1. Introduction
Cirrhosis is a chronic disease characterized by widespread fibrosis and the conversion of the liver parenchyma into abnormal nodules [
1]. Cirrhosis diagnosis is morphologic, based on the coexistence of parenchymal cell necrosis, nodular regeneration, and liver fibrosis, and it is always associated with a progressive deterioration of the functional properties of the organ [
2].
Hepatic encephalopathy (HE) is a serious neuropsychiatric complication of cirrhosis [
3]; the symptoms of this disease are characterized by alterations of psychomotor, fine motor, cognitive, intellectual and behavioral functions. Cirrhotic cardiomyopathy is another serious complication of cirrhosis, characterized by chronic cardiac dysfunction with impaired cardiac contractility in response to stress and/or diastolic failure, in the absence of known cardiac disease. Furthermore such cardiomyopathy is regardless of the causes of cirrhosis, although some etiologies (for example, alcohol abuse or iron overload) have a greater impact on myocardial function [
4,
5].
Interest in calcium sensitizing pharmacological agents has grown in recent years. These new drugs improve myocardial contraction with a mechanism of action that increases calcium sensitivity. Levosimendan is the most studied of this drug class. Recent studies have demonstrated that levosimendan is able to preserve the functionality of some organs in acute heart failure induced by septic shock [
6]. Furthermore, levosimendan has been shown to protect the liver from ischemic liver injury through its function on mitoKATP channels and through mechanisms related to nitric oxide (NO) production [
7].
Our study aimed to evaluate the effects of levosimendan on cirrhosis-induced liver fibrosis and its complications, such as cirrhotic cardiomyopathy and hepatic encephalopathy.
2. Material and Methods
NOTE: This protocol was approved by the Institutional Animal Care and Use Commitee (IACUC) of the University of Salerno based on animal use and care; agrees with the practices and principles of the European Guide for the care and use of laboratory animals, in accordance with the ethical guidelines of the European Community Council Directive (86/609/EEC). Rats are housed in specific pathogen-free conditions according to the guidelines of the Federation for Laboratory Animal Science Associations (FELASA)
Animals
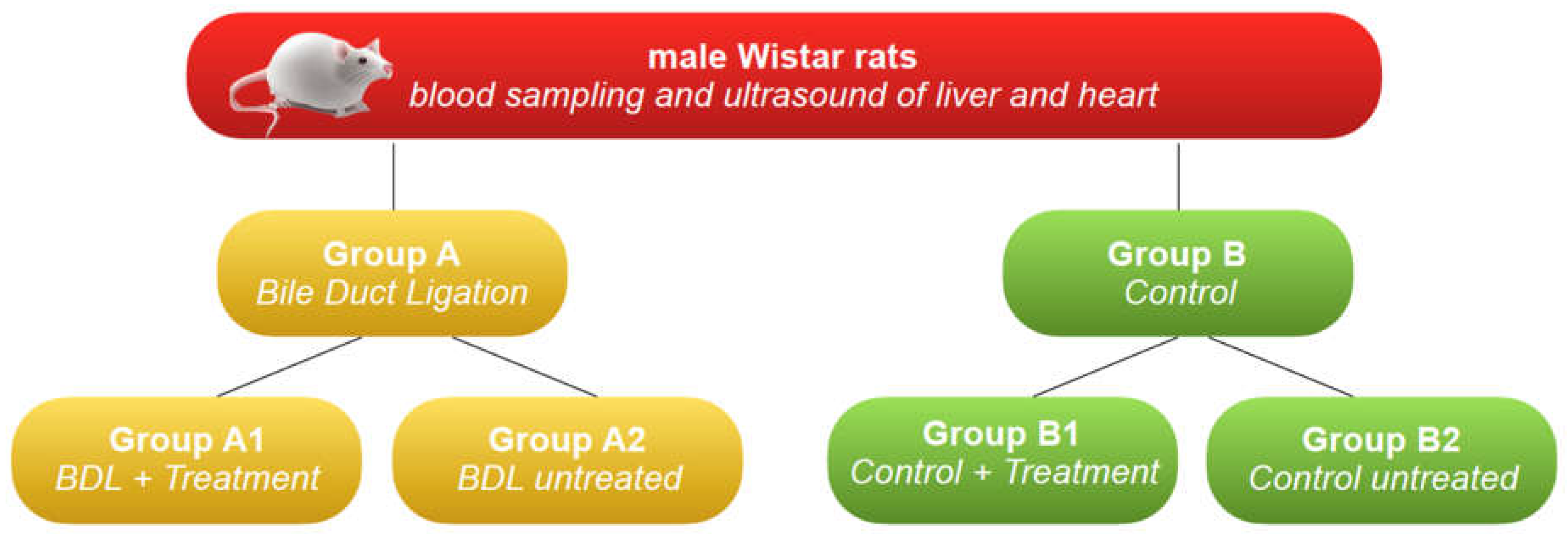
Seventy male Wistar rats with an average weight of 200g were used for this study. A week before experimentation, the animals were housed for acclimatization to 22 ± 2 ° C, humidity 55 ± 10%, and cycles with light/dark for 12 h. They had free access to water and food pellets.
Subsequently were randomized into two groups, as follows:
1. Group A: 50 rats in which liver cirrhosis was induced by bile duct ligation (BDL).
2. Group B: 20 rats (control).
Group A was further divided into two subgroups:
1. Group A1: 25 animals with cirrhosis induced by BDL were treated with an intraperitoneal injection of levosimendan.
2. Group A2: 25 animals with cirrhosis induced by BDL with no treatment.
Group B was further divided into two subgroups:
1. Group B1: 10 animals with normal liver function treated with an intraperitoneal injection of levosimendan.
2. Group B2: 10 untreated animals with normal hepatic function.
Timing of the study
T0: all animals were subjected to blood sampling and ultrasound of the liver and heart. Then, during the same anesthesia, the animals of group A were subjected to surgical ligation of the bile duct.
