4. Discussion
The prevalence of antibiotic-resistant microorganisms to multiple antibiotics coupled with the increasing healthcare cost haves propelled researchers to develop new and cost-effective antimicrobial reagents. This development has led to the resurgence of metals used in ancient times since microorganisms did not develop resistance against these metals. Combinatorial therapy joining one or more drugs, is the most exciting area of research to combat multidrug-resistant pathogens. Combinatorial therapy has a potential track record concerning a broader antimicrobial spectrum, synergistic effect, and reduced risk for emerging resistance during therapy compared to monotherapy. In the absence of evidence-based treatment options, combinations are increasingly employed to enhance the antibacterial effects of available drugs [23]. However, due to the continuous misuse of antibiotics, the development of resistance and adverse effects associated with it can occur. A case study conducted by Paul et al. has compared the monotherapy’s effectiveness with combinatorial therapy consisting of aminoglycoside and β-lactam in patients with sepsis where bacterial resistance was frequently encountered [24]. They found that the continual use of combinational therapy was not associated with mortality but led to nephrotoxicity in patients.
Combinational therapy is considered a double-edged sword, so its usage must be strictly restricted to multi-drug-resistant bacteria [25]. Streptomycin-resistant bacteria have been treated with streptomycin conjugated metal nanoparticles in place of β-lactam. The following observations have been made from this study:
The mechanism of action of metal nanoparticles is similar to β -lactam antibiotics against bacteria.
The enhanced potential of combinational therapy consists of aminoglycoside antibiotics with metal nanoparticles to treat aminoglycoside-resistant bacteria.
Therefore, an attempt has been made in the present investigation to evaluate the antibacterial activity of bare metal nanoparticles [26,27,28] and metal nanoparticles conjugated with streptomycin.
The results obtained in the present investigation for the first time demonstrated enhanced bactericidal activity of the green synthesized silver (AgNP), gold (AuNP), and platinum (PtNP) nanoparticles when they were conjugated with the streptomycin compared to their free forms. Generally, such conjugation needs the process of functionalization and high energy requirements. Ramulu et al. (2015) [29] synthesized the silver nanoparticles after the reduction of 2 mM silver nitrate with 0.6 mM NaBH4, followed by biofunctionalization with antibacterial peptide polymyxin–B. Similarly, [30] have synthesized aminoglycoside gold nanoparticles by adding trisodium citrate to 1mM chloroauric acid, followed by adding 3 mM drug to the citrate-capped gold nanoparticles under stirring conditions for two h continuously. Hydrolysable linkers have been used to conjugate Human Serum Albumin nanoparticles with two antineoplastic agents, imatinib and 5-fluorouracil [31]. 1-Ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDC) has been used as an agent to link the 5-fluorouracil with green synthesized gold nanoparticles synthesized from the peel extract of Punica granatum with folic acid [32]. In the present study, no such chemical interference has been utilized as a functionalizing agent in determining the bio-conjugated nanoparticles’ bactericidal activity of the bioconjugated nanoparticles to avoid the adverse effect of chemicals [33].
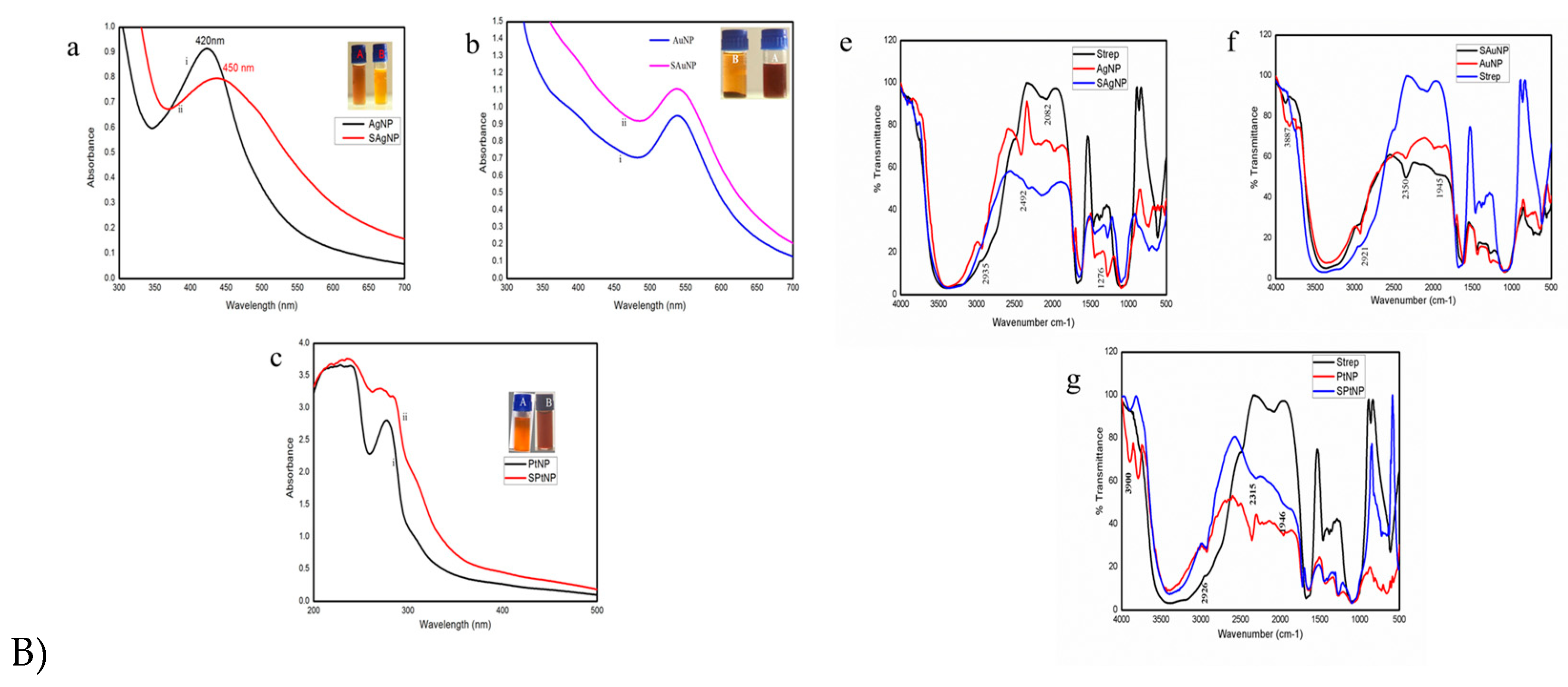
The UV-VIS spectral analysis of the streptomycin conjugated metal nanoparticles, such as SAgNP, SAuNP, and SPtNP, has suggested that the metal nanoparticles’ integrity was not compromised even after the bio-fabrication with the drug. The decreased aggregation observed with the streptomycin conjugated metal nanoparticles further points to the presence of streptomycin on the surface of metal nanoparticles. Adsorption appears to be the interaction between the antibiotics and nanoparticles, as is evident from the redshift in the surface plasmon resonance in the spectrum. This phenomenon indicates the formation of the larger nanoparticles; however, the shift is less than ~3 nm in the case of SPtNP (
Figure 2c), 10 nm in the case of SAuNP (
Figure 2b), and 30 nm in the case of SAgNP (
Figure 2a) when compared to their bare nanoparticles. A similar observation was made by Debalina et al. [34] when the gold nanoparticles synthesized with sodium borohydride as the reducing agent was conjugated with ampicillin, streptomycin, and kanamycin. The surface plasmon resonance observed for the bare gold nanoparticles was 526 nm. The conjugation of antibiotics with nanoparticles leads to the formation of larger particles, as evidenced by the color change from red wine to purple. The redshift observed suggested the adsorption of the antibiotics onto the surface of the nanoparticles. It is well known that an antibiotic molecule’s the presence of hydroxyl and amine groups of an antibiotic molecule can easily protonate in an acidic or neutral solution, transfer the electrons to the metal ions and form metal-amine complexes via simple amine chemistry [35].
