Submitted:
15 February 2023
Posted:
16 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
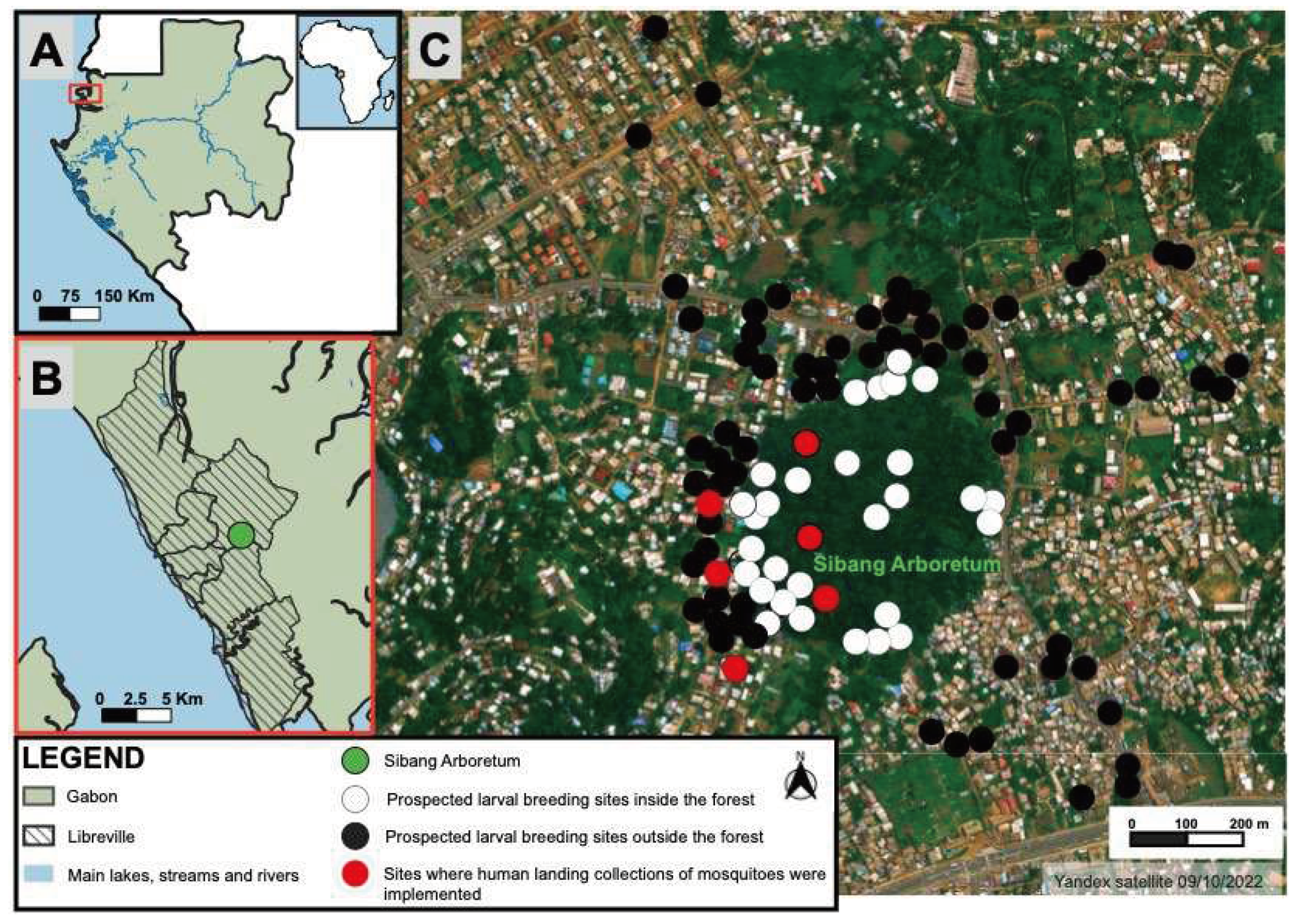
2.1. Study area
2.2. Larval sampling
2.3. Human landing collection
2.4. Data analysis
3. Results
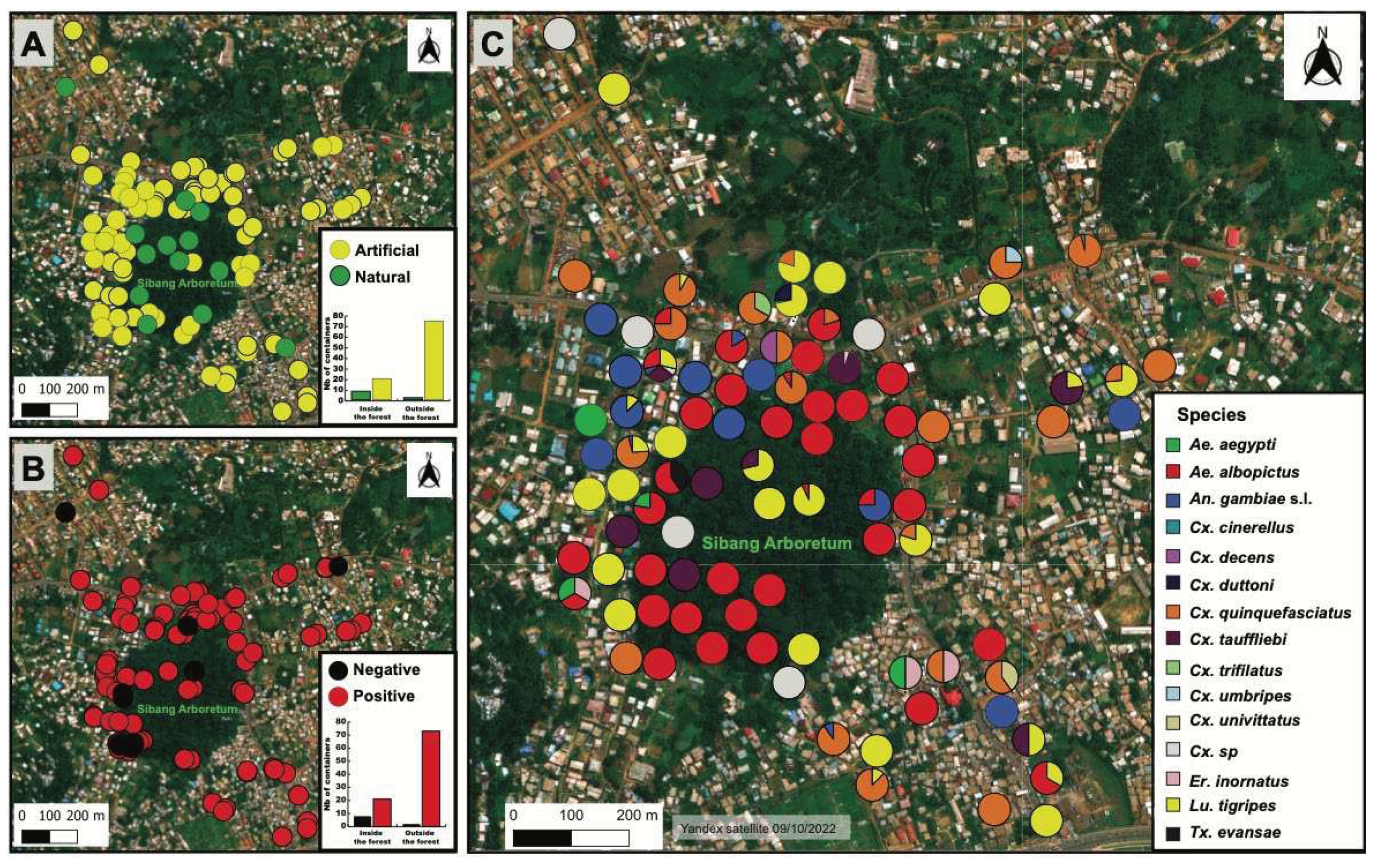
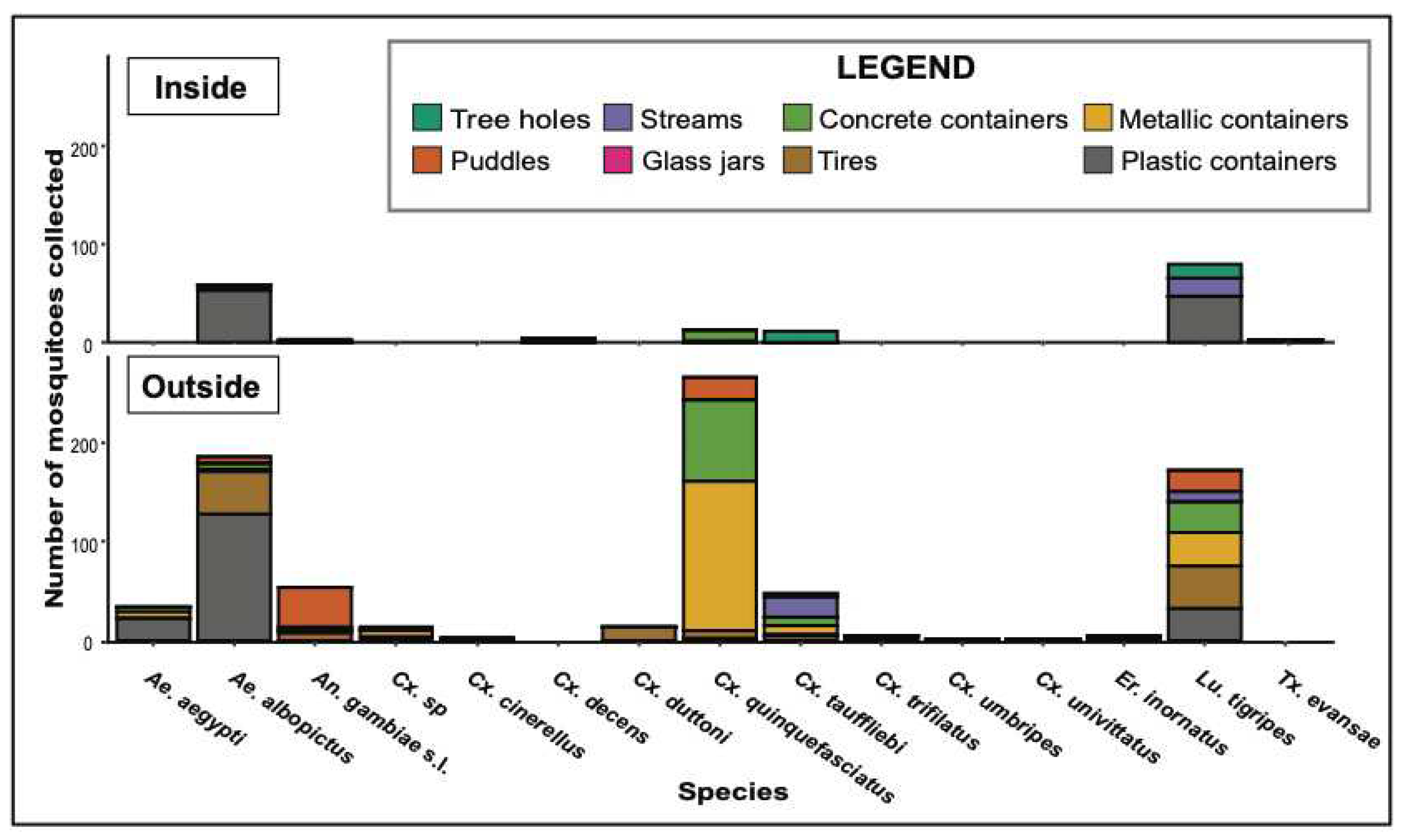
3.1. Typology and positivity of larval habitats inside and outside the forest
3.2. Mosquito species composition and diversity
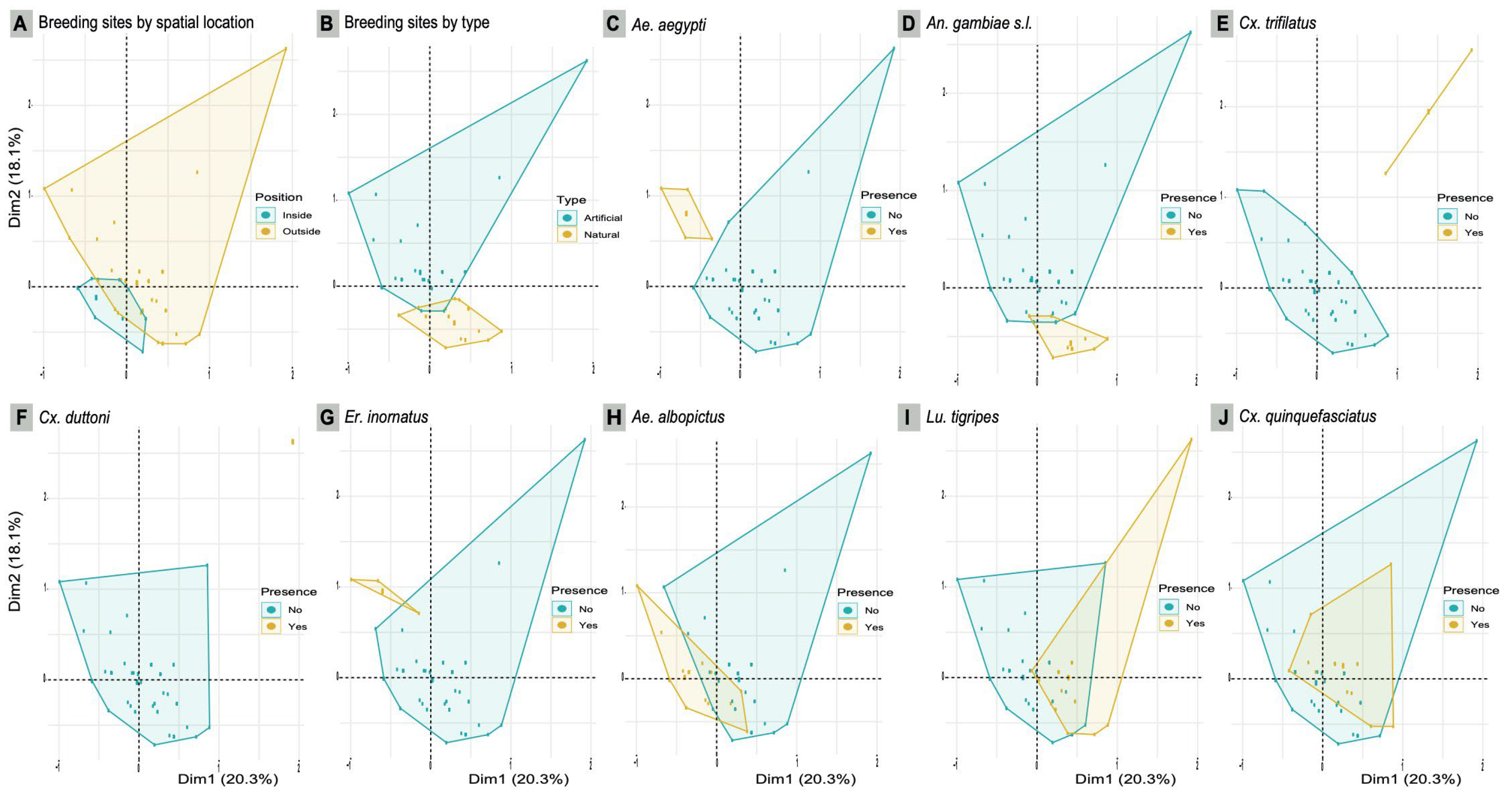
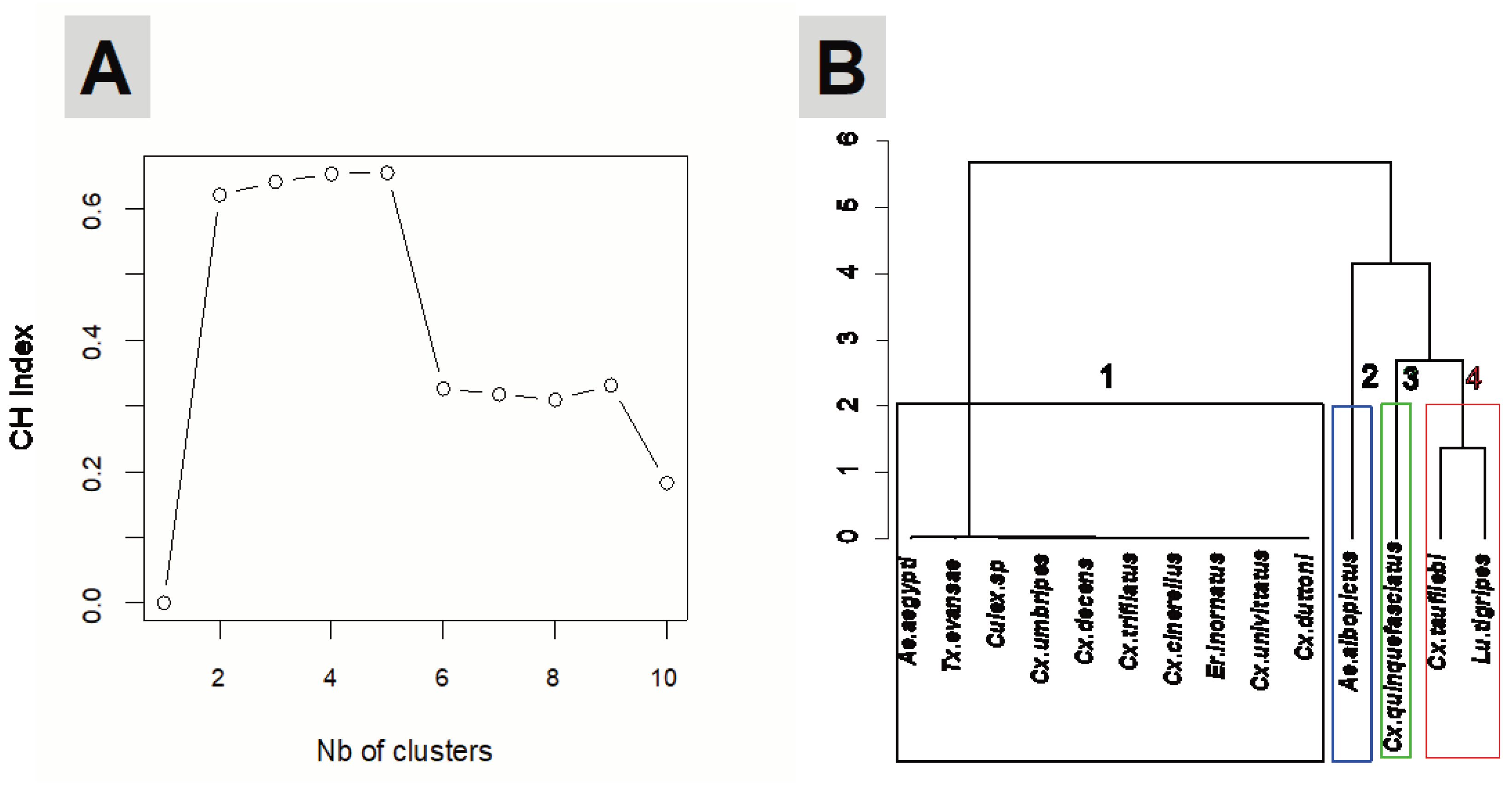
3.3. Larval habitat typology, similarity and species culsturing
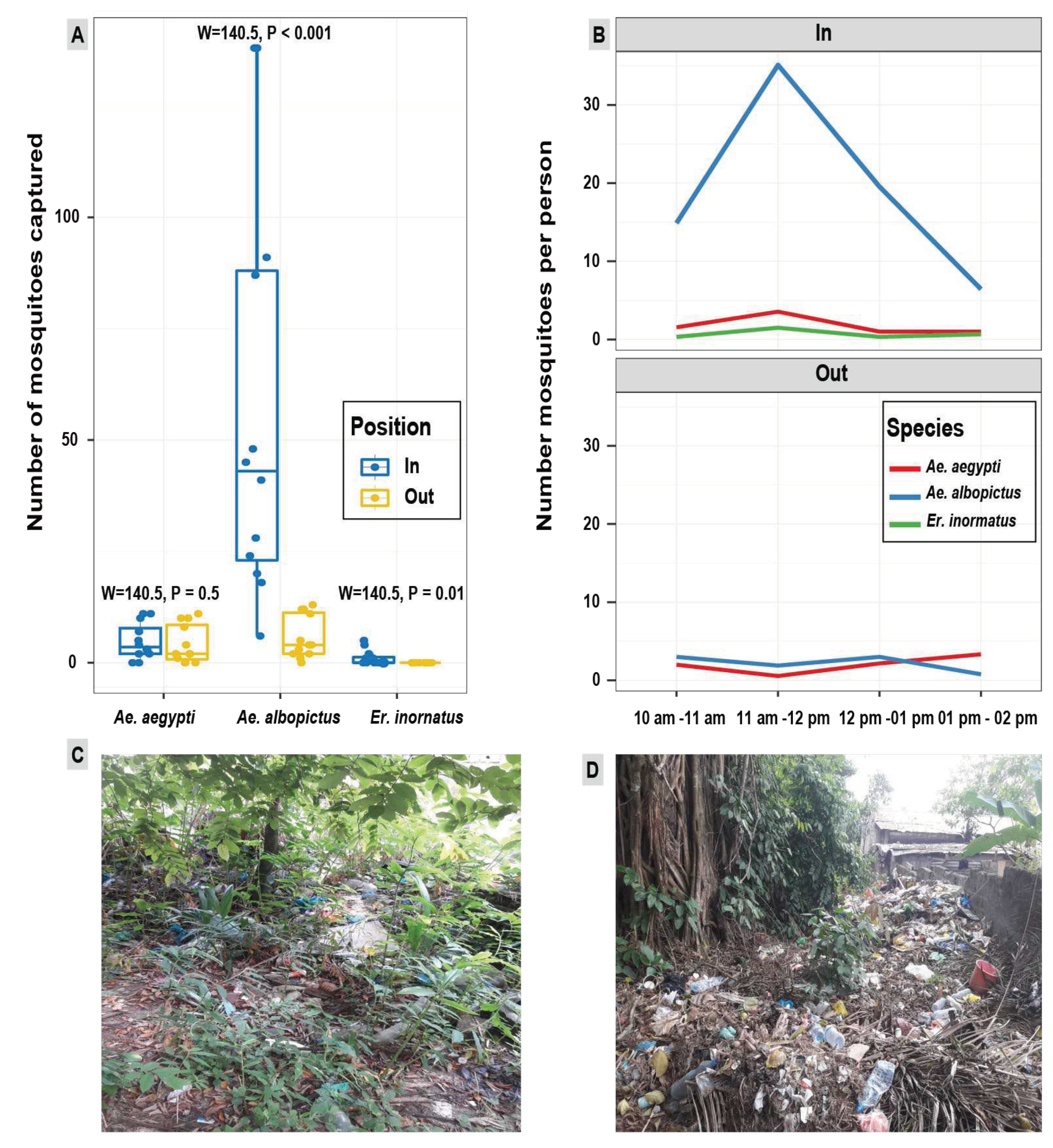
3.4. Biting patterns of mosquito species
4. Discussion
4.1. Mosquito communities in the urban forested area of Sibang
4.2. The mosquito proliferation drivers in the urban forested area of Sibang
4.3. Mosquito aggressiveness in the urban forested area of Sibang