T1 (day 14): the animals of all groups (groups A and B) were subjected to blood sampling and ultrasound of the liver and heart, and the animals of groups A1 and B1 were treated with an intraperitoneal injection of levosimendan.
T2 (day 19), all animals (group A1, A2, B1, B2) were subjected to blood sampling and ultrasound of the liver and heart; then, the animals were subjected to liver and brain microdialysis and subsequently euthanized. (
Figure 1)
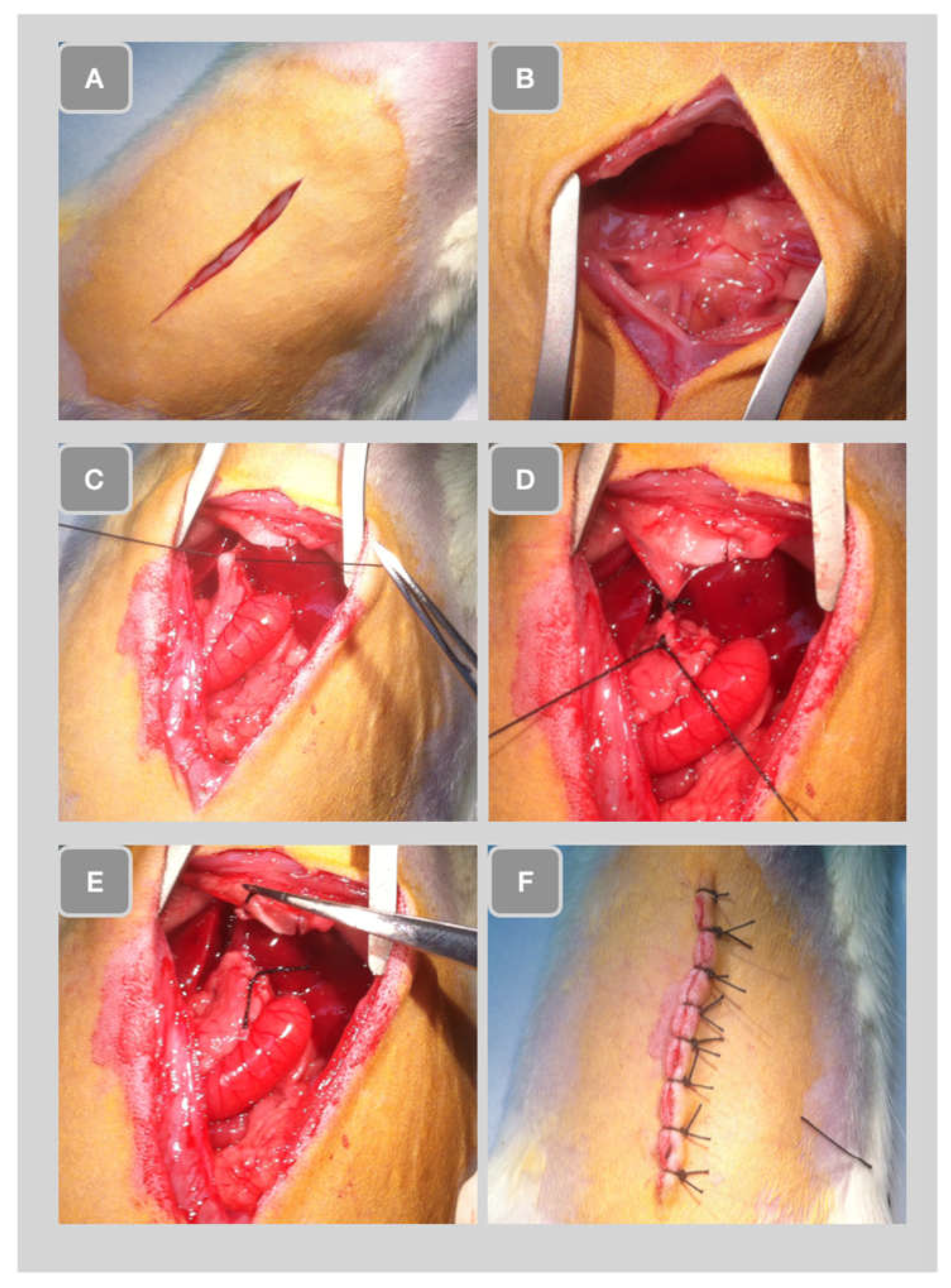
Ligature of the common bile duct
Surgery for ligation of the common bile duct was performed following our protocol [
8]. The previously shaved abdominal wall was cut along the midline (
Figure 2A). Subsequently, the hepatic lobules were pulled down and the intestine gently extracted to uncover the bile duct (
Figure 2B). The extrahepatic common bile duct was then ligated (
Figure 2C). Subsequently, a second ligature was introduced approximately 1 cm apart (
Figure 2D) and the bile duct was cut between the two ligatures (
Figure 2E). Finally, the liver was repositioned in the abdominal cavity, the abdomen was closed with stitches (
Figure 2F).
Treatment with levosimendan
At T1 time, after induction of liver cirrhosis proven by ultrasonography and blood sampling, animals were subjected to treatment with levosimendan :
1. Group A1: 25 rats with liver cirrhosis induced by bile duct ligation
2 . Group B1: 10 rats with normal liver function
Levosimendan (Simdax, Orion Pharma) diluted in 10 ml of 0.5% dextrose was administered intraperitoneal injection in a single dose of 12 micrograms/kg ( 0.25 mL /animal).
