Submitted:
21 February 2023
Posted:
22 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction:
2. Methods
3. Genomic-Proteomic Context
3.1. Genetic location of BCL-2 Family Member Gene
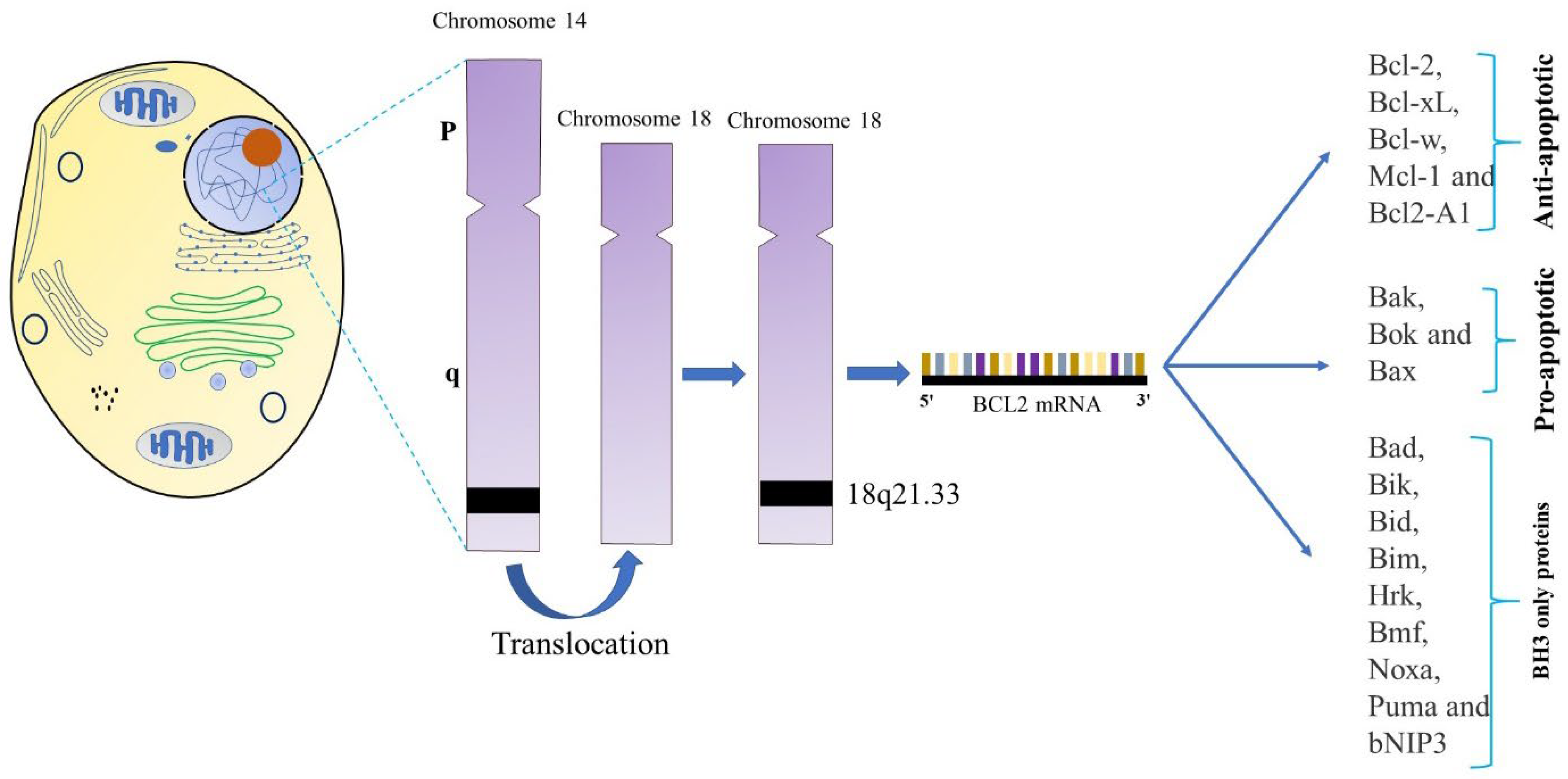
| Approved gene symbol | Approved Gene name | Aliases | Chromosomal Location | References |
|---|---|---|---|---|
| BCL-2 | BCL-2 apoptosis regulator | BCL-2, PPP1R50 |
18q21.33 | [31], [32] |
| BCL-2L1 | BCL-2 like 1 | BCLX, BCL-2L, BCL-X, BCL-XL, BCL-XS, PPP1R52 |
20q11.21 | [1] |
| BCL-2L2 | BCL-2 like 2 | KIAA0271, BCL-W, PPP1R51 |
14q11.2 | [36] |
| MCL1 | MCL1 apoptosis regulator, BCL-2 family member | BCL-2L3, Mcl-1 |
1q21.2 | [37] |
| BAX | BCL-2 associated X, apoptosis regulator | BCL-2L4 | 19q13.3 q13.4 | [38] |
| BAK1 | BCL-2 antagonist/killer 1 | BCL-2L7, BAK |
6p21.31 | [39,40] |
| BOK | BCL-2 family apoptosis regulator BOK | BCL-2L9, BOKL, MGC4631 |
2q37.3 | [39] |
| BCL-2L10 | BCL-2 like 10 | Diva, Boo, BCL-B |
15q21.2 | [39] |
| BIK | BCL-2-interacting killer (apoptosis-inducing) | NBK, BBC1 | 22q13.31 | [41] |
| PMAIP1 | Phorbol-12-myristate-13- acetate-induced protein 1 | NOXA, APR |
18q21 / (18q21.31) | [42,40] |
| BCL-2A1 | BCL-2-related protein A1 | GRS, BFL1, BCL-2L5, HBPA1 | 15q24.3 | [43] |
| BBC3 | BCL-2 binding component 3 | JFY1 , PUMA | 19q13.3-q13.4 | [44] |
| BNIP2 | BCL-2 interacting protein 2 | Nip2, BNIP-2 |
15q22.2 | [39] |
| HRK | Harakiri, BCL-2 interacting protein (contains only BH3 domain) | DP5 | 12q24.2 | [39] |
| BMF | BCL-2 modifying factor | 15q14 | [45] |
3.2. Structures of BCL-2 family members
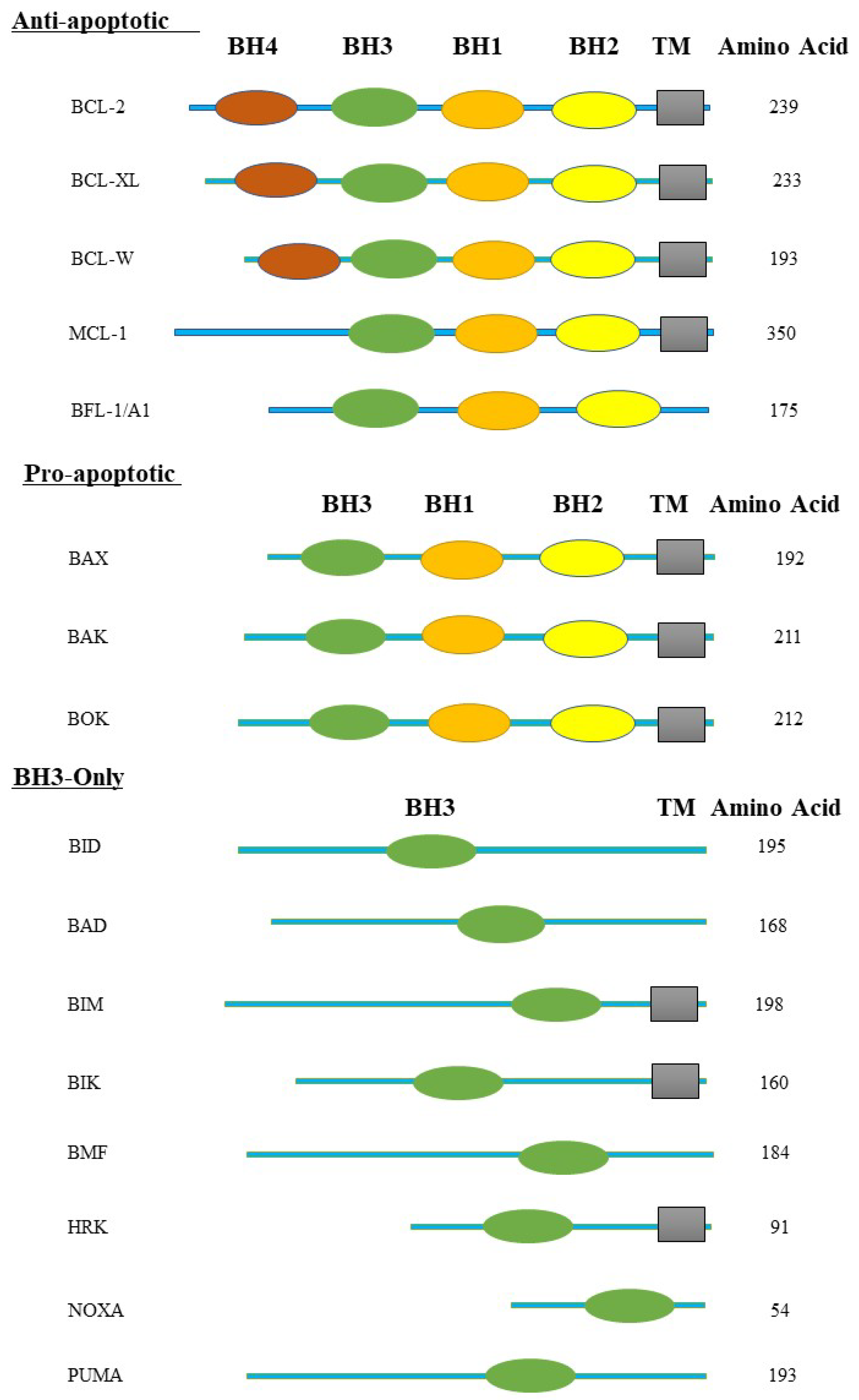
3.2. Gene Expression of BCL-2 Family Protein
| BCL-2 protein | Subcellular localization | Function | Reference |
| BCL-2 | Consistently linked to the MOM and/or ER membrane. | Both apoptosis effectors and BH3 only proteins are inhibited. | [34] [53] |
| BCL-XL | Located in the nucleus envelope, endoplasmic reticulum (ER), and mitochondrial membrane surfaces. | Bind to BAX or BAK to prevent apoptosis. | [19] [54] |
| MCL-1 | Placement to the outer mitochondrial membrane (OMM), inner mitochondrial membrane (IMM). | BH3-only proteins are inhibited as well as apoptotic effectors. Has an impact on cellular health and development in addition to its known pro-survival activities. | [55] |
| BCL-W | Loosely connected to the mitochondrial membrane and cytosol. | Inhibits BH3-only proteins as well as apoptotic effectors. | [19] [34] [36] |
| A1/Bfl-1 | Identified on the Golgi apparatus, cytosol, nuclear envelope, and ER membranes. | opposes the activation of caspase and prevents the release of proapoptotic cytochrome c from mitochondria. | [1] [56] |
| BAK | Always present on the OMM. | Oligomerizes to create holes in the OMM, which is considered to enhance MOMP. | [57] |
| BAX | Present on the OMM, often present in the cytoplasm as an inactive monomer. | Oligomerizes to create holes in the OMM, which is considered to enhance MOMP. | [57] |
| BOK | Detected on the ER membranes, nuclear outer membrane, and Golgi apparatus. | Depends on BAX and BAK to promote apoptosis. | [58] |
| BAD | Both the cytosol and the mitochondrial membrane | BAD is referred to as a "indirect" promoter of apoptosis because it causes cell death through inhibiting antiapoptotic proteins. | [59] |
| BIM | Nucleus envelope, endoplasmic reticulum (ER), cytoplasm, and mitochondrial outer membrane (MOM). | Apoptotic induction, it also controls mitochondrial activity and cellular metabolism. | [56] [60] |
| PUMA | Placement on the mitochondrial outer membrane (MOM), | Crucial p53-dependent apoptosis mediator. Activates BAK and BAX, resulting in MOMP. | [19] [61] |
| BIK | Mostly found in the ER membrane | Directly impacts BCL-2 and BCL-2-like 1 | [1] [62] |
4. Apoptotic role of BCL-2 Family
4.1. Models of BAX and BAK Activation
4.2. Pore Formation by BAX and BAK
4.3. BH3-Only Proteins Stimulate Apoptotic Pathway
5. Anti-apoptotic role of BCL-2 Family
5.1. BCL-2
5.2. BCL-XL
5.3. MCL-1
5.4. BFL-1
5.5. BCL-W
6. The Apoptosis Pathway
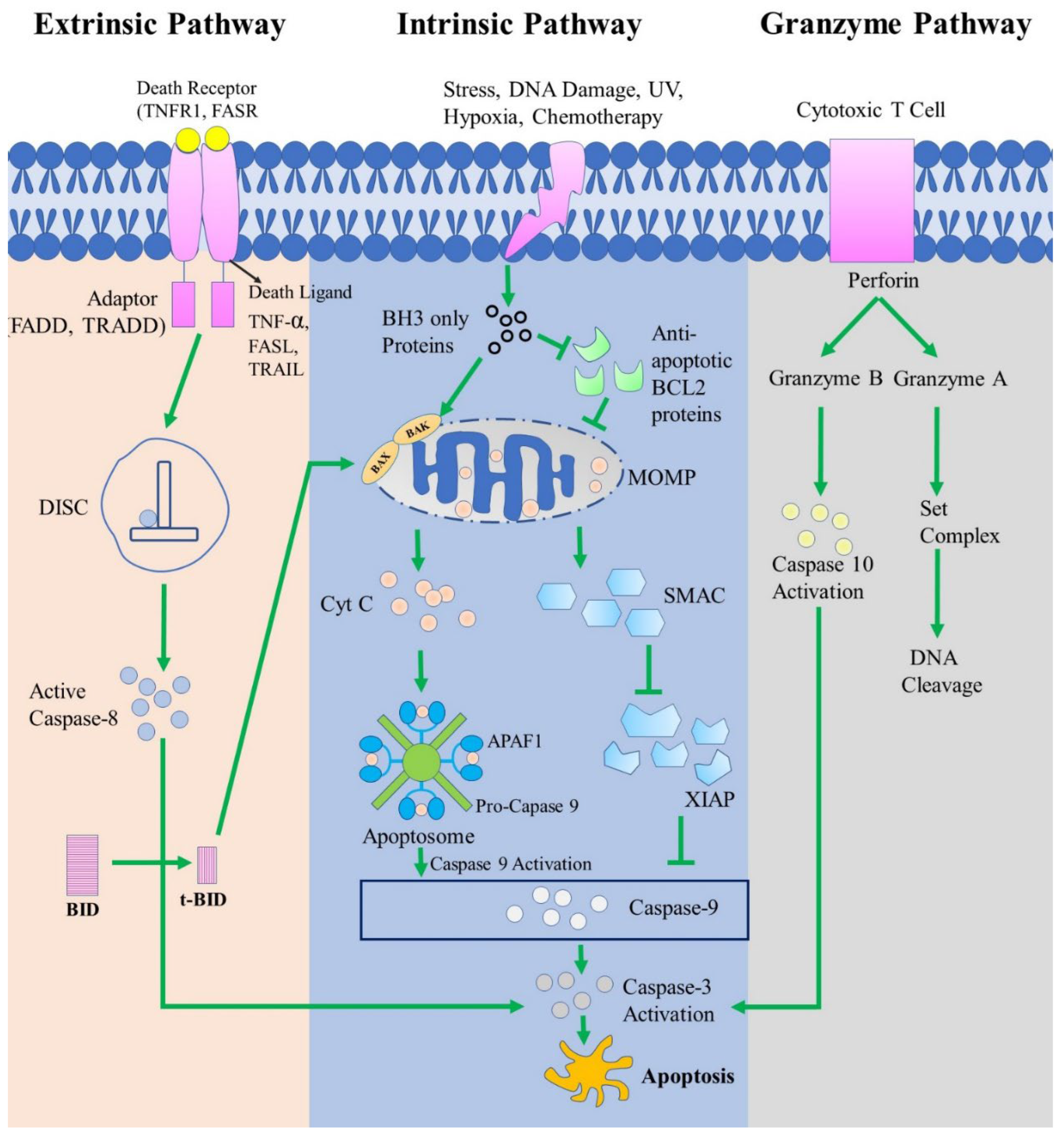
7. The Relationship Between BCL-2 Protein Family and P53
8. Potential Biomarkers
8.1. P53
8.2. ABT-737
8.3. BCR-ABL
8.4. PUMA and NOXA
9. Therapeutic Potential of BCL-2 Family Proteins
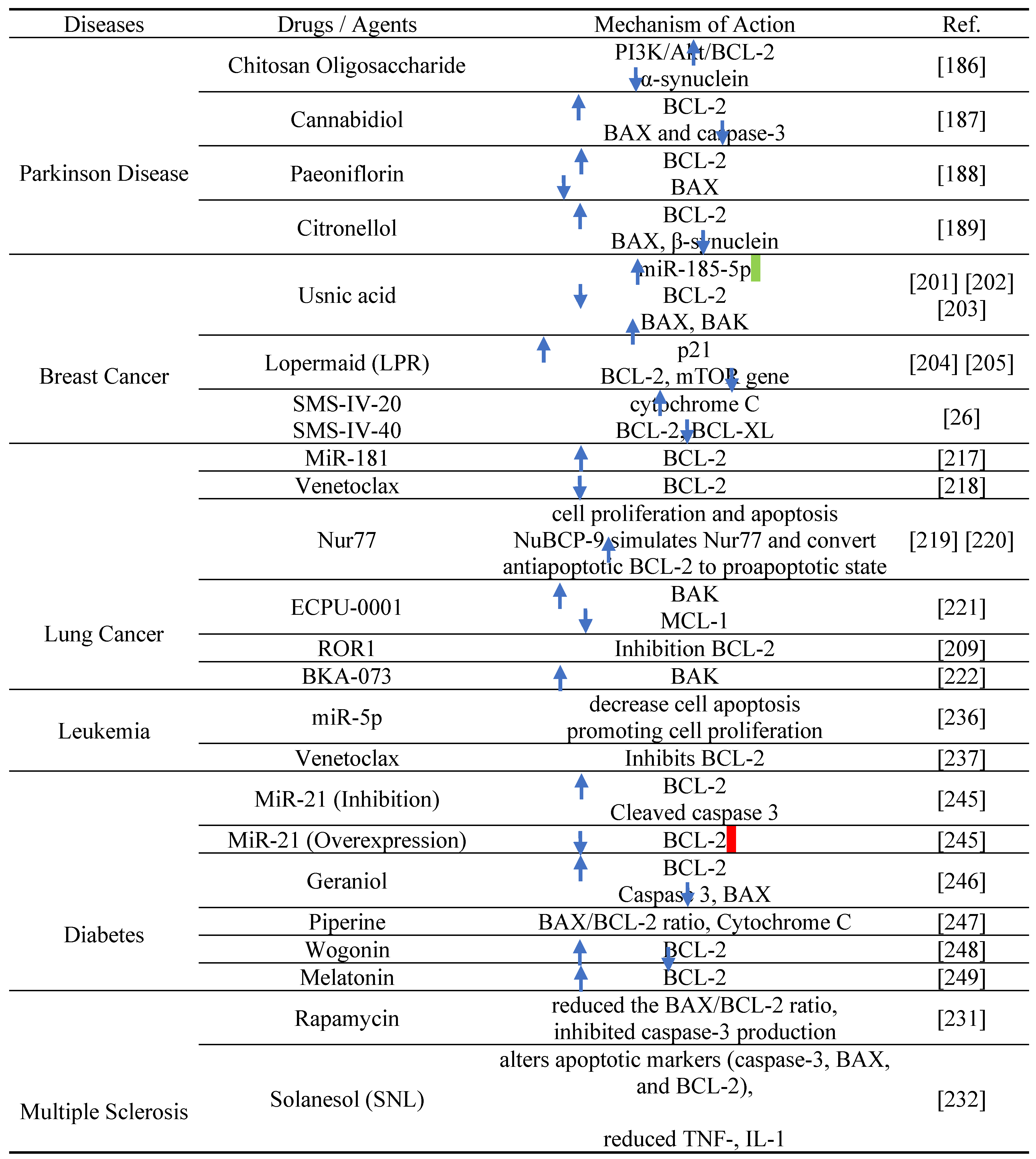
10. BCL-2 proteins as therapeutic target in diseases
10.1. Parkinson disease
10.2. Breast Cancer
10.3. Lung cancer
10.4. Multiple sclerosis (MS)
10.5. Leukemia
10.6. Diabetes
Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgment
Conflicts of Interest
Additional Information
Ethical Approval
Consent for Publication
Abbreviations
| BCL-2 | B Cell Lymphoma Protein 2 |
