1. Introduction
Oxidative stress of cells and tissues causes aging in human beings. Several oxidizing agents are produced by endogenous and exogenous processes. Those agents are called Reactive Oxygen Species (ROS). Oxidizing stress and aging are related to several diseases, such as diabetes, cancer, cardiovascular illnesses, and others [1]. The antioxidant compounds eliminate the free radicals of ROS, through hydrogen atom transfer (HAT), single electron transfer (SET), or chelation using transition metals [2]. Those compounds can be sorted in two classes: radicals and non-radicals.
Phenolic compounds, carotenoids, and vitamins are some metabolites from fruits and vegetables responsible for antioxidant capacity. [3]. Roselle Hibiscus is a tropical plant also known as rozelle, sorrel, red sorrel, Jamaican sorrel, Indian sorrel, Guinea sorrel, sour-sour, queensland jelly plant, jelly okra, lemon bush, and Florida cranberry [4]. Water-based roselle calyxes extracts are used worldwide to prepare beverages of good taste and antioxidant properties [5]. The antioxidant properties are related to the content of organic acids, flavonoids, and phenolic acids [6,7,8]. Roselle extracts show functional properties and are interesting for nutraceutical product development. Those bioactive products can reduce the risk for some illnesses and improve some organ functions and overall health. Roselle is cytocompatible and it can even replace dyes for histological staining [9].
Previous studies have evaluated different solvents [10], including water [11]. Also, different solid/solvent ratios are reported from 1-10 [12,13] to 100 or greater [14]. Most previous articles had focused on extraction time and stability of molecules [15]anthocyanin yield [16], or some specific bioactivities such as enzymatic inhibition [17] In this work we are optimizing the antioxidant capacity. A Box-Behnken Design is used for such a purpose. This information is useful to reduce cost and time, and to obtain more functional foods. This work aims to optimize the antioxidant activity of extracts from H. sabdariffa calyxes in order to produce nutraceutical products.
2. Results and discussion
Solid-liquid extraction is a separation process used for transferring solutes from a solid matrix to a solvent. This technique is used to obtain bioactive compounds from plants. The efficiency of solid-liquid extraction is related to many factors such as temperature, solvent composition, stirring speed, solid-liquid rate, time, particle size, pH, and others. Four variables shown in
Table 1 were selected based on a bibliographic review [18,19,20,21,22].
BBD was utilized to find the best extraction conditions for hibiscus roselle, in order to optimize the antioxidant capacity. The experimental values obtained in this work were used to obtain the second-order empirical coefficients for each variable. Only significant coefficients (P-value <0.05) for both the variables and the interactions were included in the model. Eq 1 describes the overall polynomial model explaining the antioxidant capacity in terms of ethanol:water, temperature (°C), time (min), and Solid/Solvent ratio, described as X1, X2, X3, and X4, respectively.
Results of ANOVA test can be found in
Table 2 P-value, and F-value for the regression (Eq. 1) were < 0,0001 and 36,190, respectively.
Analysis of variance was realized for the adjusted model for the antioxidant capacity of Hibiscus extracts. The determination coefficient (R2) of the model is 0.940. The R2 value confirms the regression model explains well the actual behavior of the system [23]. The adjusted R2 is 0.9241. Although it is smaller than the regular determination coefficient, both values are very close to each other. It means the values predicted by the model are a good representation of the experimental results [24].
A lack-of-fit test showed a P-value of 0.108. It is higher than 0.05, and it means there is no evidence of a lack of fit. The model is an appropriate representation of the relationship between the experimental factors and the response variable [23].
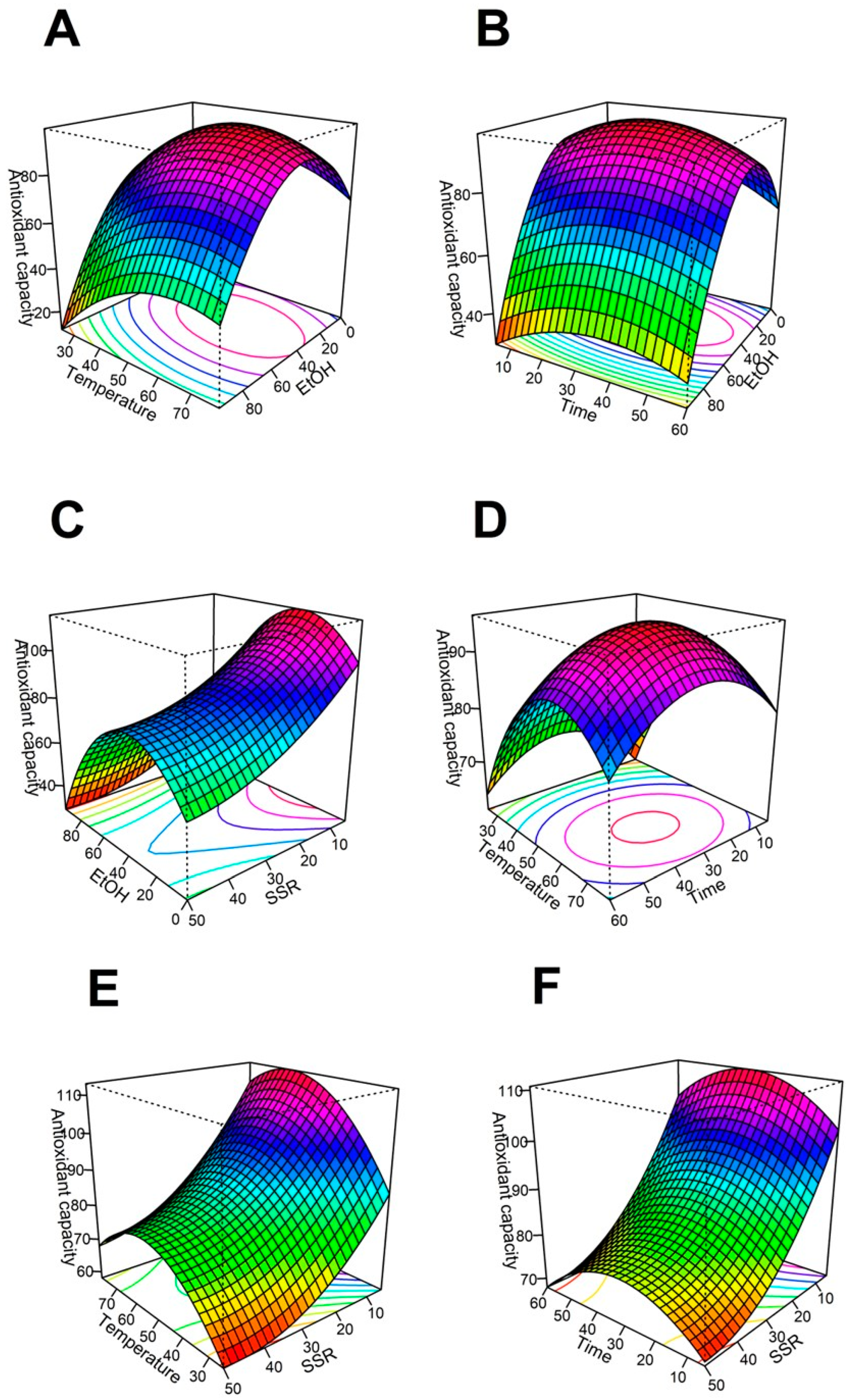
The regression model predicts the effect of the four variables on the antioxidant capacity after the extraction process. The relationship between dependent and independent variables is illustrated through the surface 3D graphs generated from the model (
