Submitted:
14 February 2023
Posted:
15 February 2023
You are already at the latest version
Abstract
Keywords:
Background Statement
1. Introduction
2. Physical and Chemical Properties of Human and Livestock Urine and Feces
| Category | Moisture content (%) |
Weight (kg) | N (kg) | K (kg) | P (kg) |
Volatile solids (kg) | Total solids (kg) | Biological oxygen demand, (BOD) (kg) |
|---|---|---|---|---|---|---|---|---|
| Poultry manures | ||||||||
| Broilers | 74 | 40.00 | 0.44 | 0.25 | 0.13 | 7.72 | 9.99 | 2.41 |
| Duck | 74 | 46.31 | 0.45 | 0.23 | 0.16 | 7.26 | 12.26 | 2.04 |
| Layers | 75 | 25.88 | 0.50 | 0.18 | 0.15 | 5.00 | 6.81 | 1.51 |
| Swine manure | ||||||||
| Boar | 90 | 8.63 | 0.06 | 0.04 | 0.03 | 0.77 | 0.86 | 0.30 |
| Gestating sow | 90 | 11.34 | 0.07 | 0.05 | 0.03 | 1.04 | 1.14 | 0.38 |
| Lactating sow | 90 | 26.79 | 0.20 | 0.13 | 0.06 | 2.45 | 2.68 | 0.91 |
| Beef manure | ||||||||
| Finishing cattle | 92 | 29.51 | 0.16-0.23 | 0.11 | 0.02-0.03 | 1.95 | 2.36 | 0.45 |
| Beef cow in confinement | 88 | 47.22 | 0.16 | 0.11 | 0.04 | 5.00 | 5.90 | 1.14 |
| Growing calf in confinement | 88 | 34.96 | 0.20 | 0.13 | 0.04 | 3.50 | 4.18 | 0.77 |
| Human | ||||||||
| Human feces | 72 | 0.225 | 0.01 | 0.004 | 0.01 | - | - | 5.48 |
| Human urine | 95 | 0.12 | 0.02 | 0.005 | 0.005 | - | - | 1.83 |
| Waste types | Proximate analysis | Ultimate analysis | Higher heating value | References | |||||
|---|---|---|---|---|---|---|---|---|---|
| Volatile matter (wt.%) |
Fixed carbon (wt.%) |
Ash content (wt.%) |
C (wt.%) |
S (wt.%) |
O (wt.%) |
H (wt.%) |
HHV (MJ/kg) | ||
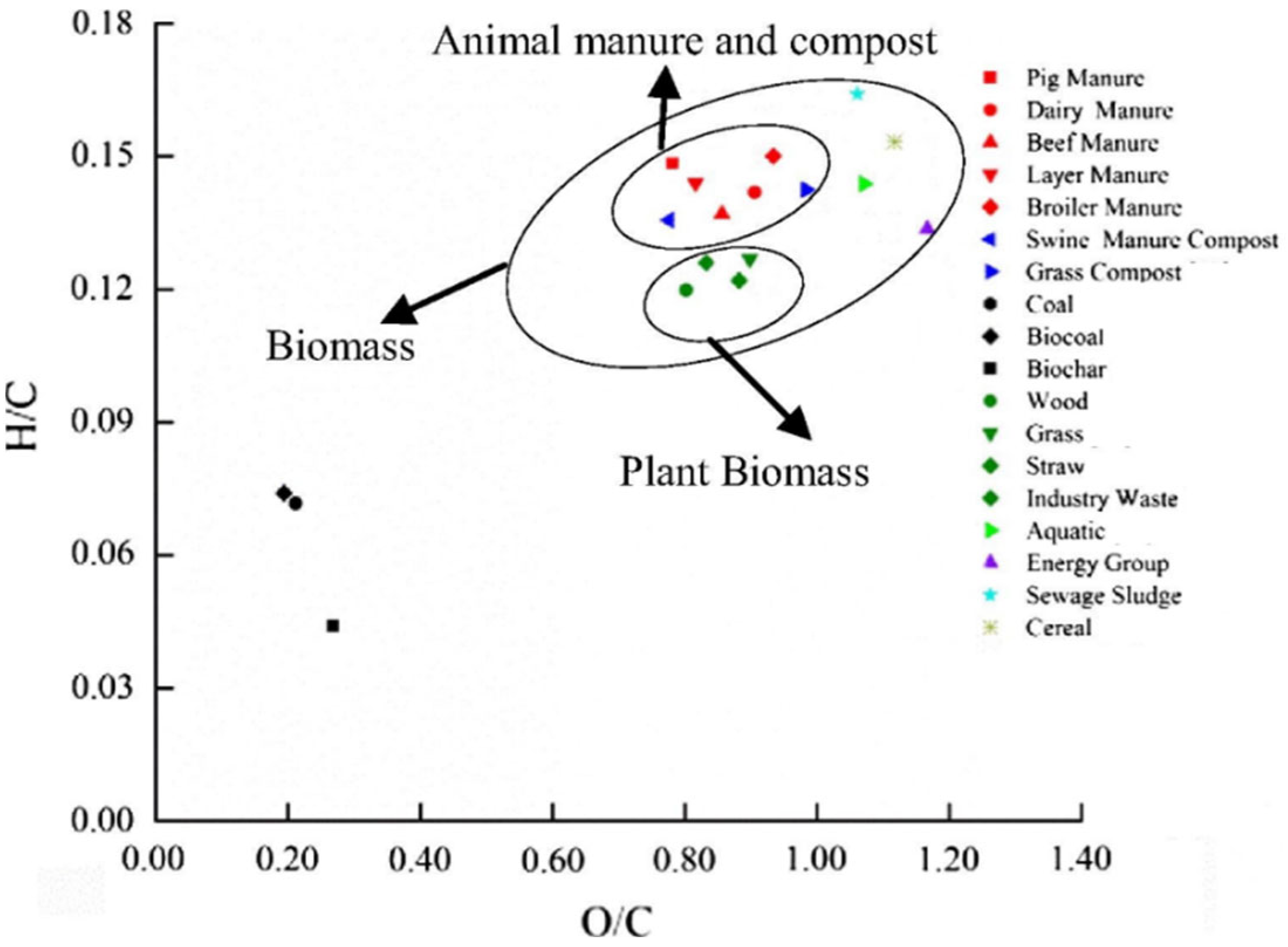
| Chicken manure | 65.6 | 12.9 | 21.7 | 35.6 | 1.5 | 35.5 | 4.6 | 13.2 | Hussein et al. [26] |
| Human feces | 50.2 | 25.1 | 14.8 | 43.5 | 0.7 | 30.1 | 6.4 | 19.3 | Yacob et al. [27] |
| Horse manure | 70.4 | 11 | 10.5 | 46.1 | 0.2 | 53.1 | 5.4 | 22.5 | Nitsche et al. [28] Chong et al. [29] |
| Pig manure | - | - | 22.3 | 40.4 | 0.4 | 50.6 | 6.3 | 13.7 | Wu et al. [30] |
| Cattle manure | - | - | 7.2 | 35.4 | - | 57.5 | 4.7 | 15.2 | Nazrul et al. [31] |
3. Thermochemical Conversion of Human and Animal Waste
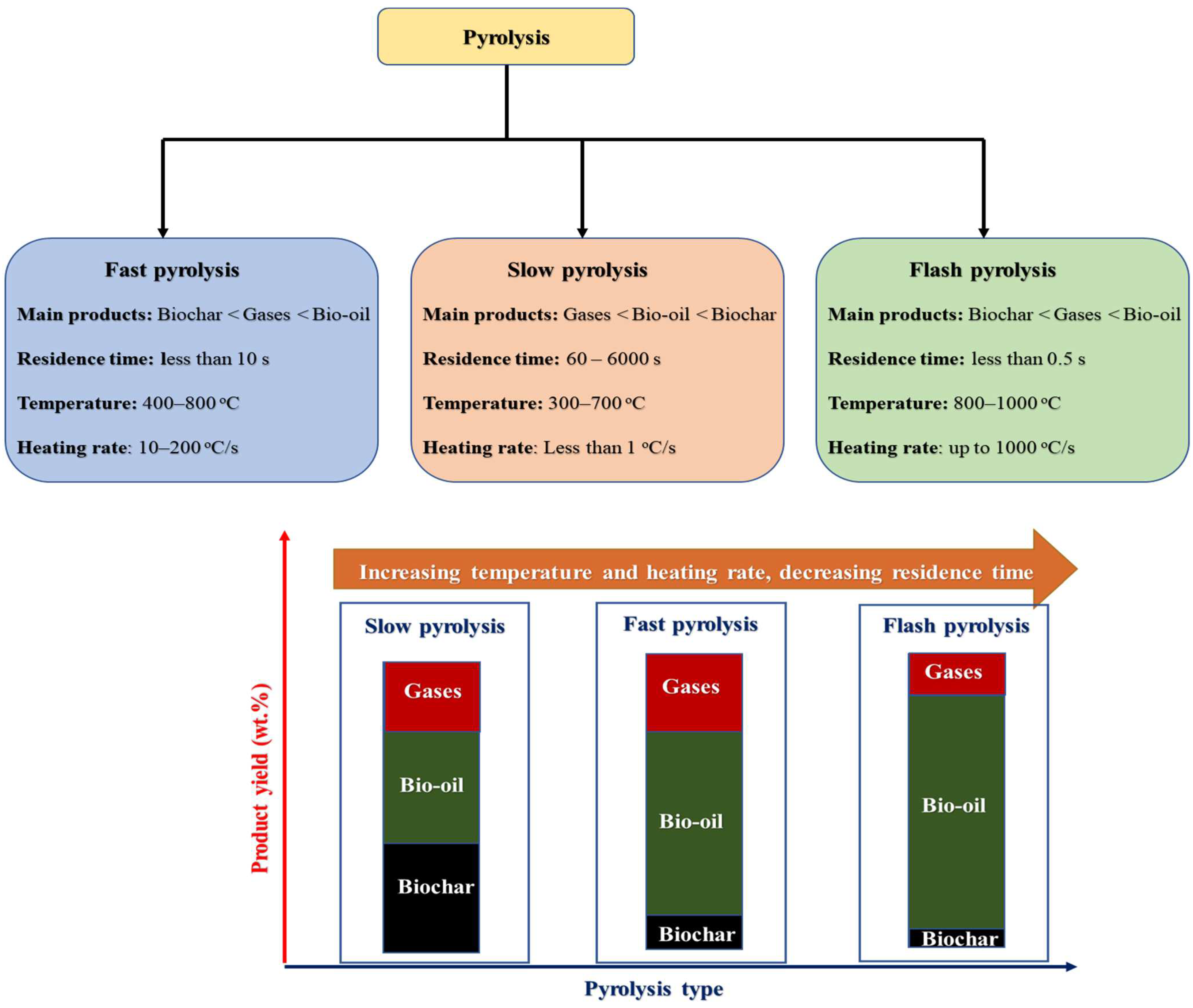
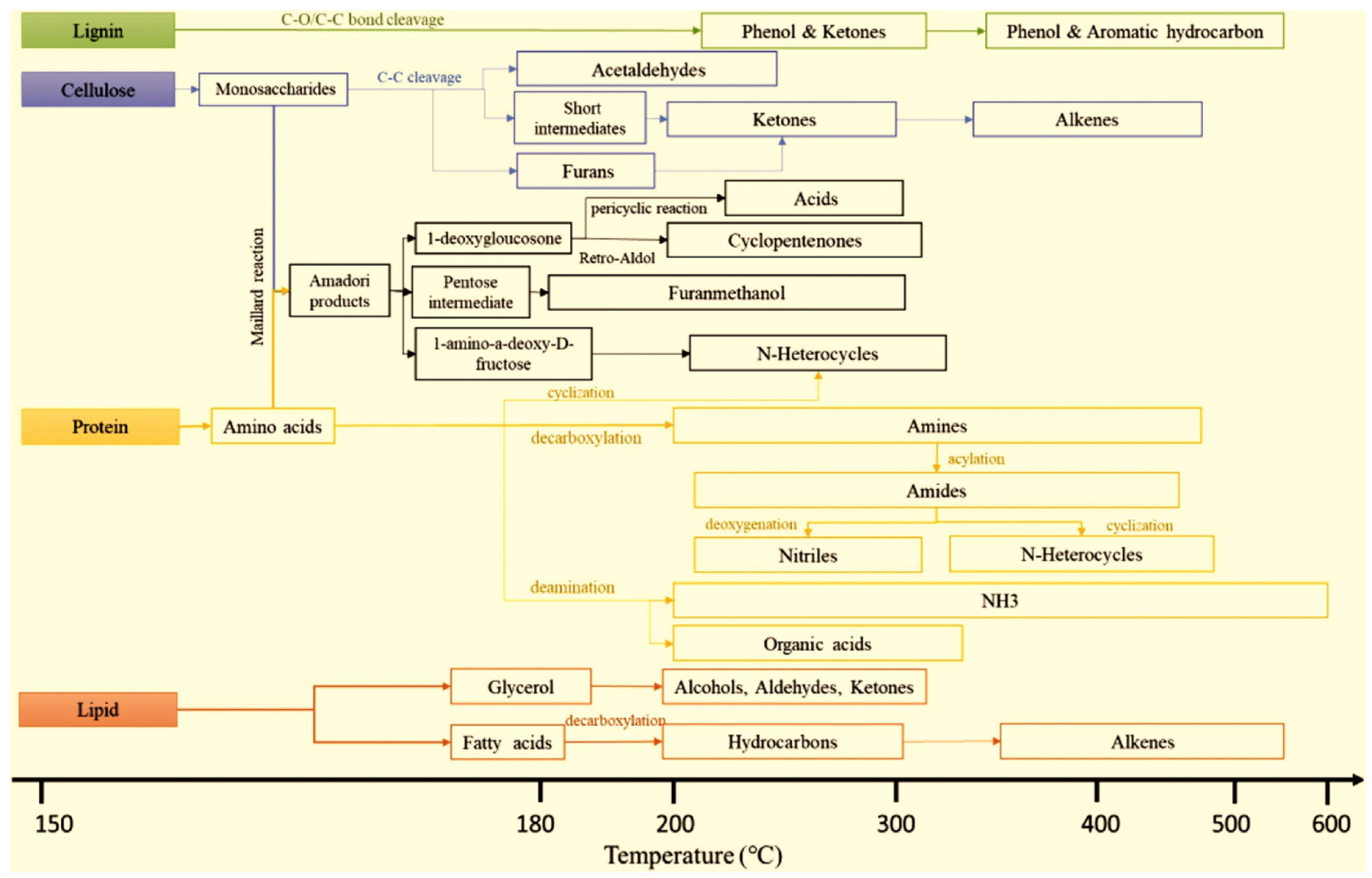
3.1. Pyrolysis
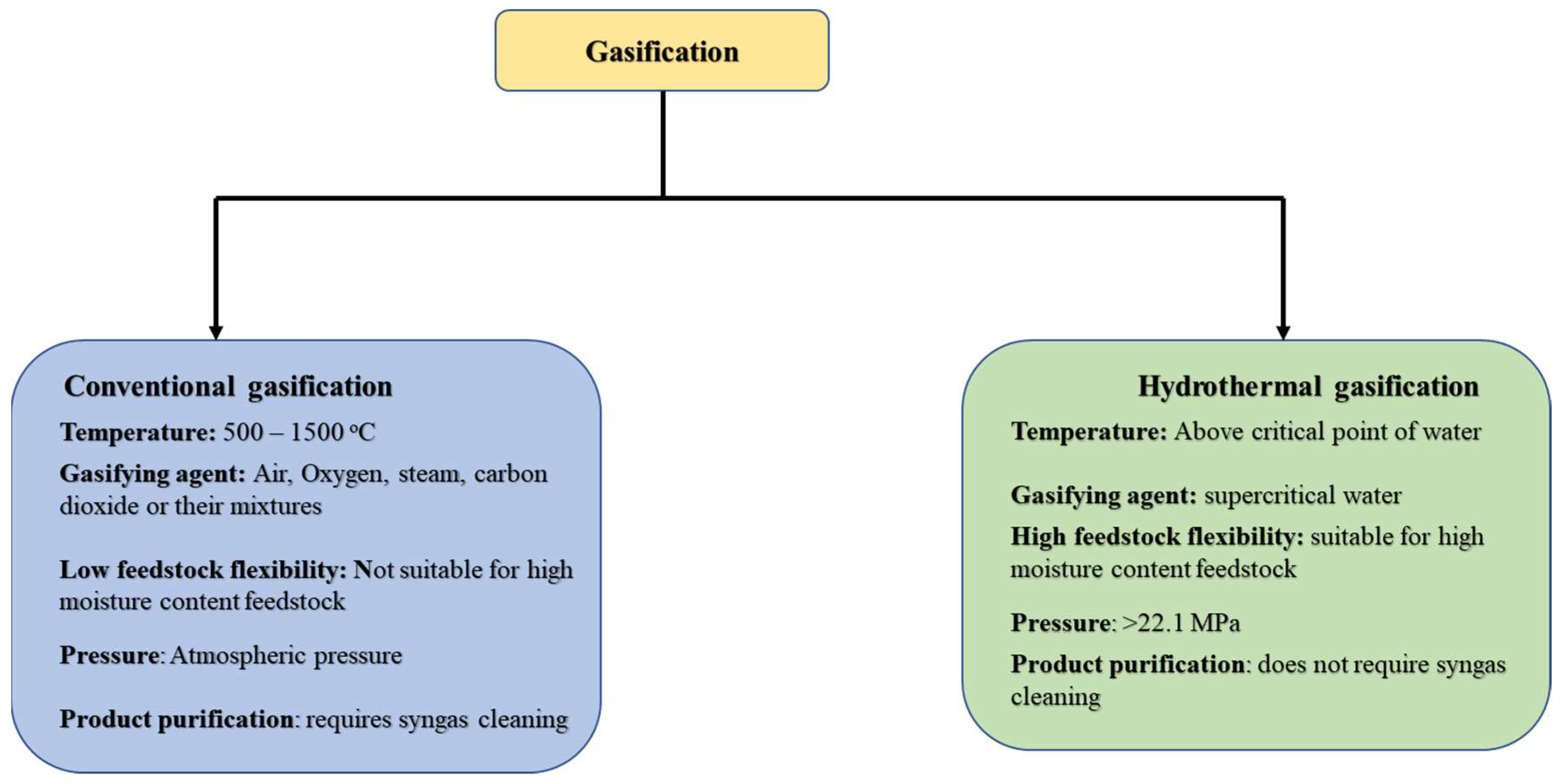
3.2. Gasification
| Type of gasification and key products | Key findings | References |
| Gasification type: Conventional fluidized bed gasification. Feedstock: Poultry litter Study focus: Parametric studies and process optimization. To investigate the effect of adding limestone (CaCO3), different gasifying agent and temperature on product gas yield and cold gas efficiency during gasification. |
|
Pandey et al. [79] |
| Gasification type: Conventional gasification Feedstock: Chicken manure Study focus: Co-gasification and catalytic studies. Study the synergistic effect of gasifying petroleum coke and chicken manure while the chicken manure is a catalyst. |
|
Liu et al. [69] |
| Gasification type: Conventional gasification Feedstock: Human faces Study focus: Thermodynamic and energy analysis with Aspen plus simulation. Explored the viability of human feces as a raw material for gasification. Estimation of the quantity of energy that could be produced from human feces. |
|
Onabanjo et al. [70] |
| Gasification type: Conventional gasification Feedstock: Chicken manure Study focus: Parametric studies The effect of gasifying media (air, steam, carbon dioxide, and nitrogen) and temperatures ranging from 600 oC to 1000 oC on the pyrolysis and gasification of chicken manure. |
