Submitted:
18 February 2023
Posted:
22 February 2023
You are already at the latest version
Abstract
Keywords:
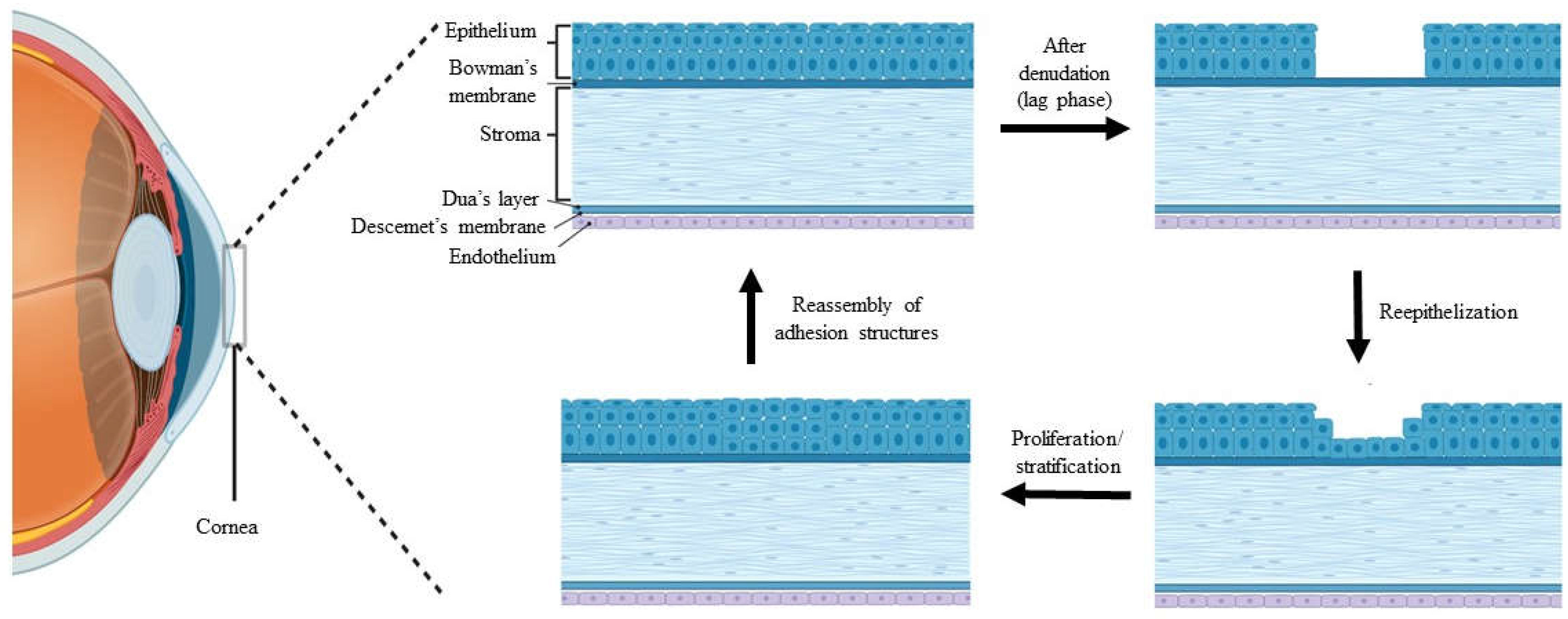
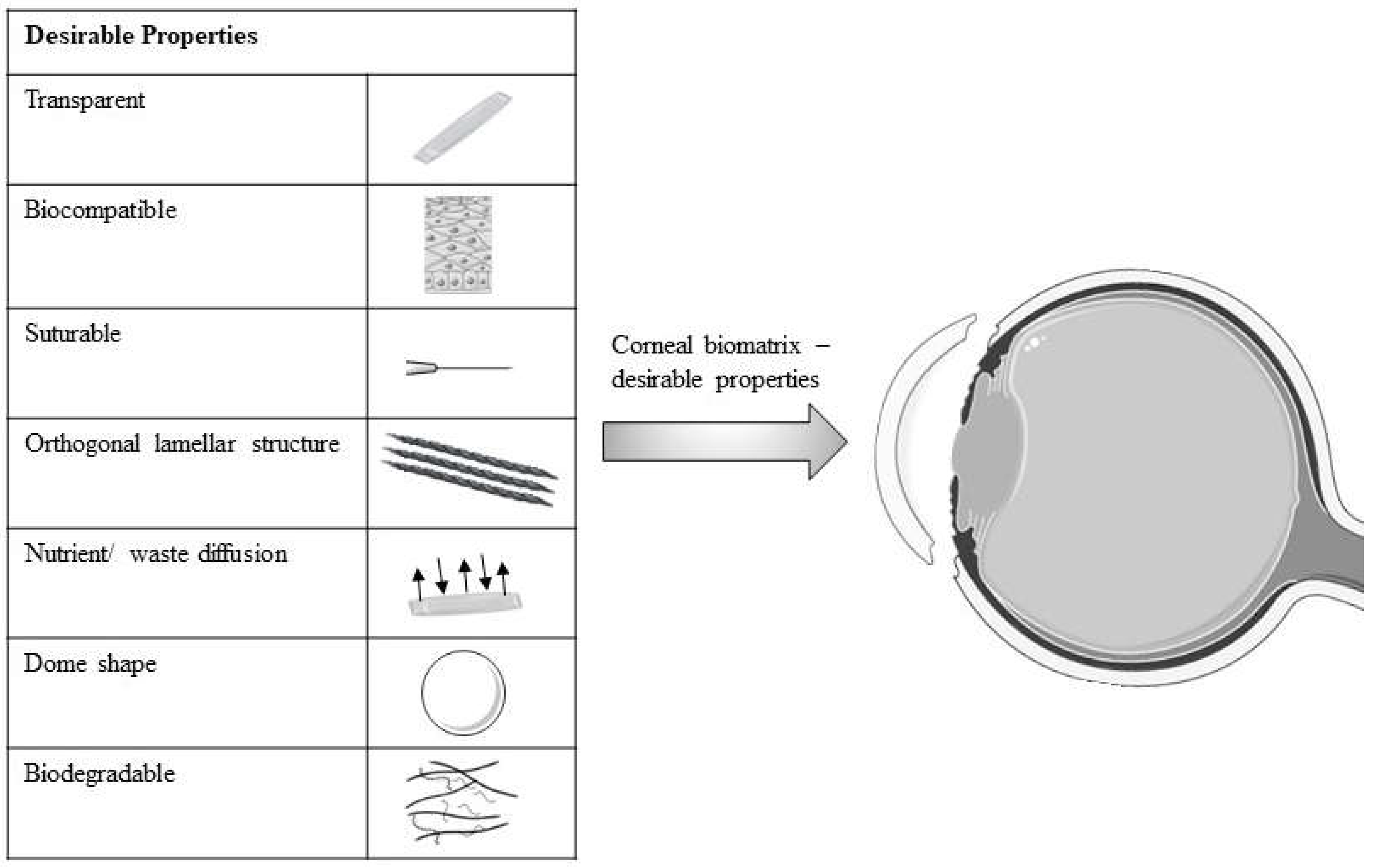
1. Introduction
2. Materials and Methods
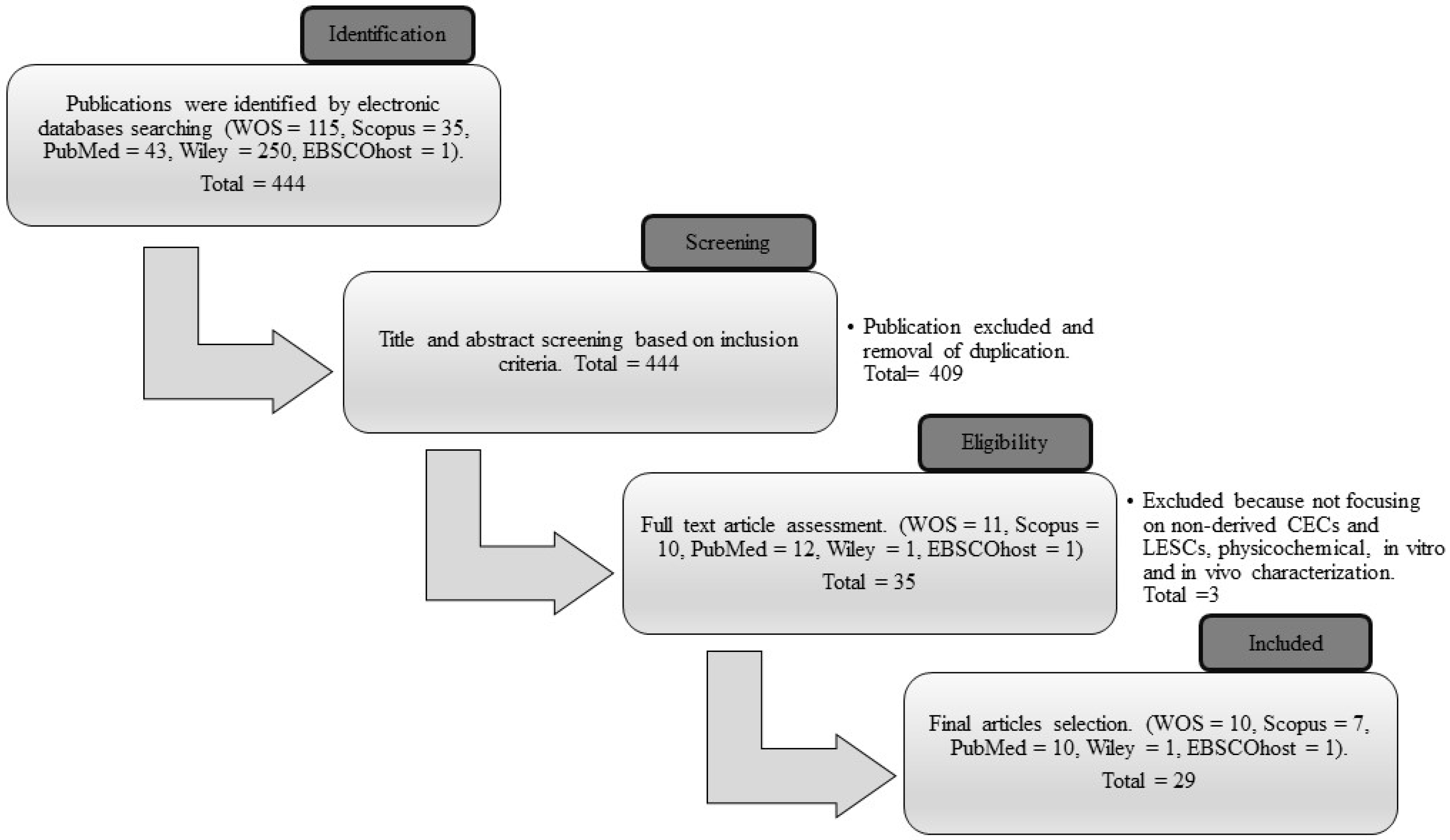
2.1. Search Strategy
2.2. Criteria of Selection
2.3. Management of Data Extraction Table
3. Results
3.1. Searching Result
3.2. Study Characteristics
4. Discussion
4.1. New Bioresource and Its Efficacy on CECs and LESCs
4.2. Physical Modification of Biomatrix
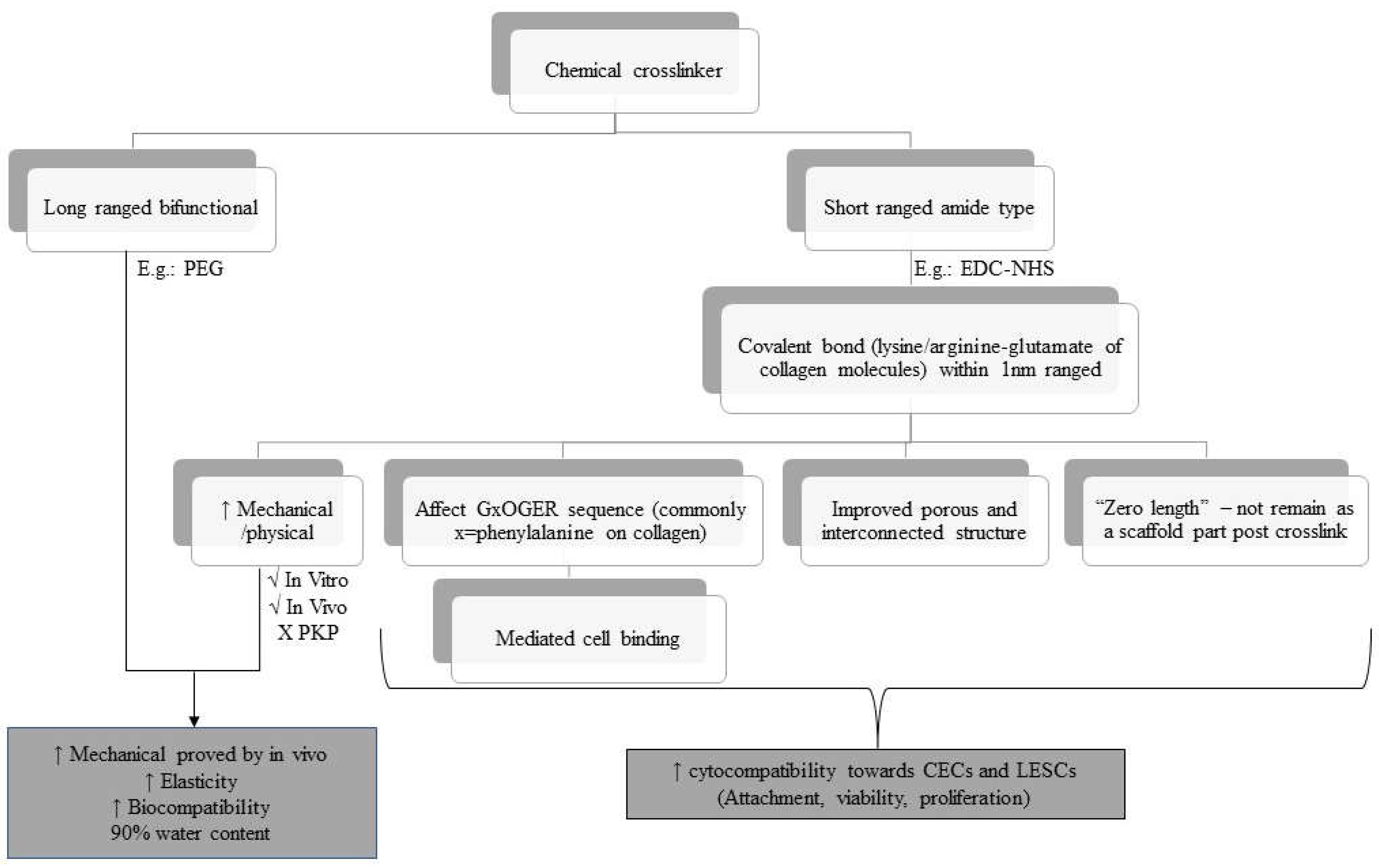
4.3. Crosslinking Effect on the Biocompatibility of the Construct towards CECs/LESCs
4.4. Interaction of CECs/LESCs with Other Cells and Molecules in a Collagen Biomatrix
4.5. Collagen as a Subtitute for Biomatrix
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| 3D-BDCS | 3D bioprinting decellularized collagen sheet |
| A2-P | L-ascorbic acid 2-phosphate |
| aCM | Acellular conjunctiva matrix |
| AHDCS | Adult human derived corneal stromal |
| APCS-gel | Acellular porcine corneal stroma hydrogels |
| BC1-gels-PEG- NHS | Bovine collagen type 1 hydrogel crosslinked to PEG-NHS |
| BMP | Bone morphogenetic protein |
| BPC | Bioengineered porcine collagen |
| CAT | Chloramphenicol acetyltransferase |
| CECs | Corneal epithelial cells |
| CK | Cytokeratin |
| CLP | Collagen-like peptide |
| CLP-12 EDC | Collagen-like peptide type 12 crosslinked with 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride |
| CLP-PEG-EDC-NHS | Collagen-like peptide crosslinked with 1–Ethyl–3-(3-dimethylaminopropyl) carbodiimide-N-hydroxy succinimide and conjugated to the polyethene glycol |
| CMP | Collagen mimetic peptides |
| COLLEN | Decellularized corneal lenticule embedded compressed collagen |
| ColMA | Collagen Methacrylate |
| CSSCs | Corneal stromal stem cells |
| CV | Collagen vitrigel |
| dAM | Denuded amniotic membrane |
| dCL | Decellularized corneal lenticule |
| dFib | Diseased fibroblasts |
| DHC | Decellularized human corneal tissue remnants |
| DMTMM | 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium chloride |
| ECM | Extracellular matrix |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride |
| EDC-NHS | 1–Ethyl–3-(3-dimethylaminopropyl) carbodiimide-N-hydroxysuccinimide |
| FGF | Fibroblast growth factor |
| FN | Fibronectin |
| FSC-PE | Fish scale collagen coated with polyethene |
| FTIR | Fourier-transform infrared spectroscopy |
| H&E | Haematoxylin and eosin stain |
| HA-DOPA | Dopamine hydrazone scaffold- crosslinked hyaluronic acid |
| hADSCs | Human adipose-derived mesenchymal stem cells |
| HAM | Human amniotic membrane |
| hCECs | Human corneal epithelial cells |
| hLESCs | Human limbal epithelial stem cells |
| hLF | Human limbal fibroblast |
| Hyp | Hydroxyproline |
| IF | Immunofluorescence |
| IHC | Immunohistochemistry |
| ihCECs | Immortalized human corneal epithelial cells |
| K | Keratin |
| LEPC | Limbal epithelial progenitor cells |
| LESCs | Limbal epithelial stem cells |
| LM | Limbal melanocytes |
| LMSC | Limbal mesenchymal stromal cells |
| LSCD | Limbal stem cell deficiency |
| MMP | Matrix metalloproteinases |
| MPC | Methacyloyloxyethyl phosphorylcholine |
| MTT | Tetrazolium salt (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NHS | N-hydroxysuccinimide |
| NS | Naproxen sodium |
| OA-gels-CIV | Oxidized alginate hydrogel incorporated with collagen IV |
| OCT | Optical coherence tomography |
| PE | Polyethene |
| PEG | polyethene glycol |
| PKP | Penetrating keratoplasty |
| PLA | Poly-L/DL- lactic acid |
| PLGA | Poly lactic-co-glycolic acid |
| RAFT TE | Real Architecture for 3-Dimensional Tissue Equivalent |
| RAFT TE-CI | Real Architecture for 3-Dimensional Tissue Equivalent treated with collagenase I |
| RAFT TE-NT | Real Architecture for 3-Dimensional Tissue Equivalent treated without treatment. |
| RAFT TE-PBS | Real Architecture for 3-Dimensional Tissue Equivalent treated with phosphate-buffered saline |
| RAFT-TE | Real Architecture for 3-Dimensional Tissue Equivalent |
| rCECs | Rabbit corneal epithelial cells |
| RHC | Recombinant human collagen |
| RHCI | Recombinant human collagen type I |
| RHCIII | Recombinant human collagen type III |
| RTCI-gels | Rat tail collagen type I hydrogel |
| RT-PCR | Reverse transcription polymerase chain reaction |
| SCF | Stem cell factor |
| SDS | Sodium dodecyl-sulphate |
| SEM | Scanning electron microscope |
| SIRC | Statens Seruminstitut Rabbit Cornea |
| TEM | Transmission electron microscopy |
| TGF-β1 | Transforming growth factor beta 1 |
| TKE2 | Mouse corneal epithelial stem/progenitor cells |
| TMSCs | Human turbinate-derived mesenchymal stem cells |
| WOS | Web of Science |
| YAP | Yes-associated protein |
References
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. Journal of Cataract & Refractive Surgery 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Maurice, D.M. The cornea and sclera. In Vegetative Physiology and Biochemistry, 1st ed.; Davson, H., Ed.; Elsevier: Amsterdam, Netherlands, 1962; Volume 1, pp. 289–368. [Google Scholar] [CrossRef]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian journal of ophthalmology 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, C.E.; Rnjak-Kovacina, J.; Kaplan, D.L. Corneal tissue engineering: recent advances and future perspectives. Tissue Engineering Part B: Reviews 2015, 21, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Espana, E.; Ti, S.; Grueterich, M.; Touhami, A.; Tseng, S. Corneal stromal changes following reconstruction by ex vivo expanded limbal epithelial cells in rabbits with total limbal stem cell deficiency. British journal of ophthalmology 2003, 87, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Gipson, I.K.; Stepp, M.A. Anatomy and cell biology of the cornea, superficial limbus, and conjunctiva. Albert and Jakobiec's Principles and Practice of Ophthalmology 2022, 3–30. [CrossRef]