The FT-IR spectrum is the fingerprint for identifying unknown compounds compared to previous reference spectra [36]. The alteration in frequency and intensity observed between the bare metal nanoparticles and streptomycin conjugated metal nanoparticles is due to the change in the atom vibration. Nirmala and Pandian [30] have suggested that the drug aminoglycoside contains active groups which can efficiently react with gold nanoparticles by chelation and get adsorbed onto the surface. The antimicrobial entity comes near the nanogold core surrounded by aminoglycoside drug moieties. In the present study, spectral analysis of streptomycin and streptomycin conjugated metal nanoparticles (SAgNP, SAuNP, and SPtNP) suggests a possible alteration in the vibration of the atom, and the change in the –NH band stretch indicates the involvement of the amine group of streptomycin in the conjugation process where the chemical structure of the drug left unaltered. However exact nature of events during the conjugation process in the present study is far from satisfactory and warrants rigorous analyses to explain the phenomenon behind the conjugation.
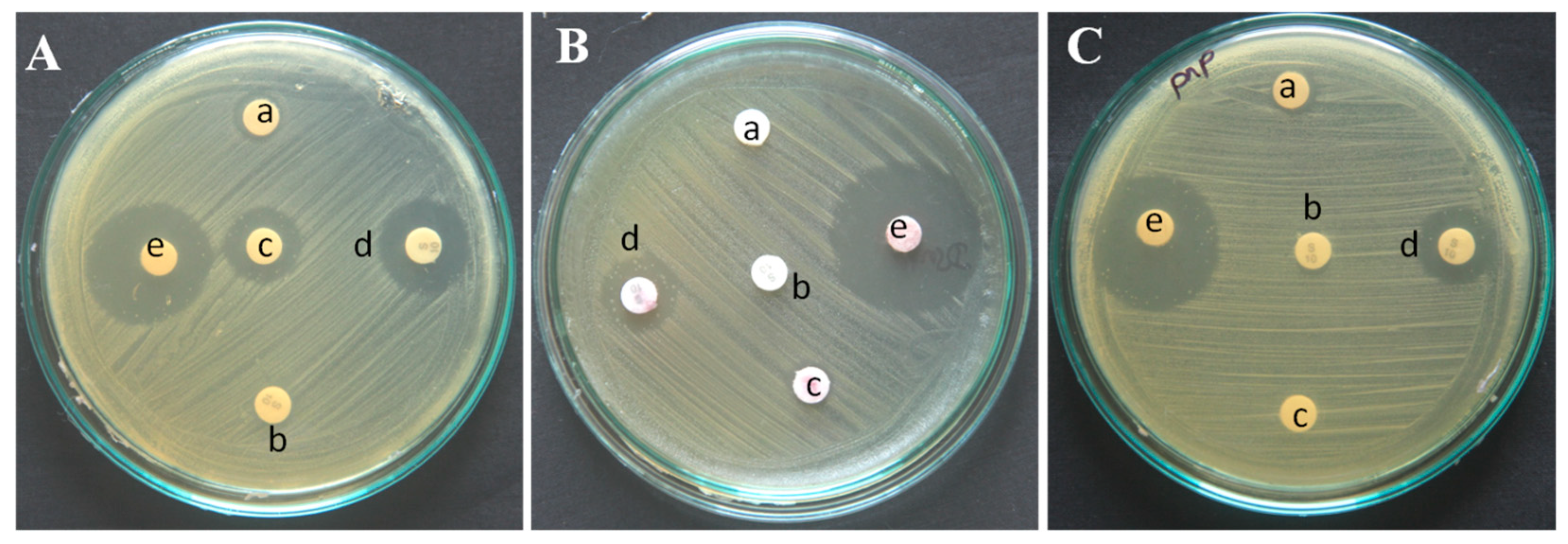
The antibacterial activity of bare green synthesized nanoparticles (AgNP, AuNP, and PtNP), streptomycin preloaded disk impregnated with green synthesized metal nanoparticles, and streptomycin conjugated metal nanoparticles (SAgNP, SAuNP, and SPtNP) have been performed against the streptomycin-resistant
Bacillus sp. by disk diffusion method. There was a remarkable increase in antibacterial activity when the antibiotic streptomycin was conjugated with a silver nanoparticle (22 mm); however, the antibacterial activity observed with a streptomycin preloaded disk impregnated with silver nanoparticle showed only a marginal increase in activity (16 mm) from the bare silver nanoparticles (14 mm). In terms of percentage, the highest (37.5%) antibacterial activity was observed with streptomycin conjugated silver nanoparticles (SAgNP), followed by streptomycin preloaded disk impregnated with silver nanoparticles (14%) over the bare silver nanoparticles. Interestingly, the streptomycin conjugated gold (SAuNP) and platinum (SPtNP) nanoparticles have shown a 100 and 76% increase in antibacterial activity, respectively, when compared to the activity shown by the same nanoparticles when added to streptomycin preloaded discs. Thus, it is evident that the enhancement of the antibacterial activity is due to the conjugation of streptomycin onto the nanoparticles (
Table 1).
One essential property of any antibacterial agent is determining its minimal inhibitory concentration, allowing it to be used in a clinical setup. The minimum inhibitory concentration (MIC) is defined as the lowest concentration of an antimicrobial agent that inhibits the visible growth of bacteria. This information will be quite valuable during the antibacterial agent’s research and development phase of an antibacterial agent to determine the appropriate concentrations required in the final product. These drug concentrations are needed to produce the effect; ordinarily several hundred to a thousand times less than the concentration found in the final finished dosage form. The minimum bactericidal concentration (MBC) is the lowest concentration of an antibacterial agent required to kill a bacterium over a fixed period of 18 or 24 h, under a specific set of conditions. It can be determined by sub-growing the bacterium previously incubated with the antibiotics on an agar medium after appropriate dilutions.
It has been observed in the present study that the streptomycin-resistant
Bacillus sp. was found to be highly resistant to free streptomycin and bare gold and platinum nanoparticles (
Figure S1). However, the same bacterium became susceptible when treated with the bare and streptomycin conjugated metal nanoparticles in a dose-dependent manner (
Table S1). Among the conjugated nanoparticles, the gold nanoparticle with streptomycin showed the lowest MIC (0.106 ppm) followed by SAgNP (0.187 ppm) and SPtNP (34.0 ppm).
The turbidity caused by the insoluble compounds during broth dilution has been observed to lead to false-positive results. Therefore, the MBC was determined by culturing on Muller Hinton agar medium. The agar medium incorporated with a particular concentration of test compound where no visible growth occurred was determined as the minimum bactericidal concentration (MBC). The MBC’s of AgNP, SAgNP, SAuNP, and SPtNP was found to be 0.65, 0.375, 0.2135, and 68 ppm, respectively.