Echocardiography
Echocardiograms were performed in animals at T0, T2, and after four weeks of surgery, after injection of levosimendan or placebo. The animals were anesthetized and placed on the heated bed Vevo 2100 VisualSonics. This diagnostic test was used to acquire information about the parameters of cardiac contractilities, such as Ejection Fraction (EF%), stroke volume, cardiac output, and signs of pulmonary hypertension typical of cirrhotic disease.
Blood sampling
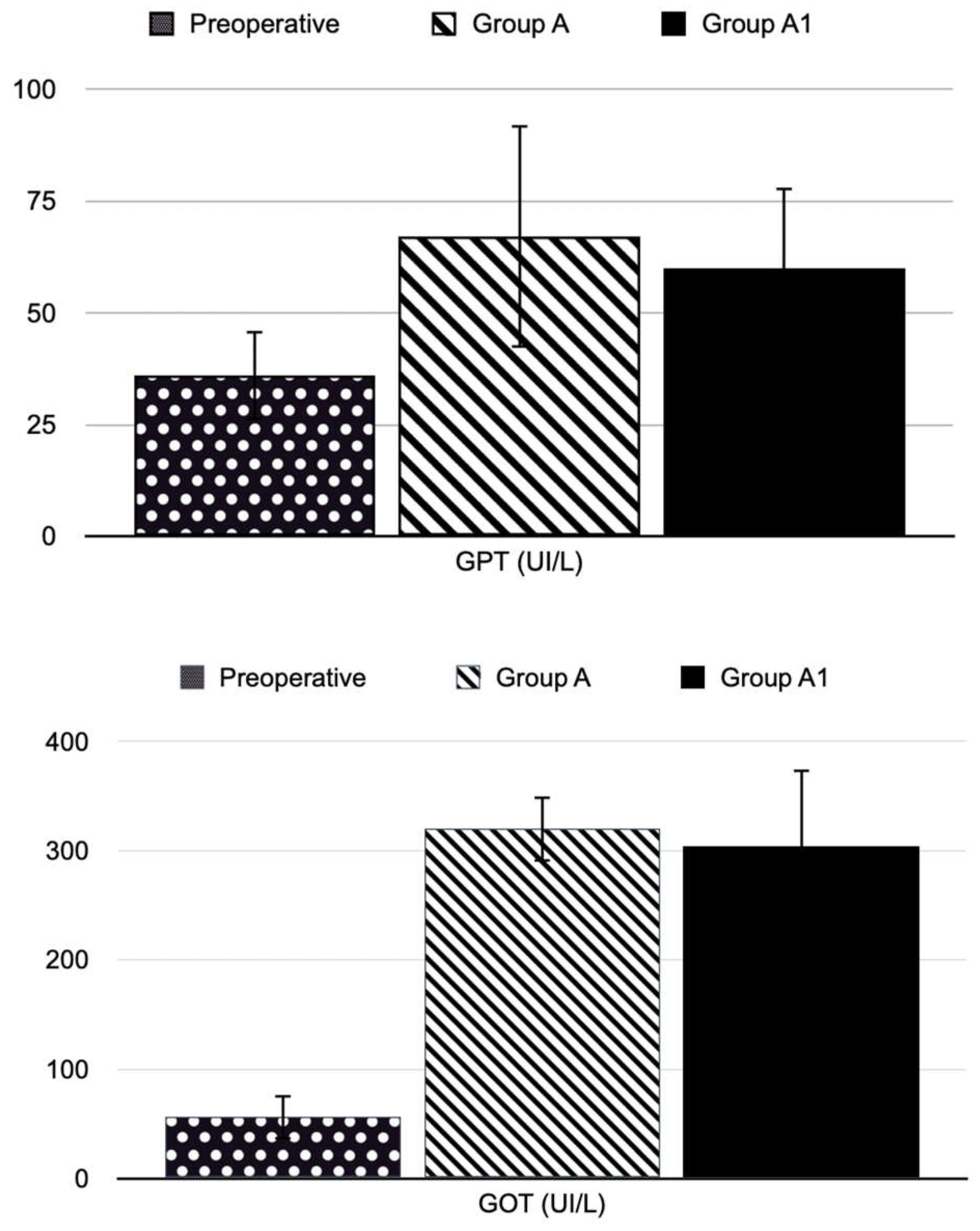
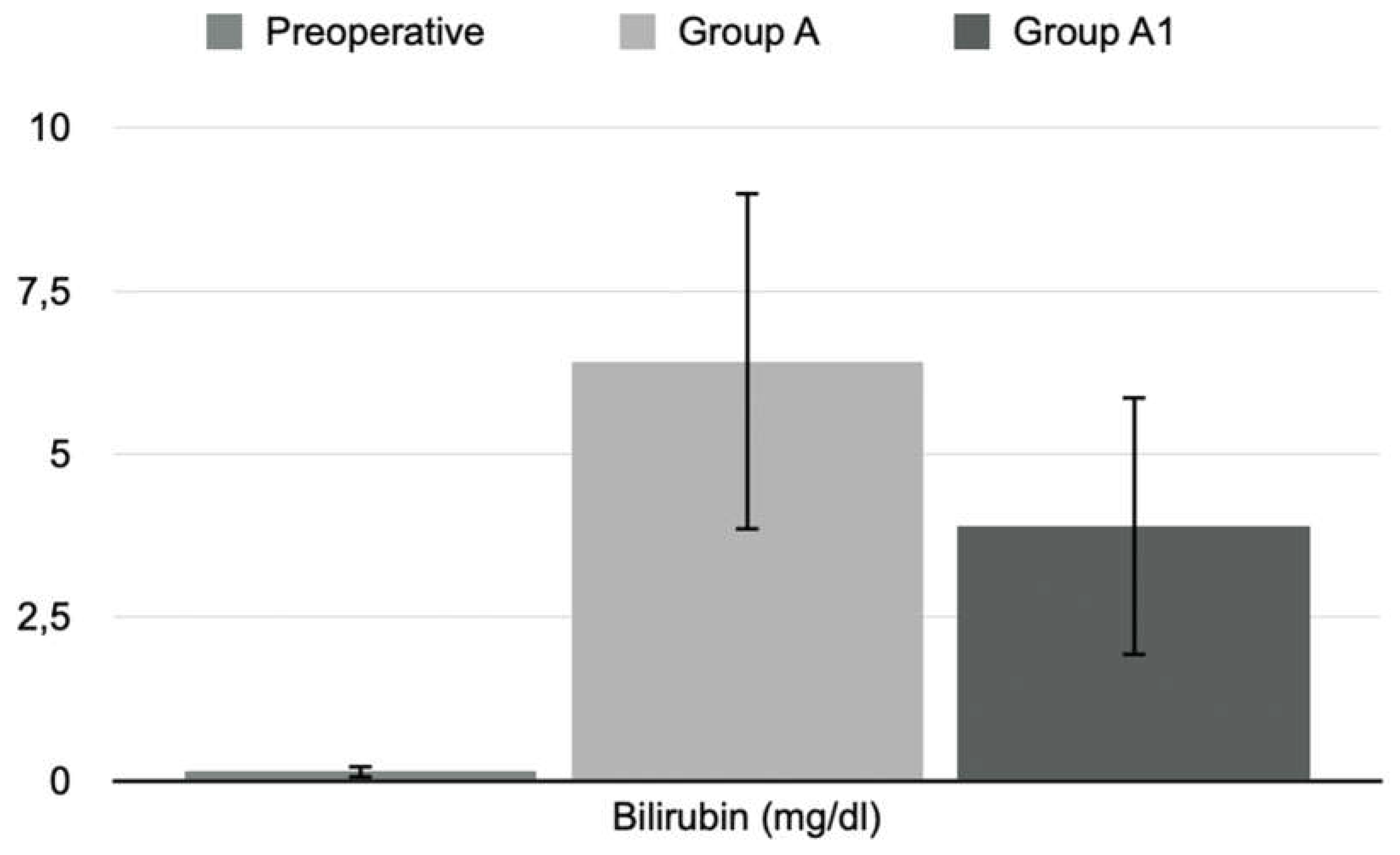
The blood sampling was performed at times T1, T2, and T3. The animals were anesthetized and placed in sternal recumbency. We previously put a peripheral (heparinized, 24G) venous catheter in the lateral tail vein. A tourniquet facilitated this procedure at the base of the tail. We collected a sample of 600 microliters of blood in a test tube with a gel separator for each rat. The sample was centrifuged at 2700 rpm for 15 minutes at 4 °C. The collected plasma was aliquoted in Eppendorf 2 ml and used to determine GOT, GPT, and total bilirubin.
Microdialysis
The rats at T3 were subjected to liver and brain microdialysis as follows:
1. Group A1: 25 rats with liver cirrhosis induced by bile duct ligation treated with levosimendan
2. Group A2: 25 rats with liver cirrhosis induced by bile duct ligation, no levosimendan
3. Group B1: 10 rats with normal liver function treated with levosimendan
4. Group B2: 10 rats not treated with levosimendan and with normal hepatic function.
Histological analysis