4.4. Mosquito aggressiveness in the urban forested area of Sibang
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brady, O.J.; Hay, S.I. The global expansion of dengue: how Aedes aegypti mosquitoes enabled the first pandemic arbovirus. Annu Rev Entomol. 2020, 65, 191–208. [Google Scholar] [CrossRef]
- Hoerauf, A.; Pfarr, K.; Mand, S.; et al. Filariasis in Africa—treatment challenges and prospects. Clin Microbiol Infect. 2011, 17, 977–985. [Google Scholar] [CrossRef]
- Rebollo, M.P.; Bockarie, M.J. Can lymphatic filariasis be eliminated by 2020? Trends Parasitol. 2017, 33, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Charlier, C.; Vasilakis, N.; et al. Zika, chikungunya, and other emerging vector-borne viral diseases. Annu Rev Med. 2018, 69, 395–408. [Google Scholar] [CrossRef] [PubMed]
- WHO World malaria report. 2021. p. 2–5.
- Eder, M.; Cortes, F.; Teixeira de Siqueira Filha, N.; et al. Scoping review on vector-borne diseases in urban areas: transmission dynamics, vectorial capacity and co-infection. Infect Dis Poverty. 2018, 7, 1–24. [Google Scholar] [CrossRef]
- Koukouikila-Koussounda, F.; Ntoumi, F. Malaria epidemiological research in the Republic of Congo. Malar J. 2016, 15, 1–11. [Google Scholar] [CrossRef]
- Mbohou, C.N.; Foko, L.P.K.; Nyabeyeu, H.N.; et al. Malaria screening at the workplace in Cameroon. PLoS One. 2019, 14, e0225219. [Google Scholar] [CrossRef]
- Messina, J.P.; Taylor, S.M.; Meshnick, S.R.; et al. Population, behavioural and environmental drivers of malaria prevalence in the Democratic Republic of Congo. Malar J. 2011, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Olivier, B.M.B.; Eliezer, M.P.; Ngatimo, E.V.; et al. Influence of climate variability on the dynamics of malaria transmission among children in Bangui, health challenges in central African Republic. Open J Pediatr. 