It has become essential to distinguish a compound’s bactericidal or bacteriostatic activity of a compound. Woods and Washington [37] have described an antimicrobial agent as bactericidal when the MBC/MIC ratio is less than or equal to 4, and bacteriostatic when the ratio is greater or equal to 16. Thus, the MBC/MIC ratio is a parameter that reflects the bactericidal capacity of the analyzed compound [38]. In the present study, the tolerance level of the test bacterium was determined by analyzing the ratio of MBC/MIC, and the ratio of all the analyzed antimicrobial agents such as AgNP, SAgNP, SAuNP, and SPtNP was found to be surprisingly 2, i.e., the MBC is double the concentration of MIC, suggesting that the compound could be potential antibacterial agents against streptomycin-resistant Bacillus sp. Whereas SAuNP and SPtNP exhibited antibacterial activity only after conjugating the streptomycin with the bare nanoparticles, suggesting that the nanoconjugates increased the antibacterial property of streptomycin by acting as a nanocarrier of the drug to the target site. The results obtained in the present study have been in line with the observations made by Das et al. [38]; they evaluated the antibacterial activity of silver nanoparticles synthesized from the leaf extract of O. gratissimum against multidrug-resistant E. coli and S. aureus strains. The obtained MIC for E. coli was 4 μg/ml and for S. aureus was 8 μg/ml, and the MBC was two times higher than the MIC, which was 8 and 16 μg/ml, respectively, for the organisms.
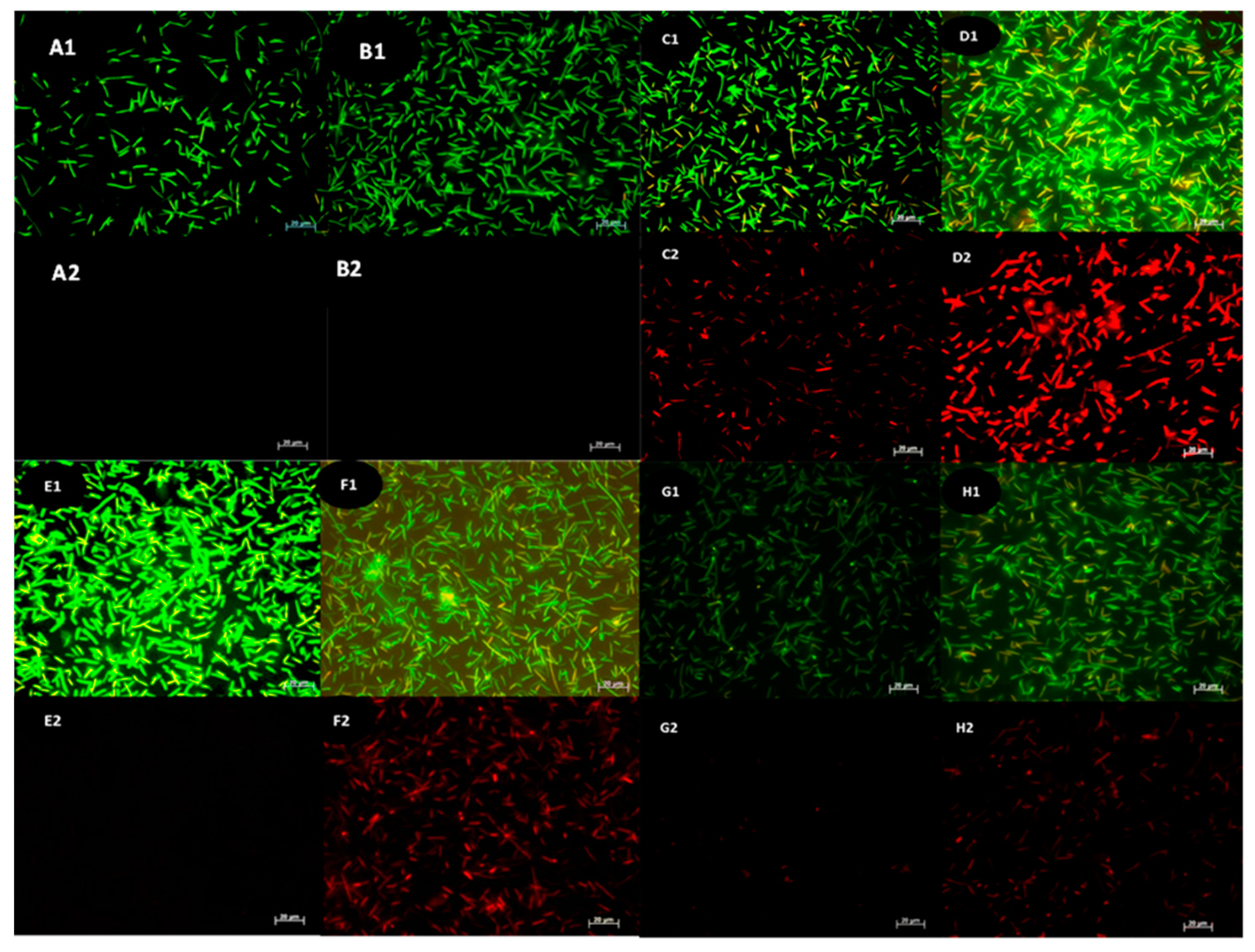
After treatments, the intact and compromised cells can be distinguished by staining the treated cells with AO/PI staining and viewing them under a fluorescent microscope. The membrane integrity of the bacteria treated with the antimicrobial test compounds has also been analyzed using acridine orange and propidium iodide staining [39]. The stains used were the membrane-impermeable PI causing red fluorescence in cells with compromised membranes. AO is a cell-permeable nucleic acid binding green fluorescent dye that enters the cells regardless of whether they are intact or compromised. The live/undamaged cells will appear green, whereas bacteria with compromised cell walls will appear reddish-orange, and the dead cells will appear red. The streptomycin-resistant Bacillus sp was treated with the respective MIC of tested antimicrobial compounds. The cells with compromised cell walls allow the PI stain to get through the cell wall due to the lack of cell wall integrity, and thus the PI stains the nucleic acid (DNA) and appears red when viewed under a fluorescence microscope. The number of red fluorescent cells was higher in the case of SAgNP, SAuNP, and SPtNP treated cells than in AgNP treatment.