Figure 1). The optimal points from the 3D graphs are the highest antioxidant capacity from the subset of conditions considered within the graph.
2.1. Effect of the ethanol content on the antioxidant capacity
(
Figure 1(A), 1(B), &12(C)) show the effect of increasing the ethanol content of the solvent on the extraction, respecting the solid:solvent ratio, the temperature, and the time, respectively. For either (
Figure 1(A), 1(B), or 1(C)), the extraction efficiency of antioxidants starts growing when the ethanol concentration in the solvent increases from 0 to 34.5% ethanol. However, the antioxidant extraction decreases its efficiency when the ethanol concentration increases by more than 34.5% in the extraction solvent. When the ethanol:water ratio is fixed at 34.5:65.5, the maximum antioxidant capacity is reached at a low solid solvent ratio, intermediate values of temperature, and intermediate values of time.
Figure 1.
Surface-response analysis of the effect of EtOH (%), Temperature (°C), Time (min), and SSR (g/500 mL) on the antioxidant capacity (µmol TE/gDM). (A) SSR vs EtOH, (B) EtOH vs temperature, (C) EtOH vs Time, (D) Time vs SSR, (E) Temperature vs SSR, & (F) Temperature vs Time. Abbreviations: TE Trolox equivalents, DM dry mass, SSR: solid solvent ratio, EtOH (mass % ethanol in solvent, the remaining percent corresponds to water).
Figure 1.
Surface-response analysis of the effect of EtOH (%), Temperature (°C), Time (min), and SSR (g/500 mL) on the antioxidant capacity (µmol TE/gDM). (A) SSR vs EtOH, (B) EtOH vs temperature, (C) EtOH vs Time, (D) Time vs SSR, (E) Temperature vs SSR, & (F) Temperature vs Time. Abbreviations: TE Trolox equivalents, DM dry mass, SSR: solid solvent ratio, EtOH (mass % ethanol in solvent, the remaining percent corresponds to water).
Low antioxidant capacity of the extracts is obtained when the ethanol concentration is high in the extractant phase [25]. These results may confirm the high efficiency of water:ethanol as a solvent to evaluate the antioxidant capacity of
H. Sabdariffa. Antioxidants found in literature for hibiscus aqueous-ethanolic extracts are: organic acids, phenolic acids, flavonoids, and anthocyanins [14]. Those compounds are soluble in hydroalcoholic mixtures containing equal amounts of ethanol and water, but their solubility decreases when ethanol concentration is near the azeotrope. In our results, the best antioxidant capacity is obtained when the solvent is composed of a mixture of water and organic solvent (
Table 1). The combination of those solvents dissolves a wide range of phenolic compounds [26]. The dipoles from phenolic compounds (such as delphinidin-3-O-sambubioside, and cyanidine-3-O-sambubioside) interact with the dipoles from ethanol and water, yielding a higher extraction rate [27].
The optimal ethanol concentration in the solvent is 34.5%. There is no additional improvement in antioxidant capacity when the ethanol concentration increases above 34.5%. This behavior is due to the average affinity of the antioxidant compounds. Nonetheless, a higher water concentration could promote the degradation of anthocyanidins [28] because Flavylium cation stability. This ion is the predominant form of the anthocyanins in acidic medium. Flavylium ion is susceptible to nucleophilic attack of water, and after reacting, it generates a pseudo hemiketal with reduced antioxidant capacity [29].
2.2. Effect of extraction temperature on the antioxidant capacity
Temperature is an important parameter in the extraction of Hibiscus. (
Figure 1 B, E, and F) contain the effects of the temperature, solid-solvent ratio, and time on the antioxidant capacity of the extract. The highest antioxidant capacity is reached at 60°C, combined with low solid/solvent ratios and medium values for % ethanol in solvent and time. Similar behavior was reported [30]. They found that the total phenolics and the antioxidant capacity of the extract decays when the temperature was increased to 90°C. The high temperatures can decompose or modify the thermosensitive bioactive compounds, such as polyphenols and other antioxidants [31]. Also, very high temperatures can accelerate the co-extraction of other non-active components such as sugars and fiber. Then, the antioxidant concentration in the extract would decrease [32].
