Introduction
Liver cirrhosis leads to clinically significant portal hypertension (CSPH) in 40-70% of patients, increasing the risk of decompensation and death [
1,
2]. TIPS (transjugular intrahepatic portosystemic shunt) has been shown to effectively reduce the degree of portal hypertension and treat complications such as recurrent/refractory ascites and bleeding from gastroesophageal varices [
2]. Cirrhosis and portal hypertension often result in malnutrition and sarcopenia, due to factors such as reduced caloric intake and increased catabolism [
3,
4]. 80% of decompensated cirrhosis patients experience sarcopenia that further worsens with liver decompensation [
5]. These conditions raise the risk of increased mortality and decompensation [
6,
7]. Interestingly, even without impaired liver function, portal hypertension alone may increase the risk of sarcopenia [
8]. Given this relationship, TIPS placement often occurs in a context of malnutrition. Sarcopenia has been shown to be associated with hepatic encephalopathy (HE), acute on chronic liver failure (ACLF), and mortality following TIPS placement [
9,
10,
11,
12,
13]. As a result, sarcopenia is now considered a relative contraindication to TIPS placement in current guidelines [
2]. On the other hand, recent evidence suggests that TIPS placement may improve the nutritional status of cirrhotic patients, by increasing the fat-free muscle mass and reducing visceral fat [
10,
14,
15]. However, the optimal timing for TIPS placement in sarcopenic cirrhotic patients remains unknown. The purpose of this systematic review is to examine the current literature and determine the true impact of TIPS placement on the nutritional status of cirrhotic patients.
Materials and Methods
The PICO framework was followed to define the review question’s key elements [
16]. The reporting of this systematic review and meta-analysis follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (
http://www.prisma-statement.org) [
17].
Literature Search
The MEDLINE (Pubmed), Scopus, Web of Science, and Cochrane library databases were comprehensively searched from inception to December 14, 2022. A literature review was created using the following terms (with corresponding variations): ((cirrhosis[MeSH Terms] OR (ascites) OR (hepatic encephalopathy) OR (cirrhotics)) AND (Portosystemic Shunt, Transjugular Intrahepatic[Mesh Terms]) AND ((Nutritional Status[MeSH Terms]) OR (nutritional) OR (albumin) OR (BMI) OR (total body mass) OR sarcopenia OR (body composition) OR (body weight))). Medical Subject Headings (MESH) were used to increase the precision and efficiency of the search. Additionally, we manually checked the reference lists of the included studies (or relevant review articles) and performed a backward citation analysis.
Criteria for the Selection of Studies
Inclusion criteria for the studies consisted of evaluations of patients diagnosed with liver cirrhosis who underwent transjugular intrahepatic portosystemic shunt (TIPS) placement, regardless of the indication for the procedure. Additionally, the studies were required to assess changes in nutritional status of the patients prior to and following TIPS insertion. The exclusion criteria for the studies consisted of any abstracts, studies that utilized animal models, case reports, and correspondence letters. Additionally, the scope of the literature search was restricted to articles written in the English, German, Italian and Spanish languages.
Study Selection, Data Extraction and Data Calculation
A standardized data extraction sheet was developed, and subsequently underwent pilot testing to ensure its reliability. Two investigators independently performed a literature review, utilizing titles and abstracts as initial screening criteria (JG, SK). Eligible full-text articles were subsequently assessed, and relevant data were extracted by the investigators (JG, SK). The extracted information was then reciprocally compared between the investigators (JG, LP, SD, SK). Any discrepancies in study selection or data extraction were resolved through a consensus process, with the assistance of a senior hepatologist (MM).
The following pre-determined data points were extracted from each eligible study: (1) First author's name, (2) Year of publication, (3) Study design, (4) Time frame of the study, (5) Patient exclusion criteria, (6) Sample size, (7) Characteristics of the study participants, (8) Indication for TIPS procedure, (9) Characteristics of the TIPS procedure, (10) Portal pressure gradient post-TIPS procedure, (11) Follow-up duration, (12) Measures of evaluating nutritional status, and (13) Change in nutritional status. In instances where the authors did not directly report the change in nutritional status, it was calculated from the available information, if feasible.