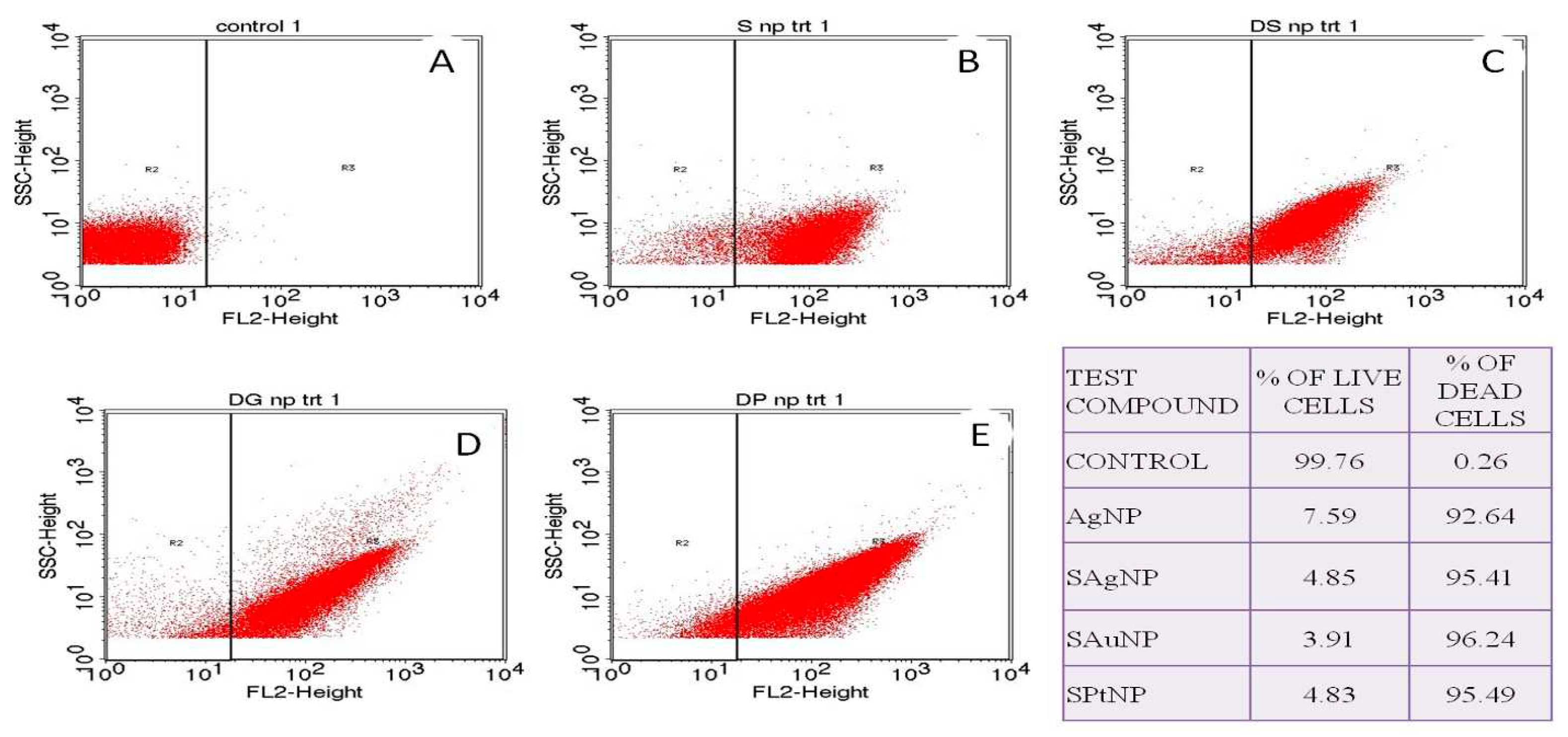
Accurate determination of live and dead bacteria is vital in determining the dosage level of antibiotics. The traditional culture-based viable test does not provide real-time results needed in applications such as industrial manufacturing. However, the enumeration of accurate live and dead cells is now possible with flow cytometric analyses. The nucleic acid leaked out due to the treatment will be freely available for the PI dye for binding, leaving the cells available to emit red fluorescence, and thus enumeration of the dead cells is possible by flow cytometric technique. In the present study, the cells were treated with their MIC concentration, and the numbers of dead cells were enumerated, and 96% of cells were found dead at 0.106 ppm, 95% at 0.18, and 34 ppm for SAgNP and SPtNP, respectively. About 92% of dead cells were found at 0.325 ppm in respect of AgNP treatment.
Scanning electron micrographs representing the ultrastructure of cells are a powerful method for understanding the effect of nanoparticles and have been widely described in the literature. An earlier observation made by Das et al. [38,40] has shown damaged membranes of bacterial cells when treated with silver nanoparticles synthesized from the extract of O. gratissimum, which resulted in enhanced plasma membrane permeability of the plasma membrane. This permeability has led to the leakage of cellular content and the death of the cells [41].
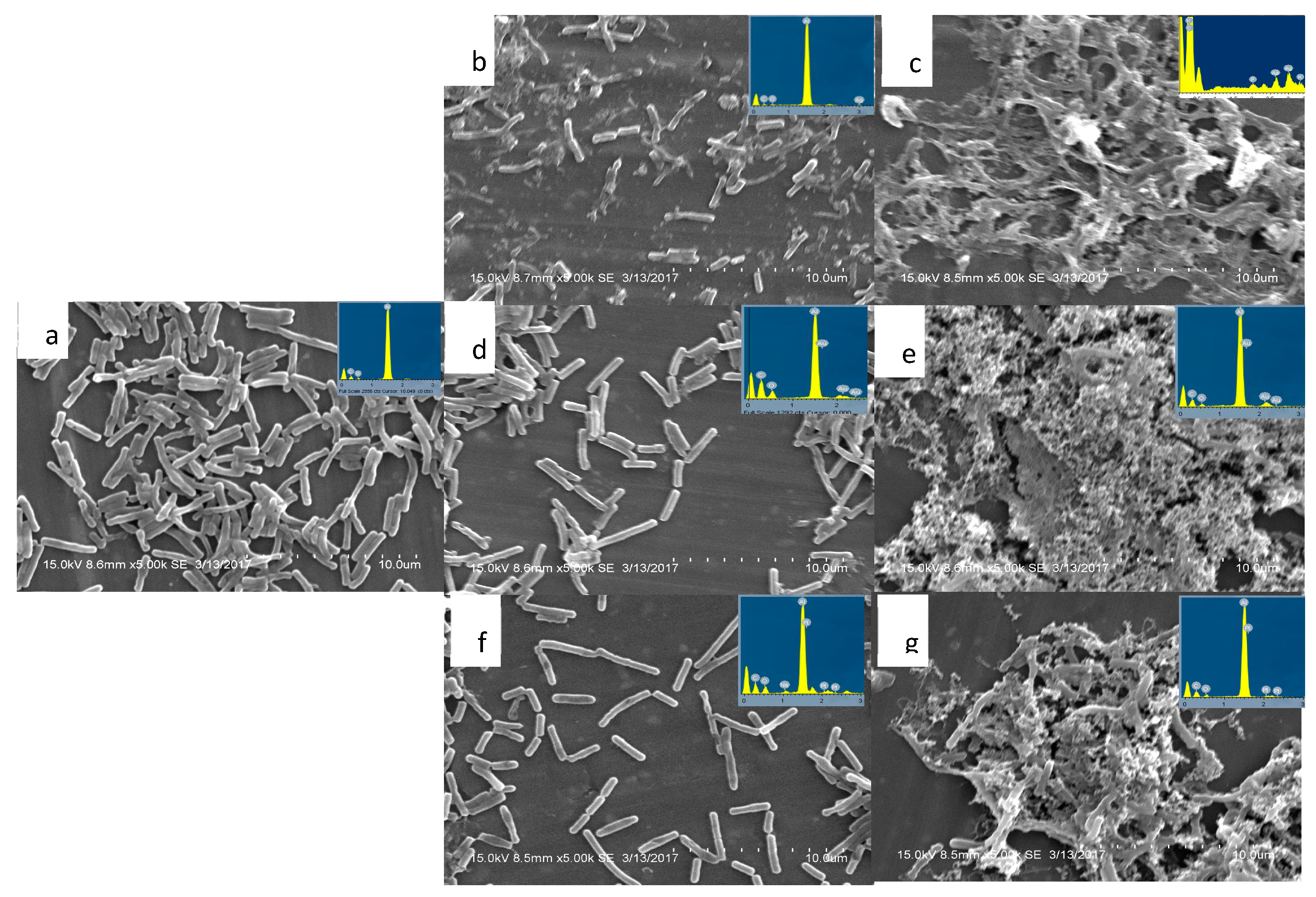
The present study has also attempted to evaluate the structural changes after challenging the bacterium with antibiotic-conjugated nanoparticles using electron microscopic analysis. The cells treated with silver nanoparticles showed extensive damage to the structure (
Figure 5b). Most cells were disintegrated and lysed. The cells treated with gold (AuNP) and platinum (PtNP) nanoparticles, however, did not have any effect on the integrity of the structure (
Figure 5b,d).
