentrations and virus activity in inlets and return pipes.
6. Conclusions and Future Perspectives
It is crucial for the prevention of airborne transmission diseases to develop various aerosol viral particle collection devices based on the fundamental mechanisms in aerosol science. To deal with the hazards caused by aerosol virus, such as COVID-19, multiple primary mechanisms enhancement techniques and sensitive detection methods should be used to make aerosol particle collection and detection devices with higher performance.
Current aerosol particle collection methods vary greatly, and uniform standards are lacking and difficult to establish. For example, almost all of devices mentioned in the above-mentioned literature claim high flow rates. However, the flow rates of at least thousands liter per minutes, according to the comparisons in this review, maybe considered as high flow rates. Beside the flow rate, another critical metric is enrichment capacity which indicates the particle concentration of the liquid sample exported by the device. The low-flow devices are still valuable in applications such as individual monitoring and air purification, which require quite high collection efficiency. This specific requirement may be met by particle amplifiers and filters with ultra-high efficiency.
Current epidemic control methods rely on monitoring individuals for nucleic acids and antigens. This means that when we had public social events, the environmental biosecurity of their rooms is marked with a random or mystery box. Only if an infection is present can testing and symptomatic treatment be performed. Indoor ambient air monitoring is becoming increasingly important as viruses continue to mutate and bacteria and fungi continue to pose a risk. The flow and efficiency of sample and collect will have an impact on the scenario of equipment application, and this paper examines the existing principles and their corresponding scenarios. This paper summarizes the sensitivity, specificity, and detection time of the detection techniques themselves, which can provide future research directions.
Author Contributions
Y.C., Y.W. and W.L. contributed equally to this review. Conceptualization, Y.C., Y.W. and R. C.; methodology, Y.C., Y. W. and W. L.; validation, Y.C., Y.W., W.L., Z.W. S.T. and R.C.; formal analysis, R.C.; investigation, Y.C., Y.W. and W.L.; resources, Z.W., S.T. and R.C.; data curation, Y.C., Y.W. and W.L.; writing—original draft preparation, Y.C., Y.W. and W.L.; writing—review and editing, Y.C., Y.W. and R.C.; visualization, Y.C., Y.W., W.L. and R.C.; supervision, S.T. and R.C.; project administration, Y.W.; funding acquisition, S.T. and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 21777036), and Beijing Academy of Science and Technology (11000022T000000445556, 11000022T000000468169).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, K. H.; Kabir, E.; Jahan, S. A. , Airborne bioaerosols and their impact on human health. J. Environ. Sci. (China) 2018, 67, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, C. C.; Prather, K. A.; Sznitman, J.; Jimenez, J. L.; Lakdawala, S. S.; Tufekci, Z.; Marr, L. C. , Airborne transmission of respiratory viruses. Science 2021, 373. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yin, H.; Cole, I. S.; Shen, S.; Zhou, X.; Wang, Y.; Tang, S. , Exposure, assessment and health hazards of particulate matter in metal additive manufacturing: A review. Chemosphere 2020, 259, 127452. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C. C.; Li, C. S. , Collection efficiencies of aerosol samplers for virus-containing aerosols. J. Aerosol Sci. 2005, 36, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hu, B.; Liu, Y.; Xu, J.; Yang, G.; Xu, D.; Chen, C. , Beyond PM2.5: The role of ultrafine particles on adverse health effects of air pollution. Biochim. Biophys. Acta 2016, 1860, 2844–55. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Qi, X.; Chen, H.; Li, X.; Zhang, Z.; Wang, H.; Sun, L.; Zhang, L.; Guo, J.; Morawska, L.; Grinshpun, S. A.; Biswas, P.; Flagan, R. C.; Yao, M. , Coronavirus Disease 2019 Patients in Earlier Stages Exhaled Millions of Severe Acute Respiratory Syndrome Coronavirus 2 Per Hour. Clin. Infect. Dis. 2021, 72, e652–e654. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, W. G.; Blachere, F. M.; Thewlis, R. E.; Vishnu, A.; Davis, K. A.; Cao, G.; Palmer, J. E.; Clark, K. E.; Fisher, M. A.; Khakoo, R.; Beezhold, D. H. , Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS One 2010, 5, e15100. [Google Scholar] [CrossRef] [PubMed]
- Scheuch, G. , Breathing Is Enough: For the Spread of Influenza Virus and SARS-CoV-2 by Breathing Only. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Chao, C. Y. H.; Wan, M. P.; Morawska, L.; Johnson, G. R.; Ristovski, Z. D.; Hargreaves, M.; Mengersen, K.; Corbett, S.; Li, Y.; Xie, X.; Katoshevski, D. , Characterization of expiration air jets and droplet size distributions immediately at the mouth opening. J. Aerosol Sci. 2009, 40, 122–133. [Google Scholar] [CrossRef]
- Damit, B.; Wu, C.-Y.; Yao, M. , Ultra-high temperature infrared disinfection of bioaerosols and relevant mechanisms. J. Aerosol Sci. 2013, 65, 88–100. [Google Scholar] [CrossRef]
- Robotto, A.; Quaglino, P.; Lembo, D.; Morello, M.; Brizio, E.; Bardi, L.; Civra, A. , SARS-CoV-2 and indoor/outdoor air samples: a methodological approach to have consistent and comparable results. Environ. Res. 2021, 195, 110847. [Google Scholar] [CrossRef]
- Lovelace, B.; Higgins-Dunn, N.; Feuer, W. J. U. C. , WHO considers ‘airborne precautions’ for medical staff after study shows coronavirus can survive in air. 2020.