A low concentration is also inconvenient in order to obtain an antioxidant-rich extract. The cell wall from hibiscus is weakened when the temperature is increased. Then, the cellular components, and the chemical compounds have more interaction with the solvents. [33]. Results from (
Figure 1B, E, & F) show that the best temperature for extraction of antioxidants from Hibiscus is 60°C. Commonly, Hibiscus calyxes are boiled to obtain infusions. Although our results suggest Hibiscus should not be boiled because that process decreases its quality.
2.3. Effect of extraction time on the antioxidant capacity
(Figure 1 C, D, and F) show the effect of the time on the antioxidant capacity, respecting the % of ethanol in the solvent (
Figure 1C), the solid/solvent ratio (
Figure 1D), and the temperature (
Figure 1F). There are no significant differences in the antioxidant activity when the time changes between the limits included in this study. However, prolonged extraction times at high temperatures can decompose and oxidize phenolic compounds, and consequently, the antioxidant capacity is reduced [34].
The extract with the best antioxidant capacity was reached when the extraction temperature was kept at 60°C for 33 min. According to Ramírez et al. [20], the concentration of polyphenolic compounds increases with the extraction time, at the appropriate temperature. The compounds need time to migrate to the solvent [34].
2.4. Effect of the solid/solvent ratio on the antioxidant capacity
The solid/solvent ratio is an important parameter during the extraction process. The solid is the mass of powdered calyxes from H. sabdariffa, and the solvent is the hydroalcoholic mixture. (
Figure 1A, D, & E) show the surface response graph for 500 mL total volume and variable solid/solvent ratio. For example, 5g of hibiscus extracted with 500 mL of solvent represents a 1/100 ratio. The maximum antioxidant capacity was reached at this condition (1/100 solid/solvent). The antioxidant activity increases with the decrease in the solid/solvent ratio until it reaches the optimal value. Similar results were seen by Tan et al. [35]. A greater concentration gradient increases the diffusion rate when the solvent amount increases, according to the mass transfer principle. This is consistent with the principle of mass transfer [36]. The trend can be reversed at some point by the dilution rate. Although this behavior is not observed within the conditions included in this study.
2.5. Contribution of total polyphenolic compounds (TPC) and anthocyanins (ACs) to antioxidant activity
The two main ACs (delphinidin -3-O-sambubioside and cyanidine-3-O-sambubioside), and the TPC were determined for each extract. The ACs selected were found as main components of Hibiscus aqueous extracts by Segura et al. [37].
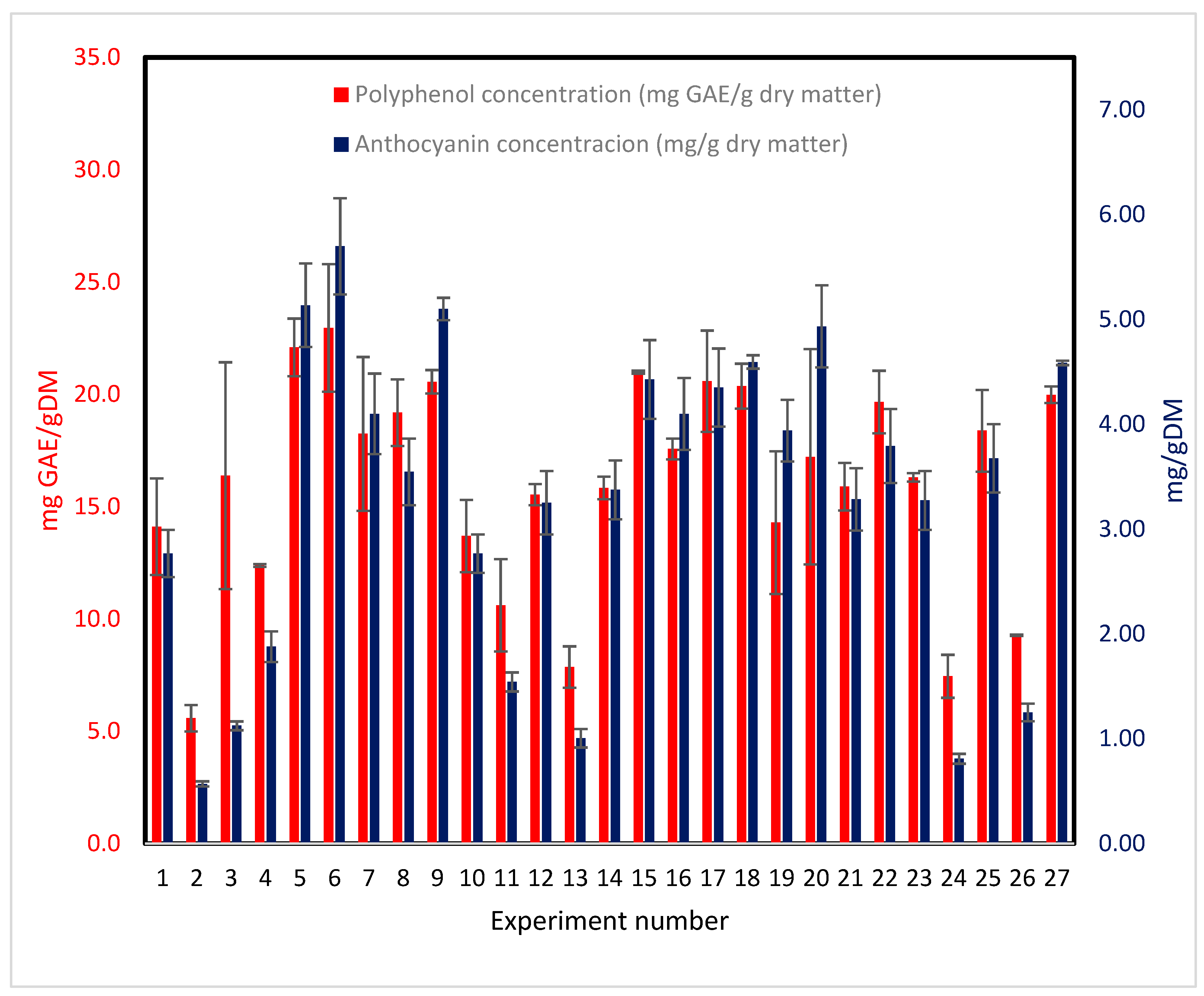
According to Ramírez et al. [20], ACs handle 51% of antioxidant capacity. (
Figure 2) shows TPC and ACs for the 26 experiments included in the BBD. TPC values are higher than ACs as expected. Although both TPC and ACs follow the same trend, both are related to antioxidant capacity. Also, the experiment runs showing lower content of both TPC and ACs were extracted with 95% ethanol. It means that ethanol is not a good solvent for extracting phenolic compounds.
