1. Introduction
Cardiac arrhythmias encompass a wide range of abnormalities with a prevalence of 1.5% to 5% in the general population. Among these arrhythmic disorders, atrial fibrillation is the most common and frequently associated with a high morbidity and mortality [1]. However, the origins and clinical apparition of cardiac arrhythmias can be diverse as evidenced by the variety of different genes which have been linked to these conditions.
Mechanical forces play a critical role in a large variety of biological processes. Various cellular sensors exist and are present in most organs including the heart, where they have been shown to be involved in mechano-electrical feedback, a mechanism which regulates cardiac electrical activity in response to mechanical force [2]. Among the mechanosensors present in the heart, ion channels play a central role in this process as they can both sense mechanical forces and trigger electrical responses. Several reports demonstrate the expression of different mechanosensitive ion channels within the heart, in particular in sinoatrial node (SAN) cells which are responsible for generating cardiac rhythm [3]. Moreover, SAN cells not only express mechanosensitive ion channels that might regulate their electrical activity, but some of the main actors that are involved in the generation of the action potential (AP) are themselves mechanically modulated and can cause AP shortening in response to mechanical forces [3]. This is also the case for cardiomyocytes in general where mechanosensitive ion channels can regulate AP duration [2].
Although the underlying mechanisms remain unknown, the presence of mechanosensitive ion channels in cardiomyocytes and the potential role of mechanical forces in regulating cardiac rhythm have frequently been associated with the generation of cardiac arrhythmias. For example, blocking mechanosensitve ion channels with GsMTx4, a peptide isolated from spider venom, can alleviate atrial fibrillation in rabbit models [4]. This also suggests that overactivation of mechanosensitve ion channels could play a causal role in cardiac arrhythmias.
The mechanosensitive ion channel PIEZO1 is expressed in both cardiomyocytes and SAN cells [3,5]. Recently it has been demonstrated that PIEZO1 is involved in Ca2+ homeostasis and signaling in mouse cardiomyocytes and that loss of PIEZO1 specifically in cardiomyocytes leads to impaired cardiac function [5]. More interestingly, it appears that the overexpression of PIEZO1 induces severe heart failure and arrhythmia, indicating that PIEZO1 may be associated with cardiac arrhythmias [5]. However, in human patients suffering from cardiac arrhythmias, it is unlikely that this condition is caused by an overexpression of PIEZO1 specifically in cardiomyocytes. A more logical scenario is that certain genetic variants in PIEZO1 could lead to a gain of function and subsequent cardiac arrhythmia. Indeed, gain of function variants in PIEZO1 have been identified in patients who suffer from xerocytosis, a rare hemolytic anemia which affects red blood cell physiology [6]. However, at present no analysis has been performed to determine whether PIEZO1-induced xerocytosis patients are more susceptible to cardiac arrhythmias.
Zebrafish have proven to be a useful model to study cardiac development and disease [7]. Indeed, when compared with mice, zebrafish cardiac electrophysiology and heart rate are more similar to humans [8]. Here we demonstrate, using a combination of pharmacology and an array of different cardiac electrical analyses, that prolonged Piezo1 activation results in cardiac arrhythmia in zebrafish.
The introduction should briefly place the study in a broad context and highlight why it is important. It should define the purpose of the work and its significance. The current state of the research field should be carefully reviewed and key publications cited. Please highlight controversial and diverging hypotheses when necessary. Finally, briefly mention the main aim of the work and highlight the principal conclusions. As far as possible, please keep the introduction comprehensible to scientists outside your particular field of research. References should be numbered in order of appearance and indicated by a numeral or numerals in square brackets—e.g., [1] or [2,3], or [4–6]. See the end of the document for further details on references.
2. Results
2.1. Subsection Piezo1 Is Expressed in Zebrafish Cardiomyocytes
Previous studies in mammals indicate that
Piezo1 is expressed in cardiomyocytes and plays a critical role in Ca
2+ homeostasis and signaling [5]. To determine whether
piezo1 is expressed in zebrafish cardiomyocytes, we performed a transcriptomic analysis at the single cell level on adult zebrafish hearts. Because cardiomyocytes are largely absent from single-cell data (their size and fragility are not compatible with scRNAseq pipelines) [11] and since ion channels in general are not highly expressed and are often difficult to detect using standard techniques such as
in situ hybridization or immunohistochemistry, we adopted a single nuclei RNA sequencing (snRNAseq) strategy. Unbiased clustering of ventricular nuclei revealed five distinct clusters (
Figure 1A). Each cluster corresponded to a distinct cell type and included
tnnt2+ cardiomyocytes,
spock3+ endothelial cells,
itga2b+ thrombocytes,
csf1ra+ myeloid cells and
tbx18+ fibroblasts (
Figure 1B). Next, we analysed this data set for the expression of the 2 zebrafish
piezo1 othologs-
piezo1a [12] and
piezo1b [13]. In this manner, we were able to detect expression of both
piezo1 orthologs within the cardiomyocyte population (
Figure 1C). This data indicates that, like mammals,
piezo1 is expressed in zebrafish cardiomyocytes.