Risk of Bias Assessment
The risk of bias is evaluated using the ROBINS-I guidelines, which identify seven domains and classify the risk as low, moderate, serious, or critical. A low risk of bias indicates that the study is similar to a well-conducted randomized trial, while a critical risk indicates significant problems with the study [
18].
Results
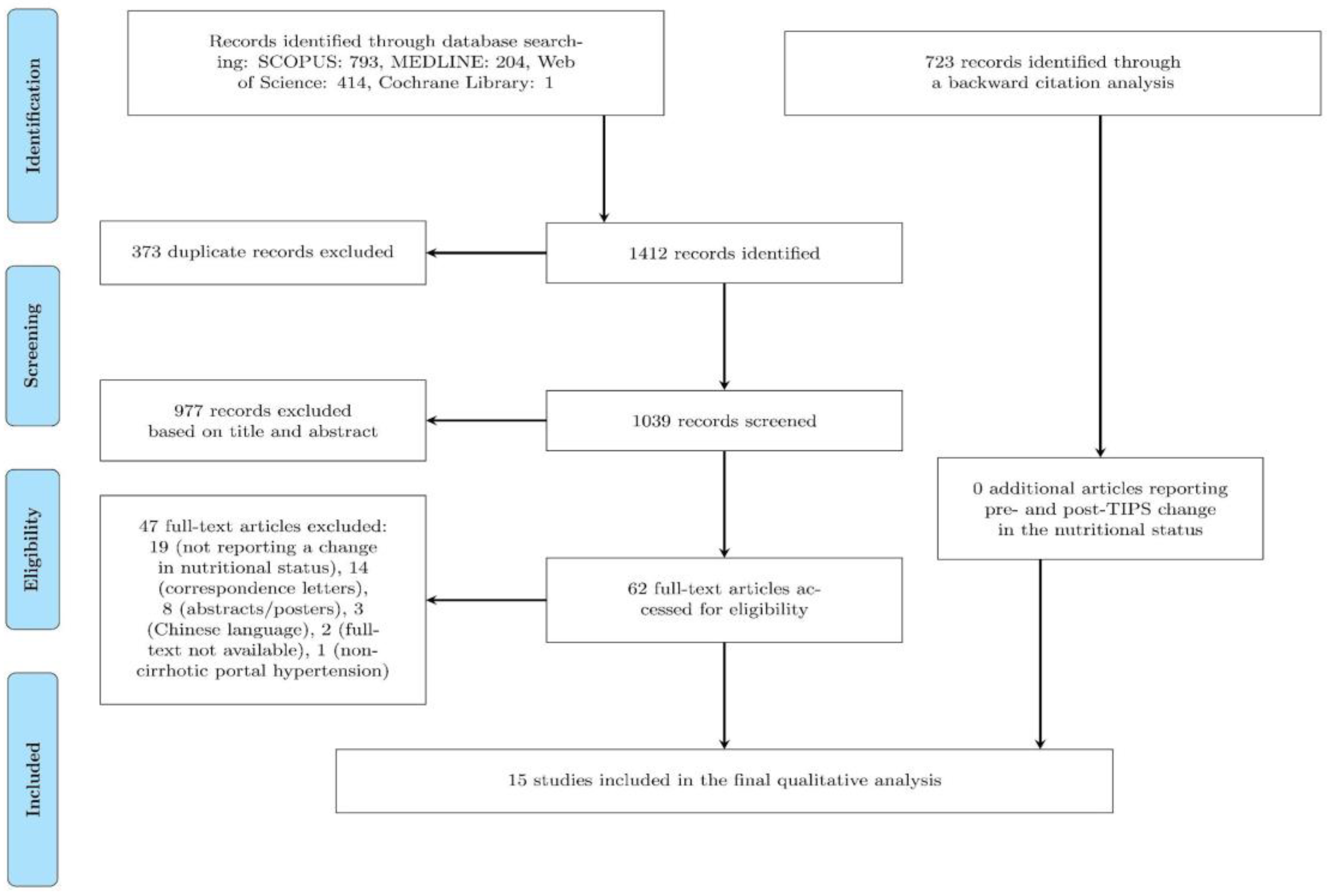
A comprehensive search of four major medical databases (MEDLINE, Scopus, Web of Science, and Cochrane) yielded a total of 1412 records. 373 duplicate records were subsequently removed. Of the remaining 1039 records, 62 were evaluated in full-text, and 57 were excluded based on pre-determined exclusion criteria (as detailed in
Figure 1). Additionally, 723 additional records were identified through backward citation analysis. In total, 15 records were finally included in the present study (as illustrated in
Figure 1).