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N. K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; Liu, X.; Xu, K.; Ho, K. F.; Kan, H.; Fu, Q.; Lan, K. , Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef]
- Kim, H. R.; An, S.; Hwang, J. , High air flow-rate electrostatic sampler for the rapid monitoring of airborne coronavirus and influenza viruses. J. Hazard Mater. 2021, 412, 125219. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. R.; An, S.; Hwang, J. , Aerosol-to-Hydrosol Sampling and Simultaneous Enrichment of Airborne Bacteria For Rapid Biosensing. ACS Sens. 2020, 5, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Piri, A.; Kim, H. R.; Hwang, J. , Prevention of damage caused by corona discharge-generated reactive oxygen species under electrostatic aerosol-to-hydrosol sampling. J. Hazard Mater. 2020, 384, 121477. [Google Scholar] [CrossRef] [PubMed]
- Piri, A.; Kim, H. R.; Park, D. H.; Hwang, J. , Increased survivability of coronavirus and H1N1 influenza virus under electrostatic aerosol-to-hydrosol sampling. J. Hazard Mater. 2021, 413, 125417. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Mainelis, G. , Utilization of natural electrical charges on airborne microorganisms for their collection by electrostatic means. J. Aerosol Sci. 2006, 37, 513–527. [Google Scholar] [CrossRef]
- Tan, M. M.; Shen, F. X.; Yao, M. S.; Zhu, T. , Development of an Automated Electrostatic Sampler (AES) for Bioaerosol Detection. Aerosol Sci. and Tech. 2011, 45, 1154–1160. [Google Scholar] [CrossRef]
- Kim, H. R.; An, S.; Hwang, J. , An integrated system of air sampling and simultaneous enrichment for rapid biosensing of airborne coronavirus and influenza virus. Biosens. Bioelectron. 2020, 170, 112656. [Google Scholar] [CrossRef]
- Sung, G.; Kim, H. U.; Shin, D.; Shin, W. G.; Kim, T. , High Efficiency Axial Wet Cyclone Air Sampler. Aerosol Air Qual. Res. 2018, 18, 2529–2537. [Google Scholar] [CrossRef]
- Sung, G.; Ahn, C.; Kulkarni, A.; Shin, W. G.; Kim, T. , Highly efficient in-line wet cyclone air sampler for airborne virus detection. J. Mech. Sci. Tech. 2017, 31, 4363–4369. [Google Scholar] [CrossRef]
- Lim, J. H.; Park, D.; Yook, S. J. , Development of a multi-slit virtual impactor as a high-volume bio-aerosol sampler. Sep. Purif. Technol. 2020, 250. [Google Scholar] [CrossRef]
- Lim, J. H.; Nam, S. H.; Kim, J.; Kim, N. H.; Park, G. S.; Maeng, J. S.; Yook, S. J. , High-volume sampler for size-selective sampling of bioaerosols including viruses. Atmos. Environ. (1994) 2021, 265, 118720. [Google Scholar] [CrossRef]
- Hong, S.; Bhardwaj, J.; Han, C. H.; Jang, J. , Gentle Sampling of Submicrometer Airborne Virus Particles using a Personal Electrostatic Particle Concentrator. Environ. Sci. Technol. 2016, 50, 12365–12372. [Google Scholar] [CrossRef]
- McDevitt, J. J.; Koutrakis, P.; Ferguson, S. T.; Wolfson, J. M.; Fabian, M. P.; Martins, M.; Pantelic, J.; Milton, D. K. , Development and Performance Evaluation of an Exhaled-Breath Bioaerosol Collector for Influenza Virus. Aerosol Sci. Technol. 2013, 47, 444–451. [Google Scholar] [CrossRef]
- Priyamvada, H.; Kumaragama, K.; Chrzan, A.; Athukorala, C.; Sur, S.; Dhaniyala, S. , Design and evaluation of a new electrostatic precipitation-based portable low-cost sampler for bioaerosol monitoring. Aerosol Sci. Technol. 2020, 55, 24–36. [Google Scholar] [CrossRef]
- Ma, Z. S.; Zheng, Y. H.; Cheng, Y. N.; Xie, S.; Ye, X. Y.; Yao, M. S. , Development of an integrated microfluidic electrostatic sampler for bioaerosol. J. Aerosol Sci. 2016, 95, 84–94. [Google Scholar] [CrossRef]
- Lin, X. T.; Hsu, N. Y.; Wang, J. R.; Chen, N. T.; Su, H. J.; Lin, M. Y. , Development of an efficient viral aerosol collector for higher sampling flow rate. Environ. Sci. Pollut. Res. Int. 2018, 25, 3884–3893. [Google Scholar] [CrossRef]
- Novosselov, I. V.; Gorder, R. A.; Van Amberg, J. A.; Ariessohn, P. C. , Design and Performance of a Low-Cost Micro-Channel Aerosol Collector. Aerosol Sci. Technol. 2014, 48, 822–830. [Google Scholar] [CrossRef]
- Yeh, Y. T.; Tang, Y.; Sebastian, A.; Dasgupta, A.; Perea-Lopez, N.; Albert, I.; Lu, H.; Terrones, M.; Zheng, S. Y. , Tunable and label-free virus enrichment for ultrasensitive virus detection using carbon nanotube arrays. Sci. Adv. 2016, 2, e1601026. [Google Scholar] [CrossRef]
- Hong, T. C.; Mai, Q. L.; Cuong, D. V.; Parida, M.; Minekawa, H.; Notomi, T.; Hasebe, F.; Morita, K. , Development and evaluation of a novel loop-mediated isothermal amplification method for rapid detection of severe acute respiratory syndrome coronavirus. J. Clin. Microbiol. 2004, 42, 1956–61. [Google Scholar] [PubMed]
- Coelho, B. J.; Veigas, B.; Aguas, H.; Fortunato, E.; Martins, R.; Baptista, P. V.; Igreja, R. , A Digital Microfluidics Platform for Loop-Mediated Isothermal Amplification Detection. Sensors (Basel) 2017, 17. [Google Scholar] [CrossRef]
- Liu, Y. C.; Lu, B. Y.; Tang, Y. D.; Du, Y.; Li, B. L. , Real-time gene analysis based on a portable electrochemical microfluidic system. Electrochem. Commun. 2020, 111. [Google Scholar] [CrossRef]
- Xiong, H.; Ye, X.; Li, Y.; Wang, L.; Zhang, J.; Fang, X.; Kong, J. , Rapid Differential Diagnosis of Seven Human Respiratory Coronaviruses Based on Centrifugal Microfluidic Nucleic Acid Assay. Anal. Chem. 2020, 92, 14297–14302. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Liu, C.; Deng, J.; Han, Z.; Zhang, L.; Chen, Q.; Sun, J. , A fully automated centrifugal microfluidic system for sample-to-answer viral nucleic acid testing. Sci. China Chem. 2020, 63, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Mayuramart, O.; Nimsamer, P.; Rattanaburi, S.; Chantaravisoot, N.; Khongnomnan, K.; Chansaenroj, J.; Puenpa, J.; Suntronwong, N.; Vichaiwattana, P.; Poovorawan, Y.; Payungporn, S. , Detection of severe acute respiratory syndrome coronavirus 2 and influenza viruses based on CRISPR-Cas12a. Exp. Biol. Med. (Maywood) 2021, 246, 400–405. [Google Scholar] [CrossRef]
- Qin, P.; Park, M.; Alfson, K. J.; Tamhankar, M.; Carrion, R.; Patterson, J. L.; Griffiths, A.; He, Q.; Yildiz, A.; Mathies, R.; Du, K. , Rapid and Fully Microfluidic Ebola Virus Detection with CRISPR-Cas13a. ACS Sens. 2019, 4, 1048–1054. [Google Scholar] [CrossRef]
- Li, D. W.; Ling, S.; Wu, H. S.; Yang, Z. Q.; Lv, B. CRISPR/Cas12a-based biosensors for ultrasensitive tobramycin detection with single- and double-stranded DNA activators. Sensor. Actuat. B-Chem. 2022, 355.