Figure 2.
Total polyphenolic compounds and anthocyanins for individual extraction experiments from H. sabdariffa. Error bars represent standard deviation. Tuckey test results for the 27 experiments is shown in Supplementary Tables S1 (TPC) and S2 (PAC).
Figure 2.
Total polyphenolic compounds and anthocyanins for individual extraction experiments from H. sabdariffa. Error bars represent standard deviation. Tuckey test results for the 27 experiments is shown in Supplementary Tables S1 (TPC) and S2 (PAC).
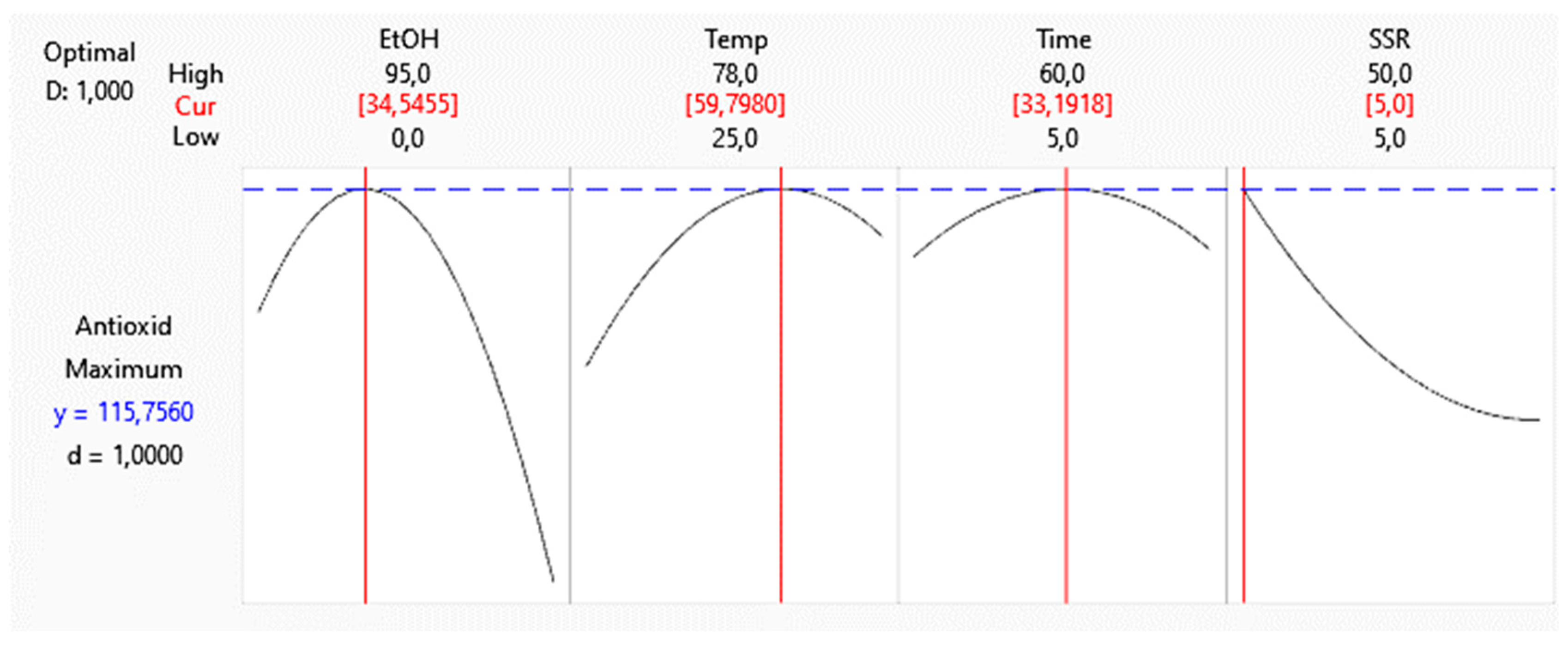
Figure 3.
Optimized variables of the extraction of antioxidant compounds from Hibiscus sabdariffa calyxes.
Figure 3.
Optimized variables of the extraction of antioxidant compounds from Hibiscus sabdariffa calyxes.
