Introduction
Smokefree laws have been implemented in public buildings and in private businesses in most EU countries 1,2. Their aims, in general, have been to protect workers and customers from exposure to secondhand smoke (SHS) and to improve health.
They have been successful in reducing exposure to SHS, improving health, reducing illness and denormalising smoking 3–6.
In entertainment venues, such as pubs, bars and clubs, allowance is usually made in the laws to permit an area outside the main premises, or terraces, where smoking is allowed provided these areas are separate and are not complete buildings to allow increased ventilation. The details of the laws vary and result in variable exposures.
However, it has become obvious in many instances that these smoking areas allow the accumulation of SHS and cannot be considered safe 7,8. Since we now also accept that there is no safe level of SHS exposure9 it can be expected that the exposure in those areas causes adverse health effects in the long term. Nasal and oral sensory symptoms have been observed and lung function measurements have shown deterioration and heart monitoring has shown variation in heart rhythm in exposed non-smokers and increase in myocardial infarction and stroke have been recorded from long term exposure 10–13.
Present knowledge suggests that acute adverse SHS effects are the most likely to be seen in the respiratory system, upper or lower, or cardiovascular systems and subjects with underlying disease may be more likely to be more susceptible to acute effects but are at increased risk of adverse long-term effects from SHS exposure 14.
Over the past two decades, scientific evidence has accumulated linking SHS exposure to adverse health outcomes, including respiratory outcomes in children and adults, acute cardiovascular effects, and lung cancer 15–19. However, knowledge about acute health effects of SHS on respiratory disease patients is scarce 15,20.
Chronic respiratory diseases cause an important worldwide health burden. It was estimated that in 2017, they were the third leading cause of death, behind cardiovascular diseases and neoplasms. Globally, there were 3 914 196 deaths due to chronic respiratory diseases in 2017, an increase of 18.0% since 1990 21.
With these known long-term effects, we decided to monitor acute exposure to SHS and acute breathing responses of subjects with known doctor-diagnosed common respiratory diseases, asthma and COPD. Because of the possible, but unknown, acute effects, only subjects who routinely visited outside smoking areas as part of their normal social life were considered.
