1. Introduction
Novel coronavirus infection (COVID-19), caused by SARS-CoV-2, was characterized as a pandemic by WHO in March, 2020 [31].
There is a growing number of publications dedicated to the interactions between SARS-CoV-2 and various human tissues and organs. COVID-19, unlike other respiratory viruses, often causes cardiovascular complications, i.e. myocarditis, arrhythmia, cardiac failure, thromboembolism and disseminated intravascular coagulation etc [12].
Patients often develop arterial thromboembolic complications during or after COVID-19 infection [1]. These complications may cause acute myocardial infarction or acute ischemic stroke [9,11]. Hence, one may suppose that SARS-CoV-2 is able to change the status of atherosclerotic plaques [22].
The similarities between pathogenetic mechanisms of atherosclerosis and COVID-19, the data about higher frequencies of cardiovascular complications in COVID-19 and post-COVID19 disorders, and the importance of vasa vasorum in atherogenesis - call for investigation of pathomorphology of vessel walls in these diseases [5,6].
2. Case presentation
The patient, a 69-year old woman with arterial hypertension, survived COVID-19, of a moderate severity, in April of 2022. In May 2022 she developed acute ischemic stroke and, despite being given medication, died of progressive brain edema.
During postmortem examination of aorta and large arteries multiple atherosclerotic plaques were found, with intraplaque hemorrages, necroses, and thrombotic masses on their surface.
Samples of aorta were taken to perform pathohistological examination, fixed in 10% neutral buffered formalin and embedded in paraffin. Standard H&E, alcian blue and Van Gieson stainings were performed.
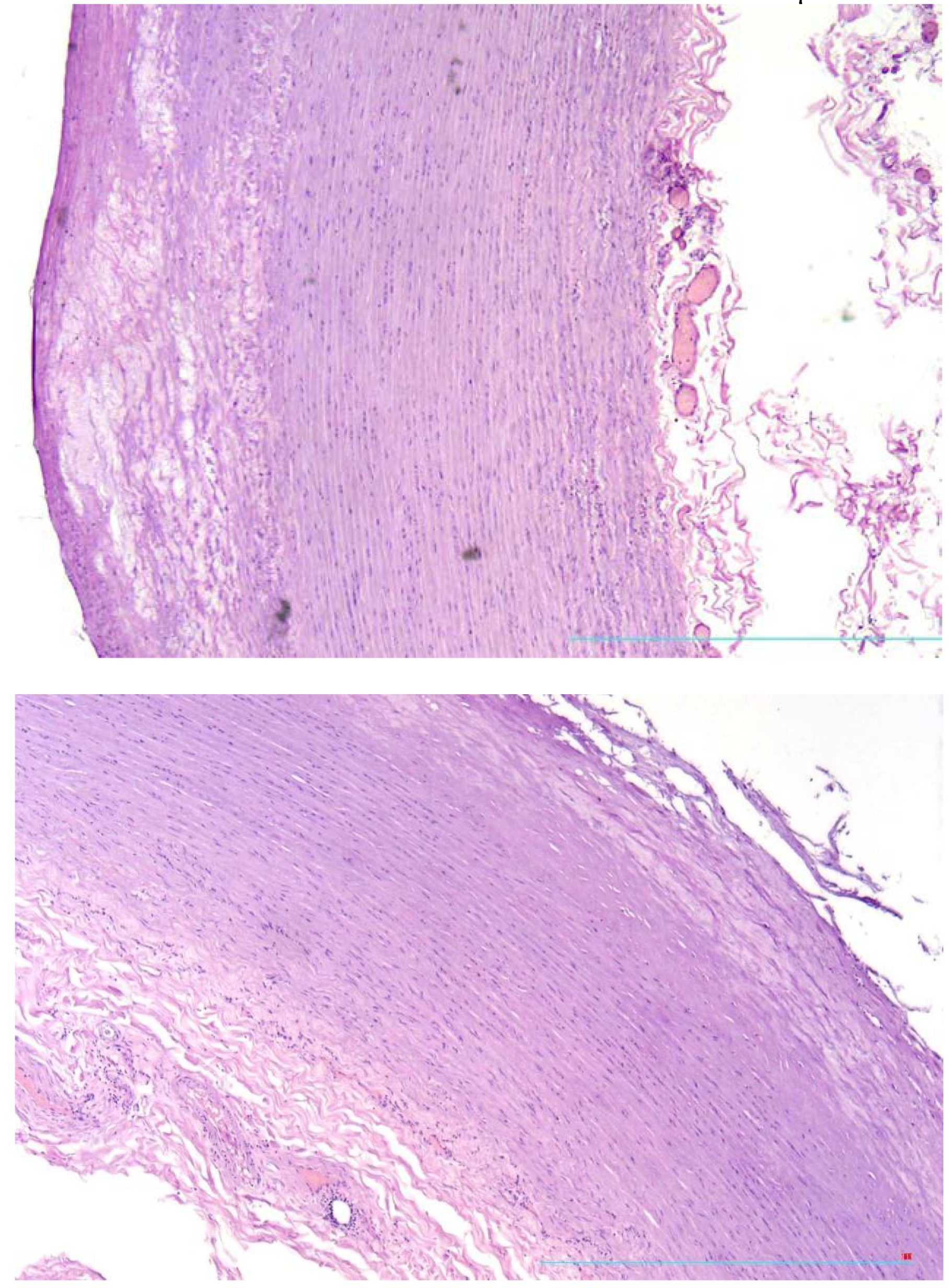
While microscopic examination of the aorta there were found atherosclerotic plaques with thin fibrous cap and relatively vast lipid deposits (
Figure 1a) and plaques with tears and fissures of fibrous caps (
Figure 1b).