| BCL-X | BCL-2-Like 1 |
| BCL-XL | B-Cell Lymphoma-Extra Large |
| BCL-XS | B-Cell Lymphoma-Extra Short |
| BCL-W | BCL-2-Like 2 Protein |
| BAG | BCL-2-Associated Athanogene |
| BCL-10 | B Cell Lymphoma Protein 10 |
| BAX | BCL-2-Associated X Protein |
| BAK | BCL-2 Antagonist Killer 1 |
| BID | BH3-Interacting Domain |
| BAD | BCL-2 Antagonist of Cell Death |
| BIM | BCL-2-Interacting Protein |
| BFL1 | BCL2-Related Protein A1 |
| BIK | BCL-2-Interacting Killer |
| BLK | BIK-Like Killer Protein |
| BH | BCL2 Homology Region |
| PUMA | P53 Upregulated Modulator of Apoptosis |
| BBC3 | BCL2 Binding Component 3 |
| APAF-1 | Apoptosis Protease-Activating Factor-1 |
| IAP | Inhibitor of Apoptosis Proteins |
| AIF | Apoptosis-Inducing Factor |
| CAD | Caspase-Activated Dnase |
| DR3 | Death Receptor 3 |
| DR6 | Death Receptor 6 |
| MOM | Mitochondrial Outer Membrane |
| MOMP | MOM Permeabilization |
| SMAC | Second Mitochondria-Derived Activator of Caspase |
| DISC | Death-Inducing Signaling Complex |
| TNFR | Tumor Necrosis Factor Receptor |
| TNFR1 | Tumor Necrosis Factor Receptor 1 |
| TRAILR | TNF-Related Apoptosis-Inducing Ligand Receptor |
| HRK | Harakiri |
| MCL1 | Myeloid Cell Leukemia 1 |
| MCL1L | MCL1 Long Form |
| MCL1S | MCL1 Short Form |
| BMF | BCL2-Modifying Factor |
| BNIP | BCL2 and the Nineteen Kda Interacting Protein |
| AML | Acute Myeloid Leukemia |
| BOO | BCL2 Homologue of the Ovary |
| CLL | Chronic Lymphocytic Leukemia |
| AIF | Apoptosis-Inducing Factor |
| IL | Interleukin |
| NMR | Nuclear Magnetic Resonance |
| TM | Transmembrane |
| PEST | Sequence Enriched in Proline/Glutamic Acid/Serine/Threonine |
| STAT3 | Signal Transducers and Activators of Transcription |
| HCC | Hepatocellular Carcinoma |
| DLBCL | Diffuse Large B-Cell Lymphoma |
| XIAP | X-linked Inhibitor of Apoptosis Protein |
| FADD | Fas-Associated Death Domain |
| DISC | Death-Inducing Signaling Complex |
| tBAD | Truncated BAD |
| BCC | Basal Cell Carcinoma |
| CLL | Chronic Lymphocytic Leukemia |
| AHSCT | Autologous Hematopoietic Stem Cell Transplantation |
| EAE | Autoimmune Encephalomyelitis |
| ALL | Acute Lymphoblastic Leukemia |
| AML | Acute Myeloblastic Leukemia |
| CLL | Chronic Lymphocytic Leukemia |
| CML | Chronic Myeloid Leukemia |
| CLL | Chronic Lymphocytic Leukemia |
| T1DM | Type 1 Diabetes Mellitus |
| T2DM | Type 2 Diabetes Mellitus |
References
- W. A. Siddiqui, A. Ahad, and H. Ahsan, “The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update,” Archives of Toxicology, vol. 89, no. 3. Springer Verlag, pp. 289–317, Feb. 2015. [CrossRef]
- K. Labbé and M. Saleh, “Cell death in the host response to infection,” Cell Death Differ., vol. 15, no. 9, pp. 1339–1349, 2008. [CrossRef]
- S. Arandjelovic and K. S. Ravichandran, “Phagocytosis of apoptotic cells in homeostasis,” Nat. Immunol., vol. 16, no. 9, pp. 907–917, 2015. [CrossRef]
- I. K. H. Poon, C. D. Lucas, A. G. Rossi, and K. S. Ravichandran, “Apoptotic cell clearance: basic biology and therapeutic potential,” Nat. Rev. Immunol., vol. 14, no. 3, pp. 166–180, Mar. 2014. [CrossRef]
- S. Goldar, M. S. Khaniani, S. M. Derakhshan, and B. Baradaran, “Molecular mechanisms of apoptosis and roles in cancer development and treatment,” Asian Pacific J. Cancer Prev., vol. 16, no. 6, pp. 2129–2144, 2015. [CrossRef]
- A. Hazafa et al., “Humanin: A mitochondrial-derived peptide in the treatment of apoptosis-related diseases,” Life Sci., vol. 264, p. 118679, 2021. [CrossRef]
- J. A. Glab, Z. Cao, and H. Puthalakath, Bcl-2 family proteins, beyond the veil, 1st ed., vol. 351. Elsevier Inc., 2020. [CrossRef]
- S. Fulda, “Targeting apoptosis for anticancer therapy,” Semin. Cancer Biol., vol. 31, pp. 84–88, 2015. [CrossRef]
- H. Walczak, “Death receptor-ligand systems in cancer, cell death, and inflammation,” Cold Spring Harb. Perspect. Biol., vol. 5, no. 5, 2013. [CrossRef]
- M. E. Guicciardi and G. J. Gores, “Life and death by death receptors,” FASEB J., vol. 23, no. 6, pp. 1625–1637, 2009. [CrossRef]
- D. R. Green, “The Mitochondrial Pathway of Apoptosis Part II: The BCL-2 Protein Family,” Cold Spring Harb. Perspect. Biol., vol. 14, no. 6, p. a041046, Jun. 2022. [CrossRef]
- X. Luo, K. L. O’Neill, and K. Huang, “The third model of Bax/Bak activation: a Bcl-2 family feud finally resolved?,” F1000Research, vol. 9, 2020. [CrossRef]
- H. M. Al-Aamri, H. R. Irving, C. Bradley, and T. Meehan-Andrews, “Intrinsic and extrinsic apoptosis responses in leukaemia cells following daunorubicin treatment,” BMC Cancer, vol. 21, no. 1, pp. 1–10, 2021. [CrossRef]
- I. Kapoor, J. Bodo, B. T. Hill, E. D. Hsi, and A. Almasan, “Targeting BCL-2 in B-cell malignancies and overcoming therapeutic resistance,” Cell Death Dis., vol. 11, no. 11, 2020. [CrossRef]