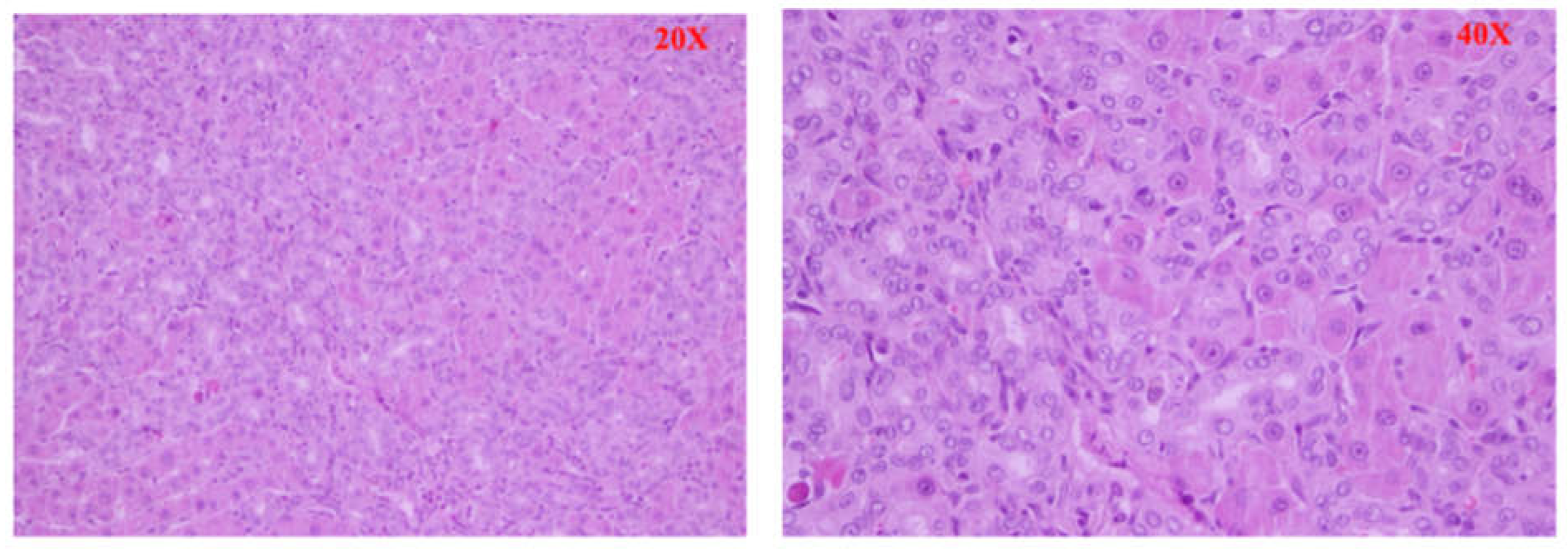
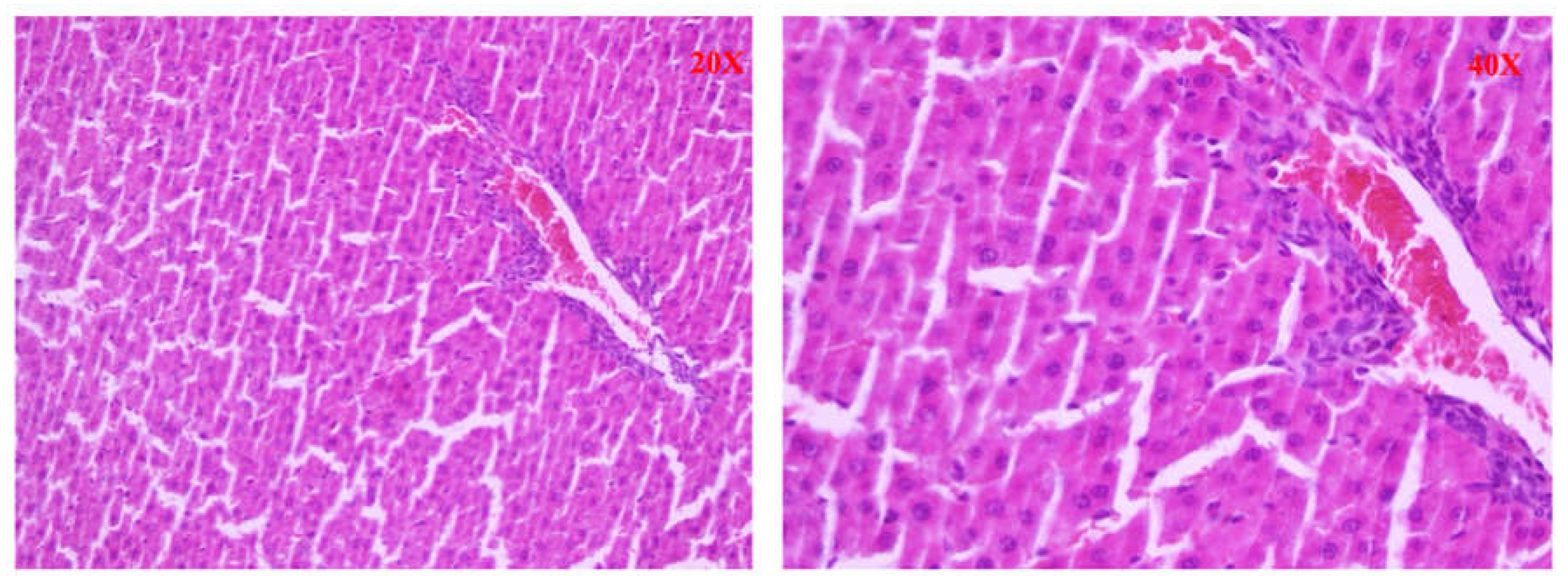
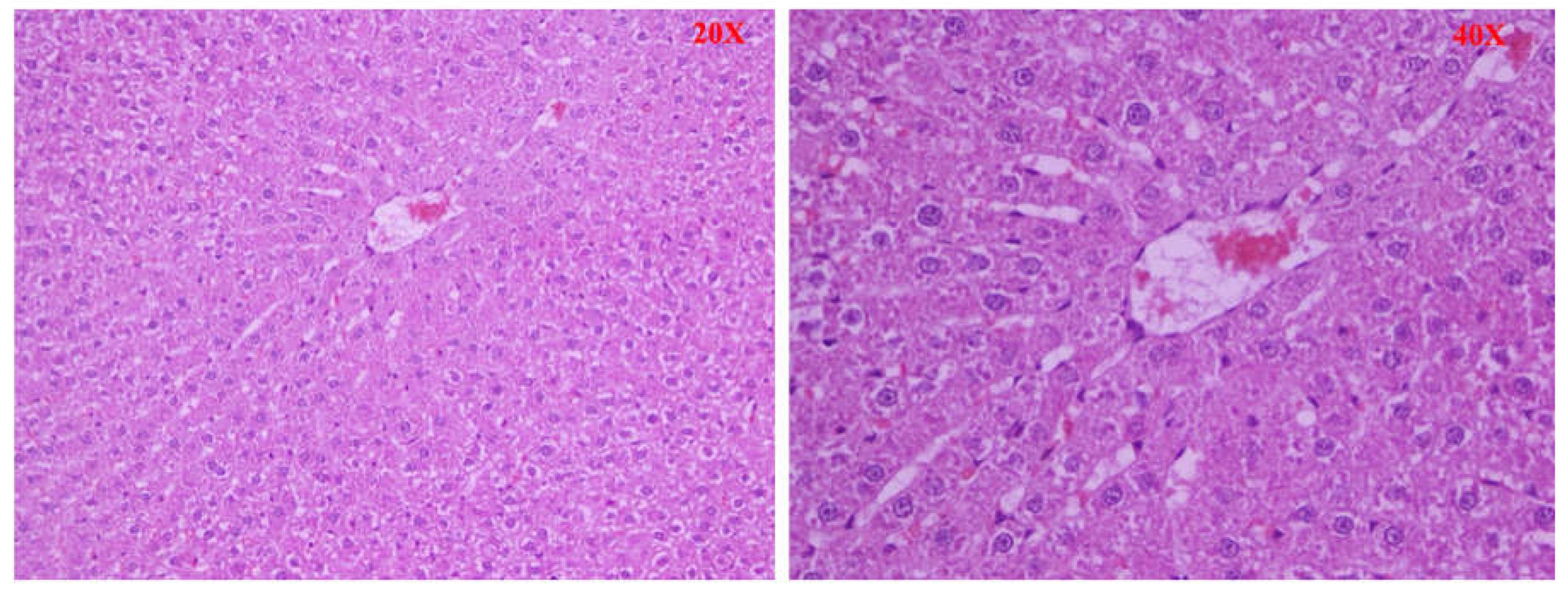
After euthanasia, a liver sample was quickly removed from the animal, rested on support, and covered with synthetic resin for inclusion (OCT). The whole was placed at a variable temperature between -15 ° C and - 30 ° C to ensure a time of slow freezing and morphological conservation optimal, and stored at - 80 ° C. The levy was cut into frozen sections using a cryostat and stained with hematoxylin and eosin. The stages of liver fibrosis were classified according to the Scheuer classification [
9]: no fibrosis (stage 0) fibrosis confined to the portal tracts (stage 1); periportal fibrosis or portal architecture with intact (stage 2); septal fibrosis with structural distortion but not evident cirrhosis (stage 3 ); probable or definite cirrhosis (stage 4).
Statistical Analysis
The number of experimental rats was calculated to reach the minimum for the analysis of variance, performed using the STAT PLUS software, to obtain a statistical difference of 0.05 at power analysis. Data are presented as mean (SEM) unless otherwise stated. A P value <0.05 was considered statistically significant.
4. Discussion
Cirrhosis affects more than 5% of the world's population, mainly adults.The increased knowledge of the etiology and pathophysiology of chronic hepatitis and cirrhosis has allowed the setting of prevention, based both on programs against alcohol abuse and on the vaccination against hepatitis B and the application of several actions to prevent the parenteral transmission of hepatitis viruses [