- Johnston, R. K.; Seamon, K. J.; Saada, E. A.; Podlevsky, J. D.; Branda, S. S.; Timlin, J. A.; Harper, J. C. , Use of anti-CRISPR protein AcrIIA4 as a capture ligand for CRISPR/Cas9 detection. Biosens. Bioelectron. 2019, 141, 111361. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, R.; Huang, M.; Shen, J.; Shan, Y.; Xing, D. , CRISPR/Cas13a Powered Portable Electrochemiluminescence Chip for Ultrasensitive and Specific MiRNA Detection. Adv. Sci. (Weinh) 2020, 7, 1903661. [Google Scholar] [CrossRef]
- Kaminski, M. M.; Abudayyeh, O. O.; Gootenberg, J. S.; Zhang, F.; Collins, J. J. , CRISPR-based diagnostics. Nat. Biomed. Eng. 2021, 5, 643–656. [Google Scholar] [CrossRef]
- Coarsey, C.; Coleman, B.; Kabir, M. A.; Sher, M.; Asghar, W. , Development of a Flow-Free Magnetic Actuation Platform for an Automated Microfluidic ELISA. RSC Adv. 2019, 9, 8159–8168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, Y.; Fu, Q.; Lin, M.; He, J.; He, S.; Yang, M.; Chen, S.; Zhou, J. , Reciprocating-flowing on-a-chip enables ultra-fast immunobinding for multiplexed rapid ELISA detection of SARS-CoV-2 antibody. Biosens. Bioelectron. 2021, 176, 112920. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y. F.; Wang, W. H.; Hong, Y. W.; Yuan, R. Y.; Chen, K. H.; Huang, Y. W.; Lu, P. L.; Chen, Y. H.; Chen, Y. A.; Su, L. C.; Wang, S. F. , Simple Strategy for Rapid and Sensitive Detection of Avian Influenza A H7N9 Virus Based on Intensity-Modulated SPR Biosensor and New Generated Antibody. Anal. Chem. 2018, 90, 1861–1869. [Google Scholar] [CrossRef]
- Usachev, E. V.; Usacheva, O. V.; Agranovski, I. E. , Surface plasmon resonance-based real-time bioaerosol detection. J. Appl. Microbiol. 2013, 115, 766–73. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Fu, Y.; Xu, M.; Su, L.; Cao, L.; Xu, J.; Cheng, X. , Evaluation of a PCR/ESI-MS platform to identify respiratory viruses from nasopharyngeal aspirates. J. Med. Virol. 2015, 87, 1867–71. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Downard, K. M.; Wong, J. W. , FluClass: A novel algorithm and approach to score and visualize the phylogeny of the influenza virus using mass spectrometry. Anal. Chim. Acta 2015, 895, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Lednicky, J. A.; Wu, C. Y. , Collection, particle sizing and detection of airborne viruses. J. Appl. Microbiol. 2019, 127, 1596–1611. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, A.; Sakagami, M.; Byron, P. R. , Cascade impactor practice for a high dose dry powder inhaler at 90 L/min: NGI versus modified 6-stage and 8-stage ACI. J. Pharm. Sci. 2009, 98, 1028–39. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, J.; Veillette, M.; Mbareche, H.; Duchaine, C. , Re-aerosolization in liquid-based air samplers induces bias in bacterial diversity. Aerosol Sci. Technol. 2019, 53, 1244–1260. [Google Scholar] [CrossRef]
- Taghvaee, S.; Mousavi, A.; Sowlat, M. H.; Sioutas, C. , Development of a novel aerosol generation system for conducting inhalation exposures to ambient particulate matter (PM). Sci. Total Environ. 2019, 665, 1035–1045. [Google Scholar] [CrossRef]
- Penner, T.; Meyer, J.; Dittler, A. , Oleophilic and oleophobic media combinations - Influence on oil mist filter operating performance. Sep. Purif. Technol. 2021, 261. [Google Scholar] [CrossRef]
- Komatsu, N.; Kadota, N.; Kimura, T.; Kikuchi, Y.; Arikawa, M. , Remarkable improvement in efficiency of filtration method for fullerene purification. Fuller. Nanotub. Car. N. 2007, 15, 217–226. [Google Scholar] [CrossRef]
- Gao, G.-H.; Lei, Y.-H.; Dong, L.-H.; Liu, W.-C.; Wang, X.-F.; Chang, X.-T.; Liu, T.; Yin, Y.-S.; Ajayan, P. M. , Synthesis of Nanocomposites of Silver Nanoparticles with Medical Stone and Carbon Nanotubes for Their Antibacterial Applications. Mater. Express 2012, 2, 85–93. [Google Scholar] [CrossRef]
- Chai, M.; Lu, M.; Keener, T.; Khang, S.-J.; Chaiwatpongsakorn, C.; Tisch, J. , Using an improved electrostatic precipitator for poultry dust removal. J. Electrostat. 2009, 67, 870–875. [Google Scholar] [CrossRef]
- Jemaa, N.; Paleologou, M.; Thompson, R.; Richardson, B.; Brown, C.; Sheedy, M. Chloride removal from the kraft recovery boiler ESP dust using the precipitator dust purification (PDP) system - High efficiency is reported. Pulp & Pap. -Canada 1999, 100, 46-53.