In the present study, a total of 15 studies were included (
Table 1, more detailed in Supplementary
Table 1), which were published between 1998 and 2022 and which altogether followed 850 patients. Of these, 7 studies (47%) were retrospective in design [
13,
14,
19,
20,
21,
22,
23], 3 studies (20%) were prospective [
24,
25,
26], and 5 studies (33%) did not disclose this information [
15,
27,
28,
29,
30]. In the majority of the studies, the sample population consisted of all consecutive patients, with exclusion criteria based on cardiac, renal, hepatic, or pulmonary function, as well as a history of malignancy. The most frequent underlying chronic liver disease was alcohol-related liver disease (in 10 out of 15 included studies; 67%). The sample sizes of the included studies varied between 11 and 224 patients, and the mean or median age of the patients varied between 54.1 and 60 years, with male patients predominating in all studies. The indications for TIPS insertion were refractory ascites and variceal bleeding. The most frequently used stent material was polytetrafluoroethylene (PTFE), however, some older studies employed bare metallic stents. The proportion of patients with TIPS dysfunction was reported infrequently [
19,
20,
26]. In instances of dysfunction detection, intervention in the form of TIPS revision was implemented to re-establish patency. Non-responsive cases were excluded from the original studies. The post-TIPS portal pressure gradient varied between studies, ranging from 6.0 to 15.5 mmHg. Furthermore, the time point at which the change in nutritional status was measured varied between 2 and 36 months after TIPS insertion.
The subsequent section of the results is divided into three distinct sub-sections. The first sub-section presents the changes in Body Mass Index (BMI), weight (W), and/or body cell mass (BCM). The second and third sub-sections present the changes in body composition with regards to alterations in muscle and fat tissue, respectively.