Neovascularisation was found in the zones of aortal wall, corresponding to the plaques.
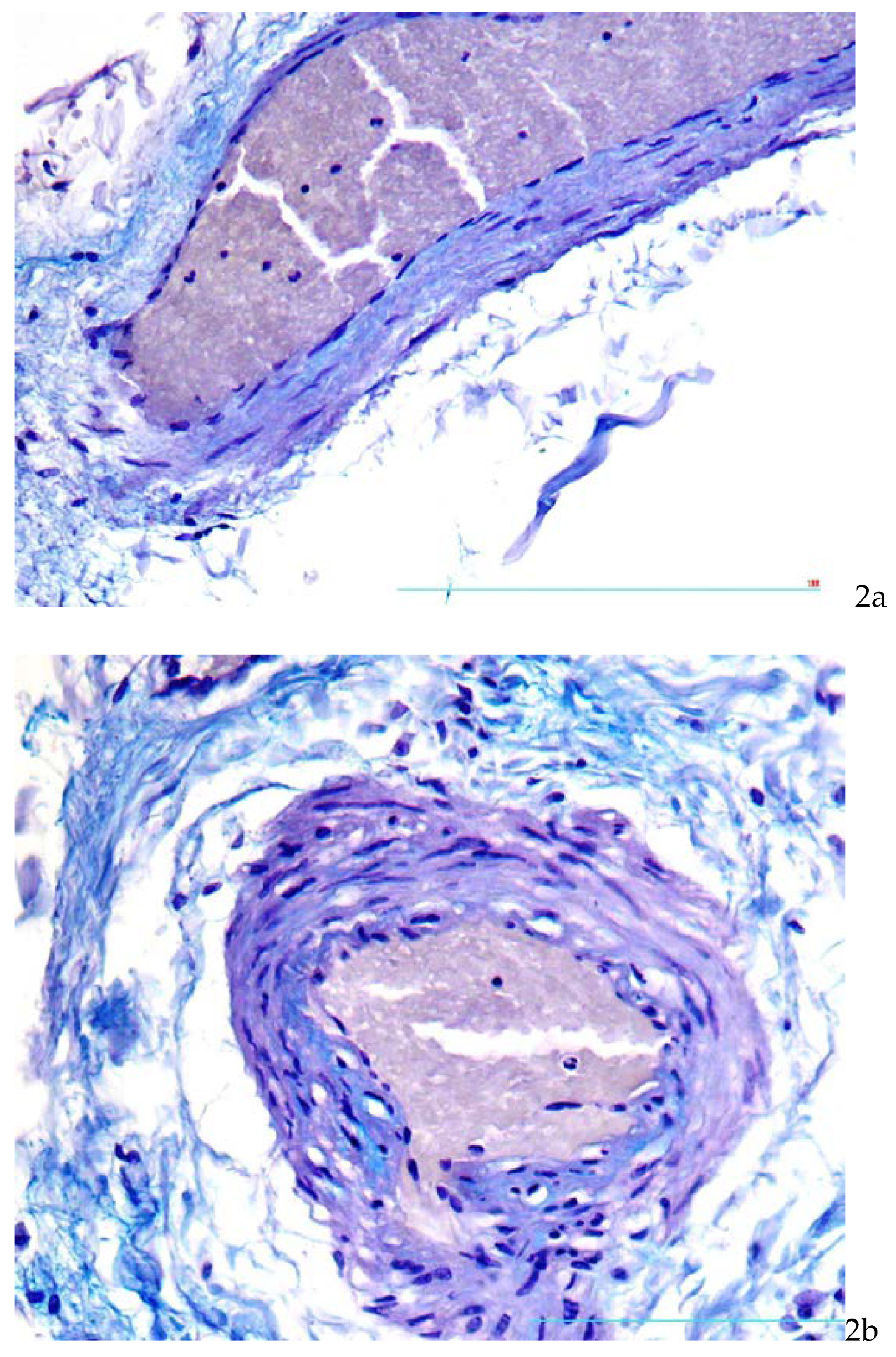
Also in the zones corresponding to unstable plaques were found moderate thickening of
vasa vasorum walls due to focal deposition of glycosoaminoglycans (
Figure 2a,b).