|
Hussein et al. [71] |
| Gasification type: Conventional gasification Feedstock: Cattle manure Study focus: Parametric studies The viability of a two-step gasification route for producing hydrogen gas was examined by studying the temperature impact on biochar characteristics and product distribution. |
|
Xin et al. [80] |
| Gasification type: Hydrothermal gasification Feedstock: Horse manure Catalyst: Homogeneous alkali catalyst including NaOH, Na2CO3 and K2CO3. Study focus: Parametric studies Explored the effect of reaction temperature (400–600 °C), biomass-to-water ratio (1:5 and 1:10) and reaction time (15–45 min) at a pressure range of 23–25 MPa on product yield during horse manure gasification in supercritical water. |
|
Nanda et al. [81] |
| Gasification type: Conventional gasification Feedstock:Pig manure compost Heterogeneous catalyst: Ni/Al2O3, Ni-loaded brown coal char. Study focus: Catalytic effect of supported Ni catalyst during gasification and parametric studies. |
|
Xiao et al. [82] |
| Gasification type: Hydrothermal gasification Feedstock: Chicken manure Catalyst: K2CO3 Study focus: Parametric studies, kinetics, and reaction mechanism evaluation. |
|
Liu et al. [77] |
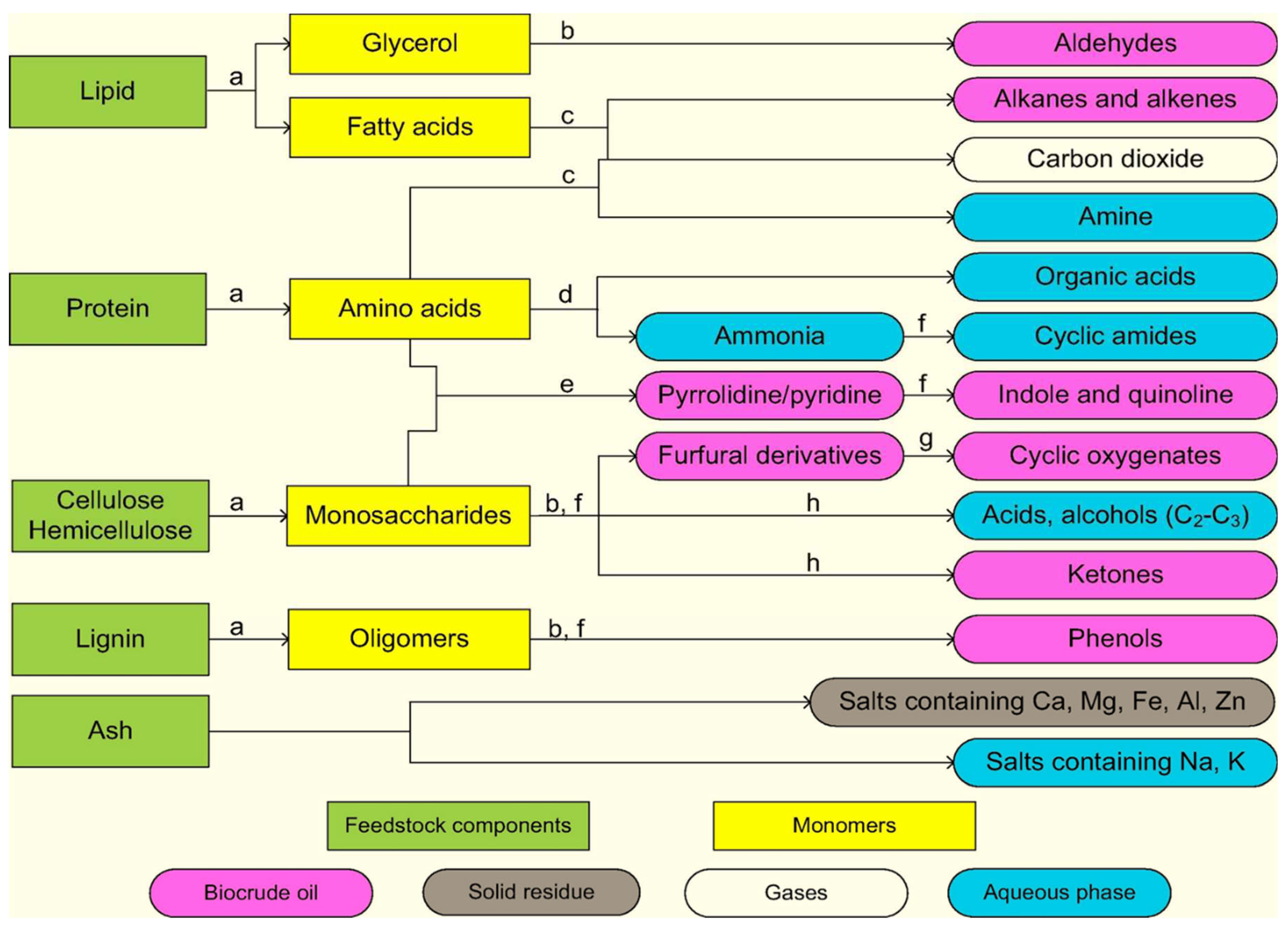
3.3. Liquefaction
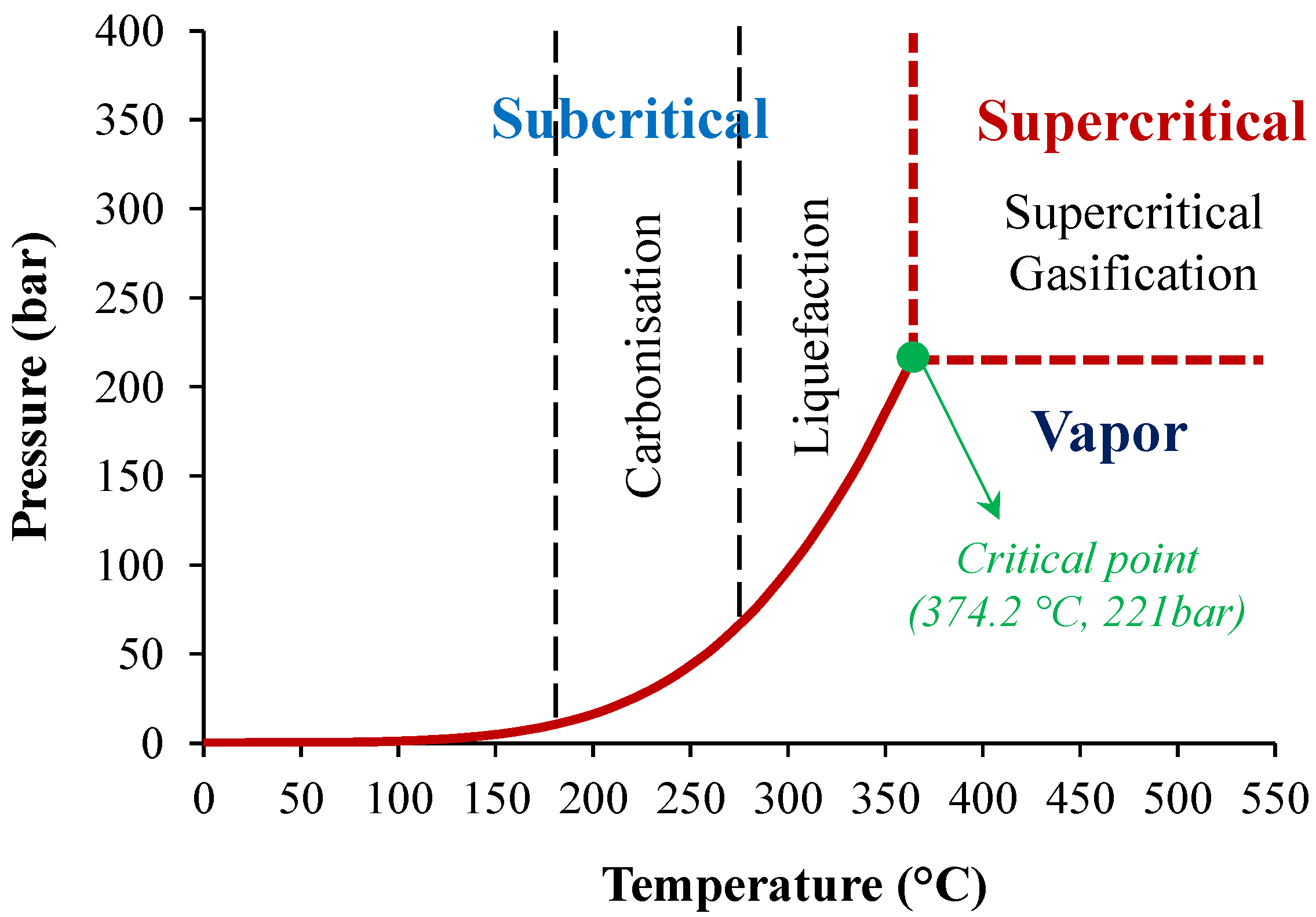
3.4. Hydrothermal Carbonization
| Aim of study | Key findings | Authors |
|---|---|---|
| To examine the effects of temperature, holding time, and catalyst on the product distribution of a Ni-Tm/TiO2-catalyzed liquefaction of human feces. |
|
Wang et al. [109] |
| The feasibility of using hydrothermal liquefaction to produce energy (biocrude oil), recover nutrients and metals from human feces at specific retention times, temperatures, and total solid contents. |
|
Lu et al. [11] |
| Nutrient recovery and energy production from the decomposition of animal manure, sewage sludge, and fish sludge with or without K2CO3 (catalyst) under subcritical (350 oC) and supercritical conditions (450 oC). |
|
Conti et al. [90] |
| Studied the influence of temperatures, solvent filling rates, and solid-liquid rates on the composition and yield of bio-oil derived from pig manure are examined. |
|
Wu et al. [92] |
| To compare the hydrothermal liquefaction of dairy manure, broiler manure, dairy manure, laying hen manure, swine manure, and beef manure. |
|
Li et al. [110] |
| Explored the possibilities of converting camel manure into bio-oil and upgrading to drop—in fuel via hydrothermal liquefaction. |
|
Alherbawi et al. [111] |
| Studied the synergistic effect during the co-liquefaction of corn cob and cattle manure. |
|
He et al. [112] |
4. Biological Conversion of Human and Animal Waste
4.1. Anaerobic Digestion
4.2. Fermentation
5. Nutrient and Fertilizer Recovery from Human and Animal Waste
5.1. Overview of Nutrient Recovery Technologies
5.1.1. Selective Adsorption
5.1.2. Struvite precipitation
5.1.3. Ammonia Stripping
5.1.4. Evaporation
6. Bibliometric Research Trends on Resource Recovery from Human and Animal Waste
7. Conclusions and Future Research Directions
- Developing effective and safe methods for processing human and animal waste is needed. There is a need for safe and effective methods for processing hazardous waste to optimize nutrients and resource recovery.