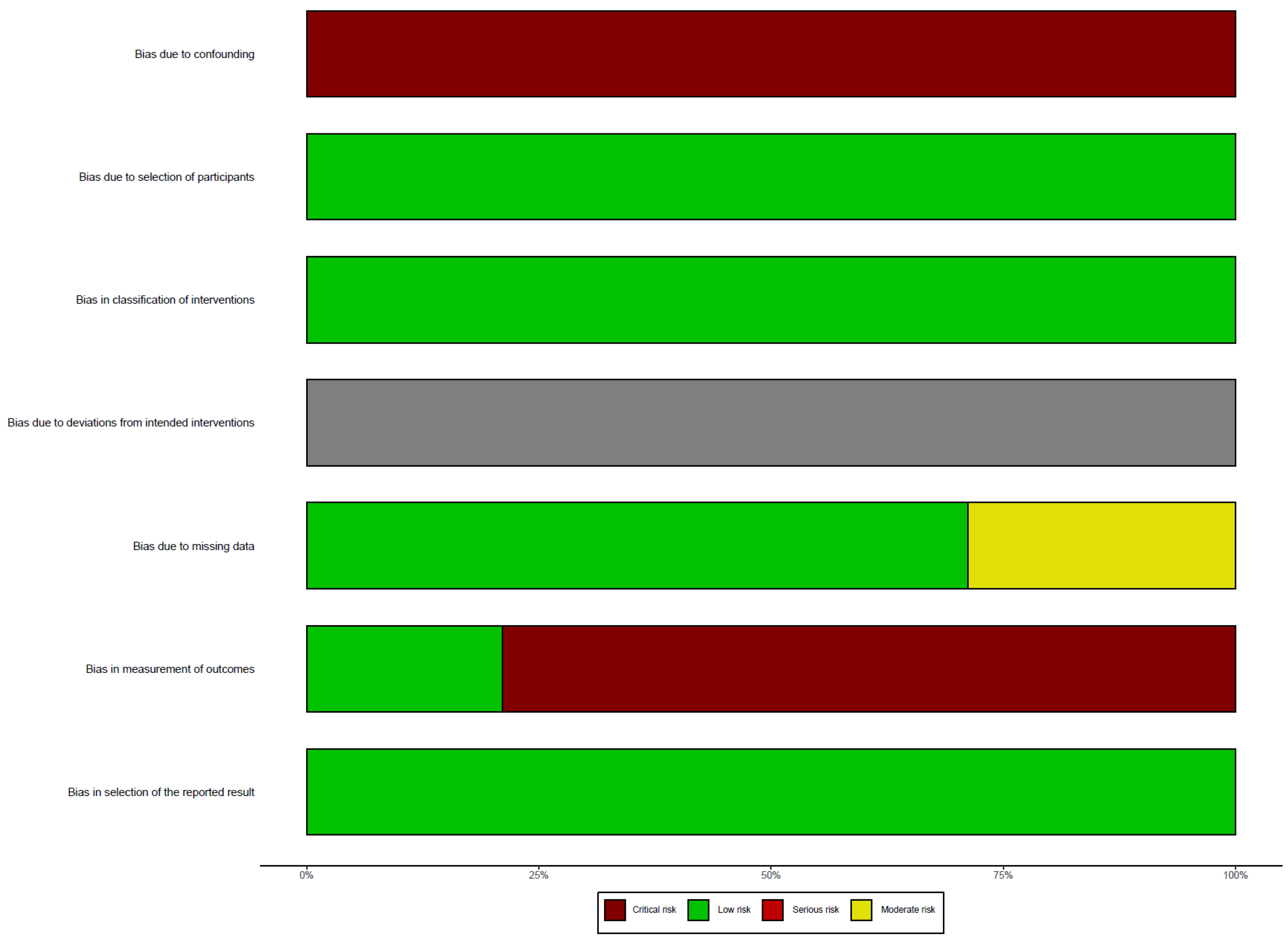
Risk of Bias Assessment
In this systematic review, we did not assess the risk of bias related to deviations from intended interventions. We found a critical risk of bias due to confounding, as none of the studies controlled for variables such as caloric intake or the frequency and intensity of physical exercise. On the other hand, the risk of bias due to participant selection was low, as all eligible and consecutive patients were included. The risk of bias related to the classification of interventions was also low. The risk of bias due to missing data was moderate in some studies, as a number of patients were lost to follow-up. However, the risk of bias related to the measurement of outcomes was critical, as most evaluators of nutritional status were not blinded to the intervention. Lastly, we did not detect any evidence of selective reporting of results (
Figure 2).
Body Mass Index, Weight, and/or Body Cell Mass
In total, 11 studies reported on changes in Body Mass Index (BMI, kg/m²), weight (W, kg), and/or body cell mass (BCM, kg) [
15,
20,
22,
23,
24,
25,
26,
27,
28,
29,
30] (
Table 1, more detailed in Supplementary
Table 1). These measures were evaluated in patients with no ascites (following peritoneal paracentesis) or were adjusted for ascites volume. Of these studies, six reported a significant improvement in BMI or W [
20,
22,
23,
24,
25,
27], with two studies noting improvement in BCM only (the increase in BMI/weight was in these cases statistically not significant) [
28,
30]. Two studies that specifically distinguished between underweight or sarcopenic patients and those who were overweight or nonsarcopenic reported a significant improvement in nutritional status in the former group [
20,
26]. Finally, only one study failed to report an improvement in such measures [
15].