2022, 12, 461–475. [Google Scholar] [CrossRef]
- Tewara, M.A.; Mbah-Fongkimeh, P.N.; Dayimu, A.; et al. Small-area spatial statistical analysis of malaria clusters and hotspots in Cameroon; 2000–2015. BMC Infect Dis. 2018, 18, 1–15. [Google Scholar] [CrossRef]
- Leroy, E.M.; Nkoghe, D.; Ollomo, B.; et al. Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg Infect Dis. 2009, 15, 591. [Google Scholar] [CrossRef]
- Nana-Ndjangwo, S.M.; Djiappi-Tchamen, B.; Mony, R.; et al. Assessment of Dengue and Chikungunya Infections among Febrile Patients Visiting Four Healthcare Centres in Yaoundé and Dizangué, Cameroon. Viruses. 2022, 14, 2127. [Google Scholar] [CrossRef]
- Ushijima, Y.; Abe, H.; Mbadinga, M.J.; et al. Re-emergence of dengue, chikungunya, and Zika viruses in 2021 after a 10-year gap in Gabon. IJID Reg. 2022, 5, 68–71. [Google Scholar] [CrossRef]
- Vairo, F.; Aimè Coussoud-Mavoungou, M.P.; Ntoumi, F.; et al. Chikungunya outbreak in the Republic of the Congo, 2019—epidemiological, virological and entomological findings of a South-North Multidisciplinary Taskforce Investigation. Viruses. 2020, 12, 1020. [Google Scholar] [CrossRef]
- Gubler, D.J.; Clark, G.G. Community involvement in the control of Aedes aegypti. Acta Trop. 1996, 61, 169–179. [Google Scholar] [CrossRef]
- Lindsay, S.W.; Wilson, A.; Golding, N.; et al. Improving the built environment in urban areas to control Aedes aegypti-borne diseases. Bull World Health Organ. 2017, 95, 607. [Google Scholar] [CrossRef]
- Mathey, J.; Rößler, S.; Lehmann, I. Urban green spaces: potentials and constraints for urban adaptation to climate change. Resilient Cities. Springer; 2011. p. 479–485.