The exact mechanism by which the nanoparticles penetrate the bacteria is not fully understood. However, according to Morones et al. [42], silver nanoparticles can damage the cells by interacting with phosphorous and sulfur-containing compounds such as DNA and regulating enzymes. In the present study, the silver nanoparticles (AgNP) alone have shown the potential to damage the bacterial cells by creating small pits on the surface and penetrating the cells, which causes the leakage of intracellular compounds and eventually leads to the death of the bacteria. The failure of gold (AuNP) and platinum (PtNP) nanoparticles may be due to the lack of affinity towards phosphorus and sulfur-containing compounds compared to silver. The gold /platinum nanoparticles are higher-order metals used as biocompatible catalyst and drug delivery agents because of their inertness. However, the inert gold and platinum nanoparticles, when conjugated with streptomycin, have acquired remarkable antibacterial activity and were able to lyse the cells (
Figure 5e,g), ultimately leading to death. In general, antibiotic resistance against the aminoglycoside class of antibiotics by bacteria can be trespassed by combining with the β-lactam class of drugs. With this crucial point in mind, we can understand the mechanism of the nanoparticle, where the nanoparticle acts as the agent to damage the bacteria’s cell wall of the bacteria and carries the streptomycin inside the cells, and then it acts on the protein machinery leading to the death of the bacteria.
One of the most critical challenges in the growing field of nanomedicine is the behavioral nature of nanoparticles inside the cells. Metabolic and immunological responses induced by the nanoparticles are still under investigation; however, they may act as checkpoints for the growth of nanomedicine. Nanotoxicology has been described as the discipline that deciphers the molecular events that regulate nanoparticle bioaccumulation and toxicity [43]. There must be enhanced vigilance on the potential secondary effects generated by using nanomaterials in nanomedicine. Blood is the major component in humans that serves as the primary route for the translocation of foreign bodies into cells, tissues, and organs. Therefore, its compatibility with foreign bodies assumes significance in developing materials like nanoparticles into effective antimicrobial agents.
RBC is an essential component of the blood circulatory system since it plays ais crucial role in transporting oxygen to the body tissues. Any malfunction or disruption of the RBC might pose a risk to the health; therefore, the need to test the influence of the nanoparticles on RBC is inevitable. Though it is considered to be the most crucial area of research, there have been only a few reports available on the hemocompatible nature of the nanoparticles, such as silica [44],[45], TiO2 [46], and polymer nanoparticles [47].
In the present study, an attempt has been made to assess the hemocompatible nature of the bare and streptomycin conjugated silver, gold, and platinum nanoparticles synthesized from the rind extract of
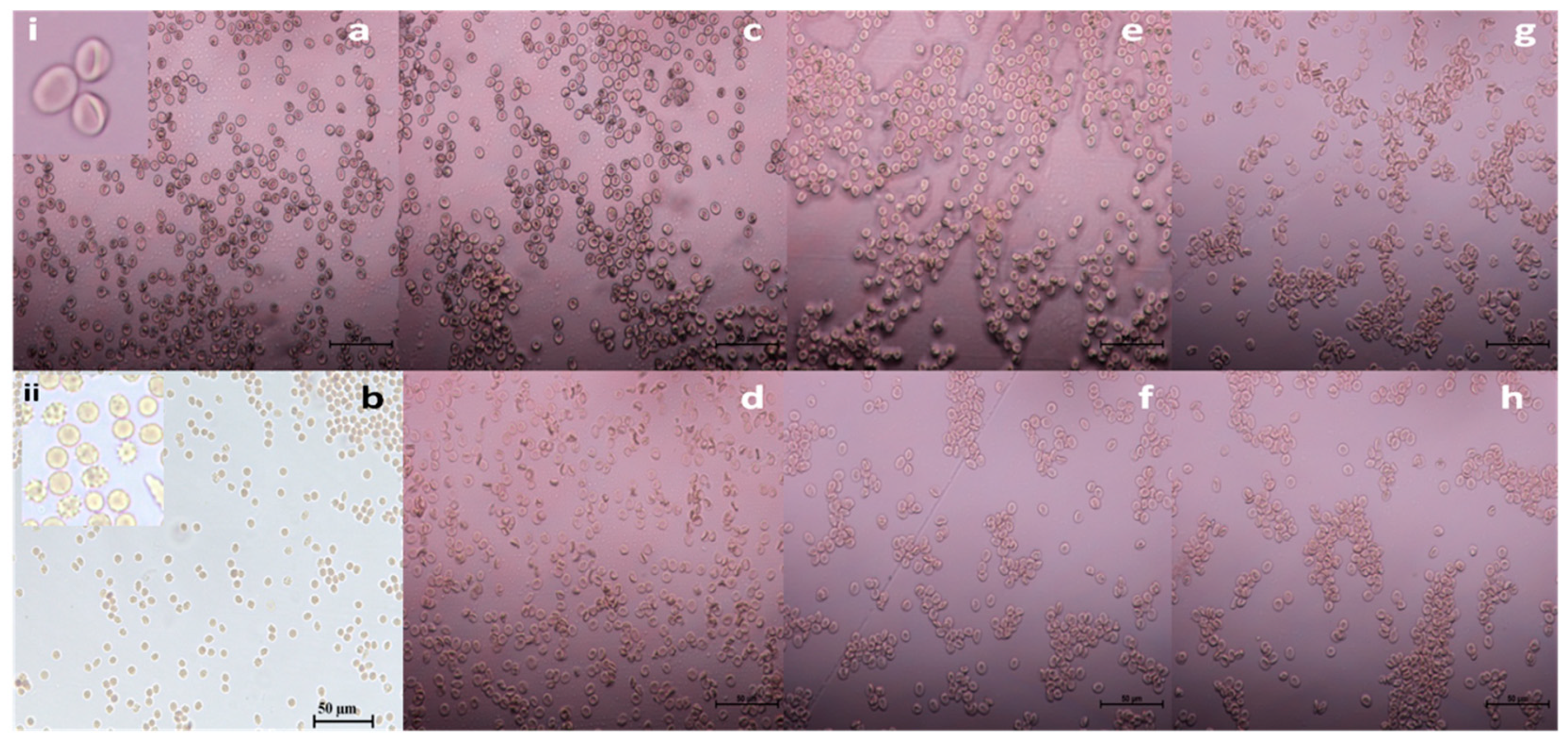
G. mangostana fruit. It has been observed that no hemolysis was induced either by the bare nanoparticles or streptomycin conjugated nanoparticles (
Figure S8).