Three countries with statutory comprehensive smokefree laws, which have been in place for varying lengths of time, Czechia, Ireland and Spain were selected.
Ireland introduced its comprehensive Smokefree laws in 2004 and was the first country in the world to do so; Spain introduced its Smokefree laws initially in 2008 and strengthened them in 2012;and Czechia introduced its comprehensive laws in 2016 22–24. These countries allow a geographic spread in the EU. Their laws also allow smoking in special areas in a variety of structures which are outside the main premises.
Methods
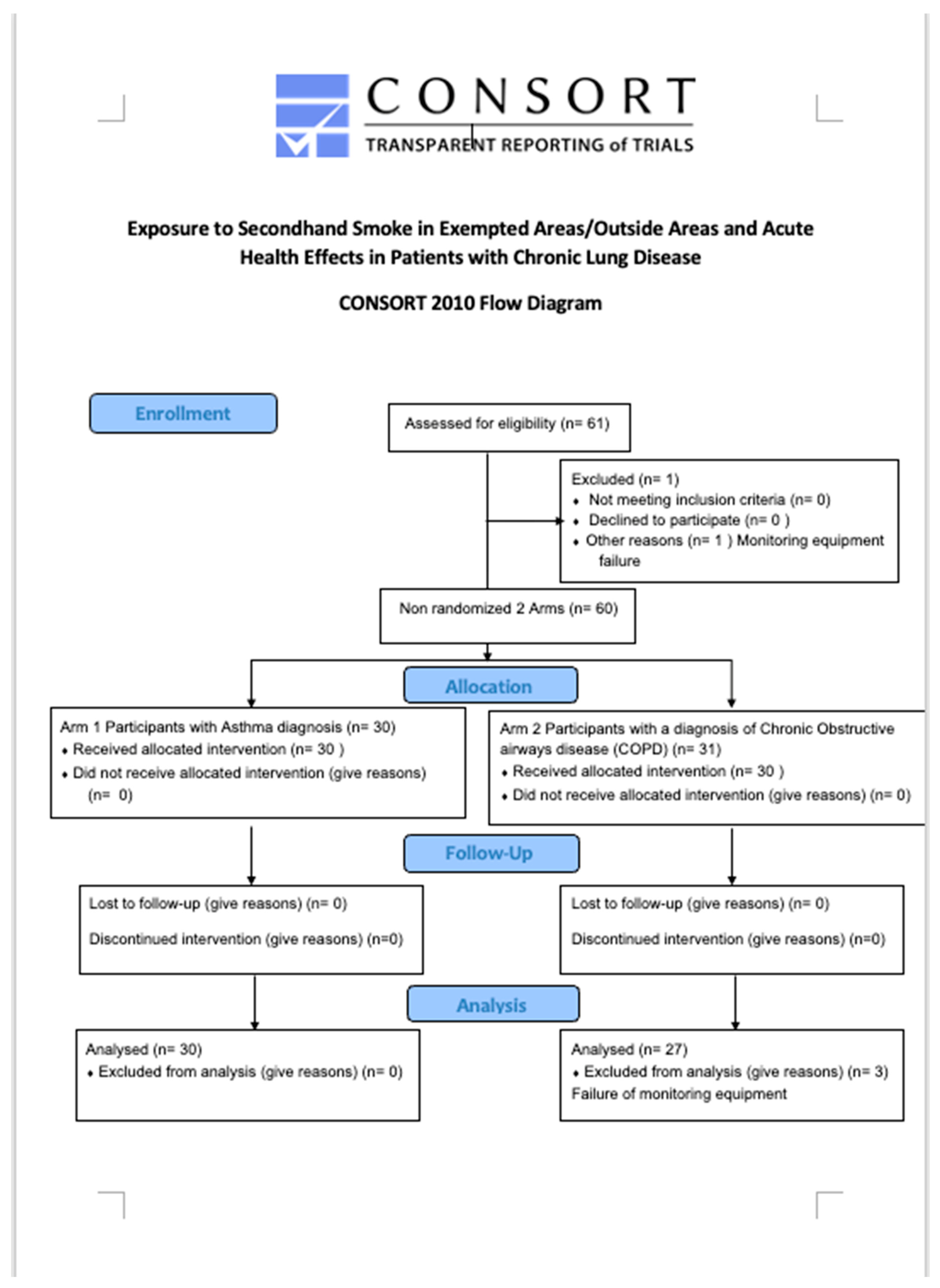
The study is an open, multi-centre, non-randomised, interventional study model, clinical trial of the acute effects of exposure to SHS in outside smoking areas in 3 EU countries with comprehensive smokefree laws. All 60 patients (
Figure 1, Consort Flow Diagram) were assessed in a similar manner with personal monitoring of particle exposure to PM 2.5 and breathing pattern on a visit, of at least one-hour duration, to an outside area of a pub. All measurements reported were made with the subjects resting for at least 15 minutes before visiting the venue and during exposure to SHS in a legal outside smoking area.
Ethical approval was awarded in Ireland by Dublin Institute of Technology, Research Ethics Committee (Approval Ref 13.103); in Spain by Comité de Ética de La Investigación con Medicamentos del Hospital Universitario de la Princesa, Madrid (Nº de Registro: 3221); and in Czechia by the Ethics Committee of the Regional Hospital in Liberic (Ref No. EK/22/2018). (
Supplementary File S1 uploaded)
Recruitment
Preliminary discussions were held with patient representative groups in Ireland and following these discussions it was decided that recruitment through contact with established chest clinics would be more appropriate than direct approach to patients for safety and consent considerations.
The study was discussed in each of the three countries, Chechia, Spain and Ireland with hospital staff and copies of the full protocol were made available as well as patient information leaflets and copies of consent forms.
Criteria for eligibility
Minimum age 18 years, sex all, doctor-diagnosed COPD patients who were current or ex-smokers, or doctor-diagnosed asthma, irrespective of smoking history. Fully ambulant patients.