- Cardello, N.; Volckens, J.; Tolocka, M. P.; Wiener, R.; Buckley, T. J. , Technical Note: Performance of a Personal Electrostatic Precipitator Particle Sampler. Aerosol Sci. Technol. 2002, 36, 162–165. [Google Scholar] [CrossRef]
- Kawada, Y.; Shimizu, H. , Reduction of Suspended Particles in Closed Space with Electrostatic Precipitator. Electr. Commun. Jap. 2017, 100, 32–40. [Google Scholar] [CrossRef]
- Viner, A. S.; Lawless, P. A.; Ensor, D. S.; Sparks, L. E. , Ozone generation in DC-energized electrostatic precipitators. IEEE T. Ind. Appl. 1992, 28, 504–512. [Google Scholar] [CrossRef]
- Yu, H.; Afshar-Mohajer, N.; Theodore, A. D.; Lednicky, J. A.; Fan, Z. H.; Wu, C. Y. , An efficient virus aerosol sampler enabled by adiabatic expansion. J. Aerosol Sci. 2018, 117, 74–84. [Google Scholar] [CrossRef]
- Li, J.; Leavey, A.; Wang, Y.; O'Neil, C.; Wallace, M. A.; Burnham, C. D.; Boon, A. C.; Babcock, H.; Biswas, P. , Comparing the performance of 3 bioaerosol samplers for influenza virus. J. Aerosol Sci. 2018, 115, 133–145. [Google Scholar] [CrossRef]
- Burton, N. C.; Grinshpun, S. A.; Reponen, T. , Physical collection efficiency of filter materials for bacteria and viruses. Ann. Occup. Hyg. 2007, 51, 143–51. [Google Scholar]
- Su, Y. Y.; Wang, W.; Wang, W. L.; Zhai, L. H.; Shen, X. P.; Xu, J.; Li, Z. M. , Re-evaluation of BioSampler and its improvement for on-line, time-resolved monitoring of environmental coarse aerosol. Atmos. Environ. 2020, 225. [Google Scholar] [CrossRef]
- Ratnesar-Shumate, S.; Bohannon, K.; Williams, G.; Holland, B.; Krause, M.; Green, B.; Freeburger, D.; Dabisch, P. , Comparison of the performance of aerosol sampling devices for measuring infectious SARS-CoV-2 aerosols. Aerosol Sci. Technol. 2021, 55, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Kutter, J. S.; de Meulder, D.; Bestebroer, T. M.; Mulders, A.; Fouchier, R. A. M.; Herfst, S. , Comparison of three air samplers for the collection of four nebulized respiratory viruses - Collection of respiratory viruses from air. Indoor Air 2021, 31, 1874–1885. [Google Scholar] [CrossRef]
- Dybwad, M.; Skogan, G.; Blatny, J. M. , Comparative Testing and Evaluation of Nine Different Air Samplers: End-to-End Sampling Efficiencies as Specific Performance Measurements for Bioaerosol Applications. Aerosol Sci. Technol. 2014, 48, 282–295. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Thajudeen, T.; Ouyang, H.; Hogan, C. J. , The unipolar diffusion charging of arbitrary shaped aerosol particles. J. Aerosol Sci. 2013, 64, 60–80. [Google Scholar] [CrossRef]
- Alonso, M.; Huang, C. H. , High-efficiency electrical charger for nanoparticles. J. Nanopart. Res. 2015, 17. [Google Scholar] [CrossRef]
- Park, J.; Kim, C.; Jeong, J.; Lee, S. G.; Hwang, J. , Design and evaluation of a unipolar aerosol charger to generate highly charged micron-sized aerosol particles. J. Electrostat. 2011, 69, 126–132. [Google Scholar] [CrossRef]
- Shin, W. G.; Qi, C. L.; Wang, J.; Fissan, H.; Pui, D. Y. H. , The effect of dielectric constant of materials on unipolar diffusion charging of nanoparticles. J. Aerosol Sci. 2009, 40, 463–468. [Google Scholar] [CrossRef]
- Kalaiselvan, P.; Yasir, M.; Kuppusamy, R.; Willcox, M.; Vijay, A. K. , Ability of Essential Oil Vapours to Reduce Numbers of Culturable Aerosolised Coronavirus, Bacteria and Fungi. Antibiotics (Basel) 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, Y.; Yamaguchi, M.; Kadota, C.; Hasan, M. A.; Kabir, M. H.; Shoham, D.; Murakami, H.; Takehara, K. , Rapid in vitro virucidal activity of slightly acidic hypochlorous acid water toward aerosolized coronavirus in simulated human-dispersed droplets. Virus Res. 2022, 311, 198701. [Google Scholar] [CrossRef]
- Jung, J. H.; Hwang, G. B.; Lee, J. E.; Bae, G. N. , Preparation of airborne Ag/CNT hybrid nanoparticles using an aerosol process and their application to antimicrobial air filtration. Langmuir 2011, 27, 10256–64. [Google Scholar] [CrossRef]
- Li, J.; Ha, N. S.; Liu, T.; van Dam, R. M.; cj' Kim, C. J. , Ionic-surfactant-mediated electro-dewetting for digital microfluidics. Nature 2019, 572, 507–510. [Google Scholar] [CrossRef]
- Lu, H.; Lu, L. , Effects of rib spacing and height on particle deposition in ribbed duct air flows. Build. Environ. 2015, 92, 317–327. [Google Scholar] [CrossRef]
- Hemmati, Y.; Rafee, R. , Effects of the shape and height of artificial 2D roughness elements on deposition of nano and microparticles in the turbulent gas flow inside a horizontal channel. J. Aerosol Sci. 2018, 122, 45–58. [Google Scholar] [CrossRef]
- Gao, R.; Li, A. , Dust deposition in ventilation and air-conditioning duct bend flows. Energ. Convers. Manage. 2012, 55, 49–59. [Google Scholar] [CrossRef]
- Zhong, X. L.; Fu, S. C.; Chan, K. C.; Chao, C. Y. H. , Experimental study of particle deposition on patterned microstructured surfaces in a chamber environment. J. Aerosol Sci. 2021, 157. [Google Scholar] [CrossRef]
- Dritselis, C. D. , Numerical study of particle deposition in a turbulent channel flow with transverse roughness elements on one wall. Int. J. Multiphas. Flow 2017, 91, 1–18. [Google Scholar] [CrossRef]
- Li, A.; Ahmadi, G. , Computer-Simulation of Deposition of Aerosols in a Turbulent Channel Flow with Rough Walls. Aerosol Sci. Technol. 1993, 18, 11–24. [Google Scholar] [CrossRef]
- Lecrivain, G.; Sevan, D. M.; Thomas, B.; Hampel, U. , Numerical simulation of multilayer deposition in an obstructed channel flow. Adv. Powder Technol. 2014, 25, 310–320. [Google Scholar] [CrossRef]
- Sun, K.; Lu, L. , Particle flow behavior of distribution and deposition throughout 90 degrees bends: Analysis of influencing factors. J. Aerosol Sci. 2013, 65, 26–41. [Google Scholar] [CrossRef]
- Yeh, Y. T.; Gulino, K.; Zhang, Y.; Sabestien, A.; Chou, T. W.; Zhou, B.; Lin, Z.; Albert, I.; Lu, H.; Swaminathan, V.; Ghedin, E.; Terrones, M. , A rapid and label-free platform for virus capture and identification from clinical samples. Proc. Natl. Acad. Sci. U S A 2020, 117, 895–901. [Google Scholar] [CrossRef]