2.6. Study of a formula prepared using the optimized Hibiscus extract as a base component
Nutraceutical products can prevent or treat several diseases. Nutraceutical intake is recommended as complementary or alternative treatments [38]. Hibiscus is commercially utilized to prepare hot beverages, mainly. For this reason, in this work, a bioactive nutraceutical product was formulated using Hibiscus extract. When the extract is obtained using the optimized conditions, as shown in
Figure 3, it showed an antioxidant capacity of 103,36 µmol TE / g DM, with a prediction error of 10.71 % when compared to the prediction value obtained in the statistical analysis whose result 115,76 µmol TE / g DM is shown in
Figure 3. The hibiscus dried extract was dried by atomization and blended with a non-caloric sweetener and polydextrose. This procedure helps to preserve the stability of the bioactive compounds and requires less time than freeze-drying or vacuum-drying [39]. The drying process have some advantages: the powder is lighter, its volume is decreased, then it is easier to handle and transport. The spray-drying technique produces a microbiological and oxidative resistant powder. The method is simple, easily automatable, and fast. However, the atomization drying yield is 30% lower than freeze dryer, but the process still can be improved by optimizing some parameters such as the inlet temperature, the feeding flux, and the outlet temperature. The proximate analysis of the resulting powder shows 1.14 µg/g of total sugars, 3.81 Kcal/g, and 0.258 mg/g of sodium. There is not a standard daily consumption of antioxidants, however, the United States Department of Agriculture (USDA) recommends ingestion of 3000-5000 µmol TE [40].
A mass of 5 g of the previously prepared formula can be dissolved in 100 mL of water. The drink showed an antioxidant capacity of 495 µmol TE. Our antioxidant activities are close to the shown by other reference herbal infusions such as the green tea [41]. Although, most ready-to-drink formulas are supplemented with ascorbic acid and other antioxidant compounds [42]. The formula proposed in this study is just supplemented with stevia such as sweetener and polydextrose for flavor stabilization. The only source of antioxidants in the formulation proposed is the herbal extract.
Some of the advantages of a dry powder formula are the antioxidant activity is not affected by the preparation conditions [42], the humidity content is very low, the product is long time stable [43], and the phytochemical characteristics are preserved.
3. Materials and Methods
3.1. Plant material preparation and maceration
Dry whole roselle calyxes were purchased from Doña Rosa Food Products (San Isidro, Heredia, Costa Rica). The material was freeze-dried in a Freezone 2.5 Plus (from Labconco Corp., Kansas City, MO), and ground to 2 mm in a cutting mill SM100 (from Restsch GmbH, Germany). Samples were stored at room temperature.
A double jacket laboratory reactor model LR-2.ST (from IKA WERKE, Germany) was equipped with a circulating thermostat Ecoline E306 (from LAUDA, Germany) was used for the extraction procedure. The reaction flask was equipped with a glass condenser and deflectors. Mechanical agitation was kept at 100 rpm. Different ratios of the previously grounded material and solvent were tested. The reactor was filled with 500 mL of the selected solvent and the corresponding previously-ground roselle calyxes. Specific extraction times, temperature, solvent composition, and solid/solvent ratio are explained in the experimental design section. The extracts were directly used for further analyses.
3.2. Antioxidant activity determination
The antioxidant activity was measured by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) antiradical test. We followed the 96-well microplate protocol [44] 30 µL of each extracted sample was mixed with 270 µL of a 0,04 mg/mL DPPH solution in 80% methanol. After 20 min of incubation at room temperature, absorbance was measured at 515 nm in a microplate reader Synergy HT Multi-Mode (from BioTek Instruments, EUA). A standard curve ranging from 0 to 250 µmol/mL 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) was used. Sample antioxidant activity was reported as Trolox Equivalents per gram of Dry Mass (µmol TE/DM).
The ORAC procedure used an automated plate reader Synergy HT Multi-Mode (from BioTek Instruments, EUA) [45] Analyses were conducted in phosphate buffer pH 7,4. Peroxyl radical was generated using 2,2-azobis(2-amidino-propane) dihydrochloride which was prepared fresh for each run. The standard curve was linear between 0 and 125 µmol/L Trolox. Fluorescein was used as the substrate. Fluorescence conditions were as follows excitation at 485 nm at emission at 520 nm. Sample antioxidant activity was reported as Trolox Equivalents per gram of Dry Mass (µmol TE/DM).
3.3. Box-Behnken Design (BBD)
Box-Behnken design was used. It comprises 27 experiments with 3 central points, and 4 variables at three levels. Variables were the solvent composition (ethanol:water ratio), the temperature, the time, and the solid:solvent ratio (ground roselle mass and volume of ethanol/water).
Table 1 explains the levels for each variable selected. The response variable is the Antioxidant Activity determined by the DPPH method.
Table 3.
Independent variables and levels for Box Behnken Experimental Design.
Table 3.
Independent variables and levels for Box Behnken Experimental Design.
| Factor |
Symbol |
Level |
| -1 |
0 |
1 |
| Ethanol:water |
X1
|
5:95 |
50:50 |
0:100 |
| Temperature (°C) |
X2
|
25 |
50 |
78 |
| Time (min) |
X3
|
5 |
30 |
60 |
| Solid/Solvent ratio |
X4
|
1/100 |
1/50 |
1/10 |
3.4. Total Phenolic Content (TPC) determination
TPC was determined by employing colorimetry using the Folin-Ciocalteu method. We follow the 96-well microplate procedure developed by Sánchez-Rangel et al. (2013) [47], with minor modifications. 30 µL of the sample was mixed in a well with 200 µL of distilled water, 15 µL of Folin-Ciocalteu reagent, and 50 µL of 20% Na2CO3 solution. Then, the mixture was incubated for 20 min while mixing in a Synergy HT Multi-Detection Microplate Reader (BioTek Instruments) at 40 °C. Finally, absorbance was measured at 755 nm, against a standard curve of 0.000, 0.020, 0.040, 0.060, 0.080, and 0.120 mg/1 mL of gallic acid.