Exclusion criteria
Under 18 years, on oxygen therapy, pregnant, currently undergoing treatment for an acute exacerbation of their primary condition.
Consent
Informed written consent was obtained from each subject at a specially arranged visit to the centre, where the study was explained and each patient was given written information.
The voluntary nature of their consent was stressed and their right to withdraw at any stage was explained. It was established at interview that it was usual practice for each participating patient to visit outside smoking areas of pubs and bars in their usual social life.
Group assignment
It was explained that this study followed an interventional model with single group assignment and that there was no randomisation.
Details of the intervention
Monitoring devices: AirSpeck monitors employ a light-scattering nephelometer for recording real-time PM2.5 concentration data at 10-second intervals 25. RESpeck monitors ,are light-weight - 17gms (incl. battery) unobtrusive devices, which use an encapsulated tri-axial accelerometer to identify the personal mode of the subject when wearing the device, i.e., stationary, lying or mobile which is then used to derive a reliable measure of activity, of respiratory rate and geolocation26. Each pair of sensor readings were communicated wirelessly, using Bluetooth connectivity, to a smartphone where it was GPS-stamped for later onward transmission to a secure server dashboard for display and later offline analysis. All the exposure measurements, for each of the 5 AirSpeck monitors used, were adjusted according to the calibration factor derived, in experimental studies in the Edinburgh laboratory and the National Physical Laboratory,Postcode:TW110LW. The data were analysed in consultation with Edinburgh University colleagues, DF and DKA.
National research partners in Spain and Czechia were trained by the Irish research team in the use of AirSpeck and RESpeck monitors.
Patient Protocol and Training
Each patient visited the local national study centre on two occasions. During the first visit, the study was explained to the participants, both in written (information sheet) and oral communication, they completed a recruitment questionnaire to ascertain personal smoking status, other sources of exposure, average weekly attendance and SHS exposure in hospitality premises, and experience of respiratory symptoms. All consented patients were trained in the use of monitoring equipment
Diary cards were demonstrated and explained to the patients and they were asked to fill in details at the first visit, medication consumed, any symptoms (e.g., cough, wheeze), doctor or hospital visits, exposure to SHS and number of cigarettes smoked (if any).
Diary card entries were also made on the day of exposure and included a description of premises visited; number of smokers present during exposure time as well as any change in their use of medication required during the 24hour period.
The participants were also asked to note the time and date when exposure to SHS occurred in outside areas.
Venues
At least one visit to an outdoor smoking area was scheduled during a one-hour visit to a premises. An outdoor smoking area was defined as: a place or premises, or part of a place or premises that, is fully uncovered by any roof, fixed or mobile, or an outdoor place or premises that is covered by a roof, so long as not more than 50% of the perimeter (outside) is covered by a wall, windows, gate or similar.
Study subjects were asked to spend at least 15 minutes in the outdoor smoking area, a preferable time of 30-60 mins and 15 minutes at rest was desirable.
Measurements
Patients wore the personal monitors for 24 hours to continuously measure exposure to particulate matter PM2.5, with continuous geolocalisation monitoring (AirSpeck) and a RESpeck monitor to measure breathing rate, to detect activity and any acute changes in breathing before and during exposure to SHS. To have a standardised period for measurement of breathing rates, we selected a period of 7 minutes when the patient was at rest before the exposure to SHS, as defined by the RESpeck measurements, and PM2.5 was less than 10 µg/m3 and compared it to breathing rates for 7 minutes at rest during the exposure and PM 2.5 was greater than 10 µg/m3.
At the second study centre visit on the day post exposure all data recorded by the devices were downloaded and checked and any diary card anomalies were addressed and clarified with the patient.
Routine pulmonary function tests consisting of forced expiratory volume in the first second (FEV1) forced vital capacity (FVC) and Peak expiratory Flow rate (PEFR) were measured at the study centre pre- and post-exposure to SHS within 24 hours (and are reported elsewhere) (19).