- Rehn, A.; Braun, P.; Knupfer, M.; Wolfel, R.; Antwerpen, M. H.; Walter, M. C. , Catching SARS-CoV-2 by Sequence Hybridization: a Comparative Analysis. mSystems 2021, 6, e0039221. [Google Scholar] [CrossRef]
- Chu, H. W.; Lai, C. S.; Ko, J. Y.; Harroun, S. G.; Chuang, C. I.; Wang, R. Y. L.; Unnikrishnan, B.; Huang, C. C. , Nanoparticle-Based LDI-MS Immunoassay for the Multiple Diagnosis of Viral Infections. ACS Sens. 2019, 4, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Wylezich, C.; Calvelage, S.; Schlottau, K.; Ziegler, U.; Pohlmann, A.; Hoper, D.; Beer, M. , Next-generation diagnostics: virus capture facilitates a sensitive viral diagnosis for epizootic and zoonotic pathogens including SARS-CoV-2. Microbiome. 2021, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Frias, I. A. M.; Vega Gonzales Gil, L. H.; Cordeiro, M. T.; Oliveira, M. D. L.; Andrade, C. A. S. , Self-Enriching Electrospun Biosensors for Impedimetric Sensing of Zika Virus. ACS Appl. Mater. Interfaces 2022, 14, 41–48. [Google Scholar] [CrossRef]
- Bai, Z.; Wei, H.; Yang, X.; Zhu, Y.; Peng, Y.; Yang, J.; Wang, C.; Rong, Z.; Wang, S. , Rapid Enrichment and Ultrasensitive Detection of Influenza A Virus in Human Specimen using Magnetic Quantum Dot Nanobeads Based Test Strips. Sens. Actuat. B Chem. 2020, 325, 128780. [Google Scholar] [CrossRef]
- Dzuvor, C. K. O.; Tettey, E. L.; Danquah, M. K. , Aptamers as promising nanotheranostic tools in the COVID-19 pandemic era. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1785. [Google Scholar] [CrossRef]
- Panigaj, M.; Johnson, M. B.; Ke, W.; McMillan, J.; Goncharova, E. A.; Chandler, M.; Afonin, K. A. , Aptamers as Modular Components of Therapeutic Nucleic Acid Nanotechnology. ACS Nano 2019, 13, 12301–12321. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Wu, J.; Gu, J.; Shen, L.; Mao, L. , Application of Aptamers in Virus Detection and Antiviral Therapy. Front. Microbiol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Yu, K. P.; Chen, Y. P.; Gong, J. Y.; Chen, Y. C.; Cheng, C. C. , Improving the collection efficiency of the liquid impinger for ultrafine particles and viral aerosols by applying granular bed filtration. J. Aerosol Sci. 2016, 101, 133–143. [Google Scholar] [CrossRef]
- Pyankov, O. V.; Usachev, E. V.; Pyankova, O.; Agranovski, I. E. , Inactivation of Airborne Influenza Virus by Tea Tree and Eucalyptus Oils. Aerosol Sci. Technol. 2012, 46, 1295–1302. [Google Scholar] [CrossRef]
- Huang, R.; Pyankov, O. V.; Yu, B.; Agranovski, I. E. , Inactivation of Fungal Spores Collected on Fibrous Filters byMelaleuca alternifolia(Tea Tree Oil). Aerosol Sci. Technol. 2010, 44, 262–268. [Google Scholar] [CrossRef]
- Borges, J. T.; Nakada, L. Y. K.; Maniero, M. G.; Guimaraes, J. R. , SARS-CoV-2: a systematic review of indoor air sampling for virus detection. Environ. Sci. Pollut. Res. Int. 2021, 28, 40460–40473. [Google Scholar] [CrossRef] [PubMed]
- Cassedy, A.; Parle-McDermott, A.; O'Kennedy, R. , Virus Detection: A Review of the Current and Emerging Molecular and Immunological Methods. Front. Mol. Biosci. 2021, 8, 637559. [Google Scholar] [CrossRef]
- Lee, S. H.; Kim, S. W.; Kang, J. Y.; Ahn, C. H. , A polymer lab-on-a-chip for reverse transcription (RT)-PCR based point-of-care clinical diagnostics. Lab Chip 2008, 8, 2121–7. [Google Scholar] [CrossRef] [PubMed]
- Oshiki, M.; Miura, T.; Kazama, S.; Segawa, T.; Ishii, S.; Hatamoto, M.; Yamaguchi, T.; Kubota, K.; Iguchi, A.; Tagawa, T.; Okubo, T.; Uemura, S.; Harada, H.; Kobayashi, N.; Araki, N.; Sano, D. , Microfluidic PCR Amplification and MiSeq Amplicon Sequencing Techniques for High-Throughput Detection and Genotyping of Human Pathogenic RNA Viruses in Human Feces, Sewage, and Oysters. Front. Microbiol. 2018, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Miao, B.; Li, Z.; Sun, Z.; Peng, N. , Sample-to-Answer Hepatitis B Virus DNA Detection from Whole Blood on a Centrifugal Microfluidic Platform with Double Rotation Axes. ACS Sens. 2019, 4, 2738–2745. [Google Scholar] [CrossRef]
- Ma, Y. D.; Chen, Y. S.; Lee, G. B. Locked nucleic acid molecular beacon for multiplex detection of loop mediated isothermal amplification. Sensor. Actuat. B: Chem. 2018, 268, 255-263.
- Kouguchi, Y.; Fujiwara, T.; Teramoto, M.; Kuramoto, M. , Homogenous, real-time duplex loop-mediated isothermal amplification using a single fluorophore-labeled primer and an intercalator dye: Its application to the simultaneous detection of Shiga toxin genes 1 and 2 in Shiga toxigenic Escherichia coli isolates. Mol. Cell. Probe. 2010, 24, 190–5. [Google Scholar] [CrossRef]
- Gadkar, V. J.; Goldfarb, D. M.; Gantt, S.; Tilley, P. A. G. , Real-time Detection and Monitoring of Loop Mediated Amplification (LAMP) Reaction Using Self-quenching and De-quenching Fluorogenic Probes. Sci. Rep. 2018, 8, 5548. [Google Scholar] [CrossRef]
- Mashooq, M.; Kumar, D.; Niranjan, A. K.; Agarwal, R. K.; Rathore, R. , Development and evaluation of probe based real time loop mediated isothermal amplification for Salmonella: A new tool for DNA quantification. J. Microbiol. Meth. 2016, 126, 24–9. [Google Scholar] [CrossRef]
- Howard, R. L.; French, D. J.; Richardson, J. A.; O'Neill, C. E.; Andreou, M. P.; Brown, T.; Clark, D.; Clarke, I. N.; Holloway, J. W.; Marsh, P.; Debenham, P. G. , Rapid detection of diagnostic targets using isothermal amplification and HyBeacon probes--a homogenous system for sequence-specific detection. Mol. Cell. Probe. 2015, 29, 92–8. [Google Scholar] [CrossRef] [PubMed]
- Bakthavathsalam, P.; Longatte, G.; Jensen, S. O.; Manefield, M.; Gooding, J. J. Locked nucleic acid molecular beacon for multiplex detection of loop mediated isothermal amplification. Sensor. Actuat. B: Chem. 2018, 268, 255-263.