- It is imperative to examine the most effective ways to use the recovered resources. Once resources have been recovered from human or animal waste, there is a need to determine the most effective ways to use them, such as for agricultural purposes or as a source of energy, while considering the environmental impacts of different utilization methods.
- Understanding the potential impacts of using recovered resources: It is important to understand any potential negative impacts of using recovered resources on the environment on a large scale. Usually, a cradle-to-grave lifecycle assessment should be performed.
- Developing technologies for the on-site recovery of resources: There is a need for technologies that can be used to recover resources from feces on-site, such as at a wastewater treatment plant or in a portable system. Offsite or district waste processing facilities with improved heat optimization and materials recovery could also be a viable alternative.
References
- Krounbi, L.; Enders, A.; van Es, H.; Woolf, D.; van Herzen, B.; Lehmann, J. Biological and Thermochemical Conversion of Human Solid Waste to Soil Amendments. Waste Management 2019, 89, 366–378. [CrossRef]
- WHO Population Practising Open Defecation (%).
- Hunter, B.; Deshusses, M.A. Science of the Total Environment Resources Recovery from High-Strength Human Waste Anaerobic Digestate Using Simple Nitri Fi Cation and Denitri Fi Cation Fi Lters. 2019, 1–9. [CrossRef]
- Hart, C.A.; Umar, L.W. Diarrhoeal Disease.
- Hunter, B.; Deshusses, M.A. Science of the Total Environment Resources Recovery from High-Strength Human Waste Anaerobic Digestate Using Simple Nitri Fi Cation and Denitri Fi Cation Fi Lters. 2019, 1–9. [CrossRef]
- Szogi, A.A.; Vanotti, M.B.; Ro, K.S. Methods for Treatment of Animal Manures to Reduce Nutrient Pollution Prior to Soil Application. 2015, 47–56. [CrossRef]
- Orner, K.D.; Mihelcic, J.R. A Review of Sanitation Technologies to Achieve Multiple Sustainable Development Goals That Promote Resource Recovery. Environ Sci (Camb) 2018, 4, 16–32. [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. A Review on Subcritical and Supercritical Water Gasification of Biogenic, Polymeric and Petroleum Wastes to Hydrogen-Rich Synthesis Gas. Renewable and Sustainable Energy Reviews 2020, 119, 109546. [CrossRef]
- Okolie, J.A.; Epelle, E.I.; Tabat, M.E.; Orivri, U.; Amenaghawon, A.N.; Okoye, P.U.; Gunes, B. Waste Biomass Valorization for the Production of Biofuels and Value-Added Products: A Comprehensive Review of Thermochemical, Biological and Integrated Processes. Process Safety and Environmental Protection 2022, 159, 323–344. [CrossRef]
- Lu, J.; Zhang, J.; Zhu, Z.; Zhang, Y.; Zhao, Y.; Li, R.; Watson, J.; Li, B.; Liu, Z. Simultaneous Production of Biocrude Oil and Recovery of Nutrients and Metals from Human Feces via Hydrothermal Liquefaction. Energy Convers Manag 2017, 134, 340–346. [CrossRef]
- Lu, J.; Zhang, J.; Zhu, Z.; Zhang, Y.; Zhao, Y.; Li, R.; Watson, J.; Li, B.; Liu, Z. Simultaneous Production of Biocrude Oil and Recovery of Nutrients and Metals from Human Feces via Hydrothermal Liquefaction. Energy Convers Manag 2017, 134, 340–346. [CrossRef]
- Min, K.J.; Park, K.Y. Economic Feasibility of Phosphorus Recovery through Struvite from Liquid Anaerobic Digestate of Animal Waste. Environmental Science and Pollution Research 2021, 28, 40703–40714. [CrossRef]
- Onwosi, C.O.; Igbokwe, V.C.; Ezugworie, F.N. Decentralized Anaerobic Digestion Technology for Improved Management of Human Excreta in Nigeria. 2022, 137–163. [CrossRef]
- Ihoeghian, N.A.; Amenaghawon, A.N.; Ajieh, M.U.; Oshoma, C.E.; Ogofure, A.; Erhunmwunse, N.O.; Edosa, V.I.O.; Tongo, I.; Obuekwe, I.S.; Isagba, E.S.; et al. Anaerobic Co-Digestion of Cattle Rumen Content and Food Waste for Biogas Production: Establishment of Co-Digestion Ratios and Kinetic Studies. Bioresour Technol Rep 2022, 18, 101033. [CrossRef]
- Liu, H.; Li, X.; Zhang, Z.; Nghiem, L.D.; Wang, Q. Urine Pretreatment Significantly Promotes Methane Production in Anaerobic Waste Activated Sludge Digestion. Science of The Total Environment 2022, 853, 158684. [CrossRef]
- Oa, Y.L.S. Resource-Recovery Processes from Animal Waste as Best Available Technology. J Mater Cycles Waste Manag 2015. [CrossRef]
- Orner, K.D.; Mihelcic, J.R. A Review of Sanitation Technologies to Achieve Multiple Sustainable Development Goals That Promote Resource Recovery. Environ Sci (Camb) 2018, 4, 16–32. [CrossRef]
- Ranjan, P.; Daya, R.; Pandey, S.; Haynes, M.; Caitlin, P.; Luís, H.; Manuel, C.; Umapathi, R.; Mukherjee, S.; Panigrahi, S. Sustainable Valorisation of Animal Manures via Thermochemical Conversion Technologies : An Inclusive Review on Recent Trends. Waste Biomass Valorization 2022. [CrossRef]
- Olugasa, T.T.; Odesola, I.F.; Oyewola, M.O. Energy Production from Biogas: A Conceptual Review for Use in Nigeria. Renewable and Sustainable Energy Reviews 2014, 32, 770–776. [CrossRef]
- Adebayo, G.; Malomo, A. Sustainable Animal Animal Manure Manure Management Management Strategies Strategies and Practices. 2018. [CrossRef]
- Zseni, A. Human Excreta Management : Human Excreta as an Important Base of Sustainable Agriculture. 2015.
- Chen, W.T.C.H.H. Pyrolytic Conversion of Horse Manure into Biochar and Its Thermochemical and Physical Properties. Waste Biomass Valorization 2015. [CrossRef]
- Afolabi, I.C.; Emmanuel, E.I.; Gunes, B.; Okolie, J.A. Data-Driven Machine Learning Approach for Predicting the Higher Heating Value of Different Biomass Classes. SSRN Electronic Journal 2022, 4, 1227–1241. [CrossRef]
- Shen, X.; Huang, G.; Yang, Z.; Han, L. Compositional Characteristics and Energy Potential of Chinese Animal Manure by Type and as a Whole. Appl Energy 2015, 160, 108–119. [CrossRef]
- Shen, X.; Huang, G.; Yang, Z.; Han, L. Compositional Characteristics and Energy Potential of Chinese Animal Manure by Type and as a Whole. Appl Energy 2015, 160, 108–119. [CrossRef]
- Hussein, M.S.; Burra, K.G.; Amano, R.S.; Gupta, A.K. Temperature and Gasifying Media Effects on Chicken Manure Pyrolysis and Gasification. Fuel 2017, 202, 36–45. [CrossRef]
- Yacob, T.W.; Chip, R.; Linden, K.G.; Weimer, A.W. Pyrolysis of Human Feces : Gas Yield Analysis and Kinetic Modeling. Waste Management 2018, 79, 214–222. [CrossRef]
- Nitsche, M.; Hensgen, F.; Wachendorf, M. Energy Generation from Horse Husbandry Residues by Anaerobic Digestion, Combustion, and an Integrated Approach. 2017. [CrossRef]
- Chong, C.T.; Mong, G.R.; Ng, J.H.; Chong, W.W.F.; Ani, F.N.; Lam, S.S.; Ong, H.C. Pyrolysis Characteristics and Kinetic Studies of Horse Manure Using Thermogravimetric Analysis. Energy Convers Manag 2019, 180, 1260–1267. [CrossRef]
- Wu, Q.; Wang, H.; Zheng, X.; Liu, F.; Wang, A. Thermochemical Liquefaction of Pig Manure : Factors in Fl Uencing on Oil. 2020, 264. [CrossRef]
- Nazrul, M.; Park, I.J.; Korea, B.Á.E.Á.S. A Short Review on Hydrothermal Liquefaction of Livestock Manure and a Chance for Korea to Advance Swine Manure to Bio-Oil Technology. J Mater Cycles Waste Manag 2016. [CrossRef]
- Salana, S.; Banerji, T.; Kumar, A.; Singh, E.; Kumar, S. Resource Recovery-Oriented Sanitation and Sustainable Human Excreta Management. 2021, 109–136.
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The Characterization of Feces and Urine : A Review of the Literature to Inform Advanced Treatment Technology. 2015, 1827–1879. [CrossRef]
- Salana, S.; Banerji, T.; Kumar, A.; Singh, E.; Kumar, S. Resource Recovery-Oriented Sanitation and Sustainable Human Excreta Management. 2021, 109–136.
- Biswas, J.K.; Rana, S.; Meers, E. Bioregenerative Nutrient Recovery from Human Urine : Closing the Loop in Turning Waste into Wealth.
- Simha, P.; Ganesapillai, M. Ecological Sanitation and Nutrient Recovery from Human Urine: How Far Have We Come? A Review. Sustainable Environment Research 2017. [CrossRef]
- Simha, P.; Ganesapillai, M. Ecological Sanitation and Nutrient Recovery from Human Urine: How Far Have We Come? A Review. Sustainable Environment Research 2017. [CrossRef]
- Shanableh, A.; Abdallah, M.; Tayara, A.; Ghenai, C.; Kamil, M.; Inayat, A.; Shabib, A. Experimental Characterization and Assessment of Bio- and Thermo-Chemical Energy Potential of Dromedary Manure. Biomass Bioenergy 2021, 148, 106058. [CrossRef]
- Okolie, J.A.; Epelle, E.I.; Tabat, M.E.; Orivri, U.; Amenaghawon, A.N.; Okoye, P.U.; Gunes, B. Waste Biomass Valorization for the Production of Biofuels and Value-Added Products: A Comprehensive Review of Thermochemical, Biological and Integrated Processes. Process Safety and Environmental Protection 2022, 159, 323–344. [CrossRef]
- Perera, S.M.H.D.; Wickramasinghe, C.; Samarasiri, B.K.T.; Narayana, M. Modeling of Thermochemical Conversion of Waste Biomass—a Comprehensive Review. 2021, 32, 1481–1528. [CrossRef]
- Jha, S.; Nanda, S.; Acharya, B.; Dalai, A.K. A Review of Thermochemical Conversion of Waste Biomass to Biofuels. 2022, 1–23.