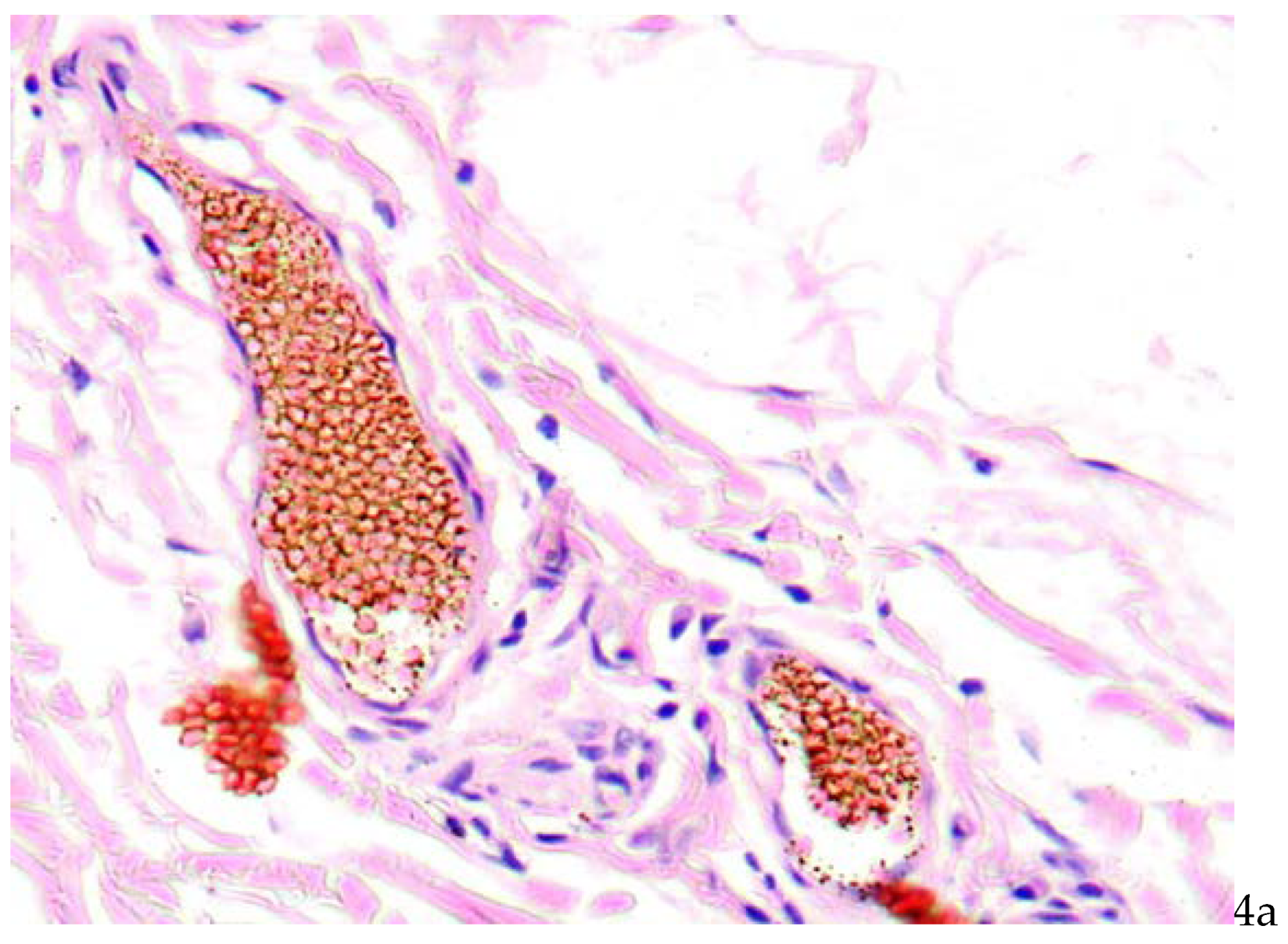
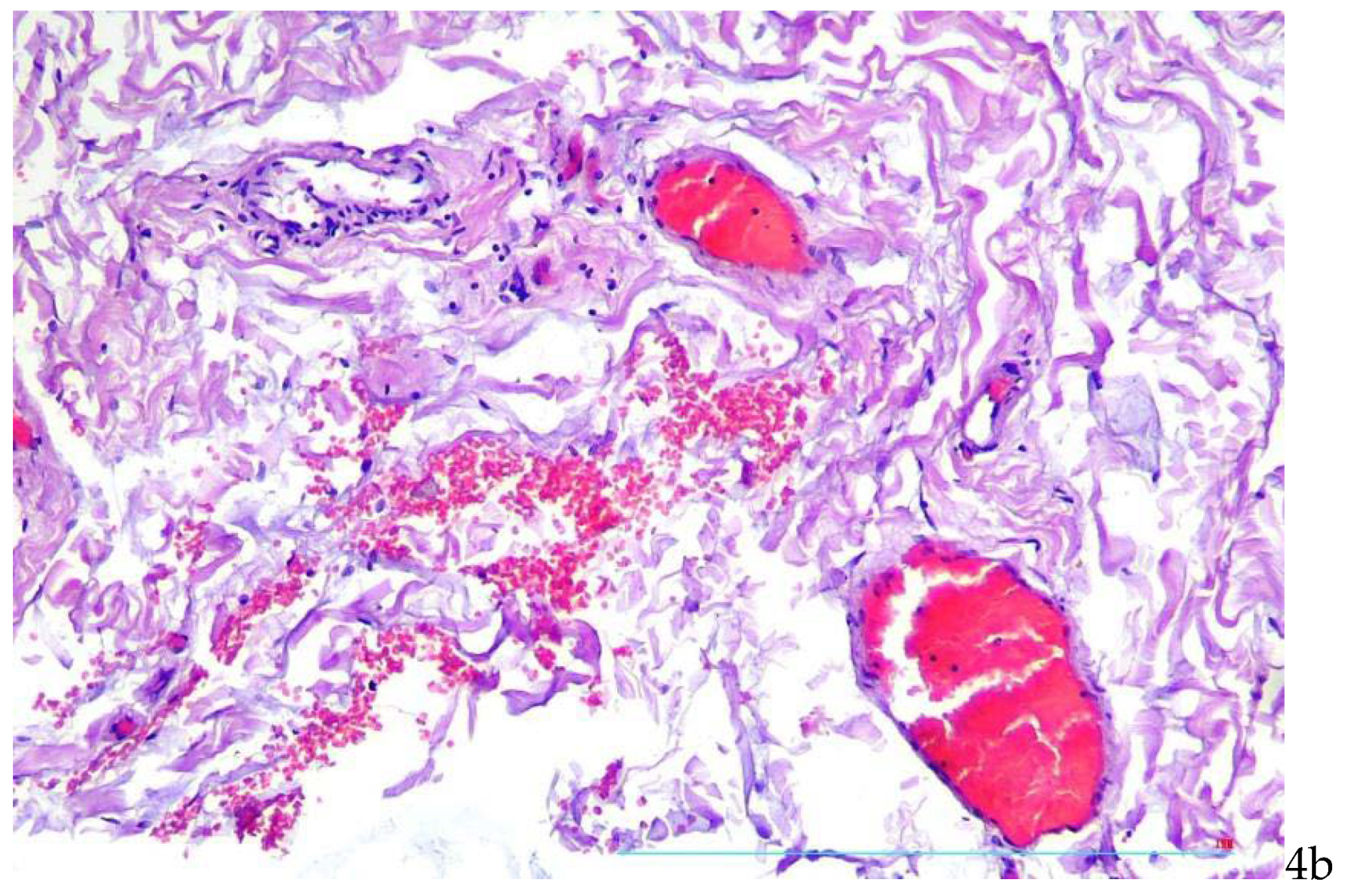
There were found stasis of erythrocytes and red thrombi