- Lu, L.; Reinach, P.S.; Kao, W.W. Corneal epithelial wound healing. Experimental biology and medicine 2001, 226, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Krachmer, J.; Mannis, M.; Holland, E. Cornea, Volume Two: Surgery of the Cornea and Conjuctiva, 2nd ed.; Elsevier Mosby, Maryland Height, Missouri, United States, 2005; pp. 997–1011. [CrossRef]
- Bergmanson, J.P. Anatomy and physiology of the cornea and related structures. In Contact Lens, 6th ed.; Anthony, J.P.; Lynne, S. Eds.; Elsevier Mosby, Maryland Height, Missouri, United States, 2019; pp. 33–43. [CrossRef]
- Downie, L.E.; Bandlitz, S.; Bergmanson, J.P.; Craig, J.P.; Dutta, D.; Maldonado-Codina, C.; Ngo, W.; Siddireddy, J.S.; Wolffsohn, J.S. BCLA CLEAR-Anatomy and physiology of the anterior eye. Contact Lens and Anterior Eye 2021, 44, 132–156. [Google Scholar] [CrossRef] [PubMed]
- Fagerholm, P. Wound healing after photorefractive keratectomy. Journal of Cataract & Refractive Surgery 2000, 26, 432–447. [Google Scholar] [CrossRef]
- Kuo, I.C. Corneal wound healing. Current opinion in ophthalmology 2004, 15, 311–315. [Google Scholar] [CrossRef]
- Liu, C.Y.; Kao, W.W. Corneal Epithelial Wound Healing. Progress in molecular biology and translational science 2015, 134, 61–71. [Google Scholar] [CrossRef]
- Park, M.; Richardson, A.; Pandzic, E.; Lobo, E.P.; Whan, R.; Watson, S.L.; Lyons, J.G.; Wakefield, D.; Di Girolamo, N. Visualizing the Contribution of Keratin-14(+) Limbal Epithelial Precursors in Corneal Wound Healing. Stem Cell Reports 2019, 12, 14–28. [Google Scholar] [CrossRef]
- Choi, J.S.; Joo, C.K. Wakayama symposium: new therapies for modulation of epithelialization in corneal wound healing. The ocular surface 2013, 11, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Notara, M.; Lentzsch, A.; Coroneo, M.; Cursiefen, C. The role of limbal epithelial stem cells in regulating corneal (lymph) angiogenic privilege and the micromilieu of the limbal niche following UV exposure. Stem cells international 2018, 2018, 8620172. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.; González, S.; Roberts, J.S.; Robertson, S.Y.; Ruiz, M.; Zheng, J.; Deng, S.X. Human limbal epithelial stem cell regulation, bioengineering and function. Progress in retinal and eye research 2021, 85, 100956. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Xu, J.; Deng, S.X. The diagnosis of limbal stem cell deficiency. The ocular surface 2018, 16, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Joseph, A.; Shanmuganathan, V.; Jones, R. Stem cell differentiation and the effects of deficiency. Eye 2003, 17, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Potter, V.J.; Karamichos, D.; Lee, D.J. Ocular complications of diabetes and therapeutic approaches. BioMed research international 2016, 2016, 3801570. [Google Scholar] [CrossRef]
- Córdoba, A.; Mejía, L.F.; Mannis, M.J.; Navas, A.; Madrigal-Bustamante, J.A.; Graue-Hernandez, E.O. Current global bioethical dilemmas in corneal transplantation. Cornea 2020, 39, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Behlau, I.; Martin, K.V.; Martin, J.N.; Naumova, E.N.; Cadorette, J.J.; Sforza, J.T.; Pineda, R.; Dohlman, C.H. Infectious endophthalmitis in Boston keratoprosthesis: incidence and prevention. Acta ophthalmologica 2014, 92, 546–555. [Google Scholar] [CrossRef]
- Duan, X.; Sheardown, H. Dendrimer crosslinked collagen as a corneal tissue engineering scaffold: mechanical properties and corneal epithelial cell interactions. Biomaterials 2006, 27, 4608–4617. [Google Scholar] [CrossRef]
- Zhang, B.; Xue, Q.; Hu, H.-y.; Yu, M.-f.; Gao, L.; Luo, Y.-c.; Li, Y.; Li, J.-t.; Ma, L.; Yao, Y.-f. Integrated 3D bioprinting-based geometry-control strategy for fabricating corneal substitutes. Journal of Zhejiang University-Science B 2019, 20, 945–959. [Google Scholar] [CrossRef]
- Jameson, J.F.; Pacheco, M.O.; Nguyen, H.H.; Phelps, E.A.; Stoppel, W.L. Recent Advances in Natural Materials for Corneal Tissue Engineering. Bioengineering 2021, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M.; Fernandez-Perez, J.; Masterton, S.; Madden, P.W.; Bhattacharjee, P. Designing-scaffolds for corneal regeneration. Advanced Functional Materials 2020, 30, 1908996. [Google Scholar] [CrossRef]
- Hong, H.; Huh, M.-I.; Park, S.M.; Lee, K.-P.; Kim, H.K.; Kim, D.S. Decellularized corneal lenticule embedded compressed collagen: toward a suturable collagenous construct for limbal reconstruction. Biofabrication 2018, 10, 045001. [Google Scholar] [CrossRef] [PubMed]
- Man, R.C.; Yong, T.K.; Hwei, N.M.; Halim, W.H.W.A.; Zahidin, A.Z.M.; Ramli, R.; Saim, A.B.; Idrus, R.B.H. Corneal regeneration by induced human buccal mucosa cultivated on an amniotic membrane following alkaline injury. Molecular vision 2017, 23, 810. [Google Scholar] [PubMed]
- Rohaina, C.M.; Then, K.Y.; Ng, A.M.H.; Halim, W.H.W.A.; Zahidin, A.Z.M.; Saim, A.; Idrus, R.B. Reconstruction of limbal stem cell deficient corneal surface with induced human bone marrow mesenchymal stem cells on amniotic membrane. Translational Research 2014, 163, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Rama, P.; Bonini, S.; Lambiase, A.; Golisano, O.; Paterna, P.; De Luca, M.; Pellegrini, G. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation 2001, 72, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. New England journal of medicine 2010, 363, 147–155. [Google Scholar] [CrossRef]
- Alaminos, M.; Sánchez-Quevedo, M.D.C.; Munoz-Ávila, J.I.; Serrano, D.; Medialdea, S.; Carreras, I.; Campos, A. Construction of a complete rabbit cornea substitute using a fibrin-agarose scaffold. Investigative ophthalmology & visual science 2006, 47, 3311–3317. [Google Scholar] [CrossRef]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. The Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Behaegel, J.; Ní Dhubhghaill, S.; Koppen, C.; Zakaria, N. Safety of cultivated limbal epithelial stem cell transplantation for human corneal regeneration. Stem cells international 2017, 2017, 6978253. [Google Scholar] [CrossRef]