- J. T. Opferman and A. Kothari, “Anti-apoptotic BCL-2 family members in development,” Cell Death Differ. 2018 251, vol. 25, no. 1, pp. 37–45, Nov. 2017. [CrossRef]
- B. Antonsson and J. Martinou, “MINIREVIEW The Bcl-2 Protein Family,” vol. 57, p. 4839, 2000. [CrossRef]
- C. M. Croce and J. C. Reed, “Finally, an apoptosis-targeting therapeutic for cancer,” Cancer Res., vol. 76, no. 20, pp. 5914–5920, 2016. [CrossRef]
- C. F. A. Warren, M. W. Wong-Brown, and N. A. Bowden, “BCL-2 family isoforms in apoptosis and cancer,” Cell Death and Disease, vol. 10, no. 3. Nature Publishing Group, Mar. 2019. [CrossRef]
- J. Kale, E. J. Osterlund, and D. W. Andrews, “BCL-2 family proteins: Changing partners in the dance towards death,” Cell Death and Differentiation, vol. 25, no. 1. Nature Publishing Group, pp. 65–80, 2018. [CrossRef]
- E. L.Omonosova and G. C.Hinnadurai, “BH3-only proteins in apoptosis and beyond: An overview,” Oncogene, vol. 27, no. S1, pp. S2–S19, 2008. [CrossRef]
- S. D’Aguanno and D. Del Bufalo, “Inhibition of Anti-Apoptotic Bcl-2 Proteins in Preclinical and Clinical Studies: Current Overview in Cancer,” Cells, vol. 9, no. 5. NLM (Medline), May 2020. [CrossRef]
- D. M. Jang, E. K. Oh, H. Hahn, H. S. Kim, and B. W. Han, “Structural insights into apoptotic regulation of human Bfk as a novel Bcl-2 family member,” Comput. Struct. Biotechnol. J., vol. 20, pp. 745–756, 2022. [CrossRef]
- H. Dai, W. Meng, and S. Kaufmann, “BCL2 Family, Mitochondrial Apoptosis, and Beyond,” Cancer Transl. Med., vol. 2, no. 1, p. 7, 2016. [CrossRef]
- A. Strasser, “The role of BH3-only proteins in the immune system,” Nat. Rev. Immunol., vol. 5, no. 3, pp. 189–200, 2005. [CrossRef]
- M. R. Schnorenberg, J. A. Bellairs, R. Samaeekia, H. Acar, M. V. Tirrell, and J. L. LaBelle, “Activating the intrinsic pathway of apoptosis using BIM BH3 peptides delivered by peptide amphiphiles with endosomal release,” Materials (Basel)., vol. 12, no. 16, 2019. [CrossRef]
- H. C. Chen et al., “An interconnected hierarchical model of cell death regulation by the BCL-2 family,” Nat. Cell Biol., vol. 17, no. 10, pp. 1270–1281, 2015. [CrossRef]
- A. Hantusch, M. Rehm, and T. Brunner, “Counting on Death – Quantitative aspects of Bcl-2 family regulation,” FEBS J., vol. 285, no. 22, pp. 4124–4138, 2018. [CrossRef]
- J. M. Adams, “BAX and BAK become killers without a BH3 trigger,” Cell Res., vol. 29, no. 12, pp. 967–968, 2019. [CrossRef]
- E. C. Donaldson, “Chapter 1 股関節 概念 Chapter 1 股関節,” An Autom. Irrig. Syst. Using Arduino Microcontroller, vol. 1908, no. January, pp. 2–6, 2011. [CrossRef]
- A. Ashkenazi, W. J. Fairbrother, J. D. Leverson, and A. J. Souers, “From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors,” Nature Reviews Drug Discovery, vol. 16, no. 4. Nature Publishing Group, pp. 273–284, Apr. 2017. [CrossRef]
- R. J. Youle and A. Strasser, “The BCL-2 protein family: Opposing activities that mediate cell death,” Nature Reviews Molecular Cell Biology, vol. 9, no. 1. pp. 47–59, Jan. 2008. [CrossRef]
- S. Choudhury, “A comparative analysis of BCL-2 family,” Bioinformation, vol. 15, no. 4, pp. 299–306, 2019. [CrossRef]
- A. R. D. Delbridge, S. Grabow, A. Strasser, and D. L. Vaux, “Thirty years of BCL-2: Translating cell death discoveries into novel cancer therapies,” Nature Reviews Cancer, vol. 16, no. 2. Nature Publishing Group, pp. 99–109, Feb. 2016. [CrossRef]
- H. Dai, W. Meng, and S. Kaufmann, “BCL2 Family, Mitochondrial Apoptosis, and Beyond,” undefined, vol. 2, no. 1, p. 7, 2016. [CrossRef]
- S. Thomas et al., “Targeting the Bcl-2 family for cancer therapy,” Expert Opinion on Therapeutic Targets, vol. 17, no. 1. pp. 61–75, Jan. 2013. [CrossRef]
- M. L. Hartman and M. Czyz, “BCL-w: apoptotic and non-apoptotic role in health and disease,” Cell Death and Disease, vol. 11, no. 4. Springer Nature, Apr. 2020. [CrossRef]
- C. Akgul, “Mcl-1 is a potential therapeutic target in multiple types of cancer,” Cellular and Molecular Life Sciences, vol. 66, no. 8. pp. 1326–1336, Apr. 2009. [CrossRef]
- D. Bhatt et al., “BCL-2 (-938C>A), BAX (-248G>A), and HER2 Ile655Val Polymorphisms and Breast Cancer Risk in Indian Population,” J. Oncol., vol. 2021, 2021. [CrossRef]
- H. Thomadaki and A. Scorilas, “BCL2 family of apoptosis-related genes: Functions and clinical implications in cancer,” Critical Reviews in Clinical Laboratory Sciences, vol. 43, no. 1. pp. 1–67, Jan. 2006. [CrossRef]
- S. Sethi, M. S. Benninger, M. Lu, S. Havard, and M. J. Worsham, “Noninvasive Molecular Detection of Head and Neck Squamous Cell Carcinoma An Exploratory Analysis,” 2009.