in vasa vasorum lumina (
Figure 4a,b).
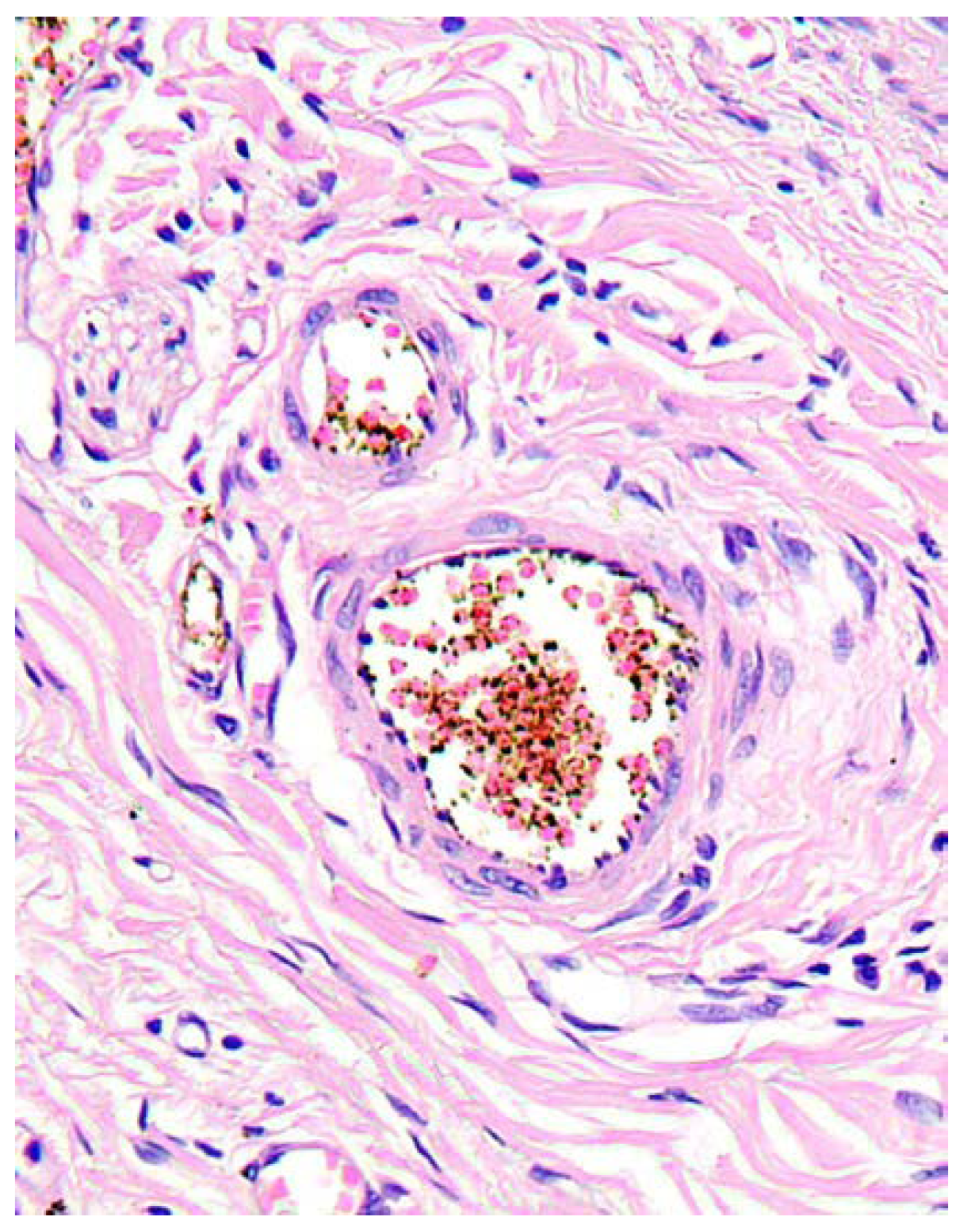
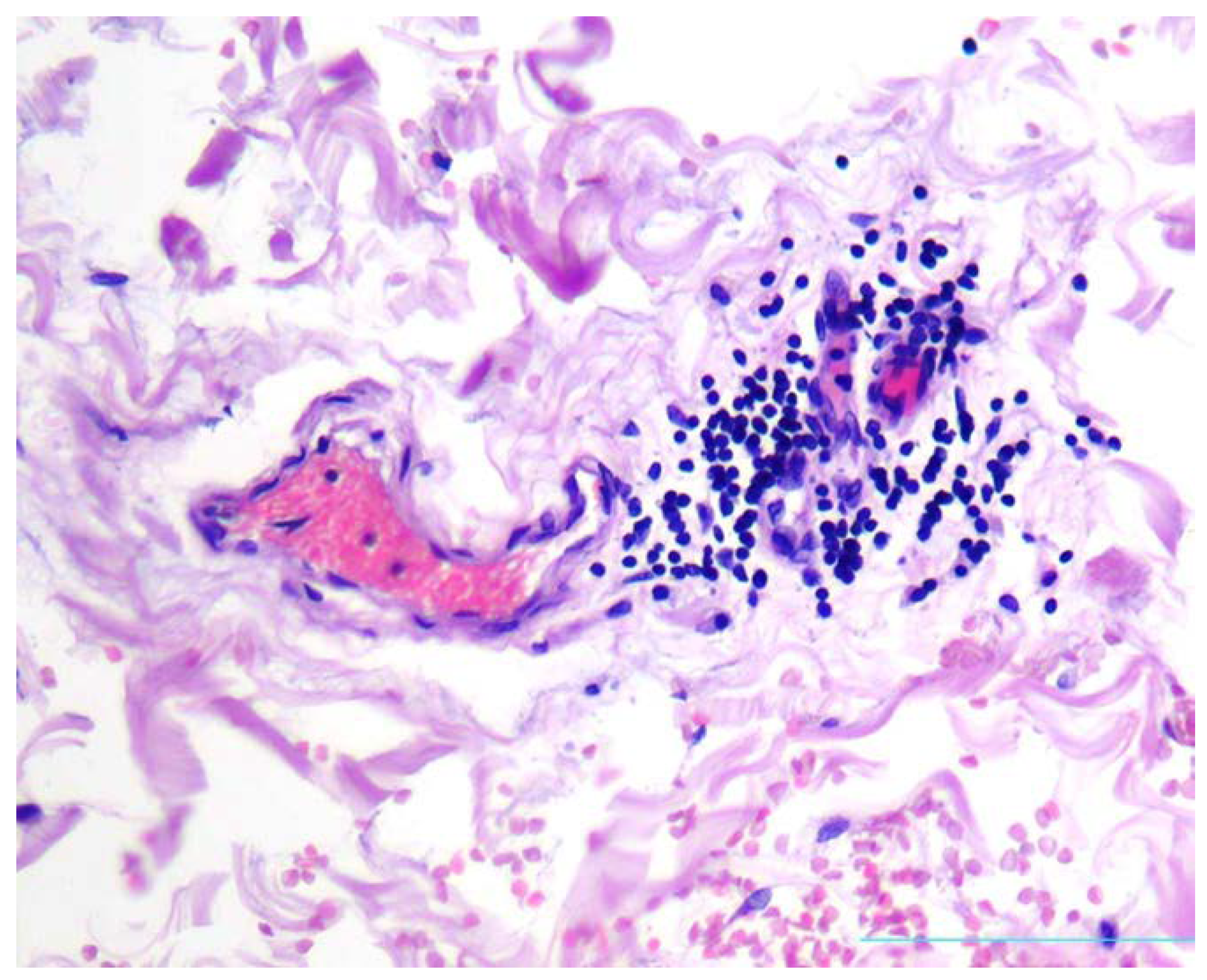
In aortal adventitia, mild to moderate lymphocytic infiltrate was present in the loci corresponding to focal oedema of vasa vasorum (
Figure 5).