- Chae, J.J.; McIntosh, A.W.; Espinoza, F.A.; Mulreany, D.G.; Ng, S.; Takezawa, T.; Trexler, M.M.; Schein, O.D.; Chuck, R.S.; Elisseeff, J.H. Regeneration of corneal epithelium utilizing a collagen vitrigel membrane in rabbit models for corneal stromal wound and limbal stem cell deficiency. Acta ophthalmologica 2015, 93, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Beuerman, R.W.; Chan-Park, M.; Cheng, Z.; Ang, L.P.; Tan, D.T. Enhancement of the mechanical and biological properties of a biomembrane for tissue engineering the ocular surface. Annals-Academy of Medicine Singapore 2006, 35, 210–214. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Yang, L.; Zeng, Y.; Gao, X.; Xu, H. Poly (ethylene glycol)-modified silk fibroin membrane as a carrier for limbal epithelial stem cell transplantation in a rabbit LSCD model. Stem cell research & therapy 2017, 8, 1–19. [Google Scholar] [CrossRef]
- Dang, X.; Li, Y.; Yang, M. Biodegradable waterborne polyurethane grafted with gelatin hydrolysate via solvent-free copolymerization for potential porous scaffold material. Journal of the mechanical behavior of biomedical materials 2019, 92, 79–89. [Google Scholar] [CrossRef] [PubMed]
- de la Mata, A.; Nieto-Miguel, T.; López-Paniagua, M.; Galindo, S.; Aguilar, M.R.; Garcia-Fernandez, L.; Gonzalo, S.; Vázquez, B.; San Román, J.; Corrales, R.M. Chitosan–gelatin biopolymers as carrier substrata for limbal epithelial stem cells. Journal of Materials Science: Materials in Medicine 2013, 24, 2819–2829. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.; De Bank, P.A.; Luetchford, K.A.; Acosta, F.R.; Connon, C.J. Oxidized alginate hydrogels as niche environments for corneal epithelial cells. Journal of Biomedical Materials Research Part A 2014, 102, 3393–3400. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Qu, Y.; Hua, X.; Zhang, L.; Liu, Z.; Pflugfelder, S.; Li, D. A hyaluronan hydrogel scaffold-based xeno-free culture system for ex vivo expansion of human corneal epithelial stem cells. Eye 2017, 31, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; AbuSamra, D.B.; Chivu, A.; Argüeso, P.; Dohlman, C.H.; Patra, H.K.; Chodosh, J.; González-Andrades, M. Optimization of collagen chemical crosslinking to restore biocompatibility of tissue-engineered scaffolds. Pharmaceutics 2021, 13, 832. [Google Scholar] [CrossRef]
- Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in vitro characterization of cross-linked collagen–gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. International journal of biological macromolecules 2019, 126, 620–632. [Google Scholar] [CrossRef]
- Palchesko, R.N.; Carrasquilla, S.D.; Feinberg, A.W. Natural biomaterials for corneal tissue engineering, repair, and regeneration. Advanced healthcare materials 2018, 7, 1701434. [Google Scholar] [CrossRef]
- Crabb, R.A.; Chau, E.P.; Decoteau, D.M.; Hubel, A. Microstructural characteristics of extracellular matrix produced by stromal fibroblasts. Annals of biomedical engineering 2006, 34, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Chivu, A.; AbuSamra, D.B.; Saha, A.; Chowdhuri, S.; Pramanik, B.; Dohlman, C.H.; Das, D.; Argüeso, P.; Rajaiya, J. Crosslinker-free collagen gelation for corneal regeneration. Scientific Reports 2022, 12, 9180. [Google Scholar] [CrossRef] [PubMed]
- Varghese, T.; Jacob, J. Fish Collagen as a Potential Biocompatible Composite with Biomedical Applications. Journal of Science, Technology and Management 2019, 12, 47–51. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, S.; Mao, B.; Chen, J.; Zhang, Z. Cornea-stroma-mimicking films derived from cellulose nanocrystal templating fibrous collagen as therapeutic contact lenses. ACS Sustainable Chemistry & Engineering 2019, 7, 12248–12260. [Google Scholar] [CrossRef]
- Cheema, U.; Brown, R.A. Rapid fabrication of living tissue models by collagen plastic compression: understanding three-dimensional cell matrix repair in vitro. Advances in wound care 2013, 2, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Le, P.; Fernandes-Cunha, G.M.; Heilshorn, S.C.; Myung, D. Bio-orthogonally crosslinked hyaluronate-collagen hydrogel for suture-free corneal defect repair. Biomaterials 2020, 255, 120176. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Cunha, G.M.; Chen, K.M.; Chen, F.; Le, P.; Han, J.H.; Mahajan, L.A.; Lee, H.J.; Na, K.S.; Myung, D. In situ-forming collagen hydrogel crosslinked via multi-functional PEG as a matrix therapy for corneal defects. Scientific reports 2020, 10, 16671. [Google Scholar] [CrossRef] [PubMed]
- Kishore, V.; Iyer, R.; Frandsen, A.; Nguyen, T.-U. In vitro characterization of electrochemically compacted collagen matrices for corneal applications. Biomedical Materials 2016, 11, 055008. [Google Scholar] [CrossRef]
- Ji, P.; Zhang, C.; Kong, Y.; Liu, H.; Guo, J.; Shi, L.; Yang, H.; Gu, Z.; Liu, Y. Collagen Film with Bionic Layered Structure and High Light Transmittance for Personalized Corneal Repair Fabricated by Controlled Solvent Evaporation Technique. Journal of Functional Biomaterials 2022, 13, 52. [Google Scholar] [CrossRef]
- Qin, L.; Gao, H.; Xiong, S.; Jia, Y.; Ren, L. Preparation of collagen/cellulose nanocrystals composite films and their potential applications in corneal repair. Journal of Materials Science: Materials in Medicine 2020, 31, 55. [Google Scholar] [CrossRef]
- Hancox, Z.; Keshel, S.H.; Yousaf, S.; Saeinasab, M.; Shahbazi, M.-A.; Sefat, F. The progress in corneal translational medicine. Biomaterials Science 2020, 8, 6469–6504. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Ji, S.; Luo, P.; Liu, H.; Zhu, S.; Wang, G.; Zhou, P.; Xiao, S.; Xia, Z. Accelerated expansion of epidermal keratinocyte and improved dermal reconstruction achieved by engineered amniotic membrane. Cell transplantation 2013, 22, 1831–1844. [Google Scholar] [CrossRef] [PubMed]