- Rowles, L.S.; Morgan, V.L.; Li, Y.; Zhang, X.; Watabe, S.; Stephen, T.; Lohman, H.A.C.; Desouza, D.; Hallowell, J.; Cusick, R.D.; et al. Financial Viability and Environmental Sustainability of Fecal Sludge Treatment with Pyrolysis Omni Processors. ACS Environmental Au 2022, 2, 455–466. [CrossRef]
- Selim, O.M.; Amano, R.S. Co-Pyrolysis of Chicken and Cow Manure. Journal of Energy Resources Technology, Transactions of the ASME 2021, 143. [CrossRef]
- Beik, F.; Williams, L.; Brown, T.; Wagland, S.T. Development and Prototype Testing of a Novel Small-Scale Pyrolysis System for the Treatment of Sanitary Sludge. Energy Convers Manag 2023, 277, 116627. [CrossRef]
- Wang, Q.; Ren, X.; Sun, Y.; Zhao, J.; Awasthi, M.K.; Liu, T.; Li, R.; Zhang, Z. Improvement of the Composition and Humification of Different Animal Manures by Black Soldier Fly Bioconversion. J Clean Prod 2021, 278, 123397. [CrossRef]
- Cantrell, K.; Ro, K.; Mahajan, D.; Anjom, M.; Hunt, P.G. Role of Thermochemical Conversion in Livestock Waste-to-Energy Treatments : Obstacles and Opportunities. 2007, 8918–8927.
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Bioresource Technology Impact of Pyrolysis Temperature and Manure Source on Physicochemical Characteristics of Biochar. Bioresour Technol 2012, 107, 419–428. [CrossRef]
- Lee, D.J.; Jung, S.; Jeong, K.H.; Lee, D.H.; Lee, S.H.; Park, Y.K.; Kwon, E.E. Catalytic Pyrolysis of Cow Manure over a Ni/SiO2 Catalyst Using CO2 as a Reaction Medium. Energy 2020, 195, 117077. [CrossRef]
- Krounbi, L.; Enders, A.; Es, H. Van; Woolf, D.; Herzen, B. Van; Lehmann, J. Biological and Thermochemical Conversion of Human Solid Waste to Soil Amendments. Waste Management 2019, 89, 366–378. [CrossRef]
- Yacob, T.W.; Chip, R.; Linden, K.G.; Weimer, A.W. Pyrolysis of Human Feces : Gas Yield Analysis and Kinetic Modeling. Waste Management 2018, 79, 214–222. [CrossRef]
- Mong, G.R.; Chong, C.T.; Ng, J.H.; Chong, W.W.F.; Ong, H.C.; Tran, M.V. Multivariate Optimisation Study and Life Cycle Assessment of Microwave-Induced Pyrolysis of Horse Manure for Waste Valorisation and Management. Energy 2021, 216, 119194. [CrossRef]
- Liu, X.; Li, Z.; Zhang, Y.; Feng, R.; Mahmood, I.B. Characterization of Human Manure-Derived Biochar and Energy-Balance Analysis of Slow Pyrolysis Process. Waste Management 2014, 34, 1619–1626. [CrossRef]
- Zhou, S.; Liang, H.; Han, L.; Huang, G.; Yang, Z. The Influence of Manure Feedstock, Slow Pyrolysis, and Hydrothermal Temperature on Manure Thermochemical and Combustion Properties. Waste Management 2019, 88, 85–95. [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A Review on Thermal and Catalytic Pyrolysis of Plastic Solid Waste (PSW). J Environ Manage 2017, 197, 177–198. [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A Review on Thermal and Catalytic Pyrolysis of Plastic Solid Waste (PSW). J Environ Manage 2017, 197, 177–198. [CrossRef]
- Bleuler, M.; Gold, M.; Strande, L.; Schönborn, A. Pyrolysis of Dry Toilet Substrate as a Means of Nutrient Recycling in Agricultural Systems: Potential Risks and Benefits. Waste Biomass Valorization 2021, 12, 4171–4183. [CrossRef]
- Koutcheiko, S. Preparation and Characterization of Activated Carbon Derived from the Thermo-Chemical Conversion of Chicken Manure. 2007, 98, 2459–2464. [CrossRef]
- Emrah, A.; Polat, R.; Ozbay, G. Engineering Science and Technology, an International Journal Pyrolysis of Goat Manure to Produce Bio-Oil. Engineering Science and Technology, an International Journal 2018. [CrossRef]
- Elkasabi, Y.; Mullen, C.A.; Pighinelli, A.L.M.T.; Boateng, A.A. Hydrodeoxygenation of Fast-Pyrolysis Bio-Oils from Various Feedstocks Using Carbon-Supported Catalysts. Fuel Processing Technology 2014, 123, 11–18. [CrossRef]
- Pandey, D.S.; Katsaros, G.; Lindfors, C.; Leahy, J.J.; Tassou, S.A. Fast Pyrolysis of Poultry Litter in a Bubbling Fluidised Bed Reactor: Energy and Nutrient Recovery. Sustainability (Switzerland) 2019, 11, 2533. [CrossRef]
- Mong, G.R.; Chong, C.T.; Ng, J.H.; Chong, W.W.F.; Lam, S.S.; Ong, H.C.; Ani, F.N. Microwave Pyrolysis for Valorisation of Horse Manure Biowaste. Energy Convers Manag 2020, 220, 113074. [CrossRef]
- Lee, D.J.; Jung, S.; Jang, Y.N.; Jo, G.; Park, S.H.; Jeon, Y.J.; Park, Y.K.; Kwon, E.E. Offering a New Option to Valorize Hen Manure by CO2-Assisted Catalytic Pyrolysis over Biochar and Metal Catalysts. Journal of CO2 Utilization 2020, 42, 101344. [CrossRef]
- He, S.; Cao, C.; Wang, J.; Yang, J.; Cheng, Z.; Yan, B.; Pan, Y.; Chen, G. Pyrolysis Study on Cattle Manure: From Conventional Analytical Method to Online Study of Pyrolysis Photoionization Time-of-Flight Mass Spectrometry. J Anal Appl Pyrolysis 2020, 151, 104916. [CrossRef]
- Ren, C.; Guo, S.; Wang, Y.; Liu, S.; Du, M.; Chen, Y.; Guo, L. Thermodynamic Analysis and Optimization of Auto-Thermal Supercritical Water Gasification Polygeneration System of Pig Manure. Chemical Engineering Journal 2022, 427, 131938. [CrossRef]
- Onabanjo, T.; Patchigolla, K.; Wagland, S.T.; Fidalgo, B.; Kolios, A.; McAdam, E.; Parker, A.; Williams, L.; Tyrrel, S.; Cartmell, E. Energy Recovery from Human Faeces via Gasification: A Thermodynamic Equilibrium Modelling Approach. Energy Convers Manag 2016, 118, 364–376. [CrossRef]
- Parthasarathy, P.; Fernandez, A.; Al-Ansari, T.; Mackey, H.R.; Rodriguez, R.; McKay, G. Thermal Degradation Characteristics and Gasification Kinetics of Camel Manure Using Thermogravimetric Analysis. J Environ Manage 2021, 287, 112345. [CrossRef]
- Sobamowo, G.M.; Ojolo, S.J. Techno-Economic Analysis of Biomass Energy Utilization through Gasification Technology for Sustainable Energy Production and Economic Development in Nigeria. Journal of Energy 2018, 2018, 1–16. [CrossRef]
- Jia, J.; Shu, L.; Zang, G.; Xu, L.; Abudula, A.; Ge, K. Energy Analysis and Techno-Economic Assessment of a Co-Gasification of Woody Biomass and Animal Manure, Solid Oxide Fuel Cells and Micro Gas Turbine Hybrid System. Energy 2018, 149, 750–761. [CrossRef]
- Liu, M.; Li, F.; Liu, H.; Wang, C. Synergistic Effect on Co-Gasification of Chicken Manure and Petroleum Coke : An Investigation of Sustainable Waste Management. Chemical Engineering Journal 2020, 128008. [CrossRef]
- Onabanjo, T.; Patchigolla, K.; Wagland, S.T.; Fidalgo, B.; Kolios, A.; McAdam, E.; Parker, A.; Williams, L.; Tyrrel, S.; Cartmell, E. Energy Recovery from Human Faeces via Gasification: A Thermodynamic Equilibrium Modelling Approach. Energy Convers Manag 2016, 118, 364–376. [CrossRef]
- Hussein, M.S.; Burra, K.G.; Amano, R.S.; Gupta, A.K. Temperature and Gasifying Media Effects on Chicken Manure Pyrolysis and Gasification. Fuel 2017, 202, 36–45. [CrossRef]
- Xin, Y.; Cao, H.; Yuan, Q.; Wang, D. Two-Step Gasification of Cattle Manure for Hydrogen-Rich Gas Production : Effect of Biochar Preparation Temperature and Gasification Temperature. Waste Management 2017. [CrossRef]
- Nanda, S.; Dalai, A.K.; Gökalp, I.; Kozinski, J.A. Valorization of Horse Manure through Catalytic Supercritical Water Gasification. Waste Management 2016, 52, 147–158. [CrossRef]
- Tavasoli, A.; Aslan, M.; Salimi, M.; Balou, S.; Pirbazari, S.M.; Hashemi, H.; Kohansal, K. Influence of the Blend Nickel/Porous Hydrothermal Carbon and Cattle Manure Hydrochar Catalyst on the Hydrothermal Gasification of Cattle Manure for H2 Production. Energy Convers Manag 2018, 173, 15–28. [CrossRef]
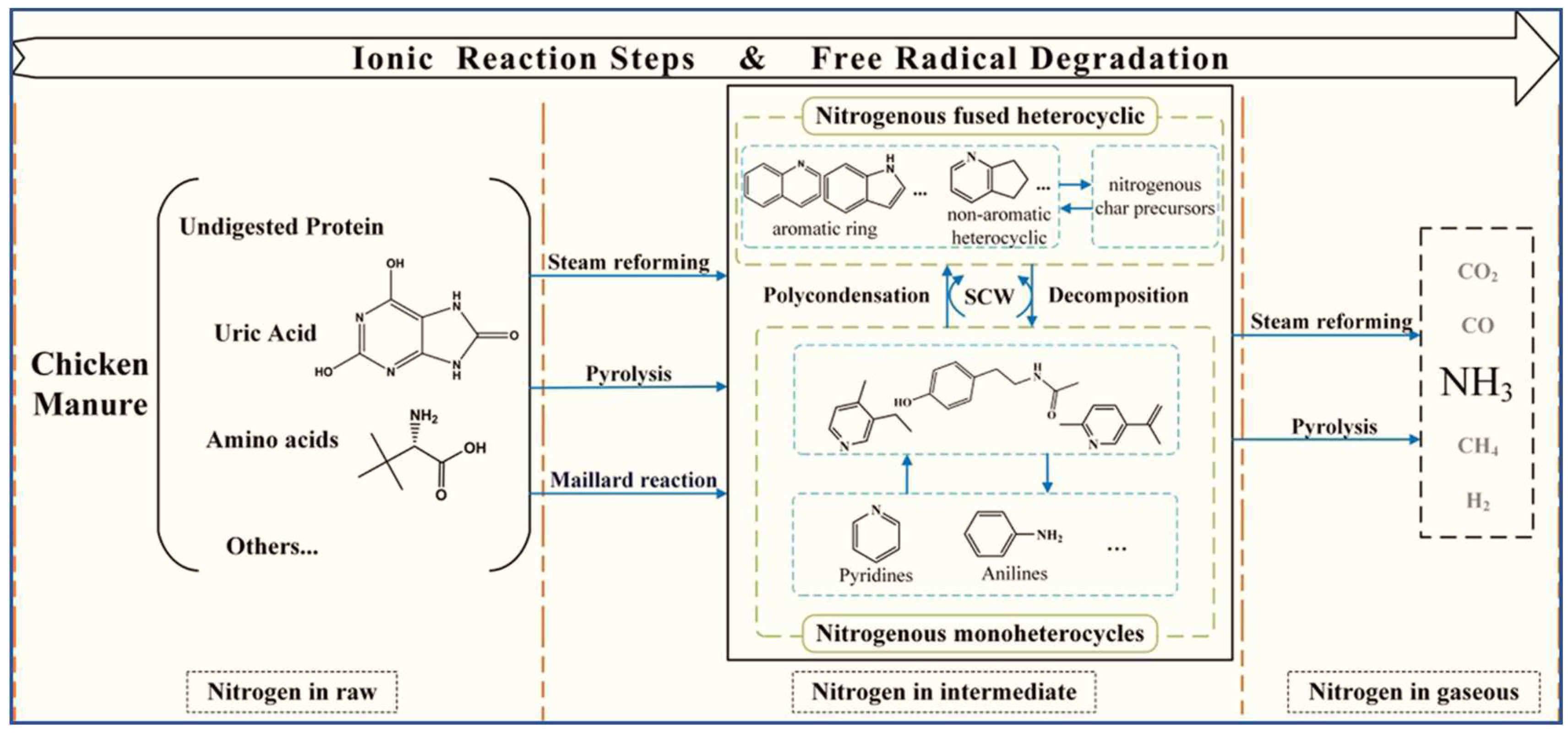
- Liu, S.; Cao, W.; Wang, Y.; Wei, W.; Li, L.; Jin, H.; Guo, L. Characteristics and Mechanisms of Nitrogen Transformation during Chicken Manure Gasification in Supercritical Water. Waste Management 2022, 153, 240–248. [CrossRef]
- de Blasio, C. Notions of Biomass Gasification. Green Energy and Technology 2019, 307–334.