- S. Verma, M. L. Budarf, B. S. Emanuel, and G. Chinnadurai, “Structural analysis of the human pro-apoptotic gene Bik: Chromosomal localization, genomic organization and localization of promoter sequences,” 2000.
- Y. Sun and D. W. Leaman, “Involvement of noxa in cellular apoptotic responses to interferon, double-stranded RNA, and virus infection,” J. Biol. Chem., vol. 280, no. 16, pp. 15561–15568, Apr. 2005. [CrossRef]
- M. Vogler, “BCL2A1: The underdog in the BCL2 family,” Cell Death and Differentiation, vol. 19, no. 1. pp. 67–74, Jan. 2012. [CrossRef]
- F. K. Ahmad, S. Deris, and H. Othman, “Improved Bayesian Network Structure Learning for Breast Cancer Prognosis,” 2010.
- H. Puthalakath et al., “Bmf: A Proapoptotic BH3-Only Protein Regulated by Interaction with the Myosin V Actin Motor Complex, Activated by Anoikis.”.
- A. M. Petros, E. T. Olejniczak, and S. W. Fesik, “Structural biology of the Bcl-2 family of proteins,” Biochimica et Biophysica Acta - Molecular Cell Research, vol. 1644, no. 2–3. pp. 83–94, Mar. 2004. [CrossRef]
- P. E. Czabotar, G. Lessene, A. Strasser, and J. M. Adams, “Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy,” Nature Reviews Molecular Cell Biology, vol. 15, no. 1. pp. 49–63, Jan. 2014. [CrossRef]
- M. Li, D. Wang, J. He, L. Chen, and H. Li, “Bcl-XL: A multifunctional anti-apoptotic protein,” Pharmacological Research, vol. 151. Academic Press, Jan. 2020. [CrossRef]
- K. Papadopoulos, “Targeting the Bcl-2 Family in Cancer Therapy,” Semin. Oncol., vol. 33, no. 4, pp. 449–456, Aug. 2006. [CrossRef]
- G. F. Perini, G. N. Ribeiro, J. V. Pinto Neto, L. T. Campos, and N. Hamerschlak, “BCL-2 as therapeutic target for hematological malignancies,” J. Hematol. Oncol., vol. 11, no. 1, pp. 1–15, 2018. [CrossRef]
- J. T. Opferman and A. Kothari, “Anti-apoptotic BCL-2 family members in development,” Cell Death and Differentiation, vol. 25, no. 1. Nature Publishing Group, pp. 37–45, 2018. [CrossRef]
- B. Leibowitz and J. Yu, “Mitochondrial signaling in cell death via the Bcl-2 family,” Cancer Biology and Therapy, vol. 9, no. 6. Landes Bioscience, pp. 417–422, Mar. 2010. [CrossRef]
- A. Shamas-Din, J. Kale, B. Leber, and D. W. Andrews, “Mechanisms of action of Bcl-2 family proteins,” Cold Spring Harb. Perspect. Biol., vol. 5, no. 4, pp. 1–21, Apr. 2013. [CrossRef]
- F. Zhou, Y. Yang, and D. Xing, “Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis,” FEBS J., vol. 278, no. 3, pp. 403–413, Feb. 2011. [CrossRef]
- A. Bolomsky et al., “MCL-1 inhibitors, fast-lane development of a new class of anti-cancer agents,” Journal of Hematology and Oncology, vol. 13, no. 1. BioMed Central Ltd, Dec. 2020. [CrossRef]
- N. Popgeorgiev, L. Jabbour, and G. Gillet, “Subcellular localization and dynamics of the Bcl-2 family of proteins,” Frontiers in Cell and Developmental Biology, vol. 6, no. FEB. Frontiers Media S.A., Feb. 2018. [CrossRef]
- J. Lindsay, M. D. Esposti, and A. P. Gilmore, “Bcl-2 proteins and mitochondria-Specificity in membrane targeting for death,” Biochimica et Biophysica Acta - Molecular Cell Research, vol. 1813, no. 4. pp. 532–539, Apr. 2011. [CrossRef]
- N. Echeverry, D. Bachmann, F. Ke, A. Strasser, H. U. Simon, and T. Kaufmann, “Intracellular localization of the BCL-2 family member BOK and functional implications,” Cell Death Differ., vol. 20, no. 6, pp. 785–799, Jun. 2013. [CrossRef]
- A. C. Craik et al., “The BH3-only protein bad confers breast cancer taxane sensitivity through a nonapoptotic mechanism,” Oncogene, vol. 29, no. 39, pp. 5381–5391, Sep. 2010. [CrossRef]
- M. Miani, B. Elvira, and E. N. Gurzov, “Sweet Killing in Obesity and Diabetes: The Metabolic Role of the BH3-only Protein BIM,” Journal of Molecular Biology, vol. 430, no. 18. Academic Press, pp. 3041–3050, Sep. 2018. [CrossRef]
- P. Hikisz and Z. M. Kiliańska, “Puma, a critical mediator of cell death - one decade on from its discovery,” Cellular and Molecular Biology Letters, vol. 17, no. 4. BioMed Central Ltd., pp. 646–669, 2012. [CrossRef]
- J. Hatok and P. Racay, “Bcl-2 family proteins: Master regulators of cell survival,” Biomolecular Concepts, vol. 7, no. 4. Walter de Gruyter GmbH, pp. 259–270, Aug. 2016. [CrossRef]
- C. B. Karim et al., “Structural Mechanism for Regulation of Bcl-2 protein Noxa by phosphorylation,” Sci. Rep., vol. 5, Sep. 2015. [CrossRef]
- M. Fricker, J. O’Prey, A. M. Tolkovsky, and K. M. Ryan, “Phosphorylation of Puma modulates its apoptotic function by regulating protein stability,” Cell Death Dis., vol. 1, no. 7, Jul. 2010. [CrossRef]
- M. Hassan, H. Watari, A. Abualmaaty, Y. Ohba, and N. Sakuragi, “Apoptosis and molecular targeting therapy in cancer,” BioMed Research International, vol. 2014. Hindawi Limited, 2014. [CrossRef]
- C. M. Pfeffer and A. T. K. Singh, “Apoptosis: A target for anticancer therapy,” International Journal of Molecular Sciences, vol. 19, no. 2. MDPI AG, Feb. 2018. [CrossRef]
- R. Roufayel, “Regulation of stressed-induced cell death by the Bcl-2 family of apoptotic proteins,” Molecular Membrane Biology, vol. 33, no. 6–8. Taylor and Francis Ltd, pp. 89–99, Nov. 2016. [CrossRef]
- E. Khodapasand, N. Jafarzadeh, F. Farrokhi, B. Kamalidehghan, and M. Houshmand, “Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer?,” Iran. Biomed. J., vol. 19, no. 2, pp. 69–75, 2015. [CrossRef]
- A. R. D. Delbridge and A. Strasser, “The BCL-2 protein family, BH3-mimetics and cancer therapy,” Cell Death and Differentiation, vol. 22, no. 7. Nature Publishing Group, pp. 1071–1080, Jul. 2015. [CrossRef]
- R. W. Birkinshaw and P. E. Czabotar, “The BCL-2 family of proteins and mitochondrial outer membrane permeabilisation,” Seminars in Cell and Developmental Biology, vol. 72. Elsevier Ltd, pp. 152–162, Dec. 2017. [CrossRef]
- R. Valentin, S. Grabow, and M. S. Davids, “The rise of apoptosis: targeting apoptosis in hematologic malignancies,” 2018.
- N. Volkmann, F. M. Marassi, D. D. Newmeyer, and D. Hanein, “The rheostat in the membrane: BCL-2 family proteins and apoptosis,” Cell Death and Differentiation, vol. 21, no. 2. pp. 206–215, Feb. 2014. [CrossRef]
- S. Arulananda, E. F. Lee, W. D. Fairlie, and T. John, “The role of BCL-2 family proteins and therapeutic potential of BH3-mimetics in malignant pleural mesothelioma,” Expert Review of Anticancer Therapy, vol. 21, no. 4. Taylor and Francis Ltd., pp. 413–424, 2021. [CrossRef]
- X. Luo, K. L. O’Neill, and K. Huang, “The third model of Bax/Bak activation: A Bcl-2 family feud finally resolved?,” F1000Research, vol. 9. F1000 Research Ltd, 2020. [CrossRef]
- D. Westphal, R. M. Kluck, and G. Dewson, “Building blocks of the apoptotic pore: How Bax and Bak are activated and oligomerize during apoptosis,” Cell Death and Differentiation, vol. 21, no. 2. pp. 196–205, Feb. 2014. [CrossRef]
- K. Huang, by Kai Huang, R. Singh, K. Johnson, and T. Bessho, “Mechanism of Bax/Bak Activation in Apoptotic Signaling Mechanism of Bax/Bak Activation in Apoptotic Signaling MECHANISM OF BAX/BAK ACTIVATION IN APOPTOTIC SIGNALING.”.