- Aronson, M.F.; Lepczyk, C.A.; Evans, K.L.; et al. Biodiversity in the city: key challenges for urban green space management. Front Ecol Environ. 2017, 15, 189–196. [Google Scholar] [CrossRef]
- Bergero, P.E.; Ruggerio, C.A.; Lombardo Berchesi, R.J.; et al. Dispersal of Aedes aegypti: field study in temperate areas using a novel method. 2013. [Google Scholar]
- Hayden, M.H.; Uejio, C.K.; Walker, K.; et al. Microclimate and human factors in the divergent ecology of Aedes aegypti along the Arizona, US/Sonora, MX border. EcoHealth. 2010, 7, 64–77. [Google Scholar] [CrossRef]
- Hendy, A.; Hernandez-Acosta, E.; Chaves, B.A.; et al. Into the woods: Changes in mosquito community composition and presence of key vectors at increasing distances from the urban edge in urban forest parks in Manaus, Brazil. Acta Trop. 2020, 206, 105441. [Google Scholar] [CrossRef]
- Chevalier, J.F.; Nguema Maganga, V.; Assoumou, S. Les forêt du Gabon en 2008 [Internet]. MEFEPPN; 2008. p. 61–73. Report No.: 3. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjphuTlv7j8AhVEgP0HHdyKDFwQFnoECAkQAw&url=https%3A%2F%2Fwww.observatoire-comifac.net%2Ffile%2FeyJtb2RlbCI6IkFwcFxcTW9kZWxzXFxDYXRhbG9ndWVcXE1vZHVsZXNcXEZpbGUiLCJmaWVsZCI6ImRvY3VtZW50X2ZpbGUiLCJpZCI6NjU4fQ&usg=AOvVaw1fNKVWC7RI-UT7ZhkRMauD.
- Grard, G.; Caron, M.; Mombo, I.M.; et al. Zika virus in Gabon (Central Africa)–2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014, 8. [Google Scholar] [CrossRef]
- M’bondoukwé, N.P.; Kendjo, E.; Mawili-Mboumba, D.P.; et al. Prevalence of and risk factors for malaria, filariasis, and intestinal parasites as single infections or co-infections in different settlements of Gabon, Central Africa. Infect Dis Poverty. 2018, 7, 1–17. [Google Scholar] [CrossRef]
- Ushijima, Y.; Abe, H.; Nguema Ondo, G.; et al. Surveillance of the major pathogenic arboviruses of public health concern in Gabon, Central Africa: increased risk of West Nile virus and dengue virus infections. BMC Infect Dis. 2021, 21, 1–11. [Google Scholar]
- Mayaux, P.; Bartholomé, E.; Fritz, S.; et al. A new land-cover map of Africa for the year 2000. J Biogeogr. 2004, 31, 861–877. [Google Scholar] [CrossRef]
- Cordier, S. Sibang, l’histoire d’un arboretum en Afrique. Nîmes Lett L’OCIM. 2000, 19–27.
- Edwards, F.W. Mosquitoes of the Ethiopian Region. III.-Culicine adults and pupae. Mosquitoes Ethiop Reg III-Culicine Adults Pupae. 1941. [Google Scholar]
- Huang, Y.-M. The subgenus Stegomyia of Aedes in the Afrotropical Region with keys to the species (Diptera: Culicidae). Zootaxa. 2004, 700, 1–120. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The mathematical theory of information. Urbana Univ Ill Press. 1949, 97, 128–164. [Google Scholar]
- Magurran, A.E. Measuring biological diversity. Curr Biol. 2021, 31, R1174–R1177. [Google Scholar] [CrossRef]
- Hennig, C. fpc: flexible procedures for clustering (Version 2.2-9). 2020. [Google Scholar]
- Caliński, T.; Harabasz, J. A dendrite method for cluster analysis. Commun Stat-Theory Methods. 1974, 3, 1–27. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: an R package for multivariate analysis. J Stat Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Koumba, A.A.; Koumba, C.R.Z.; Nguema, R.M.; et al. Distribution spatiale et saisonnière des gîtes larvaires des moustiques dans les espaces agricoles de la zone de Mouila, Gabon. Int J Biol Chem Sci. 2018, 12, 1754–1769. [Google Scholar] [CrossRef]
- Paupy, C.; Ollomo, B.; Kamgang, B.; et al. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of Dengue and Chikungunya in central Africa. Vector-Borne Zoonotic Dis. 2010, 10, 259–266. [Google Scholar] [CrossRef]
- Sevidzem, S.L.; Pamba, R.; Koumba, A.A.; et al. Typology of breeding sites and species diversity of culicids (Diptera: Culicidae) in Akanda and its environs (North West, Gabon). Eur J Biol Biotechnol. 2020, 1. [Google Scholar] [CrossRef]
- Djoufounna, J.; Mayi, M.P.A.; Bamou, R.; et al. Larval habitats characterization and population dynamics of Culex mosquitoes in two localities of the Menoua Division, Dschang and Santchou, West Cameroon. J Basic Appl Zool. 2022, 83, 1–11. [Google Scholar] [CrossRef]
- Kamgang, B.; Happi, J.Y.; Boisier, P.; et al. Geographic and ecological distribution of the dengue and chikungunya virus vectors Aedes aegypti and Aedes albopictus in three major Cameroonian towns. Med Vet Entomol. 2010, 24, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Ngoagouni, C.; Kamgang, B.; Nakouné, E.; et al. Invasion of Aedes albopictus (Diptera: Culicidae) into central Africa: what consequences for emerging diseases? Parasit Vectors. 2015, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Nazni, W.; Lee, H.; et al. Mixed breeding of Aedes aegypti (L.) and Aedes albopictus Skuse in four dengue endemic areas in Kuala Lumpur and Selangor, Malaysia. Trop Biomed. 2006, 23, 224–227. [Google Scholar]