2.2. Yoda1 Increases Zebrafish Piezo1 Activation Kinetics
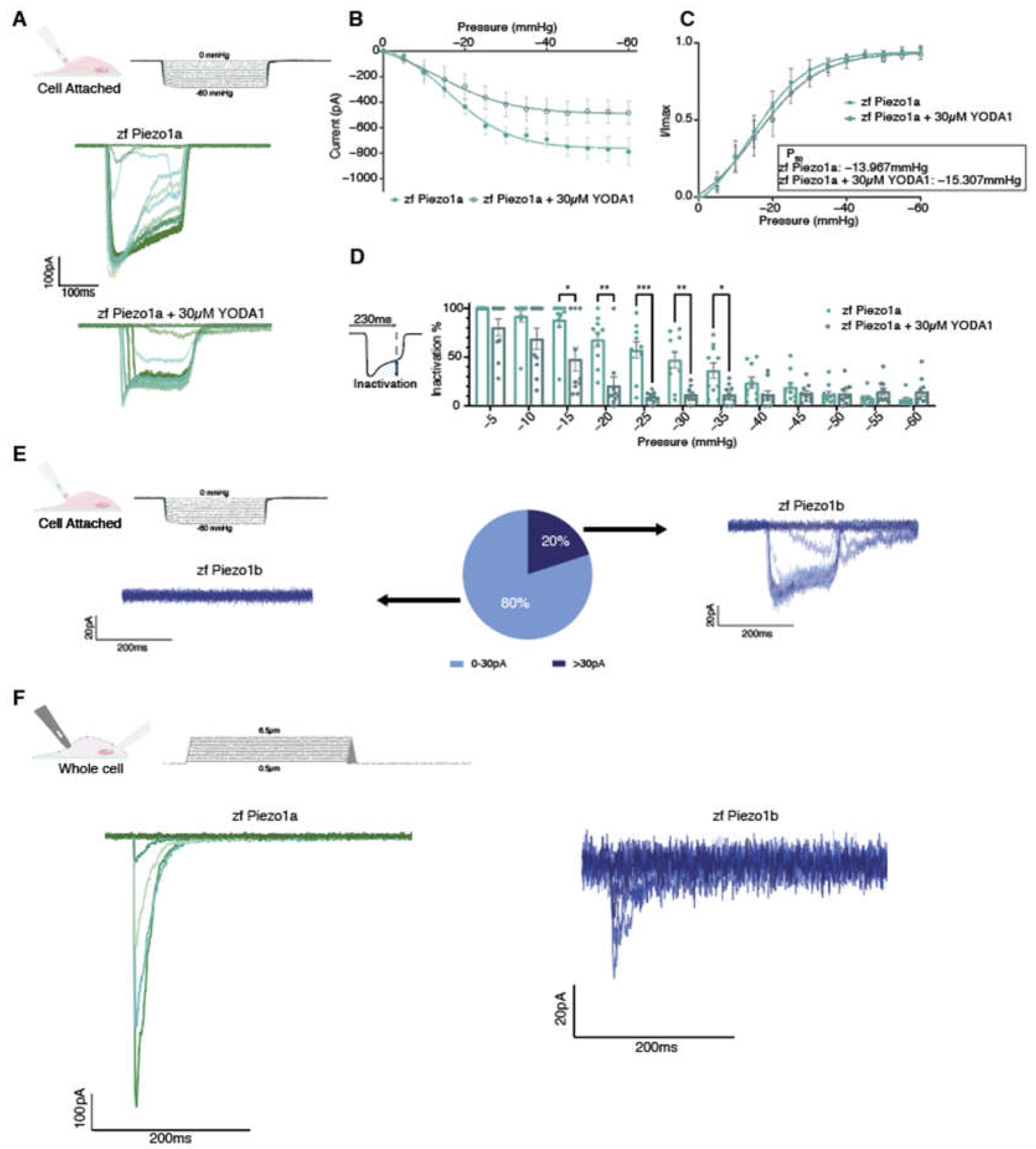
The chemical compound Yoda1 was previously identified as an activator of mammalian PIEZO1 ion channels, affecting the mechanosensitivity and inactivation kinetics [14]. To determine whether Yoda1 also had a similar effect on the zebrafish Piezo1 orthologs, we performed electrophysiology on HEK293T cells transfected with either zebrafish
piezo1a or
piezo1b. Using a cell attached configuration in conjunction with negative pressure pulses, we could readily detect Piezo1a inward currents in response to mechanical stimulation (
Figure 2A). The current-pressure relationship obtained in the cell attached configuration revealed an average P
50 of -13.967mmHg suggesting zfPiezo1a has a higher sensitivity to mechanical stimulation compared to previous reports for mammalian PIEZO1 (
Figure 2B,C). Next,
piezo1a transfected cells were treated with 30µM of Yoda1, a concentration that has previously been shown to activate mammalian PIEZO1 channels following mechanical stimulation [15]. In this manner, we found that Yoda1 had a similar effect on zebrafish Piezo1a, resulting in a delayed inactivation of the currents in comparison to non-treated cells (
Figure 2B–D). Interestingly and unlike its mammalian orthologs, Piezo1a sensitivity to mechanical stimulation was not affected by Yoda1 treatment. While Piezo1a mechanically activated currents were easily detected in cell attached configuration, Piezo1b currents were detected in a minority of cells under the same conditions (
Figure 2E). To overcome this shortcoming, we switched to a whole cell approach in conjunction with a cell-poking piezo device which revealed a small, mechanically activated inward current in cells expressing Piezo1b compared to Piezo1a (
Figure 2F) [14,16]. Altogether, these results indicate that both zebrafish Piezo1 orthologs are mechanically activated and that Yoda1 enhances Piezo1a activation kinetics.