Also in the zones corresponding to
vasa vasorum with thrombi intima of the aorta was demonstrating signs of endotheliitis, including moderate nuclear polymorphism and oedema of cytoplasm of endothelial cells (
Figure 6).
3. Discussion
Thus, clinical and pathomorphological characteristics of the case are consistent with the hypothesis that vasa vasorum alteration may be a common link in pathogenesis of COVID-19 and atherosclerosis, regarding arterial damage progression. Severe cardiovascular complications of COVID-19 may be associated with progressive viral alteration of endothelium, including that of vasa vasorum.
The impact of SARS-CoV-2 on the blood vessels is mediated by angiotensin-converting enzyme 2 (ACE2) expressed on the surface of the cells, interacting with the virus [18,28]. ACE2 is plentifully expressed not only on alveolocytes and small intestine enterocytes, but also on endotheliocytes, both arterial and venous [15]. Hyperexpression of ACE2 during COVID-19 infection may cause endothelial dysfunction and alteration of endotheliocytes [25], which is associated with cardiac failure, acute coronary syndrome and other multiorganic thrombovascular complications [24].
Alteration of cardiovascular system by SARS-CoV2 may be caused not only by direct primary action of the virus itself, but also may result from secondary alteration, induced by excessive activity of proinflammatory mediators. There is certain similarity found between proinflammatory changes, characteristic for COVID-19, and those typical for atherosclerosis [26].
SARS-CoV-2 binds the membrane ACE2 of the endothelial cells. They express receptors of interleukin -6 [23] and interleukin-2 [17] (IL-6R and IL-2R).Direct and indirect action of multiple cytokines, e.g. IL-1beta, IL-6, IL-2R, TNF [22] , which are abundant in blood of COVID-19 patients, with endotheliocytes induces further alteration of endothelium. Continuous endothelial damage results in additional secretion of pro-inflammatory autacoids, enhancing systemic action of pro-inflammatory mediators which may cause hemodynamic shock [26].
Atherosclerosis is a chronic inflammatory disease, which develops in elastic and muscular-elastic arteries, whilst COVID-19 is regarded as a viral infection, which firstly affects the lungs, and later also cardiovascular system. Hypoxemia, oxidative stress and elevated pulmonary pressure, which is caused by pulmonary thrombosis, induce cardiac dysfunction and heart failure [4]. Severe tissue hypoxia induces production of proinflammatory mediators by necrobiotic cells, which results in failure of inflammatory focal barriers and systemic correlates of inflammatory process. In extreme degree of hyperautacoidemia and excessive systemic action of inflammatory mediators “cytokine storm” follows, resulting in distributive shock, which aggravates hypoxia [4,21].
Atherosclerotic plaques are the main pathomorphological elements of atherosclerosis. In clinical terms they may be either stable or unstable.
The state of atherosclerotic plaque according to forensic and post-mortem studies significantly affects the risk of sudden cardiac death, although thrombogenic and vasospastic events in post-COVID patients also depend on many external factors [14,20]
In pathophysiologic sense it means to be either in a status of an inflammatory focus effectively isolated by the functional and structural barriers, or alternatively – in a status of inflammatory focus with barriers failed and proinflammatory signals emitted to circulation and neighboring areas. The main attributes of stable plaques are thick, firm, collagen-rich fibrous caps, smaller quantities of active macrophages and extracellular lipids. Unstable plaques, on the contrary, have thin fibrous caps, large lipid nuclei, many active macrophages and demonstrate signs of active inflammation.
The development of atherosclerotic plaques is a complicated process, including multiple cascades, where take a part both pro- and antiinflammatory mediators secreted by endothelial cells, leukocytes, platelets, foam cells and mast cells [1,3]. Insatiable active plaques disturb the blood flow not only biomechanically, due to their geometry, but also because of being a source of thrombogenic and vasoconstrictive inflammatory autacoids of lipid and peptide structure secreted by plaque cells [7,8]. Hence, both in COVID-19 and in atherosclerosis one of the essential matters is the integrity of barriers delimiting the spheres of action for local autacoids of para-, juxta- and autocrine action (like cytokines), and preventing them from excessive systemic spread and jeopardizing of neuroendocrine regulation supporting the whole-body vital functions [18].
Endothelial injury, dyslipidemias and shear stress followed by flow-mediated changes, belong to the main causes of plaque formation and progression of atherosclerosis [15].
At the moment there are two main concepts of atherogenesis: the common “inside-out” concept and the opposite “outside-in” one [18,28].