3.5. Identification and quantification of anthocyanins
Two anthocyanins (Delphinidin-3-O-sambubioside and cyanidin-3-O-sambubioside) were quantified using HPLC-DAD Ultimate 3000 (from Term Scientific Instruments, EUA). Those compounds were previously isolated from roselle and characterized by Segura et al. [37]. The separation was performed using a C18 Dionex Acclaim (250 x 4.0 mm, 5 µm) column. A constant solvent flux of 0.8 mL/min was utilized. A binary pump filled with aqueous 0.01% TFA (trifluoroacetic acid) and acetonitrile as mobile phase components were used. Initially, the pump was set to keep acetonitrile constant at 10% from time 0 to 8 min, then it was linearly increased to 50% (10 min) and finally, increased to 95% (13 min). The diluted TFA completes the remaining sambubioside and cyanidin-3-O-sambubioside (from Sigma-Aldrich, MO) as standard, and read at 530 nm. Chromatographs are shown in (
Figure S1).
3.6. Preparation and characterization of a nutraceutical drink using the optimized roselle extract as raw material
The optimized roselle extract was concentrated in a rotatory evaporator B-490, the concentrated extract was dried out into a Mini Spray Dryer B-290 (both from Büchi Corporation, Flawil, Switzerland). Finally, the powder of the most antioxidant extract was supplemented with stevia (as sweetener) and polydextrose (vehicle), in a mass ratio of 1:1:3 (extract:stevia:polydextrose).
The caloric content of the final product was determined using a bomb calorimeter model C 200 (from IKA WERKE, Germany), according to the protocol DIN 51900-1 (DIN, 2000). Total carbohydrates were determined by the colorimetric phenol-sulfuric acid method [49]. Sodium was determined by atomic absorption following the AOAC 963.09 procedure [50].
3.7. Statistical analysis
All samples were quantified in a duplicate, and the got results were expressed as mean ± standard deviation. Data processing and statistical analysis (mean value) were performed using Microsoft Excel 2019. Response surface design and statistical analysis of the model were performed through an ANOVA. Minitab 19 version was used for those purposes.
4. Conclusions
A viable method for extraction of H. sabdariffa calyxes was developed after optimization of multivariate experimental conditions. The optimal extraction conditions for antioxidant capacity were found after the development of a Box-Behnken experimental design.
The extraction method decreased the extraction time to 33 min, using 5g of sample, and 500 mL of 34.5% ethanol as solvent, at 60 °C. The method employs a reduced concentration of organic solvent and uses a renewable organic solvent. Thus, this method can be considered environmentally friendly.
Ethanol concentration in the solvent is considered the most important variable for the extraction of Hibiscus calyxes. The optimal ethanol concentration was found to be 34.5%. No significant improvement is observed in the extraction when ethanol concentration increases above that value. The reason is the basic principle of extraction, known as affinity.
Nutraceutical beverages can promote a healthy life and prevent diseases. These beverages are functional and low-caloric alternatives.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, G.R. and VA.; methodology VA and MV; software, MV AND P.J.; validation, M.J, P.J and O.A..; formal analysis, M.V.; investigation, G.R, V.A and P.J..; resources, G.R. and V.A..; data curation, M.J, V.A. and P.J.; writing—original draft preparation, M.J., P.J. and V.A.; writing—review and editing, M.J., P.J. and V.A.; visualization, M.J.; supervision, O.A.; project administration, V.A..; funding acquisition, G.R. and V.A. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: This research was funded by Universidad Nacional, grant number SIA263-18 and the publication was funded by “Fondo para apoyo a la divulgación del conocimiento generado en la UNA”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting reported results are available upon request, please contact victor.alvarez.valverde@una.ac.cr.
Acknowledgments
We thank Bach. Adrián Cerdas-Pereira for helping to proofread the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the extracts or plant material are available from the authors.
References
- Losada-Barreiro, S.; Bravo-Díaz, C. Free Radicals and Polyphenols: The Redox Chemistry of Neurodegenerative Diseases. Eur J Med Chem 2017, 133, 379–402. [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. Antioxidants 2019, 1–28. [CrossRef]
- Wong, S.P.; Leong, L.P.; William Koh, J.H. Antioxidant Activities of Aqueous Extracts of Selected Plants. Food Chem 2006, 99, 775–783. [CrossRef]
- Shruthi, V.H.; Ramachandra, C.T.; Nidoni, U.; Hiregoudar, S.; Naik, N.; Kurubar, A.R. Roselle (Hibiscus Sabdariffa L.) as a Source of Natural Colour : A Review. Plant Arch 2016, 16, 515–522.