2.3. Prolonged Piezo1 Activation In Vivo Causes Cardiac Arrhythmia
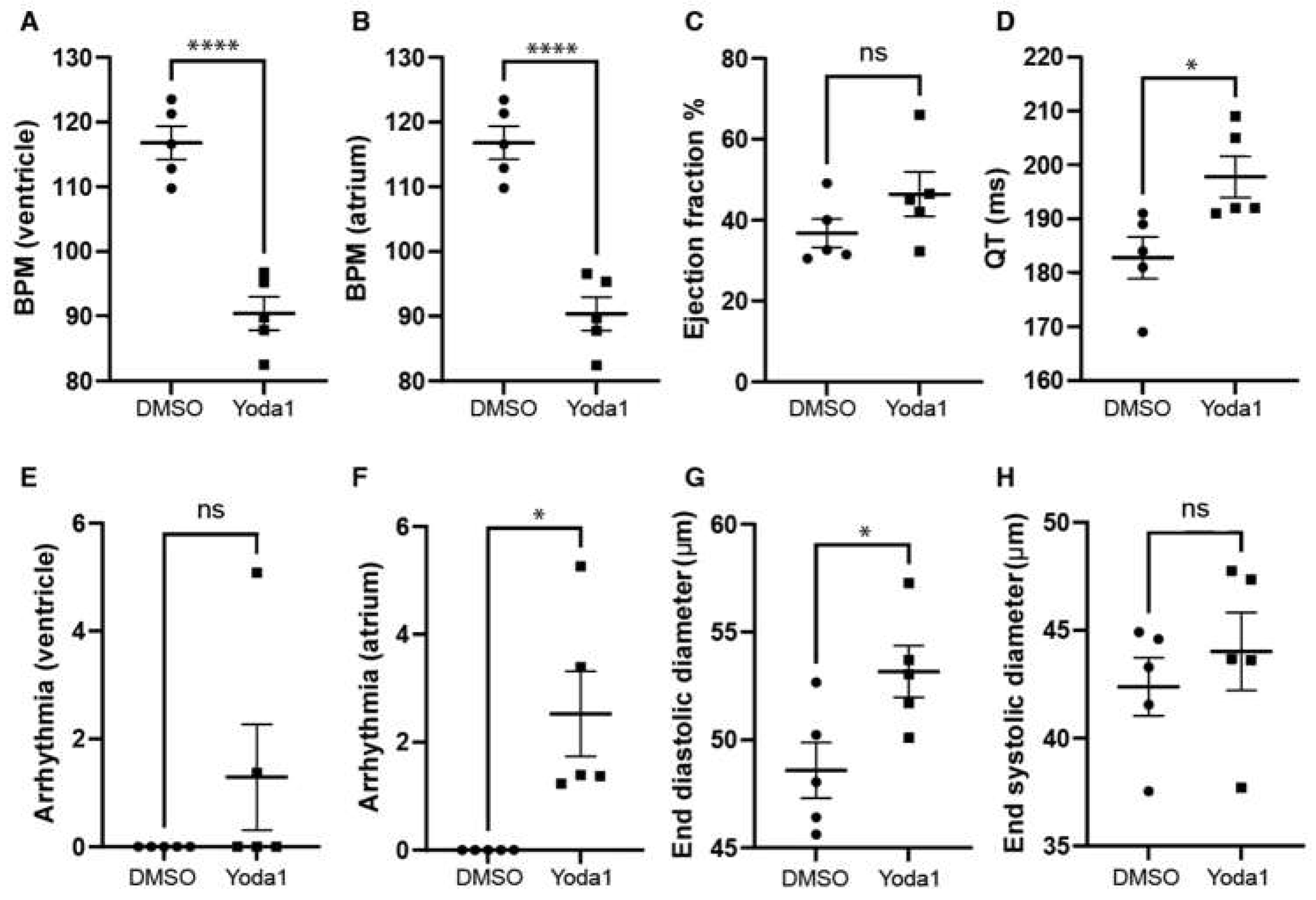
We next investigated whether prolonged Piezo1 activation could play a role in cardiac physiology. To achieve this, we treated 4 days post fertilization (dpf) transgenic myl7:EGFP zebrafish larvae with 50 µM Yoda1. The transgenic myl7:EGFP zebrafish line expresses GFP specifically in cardiomyocytes which allows us to record high definition movies and perform accurate downstream analyses. Following Yoda1 treatment, larvae were mounted in low melting point agarose and 1 minute movies of the beating heart were captured with a high speed camera. Movies were subsequently analysed using ZeCardioTM software (Videos S1 and S2). In this manner, we were able to determine that treatment with Yoda1 resulted in significant bradycardia which affected both the ventricle and atrium (
Figure 3A,B). We also observed a significant increase in the QT interval (
Figure 3D) and the prevalence of atrial fibrillation (no significant arrhythmia was observed in the ventricle) (
Figure 3F). Lastly, the end diastolic ventricular diameter appears to be significantly larger than controls following Yoda1 treatment (
Figure 3G).