Statistical Analysis
Baseline characteristics of the participating patients by their diagnosis were compared using descriptive statistics (mean, standard deviation (SD), median, interquartile range (IQR)
and percentages as appropriate). Student t-test for continuous variables and Chi-square test for categorical variables were used to determine whether there was a difference in breathing rates on the variables of interest and a two-tailed p-value, less than 0.05 significance threshold, was chosen for all tests. Stata v16 (Stata Corp LP, College Station, TX, USA) was used for the statistical analysis.
Results
Table 1 shows the demographic characteristics of the 60 patients. The COPD participants were older (age 63.3±10.2 yrs.) than the asthmatics (46.9±18.7 yrs.), there were more women (n=35) than men (n=25). 21 patients of the COPD group (70.0%) were current smokers as were 8 of the asthmatics (26.7%) while 15 of the 60 (25.0%) were ex-smokers. 16 of the asthmatics (53.3%) had never smoked.
Exposure levels
PM 2.5 levels varied wildly within the smoking areas depending mainly on the number of walls (
Table 1) in the facility and less on the number of smokers.
Table 2 shows the mean and median PM 2.5 (µg/m
3) exposure for all subjects during their visits to an outdoor smoking area (SHS exposure) and for the rest of the 24- hour period (not in SHS area). While the level of exposure was greater in the SHS areas many patients also had high exposure during the whole observed periods. It is of note that 29 of the patients were smokers.
Individual venue measurements of PM2.5 were highly variable, one area reached 2500 µg/m3 for a short period during venue exposure and was sustained for 15 minutes at ≥ 2000 µg/m3, PM2.5 levels in 4 premises were ≥ 500 (1933-539) µg/m3 ,in 8 premises ≥ 200(480-203) µg/m3,in 9 premises ≥ 100 (170-108) µg/m3 ,in 8 premises ≥ 40 (80.5- 40.1) µg/m3,in 9 premises ≥ 25 µg/m3 ,in 10 premises, ≥ 10 µg/m3 ,in 8 premises, and ≤10 µg/m3 in 3 premises, which had only a single wall.
Breathing and respiratory assessments: Mean Br (
Table 3) tended to be lower during exposure to SHS ranging from 17.88-28.58 in the non-exposed at rest period and 16.46 -27.56 during exposure, but the difference was not statistically significant. The pattern was similar looking at means and medians, asthma and COPD, men and women, smokers and non-smokers.
Some subjects increased their Br during exposure to SHS while others decreased it. Examining these two populations it emerged that the changes, increases and decreases, in Br were significant in asthma and COPD overall but were not significant in male asthmatics while they were significant for both genders in COPD but women had a greater increase (
Table 4). Younger age, female gender, lighter body weight, non-smokers and asthma were more commonly associated an increase in Br whereas older age, male gender, heavier body weight and COPD tended to be associated with a decrease in Br. Only female asthmatics who had a decrease in FEV, FVC or PEFR had a statistically significant increase in Br as a group (
Table 5)
Routine pulmonary function tests consisting of Forced expiratory volume in 1 second (FEV1) Forced vital capacity (FVC) and Peak expiratory Flow rate (PEFR) were measured at the study centre visit on the day of the pub visit and repeated at a second study centre visit on the day following the pub visit. There were minor changes measured in lung function but only showed a statistically significant deterioration in female asthma patients which are reported elsewhere 20.
No patient reported significant changes either of maintenance medication or unscheduled visits to hospital or doctor.
Discussion
This study shows that exposure to SHS under present legislation in legal outside areas in three EU countries with comprehensive smokefree laws results in exposure to very high SHS levels. There is no safe level of SHS 27 and chronic exposure to the SHS levels seen in this study has been shown to result in cancer, heart attacks and COPD in those who are chronically exposed 28. Removal from SHS exposure in the short- to medium-term has resulted in improvements in, not only symptoms, but also in improved pulmonary function even in asymptomatic bar workers whose pulmonary function was within normal limits 3,14,29,30. Nevertheless, such reports on acute pulmonary function effects of short-term exposure to SHS are scarce. We argued that any such effects are most likely to be of increased clinical importance to patient with already compromised airflow limitation. In that regard we opted to look for effects on breathing in patients with doctor-diagnosed asthma and COPD. We accepted only volunteer patients who normally had attended such venues where exposure to SHS was usual and who had not noticed significant ill-effects on many such previous visits to pubs or bars with outdoor areas where customers are allowed to smoke. Many of the COPD patients were still smokers or ex-smokers. Of interest also was that when we approached asthma/COPD patient organisations to discuss participation most of the members with severe disease told us that they had abandoned visits to pubs because of SHS exposure and they did not take part in the study.