10]. Nevertheless, the incidence of cirrhosis is growing due to the greater prevalence of chronic hepatitis and the lack of a certain therapy for liver fibrosis. New research perspectives and treatment strategies for fibrotic liver injury are emerging following improvements in the pathogenesis of cirrhosis.
In particular, it seems that microcirculation represents a crucial factor in the pathogenesis of liver damage, in fact changes in microcirculatory blood flow usually precede the development of parenchymal abnormalities [
11]. Microcirculatory changes can also cause ischemia and enlarge irreversibly damaged areas. Furthermore, they may be responsible for a progressive inflammatory response [
12]. Szijarto et al. previously demonstrated that microcirculation improvement reduced hepatic injury [
13]. Therefore, the quality of tissue microcirculation is closely linked to the severity of organ damage and therefore to the effectiveness of any intervention. Literature data suggest that levosimendan improves splanchnic microcirculation in septic rats [
14]. Hence, we examined the effect of levosimendan in rat models of liver cirrhosis. Our results showed that levosimendan treatment led to a highly significant improvement in cardiac contractility and consequently in hepatic microcirculation compared to the corresponding BDL group.
Furthermore, significant histological differences were found between the different groups of animals. In the BDL group (Group A), large and confluent areas of necrosis, sometimes accompanied by hemorrhage or leukocyte infiltration, were observed, while group A1 (the levosimendan-treated animals) showed a dramatically lower percentage of necrotic cells and one death cell mainly of focal type. Consistently, also less tissue bleeding and less extensive leukocyte infiltration. Moderate tissue damage in treated animals was supported by a significant decrease in serum GOT and GPT activities.
During cirrhosis of the liver, cell death is predominantly of hepatocytes and sinusoidal endothelial cells. It can occur as necrosis or apoptosis. The necrotic and apoptotic areas in our observation in the slides corresponded to the extensively damaged parts. After treatment with levosimendan, the areas of necrosis significantly decreased in size. These results are supported by literature data demonstrating that levosimendan has anti-apoptotic properties on the heart and kidney [
15,
16]. Cirrhosis leads to cell injury and reactive oxygen species generation after reoxygenation. Free radicals, in turn, cause direct tissue injury and increase oxidative stress through the formation of pro-inflammatory mediators and the infiltration and activation of macrophages, neutrophils and lymphocytes. Previous studies have shown that levosimendan treatment benevolently affects immune response [
17]. We demonstrated that treatment with levosimendan reduced the lactate level, indicative of a decrease in free radicals and improved liver antioxidant status. Histopathological analysis also showed less severe inflammatory cell infiltration in the treated groups in our study.