- Wang, C. H.; Lien, K. Y.; Wang, T. Y.; Chen, T. Y.; Lee, G. B. , An integrated microfluidic loop-mediated-isothermal-amplification system for rapid sample pre-treatment and detection of viruses. Biosens. Bioelectron. 2011, 26, 2045–52. [Google Scholar] [CrossRef] [PubMed]
- Joung, J.; Ladha, A.; Saito, M.; Kim, N. G.; Woolley, A. E.; Segel, M.; Barretto, R. P. J.; Ranu, A.; Macrae, R. K.; Faure, G.; Ioannidi, E. I.; Krajeski, R. N.; Bruneau, R.; Huang, M. W.; Yu, X. G.; Li, J. Z.; Walker, B. D.; Hung, D. T.; Greninger, A. L.; Jerome, K. R.; Gootenberg, J. S.; Abudayyeh, O. O.; Zhang, F. , Detection of SARS-CoV-2 with SHERLOCK One-Pot Testing. N. Engl. J. Med. 2020, 383, 1492–1494. [Google Scholar] [CrossRef] [PubMed]
- Myhrvold, C.; Freije, C. A.; Gootenberg, J. S.; Abudayyeh, O. O.; Metsky, H. C.; Durbin, A. F.; Kellner, M. J.; Tan, A. L.; Paul, L. M.; Parham, L. A.; Garcia, K. F.; Barnes, K. G.; Chak, B.; Mondini, A.; Nogueira, M. L.; Isern, S.; Michael, S. F.; Lorenzana, I.; Yozwiak, N. L.; MacInnis, B. L.; Bosch, I.; Gehrke, L.; Zhang, F.; Sabeti, P. C. , Field-deployable viral diagnostics using CRISPR-Cas13. Science 2018, 360, 444–448. [Google Scholar] [CrossRef]
- Patchsung, M.; Jantarug, K.; Pattama, A.; Aphicho, K.; Suraritdechachai, S.; Meesawat, P.; Sappakhaw, K.; Leelahakorn, N.; Ruenkam, T.; Wongsatit, T.; Athipanyasilp, N.; Eiamthong, B.; Lakkanasirorat, B.; Phoodokmai, T.; Niljianskul, N.; Pakotiprapha, D.; Chanarat, S.; Homchan, A.; Tinikul, R.; Kamutira, P.; Phiwkaow, K.; Soithongcharoen, S.; Kantiwiriyawanitch, C.; Pongsupasa, V.; Trisrivirat, D.; Jaroensuk, J.; Wongnate, T.; Maenpuen, S.; Chaiyen, P.; Kamnerdnakta, S.; Swangsri, J.; Chuthapisith, S.; Sirivatanauksorn, Y.; Chaimayo, C.; Sutthent, R.; Kantakamalakul, W.; Joung, J.; Ladha, A.; Jin, X.; Gootenberg, J. S.; Abudayyeh, O. O.; Zhang, F.; Horthongkham, N.; Uttamapinant, C. , Clinical validation of a Cas13-based assay for the detection of SARS-CoV-2 RNA. Nat. Biomed. Eng. 2020, 4, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Qian, C.; Pang, Y.; Li, M.; Yang, Y.; Ma, H.; Zhao, M.; Qian, F.; Yu, H.; Liu, Z.; Ni, T.; Zheng, Y.; Wang, Y. , opvCRISPR: One-pot visual RT-LAMP-CRISPR platform for SARS-cov-2 detection. Biosens. Bioelectron. 2021, 172, 112766. [Google Scholar] [CrossRef] [PubMed]
- Zitek, T. , The Appropriate Use of Testing for COVID-19. West. J. Emerg. Med. 2020, 21, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Chen, F. E.; Lee, P. W.; Trick, A. Y.; Park, J. S.; Chen, L.; Shah, K.; Mostafa, H.; Carroll, K. C.; Hsieh, K.; Wang, T. H. , Point-of-care CRISPR-Cas-assisted SARS-CoV-2 detection in an automated and portable droplet magnetofluidic device. Biosens. Bioelectron. 2021, 190, 113390. [Google Scholar] [CrossRef]
- Ramachandran, A.; Huyke, D. A.; Sharma, E.; Sahoo, M. K.; Huang, C.; Banaei, N.; Pinsky, B. A.; Santiago, J. G. , Electric field-driven microfluidics for rapid CRISPR-based diagnostics and its application to detection of SARS-CoV-2. Proc. Natl. Acad. Sci. U S A 2020, 117, 29518–29525. [Google Scholar] [CrossRef]
- Pandey, P. S.; Raghuwanshi, S. K.; Shadab, A.; Ansari, M. T. I.; Tiwari, U. K.; Kumar, S. , SPR Based Biosensing Chip for COVID-19 Diagnosis-A Review. Ieee Sens. J. 2022, 22, 13800–13810. [Google Scholar] [CrossRef]
- Yoo, H.; Shin, J.; Sim, J.; Cho, H.; Hong, S. , Reusable surface plasmon resonance biosensor chip for the detection of H1N1 influenza virus. Biosens. Bioelectron. 2020, 168, 112561. [Google Scholar] [CrossRef] [PubMed]
- Negri, P.; Kage, A.; Nitsche, A.; Naumann, D.; Dluhy, R. A. , Detection of viral nucleoprotein binding to anti-influenza aptamers via SERS. Chem. Commun. (Camb) 2011, 47, 8635–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ruan, Q.; Lei, Z. C.; Lin, S. C.; Zhu, Z.; Zhou, L.; Yang, C. , Highly Sensitive and Automated Surface Enhanced Raman Scattering-based Immunoassay for H5N1 Detection with Digital Microfluidics. Anal. Chem. 2018, 90, 5224–5231. [Google Scholar] [CrossRef] [PubMed]
- Idili, A.; Parolo, C.; Alvarez-Diduk, R.; Merkoci, A. , Rapid and Efficient Detection of the SARS-CoV-2 Spike Protein Using an Electrochemical Aptamer-Based Sensor. ACS Sens. 2021, 6, 3093–3101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, Z.; Hu, H.; Zhou, Q.; Liu, W.; Li, X.; Liu, Z.; Wang, Y.; Ma, Y. , A point-of-care selenium nanoparticle-based test for the combined detection of anti-SARS-CoV-2 IgM and IgG in human serum and blood. Lab Chip 2020, 20, 4255–4261. [Google Scholar] [CrossRef] [PubMed]
- Najjar, D.; Rainbow, J.; Sharma Timilsina, S.; Jolly, P.; de Puig, H.; Yafia, M.; Durr, N.; Sallum, H.; Alter, G.; Li, J. Z.; Yu, X. G.; Walt, D. R.; Paradiso, J. A.; Estrela, P.; Collins, J. J.; Ingber, D. E. , A lab-on-a-chip for the concurrent electrochemical detection of SARS-CoV-2 RNA and anti-SARS-CoV-2 antibodies in saliva and plasma. Nat. Biomed. Eng. 2022, 6, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Jarrold, M. F. , Applications of Charge Detection Mass Spectrometry in Molecular Biology and Biotechnology. Chem. Rev. 2022, 122, 7415–7441. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, D.; Grenga, L.; Gaillard, J. C.; Gallais, F.; Bellanger, L.; Pible, O.; Armengaud, J. , Shortlisting SARS-CoV-2 Peptides for Targeted Studies from Experimental Data-Dependent Acquisition Tandem Mass Spectrometry Data. Proteomics 2020, 20, e2000107. [Google Scholar] [CrossRef] [PubMed]