- Z. Liu, Y. Ding, N. Ye, C. Wild, H. Chen, and J. Zhou, “Direct Activation of Bax Protein for Cancer Therapy,” Med. Res. Rev., vol. 36, no. 2, pp. 313–341, Mar. 2016. [CrossRef]
- K. Huang et al., “BH3-only proteins target BCL-xL/MCL-1, not BAX/BAK, to initiate apoptosis,” Cell Res., vol. 29, no. 11, pp. 942–952, Nov. 2019. [CrossRef]
- R. J. C. Gilbert, M. D. Serra, C. J. Froelich, M. I. Wallace, and G. Anderluh, “Membrane pore formation at protein-lipid interfaces,” Trends in Biochemical Sciences, vol. 39, no. 11. Elsevier Ltd, pp. 510–516, 2014. [CrossRef]
- L. A. Gilliesa, H. Du, B. Peters, C. M. Knudson, D. D. Newmeyer, and T. Kuwana, “Visual and functional demonstration of growing Bax-induced pores in mitochondrial outer membranes,” Mol. Biol. Cell, vol. 26, no. 2, pp. 339–349, Jan. 2015. [CrossRef]
- D. Westphal et al., “Apoptotic pore formation is associated with in-plane insertion of Bak or Bax central helices into the mitochondrial outer membrane,” Proc. Natl. Acad. Sci. U. S. A., vol. 111, no. 39, pp. E4076–E4085, Sep. 2014. [CrossRef]
- R. T. Uren, S. Iyer, and R. M. Kluck, “Pore formation by dimeric Bak and Bax: an unusual pore?,” Philos. Trans. R. Soc. B Biol. Sci., vol. 372, no. 1726, 2017. [CrossRef]
- K. Cosentino and A. J. García-Sáez, “Bax and Bak Pores: Are We Closing the Circle?,” Trends in Cell Biology, vol. 27, no. 4. Elsevier Ltd, pp. 266–275, Apr. 2017. [CrossRef]
- K. Cosentino and A. J. García-Sáez, “Mitochondrial alterations in apoptosis,” Chemistry and Physics of Lipids, vol. 181. Elsevier Ireland Ltd, pp. 62–75, 2014. [CrossRef]
- A. Peña-Blanco and A. J. García-Sáez, “Bax, Bak and beyond — mitochondrial performance in apoptosis,” FEBS Journal, vol. 285, no. 3. Blackwell Publishing Ltd, pp. 416–431, Feb. 2018. [CrossRef]
- S. Bleicken, G. Jeschke, C. Stegmueller, R. Salvador-Gallego, A. J. García-Sáez, and E. Bordignon, “Structural Model of Active Bax at the Membrane,” Mol. Cell, vol. 56, no. 4, pp. 496–505, Nov. 2014. [CrossRef]
- U. Ros and A. J. García-Sáez, “More Than a Pore: The Interplay of Pore-Forming Proteins and Lipid Membranes,” J. Membr. Biol., vol. 248, no. 3, pp. 545–561, Jun. 2015. [CrossRef]
- T. Kuehl and D. Lagares, “BH3 mimetics as anti-fibrotic therapy: Unleashing the mitochondrial pathway of apoptosis in myofibroblasts,” Matrix Biology, vol. 68–69. Elsevier B.V., pp. 94–105, Aug. 2018. [CrossRef]
- J. A. Glab, G. W. Mbogo, and H. Puthalakath, “BH3-Only Proteins in Health and Disease,” in International Review of Cell and Molecular Biology, vol. 328, Elsevier Inc., 2017, pp. 163–196. [CrossRef]
- K. L. O’neill, K. Huang, J. Zhang, Y. Chen, and X. Luo, “Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane,” Genes Dev., vol. 30, no. 8, pp. 973–988, Apr. 2016. [CrossRef]
- M. Doerflinger, J. A. Glab, and H. Puthalakath, “BH3-only proteins: A 20-year stock-take,” FEBS Journal, vol. 282, no. 6. Blackwell Publishing Ltd, pp. 1006–1016, 2015. [CrossRef]
- M. Jullien, P. Gomez-Bougie, D. Chiron, and C. Touzeau, “Restoring Apoptosis with BH3 Mimetics in Mature B-Cell Malignancies,” Cells, vol. 9, no. 3. NLM (Medline), Mar. 2020. [CrossRef]
- M. Kvansakul and M. G. Hinds, “The structural biology of bh3-only proteins,” in Methods in Enzymology, vol. 544, Academic Press Inc., 2014, pp. 49–74. [CrossRef]
- K. J. Hutt, “The role of BH3-only proteins in apoptosis within the ovary,” Reproduction, vol. 149, no. 2. BioScientifica Ltd., pp. R81–R89, Feb. 2015. [CrossRef]
- H. Dai et al., “Measurement of BH3-only protein tolerance,” Cell Death Differ., vol. 25, no. 2, pp. 282–293, 2018. [CrossRef]
- H. Dai, X. W. Meng, and S. H. Kaufmann, “Mitochondrial apoptosis and BH3 mimetics,” F1000Research, vol. 5. Faculty of 1000 Ltd, 2016. [CrossRef]
- J. H. Zheng, A. Viacava Follis, R. W. Kriwacki, and T. Moldoveanu, “Discoveries and controversies in BCL-2 protein-mediated apoptosis,” FEBS Journal. Blackwell Publishing Ltd, pp. 2690–2700, Jul. 2016. [CrossRef]
- C. Correia et al., “Emerging understanding of Bcl-2 biology: Implications for neoplastic progression and treatment,” Biochimica et Biophysica Acta - Molecular Cell Research, vol. 1853, no. 7. Elsevier, pp. 1658–1671, Jul. 2015. [CrossRef]
- T. Moldoveanu, A. V. Follis, R. W. Kriwacki, and D. R. Green, “Many players in BCL-2 family affairs,” Trends in Biochemical Sciences, vol. 39, no. 3. pp. 101–111, Mar. 2014. [CrossRef]
- J. T. Opferman, “Attacking cancer’s Achilles heel: antagonism of anti-apoptotic BCL-2 family members,” FEBS Journal. Blackwell Publishing Ltd, pp. 2661–2675, Jul. 2016. [CrossRef]
- F. Edlich, “BCL-2 proteins and apoptosis: Recent insights and unknowns,” Biochem. Biophys. Res. Commun., vol. 500, no. 1, pp. 26–34, May 2018. [CrossRef]
- A. Basu, “The interplay between apoptosis and cellular senescence: Bcl-2 family proteins as targets for cancer therapy,” Pharmacology and Therapeutics, vol. 230. Elsevier Inc., Feb. 2022. [CrossRef]
- U. Anilkumar and J. H. M. Prehn, “Anti-apoptotic BCL-2 family proteins in acute neural injury,” Frontiers in Cellular Neuroscience, vol. 8, no. SEP. Frontiers Research Foundation, Sep. 2014. [CrossRef]
- A. Slomp and V. Peperzak, “Role and regulation of pro-survival BCL-2 proteins in multiple myeloma,” Frontiers in Oncology, vol. 8, no. NOV. Frontiers Media S.A., 2018. [CrossRef]
- E. F. Lee and W. Douglas Fairlie, “The structural biology of Bcl-xL,” International Journal of Molecular Sciences, vol. 20, no. 9. MDPI AG, May 2019. [CrossRef]
- C. Borrás et al., “BCL-xL, a mitochondrial protein involved in successful aging: From C. elegans to human centenarians,” Int. J. Mol. Sci., vol. 21, no. 2, 2020. [CrossRef]
- A. V. Follis et al., “Regulation of apoptosis by an intrinsically disordered region of Bcl-xL,” Nat. Chem. Biol., vol. 14, no. 5, pp. 458–465, May 2018. [CrossRef]
- S. Maji et al., “Bcl-2 Antiapoptotic Family Proteins and Chemoresistance in Cancer,” in Advances in Cancer Research, vol. 137, Academic Press Inc., 2018, pp. 37–75. [CrossRef]
- V. V. Senichkin, A. Y. Streletskaia, A. S. Gorbunova, B. Zhivotovsky, and G. S. Kopeina, “Saga of Mcl-1: regulation from transcription to degradation,” Cell Death and Differentiation, vol. 27, no. 2. Springer Nature, pp. 405–419, Feb. 2020. [CrossRef]
- G. Morciano et al., “Mcl-1 involvement in mitochondrial dynamics is associated with apoptotic cell death,” Mol. Biol. Cell, vol. 27, no. 1, pp. 20–34, Jan. 2016. [CrossRef]
- H. Wang, M. Guo, H. Wei, and Y. Chen, “Targeting MCL-1 in cancer: current status and perspectives,” Journal of Hematology and Oncology, vol. 14, no. 1. BioMed Central Ltd, Dec. 2021. [CrossRef]
- R. Cherla, Y. Zhang, L. Ledbetter, and G. Zhang, “Coxiella burnetii inhibits neutrophil apoptosis by exploiting survival pathways and antiapoptotic protein Mcl-1,” Infect. Immun., vol. 86, no. 4, Apr. 2018. [CrossRef]
- A. R. Shenoy, S. Kirschnek, and G. Häcker, “IL-15 regulates Bcl-2 family members Bim and Mcl-1 through JAK/STAT and PI3K/AKT pathways in T cells,” Eur. J. Immunol., vol. 44, no. 8, pp. 2500–2507, 2014. [CrossRef]
- W. Xiang, C. Y. Yang, and L. Bai, “MCL-1 inhibition in cancer treatment,” OncoTargets and Therapy, vol. 11. Dove Medical Press Ltd., pp. 7301–7314, 2018. [CrossRef]
- Y. Fernández-Marrero, S. Spinner, T. Kaufmann, and P. J. Jost, “Survival control of malignant lymphocytes by anti-apoptotic MCL-1,” Leukemia, vol. 30, no. 11. Nature Publishing Group, pp. 2152–2159, Nov. 2016. [CrossRef]
- L. Chen and S. Fletcher, “Mcl-1 inhibitors: a patent review,” Expert Opinion on Therapeutic Patents, vol. 27, no. 2. Taylor and Francis Ltd, pp. 163–178, Feb. 2017. [CrossRef]
- E. P. Harvey, H. S. Seo, R. M. Guerra, G. H. Bird, S. Dhe-Paganon, and L. D. Walensky, “Crystal Structures of Anti-apoptotic BFL-1 and Its Complex with a Covalent Stapled Peptide Inhibitor,” Structure, vol. 26, no. 1, pp. 153-160.e4, 2018. [CrossRef]
- C. K. Hind, M. J. Carter, C. L. Harris, H. T. C. Chan, S. James, and M. S. Cragg, “Role of the pro-survival molecule Bfl-1 in melanoma,” Int. J. Biochem. Cell Biol., vol. 59, pp. 94–102, 2015. [CrossRef]
- G. Wang, S. T. Diepstraten, and M. J. Herold, “Last but not least: BFL-1 as an emerging target for anti-cancer therapies,” Biochem. Soc. Trans., vol. 50, no. 4, pp. 1119–1128, 2022. [CrossRef]
- L. Gangoda et al., “Absence of pro-survival A1 has no impact on inflammatory cell survival in vivo during acute lung inflammation and peritonitis,” Cell Death Differ., vol. 29, no. 1, pp. 96–104, 2022. [CrossRef]
- M. Sochalska, F. Schuler, J. G. Weiss, M. Prchal-Murphy, V. Sexl, and A. Villunger, “MYC selects against reduced BCL2A1/A1 protein expression during B cell lymphomagenesis,” Oncogene, vol. 36, no. 15, pp. 2066–2073, 2017. [CrossRef]