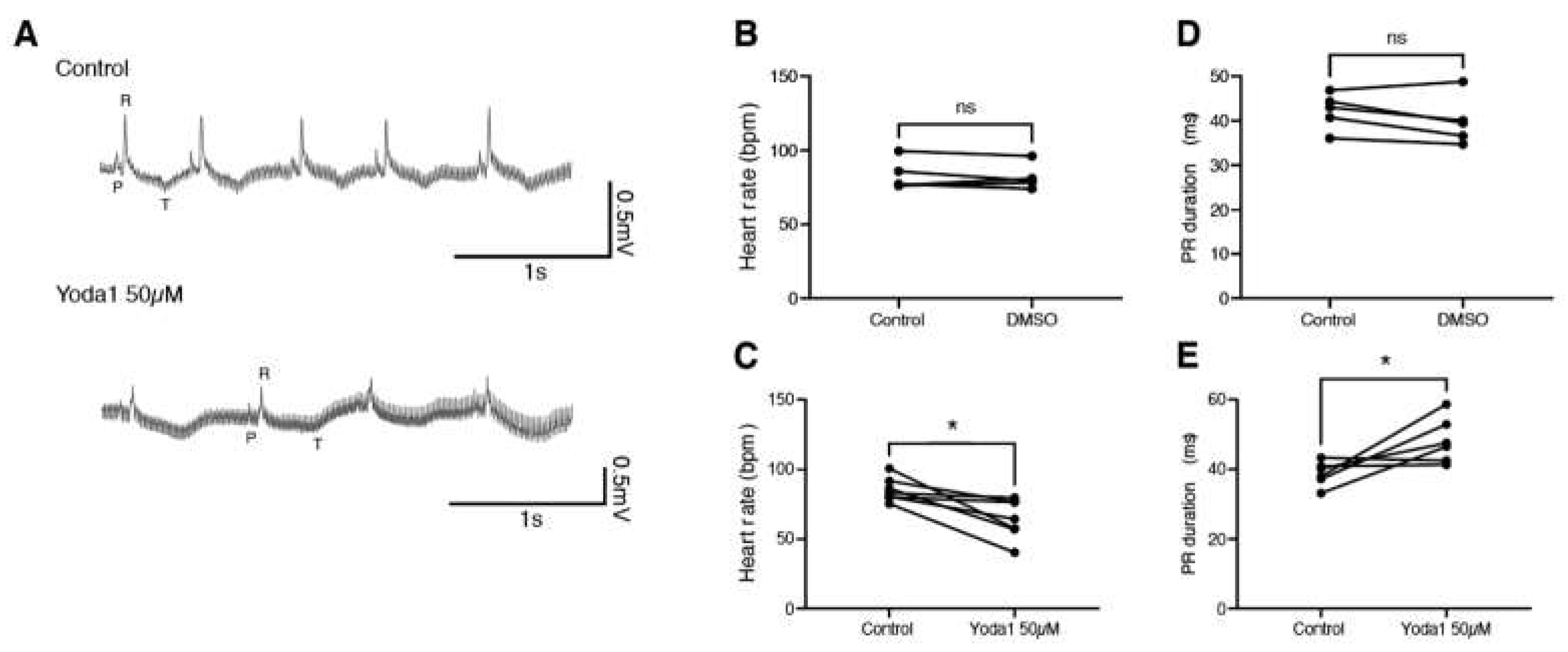
To determine whether this is conserved during adulthood, we performed electrocardiogram (ECG) recordings on adult zebrafish in the exposed heart configuration [10]. After a baseline recording, fish were treated with either DMSO or 50 µM Yoda1 for one hour. A second ECG recording was then performed and compared to the non-treated recording (
Figure 4A). We observed that, while DMSO had no effect, Yoda1 treatment significantly slowed the heart rate (
Figure 4B,C). This bradycardia was associated with an increased PR duration suggesting a conduction defect between the atrium and ventricle such as a first-degree atrioventricular block (
Figure 4 D,E).Taken together, these data indicate that Yoda1 treatment affects heart performance and indicates that prolonged Piezo1 activation results in cardiac arrhythmia.
2.4. Prolonged Piezo1 Activation In Vivo Affects the Dynamics of the Cardiac Action Potential
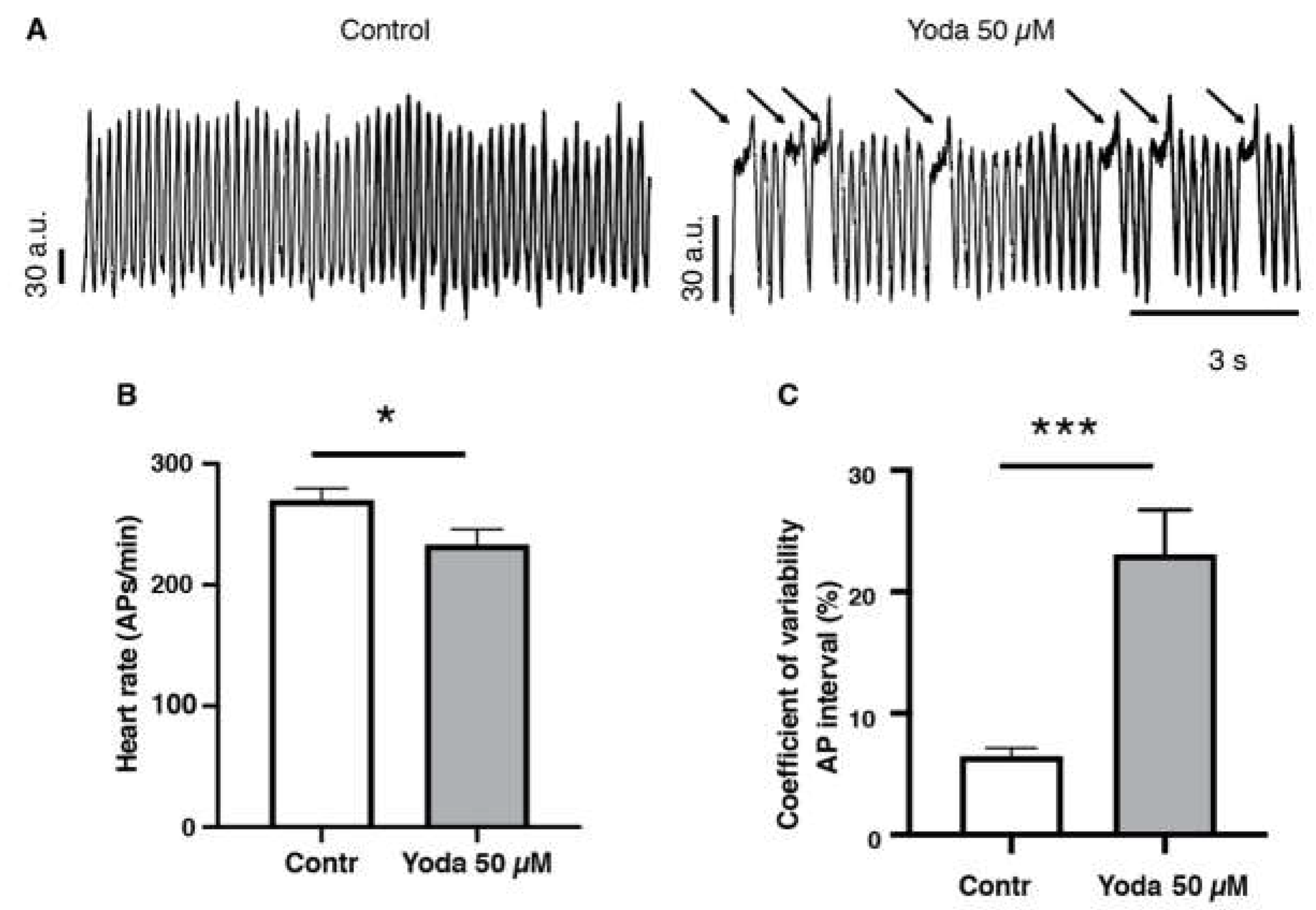
To confirm that prolonged Piezo1 activation results in cardiac arrhythmia, we adopted an optical mapping approach combined with a voltage sensitive-dye to assess the spatiotemporal dynamics of excitable events in the whole heart in vivo [17,18] (
Figure 5A and Videos S3 and S4). After prolonged incubation with 50 µM Yoda1 or DMSO followed by an incubation in the voltage sensitive dye, our recordings indicated that Yoda1 significantly affected the heart rate as evidenced by a reduced number of cardiac APs measured in the ventricular region (
Figure 5B). We also observed that heart rhythmicity was affected as Yoda1 treatment induced an increase of the coefficient of variability of the AP intervals (
Figure 5C). Moreover, we noticed that the reduction of heart rate (cardiac APs) and the increase of coefficient of variability of the AP intervals under Yoda1 treatment were caused by a remarkable prolongation in the depolarization phases that occurred in the ventricular region but not in the atria (
Figure 5A and Videos S3 and S4). Altogether, these results suggest that Piezo1 activation by Yoda1 impairs heart rhythmicity.