- Shajahan, A.; Supekar, N. T.; Gleinich, A. S.; Azadi, P. , Deducing the N- and O-glycosylation profile of the spike protein of novel coronavirus SARS-CoV-2. Glycobiology 2020, 30, 981–988. [Google Scholar] [CrossRef]
- Deyde, V. M.; Sampath, R.; Garten, R. J.; Blair, P. J.; Myers, C. A.; Massire, C.; Matthews, H.; Svoboda, P.; Reed, M. S.; Pohl, J.; Klimov, A. I.; Gubareva, L. V. , Genomic signature-based identification of influenza A viruses using RT-PCR/electro-spray ionization mass spectrometry (ESI-MS) technology. PLoS One 2010, 5, e13293. [Google Scholar] [CrossRef]
- Deyde, V. M.; Sampath, R.; Gubareva, L. V. , RT-PCR/electrospray ionization mass spectrometry approach in detection and characterization of influenza viruses. Expert. Rev. Mol. Diagn. 2011, 11, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Bryden, W. A.; Fenselau, C.; McLoughlin, M.; Haddaway, C. R.; Devin, A. P.; Caton, E. R.; Bradrick, S. S.; Miller, J. M.; Tacheny, E. A.; Lemmon, M. M.; Bogan, J. , MALDI-TOF Mass Spectrometric Detection of SARS-CoV-2 Using Cellulose Sulfate Ester Enrichment and Hot Acid Treatment. J. Proteome. Res. 2022, 21, 2055–2062. [Google Scholar] [CrossRef]
- Erdogan, R. T.; Alkhaled, M.; Kaynak, B. E.; Alhmoud, H.; Pisheh, H. S.; Kelleci, M.; Karakurt, I.; Yanik, C.; Sen, Z. B.; Sari, B.; Yagci, A. M.; Ozkul, A.; Hanay, M. S. , Atmospheric Pressure Mass Spectrometry of Single Viruses and Nanoparticles by Nanoelectromechanical Systems. ACS Nano 2022, 16, 3821–3833. [Google Scholar] [CrossRef]
- Dao Thi, V. L.; Herbst, K.; Boerner, K.; Meurer, M.; Kremer, L. P.; Kirrmaier, D.; Freistaedter, A.; Papagiannidis, D.; Galmozzi, C.; Stanifer, M. L.; Boulant, S.; Klein, S.; Chlanda, P.; Khalid, D.; Barreto Miranda, I.; Schnitzler, P.; Krausslich, H. G.; Knop, M.; Anders, S. , A colorimetric RT-LAMP assay and LAMP-sequencing for detecting SARS-CoV-2 RNA in clinical samples. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Rodel, J.; Egerer, R.; Suleyman, A.; Sommer-Schmid, B.; Baier, M.; Henke, A.; Edel, B.; Loffler, B. , Use of the variplex SARS-CoV-2 RT-LAMP as a rapid molecular assay to complement RT-PCR for COVID-19 diagnosis. J. Clin. Virol. 2020, 132, 104616. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, H.; Liu, H.; Ye, Q. , Advances in laboratory detection methods and technology application of SARS-CoV-2. J. Med. Virol. 2022, 94, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Petralia, S.; Conoci, S. , PCR Technologies for Point of Care Testing: Progress and Perspectives. ACS Sens. 2017, 2, 876–891. [Google Scholar] [CrossRef]
- Ahrberg, C. D.; Manz, A.; Neuzil, P. , Palm-Sized Device for Point-of-Care Ebola Detection. Anal. Chem. 2016, 88, 4803–7. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, R.; Li, Y.; Kong, W.; Guo, X.; Yang, Y.; Wu, F.; Liu, W.; Song, H.; Hao, R. , Rapid detection of multiple respiratory viruses based on microfluidic isothermal amplification and a real-time colorimetric method. Lab Chip 2018, 18, 3507–3515. [Google Scholar] [CrossRef] [PubMed]
- Ahrberg, C. D.; Neuzil, P. , Doubling Throughput of a Real-Time PCR. Sci. Rep. 2015, 5, 12595. [Google Scholar] [CrossRef]
- Welch, N. L.; Zhu, M.; Hua, C.; Weller, J.; Mirhashemi, M. E.; Nguyen, T. G.; Mantena, S.; Bauer, M. R.; Shaw, B. M.; Ackerman, C. M.; Thakku, S. G.; Tse, M. W.; Kehe, J.; Uwera, M. M.; Eversley, J. S.; Bielwaski, D. A.; McGrath, G.; Braidt, J.; Johnson, J.; Cerrato, F.; Moreno, G. K.; Krasilnikova, L. A.; Petros, B. A.; Gionet, G. L.; King, E.; Huard, R. C.; Jalbert, S. K.; Cleary, M. L.; Fitzgerald, N. A.; Gabriel, S. B.; Gallagher, G. R.; Smole, S. C.; Madoff, L. C.; Brown, C. M.; Keller, M. W.; Wilson, M. M.; Kirby, M. K.; Barnes, J. R.; Park, D. J.; Siddle, K. J.; Happi, C. T.; Hung, D. T.; Springer, M.; MacInnis, B. L.; Lemieux, J. E.; Rosenberg, E.; Branda, J. A.; Blainey, P. C.; Sabeti, P. C.; Myhrvold, C. , Multiplexed CRISPR-based microfluidic platform for clinical testing of respiratory viruses and identification of SARS-CoV-2 variants. Nat. Med. 2022, 28, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Weibel, D. B.; Diluzio, W. R.; Whitesides, G. M. , Microfabrication meets microbiology. Nat. Rev. Microbiol. 2007, 5, 209–18. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G. M. , The origins and the future of microfluidics. Nature 2006, 442, 368–73. [Google Scholar] [CrossRef]
- Beal, S. G.; Assarzadegan, N.; Rand, K. H. , Sample-to-result molecular infectious disease assays: clinical implications, limitations and potential. Expert. Rev. Mol. Diagn. 2016, 16, 323–41. [Google Scholar] [CrossRef] [PubMed]
- Foudeh, A. M.; Fatanat Didar, T.; Veres, T.; Tabrizian, M. , Microfluidic designs and techniques using lab-on-a-chip devices for pathogen detection for point-of-care diagnostics. Lab Chip 2012, 12, 3249–66. [Google Scholar] [CrossRef] [PubMed]