- X. Li, J. Dou, Q. You, and Z. Jiang, “Inhibitors of BCL2A1/Bfl-1 protein: Potential stock in cancer therapy,” European Journal of Medicinal Chemistry, vol. 220. Elsevier Masson s.r.l., Aug. 2021. [CrossRef]
- A. J. Huhn, R. M. Guerra, E. P. Harvey, G. H. Bird, and L. D. Walensky, “Selective Covalent Targeting of Anti-Apoptotic BFL-1 by Cysteine-Reactive Stapled Peptide Inhibitors,” Cell Chem. Biol., vol. 23, no. 9, pp. 1123–1134, 2016. [CrossRef]
- H. Flores-Romero et al., “BFL1 modulates apoptosis at the membrane level through a bifunctional and multimodal mechanism showing key differences with BCLXL,” Cell Death Differ., vol. 26, no. 10, pp. 1880–1894, 2019. [CrossRef]
- Z. O. Alabi, O. Tosin Olaoba, K. Sulaimon Ayinde, A. O. Akinyemi, and T. I. Adelusi, “Zaccheaus Oluwatayo Alabi, Olamide Tosin Olaoba , Kehinde Sulaimon Ayinde, Amos Olalekan Akinyemi and Temitope Isaac Adelusi. The Role of BFl-1 in Cancer Unravels Inhibition Mechanism,” Cancer Biol., vol. 9, no. 3, pp. 92–100, 2019. [CrossRef]
- P. Kumari and R. Rameshwari, “In silico mutational analysis to identify the role and pathogenicity of BCL-w missense variants,” J. Genet. Eng. Biotechnol., vol. 20, no. 1, 2022. [CrossRef]
- S. Huang, R. Tang, and R. Y. C. Poon, “BCL-W is a regulator of microtubule inhibitor-induced mitotic cell death,” Oncotarget, vol. 7, no. 25, pp. 38718–38730, 2016. [CrossRef]
- C. M. Adams, A. S. Kim, R. Mitra, J. K. Choi, J. Z. Gong, and C. M. Eischen, “BCL-W has a fundamental role in B cell survival and lymphomagenesis,” J. Clin. Invest., vol. 127, no. 2, pp. 635–650, Feb. 2017. [CrossRef]
- S. T. Diepstraten et al., “BCL-W is dispensable for the sustained survival of select Burkitt lymphoma and diffuse large B-cell lymphoma cell lines,” Blood Adv., vol. 4, no. 2, pp. 356–366, 2020. [CrossRef]
- R. Singh, A. Letai, and K. Sarosiek, “Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins,” Nature Reviews Molecular Cell Biology, vol. 20, no. 3. Nature Publishing Group, pp. 175–193, Mar. 2019. [CrossRef]
- T. K. Palai and S. R. Mishra, “Caspases: An apoptosis mediator,” Journal of Advanced Veterinary and Animal Research, vol. 2, no. 1. Network for the Veterinarians of Bangladesh, pp. 18–22, Mar. 2015. [CrossRef]
- D. Martinvalet, “Mitochondrial entry of cytotoxic proteases: A new insight into the granzyme B cell death pathway,” Oxidative Medicine and Cellular Longevity, vol. 2019. Hindawi Limited, 2019. [CrossRef]
- S. Zaman, R. Wang, and V. Gandhi, “Targeting the apoptosis pathway in hematologic malignancies,” Leukemia and Lymphoma, vol. 55, no. 9. Informa Healthcare, pp. 1980–1992, 2014. [CrossRef]
- M. S. Mohamed, M. K. Bishr, F. M. Almutairi, and A. G. Ali, “Inhibitors of apoptosis: clinical implications in cancer,” Apoptosis 2017 2212, vol. 22, no. 12, pp. 1487–1509, Oct. 2017. [CrossRef]
- D. Kashyap, V. K. Garg, and N. Goel, “Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis,” in Advances in Protein Chemistry and Structural Biology, vol. 125, Academic Press Inc., 2021, pp. 73–120. [CrossRef]
- L. R. de Armas and E. R. Podack, “Natural killer cytolytic activity,” Nat. Kill. Cells Basic Sci. Clin. Appl., pp. 215–227, Jan. 2010. [CrossRef]
- I. Osińska, K. Popko, and U. Demkow, “Perforin: An important player in immune response,” Central European Journal of Immunology, vol. 39, no. 1. Termedia Publishing House Ltd., pp. 109–115, 2014. [CrossRef]
- J. G. Nirmala and M. Lopus, “Cell death mechanisms in eukaryotes,” Cell Biology and Toxicology, vol. 36, no. 2. Springer, pp. 145–164, Apr. 2020. [CrossRef]
- P. C. Kam, “Apoptosis : Mechanisms and clinical implications Apoptosis : mechanisms and clinical implications,” no. December 2000, 2018.
- C. Kunst, M. Haderer, S. Heckel, S. Schlosser, and M. Müller, “The p53 family in hepatocellular carcinoma,” Transl. Cancer Res., vol. 5, no. 6, pp. 632–638, 2016. [CrossRef]
- E. Wawryk-Gawda et al., “P53 protein in proliferation, repair and apoptosis of cells,” Protoplasma, vol. 251, no. 3, pp. 525–533, 2014. [CrossRef]
- L. Zhao and S. Sanyal, “p53 Isoforms as Cancer Biomarkers and Therapeutic Targets,” Cancers (Basel)., vol. 14, no. 13, p. 3145, Jun. 2022. [CrossRef]
- S. W. Chi, “Structural insights into the transcription-independent apoptotic pathway of p53,” BMB Rep., vol. 47, no. 3, pp. 167–172, 2014. [CrossRef]
- T. Ben Safta et al., “Granzyme B–Activated p53 Interacts with Bcl-2 To Promote Cytotoxic Lymphocyte–Mediated Apoptosis,” J. Immunol., vol. 194, no. 1, pp. 418–428, 2015. [CrossRef]
- H. Mansouri, L. F. Mnango, E. P. Magorosa, E. Sauli, and E. A. Mpolya, “Ki-67, p53 and BCL-2 Expressions and their Association with Clinical Histopathology of Breast Cancer among Women in Tanzania,” Sci. Rep., vol. 9, no. 1, pp. 1–11, 2019. [CrossRef]
- R. G. Mendez-Flores et al., “Role of Bcl-2, p53, and Ki-67 expression in basal cell carcinoma and their association with aggressive and non-aggressive histological phenotypes,” Postep. Dermatologii i Alergol., vol. 39, no. 3, pp. 517–523, 2022. [CrossRef]
- N. Dashzeveg and K. Yoshida, “Cell death decision by p53 via control of the mitochondrial membrane,” Cancer Lett., vol. 367, no. 2, pp. 108–112, 2015. [CrossRef]
- X. J. Wang et al., “P53 expression correlates with poorer survival and augments the negative prognostic effect of MYC rearrangement, expression or concurrent MYC/BCL2 expression in diffuse large B-cell lymphoma,” Mod. Pathol., vol. 30, no. 2, pp. 194–203, 2017. [CrossRef]
- J. Vávrová and M. Řezáčová, “Importance of proapoptotic protein PUMA in cell radioresistance,” Folia Biol. (Czech Republic), vol. 60, no. 2, pp. 53–56, 2014.
- J. E. Choi, S. M. Woo, K. J. Min, S. H. Kang, S. J. Lee, and T. K. Kwon, “Combined treatment with ABT-737 and VX-680 induces apoptosis in Bcl-2- and c-FLIP-overexpressing breast carcinoma cells,” Oncol. Rep., vol. 33, no. 3, pp. 1395–1401, 2015. [CrossRef]
- H. Wu et al., “Ionizing Radiation Sensitizes Breast Cancer Cells to Bcl-2 Inhibitor , ABT-737 , through Regulating Mcl-1 Ionizing Radiation Sensitizes Breast Cancer Cells to Bcl-2 Inhibitor , ABT-737 , through Regulating Mcl-1,” vol. 182, no. 6, pp. 618–625. [CrossRef]
- R. Pan et al., “Inhibition of Mcl-1 with the pan-Bcl-2 family inhibitor (-)BI97D6 overcomes ABT-737 resistance in acute myeloid leukemia,” Blood, vol. 126, no. 3, pp. 363–372, 2015. [CrossRef]
- A. Robert, A. Pujals, L. Favre, J. Debernardi, and J. Wiels, “The BCL-2 family protein inhibitor ABT-737 as an additional tool for the treatment of EBV-associated post-transplant lymphoproliferative disorders,” Mol. Oncol., vol. 14, no. 10, pp. 2520–2532, 2020. [CrossRef]
- P. Gorombei et al., “Bcl-2 inhibitor abt-737 effectively targets leukemia-initiating cells with differential regulation of relevant genes leading to extended survival in a nras/bcl-2 mouse model of high risk-myelodysplastic syndrome,” Int. J. Mol. Sci., vol. 22, no. 19, pp. 1–21, 2021. [CrossRef]
- Z. Ni et al., “HCC cells with high levels of Bcl-2 are resistant to ABT-737 via activation of the ROS-JNK-autophagy pathway,” Free Radic. Biol. Med., vol. 70, pp. 194–203, 2014. [CrossRef]
- S. Kasai et al., “Bcl-2/Bcl-XL inhibitor ABT-737 sensitizes pancreatic ductal adenocarcinoma to paclitaxel-induced cell death,” Oncol. Lett., vol. 14, no. 1, pp. 903–908, 2017. [CrossRef]
- D. Hematology et al., “Targeting BCL-2 as a Therapeutic Strategy for Primary p210 BCR-ABL1 -positive B-ALL Cells,” vol. 516, pp. 511–516, 2020. [CrossRef]
- B. Z. Carter et al., “Synergistic effects of p53 activation via MDM2 inhibition in combination with inhibition of Bcl-2 or Bcr-Abl in CD34+ proliferating and quiescent chronic myeloid leukemia blast crisis cells,” Oncotarget, vol. 6, no. 31, pp. 30487–30499, 2015. [CrossRef]
- L. M. Brown, D. T. Hanna, S. L. Khaw, and P. G. Ekert, “Dysregulation of BCL-2 family proteins by leukemia fusion genes,” J. Biol. Chem., vol. 292, no. 35, pp. 14325–14333, 2017. [CrossRef]
- B. Z. Carter et al., “Combined targeting of BCL-2 and BCR-ABL tyrosine kinase eradicates chronic myeloid leukemia stem cells,” Sci. Transl. Med., vol. 8, no. 355, 2016. [CrossRef]
- C. Kurschat, A. Metz, S. Kirschnek, and G. Häcker, “Importance of Bcl-2-family proteins in murine hematopoietic progenitor and early B cells,” Cell Death Dis., vol. 12, no. 8, pp. 1–8, 2021. [CrossRef]
- N. Singh, J. Sarkar, K. V. Sashidhara, S. Ali, and S. Sinha, “Anti-tumour activity of a novel coumarin-chalcone hybrid is mediated through intrinsic apoptotic pathway by inducing PUMA and altering Bax/Bcl-2 ratio,” Apoptosis, vol. 19, no. 6, pp. 1017–1028, 2014. [CrossRef]
- H. Kobeissy, R. Hage, S. Zeinab, D. Lina, and K. Ghassan, “Crosstalk between Noxa , Bcl - 2 , and ceramide in mediating p53 - dependent apoptosis in Molt - 4 human T - cell leukemia,” Mol. Cell. Biochem., no. 0123456789, 2020. [CrossRef]