The changes in breathing rates that we recorded were complex. Nearly half of the patients increased their breathing rates and an almost equal number decreased their breathing rate at rest by comparison with resting rates during non-exposure and these changes were statistically significantly different. Responses in younger, lower weight, non-smoking and female patients with asthma were associated with increases in breathing rate while older, heavier, smoking and ex-smokers, and male patients with COPD were more likely to decrease breathing rate. This suggests that there are disease, gender, age, weight, and smoking effects in the responses but these were directional changes only which did not reach statistical significance except for female asthmatics who increased rates in line with a reduction in spirometry. This increased response in asthmatics is in line with increased bronchial responsiveness of asthmatics 31 but it did not happen in all asthmatics and not significant in males. It is known that Br is higher in women. Stewart et al. 31 also showed that the change in breathing rates lead to the possibility of hypoventilation and hyperventilation since the low and high breathing rates seen in their study are known to be associated with hypercapnia and hypoxaemia respectively.
Our findings also raise the question of possible alternative mechanisms at work 32,33. The most obvious perhaps is different regulation of breathing apart from bronchial responsiveness. We know of the blunting of the chemical drive to breathing in chronic hypoxia as regards response to carbon dioxide (CO2) 34,35 but we know much less about the effect of the various chemicals in SHS on the regulation of breathing in different disease states. The chemical content, concentration and dispersion of SHS are likely to be very different in different settings, in different countries. The dose inhaled is likely to vary widely and if the susceptibility also varies then this may account or contribute to the variance in response we saw in this study. The study was not designed to answer this question and the variation in the patient characteristics and sample size are also unsuitable to shed light on this aspect of the results.
However, the main aim of the study was to determine if SHS exposure in legal outside smoking areas was associated with measurable changes in breathing. We believe this is an important question as the rationale for smokefree bars was to protect staff and patrons from harmful exposure to SHS. This has been largely achieved in countries with comprehensive bans on smoking inside pubs but most legislation envisions an area outside the pub supplied by the owners of the pubs where smoking is allowed. It was anticipated when framing the smokefree legislation that these areas would be such that there was negligible or no exposure to SHS. Now that we know there is no safe level of long-term exposure to SHS it is especially important to know if there are significant respiratory changes due to short-term exposure. This is particularly important for patients who already have impaired pulmonary function due to disease and our results show that there are changes.
Conclusion
This study showing changes in breathing in patients exposed to SHS in outside areas of hospitality premises raises the need to abandon these designated areas and redefine a Smokefree pub as an establishment where smoking is not allowed in any part of its premises.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
LC, SK and EF had the original idea for the study. SK trained all the researchers in the use of the monitoring devices, collected the data in Ireland and was responsible for data management for all three sites. Dr Milada Sıpkov a collected the data in Czechia and Patricia Perez (PP) performed the fieldwork in Spain. Marıa Teresa Pastor (MTP) and SS carried out the statistical analysis with the supervision of TA and JBS and LC; LC wrote the first draft of the article in collaboration with JBS, TA and SK; all the authors contributed to manuscript preparation and approved its final version prior to submission. Sheila Keogan and Tamara Alonso: Joint first authorship. Joan B. Soriano and Luke Clancy: Joint senior authorship. LC and JBS are the guarantors.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 681040. BA received the support of a fellowship from “La Caixa” Foundation (ID 100010434; Fellowship code: LCF/BQ/IN17/11620013). AL was supported by a fellowship from the Italian Association for Cancer Research (AIRC). The Tobacco Control Research Group at ICO-IDIBELL (BA, EF, MF) is partly supported by the Ministry of Universities and Research, Government of Catalonia (2017SGR319). EF is partly supported by the Instituto de Salud Carlos III, Government of Spain, co-funded by the European Regional Development Fund (FEDER; INT16/00211 and INT17/00103). The work of SG was partially funded by the Italian League Against Cancer (LILT, Milan). SS was funded from Grant 167 from RCDHT. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data Availability Statement
The authors are open to data sharing of de-identified data.