Skeletal Muscle Volume, Skeletal Muscle Function
A total of 7 studies reported on changes in skeletal muscle volume [
13,
14,
15,
19,
20,
21,
24] and skeletal muscle function [
27] (
Table 1, more detailed in Supplementary
Table 1). The mid-arm muscle area (MAMA) was quantitatively determined using a flexible tape measure [
24]. To further assess muscle mass, axial computed tomography (CT) images were obtained at an L3 vertebra height through the abdomen and utilized to calculate skeletal muscle areas (SMA) [
15,
20,
21]. These muscle areas were then normalized to height, resulting in the calculation of the skeletal muscle index (SMI) [
13,
14,
20]. Additionally, both transversal right psoas muscle thickness at the umbilical level/height (TPMT/height in mm/m) and total psoas muscle area (TPMA in mm
2) were also reported in the study by Artru et al [
19]. All studies measuring changes in skeletal muscle volume reported a significant improvement, with Liu et al. confirming this trend specifically in sarcopenic patients [
20]. The SMA increased by a minimum of 6.6 cm
2 within 12 months after TIPS placement [
15,
21]. This increase was even more pronounced in sarcopenic patients, with an increase of up to 20 cm² [
20]. The improvement in SMI ranged between 2.39 and 5.8 cm²/m², with sarcopenic patients showing even greater improvement of approximately 8 cm²/m² within 10-19 months after TIPS placement [
13,
14,
20]. Additionally, both the TPMT and TPMA significantly improved within 6 months of TIPS placement [
19]. On the other hand, the study conducted by Allard et al. found that there was no improvement in measures of skeletal muscle function, such as muscle relaxation rate and muscle force [
27].
Adipose Tissue Volume
In a total of 9 studies, changes in the quantity of adipose tissue were evaluated [
14,
15,
19,
20,
24,
26,
27,
29,
30] (
Table 1, more detailed in Supplementary
Table 1). To assess adipose tissue quantity, axial computed tomography (CT) images were obtained at an L3 height through the abdomen. The volume of subcutaneous adipose tissue (SAT, adipose tissue below skin but above the parietal peritoneal lining in cm
3/3mm) and visceral adipose tissue (VAT, intraperitoneal adipose tissue in cm
3/3mm) were estimated [
15]. Furthermore, adipose tissue areas were normalized to height, resulting in the estimation of the tissue area indices – subcutaneous adipose tissue index and visceral adipose tissue index (SATI and VATI in cm
2/m
2) [
14]. Subcutaneous and visceral fat surfaces (SFA and VFA in cm
2, respectively) were also calculated [
19,
20]. Additionally, Liu et al. quantified subcutaneous fat thickness (SFT in mm) [
20]. Fat mass (% of body weight or in kg) was estimated using calorimetry [
26,
27,
29,
30]. Finally, Plauth et al. quantified mid-arm fat area using a skinfold caliper (MAFA in cm
2) [
24]. In the reviewed studies, no significant change was observed in measures of fat mass (measured using calorimetry, except from an increase in the study by Allard et al) or in mid-arm fat area [
24,
26,
27,
29,
30]. However, a significant expansion of subcutaneous fat tissue was observed in all studies [
14,
19,
20], with the exception of one study [
15]. Additionally, a notable reduction in visceral fat tissue was reported in two studies [
14,
19], while remaining unchanged in one study [
15].
Discussion
TIPS placement is a commonly utilized therapeutic intervention for individuals with liver cirrhosis and associated complications of portal hypertension, including refractory ascites and variceal bleeding. This intervention has been demonstrated to significantly improve overall survival in these patients, as evidenced by several clinical studies [
31,
32]. Despite these benefits, malnutrition, which is prevalent among cirrhotic individuals, has been linked to adverse outcomes after TIPS placement, such as hepatic encephalopathy, acute on chronic liver failure (ACLF), and mortality [
10,
11,
12]. On the other hand, some studies suggested an improvement in the nutritional status following TIPS placement in cirrhotic patients. Therefore, the aim of this systematic review was to evaluate changes in nutritional status that occur after TIPS placement in patients with liver cirrhosis.
This review analyzed data from 15 studies (comprising a total of 850 patients) that were published between 1998 and 2022. The majority of participants in these studies had alcohol-related liver disease, and the number of participants per study ranged from 11 to 224, with the majority being men. The indications for TIPS insertion were refractory ascites and variceal bleeding, the most commonly used stent material in these studies was polytetrafluoroethylene, and the post-TIPS portal pressure gradients ranged from 6.0 to 15.5 mmHg.