The hepatoprotective effect of levosimendan could be the consequence that the drug has on the hemodynamic impact. In our data set, treatment with levosimendan induced an increase in heart rate and a decrease in blood pressure as already demonstrated in the literature. [
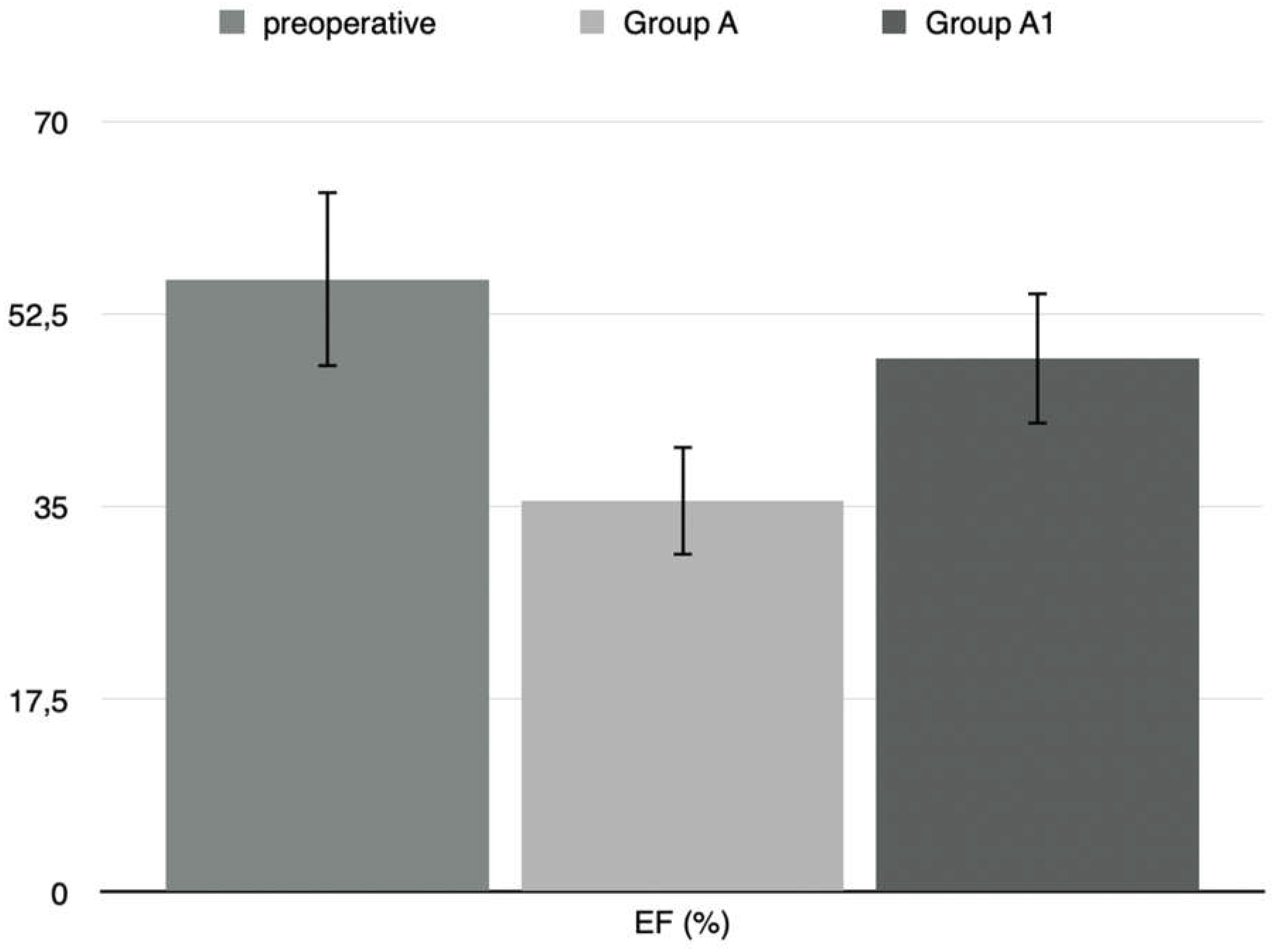
18]. In our results, Ejection fraction (EF%) in operated rats had higher preoperative values than those measured two weeks after surgery. In the treated rats group (Group A1), the ejection fraction value decreased significantly after two weeks postoperatively and was highest after treatment with levosimendan.
The anti-inflammatory, antioxidant, antiapoptotic, and anti-stun effect of levosimendan may contribute to the beneficial impacts on cirrhotic cardiomyopathy. This effect was supported by histological analysis and measurements of GPT and GOT levels and tissue viability.
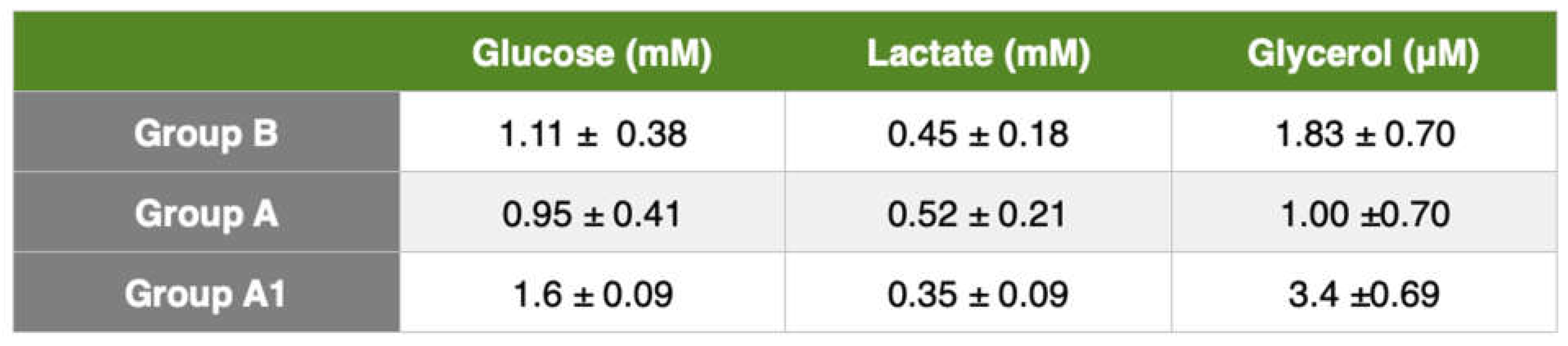
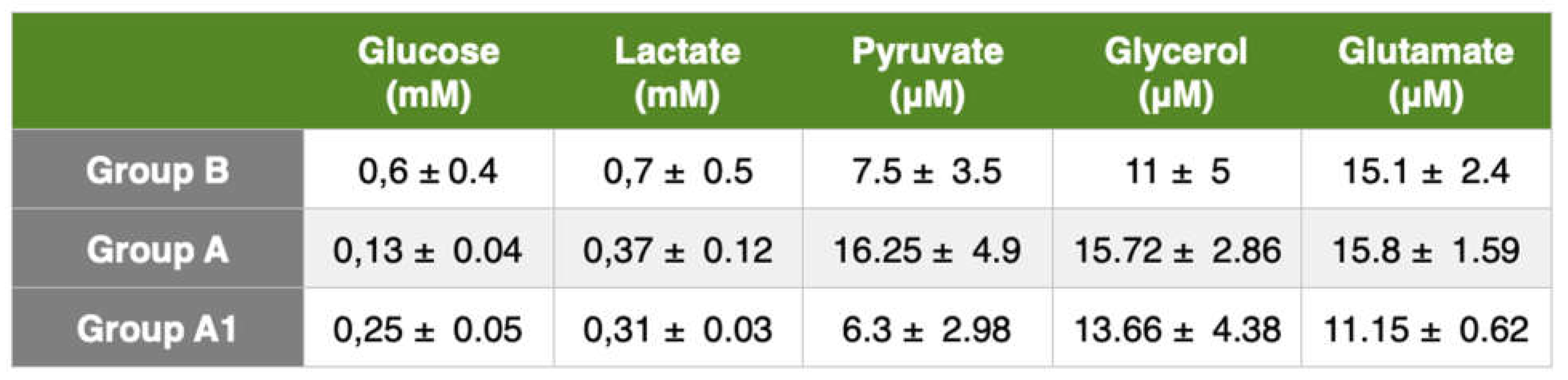
Liver cirrhosis produces fibrotic tissue and new regenerative node formation. The higher hepatic microdialysis glucose values of the control group indicate a higher glucose metabolic turnover and reflect an increase in gluconeogenesis. Such values were not found in cirrhotic rats, we believe due to the inability of the liver to increase gluconeogenesis. Plasma concentrations of lactate, glucose, and glycerol are similar in cirrhotic and healthy people [
19], so microdialysis is the only effective method for monitoring metabolic changes in the liver.
The glucose concentrations in the liver were higher in the control group than in the liver-damaged rats. This follows the findings of Petersen et al. [
19]. These authors demonstrated that low liver glycogen content could be due to a decrease in functioning hepatocytes resulting in impaired hepatic glycogen synthesis and hydrolysis [
20]. Cirrhosis is also associated with increased lipolysis with elevated plasma concentrations of non-esterified fatty acids and release of more glycerol and free fatty acids.
Johansson et al. [
21] in a study of cirrhotic patients using microdialysis, reported higher arterial and interstitial adipose tissue glycerol concentrations in cirrhotic patients than in the control group. Our study found an increase in glycerol concentration in cirrhotic rats. Other authors such as Shangraw et al. [
22] Kaye et al. [
23], and Nosadini et al. [