- Dinescu, S.; Albu Kaya, M.; Chitoiu, L.; Ignat, S.; Kaya, D.A.; Costache, M. Collagen-based hydrogels and their applications for tissue engineering and regenerative medicine. In Cellulose-Based Superabsorbent Hydrogels. Polymers and Polymeric Composites: A Reference Series; Mondal, M., Ed.; Springer International Publishing: Edinburg, Scotland, UK, 2019; pp. 1643–1664. [Google Scholar] [CrossRef]
- Xeroudaki, M.; Thangavelu, M.; Lennikov, A.; Ratnayake, A.; Bisevac, J.; Petrovski, G.; Fagerholm, P.; Rafat, M.; Lagali, N. A porous collagen-based hydrogel and implantation method for corneal stromal regeneration and sustained local drug delivery. Scientific reports 2020, 10, 16936. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.R.; Hamley, I.W.; Connon, C.J. Ex vivo expansion of limbal stem cells is affected by substrate properties. Stem cell research 2012, 8, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, R.M.; Vajda, F.; Wibowo, J.A.; Figueiredo, F.; Connon, C.J. YAP, ΔNp63, and β-catenin signaling pathways are involved in the modulation of corneal epithelial stem cell phenotype induced by substrate stiffness. Cells 2019, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Massie, I.; Kureshi, A.K.; Schrader, S.; Shortt, A.J.; Daniels, J.T. Optimization of optical and mechanical properties of real architecture for 3-dimensional tissue equivalents: towards treatment of limbal epithelial stem cell deficiency. Acta biomaterialia 2015, 24, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Then, K.Y.; Azlina, M.; Ropilah, A.; Ruszymah, B.; Rohaina, C.; Ng, M. The use of bone marrow derived mesenchymal stem cell for cornea regeneration in rabbit model. Asian Journal of Ophthalmology 2017, 15, 224–233. [Google Scholar] [CrossRef]
- Hallab, N.J.; Bundy, K.J.; O'Connor, K.; Moses, R.L.; Jacobs, J.J. Evaluation of metallic and polymeric biomaterial surface energy and surface roughness characteristics for directed cell adhesion. Tissue engineering 2001, 7, 55–71. [Google Scholar] [CrossRef]
- Wang, X.; Majumdar, S.; Ma, G.; Sohn, J.; Yiu, S.C.; Stark, W.; Al-Qarni, A.; Edward, D.P.; Elisseeff, J.H. Chondroitin sulfate–based biocompatible crosslinker restores corneal mechanics and collagen alignment. Investigative ophthalmology & visual science 2017, 58, 3887–3895. [Google Scholar] [CrossRef]
- Kang, K.B.; Lawrence, B.D.; Gao, X.R.; Luo, Y.; Zhou, Q.; Liu, A.; Guaiquil, V.H.; Rosenblatt, M.I. Micro-and nanoscale topographies on silk regulate gene expression of human corneal epithelial cells. Investigative ophthalmology & visual science 2017, 58, 6388–6398. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Systematic reviews 2021, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. JBI Evidence Implementation 2015, 13, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Sekar, S.; Katheem, M.F.; Krishnakumar, S.; Sastry, T.P. Fish scale collagen-a novel material for corneal tissue engineering. Artificial organs 2012, 36, 829–835. [Google Scholar] [CrossRef]
- Jangamreddy, J.R.; Haagdorens, M.K.; Islam, M.M.; Lewis, P.; Samanta, A.; Fagerholm, P.; Liszka, A.; Ljunggren, M.K.; Buznyk, O.; Alarcon, E.I. Short peptide analogs as alternatives to collagen in pro-regenerative corneal implants. Acta biomaterialia 2018, 69, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Qu, M.; Wang, Y.; Wang, Z.; Shi, W. Xenogeneic acellular conjunctiva matrix as a scaffold of tissue-engineered corneal epithelium. PLoS One 2014, 9, 111846. [Google Scholar] [CrossRef]
- Park, J.; Lee, K.P.; Kim, H.; Park, S.; Wijesinghe, R.E.; Lee, J.; Han, S.; Lee, S.; Kim, P.; Cho, D.W. Biocompatibility evaluation of bioprinted decellularized collagen sheet implanted in vivo cornea using swept-source optical coherence tomography. Journal of biophotonics 2019, 12, 201900098. [Google Scholar] [CrossRef] [PubMed]
- Baratta, R.O.; Del Buono, B.J.; Schlumpf, E.; Ceresa, B.P.; Calkins, D.J. Collagen Mimetic Peptides Promote Corneal Epithelial Cell Regeneration. Frontiers in Pharmacology 2021, 12, 705623. [Google Scholar] [CrossRef]
- Zhou, Q.; Guaiquil, V.H.; Wong, M.; Escobar, A.; Ivakhnitskaia, E.; Yazdanpanah, G.; Jing, H.; Sun, M.; Sarkar, J.; Luo, Y. Hydrogels derived from acellular porcine corneal stroma enhance corneal wound healing. Acta Biomaterialia 2021, 134, 177–189. [Google Scholar] [CrossRef]
- Polisetti, N.; Roschinski, B.; Schlötzer-Schrehardt, U.; Maier, P.; Schlunck, G.; Reinhard, T. A decellularized human limbal scaffold for limbal stem cell niche reconstruction. International journal of molecular sciences 2021, 22, 10067. [Google Scholar] [CrossRef]
- Sánchez-Porras, D.; Caro-Magdaleno, M.; González-Gallardo, C.; García-García, Ó.D.; Garzón, I.; Carriel, V.; Campos, F.; Alaminos, M. Generation of a biomimetic substitute of the corneal limbus using decellularized scaffolds. Pharmaceutics 2021, 13, 1718. [Google Scholar] [CrossRef]
- Chakraborty, A.; Dutta, J.; Das, S.; Datta, H. Comparison of ex vivo cultivated human limbal epithelial stem cell viability and proliferation on different substrates. International ophthalmology 2013, 33, 665–670. [Google Scholar] [CrossRef]
- Haagdorens, M.; Cėpla, V.; Melsbach, E.; Koivusalo, L.; Skottman, H.; Griffith, M.; Valiokas, R.; Zakaria, N.; Pintelon, I.; Tassignon, M.-J. In vitro cultivation of limbal epithelial stem cells on surface-modified crosslinked collagen scaffolds. Stem cells international 2019, 2019, 7867613. [Google Scholar] [CrossRef] [PubMed]