The results of the qualitative analysis showed marked improvements in muscle mass and shift fat tissue distribution after TIPS placement. However, the considerable clinical and methodological heterogeneity between studies precluded the conduct of a quantitative analysis (meta-analysis). The variability in nutritional status evaluation across studies was identified as a major contributing factor to this limitation. Furthermore, the time interval between TIPS placement and nutritional reassessment ranged from 1-3 to 19 months.
Shorter follow-up times might have limited the observation of significant changes, while longer follow-up times may have resulted in significant selection bias, as patients who had died or undergone transplantation were more likely to be excluded from the analysis. The methods used to evaluate nutritional status varied widely across studies, including the use of Body Mass Index (BMI), weight, body cell mass, skeletal muscle volume and function, and measures of adipose tissue.
There are several potential explanations for the observed changes in body composition after TIPS placement. These changes may be related to the reduction in portal hypertension [
8] and its associated effects on gut permeability, bacterial translocation, proinflammatory cytokines, and chronic inflammation [
33,
34,
35,
36]. Additionally, the TIPS procedure may improve protein-losing enteropathy and reduce frequent hospitalizations due to gastrointestinal bleeding and paracentesis, leading to improved mobility and oral intake. However, limited information is available regarding dietary changes after TIPS [
15].
Tsien et al. reported that younger age, male sex, and lower pre-TIPS muscle area were independent predictors of increased muscle mass after TIPS [
15]. Two studies found that the improvement in nutritional status was more pronounced in patients with sarcopenia or who were underweight or of normal weight, rather than in overweight or non-sarcopenic individuals [
20,
26]. These findings create a paradox, as malnutrition and sarcopenia have been associated with increased risk of adverse outcomes after TIPS placement, yet these patients are also more likely to benefit from TIPS in terms of nutritional status.
Several studies have observed alterations in adipose tissue composition following TIPS placement. There is evidence to suggest that the procedure leads to a decrease in VAT and an increase in SAT. The venous drainage of VAT occurs directly via the portal circulation to the liver. Therefore, changes in portal circulation subsequent to TIPS placement may enhance the availability of these fat deposits to be metabolized as energy sources by the liver [
37,
38]. Additionally, increased circulating levels of adipokines have been observed post-TIPS placement, potentially reflecting anabolic changes that contribute to the alterations in adipose tissue observed in patients undergoing TIPS [
30].
Conclusions
In conclusion, the results of this review indicate an improvement in ascites-free weight and body mass index. This improvement has been associated with the increase in muscle mass, as evidenced by various measures such as the skeletal muscle index or skeletal muscle area. Additionally, TIPS placement leads to a redistribution of fat mass, with a preference for subcutaneous over visceral adipose tissue. It is worth noting that sarcopenic patients are particularly likely to benefit from TIPS placement in terms of their nutritional status. This creates a paradox as malnutrition and sarcopenia have been linked to increased risk of adverse outcomes after TIPS placement, yet these patients are also more likely to benefit from TIPS placement in terms of nutrition. It is crucial to conduct well-designed prospective studies using gold-standard methods for evaluating malnutrition, in order to identify patients who would most benefit from TIPS placement, including from a nutritional perspective. These efforts could provide new pathophysiological insights into the relationship between portal hypertension, malnutrition, and TIPS placement, as well as implications for clinical practice.
Author Contributions
J.G., S.D., L.L., S.K. and M.M: substantial contributions to the conception and design. J.G., S.D., L.L and S.K.: acquisition of data. J.G., S.D., L.L., S.K. and M.M.: interpretation of data, J.G., S.D., L.L. and M.M: drafting of the article. M.M.: critical revision for important intellectual content. All authors have approved the final version manuscript.
Funding
This is a no profit study which did not receive any specific funding but was performed as part of the employment of the authors at their respective institutions.
Data Availability Statement
The data used to support these findings are all available within the article [and/or] its supplementary material.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Liver EA for TS of the. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406–60.
- de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Abraldes JG, et al. Baveno VII – Renewing consensus in portal hypertension. J Hepatol 2022;76. [CrossRef]
- Merli M, Berzigotti A, Zelber-Sagi S, Dasarathy S, Montagnese S, Genton L, et al. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol 2019;70. [CrossRef]
- Romiti A, Merli M, Martorano M, Parrilli G, Martino F, Riggio O, et al. Malabsorption and nutritional abnormalities in patients with liver cirrhosis. Italian Journal of Gastroenterology 1990;22.
- Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS One 2017;12. [CrossRef]
- Tantai X, Liu Y, Yeo YH, Praktiknjo M, Mauro E, Hamaguchi Y, et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J Hepatol 2022;76. [CrossRef]
- Montagnese S, Russo FP, Amodio P, Burra P, Gasbarrini A, Loguercio C, et al. Hepatic encephalopathy 2018: A clinical practice guideline by the Italian Association for the Study of the Liver (AISF). Digestive and Liver Disease 2019;51. [CrossRef]
- Lattanzi B, Gioia S, di Cola S, D’Ambrosio D, Nardelli S, Tavano D, et al. Prevalence and impact of sarcopenia in non-cirrhotic portal hypertension. Liver International 2019;39. [CrossRef]
- Nardelli S, Lattanzi B, Torrisi S, Greco F, Farcomeni A, Gioia S, et al. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clinical Gastroenterology and Hepatology 2017;15:934–6.
- Praktiknjo M, Clees C, Pigliacelli A, Fischer S, Jansen C, Lehmann J, et al. Sarcopenia is associated with development of acute-on-chronic liver failure in decompensated liver cirrhosis receiving transjugular intrahepatic portosystemic shunt. Clin Transl Gastroenterol 2019;10. [CrossRef]
- Yang C, Zhu X, Liu J, Shi Q, Du H, Chen Y, et al. Development and Validation of Prognostic Models to Estimate the Risk of Overt Hepatic Encephalopathy after TIPS Creation: A Multicenter Study. Clin Transl Gastroenterol 2022;13. [CrossRef]
- Nardelli S, Lattanzi B, Merli M, Farcomeni A, Gioia S, Ridola L, et al. Muscle Alterations Are Associated With Minimal and Overt Hepatic Encephalopathy in Patients With Liver Cirrhosis. Hepatology 2019;70. [CrossRef]
- Gioia S, Merli M, Nardelli S, Lattanzi B, Pitocchi F, Ridola L, et al. The modification of quantity and quality of muscle mass improves the cognitive impairment after TIPS. Liver International 2019;39. [CrossRef]
- Gioia S, Ridola L, Cristofaro L, Merli M, Faccioli J, Riggio O, et al. The improvement in body composition including subcutaneous and visceral fat reduces ammonia and hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Liver International 2021;41. [CrossRef]
- Tsien C, Shah SN, Mccullough AJ, Dasarathy S. Reversal of sarcopenia predicts survival after a transjugular intrahepatic portosystemic stent. Eur J Gastroenterol Hepatol 2013;25. [CrossRef]
- Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 2007;7:1–6.
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Reprint—preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther 2009;89:873–80.