24] found elevated plasma free fatty acid and glycerol concentrations in cirrhotic patients assuming lower hepatic clearance than in the control group. Our results, in agreement with the recent literature, showed elevated interstitial glycerol concentrations in rats with liver cirrhosis. Both the BDL and BDL+levosimendan groups had lower liver glycerol concentrations than the control group. These results show increased lipolysis rate and decreased hepatic glycerol clearance in cirrhotic rats and lower hepatic glycerol clearance in levosimendan-treated rats.
Glutamate is the major excitatory neurotransmitter in the brain. Our results showed a higher glutamate concentration in BDL rats than in the control group. This could indicate impaired glutamine-glutamate trafficking. These values are difficult to interpret because the release of glutamate from neurons is mixed with a metabolic pool of glutamate. In treated rats, glutamate tended to decrease despite normal cerebral oxygenation and [lactate/pyruvate] ratio. These findings may reflect a decrease in total brain glutamate content.
As the brain's primary energy source, glucose is an important marker of changes in brain metabolism. In our study, glucose concentrations in the microdialysate of intact rats were significantly higher compared with cirrhotic rats, and its concentrations in microdialysate in untreated rats were substantially lower than those in the liver treated with levosimendan. Cerebral blood glucose is an important marker, in fact a low systemic glucose level can cause cerebral hypoglycemia and therefore secondary brain damage.
The lactate/pyruvate ratio is a well-known marker of mitochondrial dysfunction. An increase in this ratio, as found in our results, is a sign of metabolic crises, most likely caused by mitochondrial dysfunction. The lactate/pyruvate ratio should be used in conjunction with glycerol values as a marker of cell membrane damage.
In our study, BDL rats had a higher value of lactate concentration in comparison with control rats. There was a significant decrease in microdialysate lactate concentrations in levosimendan-treated BDL rats. An increase in cerebral lactate is also observed in patients and animals with ammonia-precipitated hepatic encephalopathy [
4]. Increased cerebral lactate is associated with progression and severity of encephalopathy [
4]. Increased brain lactate concentration may also arise due to hyperlactatemia or increased glycolysis as well as increased lactate production and release or decreased lactate uptake by energy-deprived neurons. One or all of the three pathophysiological mechanisms could be implicated in the development of hepatic encephalopathy. Finally, an increase in cerebral lactate could be a marker but also a cause in the pathogenesis of cirrhotic encephalopathy.