- SNRS; Norma-Rashid, Y.; et al. Sofian–Azirun, M. Mosquitoes larval breeding habitat in urban and suburban areas, Peninsular Malaysia. Int J Bioeng Life Sci. 2011, 5, 599–603. [Google Scholar]
- Ferreira-de-Lima, V.H.; Câmara, D.C.P.; Honorio, N.A.; et al. The Asian tiger mosquito in Brazil: Observations on biology and ecological interactions since its first detection in 1986. Acta Trop. 2020, 205, 105386. [Google Scholar] [CrossRef] [PubMed]
- Pereira E da, S.; Ferreira, R.L.; Hamada, N.; et al. Trichomycete fungi (Zygomycota) associated with mosquito larvae (Diptera: Culicidae) in natural and artificial habitats in Manaus, AM Brazil. Neotrop Entomol. 2005, 34, 325–329. [Google Scholar] [CrossRef]
- Wermelinger, E.D.; de Carvalho, R.W. Methods and procedures used in Aedes aegypti control in the successful campaign for yellow fever prophylaxis in Rio de Janeiro, Brazil, in 1928 and 1929. Epidemiol E Serviços Saúde. 2016, 25, 837–844. [Google Scholar] [CrossRef]
- Appawu, M.; Quartey, S. Effect of temperature on the development and predatory behaviour of Culex (Lutzia) tigripes (Grandpre and Charmoy). Int J Trop Insect Sci. 2000, 20, 129–134. [Google Scholar] [CrossRef]
- Appawu, M.A.; Dadzie, S.K.; Quartey, S.Q. Studies on the feeding behaviour of larvae of the predaceous mosquito Culex (Lutzia) tigripes Grandpre and Chamoy (Diptera: Culicidae). Int J Trop Insect Sci. 2000, 20, 245–250. [Google Scholar] [CrossRef]
- Jackson, N. Observations on the Feeding Habits of a Predaoeous Mosquito Larva, Culex (Lutzia) tigripes Grandpré and Charmoy (Diptera). 1953. p. 153–159.
- Moirangthem, B.D.; Singh, S.N.; Singh, D.C. Lutzia tigripes (Diptera: Culicidae, Metalutzia) for the mosquito larval control: A new prospect of mosquito control. Intl J Mosq Res. 2018, 5, 1–4. [Google Scholar]
- Longo-Pendy, N.M.; Tene-Fossog, B.; Tawedi, R.E.; et al. Ecological plasticity to ions concentration determines genetic response and dominance of Anopheles coluzzii larvae in urban coastal habitats of Central Africa. Sci Rep. 2021, 11, 1–13. [Google Scholar]
- Mutuku, F.M.; Alaii, J.A.; Bayoh, M.N.; et al. Distribution, description, and local knowledge of larval habitats of Anopheles gambiae sl in a village in western Kenya. 2006. [Google Scholar]
- Simard, F.; Nchoutpouen, E.; Toto, J.C.; et al. Geographic distribution and breeding site preference of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) in Cameroon, Central Africa. J Med Entomol. 2005, 42, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Tene Fossog, B.; Ayala, D.; Acevedo, P.; et al. Habitat segregation and ecological character displacement in cryptic African malaria mosquitoes. Evol Appl. 2015, 8, 326–345. [Google Scholar] [CrossRef]
- Mnzava, A.; Monroe, A.C.; Okumu, F. Anopheles stephensi in Africa requires a more integrated response. Malar J. 2022, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Diallo, D.; Diagne, C.T.; Buenemann, M.; et al. Biodiversity pattern of mosquitoes in southeastern Senegal, epidemiological implication in arbovirus and malaria transmission. J Med Entomol. 2019, 56, 453–463. [Google Scholar] [CrossRef]
- Pereira dos Santos, T.; Roiz, D.; Santos de Abreu, F.V.; et al. Potential of Aedes albopictus as a bridge vector for enzootic pathogens at the urban-forest interface in Brazil. Emerg Microbes Infect. 2018, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kamgang, B.; Nchoutpouen, E.; Simard, F.; et al. Notes on the blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) in Cameroon. Parasit Vectors. 2012, 5, 1–4. [Google Scholar] [CrossRef]
- Delatte, H.; Desvars, A.; Bouétard, A.; et al. Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Réunion. Vector-Borne Zoonotic Dis. 2010, 10, 249–258. [Google Scholar] [CrossRef]
- Fontenille, D.; Powell, J.R. From anonymous to public enemy: how does a mosquito become a feared arbovirus vector? Pathogens. 2020, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- Pagès, F.; Peyrefitte, C.N.; Mve, M.T.; et al. Aedes albopictus mosquito: the main vector of the 2007 Chikungunya outbreak in Gabon. PLoS One. 2009, 4, e4691. [Google Scholar] [CrossRef]
- Paupy, C.; Kassa Kassa, F.; Caron, M.; et al. A chikungunya outbreak associated with the vector Aedes albopictus in remote villages of Gabon. Vector-Borne Zoonotic Dis. 2012, 12, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Diallo, M.; Laganier, R.; Nangouma, A. First record of Ae. albopictus (Skuse 1894), in Central African Republic. Trop Med Int Health 2010, 15, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Kamgang, B.; Ngoagouni, C.; Manirakiza, A. Temporal patterns of abundance of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and mitochondrial DNA analysis of Ae. albopictus in the Central African Republic. PLoS Negl Trop Dis. 2013, 7, e2590. [Google Scholar] [CrossRef] [PubMed]