Investigations undertaken to assess the hemocompatible nature of gold nanoparticles have shown that they are biocompatible and can be used as nanomedicine. It has been found that the gold nanoparticles synthesized from the extract of Zinger Officinale caused very little (0.2%) hemolysis, even at the highest concentration of 10 mg/ml. [48]. Similarly, the silver nanoparticles synthesized from β-glucan isolated from the edible mushroom, Pleurotus Florida, showed hemocompatibility despite antibacterial activity against the multi-antibiotic resistant bacteria (MAR) K. pneumonia YSI6A [49]. The RBC treated with silver nanoparticles at 15 μg/ml concentration induced only 0.68 % of hemolysis and was found to be hemocompatible. The MIC for the particular bacterium was found to be 40 μg/ml, which is ~2 times higher than the LD50 concentration however found to be compatible with RBC. The percentage of hemolysis of platinum nanoparticles synthesized from the extract of seaweed, Padina gymnospora reported as 13% for 40 μg/ml [50].
The silver nanoparticles synthesized from Catharanthus roseus have caused hemolysis at 10 μg/ml; however, the concentration from 1 to 5 μg/ml has been considered safe on RBC [51]. Srinath et al. [52] synthesized gold nanoparticles from the bacterium Brevibacillus formosus and showed them to have no hemolytic activity even at the highest concentration (150 and 200 μg/ml). The hemocompatible nature of the gold nanoparticles synthesized from the tuber extract of Curcuma mangga has also been investigated recently [53]. The percentage of hemolysis increased when the concentration of the gold nanoparticles increased, and the observed percentages were 2.7, 1.8, 2.6, 6.2, and 9.9%, with 3.13, 6.25, 12.5, 25, and 50 μg/ml, respectively.
Asharani et al. [54] have observed that the platinum and gold nanoparticles caused no hemolysis and hemagglutination, whereas silver nanoparticles caused hemolysis [55]. They synthesized four different types of nanoparticles through the chemical method and investigated the effect of those nanoparticles on the surface of human erythrocytes. It has been suggested that the membrane damage in the nanoparticle-treated cells led to the lysis of RBC, damaging the adjacent normal cell through DNA damage [56]. The results obtained in the present study are in good agreement with the above research. In the present research, the silver nanoparticles (AgNP) synthesized from the rind extract of
G. mangostana fruit alone showed hemolysis at a higher concentration (0.65 ppm). In comparison, no hemolysis occurred at a lower concentration. Gold and platinum nanoparticles were comparatively more hemocompatible than the silver nanoparticles synthesized from the same source (
Figure S8a).
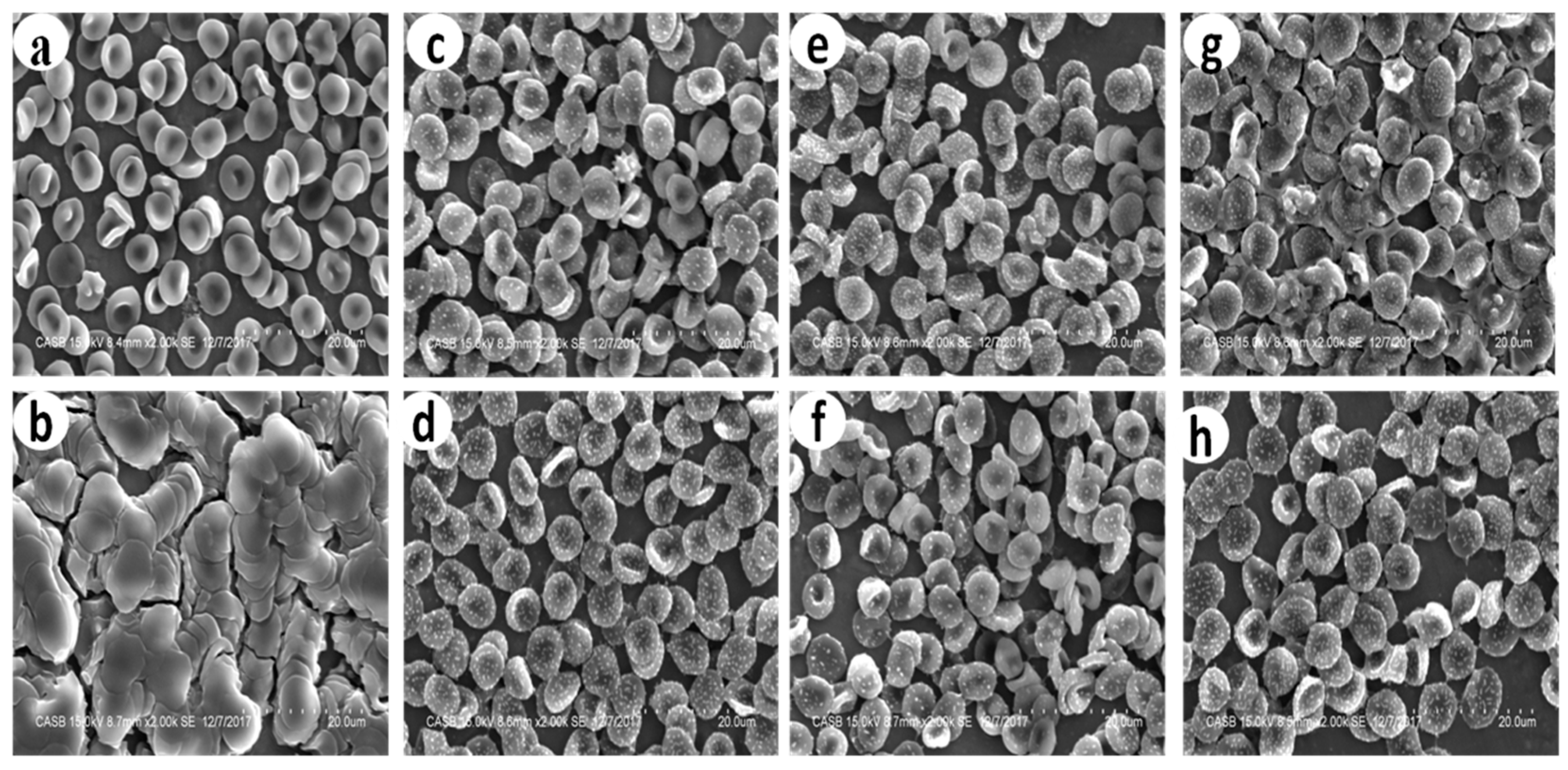
The electron microscopic analysis of cells treated with platinum nanoparticles (PtNP) revealed the presence of a few ghost cells and the slight alteration in the shape of RBC from discocyte to spherocyte (
Figure 7g). Continuous exposure might have led to the loss of the membrane’s resilience and flexibility. Further investigation is necessary to ascertain the nature of the effect before this can be taken for any clinical trial.
Interestingly, the RBC treated with bare and streptomycin conjugated gold nanoparticles (
Figure 7e,f) showed no sign of morphological disturbances, which suggests that these conjugates could be used against the multidrug-resistant bacteria without causing any effect on the normal cells (
Figure 7a).