- Liu, S.; Cao, W.; Wang, Y.; Wei, W.; Li, L.; Jin, H.; Guo, L. Characteristics and Mechanisms of Nitrogen Transformation during Chicken Manure Gasification in Supercritical Water. Waste Management 2022, 153, 240–248. [CrossRef]
- Salierno, G.; Marinelli, F.; Likozar, B.; Ghavami, N.; de Blasio, C. Supercritical Water Gasification of Glycerol: Continuous Reactor Kinetics and Transport Phenomena Modeling. Int J Heat Mass Transf 2022, 183, 122200. [CrossRef]
- Pandey, D.S.; Kwapinska, M.; Barea, A.G.; Horvat, A.; Fryda, L.E.; Rabou, L.P.L.M.; Leahy, J.J.; Kwapinski, W. Poultry Litter Gasification in a Fluidized Bed Reactor : Effects of Gasifying Agent and Limestone Addition Poultry Litter Gasification in a Fluidized Bed Reactor : Effects of Gasifying Agent and Limestone Addition. 2016. [CrossRef]
- Xin, Y.; Cao, H.; Yuan, Q.; Wang, D. Two-Step Gasification of Cattle Manure for Hydrogen-Rich Gas Production : Effect of Biochar Preparation Temperature and Gasification Temperature. Waste Management 2017. [CrossRef]
- Nanda, S.; Dalai, A.K.; Gökalp, I.; Kozinski, J.A. Valorization of Horse Manure through Catalytic Supercritical Water Gasification. Waste Management 2016, 52, 147–158. [CrossRef]
- Xiao, X.; Cao, J.; Meng, X.; Le, D.D.; Li, L.; Ogawa, Y.; Sato, K.; Takarada, T. Synthesis Gas Production from Catalytic Gasification of Waste Biomass Using Nickel-Loaded Brown Coal Char. Fuel 2013, 103, 135–140. [CrossRef]
- Nazrul, M.; Park, I.J.; Korea, B.Á.E.Á.S. A Short Review on Hydrothermal Liquefaction of Livestock Manure and a Chance for Korea to Advance Swine Manure to Bio-Oil Technology. J Mater Cycles Waste Manag 2016. [CrossRef]
- Perera, S.M.H.D.; Wickramasinghe, C.; Samarasiri, B.K.T.; Narayana, M. Modeling of Thermochemical Conversion of Waste Biomass—a Comprehensive Review. 2021, 32, 1481–1528. [CrossRef]
- Güleç, F.; Riesco, L.M.G.; Williams, O.; Kostas, E.T.; Samson, A.; Lester, E. Hydrothermal Conversion of Different Lignocellulosic Biomass Feedstocks—Effect of the Process Conditions on Hydrochar Structures. Fuel 2021, 302, 121166. [CrossRef]
- Castello, D.; Pedersen, T.H. Continuous Hydrothermal Liquefaction of Biomass : A Critical Review. 2018. [CrossRef]
- Lu, J.; Li, H.; Zhang, Y.; Liu, Z. Nitrogen Migration and Transformation during Hydrothermal Liquefaction of Livestock Manures. ACS Sustain Chem Eng 2018, 6, 13570–13578. [CrossRef]
- Güleç, F.; Riesco, L.M.G.; Williams, O.; Kostas, E.T.; Samson, A.; Lester, E. Hydrothermal Conversion of Different Lignocellulosic Biomass Feedstocks—Effect of the Process Conditions on Hydrochar Structures. Fuel 2021, 302, 121166. [CrossRef]
- Conti, F.; Toor, S.S.; Pedersen, T.H.; Seehar, T.H.; Nielsen, A.H. Valorization of Animal and Human Wastes through Hydrothermal Liquefaction for Biocrude Production and Simultaneous Recovery of Nutrients. Energy Convers Manag 2020, 216, 112925. [CrossRef]
- Conti, F.; Toor, S.S.; Pedersen, T.H.; Seehar, T.H.; Nielsen, A.H. Valorization of Animal and Human Wastes through Hydrothermal Liquefaction for Biocrude Production and Simultaneous Recovery of Nutrients. Energy Convers Manag 2020, 216, 112925. [CrossRef]
- Li, H.; Lu, J.; Zhang, Y.; Liu, Z. Hydrothermal Liquefaction of Typical Livestock Manures in China: Biocrude Oil Production and Migration of Heavy Metals. J Anal Appl Pyrolysis 2018, 135, 133–140. [CrossRef]
- Wu, Q.; Wang, H.; Zheng, X.; Liu, F.; Wang, A. Thermochemical Liquefaction of Pig Manure : Factors in Fl Uencing on Oil. 2020, 264. [CrossRef]
- Wei, L.; Sevilla, M.; Fuertes, A.B.; Mokaya, R.; Yushin, G. Hydrothermal Carbonization of Abundant Renewable Natural Organic Chemicals for High-Performance Supercapacitor Electrodes. Adv Energy Mater 2011, 1, 356–361. [CrossRef]
- Álvarez-Murillo, A.; Sabio, E.; Ledesma, B.; Román, S.; González-García, C.M. Generation of Biofuel from Hydrothermal Carbonization of Cellulose. Kinetics Modelling. Energy 2016, 94, 600–608. [CrossRef]
- Kambo, H.S.; Dutta, A. Strength, Storage, and Combustion Characteristics of Densified Lignocellulosic Biomass Produced via Torrefaction and Hydrothermal Carbonization. Appl Energy 2014, 135, 182–191. [CrossRef]
- Subedi, R.; Kammann, C.; Pelisseti, S.; Sacco, D.; Grignani, C.; Monaco, S. Recycling of Organic Residues for Agriculture: From Waste Management to Ecosystem Services. Available online: https://scholar.google.com/scholar_lookup?title=Recycling%20of%20organic%20residues%20for%20agriculture%3A%20from%20waste%20management%20to%20ecosystem%20services&author=R.%20Subedi&publication_year=2013 (accessed on 18 January 2023).
- Guangzhi, Y.; Jinyu, Y.; Yuhua, Y.; Zhihong, T.; DengGuang, Y.; Junhe, Y. Preparation and CO2 Adsorption Properties of Porous Carbon from Camphor Leaves by Hydrothermal Carbonization and Sequential Potassium Hydroxide Activation. RSC Adv 2017, 7, 4152–4160. [CrossRef]
- Liu, Z.; Zhang, F.; Hoekman, S.K.; Liu, T.; Gai, C.; Peng, N. Homogeneously Dispersed Zerovalent Iron Nanoparticles Supported on Hydrochar-Derived Porous Carbon: Simple, in Situ Synthesis and Use for Dechlorination of PCBs. ACS Sustain Chem Eng 2016, 4, 3261–3267.
- Fan, F.; Xing, X.; Shi, S.; Zhang, X.; Zhang, X.; Li, Y.; Xing, Y. Combustion Characteristic and Kinetics Analysis of Hydrochars. Nongye Gongcheng Xuebao/Transactions of the Chinese Society of Agricultural Engineering 2016, 32, 219–224.
- Hao, W.; Björkman, E.; Lilliestråle, M.; Hedin, N. Activated Carbons for Water Treatment Prepared by Phosphoric Acid Activation of Hydrothermally Treated Beer Waste. Ind Eng Chem Res 2014, 53, 15389–15397.
- Titirici, M.M.; Antonietti, M.; Baccile, N. Hydrothermal Carbon from Biomass: A Comparison of the Local Structure from Poly- to Monosaccharides and Pentoses/Hexoses. Green Chemistry 2008, 10, 1204–1212. [CrossRef]
- Yahav Spitzer, R.; Mau, V.; Gross, A. Using Hydrothermal Carbonization for Sustainable Treatment and Reuse of Human Excreta. J Clean Prod 2018, 205, 955–963. [CrossRef]
- Afolabi, O.O.D.; Sohail, M.; Thomas, C.P.L. Microwave Hydrothermal Carbonization of Human Biowastes. Waste Biomass Valorization 2015, 6, 147–157.
- Danso-Boateng, E.; Holdich, R.G.; Martin, S.J.; Shama, G.; Wheatley, A.D. Process Energetics for the Hydrothermal Carbonisation of Human Faecal Wastes. Energy Convers Manag 2015, 105, 1115–1124. [CrossRef]
- Czerwińska, K.; Śliz, M.; Wilk, M. Hydrothermal Carbonization Process: Fundamentals, Main Parameter Characteristics and Possible Applications Including an Effective Method of SARS-CoV-2 Mitigation in Sewage Sludge. A Review. Renewable and Sustainable Energy Reviews 2022, 154, 111873. [CrossRef]
- Jang, E.S.; Ryu, D.Y.; Kim, D. Hydrothermal Carbonization Improves the Quality of Biochar Derived from Livestock Manure by Removing Inorganic Matter. Chemosphere 2022, 305, 135391. [CrossRef]
- Qaramaleki, S. v.; Villamil, J.A.; Mohedano, A.F.; Coronella, C.J. Factors Affecting Solubilization of Phosphorus and Nitrogen through Hydrothermal Carbonization of Animal Manure. ACS Sustain Chem Eng 2020, 8, 12462–12470.
- Ghavami, N.; Özdenkçi, K.; Chianese, S.; Musmarra, D.; de Blasio, C. Process Simulation of Hydrothermal Carbonization of Digestate from Energetic Perspectives in Aspen Plus. Energy Convers Manag 2022, 270, 116215. [CrossRef]
- Wang, W.; Yang, L.; Yin, Z.; Kong, S.; Han, W.; Zhang, J. Catalytic Liquefaction of Human Feces over Ni-Tm/TiO2 Catalyst and the Influence of Operating Conditions on Products. Energy Convers Manag 2018, 157, 239–245. [CrossRef]
- Li, H.; Lu, J.; Zhang, Y.; Liu, Z. Hydrothermal Liquefaction of Typical Livestock Manures in China: Biocrude Oil Production and Migration of Heavy Metals. J Anal Appl Pyrolysis 2018, 135, 133–140. [CrossRef]
- Alherbawi, M.; Parthasarathy, P.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Potential of Drop-in Biofuel Production from Camel Manure by Hydrothermal Liquefaction and Biocrude Upgrading: A Qatar Case Study. Energy 2021, 232, 121027. [CrossRef]
- He, S.; Wang, J.; Cheng, Z.; Dong, H.; Yan, B.; Chen, G. Synergetic Effect and Primary Reaction Network of Corn Cob and Cattle Manure in Single and Mixed Hydrothermal Liquefaction. J Anal Appl Pyrolysis 2021, 155, 105076. [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic Co-Digestion of Animal Manures and Lignocellulosic Residues as a Potent Approach for Sustainable Biogas Production. Renewable and Sustainable Energy Reviews 2017, 79, 308–322. [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic Co-Digestion of Animal Manures and Lignocellulosic Residues as a Potent Approach for Sustainable Biogas Production. Renewable and Sustainable Energy Reviews 2017, 79, 308–322. [CrossRef]
- Lee, S.Y.; Sankaran, R.; Chew, K.W.; Tan, C.H.; Krishnamoorthy, R. BMC Energy Waste to Bioenergy : A Review on the Recent Conversion Technologies. 2019, 1–22.
- Zhang, Y.; Cui, Y.; Chen, P.; Liu, S. Gasi Fi Cation Technologies and Their Energy Potentials; Elsevier B.V., 2019; ISBN 9780444642004.
- Lee, S.Y.; Sankaran, R.; Chew, K.W.; Tan, C.H.; Krishnamoorthy, R. BMC Energy Waste to Bioenergy : A Review on the Recent Conversion Technologies. 2019, 1–22.
- Karki, R.; Chuenchart, W.; Surendra, K.C.; Shrestha, S.; Raskin, L.; Sung, S.; Hashimoto, A.; Kumar Khanal, S. Anaerobic Co-Digestion: Current Status and Perspectives. Bioresour Technol 2021, 330, 125001. [CrossRef]
- Dalke, R.; Demro, D.; Khalid, Y.; Wu, H.; Urgun-Demirtas, M. Current Status of Anaerobic Digestion of Food Waste in the United States. Renewable and Sustainable Energy Reviews 2021, 151, 111554. [CrossRef]
- Ilo, O.P.; Simatele, M.D.; Nkomo, S.L.; Mkhize, N.M.; Prabhu, N.G. Methodological Approaches to Optimising Anaerobic Digestion of Water Hyacinth for Energy Efficiency in South Africa. Sustainability 2021, 13, 6746. [CrossRef]
- Ripoll, V.; Agabo-García, C.; Solera, R.; Perez, M. Anaerobic Digestion of Slaughterhouse Waste in Batch and Anaerobic Sequential Batch Reactors. Biomass Convers Biorefin 2022, 1–12. [CrossRef]
- Rajendran, K.; Mahapatra, D.; Venkatraman, A.V.; Muthuswamy, S.; Pugazhendhi, A. Advancing Anaerobic Digestion through Two-Stage Processes: Current Developments and Future Trends. Renewable and Sustainable Energy Reviews 2020, 123, 109746. [CrossRef]
- Donkor, K.O.; Gottumukkala, L.D.; Lin, R.; Murphy, J.D. A Perspective on the Combination of Alkali Pre-Treatment with Bioaugmentation to Improve Biogas Production from Lignocellulose Biomass. Bioresour Technol 2022, 351, 126950. [CrossRef]
- Awosusi, A.; Sethunya, V.; Matambo, T. Synergistic Effect of Anaerobic Co-Digestion of South African Food Waste with Cow Manure: Role of Low Density-Polyethylene in Process Modulation. Mater Today Proc 2021, 38, 793–803. [CrossRef]
- Ihoeghian, N.A.; Amenaghawon, A.N.; Ajieh, M.U.; Oshoma, C.E.; Ogofure, A.; Erhunmwunse, N.O.; Edosa, V.I.O.; Tongo, I.; Obuekwe, I.S.; Isagba, E.S.; et al. Anaerobic Co-Digestion of Cattle Rumen Content and Food Waste for Biogas Production: Establishment of Co-Digestion Ratios and Kinetic Studies. Bioresour Technol Rep 2022, 18, 101033. [CrossRef]
- Ma, G.; Ndegwa, P.; Harrison, J.H.; Chen, Y. Methane Yields during Anaerobic Co-Digestion of Animal Manure with Other Feedstocks: A Meta-Analysis. Science of the Total Environment 2020, 728, 138224. [CrossRef]
- Ma, G.; Ndegwa, P.; Harrison, J.H.; Chen, Y. Methane Yields during Anaerobic Co-Digestion of Animal Manure with Other Feedstocks: A Meta-Analysis. Science of the Total Environment 2020, 728, 138224. [CrossRef]
- Adjama, I.; Sarfo, N.; Derkyi, A.; Uba, F.; Akolgo, G.A.; Opuko, R. Anaerobic Co-Digestion of Human Feces with Rice Straw for Biogas Production : A Case Study in Sunyani. 2022, 2022.