Conflicts of Interest
The authors declare no competing interest.
Ethical Approval
Ethical approval was awarded, in Ireland by Dublin Institute of Technology, Research Ethics Committee (Approval Ref 13.103), in Spain by El Comité de Ética de La Investigación con Medicamentos del Hospital Universitario de la Princesa, Madrid, Nº de Registro: 3221 and, in Czechia by the Ethics Committee of the Regional Hospital in Liberic (Ref No. EK/22/2018 IRB letter (Supplementary file 1 uploaded). The study protocol (Supplementary file 2 uploaded) was registered on the ClinicalTrials.gov ID: NCT03074734
References
- Martínez-Sánchez JM, Fernández E, Fu M, et al. Smoking Behaviour, Involuntary Smoking, Attitudes towards Smoke-Free Legislations, and Tobacco Control Activities in the European Union. PLoS ONE. Published online 2010. [CrossRef]
- Sunday S, Kabir Z. Impact of Carers’ Smoking Status on Childhood Obesity in the Growing up in Ireland Cohort Study. International Journal of Environmental Research and Public Health. Published online 2019. [CrossRef]
- Goodman P, Agnew M, McCaffrey M, Paul G, Clancy L. Effects of the Irish smoking ban on respiratory health of bar workers and air quality in Dublin pubs. American Journal of Respiratory and Critical Care Medicine. Published online 2007. [CrossRef]
- Frazer K, Callinan JE, Mchugh J, et al. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database of Systematic Reviews. Published online 2016. [CrossRef]
- Rashid A, Manan AA, Yahya N, Ibrahim L. The support for smoke free policy and how it is influenced by tolerance to smoking - Experience of a developing country. PLoS ONE. Published online 2014. [CrossRef]
- EDK, - KL, - KS, et al. - Youth risk behavior surveillance - United States, 2009.; - Morbidity and mortality weekly report. Surveillance summaries (Washington, D.C. : 2002). - Morbidity & Mortality Weekly ReportSurveillance Summaries. Published online 2010.
- International Agency for Research on Cancer (IARC). Handbooks of Cancer Prevention, Tobacco Control, Vol. 13: Evaluating the Effectiveness of Smoke-Free Policies.; 2009.
- López MJ, Fernández E, Gorini G, et al. Exposure to secondhand smoke in terraces and other outdoor areas of hospitality venues in eight European countries. Published online 2012.
- Centers for Disease Control and Prevention (CDC. Disparities in secondhand smoke exposure--United States, 1988-1994 and 1999-2004. MMWR Morbidity and mortality weekly report. 2008;57(27):744-747.
- Strulovici-Barel Y, Omberg L, O’Mahony M, et al. Threshold of biologic responses of the small airway epithelium to low levels of tobacco smoke. American Journal of Respiratory and Critical Care Medicine. Published online 2010. [CrossRef]
- Xu X, Li B. Exposure-response relationship between passive smoking and adult pulmonary function. American journal of respiratory and critical care medicine. 1995;151(1):41-46.
- Eisner MD, Wang Y, Haight TJ, Balmes J, Hammond SK, Tager IB. Secondhand Smoke Exposure, Pulmonary Function, and Cardiovascular Mortality. Annals of Epidemiology. Published online 2007. [CrossRef]
- White JR, Froeb HF. Small-Airways Dysfunction in Nonsmokers Chronically Exposed to Tobacco Smoke. New England Journal of Medicine. Published online 1980. [CrossRef]
- Stallings-Smith S, Zeka A, Goodman P, Kabir Z, Clancy L. Reductions in cardiovascular, cerebrovascular, and respiratory mortality following the national Irish smoking ban: interrupted time-series analysis. PloS one. 2013;8(4):e62063.