5. Conclusions
Bio-fabrication of streptomycin onto green synthesized noble metal nanoparticles synthesized from the aqueous rind extract of G. mangostana fruit has been achieved, which could be considered the most cost-effective drug modification methodology. The streptomycin conjugated nanoparticles were characterized using UV-VIS and FT-IR spectral analyses. The addition of streptomycin to the nanoparticles caused a solution color change and showed a shift in the absorption spectra from 420 to 450 nm for SAgNP, 540 to 545 nm for SauNP, and 260 to 263 nm for SPtNP when analysed with UV-VIS spectroscopy. The shift in the wavelength confirms the successful conjugation between the streptomycin and the metal nanoparticles. The FT-IR spectra obtained for sAgNP, SAuNP, and SPtNP have shown that the –NH stretching of the streptomycin was responsible for attachment on the surface of the metal nanoparticle and maintaining the chemical structure of the drug even after conjugation. The antibacterial activity of the streptomycin-conjugated metal nanoparticles (sAgNP, SAuNP & SPtNP) and the streptomycin preloaded disk impregnated with nanoparticles was evaluated by the disk diffusion method. The streptomycin conjugated gold nanoparticle (SAuNP) showed a 100 % antibacterial activity compared with the AuNP alone, while the streptomycin conjugated silver and platinum nanoparticles showed a 22 and 76 % increase, respectively. The minimum inhibitory concentration of AgnP and SAgNP was determined as 0.325 and 0.187 ppm, respectively. The MIC value observed with streptomycin conjugated silver nanoparticles was 46% lesser when compared with the value observed with bare nanoparticles. The MIC of streptomycin conjugated gold and platinum nanoparticles were found to be 0.1067 and 34.0 ppm, respectively. The minimum bactericidal concentration of AgNP, sAgNP, SAuNP, and SPtNP were determined as 0.65, 0.375, 0.2135, and 68 ppm, respectively. The test compounds’ respective MIC and MBC values determined the tolerance level of streptomycin-resistant Bacillus sp. The ratio of MBC/MIC for AgNP, SAgNP, SAuNP, and SPtNP was 2.0, suggesting that these compounds are bactericidal in nature. After treating them with streptomycin conjugated nanoparticles, the number of live and dead bacteria after treating them with streptomycin conjugated nanoparticles was calculated using flow cytometric analysis. 96% of cells were found dead upon treatment with SAuNP, while 92 and 95 cells were found dead upon treatment with AgNP and SAgNP, respectively. About 95% of cells were found dead upon treatment with SPtNP. The cells treated with streptomycin conjugated nanoparticles showed lysis and fragmentation, suggesting that the streptomycin conjugated nanoparticles could be an ideal agent for treating drug-resistant bacteria in a clinical setup. The hemocompatibility of bare and streptomycin conjugated metal nanoparticles was evaluated with human erythrocytes. No hemolysis was observed when the RBC was treated with silver nanoparticles at its MIC of 0.325 ppm. Cells treated with higher concentrations (0.65 ppm) were rendered hemolytic to 28%. No hemolysis was observed when the cells were treated with AuNP, PtNP, SAgNP, SAuNP, and SPtNP, even when they were used at their highest concentration. Light microscopic analysis of RBC treated with the test compounds showed no sign of aggregation, while the formation of many ghost cells was observed in the cells treated with Triton X-100. Scanning electron microscopic analysis of RBC treated with bare and streptomycin conjugated nanoparticles has shown the characteristic biconcave cells with smooth surfaces suggesting the hemocompatibility nature of the test compounds.
This present investigation opens a new avenue of research for the cost-effective green synthesis of metal nanoparticles from agricultural waste, which can be considered as the value-added product, and the conjugation of the drug onto the surface of the metal nanoparticles without any harmful interfacing agents projects this study as a unique and novelty. Moreover, the emergence of multidrug resistance in pathogenic organisms has caused growing concern, especially among healthcare providers, which necessitated the development of new antimicrobial compounds. Antibiotic resistance keeps increasing in frequency, with all major classes of antibiotics used to treat a wide variety of diseases. The development of resistance against metal nanoparticles is more challenging for any pathogen. Thus, an effort has been made in the present investigation and proves a new formulation of drugs by conjugating the commercial drug with nanoparticles to treat microbial resistance.
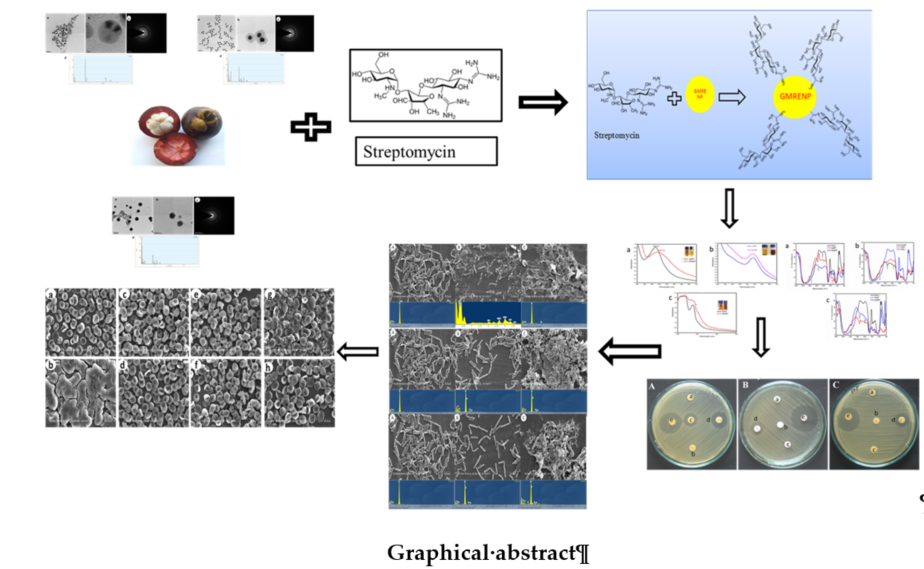
Figure 1.
A) Schematic representation of bioconjugation of the drug Streptomycin onto the surface of green synthesized metal nanoparticles from the rind extract of Garcinia mangostana Linn. (AgNP, AuNP, and PtNP). B) UV-VIS spectral analysis of streptomycin conjugated onto the surface of silver (1. B) a-SAgNP), gold (1. B) b-SAuNP), and platinum (1. B) c-PtNP) nanoparticles [Inset: Turbidity induced by conjugation of streptomycin onto the nanoparticles (B) compared to bare nanoparticles alone (A)]; FT-IR spectral analysis of streptomycin, silver nanoparticles (AgNP), and nanoparticles conjugated with streptomycin (SAgNP) 1. B)-e; GNPs 1. B)-f; PtNPs 1. B)-g.
Figure 1.
A) Schematic representation of bioconjugation of the drug Streptomycin onto the surface of green synthesized metal nanoparticles from the rind extract of Garcinia mangostana Linn. (AgNP, AuNP, and PtNP). B) UV-VIS spectral analysis of streptomycin conjugated onto the surface of silver (1. B) a-SAgNP), gold (1. B) b-SAuNP), and platinum (1. B) c-PtNP) nanoparticles [Inset: Turbidity induced by conjugation of streptomycin onto the nanoparticles (B) compared to bare nanoparticles alone (A)]; FT-IR spectral analysis of streptomycin, silver nanoparticles (AgNP), and nanoparticles conjugated with streptomycin (SAgNP) 1. B)-e; GNPs 1. B)-f; PtNPs 1. B)-g.