- Kaur, H.; Kommalapati, R.R. Optimizing Anaerobic Co-Digestion of Goat Manure and Cotton Gin Trash Using Biochemical Methane Potential (BMP) Test and Mathematical Modeling. SN Appl Sci 2021, 3, 1–14.
- Alfa, M.I.; Owamah, H.I.; Onokwai, A.O.; Gopikumar, S.; Oyebisi, S.O.; Kumar, S.S.; Bajar, S.; Samuel, O.D.; Ilabor, S.C. Evaluation of Biogas Yield and Kinetics from the Anaerobic Co-Digestion of Cow Dung and Horse Dung: A Strategy for Sustainable Management of Livestock Manure. Energy, Ecology and Environment 2020 6:5 2020, 6, 425–434. [CrossRef]
- Hamzah, A.F.A.; Hamzah, M.H.; Man, H.C.; Jamali, N.S.; Siajam, S.I.; Show, P.L. Biogas Production Through Mono- and Co-Digestion of Pineapple Waste and Cow Dung at Different Substrate Ratios. Bioenergy Res 2022, 1, 1–12. [CrossRef]
- Arifan, F.; Sumardiono, S. EFFECTIVENESS ANALYSIS OF ANAEROBIC DIGESTION METHOD IN MAKING BIOGAS FROM ANIMAL MANURE AND. 2021, 16, 84–94.
- Arifan, F.; Sumardiono, S. EFFECTIVENESS ANALYSIS OF ANAEROBIC DIGESTION METHOD IN MAKING BIOGAS FROM ANIMAL MANURE AND. 2021, 16, 84–94.
- Silwadi, M.; Mousa, H.; AL-Hajji, B.Y.; AL-Wahaibi, S.S.; AL-Harrasi, Z.Z. Enhancing Biogas Production by Anaerobic Digestion of Animal Manure. Int J Green Energy 2022. [CrossRef]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A Comprehensive Review on Food Waste Anaerobic Digestion: Research Updates and Tendencies. Bioresour Technol 2018, 247, 1069–1076. [CrossRef]
- Xu, H.; Chang, J.; Wang, H.; Liu, Y.; Zhang, X.; Liang, P.; Huang, X. Enhancing Direct Interspecies Electron Transfer in Syntrophic-Methanogenic Associations with (Semi)Conductive Iron Oxides: Effects and Mechanisms. Science of The Total Environment 2019, 695, 133876. [CrossRef]
- Lv, N.; Zhao, L.; Wang, R.; Ning, J.; Pan, X.; Li, C.; Cai, G.; Zhu, G. Novel Strategy for Relieving Acid Accumulation by Enriching Syntrophic Associations of Syntrophic Fatty Acid-Oxidation Bacteria and H2/Formate-Scavenging Methanogens in Anaerobic Digestion. Bioresour Technol 2020, 313, 123702. [CrossRef]
- Wang, P.; Peng, H.; Adhikari, S.; Higgins, B.; Roy, P.; Dai, W.; Shi, X. Enhancement of Biogas Production from Wastewater Sludge via Anaerobic Digestion Assisted with Biochar Amendment. Bioresour Technol 2020, 309, 123368. [CrossRef]
- Zhang, L.; Tsui, T.H.; Loh, K.C.; Dai, Y.; Tong, Y.W. Effects of Plastics on Reactor Performance and Microbial Communities during Acidogenic Fermentation of Food Waste for Production of Volatile Fatty Acids. Bioresour Technol 2021, 337, 125481. [CrossRef]
- Arif, S.; Liaquat, R.; Adil, M. Applications of Materials as Additives in Anaerobic Digestion Technology. Renewable and Sustainable Energy Reviews 2018, 97, 354–366. [CrossRef]
- Sitthi, S.; Hatamoto, M.; Watari, T.; Yamaguchi, T. Enhancing Anaerobic Syntrophic Propionate Degradation Using Modified Polyvinyl Alcohol Gel Beads. Heliyon 2020, 6, e05665. [CrossRef]
- Zhao, J.; Li, Y.; Euverink, G.J.W. Effect of Bioaugmentation Combined with Activated Charcoal on the Mitigation of Volatile Fatty Acids Inhibition during Anaerobic Digestion. Chemical Engineering Journal 2022, 428, 131015. [CrossRef]
- Lu, X.; Wang, H.; Ma, F.; Zhao, G.; Wang, S. Improved Process Performance of the Acidification Phase in a Two-Stage Anaerobic Digestion of Complex Organic Waste: Effects of an Iron Oxide-Zeolite Additive. Bioresour Technol 2018, 262, 169–176. [CrossRef]
- Zhang, J.; Cui, Y.; Zhang, T.; Hu, Q.; Wah Tong, Y.; He, Y.; Dai, Y.; Wang, C.H.; Peng, Y. Food Waste Treating by Biochar-Assisted High-Solid Anaerobic Digestion Coupled with Steam Gasification: Enhanced Bioenergy Generation and Porous Biochar Production. Bioresour Technol 2021, 331, 125051. [CrossRef]
- Adjama, I.; Sarfo, N.; Derkyi, A.; Uba, F.; Akolgo, G.A.; Opuko, R. Anaerobic Co-Digestion of Human Feces with Rice Straw for Biogas Production : A Case Study in Sunyani. 2022, 2022.
- Eduok, S.; John, O.; Ita, B.; Inyang, E.; Coulon, F. Enhanced Biogas Production From Anaerobic Co-Digestion of Lignocellulosic Biomass and Poultry Feces Using Source Separated Human Urine as Buffering Agent. 2018, 6, 1–9. [CrossRef]
- Pan, J.; Ma, J.; Liu, X.; Zhai, L.; Ouyang, X.; Liu, H. Effects of Different Types of Biochar on the Anaerobic Digestion of Chicken Manure. Bioresour Technol 2019, 275, 258–265. [CrossRef]
- Kizito, S.; Wu, S.; Kipkemoi Kirui, W.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of Slow Pyrolyzed Wood and Rice Husks Biochar for Adsorption of Ammonium Nitrogen from Piggery Manure Anaerobic Digestate Slurry. Science of The Total Environment 2015, 505, 102–112. [CrossRef]
- Recebli, Z.; Selimli, S.; Ozkaymak, M.; Gonc, O. BIOGAS PRODUCTION FROM ANIMAL MANURE. 2015, 10, 722–729.
- Zlateva, P.; Terziev, A.; Yordanov, K. Study of Regime Parameters of the Fermenter in the Production of Biogas from Animal Liquid Waste Materials. 2021, 02010.
- Andreev, N.; Ronteltap, M.; Boincean, B.; Wernli, M.; Zubcov, E.; Bagrin, N.; Borodin, N.; Lens, P.N.L. Lactic Acid Fermentation of Human Urine to Improve Its Fertilizing Value and Reduce Odour Emissions. J Environ Manage 2017, 198, 63–69. [CrossRef]
- Andreev, N.; Ronteltap, M.; Boincean, B.; Lens, P.N.L. Lactic Acid Fermentation of Human Excreta for Agricultural Application. J Environ Manage 2018, 206, 890–900. [CrossRef]
- Lee, D.J.; Yim, J.H.; Jung, S.; Jang, M.S.; Jeong, G.T.; Jeong, K.H.; Lee, D.H.; Kim, J.K.; Tsang, Y.F.; Jeon, Y.J.; et al. Valorization of Animal Manure: A Case Study of Bioethanol Production from Horse Manure. Chemical Engineering Journal 2021, 403, 126345. [CrossRef]
- Eduok, S.; John, O.; Ita, B.; Inyang, E.; Coulon, F. Enhanced Biogas Production From Anaerobic Co-Digestion of Lignocellulosic Biomass and Poultry Feces Using Source Separated Human Urine as Buffering Agent. 2018, 6, 1–9. [CrossRef]
- Jimenez, J.; Bott, C.; Love, N.; Bratby, J. Source Separation of Urine as an Alternative Solution to Nutrient Management in Biological Nutrient Removal Treatment Plants. Water Environment Research 2015, 87, 2120–2129. [CrossRef]
- Koffi Konan, F.; Kouame, M.K.; Author, C.; Koffi Félix, K.; Nazo Edith, K.-K.; Théophile, G.; Kotchi Yves, B.; Kouamé Martin, K.; Yao Francis, K.; Kablan, T. Improving Anaerobic Biodigestion of Manioc Wastewater with Human Urine as Co-Substrate. Int J Innov Appl Stud 2013, 2, 335–343.
- Twizerimana, M.; M’arimi, M.; Nganyi, E.O.; Marimi, M.; Bura, X. Biogas Production from Co-Digestion of Cotton Yarn Waste and Human Urine. Article in Journal of Energy Research and Reviews 2020. [CrossRef]
- Giwa, A.S.; Xu, H.; Chang, F.; Wu, J.; Li, Y.; Ali, N.; Ding, S.; Wang, K. Effect of Biochar on Reactor Performance and Methane Generation during the Anaerobic Digestion of Food Waste Treatment at Long-Run Operations. J Environ Chem Eng 2019, 7, 103067. [CrossRef]
- Wienchol, P.; Szle, A.; Ditaranto, M. Waste-to-Energy Technology Integrated with Carbon Capture e Challenges and Opportunities. 2020, 198. [CrossRef]
- Liu, X.Y.; Wang, J.J.; Nie, J.M.; Wu, N.; Yang, F.; Yang, R.J. Biogas Production of Chicken Manure by Two-Stage Fermentation Process. 2018, 01048, 5–8.
- Wienchol, P.; Szle, A.; Ditaranto, M. Waste-to-Energy Technology Integrated with Carbon Capture e Challenges and Opportunities. 2020, 198. [CrossRef]
- Zlateva, P.; Terziev, A.; Yordanov, K. Study of Regime Parameters of the Fermenter in the Production of Biogas from Animal Liquid Waste Materials. 2021, 02010.
- Recebli, Z.; Selimli, S.; Ozkaymak, M.; Gonc, O. BIOGAS PRODUCTION FROM ANIMAL MANURE. 2015, 10, 722–729.
- Zhang, A.; He, J.; Shen, Y.; Xu, X.; Liu, Y.; Li, Y.; Wu, S.; Xue, G.; Li, X.; Makinia, J. Enhanced Degradation of Glucocorticoids, a Potential COVID-19 Remedy, by Co-Fermentation of Waste Activated Sludge and Animal Manure: The Role of Manure Type and Degradation Mechanism. Environ Res 2021, 201, 111488. [CrossRef]
- Andreev, N.; Ronteltap, M.; Boincean, B.; Wernli, M.; Zubcov, E.; Bagrin, N.; Borodin, N.; Lens, P.N.L. Lactic Acid Fermentation of Human Urine to Improve Its Fertilizing Value and Reduce Odour Emissions. J Environ Manage 2017, 198, 63–69. [CrossRef]
- Lee, D.J.; Yim, J.H.; Jung, S.; Jang, M.S.; Jeong, G.T.; Jeong, K.H.; Lee, D.H.; Kim, J.K.; Tsang, Y.F.; Jeon, Y.J.; et al. Valorization of Animal Manure: A Case Study of Bioethanol Production from Horse Manure. Chemical Engineering Journal 2021, 403, 126345. [CrossRef]
- Svetlitchnyi, V.A.; Kensch, O.; Falkenhan, D.A.; Korseska, S.G.; Lippert, N.; Prinz, M.; Sassi, J.; Schickor, A.; Curvers, S. Single-Step Ethanol Production from Lignocellulose Using Novel Extremely Thermophilic Bacteria. Biotechnol Biofuels 2013, 6, 1–15. [CrossRef]
- Doreswamy, R.; Deb, R.; De, S. Potential Use of Piggery Excreta as a Viable Source of Bioethanol Production. J Clean Prod 2021, 316, 128246. [CrossRef]
- Yan, Q.; Liu, X.; Wang, Y.; Li, H.; Li, Z.; Zhou, L.; Qu, Y.; Li, Z.; Bao, X. Cow Manure as a Lignocellulosic Substrate for Fungal Cellulase Expression and Bioethanol Production. AMB Express 2018, 8, 1–12. [CrossRef]
- Dadrasnia, A.; Y, E.H.; Ahmed, M.; Lamkaddam, I.U.; Mora, M.; Pons, S.; Llenas, L.; Williams, P.M.; Oatley-radcliffe, D.L. Sustainable Nutrient Recovery from Animal Manure : A Review of Current Best Practice Technology and the Potential for Freeze Concentration. 2021, 315. [CrossRef]
- Ruiz, D.; San Miguel, G.; Corona, B.; Gaitero, A.; Domínguez, A. Environmental and Economic Analysis of Power Generation in a Thermophilic Biogas Plant. Science of the Total Environment 2018, 633, 1418–1428. [CrossRef]
- Queiroz, R.F.A.I.D.S.; Oliveira, J.E.R.M.R.C. Enriched Animal Manure as a Source of Phosphorus in Sustainable Agriculture. International Journal of Recycling of Organic Waste in Agriculture 2019, 8, 203–210. [CrossRef]
- Martin, T.M.P.; Esculier, F.; Levavasseur, F.; Houot, S.; Martin, T.M.P.; Esculier, F.; Levavasseur, F.; Houot, S. Technology Human Urine-Based Fertilizers : A Review. Crit Rev Environ Sci Technol 2020, 0, 1–47. [CrossRef]
- Sugihara, R. Reuse of Human Excreta in Developing Countries : Agricultural Fertilization Optimization. 2020, 22, 58–64. [CrossRef]
- Moya, B.; Parker, A.; Sakrabani, R. Challenges to the Use of Fertilisers Derived from Human Excreta : The Case of Vegetable Exports from Kenya to Europe and Influence of Certification Systems. Food Policy 2019, 85, 72–78. [CrossRef]
- Ajiboye, T.O.; Ogunbiyi, O.D.; Omotola, E.O.; Adeyemi, W.J.; Agboola, O.O.; Onwudiwe, D.C. Results in Engineering Urine : Useless or Useful “ Waste ” ? 2022, 16. [CrossRef]
- Pathy, A.; Ray, J.; Paramasivan, B. Challenges and Opportunities of Nutrient Recovery from Human Urine Using Biochar for Fertilizer Applications. J Clean Prod 2021, 304, 127019. [CrossRef]
- Akpan-idiok, A.U.; Asukwo, I.; Ikpi, E. Resources, Conservation and Recycling The Use of Human Urine as an Organic Fertilizer in the Production of Okra (Abelmoschus Esculentus) in South Eastern Nigeria. Resour Conserv Recycl 2012, 62, 14–20. [CrossRef]
- Pathy, A.; Ray, J.; Paramasivan, B. Challenges and Opportunities of Nutrient Recovery from Human Urine Using Biochar for Fertilizer Applications. J Clean Prod 2021, 304, 127019. [CrossRef]
- Ajiboye, T.O.; Ogunbiyi, O.D.; Omotola, E.O.; Adeyemi, W.J.; Agboola, O.O.; Onwudiwe, D.C. Results in Engineering Urine : Useless or Useful “ Waste ” ? 2022, 16. [CrossRef]
- Vaneeckhaute, C.; Lebuf, V.; Michels, E.; Belia, E.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Nutrient Recovery from Digestate: Systematic Technology Review and Product Classification. Waste Biomass Valorization 2017, 8, 21–40. [CrossRef]
- Patel, A.; Mungray, A.A.; Mungray, A.K. Technologies for the Recovery of Nutrients, Water and Energy from Human Urine: A Review. Chemosphere 2020, 259, 127372. [CrossRef]
- Harder, R.; Wielemaker, R.; Larsen, T.A.; Zeeman, G.; Harder, R.; Wielemaker, R.; Larsen, T.A.; Zeeman, G. Technology Recycling Nutrients Contained in Human Excreta to Agriculture : Pathways, Processes, and Products. Crit Rev Environ Sci Technol 2019, 0, 1–49. [CrossRef]
- Ahmed, N.; Shim, S.; Won, S.; Ra, C. Struvite Recovered from Various Types of Wastewaters: Characteristics, Soil Leaching Behaviour, and Plant Growth. Land Degrad Dev 2018, 29, 2864–2879. [CrossRef]
- Ahmed, N.; Shim, S.; Won, S.; Ra, C. Struvite Recovered from Various Types of Wastewaters: Characteristics, Soil Leaching Behaviour, and Plant Growth. Land Degrad Dev 2018, 29, 2864–2879. [CrossRef]
- Papurello, D.; Boschetti, A.; Silvestri, S.; Khomenko, I. Real-Time Monitoring of Removal of Trace Compounds with PTR-MS: Biochar Experimental Investigation. Renew Energy 2018. [CrossRef]
- Wei, S.P.; Rossum, F. Van; Pol, G.J. Van De; Winkler, M.H. AC SC. ECSN 2018. [CrossRef]
- Masrura, S.U.; Dissanayake, P.; Sun, Y.; Ok, Y.S.; Tsang, D.C.W.; Khan, E. Technology Sustainable Use of Biochar for Resource Recovery and Pharmaceutical Removal from Human Urine : A Critical Review. Crit Rev Environ Sci Technol 2020, 0, 1–33. [CrossRef]
- Zhang, J.; She, Q.; Chang, V.W.C.; Tang, C.Y.; Webster, R.D. Mining Nutrients (N, K, P) from Urban Source-Separated Urine by Forward Osmosis Dewatering. 2014.