- S. V. Vartak et al., “Novel BCL2 inhibitor, Disarib induces apoptosis by disruption of BCL2-BAK interaction,” Biochem. Pharmacol., vol. 131, pp. 16–28, 2017. [CrossRef]
- T. J. Harford, G. Kliment, G. C. Shukla, and C. M. Weyman, “The muscle regulatory transcription factor MyoD participates with p53 to directly increase the expression of the pro-apoptotic Bcl2 family member PUMA,” Apoptosis, vol. 22, no. 12, pp. 1532–1542, 2017. [CrossRef]
- J. E. Guikema, M. Amiot, and E. Eldering, “Exploiting the pro-apoptotic function of NOXA as a therapeutic modality in cancer,” Expert Opin. Ther. Targets, vol. 21, no. 8, pp. 767–779, 2017. [CrossRef]
- W. Nakajima, M. A. Hicks, N. Tanaka, G. W. Krystal, and H. Harada, “Noxa determines localization and stability of MCL-1 and consequently ABT-737 sensitivity in small cell lung cancer,” Cell Death Dis., vol. 5, no. 2, pp. 1–10, 2014. [CrossRef]
- Y. Yang et al., “SATB1 mediates long-range chromatin interactions: A dual regulator of anti-apoptotic BCL2 and pro-apoptotic NOXA genes,” PLoS One, vol. 10, no. 9, pp. 1–15, 2015. [CrossRef]
- Y. Liu et al., “NOXA genetic amplification or pharmacologic induction primes lymphoma cells to BCL2 inhibitor-induced cell death,” Proc. Natl. Acad. Sci. U. S. A., vol. 115, no. 47, pp. 12034–12039, 2018. [CrossRef]
- A. N. Hata, J. A. Engelman, and A. C. Faber, “The BCL2 family: Key mediators of the apoptotic response to targeted anticancer therapeutics,” Cancer Discov., vol. 5, no. 5, pp. 475–487, 2015. [CrossRef]
- G. Radha and S. C. Raghavan, “BCL2: A promising cancer therapeutic target,” Biochim. Biophys. Acta - Rev. Cancer, vol. 1868, no. 1, pp. 309–314, 2017. [CrossRef]
- C. Li, G. Zhang, L. Zhao, Z. Ma, and H. Chen, “Metabolic reprogramming in cancer cells: Glycolysis, glutaminolysis, and Bcl-2 proteins as novel therapeutic targets for cancer,” World J. Surg. Oncol., vol. 14, no. 1, pp. 1–7, 2016. [CrossRef]
- A. W. Roberts, “Therapeutic development and current uses of BCL-2 inhibition,” Hematol. (United States), vol. 20, no. 1, pp. 1–9, 2020. [CrossRef]
- T. Knight, D. Luedtke, H. Edwards, J. W. Taub, and Y. Ge, “A delicate balance – The BCL-2 family and its role in apoptosis, oncogenesis, and cancer therapeutics,” Biochem. Pharmacol., vol. 162, pp. 250–261, 2019. [CrossRef]
- L. Vela and I. Marzo, “Bcl-2 family of proteins as drug targets for cancer chemotherapy: the long way of BH3 mimetics from bench to bedside,” Curr. Opin. Pharmacol., vol. 23, no. Figure 1, pp. 74–81, 2015. [CrossRef]
- E. Y. Lee et al., “Human breast cancer cells display different sensitivities to ABT-263 based on the level of survivin,” Toxicol. In Vitro, vol. 46, pp. 229–236, Feb. 2018. [CrossRef]
- T. Song, G. Chai, Y. Liu, X. Yu, Z. Wang, and Z. Zhang, “Bcl-2 phosphorylation confers resistance on chronic lymphocytic leukaemia cells to the BH3 mimetics ABT-737, ABT-263 and ABT-199 by impeding direct binding,” Br. J. Pharmacol., vol. 173, no. 3, pp. 471–483, Feb. 2016. [CrossRef]
- S. Besbes, M. Mirshahi, M. Pocard, and C. Billard, “New dimension in therapeutic targeting of BCL-2 family proteins,” Oncotarget, vol. 6, no. 15, pp. 12862–12871, 2015. [CrossRef]
- S. Cory, A. W. Roberts, P. M. Colman, and J. M. Adams, “Targeting BCL-2-like Proteins to Kill Cancer Cells,” Trends in Cancer, vol. 2, no. 8, pp. 443–460, 2016. [CrossRef]
- M. J. Roy, A. Vom, P. E. Czabotar, and G. Lessene, “Cell death and the mitochondria: Therapeutic targeting of the BCL-2 family-driven pathway,” Br. J. Pharmacol., vol. 171, no. 8, pp. 1973–1987, 2014. [CrossRef]
- R. G. Micheletti, “An update on the diagnosis and treatment of hidradenitis suppurativa,” Cutis, vol. 96, no. 6, pp. 7–12, 2015.
- S. S. Chakrabarti et al., “Identifying the mechanisms of α-synuclein-mediated cytotoxicity in Parkinson’s disease: New insights from a bioinformatics-based approach,” Future Neurol., vol. 15, no. 3, pp. 13–15, 2020. [CrossRef]
- J. Jankovic and E. K. Tan, “Parkinson’s disease: Etiopathogenesis and treatment,” J. Neurol. Neurosurg. Psychiatry, vol. 91, no. 8, pp. 795–808, 2020. [CrossRef]
- J. Liu, W. Liu, and H. Yang, “Balancing apoptosis and autophagy for parkinson’s disease therapy: Targeting BCL-2,” ACS Chem. Neurosci., vol. 10, no. 2, pp. 792–802, 2019. [CrossRef]
- A. Jan, N. P. Gonçalves, C. B. Vaegter, P. H. Jensen, and N. Ferreira, “The Prion-Like Spreading of Alpha-Synuclein in Parkinson’s Disease: Update on Models and Hypotheses,” Int. J. Mol. Sci. 2021, Vol. 22, Page 8338, vol. 22, no. 15, p. 8338, Aug. 2021. [CrossRef]
- B. Wang, L. Wang, Y. Qu, J. Lu, and W. Xia, “Chitosan oligosaccharides exert neuroprotective effects via modulating the PI3K/Akt/Bcl-2 pathway in a Parkinsonian model,” Food Funct., vol. 13, no. 10, pp. 5838–5853, May 2022. [CrossRef]
- L. Wang et al., “Cannabidiol Alleviates the Damage to Dopaminergic Neurons in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinson’s Disease Mice Via Regulating Neuronal Apoptosis and Neuroinflammation,” Neuroscience, vol. 498, pp. 64–72, Aug. 2022. [CrossRef]
- K. Guo et al., “Neuroprotective effect of paeoniflorin in the mouse model of Parkinson’s disease through α-synuclein/protein kinase C δ subtype signaling pathway,” Neuroreport, vol. 32, no. 17, pp. 1379–1387, Dec. 2021. [CrossRef]
- R. L. Jayaraj, S. Azimullah, K. A. Parekh, S. K. Ojha, and R. Beiram, “Effect of citronellol on oxidative stress, neuroinflammation and autophagy pathways in an in vivo model of Parkinson’s disease,” Heliyon, vol. 8, no. 11, p. e11434, 2022. [CrossRef]
- J. Shao, X. Liu, M. Lian, and Y. Mao, “Citronellol Prevents 6-OHDA-Induced Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis in Parkinson Disease Model of SH-SY5Y Cells via Modulating ROS-NO, MAPK/ERK, and PI3K/Akt Signaling Pathways,” Neurotox. Res., pp. 1–17, Sep. 2022. [CrossRef]
- T. Hong, J. Ding, and W. Li, “mir-7 reverses breast cancer resistance to chemotherapy by targeting MRP1 and BCL2,” Onco. Targets. Ther., vol. 12, pp. 11097–11105, 2019. [CrossRef]
- A. Srivastava and I. Jatoi, “Genetic Predisposition to Breast Cancer and Its Management,” Indian J. Surg. 2021 832, vol. 83, no. 2, pp. 273–274, May 2021. [CrossRef]
- M. Boujemaa et al., “Health influenced by genetics: A first comprehensive analysis of breast cancer high and moderate penetrance susceptibility genes in the Tunisian population,” PLoS One, vol. 17, no. 3, p. e0265638, Mar. 2022. [CrossRef]
- Y. Feng et al., “Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis,” Genes Dis., vol. 5, no. 2, pp. 77–106, 2018. [CrossRef]
- M. M. Williams and R. S. Cook, “Bcl-2 family proteins in breast development and cancer: Could Mcl-1 targeting overcome therapeutic resistance?,” Oncotarget, vol. 6, no. 6, pp. 3519–3530, 2015. [CrossRef]
- D. Merino, S. W. Lok, J. E. Visvader, and G. J. Lindeman, “Targeting BCL-2 to enhance vulnerability to therapy in estrogen receptor-positive breast cancer,” Oncogene 2016 3515, vol. 35, no. 15, pp. 1877–1887, Aug. 2015. [CrossRef]
- N. Honma et al., “Differences in clinical importance of Bcl-2 in breast cancer according to hormone receptors status or adjuvant endocrine therapy,” BMC Cancer, vol. 15, no. 1, 2015. [CrossRef]
- P. Raha, S. Thomas, K. T. Thurn, J. Park, and P. N. Munster, “Combined histone deacetylase inhibition and tamoxifen induces apoptosis in tamoxifen-resistant breast cancer models, by reversing Bcl-2 overexpression,” Breast Cancer Res., vol. 17, no. 1, pp. 1–16, 2015. [CrossRef]
- A. Pyrczak-Felczykowska et al., “Synthesis of Usnic Acid Derivatives and Evaluation of Their Antiproliferative Activity against Cancer Cells,” J. Nat. Prod., vol. 82, no. 7, pp. 1768–1778, 2019. [CrossRef]
- K. Kumar, J. P. Mishra, and R. P. Singh, “Anti-cancer efficacy and mechanisms of usnic acid,” Indian J. Pharm. Biol. Res., vol. 7, no. 03, pp. 1–4, 2019. [CrossRef]
- E. Deǧerli, V. Torun, and D. Cansaran-Duman, “MiR-185-5p response to usnic acid suppresses proliferation and regulating apoptosis in breast cancer cell by targeting Bcl2,” Biol. Res., vol. 53, no. 1, pp. 1–14, 2020. [CrossRef]
- N. Kiliç, Y. Ö. Islakoğlu, İ. Büyük, B. Gür-Dedeoğlu, and D. Cansaran-Duman, “Determination of Usnic Acid Responsive miRNAs in Breast Cancer Cell Lines,” Anticancer. Agents Med. Chem., vol. 19, no. 12, pp. 1463–1472, Nov. 2019. [CrossRef]
- R. Özben and D. Cansaran-Duman, “The expression profiles of apoptosis-related genes induced usnic acid in SK-BR-3 breast cancer cell,” Hum. Exp. Toxicol., vol. 39, no. 11, pp. 1497–1506, 2020. [CrossRef]
- “J Biochem Molecular Tox - 2021 - Elia - Loperamide potentiates doxorubicin sensitivity in triple-negative breast cancer.pdf.”.