- Patel, S. Hibiscus Sabdariffa: An Ideal yet under-Exploited Candidate for Nutraceutical Applications. Biomedicine and Preventive Nutrition 2014, 4, 23–27. [CrossRef]
- Badreldin, H.A.; Naser Al, W.; Gerald, B. Phytochemical, Pharmacological and Toxicological Aspects of Hibiscus Sabdariffa L.: A Review. Phytotherapy Research 2005, 19, 369–375. [CrossRef]
- Kao, E.S.; Yang, M.Y.; Hung, C.H.; Huang, C.N.; Wang, C.J. Polyphenolic Extract from Hibiscus Sabdariffa Reduces Body Fat by Inhibiting Hepatic Lipogenesis and Preadipocyte Adipogenesis. Food Funct 2016, 7, 171–182. [CrossRef]
- Ojulari, O.V.; Lee, S.G.; Nam, J.O. Beneficial Effects of Natural Bioactive Compounds from Hibiscus Sabdariffa L. On Obesity. Molecules 2019, 24, 1–14. [CrossRef]
- Alshamar, H.A.; Dapson, R.W. Use of Roselle Extracted from Hibiscus Sabdariffa for Histological Staining: A Critical Review and Rational Stain Formulation. Biotechnic and Histochemistry 2021, 96, 94–101. [CrossRef]
- Liu, J.Z.; Lyu, H.C.; Fu, Y.J.; Jiang, J.C.; Cui, Q. Simultaneous Extraction of Natural Organic Acid and Flavonoid Antioxidants from Hibiscus Manihot L. Flower by Tailor-Made Deep Eutectic Solvent. Lwt 2022, 163, 113533. [CrossRef]
- Afshari, K.; Samavati, V.; Shahidi, S.A. Ultrasonic-Assisted Extraction and in-Vitro Antioxidant Activity of Polysaccharide from Hibiscus Leaf. Int J Biol Macromol 2015, 74, 558–567. [CrossRef]
- Chumsri, P.; Sirichote, A.; Itharat, A. Studies on the Optimum Conditions for the Extraction and Concentration of Roselle (Hibiscus Sabdariffa Linn.) Extract. Songklanakarin Journal of Science and Technology 2008, 30, 133–139.
- Pozos, G.I.P.; Ruiz-López, M.A.; Nátera, J.F.Z.; Moya, C.Á.; Ramírez, L.B.; Silva, M.R.; Macías, R.R.; García-López, P.M.; Cruz, R.G.; Pérez, E.S.; et al. Antioxidant Capacity and Antigenotoxic Effect of Hibiscus Sabdariffa L. Extracts Obtained with Ultrasound-Assisted Extraction Process. Applied Sciences (Switzerland) 2020, 10. [CrossRef]
- Sindi, H.A.; Marshall, L.J.; Morgan, M.R.A. Comparative Chemical and Biochemical Analysis of Extracts of Hibiscus Sabdariffa. Food Chem 2014, 164, 23–29. [CrossRef]
- Tsai, P.J.; McIntosh, J.; Pearce, P.; Camden, B.; Jordan, B.R. Anthocyanin and Antioxidant Capacity in Roselle (Hibiscus Sabdariffa L.) Extract. Food Research International 2002, 35, 351–356. [CrossRef]
- Cissé, M.; Bohuon, P.; Sambe, F.; Kane, C.; Sakho, M.; Dornier, M. Aqueous Extraction of Anthocyanins from Hibiscus Sabdariffa: Experimental Kinetics and Modeling. J Food Eng 2012, 109, 16–21. [CrossRef]
- Ajiboye, T.O.; Salawu, N.A.; Yakubu, M.T.; Oladiji, A.T.; Akanji, M.A.; Okogun, J.I. Antioxidant and Drug Detoxification Potentials of Hibiscus Sabdariffa Anthocyanin Extract. Drug Chem Toxicol 2011, 34, 109–115. [CrossRef]
- Cid-Ortega, S.; Guerrero-Beltrán, J.A. Roselle Calyces (Hibiscus Sabdariffa), an Alternative to the Food and Beverages Industries: A Review. J Food Sci Technol 2015, 52, 6859–6869. [CrossRef]
- Shih, M.C.; Yang, K.T. ung; Kuo, S.T. Optimization Process of Black Soybean Natto Using Response Surface Methodology. J Food Sci 2009, 74, 294–301. [CrossRef]
- Ramirez-Rodrigues, M.M.; Plaza, M.L.; Azeredo, A.; Balaban, M.O.; Marshall, M.R. Physicochemical and Phytochemical Properties of Cold and Hot Water Extraction from Hibiscus Sabdariffa. J Food Sci 2011, 76, 429–435. [CrossRef]
- Prenesti, E.; Berto, S.; Daniele, P.G.; Toso, S. Antioxidant Power Quantification of Decoction and Cold Infusions of Hibiscus Sabdariffa Flowers. Food Chem 2007, 100, 433–438. [CrossRef]
- Cid-Ortega, S.; Guerrero-Beltrán, J.A.; Andzi Barhé, T.; Feuya Tchouya, G.R.; Sindi, H.A.; Marshall, L.J.; Morgan, M.R.A.; Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; et al. Phytochemistry, Antioxidant Capacity, Total Phenolic Content and Anti-Inflammatory Activity of Hibiscus Sabdariffa Leaves. Food Chem 2015, 77, 1055–1060. [CrossRef]
- Montgomery, D. Design and Analysis of Experiments; Ninth.; WILEY: EEUU, 2020; ISBN 978-1-119-63842-1.
- Elik, A. Response Surface Methodology Based on Central Composite Design for Optimizing Temperature-Controlled Ionic Liquid-Based Microextraction for the Determination of Histamine Residual in Canned Fish Products. Journal of Food Composition and Analysis 2021, 98, 1–10. [CrossRef]
- Sáyago-Ayerdi, S.G.; Arranz, S.; Serrano, J.; Goñi, I. Dietary Fiber Content and Associated Antioxidant Compounds in Roselle Flower (Hibiscus Sabdariffa L.) Beverage. J Agric Food Chem 2007, 55, 7886–7890. [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of Extraction Solvent on Total Phenol Content, Total Flavonoid Content, and Antioxidant Activity of Limnophila Aromatica. J Food Drug Anal 2014, 22, 296–302. [CrossRef]
- Kechinski, C.P.; Guimarães, P.V.R.; Noreña, C.P.Z.; Tessaro, I.C.; Marczak, L.D.F. Degradation Kinetics of Anthocyanin in Blueberry Juice during Thermal Treatment. J Food Sci 2010, 75, 173–176. [CrossRef]
- Moreno, J.; Peinado, R. Polyphenols. In Enological Chemistry; 2012; pp. 53–76 ISBN 9780123884381.