According to the “inside-out” concept, atherogenesis includes endothelial activation, release of proinflammatory cytokines and chemokines and activation of inflammatory cascades. The atherogenic lipoproteins (LP) are accumulated in the intima, oxidized and modified. The cellular adhesion molecules expressed on activated endothelial cells, bind circulating monocytes. The monocytes migrate into intima and then transform into macrophages, expressing LP-binding receptors. After accumulation of the excess of LP the macrophages transform into foam cells. T-lymphocytes and smooth muscle cells (SMC) from the arterial media also migrate into intima, and SMC take part in foam cells formation.
According to the “outside-in” concept of atherosclerosis, the inflammation begins in adventitial layer of the vessel and then spreads on the media and intima [19,30]. In this concept vasa vasorum play an important role in formation of atherosclerotic changes and in provocation of the complications of atherosclerosis. [10;24].
It is well known that microvasculature is being damaged in many autoimmune and viral diseases, particularly in Kawasaki disease, when lesions involve many arteries similarly involved in atherosclerosis. Interestingly, Kawasaki-like syndrome was described as a complication of COVID-19 in children. Perhaps, vasa vasorum vasculitis may be the common thread between COVID-19, autoimmune vasculitis and atherosclerosis. [2].
The presence of microthrombi in vasa vasorum indicates that vascular injury associated with COVID-19 develops in microvasculature firstly as it was demonstrated earlier by Boyle et al. [2].
On the other side, damage of vasa vasorum may possibly shift the stable atherosclerotic plaques into unstable condition, increasing the risk of development of atherosclerotic complications, which obviously happened in a described case of post-COVID ischemic stroke.
The similar central role of vasa vasorum and their viral/immunopathological damage was earlier noted not only in atherosclerosis, but also in Takayasu arteritis, Buerger’s disease, temporal arteritis, vascular form of Behcet disease and inflammatory abdominal aortic aneurysm [17], as well as in Churg-Strauss syndrome [13]. The authors cited above related these immunopathological disorders to atherosclerosis, and F. Numano [21] even coined the non-conventional term: “vasa-vasoritis”.
4. Conclusions
The amount of persons survived the COVID-19 rapidly increases all over the world, and considerable part of this cohort, especially elderly patients, presumably already suffer from atherosclerosis. Bearing in mind the data described above, one may expect that new Coronavirus infection soon will display its atherogenic and complication provoking potential and, perhaps, COVID-19 would be added to the list of atherosclerosis risk factors.
Author Contributions
Churilov L.P.: idea, research leadership, data analysis, final text editing & review. Makarova J.A.: review of literature. Malachova S.A.: review of literature, pathohistological studies. Novitskaya T.A.: materials, case description, pathohistological studies. Shapkina V.A.: English translation, text editing & review. All authors have read and agreed to the published version of the manuscript.
Funding
Work was supported with the Grant of the RF Government under agreement No. 075-15-2022-1110 dated June 30, 2022 and contains the results of scientific research at the Laboratory of Microangiopathic Mechanisms of Atherogenesis, St. Petersburg State University.
Acknowledgments
We have no conflicts of interest to disclose. The research has been approved by Ethical Committee of the L.G. Sokolov North-West Regional Research and Clinical Center, affiliated clinical base of Saint Petersburg State University. Protocol # 7, dated 8 December 2022. During this research no personal information was disclosed.
References
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Der Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [CrossRef]
- Boyle EC, Haverich A. Microvasculature dysfunction as the common thread between atherosclerosis, Kawasaki disease, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) associated multi-system inflammatory syndrome in children. Eur J Cardiothorac Surg. 2020;58(6):1109-10. [CrossRef]
- Brott, T.G.; Hobson, R.W.; Howard, G.; Roubin, G.S.; Clark, W.M.; Brooks, W.; Mackey, A.; Hill, M.D.; Leimgruber, P.P.; Sheffet, A.J.; et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N. Engl. J. Med. 2010, 363, 11–23. [CrossRef]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Daisley Jr. H, Rampersad A, Daisley M, et al. The vasa vasorum of the large pulmonary vessels are involved in COVID-19. Autops Case Rep [Internet]. 2021;11:e2021304. [CrossRef]
- Faa G, Gtrosa C, Fanni D, Barcellona D, Cerrone G, Orru G, Scano A, Marongiu F, Suri G.S, Demontis R, Nioi M, D’Aloga E, La Nasa L, Saba L. Aortic vulnerability to COVID-19: is the microvasculature of vasa vasorum a key factor? A case report and a review of the literature. Eur Rev Med Pharmacol Sci 2021; 25: 6439-6442. [CrossRef]
- Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992; 326(4):242-50. [CrossRef]
- Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2). N Engl J Med. 1992; 326(5):310-8. [CrossRef]
- Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020; 5: 811. [CrossRef]
- Haverich A, Boyle EC. Aortic dissection is a disease of the vasa vasorum. JTCVS Open. 2021; 5:30-32. [CrossRef]
- Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M, Anheim M, Meziani F. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med 2020; 382: 2268-2270. [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Hervier B, Masseau A, Bossard C, Agard C, Hamidou M. Vasa-vasoritis of the aorta and fatal myocarditis in fulminant Churg-Strauss syndrome. Rheumatology (Oxford). 2008; 47(11):1728-9. [CrossRef]
- Hogea T., Suciu B.A., Ivănescu A.D., Caras C., Chinezu L., Arbănas E.M. Russu E., Kaller R., Arbănas E.M., Mures A.V., Radu C.C. Increased Epicardial Adipose Tissue (EAT), Left Coronary Artery Plaque Morphology, and Valvular Atherosclerosis as Risks Factors for Sudden Cardiac Death from a Forensic Perspective. Diagnostics 2023, 13, 142. [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; Cheng, Z.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Indes JE, Koleilat I, Hatch AN, Choinski K, Jones DB, Aldailami H, Billett H, Denesopolis JM, Lipsitz E. Early experience with arterial thromboembolic complications in patients with COVID-19. J Vasc Surg 2021; 73: 381-389. [CrossRef]
- Krieg C, Létourneau S, Pantaleo G, Boyman O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11906-11. doi: 10.1073/pnas.1002569107. Epub 2010 Jun 14. Erratum in: Proc Natl Acad Sci U S A. 2012 Jan 3;109(1):345. [CrossRef]
- Leali M, Rossi A, Gaskill M, Sengupta S, Zhang B, Carriero A, Bachir S, Crivelli P, Paschè A, Premi E, Padovani A, Gasparotti R. Imaging of Neurologic Disease in Hospitalized Patients with COVID-19: An talian Multicenter Retrospective Observational Study. Radiology 2020; 297: E270-E273. [CrossRef]
- Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007; 75:640–648. [CrossRef]
- Mures A.V., Hălmaciu I., Arbănas E.V., Kaller R., Arbănas E.M., Budis O.A., Melinte R.M., Vunvulea V., Rares C.F., Mărginean L., Suciu B.A., Brinzaniuc K., Niculescu R., Russu E. Prognostic Nutritional Index,Controlling Nutritional Status (CONUT) Score, and Inflammatory Biomarkers as Predictors of Deep Vein Thrombosis, Acute Pulmonary Embolism, and Mortality in COVID-19 Patients. Diagnostics 2022, 12, 2757. [CrossRef]
- Numano, F. Vasa vasoritis, vasculitis and atherosclerosis. Int J Cardiol. 2000; 75 Suppl 1:S1-8; discussion S17-9. [CrossRef]
- Otifi HM, Adiga BK. Endothelial Dysfunction in Covid-19 Infection. Am J Med Sci. 2022 Apr;363(4):281-287. [CrossRef]
- Ryabkova VA, Churilov LP, Shoenfeld Y. Influenza infection, SARS, MERS and COVID-19: Cytokine storm - The common denominator and the lessons to be learned. Clin Immunol. 2021; 223:108652. [CrossRef]
- Saba L, Gerosa C, Fanni D, Marongiu F, La Nasa G, Caocci G, Barcellona D, Balestrieri A, Coghe F, Orru G, Coni P, Piras M, Ledda F, Suri JS, Ronchi A, D’Andrea F, Cau R, Castagnola M, Faa G. Molecular pathways triggered by COVID-19 in different organs: ACE2 receptor-expressing cells under attack? A review. Eur Rev Med Pharmacol Sci 2020; 24: 12609-12622. [CrossRef]
- Saba L, Gerosa C, Wintermark M, Hedin U, Fanni D, Suri JS, Balestrieri A, Faa G. Can COVID19 trigger the plaque vulnerability-a Kounis syndrome warning for ‘asymptomatic subjects’. Cardiovasc Diagn Ther 2020; 10: 1352-1355. [CrossRef]
- Sagris M , Theofilis P , Antonopoulos As , Tsioufis C , Oikonomou E , Antoniades C, Crea F , Kaski JC, Tousoulis D Inflammatory Mechanisms in COVID-19 and Atherosclerosis: Current Pharmaceutical Perspectives// Int. J. Mol. Sci. 2021, 22, 6607. [CrossRef]
- Sedding DG, Boyle EC, Demandt JAF, Sluimer JC, Dutzmann J, Haverich A, Bauersachs J. Vasa Vasorum Angiogenesis: Key Player in the Initiation and Progression of Atherosclerosis and Potential Target for the Treatment of Cardiovascular Disease. Front Immunol. 2018 Apr 17;9:706. [CrossRef]
- Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol 2020; 5: 802. [CrossRef]
- Vasuri F , Ciavarella C, Collura S , Mascoli C , Valente S , Degiovanni A , Gargiulo M , Capri M, Pasquinelli G. Adventitial Microcirculation Is a Major Target of SARS-CoV-2-Mediated Vascular Inflammation. Biomolecules 2021, 11, 1063. [CrossRef]
- Watanabe M, Sangawa A, Sasaki Y, Yamashita M, Tanaka-Shintani M, Shintaku M, Ishikawa Y. Distribution of inflammatory cells in adventitia changed with advancing atherosclerosis of human coronary artery. J Atheroscler Thromb. 2007; 14:325–331. [CrossRef]
- World Health Organization. Coronavirus Disease (COVID-19) Outbreak. Available online: https://www.who.int (accessed on 1 March 2020).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).