- Kureshi, A.K.; Drake, R.A.; Daniels, J.T. Challenges in the development of a reference standard and potency assay for the clinical production of RAFT tissue equivalents for the cornea. Regenerative Medicine 2014, 9, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.; Zaniolo, K.; Gingras, M.-È.; Couture, C.; Salesse, C.; Guérin, S.L. Functional Impact of Collagens on the Activity Directed by the Promoter of the α5 Integrin Subunit Gene in Corneal Epithelial Cells. Investigative ophthalmology & visual science 2015, 56, 6217–6232. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, Q.; Yang, Y.; Yi, J.; Wei, W.; Hong, Y.; Zhang, X.; Zhou, F.; Yao, X.; Ouyang, H. Wound dressing gel with resisted bacterial penetration and enhanced re-epithelization for corneal epithelial-stromal regeneration. Applied Materials Today 2021, 24, 101119. [Google Scholar] [CrossRef]
- Chen, J.; Lan, J.; Liu, D.; Backman, L.J.; Zhang, W.; Zhou, Q.; Danielson, P. Ascorbic acid promotes the stemness of corneal epithelial stem/progenitor cells and accelerates epithelial wound healing in the cornea. Stem Cells Translational Medicine 2017, 6, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.L.; Yang, Y.; El Haj, A.J. Corneal stromal cell plasticity: in vitro regulation of cell phenotype through cell-cell interactions in a three-dimensional model. Tissue Engineering Part A 2014, 20, 225–238. [Google Scholar] [CrossRef]
- Kureshi, A.K.; Dziasko, M.; Funderburgh, J.L.; Daniels, J.T. Human corneal stromal stem cells support limbal epithelial cells cultured on RAFT tissue equivalents. Scientific reports 2015, 5, 16186. [Google Scholar] [CrossRef]
- Massie, I.; Dale, S.B.; Daniels, J.T. Limbal fibroblasts maintain normal phenotype in 3D RAFT tissue equivalents suggesting potential for safe clinical use in treatment of ocular surface failure. Tissue Engineering Part C: Methods 2015, 21, 576–584. [Google Scholar] [CrossRef]
- de la Mata, A.; Mateos-Timoneda, M.A.; Nieto-Miguel, T.; Galindo, S.; López-Paniagua, M.; Planell, J.A.; Engel, E.; Calonge, M. Poly-l/dl-lactic acid films functionalized with collagen IV as carrier substrata for corneal epithelial stem cells. Colloids and Surfaces B: Biointerfaces 2019, 177, 121–129. [Google Scholar] [CrossRef]
- Kayıran Çelebier, S.; Bozdağ Pehlivan, S.; Demirbilek, M.; Akıncı, M.; Vural, İ.; Akdağ, Y.; Yürüker, S.; Ünlü, N. Development of an anti-inflammatory drug-incorporated biomimetic scaffold for corneal tissue engineering. Journal of Ocular Pharmacology and Therapeutics 2020, 36, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kang, K.B.; Sartaj, R.; Sun, M.G.; Zhou, Q.; Guaiquil, V.H.; Rosenblatt, M.I. Silk films with nanotopography and extracellular proteins enhance corneal epithelial wound healing. Scientific reports 2021, 11, 8168. [Google Scholar] [CrossRef] [PubMed]
- Polisetti, N.; Schmid, A.; Schlötzer-Schrehardt, U.; Maier, P.; Lang, S.J.; Steinberg, T.; Schlunck, G.; Reinhard, T. A decellularized human corneal scaffold for anterior corneal surface reconstruction. Scientific reports 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Kobayashi, T.; Hayashi, Y.; Zhang, Y.; Hara, Y.; Higashine, M.; Shiraishi, A.; Ohashi, Y. Involvement of stem cell factor and c-kit in corneal wound healing in mice. Molecular vision 2012, 18, 1505–1515. [Google Scholar] [PubMed]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of collagen I hydrogels for bioengineered tissue microenvironments: characterization of mechanics, structure, and transport. Tissue Engineering Part B: Reviews 2014, 20, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of collagen for biomaterials in skin wound healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef]
- Dyrlund, T.F.; Poulsen, E.T.; Scavenius, C.; Nikolajsen, C.L.; Thøgersen, I.B.; Vorum, H.; Enghild, J.J. Human cornea proteome: identification and quantitation of the proteins of the three main layers including epithelium, stroma, and endothelium. Journal of proteome research 2012, 11, 4231–4239. [Google Scholar] [CrossRef] [PubMed]
- Montanino, A.; Gizzi, A.; Vasta, M.; Angelillo, M.; Pandolfi, A. Modeling the biomechanics of the human cornea accounting for local variations of the collagen fibril architecture. ZAMM-Journal of Applied Mathematics and Mechanics 2018, 98, 2122–2134. [Google Scholar] [CrossRef]
- Qin, D.; Bi, S.; You, X.; Wang, M.; Cong, X.; Yuan, C.; Yu, M.; Cheng, X.; Chen, X.-G. Development and application of fish scale wastes as versatile natural biomaterials. Chemical Engineering Journal 2022, 428, 131102. [Google Scholar] [CrossRef]
- Lee, E.; Lee, W.-H.; Kaetzel, C.S.; Parry, G.; Bissell, M.J. Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proceedings of the National Academy of Sciences 1985, 82, 1419–1423. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Murphy, C.J.; McAnulty, J.F.; Raines, R.T. Peptides that anneal to natural collagen in vitro and ex vivo. Organic & biomolecular chemistry 2012, 10, 5892–5897. [Google Scholar] [CrossRef]
- Chen, B.; Jones, R.R.; Mi, S.; Foster, J.; Alcock, S.G.; Hamley, I.W.; Connon, C.J. The mechanical properties of amniotic membrane influence its effect as a biomaterial for ocular surface repair. Soft Matter 2012, 8, 8379–8387. [Google Scholar] [CrossRef]
- Foster, J.W.; Jones, R.R.; Bippes, C.A.; Gouveia, R.M.; Connon, C.J. Differential nuclear expression of Yap in basal epithelial cells across the cornea and substrates of differing stiffness. Experimental eye research 2014, 127, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Moers, K.; Steinberg, T.; Schlunck, G.; Reinhard, T.; Tomakidi, P.; Eberwein, P. Substrate elasticity as biomechanical modulator of tissue homeostatic parameters in corneal keratinocytes. Experimental cell research 2013, 319, 1889–1901. [Google Scholar] [CrossRef]
- Yazdanpanah, G.; Haq, Z.; Kang, K.; Jabbehdari, S.; l Rosenblatt, M.; Djalilian, A.R. Strategies for reconstructing the limbal stem cell niche. The ocular surface 2019, 17, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kang, K.B.; Sartaj, R.; Sun, M.G.; Zhou, Q.; Guaiquil, V.H.; Rosenblatt, M.I. Silk films with nanotopography and extracellular proteins enhance corneal epithelial wound healing. Scientific Reports 2021, 11, 8186. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, R.M.; Koudouna, E.; Jester, J.; Figueiredo, F.; Connon, C.J. Template curvature influences cell alignment to create improved human corneal tissue equivalents. Advanced Biosystems 2017, 1, 1700135. [Google Scholar] [CrossRef]