3. Discussion
Despite decades old evidence for the association of cardiac rhythm and mechnaosensitivty, the molecular mechansims of this phenomenon have remained elusive [2]. In particular, mechanosensitive ion channels, such as PIEZO1, have long been proposed as a source of mechano-electric feedback under physiological and pathophysiological conditions. The very nature of mechanosensitve ion channels ensures that they can respond rapidly to changes in mechanical force and translate these into electrical signals that can modulate cardiac performance. Pathological conditions that disrupt the normal cardiac mechano-physiology, for example myocardial infarction, could affect mechano-electric feedback and potentially result in cardiac arrhythmias. Our data indicates that both Piezo1 orthologs (Piezo1a and Piezo1b) are expressed in zebrafish cardiomyocytes, similar to reports in mammals, where they might play a role in regulating mechano-electric feedback [19]. Our data indicates that, similar to their mammalian counterparts, zebrafish Piezo1 orthologs respond to mechanical stimulation and that Piezo1a is activated by the selective agonist Yoda1, resulting in the partial increase in activation kinetics in vitro. Using this same approach, we could only record a weak Piezo1b current. This could reflect a difference in the ion selectivity or suggest that the use of HEK293T transfected cells does not recapitulate the membrane environment essential for Piezo1b activity [20]. Recently, it has been demonstrated that PIEZO1 is a bone fide cardiomyocyte mechanosensor in vivo where it translates the mechanical forces exerted on cardiomyocytes into intracellular calcium signalling and ultimately regulates cardiac performance [5]. It is also apparent that increased PIEZO1 signalling results in detrimental effects on cardiac rhythm. In particular, overexpression of Piezo1 in mouse cardiomyocytes results in severe cardiac arrhythmia. Furthermore, under pathological conditions often associated with cardiac arrhythmias, such as hypertrophic cardiomyopathy, PIEZO1 expression increases [21]. While this response presumably ensures the adaptation to the mechanical overload, it could ultimately lead to maladaption and an increased risk of arrhythmias. However, it is unlikely that human patients suffering from cardiac arrhythmia will have a cardiomyocyte specific overexpression of PIEZO1. A more likely scenario is that variants in PIEZO1 will lead to a global gain of function in activity. This is certainly the case for the hereditary xerocytosis where gain of function variants in PIEZO1 affect red blood cell physiology. Although, no data is available at present regarding the prevalence of cardiac arrhythmias in patients suffering from this condition, mice have been engineered to contain a specific gain of function PIEZO1 mutation found in these patients [22]. Interestingly, these animals develop cardiac hypertrophy and fibrosis, a problem primarily associated with dysfunctional fibroblasts. However, it should be noted that due to the extensive differences in cardiac electrophysiology between mice and humans this species is unreliable for assessing cardiac arrhythmias [23]. Zebrafish, for its part, has also proven to be a useful model to study cardiac development and disease [7]. Despite the fact that zebrafish heart only displays one ventricle and one atrium, cardiac electrophysiological properties are more similar to those of human than mice [8]. Our in vivo data, using several approaches including video analysis, optical mapping or ECG on larvae or adult zebrafish, demonstrates that prolonged global activation of zf Piezo1 results in cardiac arrhythmia associated with abberant action potential propagation in zebrafish. Although our data is in line with Piezo1 overexpression analyses in mice, we cannot rule out that the effect we observe is due to increased Piezo1 activity in another cardiac cell type, such as fibroblasts.
4. Materials and Methods
4.1. Zebrafish Husbandry
Zebrafish were maintained under standardized conditions [9] and experiments were conducted in accordance with local approval (APAFIS#2021021117336492-32684) and the European Communities directive 2010/63/EU. The Tg(myl7:EGFP) was provided by the CMR[B] Centro de Medicina Regenerativa de Barcelona.
4.2. Cell culture and Transfection
HEK293T cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) containing 4.5g/L D-Glucose, Pyruvate, 10% fetal bovine serum, 1% Penicillin/Streptomycin. For patch clamp experiments, cells were plated onto 35mm dishes and transfected using Polyplus JetPEI (Ozyme) 24h after passaging with 1.5µg of plasmid for each dish. Cells were analysed 48-72h after transfection. The plasmids used in this study, pIRES2-GFP-zfPiezo1a and pIRES2-GFP-zfPiezo1b were generated by Genscript.