- Martin, T.M.P.; Esculier, F.; Levavasseur, F.; Houot, S.; Martin, T.M.P.; Esculier, F.; Levavasseur, F.; Houot, S. Technology Human Urine-Based Fertilizers : A Review. Crit Rev Environ Sci Technol 2020, 0, 1–47. [CrossRef]
- Azuara, M.; Kersten, S.R.A.; Kootstra, A.M.J. Recycling Phosphorus by Fast Pyrolysis of Pig Manure : Concentration and Extraction of Phosphorus Combined with Formation of Value-Added Pyrolysis Products. Biomass Bioenergy 2012, 49, 171–180. [CrossRef]
- Chen Chapter 1—Introduction; Metallurgical Industry Press, 2017;
- Zhang, T.; Bowers, K.E.; Harrison, J.H.; Chen, S. Releasing Phosphorus from Calcium for Struvite Fertilizer Production from Anaerobically Digested Dairy Effluent. 2010, 82. [CrossRef]
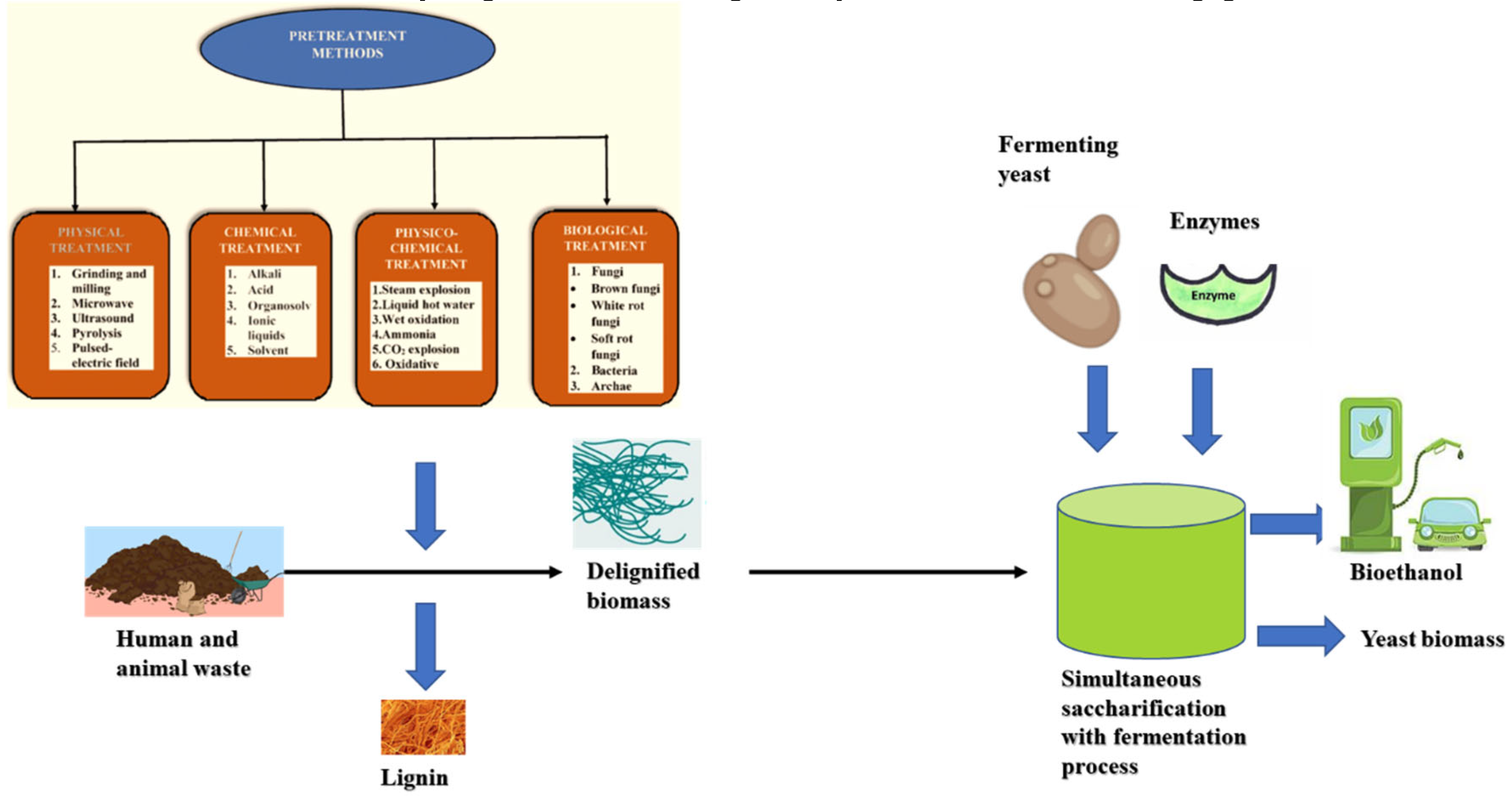
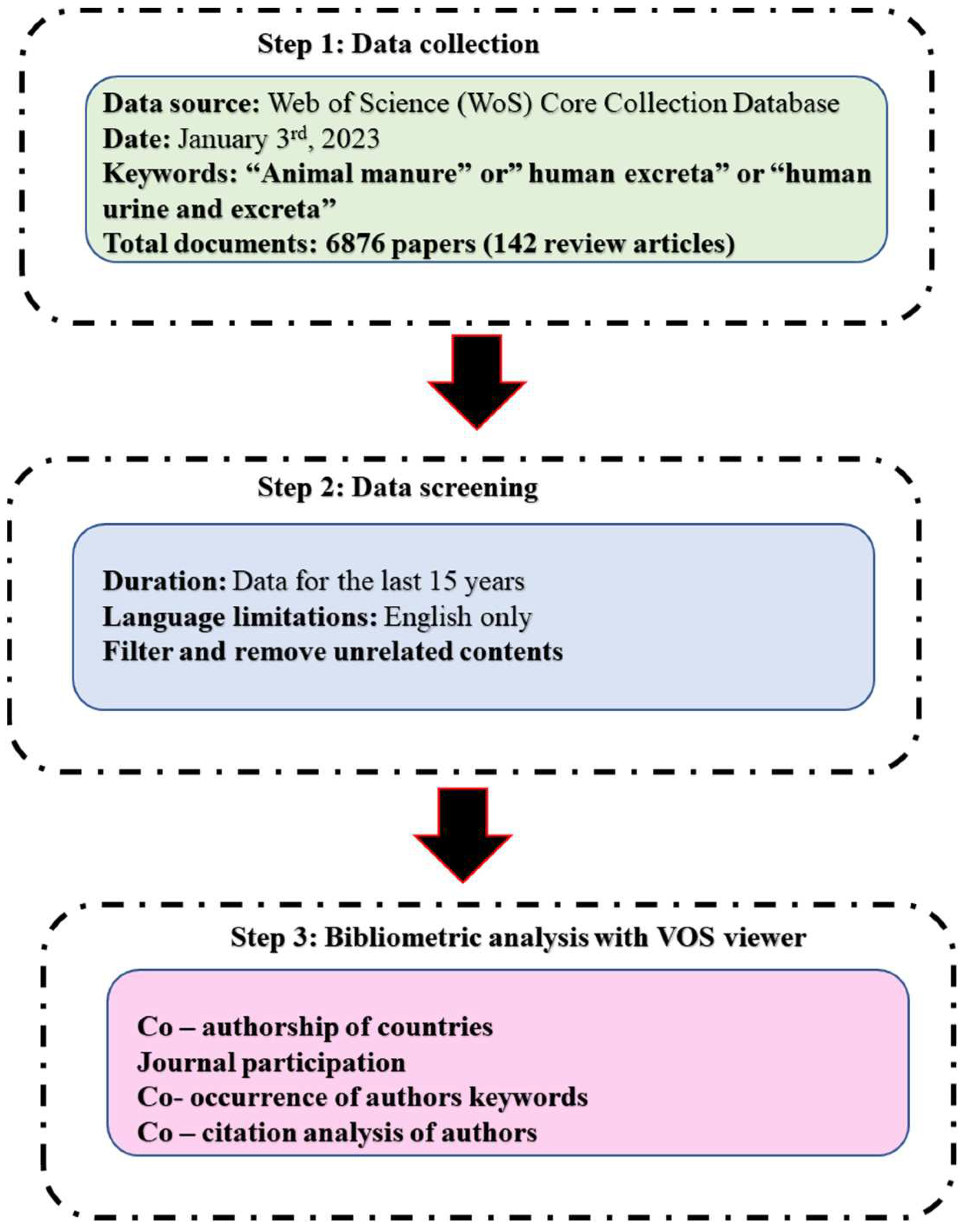
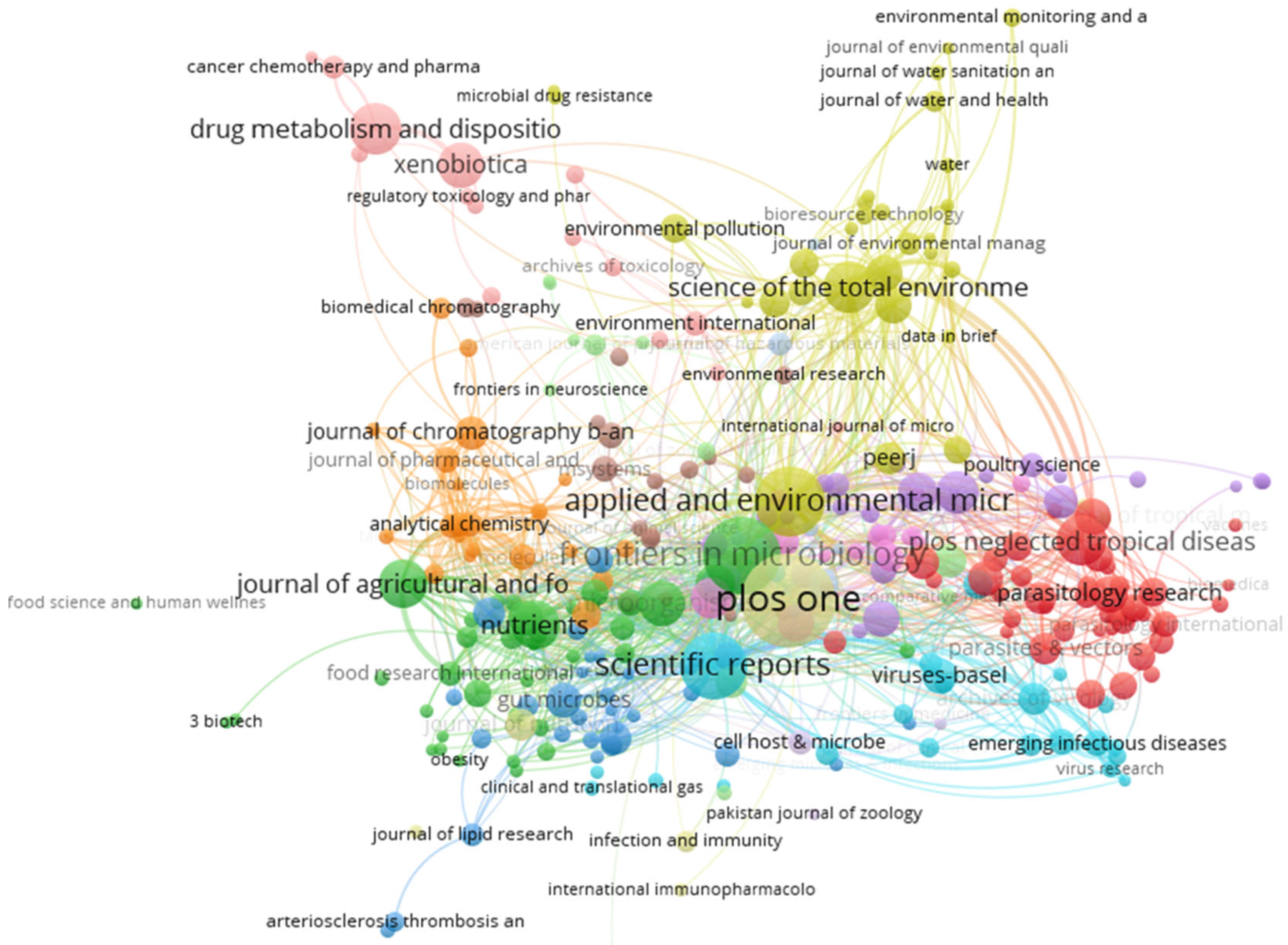
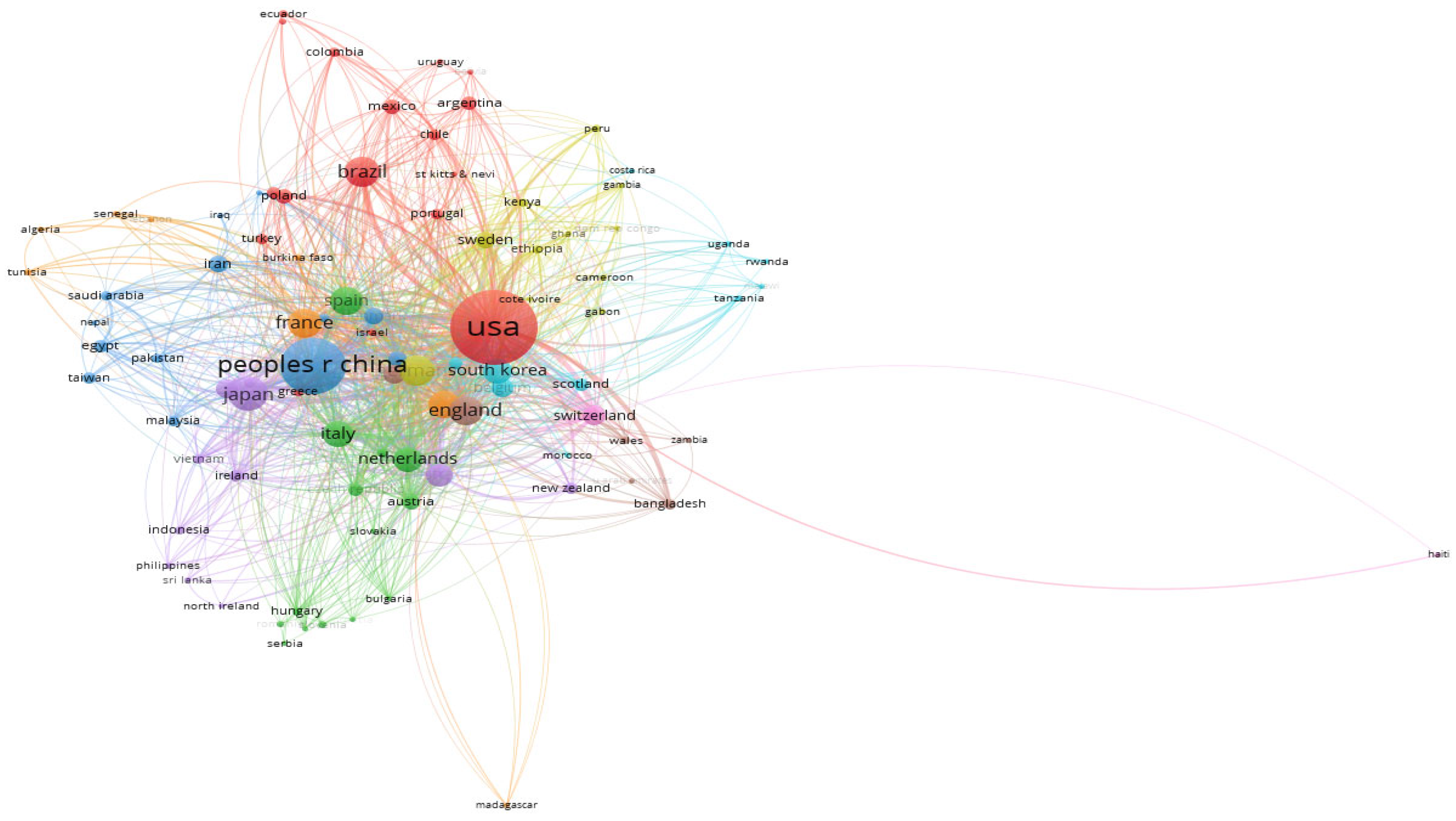
| Review title | The main issue addressed | References |
|---|---|---|
| A review of sanitation technologies to achieve multiple sustainable development goals that promote resource recovery |
|
Orner and Mihelcic [17] |
| Resource-recovery processes from animal waste as best available technology |
|
Lee and Oa. [16] |
| Sustainable Valorization of Animal Manures via Thermochemical Conversion Technologies: An Inclusive Review on Recent Trends |
|
Rout et al. [18] |
| Energy production from biogas: A conceptual review for use in Nigeria |
|
Olugasa et al. [19] |
| Sustainable Animal Manure Management Strategies and Practices |
|
Malomo et al. [20] |
| Human excreta management: human excreta as an important base ofsustainable agriculture |
|
Zseni. [21] |
| A technical review on resource recovery from human and animal waste |
|
This study |
| Type of pyrolysis and key products | Main findings | Reference |
| Feedstock: Animal manure Type: Slow pyrolysis Non-catalytic Main product: Biochar |
|
Cantrell et al. [47] |
| Feedstock: Human waste Type: Slow pyrolysis Non-catalytic Main product: Biochar |
|
Krounbi et al. [49] |
| Feedstock: Human Faces Type: Slow pyrolysis Non-catalytic Main product: Biochar |
|
Yacob et al. [50] |
| Feedstock: dry toilet substrates comprising of urine, feces, and wood chips Type: Slow pyrolysis Non-catalytic Main product: Biochar |
|
Blueler et al. [56] |
| Feedstock: Chicken manure Physical activation with CO2 to activated carbon. Type: Slow pyrolysis Catalyst: Homogeneous NaOH Main product: Biochar |
|
Koutcheiko [57] |
| Feedstock: Goat manure Type: Fast pyrolysis Non-catalytic Main product: Bio-oil |
|
Emrah et al. [58] |
| Feedstock: Equine manure Type: Fast pyrolysis Catalyst: HZSM-5 catalyst Main product: Bio-oil |
|
Elkasabi et al. [59] |
| Feedstock: Poultry litter Type: Fast pyrolysis Non-catalytic Main product: Biochar and gases |
|
Pandey et al. [60] |
| Feedstock: Horse manure Type: Microwave-assisted pyrolysis Catalyst: Activated carbon Main product: Biochar and gases |
|
Mong et al. [61] |
| Feedstock: Hen manure Type: CO2-assisted catalytic pyrolysis Catalyst: Transition metal Main product: Biochar, bio-oil and gases |
|
Lee et al. [62] |
| Authors | Aim of study | Key findings |
|---|---|---|
| Ihoeghian et al. [125] | Conversion pathway: Anaerobic digestion Investigated and established the best co-digestion ratio for cattle rumen content and food waste for synergistic biogas production |
A 50:50 ratio of cattle rumen content and food waste was recommended for biogas production Co-digestion of cattle rumen content and food waste enhanced biogas production |
| Ma et al. [127] | Conversion pathway: Anaerobic digestion Adopted the meta-analysis approach to compare the methane yield between mono-digestion and co-digestion of animal manure with other feedstock. |
Higher methane yield was obtained from the co-digestion (animal manure mixed with other feedstock) when compared to mono-digestion. |
| Adjama et al. [145] | Conversion pathway: Anaerobic digestion To investigate the proportions of anaerobic co-digestion of rice straws and human feces that will give the optimal biogas yield. |
An equal ratio of rice straws and human feces produced the highest biogas yield (61% percentage yield). |
| Arifan et al. [133] | Conversion pathway: Anaerobic digestion To study the effectiveness of co-digestion of chicken manure, cow manure, and liquid tofu waste for producing biogas. |
The best combination of feed materials that produced the optimum yield are as follow: 15% chicken manure, 70% cow manure, and 15% liquid tofu waste. |