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Online) 2016;355. [CrossRef]
- Artru F, Miquet X, Azahaf M, Labreuche J, Ntandja Wandji LC, Sergent G, et al. Consequences of TIPSS placement on the body composition of patients with cirrhosis and severe portal hypertension: a large retrospective CT-based surveillance. Aliment Pharmacol Ther 2020;52. [CrossRef]
- Liu J, Ma J, Yang C, Chen M, Shi Q, Zhou C, et al. Sarcopenia in Patients with Cirrhosis after Transjugular Intrahepatic Portosystemic Shunt Placement. Radiology 2022;303. [CrossRef]
- Jahangiri Y, Pathak P, Tomozawa Y, Li L, Schlansky BL, Farsad K. Muscle Gain after Transjugular Intrahepatic Portosystemic Shunt Creation: Time Course and Prognostic Implications for Survival in Cirrhosis. Journal of Vascular and Interventional Radiology 2019;30. [CrossRef]
- Pang N, Zhao C, Li J, Li L, Yang X, Yang M, et al. Body mass index changes after transjugular intrahepatic portosystemic shunt in individuals with cirrhosis. Nutrition 2021;84. [CrossRef]
- Trotter JF, Suhocki P v., Rockey DC. Transjugular intrahepatic portosystemic shunt (TIPS) in patients with refractory ascites: Effect on body weight and child-pugh score. American Journal of Gastroenterology 1998;93. [CrossRef]
- Plauth M, Schütz T, Buckendahl DP, Kreymann G, Pirlich M, Grüngreiff S, et al. Weight gain after transjugular intrahepatic portosystemic shunt is associated with improvement in body composition in malnourished patients with cirrhosis and hypermetabolism. J Hepatol 2004;40. [CrossRef]
- Nolte W, Wirtz M, Rossbach C, Leonhardt U, Buchwald AB, Scholz KH, et al. TIPS Implantation Raises Leptin Levels in Patients with Liver Cirrhosis. Experimental and Clinical Endocrinology and Diabetes 2003;111. [CrossRef]
- Montomoli J, Holland-Fischer P, Bianchi G, Grønbæk H, Vilstrup H, Marchesini G, et al. Body composition changes after transjugular intrahepatic portosystemic shunt in patients with cirrhosis. World J Gastroenterol 2010;16. [CrossRef]
- Allard JP, Chau J, Sandokji K, Blendis LM, Wong F. Effects of ascites resolution after successful TIPS on nutrition in cirrhotic patients with refractory ascites. American Journal of Gastroenterology 2001;96. [CrossRef]
- Holland-Fischer P, Vilstrup H, Frystyk J, Nielsen DT, Flyvbjerg A, Grøonbæk H. The IGF system after insertion of a transjugular in trahepatic porto-systemic shunt in patients with liver cirrhosis. Eur J Endocrinol 2009;160. [CrossRef]
- Holland-Fischer P, Nielsen MF, Vilstrup H, Tønner-Nielsen D, Mengel A, Schmitz O, et al. Insulin sensitivity and body composition in cirrhosis: Changes after TIPS. Am J Physiol Gastrointest Liver Physiol 2010;299. [CrossRef]
- Thomsen KL, Sandahl TD, Holland-Fischer P, Jessen N, Frystyk J, Flyvbjerg A, et al. Changes in adipokines after transjugular intrahepatic porto-systemic shunt indicate an anabolic shift in metabolism. Clinical Nutrition 2012;31. [CrossRef]
- Qi X, Jia J, Bai M, Guo X, Su C, García-Pagán JC, et al. Transjugular intrahepatic portosystemic shunt for acute variceal bleeding. J Clin Gastroenterol, vol. 49, 2015. [CrossRef]
- Salerno F, Cammà C, Enea M, Rössle M, Wong F. Transjugular Intrahepatic Portosystemic Shunt for Refractory Ascites: A Meta-analysis of Individual Patient Data. Gastroenterology 2007;133. [CrossRef]
- Liu G, Wang X, Yang T, Yan Y, Xiang T, Yang L, et al. High Interleukin-8 Levels Associated With Decreased Survival in Patients With Cirrhosis Following Transjugular Intrahepatic Portosystemic Shunt. Front Med (Lausanne) 2022;9. [CrossRef]
- Wirtz TH, Reuken PA, Jansen C, Fischer P, Bergmann I, Backhaus C, et al. Balance between macrophage migration inhibitory factor and sCD74 predicts outcome in patients with acute decompensation of cirrhosis. JHEP Reports 2021;3. [CrossRef]
- Lehmann JM, Claus K, Jansen C, Pohlmann A, Schierwagen R, Meyer C, et al. Circulating CXCL10 in cirrhotic portal hypertension might reflect systemic inflammation and predict ACLF and mortality. Liver International 2018;38. [CrossRef]
- Hayashi M, Hirose H, Senga S, Onitsuka A, Fuwa S, Mori Y, et al. Effect of portal hypertension caused by chronic high venous pressure on small-intestinal sugar absorption. Nutrition 2000;16. [CrossRef]
- Pinho CP S. Factors Associated With the Concentration of Visceral and Subcutaneous Fat. Health Care Curr Rev 2017;05. [CrossRef]
- Mittal B. Subcutaneous adipose tissue & visceral adipose tissue. Indian Journal of Medical Research 2019;149. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).