4.3. Patch Clamp Recordings
For cell attached recordings, HEK293T cells were kept in a bath solution containing (in mM): 140 KCl, 10 HEPES, 1 MgCl2 and 10 Glucose (pH 7.3 with KOH). Electrophysiological recordings were obtained using an Axopatch 200B amplifier (Axon Instruments), connected to a 1550B series Digidata (Molecular Devices). Electrodes had a resistance of 1-1.5MΩ when filled with a solution containing (in mM): 130 NaCl, 5 KCl, 10 HEPES, 1 CaCl2, 1 MgCl2 and 10 TEA-Cl (pH 7.3 with NaOH). Mechanical stimulation was applied to membrane patches using high speed pressure clamp (HSPC-1, ALA-scientific) through the pipette. The recordings were obtained at a holding potential of -80mV with pressure steps from 0 to -60mmHg (-5mmHg increments).
For whole cell recordings, HEK293T cells were kept in an extracellular solution containing (in mM): 10 glucose, 140 NaCl, 4 KCl, 2 MgCl2, 10 HEPES, 2 CaCl2, (pH 7.30). Electrodes were filled with a solution containing (in mM): 127 K-Gluconate, 10 NaCl, 5 KCl, 4 Mg-ATP, 0.4 Na-GTP, 5 Creatine P (Na), 1 CaCl2, 10 EGTA, 1 MgCl2, 10 HEPES, (pH 7.20). Mechanical stimulation was applied to cell membrane using a fire-polished glass probe. The movement of the probe was controlled by a piezo-electric microstage (PZ-150M, Burleigh). The probe had a velocity of 1µm/ms and the stimulation was applied for 200ms. A series of mechanical steps of 0.5µm increments were applied to the cells every 10s. The recordings were obtained at a holding potential of -60mV.
Recordings were acquired on Axon pCLAMP 10.6 (Molecular Devices) and analysed on Clampfit 10.6 (Molecular Devices). The protocol and fitting equation (when applicable) used are specified in each figure.
4.4. Single Nuclei RNA Sequencing
Five hearts were dissected and placed in cold PBS with heparin. Atria and outflow tracts were removed and the ventricles were opened and washed. All ventricles were pooled into a single Eppendorf tube, excess media was removed and the samples flash frozen in liquid nitrogen and stored at -80C. Nuclei were isolated as described in the 10x Genomics protocol (CG000375.Rev C). Sorting was performed on a BD FACS Melody. Nuclei suspensions were loaded on a Chromium controller (10x Genomics, Pleasanton, CA, USA) to generate single-nuclei Gel Beads-in-Emulsion (GEMs). Single-nuclei RNA-Seq libraries were prepared using Chromium Single cell 3’RNA Gel Bead and Library Kit v3.1. GEM-RT was performed in a C1000 Touch Thermal cycler with 96-Deep Well Reaction Module (Bio-Rad): 53°C for 45 min, 85°C for 5 min; held at 4°C. After RT, GEMs were broken and the single-strand cDNA was cleaned up with DynaBeads MyOne Silane Beads (Thermo Fisher Scientific). cDNA was amplified using the C1000 Touch Thermal cycler with 96-DeepWell Reaction Module: 98°C for 3 min; cycled 12: 98°C for 15 s, 63°C for 20 s, and 72°C for 1 min; 72°C for 1 min; held at 4°C. Amplified cDNA products were cleaned up with SPRI select beads. Indexed sequencing libraries were constructed following these steps: (1) fragmentation, end-repair and A-tailing and size selection with SPRIselect; (2) adapter ligation and cleanup with SPRIselect; (3) sample index PCR and size selection with SPRIselect. The barcoded sequencing libraries were quantified by quantitative PCR (KAPA Biosystems Library Quantification Kit for Illumina platforms). Sequencing libraries were loaded at 300 pM on an Illumina NovaSeq6000 using the following read length: 28 bp Read1, 8 bp I7 Index, 91 bp Read2 (experiment 1), and 28 bp Read1, 10 bp I7 Index, 10 bp I5 Index, 87 bp Read2 (experiment 2).
Image analyses and base calling were performed using the NovaSeq Control Software and the Real-Time Analysis component (Illumina). Demultiplexing was performed using the 10X Genomics software Cellranger mkfastq (v6.0.1), a wrapper of Illumina’s bcl2fastq (v2.20). The quality of the raw data was assessed using FastQC (v0.11.8) from the Babraham Institute and the Illumina software SAV (Sequencing Analysis Viewer). FastqScreen (v0.14.0) was used to estimate the potential level of contamination.
Alignment, gene expression quantification and statistical analysis were performed using Cell Ranger count on Danio rerio’s transcriptome GRCz11 (sequences and annotation were downloaded from Ensembl! on July 24th, 2019). In order to discard ambient RNA falsely identified as cells, Cell Ranger count was run a second time with the option --force-cells to force the number of cells to detect.
| Number of hearts: |
5 |
| Number of nuclei analysed: |
648 |
| Median genes per nuclei: |
722 |
4.5. Cardiac Performance Analysis
Tg(myl7:EGFP) embryos were exposed to 0.2 mM N-phenylthiourea (PTU) from the epiboly stage. At 4dpf, larvae were treated with either DMSO (vehicle) or 50 µM Yoda1 (Selleckchem) in embryo medium for 30 minutes, anesthetized using tricaine methanesulfonate at 168 mg/ml and mounted in low-melting point agarose. 1-minute videos at 67 fps were recorded using a Zeiss Discovery v20 stereomicroscope and analyzed using ZeCardioTM software (ZeClinics).