- Calderón-Colón, X.; Xia, Z.; Breidenich, J.L.; Mulreany, D.G.; Guo, Q.; Uy, O.M.; Tiffany, J.E.; Freund, D.E.; McCally, R.L.; Schein, O.D. Structure and properties of collagen vitrigel membranes for ocular repair and regeneration applications. Biomaterials 2012, 33, 8286–8295. [Google Scholar] [CrossRef]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: a multiscale deconstruction. Nature reviews Molecular cell biology 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate techniques, 3rd ed.; Academic press: Cambridge, Massachusetts, United States, 2013; pp. 259–273. [Google Scholar] [CrossRef]
- Wissink, M.; Beernink, R.; Pieper, J.; Poot, A.; Engbers, G.; Beugeling, T.; Van Aken, W.; Feijen, J. Immobilization of heparin to EDC/NHS-crosslinked collagen. Characterization and in vitro evaluation. Biomaterials 2001, 22, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Best, S.M.; Cameron, R.E. Crosslinking collagen constructs: achieving cellular selectivity through modifications of physical and chemical properties. Applied Sciences 2020, 10, 6911. [Google Scholar] [CrossRef]
- Zeeman, R.; Dijkstra, P.J.; van Wachem, P.B.; van Luyn, M.J.; Hendriks, M.; Cahalan, P.T.; Feijen, J. Successive epoxy and carbodiimide cross-linking of dermal sheep collagen. Biomaterials 1999, 20, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, J.-S.; Chansakul, T.; Yu, C.; Elisseeff, J.H.; Seungju, M.Y. Collagen mimetic peptide-conjugated photopolymerizable PEG hydrogel. Biomaterials 2006, 27, 5268–5276. [Google Scholar] [CrossRef] [PubMed]
- Doillon, C.J.; Côté, M.-F.; Pietrucha, K.; Laroche, G.; C.-Gaudreault, R. Porosity and biological properties of polyethylene glycol-conjugated collagen materials. Journal of Biomaterials Science, Polymer Edition 1995, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Rafat, M.; Li, F.; Fagerholm, P.; Lagali, N.S.; Watsky, M.A.; Munger, R.; Matsuura, T.; Griffith, M. PEG-stabilized carbodiimide crosslinked collagen–chitosan hydrogels for corneal tissue engineering. Biomaterials 2008, 29, 3960–3972. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Gu, L. Influence of crosslink density and stiffness on mechanical properties of type I collagen gel. Materials 2015, 8, 551–560. [Google Scholar] [CrossRef]
- Kang, S.J.; Kim, E.K.; Kim, H.B. Expression and distribution of extracellular matrices during corneal wound healing after keratomileusis in rabbits. Ophthalmologica 1999, 213, 20–24. [Google Scholar] [CrossRef]
- Nishida, T.; Nakamura, M.; Mishima, H.; Otori, T. Differential modes of action of fibronectin and epidermal growth factor on rabbit corneal epithelial migration. Journal of cellular physiology 1990, 145, 549–554. [Google Scholar] [CrossRef]
- Murakami, J.; Nishida, T.; Otori, T. Coordinated appearance of β1 integrins and fibronectin during corneal wound healing. The Journal of laboratory and clinical medicine 1992, 120, 86–93. [Google Scholar] [CrossRef]
- Zheng, M.; Tian, C.; Fan, T.; Xu, B. Fibronectin regulates the self-renewal of rabbit limbal epithelial stem cells by stimulating the Wnt11/Fzd7/ROCK non-canonical Wnt pathway. Experimental Eye Research 2019, 185, 107681. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R.; Timpl, R. Laminin and other basement membrane components. Annual review of cell biology 1987, 3, 57–85. [Google Scholar] [CrossRef] [PubMed]
- Nakayasu, K.; Tanaka, M.; Konomi, H.; Hayashi, T. Distribution of types I, II, III, IV and V collagen in normal and keratoconus corneas. Ophthalmic research 1986, 18. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, D.R.; Trüeb, B.; Winterhalter, K.H.; Witmer, R.; Fischer, R.W. Type VI collagen is a major component of the human cornea. FEBS letters 1986, 197, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Koulikovska, M.; Rafat, M.; Petrovski, G.; Veréb, Z.; Akhtar, S.; Fagerholm, P.; Lagali, N. Enhanced regeneration of corneal tissue via a bioengineered collagen construct implanted by a nondisruptive surgical technique. Tissue Engineering Part A 2015, 21, 1116–1130. [Google Scholar] [CrossRef]
- Kureshi, A.; Cheema, U.; Alekseeva, T.; Cambrey, A.; Brown, R. Alignment hierarchies: Engineering architecture from the nanometre to the micrometre scale. Journal of the Royal Society Interface 2010, 7, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yeh, L.-K.; Zhang, S.; Call, M.; Yuan, Y.; Yasunaga, M.; Kao, W.W.-Y.; Liu, C.-Y. Wnt/β-catenin signaling modulates corneal epithelium stratification via inhibition of Bmp4 during mouse development. Development 2015, 142, 3383–3393. [Google Scholar] [CrossRef]
- Li, X.-k.; Cai, S.-x.; Liu, B.; Xu, Z.-l.; Dai, X.-z.; Ma, K.-w.; Li, S.-q.; Yang, L.; Sung, K.P.; Fu, X.-b. Characteristics of PLGA–gelatin complex as potential artificial nerve scaffold. Colloids and Surfaces B: Biointerfaces 2007, 57, 198–203. [Google Scholar] [CrossRef]
- Campos, Y.; Almirall, A.; Fuentes, G.; Bloem, H.L.; Kaijzel, E.L.; Cruz, L.J. Tissue engineering: an alternative to repair cartilage. Tissue Engineering Part B: Reviews 2019, 25, 357–373. [Google Scholar] [CrossRef]
| Authors | Type of Biomatrix | Modification Techniques | Type of Cell | Test and Result (Physicochemical Properties) |
Test and Result (In Vitro Biocompatibility) |
Conclusion |
| Krishnan et al., 2012 [68] | FSC-PE | Decalcification and demineralization of the biomatrix, followed bycoated with PE. | Limbal tissue |
|
|
FSC-PE has good mechanical properties and supports the differentiation of LESCs and the proliferation of differentiated CECs. |