- Park, B. H.; Oh, S. J.; Jung, J. H.; Choi, G.; Seo, J. H.; Kim, D. H.; Lee, E. Y.; Seo, T. S. , An integrated rotary microfluidic system with DNA extraction, loop-mediated isothermal amplification, and lateral flow strip based detection for point-of-care pathogen diagnostics. Biosens. Bioelectron. 2017, 91, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Kenny, L. C.; Bartley, D. L. , The Performance Evaluation of Aerosol Samplers Tested with Monodisperse Aerosols. J. Aerosol Sci. 1995, 26, 109–126. [Google Scholar] [CrossRef]
- Li, S. N.; Lundgren, D. A.; Rovell-Rixx, D. , Evaluation of six inhalable aerosol samplers. AIHAJ 2000, 61, 506–16. [Google Scholar] [CrossRef]
- Sun, Y.; Lei, L.; Zhou, W.; Chen, C.; He, Y.; Sun, J.; Li, Z.; Xu, W.; Wang, Q.; Ji, D.; Fu, P.; Wang, Z.; Worsnop, D. R. , A chemical cocktail during the COVID-19 outbreak in Beijing, China: Insights from six-year aerosol particle composition measurements during the Chinese New Year holiday. Sci. Total Environ. 2020, 742, 140739. [Google Scholar] [CrossRef]
- Almatawah, Q. A.; Al-Rashidi, M. S.; Yassin, M. F.; Varghese, J. S. , Microbiological contamination of indoor and outdoor environments in a desert climate. Environ. Monit. Assess. 2022, 194, 355. [Google Scholar] [CrossRef]
- Li, M.; Wu, C. L.; Sun, L. L.; Lai, A. C. K. , A Dynamic Simulation of Indoor Polydispersion of Particles in All-Air Air-Conditioned Environment with Dynamical Particle Sources. Indoor and Built Environ. 2012, 21, 282–291. [Google Scholar] [CrossRef]
- Han, T. T.; Thomas, N. M.; Mainelis, G. , Design and development of a self-contained personal electrostatic bioaerosol sampler (PEBS) with a wire-to-wire charger. Aerosol Sci. Technol. 2017, 51, 903–915. [Google Scholar] [CrossRef]
- Suzuki, H.; Krasney, J. A. , Nitric oxide in single-breath exhalation in humans. Jpn. J. Physiol. 1997, 47, 335–9. [Google Scholar] [CrossRef] [PubMed]
- Fabian, P.; Brain, J.; Houseman, E. A.; Gern, J.; Milton, D. K. , Origin of exhaled breath particles from healthy and human rhinovirus-infected subjects. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 137–47. [Google Scholar] [CrossRef]
- Jarvis, M. C. , Aerosol Transmission of SARS-CoV-2: Physical Principles and Implications. Front. Public Health 2020, 8, 590041. [Google Scholar] [CrossRef]
- Mortazavi, H.; Beni, H. M.; Aghaei, F.; Sajadian, S. H. , SARS-CoV-2 droplet deposition path and its effects on the human upper airway in the oral inhalation. Comput. Meth. Prog. Biomed. 2021, 200, 105843. [Google Scholar] [CrossRef]
- Dietz, L.; Horve, P. F.; Coil, D. A.; Fretz, M.; Eisen, J. A.; Van Den Wymelenberg, K. , 2019 Novel Coronavirus (COVID-19) Pandemic: Built Environment Considerations To Reduce Transmission. mSystems 2020, 5. [Google Scholar] [CrossRef]
- Mahmoodzadeh, M.; Gretka, V.; Wong, S.; Froese, T.; Mukhopadhyaya, P. , Evaluating Patterns of Building Envelope Air Leakage with Infrared Thermography. Energies 2020, 13. [Google Scholar] [CrossRef]
- Pan, M.; Eiguren-Fernandez, A.; Hsieh, H.; Afshar-Mohajer, N.; Hering, S. V.; Lednicky, J.; Hugh Fan, Z.; Wu, C. Y. , Efficient collection of viable virus aerosol through laminar-flow, water-based condensational particle growth. J. Appl. Microbiol. 2016, 120, 805–15. [Google Scholar] [CrossRef]
- Guo, Z.; Wei, W.; Chen, L.; Wang, Z.; Mei, S. , Operation of Distribution Network Considering Compressed Air Energy Storage Unit and Its Reactive Power Support Capability. IEEE T. Smart Grid 2020, 11, 2954–2965. [Google Scholar] [CrossRef]
- Borro, M.; Di Girolamo, P.; Gentile, G.; De Luca, O.; Preissner, R.; Marcolongo, A.; Ferracuti, S.; Simmaco, M. Chloride removal from the kraft recovery boiler ESP dust using the precipitator dust purification (PDP) system - High efficiency is reported. Pulp & Pap. -Canada 1999, 100, 46-53.
- Setti, L.; Passarini, F.; De Gennaro, G.; Barbieri, P.; Licen, S.; Perrone, M. G.; Piazzalunga, A.; Borelli, M.; Palmisani, J.; Di Gilio, A.; Rizzo, E.; Colao, A.; Piscitelli, P.; Miani, A. , Potential role of particulate matter in the spreading of COVID-19 in Northern Italy: first observational study based on initial epidemic diffusion. BMJ Open 2020, 10, e039338. [Google Scholar] [CrossRef] [PubMed]
- Collivignarelli, M. C.; Abba, A.; Caccamo, F. M.; Bertanza, G.; Pedrazzani, R.; Baldi, M.; Ricciardi, P.; Carnevale Miino, M. , Can particulate matter be identified as the primary cause of the rapid spread of CoViD-19 in some areas of Northern Italy? Environ. Sci. Pollut. Res. Int. 2021, 28, 33120–33132. [Google Scholar] [CrossRef] [PubMed]
- Blocken, B.; van Druenen, T.; Ricci, A.; Kang, L.; van Hooff, T.; Qin, P.; Xia, L.; Ruiz, C. A.; Arts, J. H.; Diepens, J. F. L.; Maas, G. A.; Gillmeier, S. G.; Vos, S. B.; Brombacher, A. C. , Ventilation and air cleaning to limit aerosol particle concentrations in a gym during the COVID-19 pandemic. Build. Environ. 2021, 193, 107659. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).