4.6. Optical Mapping
Prior to voltage change recording, 4 dpf zebrafish larvae were incubated with DMSO or 50 µM Yoda1 (Selleckchem) and blebbistatin (8.5µM, Selleckchem) (to avoid contractions) in embryo medium for 1 hour. Larvae were then incubated in the dark for 40 minutes with the voltage sensitive probe di-4-anepps (9 µM; ThermoFisher) with DMSO or Yoda1 and blebbistatin. Next, the larvae were immobilized in the lateral position in low-melting point agarose, exposing their left side. Cardiac signals were then recorded for 30 to 60 seconds with an acquisition frequency of 100 frames/s using a 150 W halogen lighting system (SciMedia) as an excitation source, coupled with a 531/50nm excitation filter, a 580nm dichroic mirror and a 580 long-pass emission filter. For the acquisition, the Microcam3 camera with a sensor of 17.6x17.6 mm (SciMedia; Costa Mesa, CA) was coupled to two optics, the SDF PLAN FLUOR 0.3X stereo microscope objective mounted as an eyepiece lens (Olympus) and the Plan Apo 5.0 X/0.50 LWD mounted as magnification lens (Leica). Such a setting allows for an optic magnification of 16.6 X. The Microcam3 camera was connected to the BV Workbench software (ver 2.7.2, SciMedia; Costa Mesa, CA) to allow recording and successive analysis of the fluorescent signals. Before acquisition, the temperature inside the petri dish was recorded and maintained at about 27 C by a warming chamber (QE-1, Warner Instruments, Hamden, CT) including a temperature sensor , connected with a dual automatic temperature controller (TC-344B, Warner Instruments).
4.7. ECG Measurement
ECG measurements were performed in accordance with local approval (APAFIS#2021021117336492-32684) and as described in Arel & Rolland et al., [10] using the exposed heart configuration. Once a 30 seconds ECG recording was obtained, Yoda1 or DMSO were added to the water at a final concentration of 50µM for one hour and ECG were recorded again for 30 seconds. The recordings were processed using LabChart Pro v8 software (AD Instruments) and its ECG analysis module.
4.8. Data Analysis
Data were processed in GraphPad Prism 9.2.0 (GraphPad). Data are expressed as mean ± SEM with the exception of the ECG graph showing only paired individual measurements.
5. Conclusions
Considering Piezo1 calcium permeability, we could speculate that Yoda1 treatment leads to an increased Ca2+ entry following mechanical stimulation, which might affect spontaneous (pacemaker cells) and working (atrial and ventricular cells) cardiomyocytes in vivo. Furthermore, the chemical activation of Piezo1 using Yoda1 results in cardiac arrhythmias in zebrafish, at both larval and adult stages. Combined with the recent discoveries made in mammals, it is possible that PIEZO1 genetic variants with gain of function properties could be responsible for arrhythmias in patients suffering from atrial fibrillation. In summary our data encourages PIEZO1 genetic sequence analysis for patients suffering from cardiac arrhythmias.
Author Contributions
P.C., L.R., C.J. and A.F. conceived the study. L.R., C.J. and A.F interpreted the data and wrote the manuscript. A.D. was responsible for zebrafish husbandry, welfare and management. A.T., D.M. and A.F. performed and A.T. analysed the optical mapping experiments. L.R., D.M. and A.F. performed and interpreted the ECG. A.F. and C.J. performed the zebrafish hearts videos and analysis. L.R. and E.B. performed the electrophysiological experiments and analysis. A.F. and C.J. conceived and supervised the study and provided financial support. All authors read and approved the final manuscript.
Funding
This work was supported by INSERM, CNRS and the Laboratory of Excellence Ion Channel Science and Therapeutics (ANR-11-LABX-0015). Work in the C.J. lab is supported by a grant from the "la Fondation Leducq". Optical mapping equipment was supported by the Fondation pour la Recherche sur le Cerveau "Espoir en tête 2022/23" and by the University of Montpellier "Soutien à la recherche 2021".
Institutional Review Board Statement
Experiments were conducted in accordance with local approval (APAFIS#2021021117336492 v5) and the European Communities council directive 2010/63/EU.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
We would like to thank Montpellier Genomix (MGX). MGX acknowledges financial support from France Génomique National infrastructure, funded as part of "Investissement d’Avenir" program managed by Agence Nationale pour la Recherche (contract ANR-10-INBS-09). We would like to thank the iExplore Breeding and functional exploration platform for the animal core facility. We would also like to thank Matteo E. Mangoni for providing equipment. We would also like to thank the IGF zebrafish facility (part of the Montpellier ZEFIX platform) for providing and maintaining all the zebrafish lines used in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Desai, D.S.; Hajouli, S. Arrhythmias. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2022. [Google Scholar]