- Masjedi MR, Kazemi H, Johnson DC. Effects of passive smoking on the pulmonary function of adults. Thorax. Published online 1990. [CrossRef]
- International Agency for Research on Cancer (IARC). IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Published online 2004.
- Kabir Z, Manning PJ, Holohan J, Keogan S, Goodman PG, Clancy L. Second-hand smoke exposure in cars and respiratory health effects in children. European Respiratory Journal. Published online 2009. [CrossRef]
- Öberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. The Lancet. Published online 2011. [CrossRef]
- Flouris AD, Koutedakis Y. Immediate and short-term consequences of secondhand smoke exposure on the respiratory system. Current Opinion in Pulmonary Medicine. Published online 2011. [CrossRef]
- Keogan S, Alonso T, Sunday S, et al. Lung function changes in patients with chronic obstructive pulmonary disease (COPD) and asthma exposed to secondhand smoke in outdoor areas. Journal of Asthma. 2021;58(9):1169-1175.
- Soriano JB, Kendrick PJ, Paulson KR, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet Respiratory Medicine. Published online 2020. [CrossRef]
- Currie LM, Clancy L. The road to smoke-free legislation in Ireland. Addiction. 2011;106(1):15-24.
- Semple S, Creely KS, Naji A, Miller BG, Ayres JG. Secondhand smoke levels in Scottish pubs: The effect of smoke-free legislation. Tobacco Control. Published online 2007. [CrossRef]
- Kulhánek A, Lukavská K, Švancarová I, Fidesová H, Gabrhelík R. Changes in tobacco use patterns and motivation to quit related to the new smoke-free legislation in the Czech Republic. Journal of Public Health. Published online 2019. [CrossRef]
- Arvind DK, Mann J, Bates A, Kotsev K. The AirSpeck Family of Static and Mobile Wireless Air Quality Monitors. In: Proceedings - 19th Euromicro Conference on Digital System Design, DSD 2016. ; 2016. [CrossRef]
- Drummond GB, Bates A, Mann J, Arvind DK. Validation of a new non-invasive automatic monitor of respiratory rate for postoperative subjects. British Journal of Anaesthesia. Published online 2011. [CrossRef]
- Potera, C. Smoking and secondhand smoke. Study finds no level of SHS exposure free of effects. Environmental health perspectives. Published online 2010. doi: 10.1289/ehp.118-a474a. [CrossRef]
- Centers for Disease Control and Prevention (US), U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General.; 2006.
- Eagan TML, Hetland J, Aarø LE. Decline in respiratory symptoms in service workers five months after a public smoking ban. Tobacco Control. Published online 2006. [CrossRef]
- Eisner MD, Smith AK, Blanc PD. Bartenders’ respiratory health after establishment of smoke-free bars and taverns. Journal of the American Medical Association. Published online 1998. [CrossRef]
- Stewart IC, Parker A, Catterall JR, douglas NJ, Flenley DC. Effect of bronchial challenge of breathing patterns and arterial oxygenation in stable asthma. Chest. Published online 1989. [CrossRef]
- Noble MIM. Abraham Guz memorial: Still unresolved hypotheses: Lung reflexes and perceptions of breathing. Respiratory Physiology and Neurobiology. Published online 2015. [CrossRef]
- Hernandez L, Manning J, Zhang S. Voluntary control of breathing affects center of pressure complexity during static standing in healthy older adults. Gait and Posture. Published online 2019. [CrossRef]
- Flenley DC, Cooke NJ, King AJ, Leitch AG, Brash HM. The hypoxic drive to breathing during exercise in normal man and in hypoxic patients with chronic bronchitis and emphysema. Bulletin de physio-pathologie respiratoire. Published online 1973. [CrossRef]
- Robinson O, Martínez D, Aurrekoetxea JJ, et al. The association between passive and active tobacco smoke exposure and child weight status among Spanish children. Obesity. Published online 2016. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).