| Zhao et al., 2014 [70] | aCM | Xenogeneic decellularization of the conjunctiva with 0.1% sodium dodecyl-sulphate (SDS). | iCECs and primary rabbit corneal epithelial cells (rCECs). |
|
|
aCM possesses favourable physical properties and supports multi-layered CECs growth. |
| Sánchez-Porras et al., 2021 [75] | Porcine limbus | Decellularization (4 methods) and recellularization. | SIRC and hADSCs |
|
|
0.1 % SDS is the best way to decellularized limbal. This biomatrix able to regenerate the stratified epithelium. |
| Naresh et al., 2021 [88] | Decellularized human corneal tissue remnants (DHC) | Decellularization (1% sodium deoxycholate, DNAse I, and 4% dextran), followed by recellularization. | limbal epithelial progenitor cells (LEPC), limbal mesenchymal stromal cells (LMSC), and limbal melanocytes (LM) |
|
|
DHC (with 4% dextran) complete the removal of cellular component, maintain the tissue architecture, ECM composition and biocompatible with LEPCs and LMs. |
| Zhou et al., 2021 [73] |
acellular porcine corneal stroma hydrogels (APCS-gel) |
Decellularization | rCECs and rabbit corneal stromal cells (rCSCs) |
|
|
APCS-gel is suitable for CECs reconstruction by maintaining stemness and enhanced proliferation of the ocular surface. |
| Baratta et al., 2021 [72] |
CMP | Damaged collagen type 1 coated petri dish treated with CMP. | N/A |
|
N/A | CMP re-aligns the damaged collagen by enzymatic digestion. |
| Jones et al., 2012 [59] |
RTCI -gels | Compression by nylon mesh (50 µm mesh size, 134 g) for 5 min at room temperature |
hLESCs |
|
|
The compression improved the mechanical strength, surface topography and capacity of the RTC-gels to support the attachment, differentiation of LESCs and viability of differentiated CECs. |
| Gouveia et al., 2019 [60] |
RAFT TE | Treated with: collagenase I (RAFT TE-CI), Phosphate-buffered saline (PBS) (RAFT TE – PBS) or none (RAFT TE-NT). Laminin surface coating. |
hLESCs |
|
|
RAFT TE-CI supports LESCs compared to RAFT TE-PBS and RAFT TE-NT. RAFT TE-PBS and RAFT TE-NT (stiffer hydrogel supports the differentiation via mechanotransduction factor YAP and BMP4. |
| Massie et al., 2015 [61] |
RAFT TE | Different concentration and volume of collagen used. | hLESCs |
|
|
Optimal RAFT TE (0.6 ml of 3 mg/ml collagen) has suitable physical properties and supports hLESCs growth. |
| Kureshi et al., 2014 [78] |
RAFT TE | Incorporated with hLF and DMEM. 1mm wide strip defect was created on the epithelial surface on the construct (using algerbrush II corneal rust ring removal) and continue with analy sis. |
LESCs |
|
|
RAFT TE incorporated with hLF support the cultivation LESCs but is poorly differentiated and promotes wound closure. |
| Hong et al., 2018 [27] |
COLLEN | dCL embedded by compressed collagen | hCECs, rabbit LESCs |
|
|
COLLEN has suturable mechanical properties, resistant to degradation, and supports LESCs and CECs growth. |
| Jangamreddy et al., 2018 [69] | CLP-PEG-EDC-NHS & RHCIII-MPC (control) | Crosslinked to MPC & EDC-NHS. Conjugated to PEG |
ihCECs |
|
|
CLP-PEG-EDC-NHS are functionally equivalent to control, RHCIII-MPC biomatrix and biocompatible to the corneal cells. |
| Fernandes-Cunha et al., 2020 [51] |
Bovine collagen type 1 hydrogels crosslinked to PEG -NHS (BCI-gels-PEG- NHS) |
Crosslinked to NHS, Conjugated to PEG (4 or 8 arms & 4%, 8% or 16% concentration of PEG |
iCECs and corneal stromal stem cells (CSSCs) |
|
|
Mechanical properties of BC 1-gels-PEG-NHS depend on PEG’s arms and concentration. BC 1-gels-PEG-NHS support the iCECs and CSSCs proliferation, adherence and morphology compared to the non-crosslink hydrogel. |
| Haagdorens et al., 2019 [77] |
Unmodified RHCI, RHCI FN-pattern, CLP-12-EDC/NHS, CLP-12-DMTMM, CLP-12-FN-pattern, CLP-12-3D grooved, CLP-18-DMTMM. |
Different crosslinker: EDC, DMTMM. Surface modification: FN surface pattern, 3D grooved surface topography. |
iCECs primary hLESCs |
|
|
RHCI and CLP-12 DMTMM, irrespective of surface modification, support the cultivation of primary hLESCs and iCECs. The regenerated epithelium maintained similar characteristics to HAM-based cultures. |
| Xeroudaki et al., 2020 [58] |
BPC-EDC-NHS | Crosslinked with EDC-NHS Compression by compress mould method |
Primary hCECs |
|
|
BPC-EDC-NHS is transparent, has regularly arranged collagen, optimal mechanical properties and is biocompatible with CECs in vitro. |
| Chen et al., 2017 [81] |
Collagen type 1 coated 6 well plate | A2-P | TKE2 | N/A |
|
A2-P and collagen 1 enhanced the stemness and proliferation of TKE2 which depends on its regulation of ECM components including collagen I and IV. |
| Miyamoto et al., 2012 [89] |
Collagen type 1V coated dished. | Exposure to anti-SCF antibody, genistein, and Arg-Gly-Asp peptide | Mouse CECs iCECs. |
N/A |
|
SCF and c-kit play a role in the cornea wound healing by altering CECs attachment. |
| Lake et al., 2015 [79] |
Culture plates coated with 2 - 200 lg/cm2 collagen I, III, IV and VI. | Transfect a5 promoter/ chloramphenicol acetyltransferase (CAT) plasmids into CECs cultured on collagen. | hCECs rabbit CECs. |
N/A |
|
FN promoted the adhesive and migratory properties of CECs which were then altered by collagen which suppressed a5 gene expression, especially during confluence rabbit CECs. |
| Chakraborty et al., 2013 [76] |
A variety of substrates including collagen IV coated the dishes. | - | Primary hLESCs | N/A |
|
Collagen IV support the viability and proliferation of LESCs supported by the MMP-9 and MMP-2 (a key regulator of LESCs migration and proliferation). |
| Qin et al., 2021 [80] |
ColMA | Modifying collagen with methacrylate group, followed by photo crosslinking – photopolymerized in situ. | hCECs |
|
|
ColMA is a transparent biomatrix, with high-pressure overload capacity and is compatible with hCECs. |
| Wilson et al., 2014 [82] |
RTCI-gels-FN- coated-AHDCS. | FN-coating encapsulated the AHDCS, treated with transforming growth factor beta-1 (TGF-β1) media followed by wortmannin. |