- Peyronnet, R.; Nerbonne, J.M.; Kohl, P. Cardiac Mechano-Gated Ion Channels and Arrhythmias. Circ Res 2016, 118, 311–329. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Kang, C.; Mesirca, P.; Hong, J.; Mangoni, M.E.; Glukhov, A.V.; Sah, R. Electrophysiological and Molecular Mechanisms of Sinoatrial Node Mechanosensitivity. Front Cardiovasc Med 2021, 8, 662410. [Google Scholar] [CrossRef] [PubMed]
- Bode, F.; Sachs, F.; Franz, M.R. Tarantula Peptide Inhibits Atrial Fibrillation. Nature 2001, 409, 35–36. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Yin, K.; Wu, K.; Zhang, M.; Wang, S.; Cheng, H.; Zhou, Z.; Xiao, B. The Mechanosensitive Piezo1 Channel Mediates Heart Mechano-Chemo Transduction. Nat Commun 2021, 12, 869. [Google Scholar] [CrossRef] [PubMed]
- Zarychanski, R.; Schulz, V.P.; Houston, B.L.; Maksimova, Y.; Houston, D.S.; Smith, B.; Rinehart, J.; Gallagher, P.G. Mutations in the Mechanotransduction Protein PIEZO1 Are Associated with Hereditary Xerocytosis. Blood 2012, 120, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Narumanchi, S.; Wang, H.; Perttunen, S.; Tikkanen, I.; Lakkisto, P.; Paavola, J. Zebrafish Heart Failure Models. Front. Cell Dev. Biol. 2021, 9, 662583. [Google Scholar] [CrossRef] [PubMed]
- Echeazarra, L.; Hortigón-Vinagre, M.P.; Casis, O.; Gallego, M. Adult and Developing Zebrafish as Suitable Models for Cardiac Electrophysiology and Pathology in Research and Industry. Front. Physiol. 2021, 11, 607860. [Google Scholar] [CrossRef] [PubMed]
- Aleström, P.; D’Angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and Husbandry Recommendations. Lab Anim 2020, 54, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Arel, E.; Rolland, L.; Thireau, J.; Torrente, A.G.; Bechard, E.; Bride, J.; Jopling, C.; Demion, M.; Le Guennec, J.-Y. The Effect of Hypothermia and Osmotic Shock on the Electrocardiogram of Adult Zebrafish. Biology 2022, 11, 603. [Google Scholar] [CrossRef]
- Rolland, L.; Harrington, A.; Faucherre, A.; Abaroa, J.M.; Gangatharan, G.; Gamba, L.; Severac, D.; Pratlong, M.; Moore-Morris, T.; Jopling, C. The Regenerative Response of Cardiac Interstitial Cells. J Mol Cell Biol 2022, mjac059. [Google Scholar] [CrossRef]
- Faucherre, A.; Kissa, K.; Nargeot, J.; Mangoni, M.E.; Jopling, C. Piezo1 Plays a Role in Erythrocyte Volume Homeostasis. Haematologica 2014, 99, 70–75. [Google Scholar] [CrossRef]
- Faucherre, A.; Moha ou Maati, H.; Nasr, N.; Pinard, A.; Theron, A.; Odelin, G.; Desvignes, J.-P.; Salgado, D.; Collod-Béroud, G.; Avierinos, J.-F.; et al. Piezo1 Is Required for Outflow Tract and Aortic Valve Development. Journal of Molecular and Cellular Cardiology 2020, 143, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Syeda, R.; Xu, J.; Dubin, A.E.; Coste, B.; Mathur, J.; Huynh, T.; Matzen, J.; Lao, J.; Tully, D.C.; Engels, I.H.; et al. Chemical Activation of the Mechanotransduction Channel Piezo1. Elife 2015, 4, e07369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chi, S.; Guo, H.; Li, G.; Wang, L.; Zhao, Q.; Rao, Y.; Zu, L.; He, W.; Xiao, B. A Lever-like Transduction Pathway for Long-Distance Chemical- and Mechano-Gating of the Mechanosensitive Piezo1 Channel. Nat Commun 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Torrente, A.G.; Zhang, R.; Zaini, A.; Giani, J.F.; Kang, J.; Lamp, S.T.; Philipson, K.D.; Goldhaber, J.I. Burst Pacemaker Activity of the Sinoatrial Node in Sodium–Calcium Exchanger Knockout Mice. Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 9769–9774. [Google Scholar] [CrossRef] [PubMed]
- Baudot, M.; Torre, E.; Bidaud, I.; Louradour, J.; Torrente, A.G.; Fossier, L.; Talssi, L.; Nargeot, J.; Barrère-Lemaire, S.; Mesirca, P.; et al. Concomitant Genetic Ablation of L-Type Cav1.3 (A1D) and T-Type Cav3.1 (A1G) Ca2+ Channels Disrupts Heart Automaticity. Sci Rep 2020, 10, 18906. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, B.; Yuan, G.; Chen, Y.; Liang, F.; Zeng, H.; Zheng, S.; Cao, L.; Geng, D.; Zhou, S. Stretch-Activated Channel Piezo1 Is up-Regulated in Failure Heart and Cardiomyocyte Stimulated by AngII. Am J Transl Res 2017, 9, 2945–2955. [Google Scholar] [PubMed]
- Yamaguchi, Y.; Allegrini, B.; Rapetti-Mauss, R.; Picard, V.; Garçon, L.; Kohl, P.; Soriani, O.; Peyronnet, R.; Guizouarn, H. Hereditary Xerocytosis: Differential Behavior of PIEZO1 Mutations in the N-Terminal Extracellular Domain Between Red Blood Cells and HEK Cells. Front Physiol 2021, 12, 736585. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Gong, H.; Kesteven, S.; Guo, Y.; Wu, J.; Li, J.V.; Cheng, D.; Zhou, Z.; Iismaa, S.E.; Kaidonis, X.; et al. Piezo1 Is the Cardiac Mechanosensor That Initiates the Cardiomyocyte Hypertrophic Response to Pressure Overload in Adult Mice. Nat Cardiovasc Res 2022, 1, 577–591. [Google Scholar] [CrossRef]
- Bartoli, F.; Evans, E.L.; Blythe, N.M.; Stewart, L.; Chuntharpursat-Bon, E.; Debant, M.; Musialowski, K.E.; Lichtenstein, L.; Parsonage, G.; Futers, T.S.; et al. Global PIEZO1 Gain-of-Function Mutation Causes Cardiac Hypertrophy and Fibrosis in Mice. Cells 2022, 11, 1199. [Google Scholar] [CrossRef] [PubMed]
- Clauss, S.; Bleyer, C.; Schüttler, D.; Tomsits, P.; Renner, S.; Klymiuk, N.; Wakili, R.; Massberg, S.; Wolf, E.; Kääb, S. Animal Models of Arrhythmia: Classic Electrophysiology to Genetically Modified Large Animals. Nat Rev Cardiol 2019, 16, 457–475. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).