- Bagny Beilhe, L.; Arnoux, S.; Delatte, H.; et al. Spread of invasive Aedes albopictus and decline of resident Aedes aegypti in urban areas of Mayotte 2007–2010. Biol Invasions. 2012, 14, 1623–1633. [Google Scholar] [CrossRef]
- O’meara, G.F.; Evans Jr, L.F.; Gettman, A.D. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J Med Entomol. 1995, 32, 554–562. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.G.; Ubico, S.R. Arboviruses in birds. Infect Dis Wild Birds. 2007, 17, 62. [Google Scholar]
- Araujo, R.V.; Albertini, M.R.; Costa-da-Silva, A.L.; et al. São Paulo urban heat islands have a higher incidence of dengue than other urban areas. Braz J Infect Dis. 2015, 19, 146–155. [Google Scholar] [CrossRef]
- Coffinet, T.; Mourou, J.; Pradines, B.; et al. First record of Aedes albopictus in Gabon. J Am Mosq Control Assoc. 2007, 23, 471–472. [Google Scholar] [CrossRef]
- Longo-Pendy, N.M.; Boundenga, L.; Kutomy, P.O.O.; et al. Systematic Review on Diversity and Distribution of Anopheles Species in Gabon: A Fresh Look at the Potential Malaria Vectors and Perspectives. Pathogens. 2022, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Mourou, J.-R.; Coffinet, T.; Jarjaval, F.; et al. Malaria transmission in Libreville: results of a one year survey. Malar J. 2012, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Service, M. Contribution to the knowledge of the mosquitoes (Diptera, Culicidae) of Gabon. Cah ORSTOM Sér Entomol Méd Parasitol. 1976, 3, 259–263. [Google Scholar]

| Habitat nature | Inside the forest | Outside the forest | Overall |
|---|---|---|---|
| (n=28) | (N=76) | (N=104) | |
| Artificial | |||
| Glass jars | 1 (5.0%) | 1 (1.4%) | 2 (2.1%) |
| Gutters | 1 (5.0%) | 16 (21.6%) | 17 (18.1%) |
| Metallic container | 0 (0.0%) | 6 (8.1%) | 6 (6.4%) |
| Plastic container | 15 (75.0%) | 17 (23.0%) | 32 (34.0%) |
| Puddles | 0 (0.0%) | 18 (24.3%) | 18 (19.1%) |
| Tires | 0 (0.0%) | 14 (18.9%) | 14 (14.9%) |
| Wash basin | 1 (5.0%) | 1 (1.4%) | 2 (2.1%) |
| Discarded freezer | 0 (0.0%) | 1 (1.4%) | 1 (1.1%) |
| Discarded toilet bowl | 2 (10.0%) | 0 (0.0%) | 2 (2.1%) |
| Subtotal | 20 (100%) | 74 (100%) | 94 (100%) |
| Natural | |||
| Puddles | 1 (12.5%) | 0 (0.0%) | 1 (10.0%) |
| Tree holes | 5 (62.5%) | 0 (0.0%) | 5 (50.0%) |
| Streams | 2 (25.0%) | 2 (100%) | 4 (40.0%) |
| Subtotal | 8 (100%) | 2 (100%) | 10 (100%) |
| Species | Inside forest | Outside forest | Overall | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Artificial | Natural | Sub-total | Artificial | Natural | Sub-total | ||||||
| Ae. aegypti | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 27 (4.3%) | 0 (0.0%) | 27 (4.1%) | 27 (3.5%) | ||||
| Ae. albopictus | 67 (77.0%) | 3 (13.6%) | 70 (64.3%) | 188 (29.7%) | 0 (0.0%) | 188 (28.5%) | 258 (33.5%) | ||||
| An. gambiae s. l. | 0 (0.0%) | 1 (4.6%) | 1 (0.9%) | 41 (6.5%) | 0 (0.0%) | 41 (6.2%) | 42 (5.4%) | ||||
| Cx. cinerellus | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) | 0 (0.0%) | 1 (0.1%) | 1 (0.1%) | ||||
| Cx. decens | 2 (2.3%) | 0 (0.0%) | 2 (1.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.3%) | ||||
| Cx. duttoni | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 6 (0.9%) | 0 (0.0%) | 6 (0.9%) | 6 (0.8%) | ||||
| Cx. quinquefasciatus | 12 (13.8%) | 0 (0.0%) | 12 (11.0%) | 222 (35.1%) | 0 (0.0%) | 222 (33.6%) | 234 (30.4%) | ||||
| Cx. taufliebi | 0 (0.0%) | 11 (50.0%) | 11 (10.1%) | 17 (2.7%) | 19 (67.8%) | 36 (5.4%) | 47 (6.1%) | ||||
| Cx. trifilatus | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (0.5%) | 0 (0.0%) | 3 (0.5%) | 3 (0.4%) | ||||
| Cx. umbripes | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) | 0 (0.0%) | 1 (0.1%) | 1 (0.1%) | ||||
| Cx. univittatus | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 4 (0.6%) | 0 (0.0%) | 4 (0.6%) | 4 (0.5%) | ||||
| Culex sp. | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 12 (1.9%) | 1 (3.6%) | 13 (2.0%) | 13 (1.7%) | ||||
| Er. inornatus | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (0.5%) | 0 (0.0 %) | 3 (0.5%) | 3 (0.4%) | ||||
| Lu. tigripes | 6 (6.9%) | 5 (22.7%) | 11 (10.1%) | 108 (17.1%) | 8 (28.6%) | 116 (17.5%) | 127 (16.5%) | ||||
| Tx. evansae | 0 (0.0%) | 2 (9.1%) | 2 (1.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.3%) | ||||
| Overall | 87 (100%) | 22 (100%) | 109 (100%) | 633 (100%) | 28 (100%) | 661 (100%) | 770 (100%) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