Figure 2.
Antibacterial activity of the metal nanoparticles before and after conjugation with streptomycin against the streptomycin-resistant Bacillus sp. a: G. mangostana rind extract (GMRE); b: streptomycin; c: green synthesized AgNP (A), AuNP (B) and PtNP (C) nanoparticles; d: streptomycin preloaded disc impregnated with AgNP (A), AuNP (B) and PtNP (C) nanoparticles; e: streptomycin conjugated AgNP (A), AuNP (B) and PtNP (C) nanoparticles.
Figure 2.
Antibacterial activity of the metal nanoparticles before and after conjugation with streptomycin against the streptomycin-resistant Bacillus sp. a: G. mangostana rind extract (GMRE); b: streptomycin; c: green synthesized AgNP (A), AuNP (B) and PtNP (C) nanoparticles; d: streptomycin preloaded disc impregnated with AgNP (A), AuNP (B) and PtNP (C) nanoparticles; e: streptomycin conjugated AgNP (A), AuNP (B) and PtNP (C) nanoparticles.
Figure 3.
Determination of live/dead cells of Bacillus sp. after treatment with streptomycin (B1&B2), AgNP (C1&C2), SAgNP (D1&D2), AuNP (E1&E2), SAuNP (F1&F2), PtNP(G1&G2), and SPtNP (H1&H2). AO/PI dual stained cells: AO stained: control (A1) and streptomycin treated (B1) cells; AgNP (C1), SAgNP (D1), AuNP(E1), SAuNP(F1), PtNP(G1) and SPtNP(H1). Propidium iodide-stained control (A2) and streptomycin treated (B2) cells, AgNP (C2), SAgNP (D2), AuNP(E2), SAuNP(F2), PtNP(G2) and SPtNP(H2).
Figure 3.
Determination of live/dead cells of Bacillus sp. after treatment with streptomycin (B1&B2), AgNP (C1&C2), SAgNP (D1&D2), AuNP (E1&E2), SAuNP (F1&F2), PtNP(G1&G2), and SPtNP (H1&H2). AO/PI dual stained cells: AO stained: control (A1) and streptomycin treated (B1) cells; AgNP (C1), SAgNP (D1), AuNP(E1), SAuNP(F1), PtNP(G1) and SPtNP(H1). Propidium iodide-stained control (A2) and streptomycin treated (B2) cells, AgNP (C2), SAgNP (D2), AuNP(E2), SAuNP(F2), PtNP(G2) and SPtNP(H2).
Figure 4.
Fluorescence-activated cell sorting analysis of control (A), bare silver nanoparticles (MIC- 0.325ppm) (B), streptomycin conjugated, silver (MIC- 0.187ppm) (C), gold (MIC- 0.1067ppm) (D), and platinum (34ppm) (E) nanoparticles treated cells of Bacillus sp. Inset, the table shows the percentage of live and dead cells after the treatment at their respective minimum inhibitory concentration.
Figure 4.
Fluorescence-activated cell sorting analysis of control (A), bare silver nanoparticles (MIC- 0.325ppm) (B), streptomycin conjugated, silver (MIC- 0.187ppm) (C), gold (MIC- 0.1067ppm) (D), and platinum (34ppm) (E) nanoparticles treated cells of Bacillus sp. Inset, the table shows the percentage of live and dead cells after the treatment at their respective minimum inhibitory concentration.
Figure 5.
Scanning electron microscopic analysis of untreated cells of Bacillus sp. (a), cells treated with bare silver nanoparticles (b), cells treated with streptomycin conjugated silver nanoparticles (c), cells treated with bare gold nanoparticles (d), and cells treated with streptomycin conjugated gold nanoparticles (e), cells treated with bare platinum nanoparticles (f), and cells treated with streptomycin conjugated platinum nanoparticles (g) with respective EDX analysis.
Figure 5.
Scanning electron microscopic analysis of untreated cells of Bacillus sp. (a), cells treated with bare silver nanoparticles (b), cells treated with streptomycin conjugated silver nanoparticles (c), cells treated with bare gold nanoparticles (d), and cells treated with streptomycin conjugated gold nanoparticles (e), cells treated with bare platinum nanoparticles (f), and cells treated with streptomycin conjugated platinum nanoparticles (g) with respective EDX analysis.
Figure 6.
Effect of bare and streptomycin conjugated nanoparticles on the structural integrity of RBC’s (a) PBS; (b) Triton X – 100; c: AgNP (0.325 ppm); (d) SAgNP (0.187 ppm); (e) AuNP (127 ppm); (f) SAuNP (0.106 ppm), (g) PtNP (235 ppm); (h) SPtNP (34 ppm). (40X). Insets: (i) RBC with biconcave smooth surface; (ii) Appearance of ghost cells following treatment with Triton X - 100.
Figure 6.
Effect of bare and streptomycin conjugated nanoparticles on the structural integrity of RBC’s (a) PBS; (b) Triton X – 100; c: AgNP (0.325 ppm); (d) SAgNP (0.187 ppm); (e) AuNP (127 ppm); (f) SAuNP (0.106 ppm), (g) PtNP (235 ppm); (h) SPtNP (34 ppm). (40X). Insets: (i) RBC with biconcave smooth surface; (ii) Appearance of ghost cells following treatment with Triton X - 100.
Figure 7.
Effect of bare and streptomycin conjugated nanoparticles on the structural integrity of RBC’s under scanning electron microscope a: PBS; b: Triton X – 100; c: AgNP (0.325 ppm); (d) SAgNP (0.187 ppm); e: AuNP (127 ppm); f: SAuNP (0.106 ppm), g: PtNP (235 ppm); h: SPtNP (34 ppm).
Figure 7.
Effect of bare and streptomycin conjugated nanoparticles on the structural integrity of RBC’s under scanning electron microscope a: PBS; b: Triton X – 100; c: AgNP (0.325 ppm); (d) SAgNP (0.187 ppm); e: AuNP (127 ppm); f: SAuNP (0.106 ppm), g: PtNP (235 ppm); h: SPtNP (34 ppm).
Table 1.
Antibacterial activities of metal nanoparticles and streptomycin conjugated metal nanoparticles against streptomycin-resistant Bacillus sp.
Table 1.
Antibacterial activities of metal nanoparticles and streptomycin conjugated metal nanoparticles against streptomycin-resistant Bacillus sp.
| Compounds used for testing the antibacterial activity |
Zone of Inhibition (mm) |
| AgNP |
AuNP |
PtNP |
| GMRE |
NZI |
NZI |
NZI |
| Streptomycin |
NZI |
NZI |
NZI |
| Metal Nanoparticles |
14 ± 0.2 |
NZI |
NZI |
| Streptomycin preloaded disk impregnated with metal nanoparticles |
16 ± 0.1 |
13 ± 0.4 |
13 ± 0.2 |
| Streptomycin conjugated metal nanoparticle |
22 ± 0.3 |
26 ± 0.1 |
23 ± 0.2 |