AHDCS, CECs (3 different co-culture on the biomatrix: explant, transwell, and conditioned media co-cultured) |
|
|
Mutual interactions between CECs and CSSCs. A collagen hydrogel environment can retain the plasticity of CSSCs, and the mechanical properties of the cornea are defined by epithelial-stromal interactions. |
| Kureshi et al., 2015 [83] |
RAFT TE | - | Human CSSCs, hLESCs | N/A |
|
Cultivation of CSSCs support hLESCs on RAFT TE. |
| Massie et al., 2015 [84] |
RAFT TE-dFib RAFT TE-hLF |
Incorporated with hLF or dFib. | hLESCs, | N/A |
|
hLF remained quiescent while dFib maintained activated, pro-scarring phenotype properties in RAFT TE. |
| De La Mata et al., 2019 [85] |
PLA-collagen IV film | Functionalization of PLA film (70:30) | hCECs hLESCs |
|
|
PLA-collagen IV has suitable physical properties to support the attachment, viability, and proliferation of CECs and LESCs. It also maintains undifferentiated LESCs. |
| Wright et al., 2014 [40] |
Oxidized alginate hydrogel -collagen 1V (OA-gels-CIV) | Incorporated by collagen IV | Primary bovine LESCs and hCECs |
|
|
OA-gels- CIV enhanced CECs viability but does not influence LESCs viability and differentiation. |
| Kayiran Celebier et al., 2020 [86] |
PLGA- collagen I | Incorporated by collagen I | Primary rabbit CECs |
|
|
PLGA-collagen I promote CECs adhesion, viability and proliferation without causing toxic effects for at least 10 days. |
| Yuncin et al., 2021 [87] | Silk film coated collagen 1 | Nanotopography: flat, 2000, 1000, 80nm parallel ridge. Coating with ECM (including collagen I) |
Primary mouse CECs, primary rabbit CECs | N/A |
|
Collagen 1 coating and 800 nm ridge enhanced CECs growth, better cell spreading and wound recovery. |
| Authors | Type of Biomatrix | Modification Techniques | Animal Model/ Injury | Test and Result (in vivo) |
Conclusion |
| Zhao et al., 2014 [70] |
aCM | Xenogeneic decellularization of the conjunctiva with 0.1% sodium dodecyl-sulphate (SDS). | Mechanical injury by deep limbal lamellar keratectomy and chemical trauma on the CECs with n-heptanol. |
|
aCM support multilayered epithelial structure and is effective in the reconstruction of the ocular surface for the rabbit with the LSCD model compared to dAM |
| Zhou et al., 2021 [73] |
APCS-gel | Decellularization | Removal of 2 mm central corneal epithelium in mice. |
|
APCS-gel is suitable for CECs reconstruction by maintaining stemness and enhanced proliferation of the ocular surface. |
| Park et al., 2019 [71] |
3D-BDCS | Encapsulated human turbinate-derived mesenchymal stem cells (TMSCs) and followed by crosslinked in vivo. | Mechanical injury by the lamellar dissection by using a crescent knife. |
|
The changes in corneal thickness and the distributions of inflammatory cells and histology confirmed the biocompatibility of the 3D-BDCS |
| Baratta et al., 2021 [72] |
CMP | Short synthetic collagen peptide | Removal of epithelium and epithelial basement membranes of the mouse (360° lamellar keratectomy) by using an Algerbrush with a 0.5 mm burr. |
|
CMP re-aligns the damaged collagen. CMP enhanced the closure of the wound process and promoted the re-epithelization process with forming of organized epithelium layers. |
| Qin et al., 2021 [80] | ColMA | Modifying collagen with methacrylate group, followed by photocrosslinking-photopolymerized in situ. | Rabbit and pig corneal defect (partial thickness corneal defect). |
|
ColMA has a high-pressure overload capacity, a barrier against bacterial penetration, and dehydration. Nanogranules from dislodging ColMA adhere to stromal tissue promote re-epithelization, reduces myofibroblast activation and decrease scar formation. |
| Hong et al., 2018 [27] |
COLLEN-based limbal graft | dCL embedded by compressed collagen | Rabbit model of LSCD (induced by alkali burn), COLLEN was sutured onto the incised bed with 10-0 thilon nylon suture. |
|
The COLLEN-based limbal graft was successfully transplanted and verified its clinical efficacy on the ocular surface reconstruction of LSCD in a rabbit model. |
| Chae et al., 2015 [35] |
CV | Vitrification process | CV-fibrin glue group: stromal injury by lamellar keratectomy. CV-hLESCs group: LSCD induced by chemical injury. |
In the CV- fibrin glue group
CV-hLESCs group:
|
CV support CECs and prevents epithelial hypertrophy, shows no complication after implantation, and can serve as an hLESCs carrier. |
| Jangamreddy et al., 2018 [69] |
CLP- PEG- EDC NHS & RHC III-MPC (control) | Crosslinked to MPC & EDC-NHS, conjugated to PEG | Mechanical surgery of the mini pig's cornea |
|
CLP-PEG-EDC-NHS are functionally equivalent to RHCIII-MPC (control) and have pro-regenerative effects by stimulating the in-growing endogenous host cells to produce ECM via secretory extracellular vesicle. |
| Fernandes-Cunha et al., 2020 [51] |
BC1-gels-PEG- NHS |
Crosslinked to NHS, conjugated to PEG (4 or 8 arms & 4%, 8% or 16% concentration of PEG). | Mechanical injury of the cornea of adult white rabbit by lamellar keratectomy |
|
BC 1-gels-PEG- NHS is safely integrated and supports the multilayers of CECs. |
| Xeroudaki et al., 2020 [58] |
BPC-EDC-NHS | Crosslinked with EDC-NHS Compression by compress mould method |
Subcutaneous and rabbit's cornea (epithelial and stroma layer damaged) | Subcutaneous implantation.
Implantation into the rabbit corneal.
|
BPC-EDC-NHS has suitable mechanical properties, is safely integrated and is biocompatible with native corneal cells in vivo. |
| Chen et al., 2017 [81] | A2-P eye drop | A stable form of ascorbic acid. | Mechanical injury (epithelium layer) induced by using algerbrush II corneal rust remover. |
|
A2-P promoted corneal wound healing and supported viability and the proliferation of LESCs. A2-P also promoted endogenous ECM production of LESCs. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