| Edouk et al. [146] | Conversion pathway: Anaerobic digestion To compare the effectiveness of water, human urine, and sodium bicarbonate (Na2CO3) as a buffering agent for the codigestion of poultry feces and lignocellulosic biomass for the generation of biogas. |
The urine-buffered reactors produced the highest yield up to five times greater than those buffered with sodium bicarbonate and water. |
| Silwadi et al. [134] | Conversion pathway: Anaerobic digestion To compare the biogas yield and composition resulting from mono-digestion (cow, chicken, and camel) and co-digestion (mixtures of cow, chicken, and camel). |
The co-digestion gave a higher yield than the mono-digestion. Biogas yield increased 5 (co-digestion with chicken manure), 12 (co-digestion with cow manure), and 28 (co-digestion with camel manure) times when compared to mono-digestion. |
| Pan et al. [147] | Conversion pathway: Anaerobic digestion Investigated the role of wood-based biochar during AD of chicken manure. |
25% reduction in TAN accumulation. 69% increase in biogas production compared to the control. |
| Kizito et al. [148] | Conversion pathway: Anaerobic digestion Investigated the role of biochar on the removal of TAN during AD of piggery waste |
60% reduction in TAN accumulation which enhanced AD stability. |
| Recebli et al. [149] | Conversion pathway: Fermentation To compare the daily biogas production rate from poultry manure and bovine animal manure. |
Approximately 0.83 m3 and 6.33 m3 of biogas are produced daily from poultry manure and bovine animal manure, respectively. The lower heating value of the produced methane and biogas is 34000 KJ/m3 and 21000 kJ/m3 respectively. |
| Zlateva et al. [150] | Conversion pathway: Fermentation To determine the quantity of biogas and energy produced from the anaerobic fermentation of cow manure, chicken manure, and pig manure. |
It was revealed that approximately 556000 kWh per annum of energy is produced. At the same time, 55660 methane is released per annum, with pig manure, cow manure, and chicken manure contributing to the release of 7493 Nm3CH4/a, 234111 Nm3CH4/a, and 24756 Nm3CH4/a, respectively. |
| Andreev et al. [151] | Conversion pathway: Fermentation To subject human urine to lactic acid fermentation to reduce its odor and enhance its fertilizing ability. |
The pH of the treated urine ranges from 3.8-4.7 compared to the untreated which is 6.1. The ammonia composition decreases by 20-30% compared to the untreated, whose ammonia composition increases by 30% owing to hydrolysis. |
| Andreev et al. [152] | Conversion pathway: Fermentation To subject human excreta to lactic acid fermentation to reduce the loss of nutrients and the number of pathogens present in them. |
Human excreta is a promising source of nutrients via lactic acid fermentation. The nutrient loss is lowered in the presence of lactic acid with 7-10 days fermentation. |
| Adjama et al. [145] | Conversion pathway: Fermentation To investigate anaerobic fermentation chicken manure and straw mixtures in a batch reactor at a temperature of 37 °C for ten weeks |
The straw ratio of 3% gave the highest methane yield of 292.87 mLgVS-1 which is 17% greater than pure chicken manure. |
| Dong-Jun Lee et al. [153] | Conversion pathway: Fermentation Evaluate the impact of two different pretreatment methods (NaOH and H2SO4) on the bioethanol yield during horse manure fermentation. |
Alkaline/enzyme-hydrolysates showed higher bioethanol productivity (0.075 g L-1h−1) than those of acid/enzyme-hydrolysates (0.050 g L-1h−1). Fermentation of hydrolysates produced less inhibitory compounds due to the alkaline pretreatment. |
| Recovery technology | Nutrients recovered | Source | Efficiency (%) | References |
|---|---|---|---|---|
| Air stripping | Ammonium | Human urine | 90 | Wei et al. [187] |
| Struvite precipitation | Phosphorus | Human urine | 94 | Masrura et al. [188] |
| Membrane separation | Ammonium, phosphate | Human urine | Above 90 | Zhang et al. [189] |
| Bio-electrochemical systems | Ammonia | Human urine | 60 | Martin et al. [190] |
| Wet extraction | Phosphorus | Pig manure | 92-97 | Azuara et al. [191] |
| Urea hydrolysis | Phosphate | Human urine | 82 | Chen. [192] |
| Chemical precipitation | Phosphate | Dairy manure | 82 | Zhang et al. [193] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