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25. [CrossRef]
- Singh, M.; Thrimawithana, T.; Shukla, R.; Adhikari, B. Extraction and Characterization of Polyphenolic Compounds and Potassium Hydroxycitrate from Hibiscus Sabdariffa. Future Foods 2021, 4, 1–09. [CrossRef]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Microwave-Assisted Extraction for Hibiscus Sabdariffa Bioactive Compounds. J Pharm Biomed Anal 2018, 156, 313–322. [CrossRef]
- Singh, M.; Thrimawithana, T.; Shukla, R.; Adhikari, B. Extraction and Characterization of Polyphenolic Compounds and Potassium Hydroxycitrate from Hibiscus Sabdariffa. Future Foods 2021, 4, 1–09. [CrossRef]
- Roselló-Soto, E.; Martí-Quijal, F.J.; Cilla, A.; Munekata, P.E.S.; Lorenzo, J.M.; Remize, F.; Barba, F.J. Influence of Temperature, Solvent and PH on the Selective Extraction of Phenolic Compounds from Tiger Nuts by-Products: Triple-TOF-LC-MS-MS Characterization. Molecules 2019, 24. [CrossRef]
- Che Sulaiman, I.S.; Basri, M.; Fard Masoumi, H.R.; Chee, W.J.; Ashari, S.E.; Ismail, M. Effects of Temperature, Time, and Solvent Ratio on the Extraction of Phenolic Compounds and the Anti-Radical Activity of Clinacanthus Nutans Lindau Leaves by Response Surface Methodology. Chem Cent J 2017, 11, 1–11. [CrossRef]
- Tan, P.W.; Tan, C.P.; Ho, C.W. Antioxidant Properties: Effects of Solid-to-Solvent Ratio on Antioxidant Compounds and Capacities of Pegaga (Centella Asiatica). Int Food Res J 2011, 18, 557–562.
- Wong; Tan, C.P.; Ho, * Effect of Solid-to-Solvent Ratio on Phenolic Content and Antioxidant Capacities of “Dukung Anak” (Phyllanthus Niruri); 2013; Vol. 20.
- Segura-Carretero, A.; Puertas-Mejía, M.A.; Cortacero-Ramírez, S.; Beltrán, R.; Alonso-Villaverde, C.; Joven, J.; Dinelli, G.; Fernández-Gutiérrez, A. Selective Extraction, Separation, and Identification of Anthocyanins from Hibiscus Sabdariffa L. Using Solid Phase Extraction-Capillary Electrophoresis-Mass Spectrometry (Time-of-Flight/Ion Trap). Electrophoresis 2008, 29, 2852–2861. [CrossRef]
- Braithwaite, M.C.; Tyagi, C.; Tomar, L.K.; Kumar, P.; Choonara, Y.E.; Pillay, V. Nutraceutical-Based Therapeutics and Formulation Strategies Augmenting Their Efficiency to Complement Modern Medicine: An Overview. J Funct Foods 2014, 6, 82–99. [CrossRef]
- Gunjal, S.D. An Overview of Process Parameters and Spray Drying Agents Involved in Spray Drying of Herbal Extracts. Paideuma Journal 2020, XIII, 102–118.
- USDA Antioxidants and Health. URL: https://www.ars.usda.gov/news-events/news/research-news/2007/data-on-food-antioxidants-aid-research/ . (accessed on 24 03 2022).
- Kodama, D.H.; Gonçalves, A.E. de S.S.; Lajolo, F.M.; Genovese, M.I. Flavonoids, Total Phenolics and Antioxidant Capacity: Comparison between Commercial Green Tea Preparations. Ciência e Tecnologia de Alimentos 2010, 30, 1077–1082. [CrossRef]
- Prior, R.L.; Cao, G. Antioxidant Capacity and Polyphenolic Components of Teas: Implications for Altering In Vivo Antioxidant Status. Proceedings of the Society for Experimental Biology and Medicine 1999, 220, 255–261. [CrossRef]
- Intipunya, P.; Bhandari, B.R. Chemical Deterioration and Physical Instability of Food Powders; Woodhead Publishing Limited, 2010; ISBN 9781845694951.
- Vega-López, B.; Carvajal-Miranda, Y.; Brenes-Peralta, L.; Gamboa-Murillo, M.; Venegas-Padilla, J.; Rodríguez, G.; Jiménez-Bonilla, P.; Álvarez-Valverde, V. Phytonutraceutical Evaluation of Five Varieties of Tomato (Solanum Lycopersicum) during Ripening and Processing. Lwt 2022, 164, 113592. [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity (ORACFL)) of Plasma and Other Biological and Food Samples. J Agric Food Chem 2003, 51, 3273–3279. [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. The Folin-Ciocalteu Assay Revisited: Improvement of Its Specificity for Total Phenolic Content Determination. Analytical Methods 2013, 3, 1–10. [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. The Folin-Ciocalteu Assay Revisited: Improvement of Its Specificity for Total Phenolic Content Determination. Analytical Methods 2013, 3, 1–10. [CrossRef]
- DIN (German Institute for Standardization) Determining the Gross Calorific Value of Solid and Liquid Fuels Using the Bomb Calorimeter, and Calculation of Net Calorific Value - Part 1. General Information. DIN 51900-1. German Institute for Standardization. 2000.
- Nielsen, S. Food Analysis Laboratory Manual- Ash Content Determination. In Food Analysis Laboratory Manual; 2017; pp. 117–119 ISBN 978-3-319-44125-2.
- AOAC Official Methods of Analysis of the Association of Analytical Chemists International. Association of Analytical Chemists International 2000.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).