- A. Ghanem et al., “Rumex Vesicarius L. extract improves the efficacy of doxorubicin in triple-negative breast cancer through inhibiting Bcl2, mTOR, JNK1 and augmenting p21 expression,” Informatics Med. Unlocked, vol. 29, no. December 2021, p. 100869, 2022. [CrossRef]
- S. Eugin Simon et al., “New synthetic phenylquinazoline derivatives induce apoptosis by targeting the pro-survival members of the BCL-2 family,” Bioorg. Med. Chem. Lett., vol. 67, p. 128731, Jul. 2022. [CrossRef]
- P. M. de Groot, C. C. Wu, B. W. Carter, and R. F. Munden, “The epidemiology of lung cancer,” Translational Lung Cancer Research, vol. 7, no. 3. AME Publishing Company, pp. 220–233, Jun. 2018. [CrossRef]
- L. A. Byers and C. M. Rudin, “Small cell lung cancer: Where do we go from here?,” Cancer, vol. 121, no. 5, pp. 664–672, 2015. [CrossRef]
- J. Fan et al., “Bruceine D induces lung cancer cell apoptosis and autophagy via the ROS/MAPK signaling pathway in vitro and in vivo,” Cell Death Dis., vol. 11, no. 2, 2020. [CrossRef]
- G. Liu et al., “Role of autophagy and apoptosis in non-small-cell lung cancer,” Int. J. Mol. Sci., vol. 18, no. 2, 2017. [CrossRef]
- X. Song, X. Xu, J. Lu, X. Chi, Y. Pang, and Q. Li, “Lamprey Immune Protein Mediates Apoptosis of Lung Cancer Cells Via the Endoplasmic Reticulum Stress Signaling Pathway,” Front. Oncol., vol. 11, no. July, pp. 1–14, 2021. [CrossRef]
- N. Othman and N. H. Nagoor, “The role of microRNAs in the regulation of apoptosis in lung cancer and its application in cancer treatment,” Biomed Res. Int., vol. 2014, 2014. [CrossRef]
- A. R. D. Delbridge and A. Strasser, “The BCL-2 protein family, BH3-mimetics and cancer therapy,” Cell Death Differ. 2015 227, vol. 22, no. 7, pp. 1071–1080, May 2015. [CrossRef]
- S. Song et al., “ATP promotes cell survival via regulation of cytosolic [Ca2+] and Bcl-2/Bax ratio in lung cancer cells,” Am. J. Physiol. - Cell Physiol., vol. 310, no. 2, pp. C99–C114, 2016. [CrossRef]
- S. Yu, L. sheng Gong, N. feng Li, Y. feng Pan, and L. Zhang, “Galangin (GG) combined with cisplatin (DDP) to suppress human lung cancer by inhibition of STAT3-regulated NF-κB and Bcl-2/Bax signaling pathways,” Biomed. Pharmacother., vol. 97, no. May 2017, pp. 213–224, 2018. [CrossRef]
- X. Shi et al., “A critical role for the long non-coding RNA GAS5 in proliferation and apoptosis in non-small-cell lung cancer,” Mol. Carcinog., vol. 54, no. S1, pp. E1–E12, 2015. [CrossRef]
- P. Huang, B. Ye, Y. Yang, J. Shi, and H. Zhao, “MicroRNA-181 functions as a tumor suppressor in non-small cell lung cancer (NSCLC) by targeting Bcl-2,” Tumor Biol., vol. 36, no. 5, pp. 3381–3387, 2015. [CrossRef]
- T. L. Lochmann et al., “Venetoclax is effective in small-cell lung cancers with high BCL-2 expression,” Clin. Cancer Res., vol. 24, no. 2, pp. 360–369, 2018. [CrossRef]
- L. Wu and L. Chen, “Characteristics of Nur77 and its ligands as potential anticancer compounds (Review),” Mol. Med. Rep., vol. 18, no. 6, pp. 4793–4801, 2018. [CrossRef]
- M. C. Pearce et al., “Induction of apoptosis and suppression of tumor growth by Nur77-derived Bcl-2 converting peptide in chemoresistant lung cancer cells,” Oncotarget, vol. 9, no. 40, pp. 26072–26085, 2018. [CrossRef]
- R. K. Mongre et al., “Novel carbazole-piperazine hybrid small molecule induces apoptosis by targeting BCL-2 and inhibits tumor progression in lung adenocarcinoma in vitro and xenograft mice model,” Cancers (Basel)., vol. 11, no. 9, pp. 1–25, 2019. [CrossRef]
- D. Park et al., “Discovery of Small Molecule Bak Activator for Lung Cancer Therapy,” Theranostics, vol. 11, no. 17, pp. 8500–8516, 2021. [CrossRef]
- M. Marrodan, M. F. Farez, M. E. Balbuena Aguirre, and J. Correale, “Obesity and the risk of Multiple Sclerosis. The role of Leptin,” Ann. Clin. Transl. Neurol., vol. 8, no. 2, pp. 406–424, 2021. [CrossRef]
- M. Filippi et al., “EM (Nature 2018),” pp. 1–27, 2018.
- A. F. Ferreira, “Aline fernanda ferreira,” vol. 55, no. 16, pp. 1–5, 2014.
- S. E. Baranzini and J. R. Oksenberg, “The Genetics of Multiple Sclerosis: From 0 to 200 in 50 Years,” Trends Genet., vol. 33, no. 12, pp. 960–970, 2017. [CrossRef]
- B. Macchi et al., “Role of inflammation and apoptosis in multiple sclerosis: Comparative analysis between the periphery and the central nervous system,” J. Neuroimmunol., vol. 287, pp. 80–87, 2015. [CrossRef]
- S. Hagman et al., “Analysis of apoptosis-related genes in patients with clinically isolated syndrome and their association with conversion to multiple sclerosis,” J. Neuroimmunol., vol. 280, pp. 43–48, 2015. [CrossRef]
- D. H. Mahad, B. D. Trapp, and H. Lassmann, “Pathological mechanisms in progressive multiple sclerosis,” Lancet Neurol., vol. 14, no. 2, pp. 183–193, 2015. [CrossRef]
- M. Igci et al., “Gene expression profiles of autophagy-related genes in multiple sclerosis,” Gene, vol. 588, no. 1, pp. 38–46, 2016. [CrossRef]
- X. Feng, H. Hou, Y. Zou, and L. Guo, “Defective autophagy is associated with neuronal injury in a mouse model of multiple sclerosis,” Bosn. J. Basic Med. Sci., vol. 17, no. 2, pp. 95–103, 2017. [CrossRef]
- N. Sharma et al., “Neuroprotection by solanesol against ethidium bromide-induced multiple sclerosis-like neurobehavioral, molecular, and neurochemical alterations in experimental rats,” Phytomedicine Plus, vol. 1, no. 4, p. 100051, 2021. [CrossRef]
- Y. Dong et al., “Leukemia incidence trends at the global, regional, and national level between 1990 and 2017,” Exp. Hematol. Oncol., vol. 9, no. 1, pp. 1–11, Jun. 2020. [CrossRef]
- S. Ribeiro, A. M. Eiring, and J. S. Khorashad, “Genomic abnormalities as biomarkers and therapeutic targets in acute myeloid leukemia,” Cancers (Basel)., vol. 13, no. 20, pp. 1–21, 2021. [CrossRef]
- Y. Wei et al., “Targeting Bcl-2 Proteins in Acute Myeloid Leukemia,” Front. Oncol., vol. 10, no. November, pp. 1–11, 2020. [CrossRef]
- S. Zhang, Q. Zhang, G. Shi, and J. Yin, “MiR-182-5p regulates BCL2L12 and BCL2 expression in acute myeloid leukemia as a potential therapeutic target,” Biomed. Pharmacother., vol. 97, no. November 2017, pp. 1189–1194, 2018. [CrossRef]
- M. Gentile et al., “Venetoclax for the treatment of chronic lymphocytic leukemia,” Expert Opin. Investig. Drugs, vol. 26, no. 11, pp. 1307–1316, 2017. [CrossRef]
- L. Ismail, H. Materwala, and J. Al Kaabi, “Association of risk factors with type 2 diabetes: A systematic review,” Comput. Struct. Biotechnol. J., vol. 19, pp. 1759–1785, 2021. [CrossRef]
- E. N. Gurzov and D. L. Eizirik, “Bcl-2 proteins in diabetes: Mitochondrial pathways of β-cell death and dysfunction,” Trends Cell Biol., vol. 21, no. 7, pp. 424–431, 2011. [CrossRef]
- E. Pishavar and J. Behravan, “miR-126 as a Therapeutic Agent for Diabetes Mellitus,” Curr. Pharm. Des., vol. 23, no. 22, pp. 3309–3314, 2017. [CrossRef]
- L. Demirtas et al., “Apoptosis, autophagy & endoplasmic reticulum stress in diabetes mellitus,” Indian J. Med. Res., vol. 144, no. OCTOBER, pp. 515–524, 2016. [CrossRef]
- H. E. Thomas, M. D. McKenzie, E. Angstetra, P. D. Campbell, and T. W. Kay, “Beta cell apoptosis in diabetes,” Apoptosis, vol. 14, no. 12, pp. 1389–1404, 2009. [CrossRef]
- T. Tomita, “Apoptosis in pancreatic β-islet cells in Type 2 diabetes,” Bosn. J. Basic Med. Sci., vol. 16, no. 3, pp. 162–179, 2016. [CrossRef]
- E. Gokalp-Ozkorkmaz et al., “Examination of Bcl-2 and Bax Protein Levels for Determining the Apoptotic Changes in Placentas with Gestational Diabetes and Preeclampsia,” p. 1548, 2018. [CrossRef]
- E. K. Sims, A. J. Lakhter, E. Anderson-Baucum, T. Kono, X. Tong, and C. Evans-Molina, “MicroRNA 21 targets BCL2 mRNA to increase apoptosis in rat and human beta cells,” Diabetologia, vol. 60, no. 6, pp. 1057–1065, 2017. [CrossRef]
- E. F. El Azab and H. S. Mostafa, “Geraniol ameliorates the progression of high fat-diet/streptozotocin-induced type 2 diabetes mellitus in rats via regulation of caspase-3, Bcl-2, and Bax expression,” J. Food Biochem., vol. 46, no. 7, p. e14142, Jul. 2022. [CrossRef]
- Q. He et al., “Piperine is capable of improving pancreatic β-cell apoptosis in high fat diet and streptozotocin induced diabetic mice,” J. Funct. Foods, vol. 88, p. 104890, Jan. 2022. [CrossRef]
- X. qi Liu et al., “Wogonin protects glomerular podocytes by targeting Bcl-2-mediated autophagy and apoptosis in diabetic kidney disease,” Acta Pharmacol. Sin., vol. 43, no. 1, pp. 96–110, 2022. [CrossRef]
- A. H. Amin, M. A. El-Missiry, and A. I. Othman, “Melatonin ameliorates metabolic risk factors, modulates apoptotic proteins, and protects the rat heart against diabetes-induced apoptosis,” Eur. J. Pharmacol., vol. 747, pp. 166–173, 2015. [CrossRef]
- S. R. Filios and A. Shalev, “β-Cell MicroRNAs: Small but Powerful,” Diabetes, vol. 64, no. 11, pp. 3631–3644, Nov. 2015. [CrossRef]
- K. Tugay et al., “Role of microRNAs in the age-associated decline of pancreatic beta cell function in rat islets,” Diabetologia, vol. 59, no. 1, pp. 161–169, Jan. 2016. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





