Submitted:
22 February 2023
Posted:
24 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Epidemiological Association of OSA with Gout
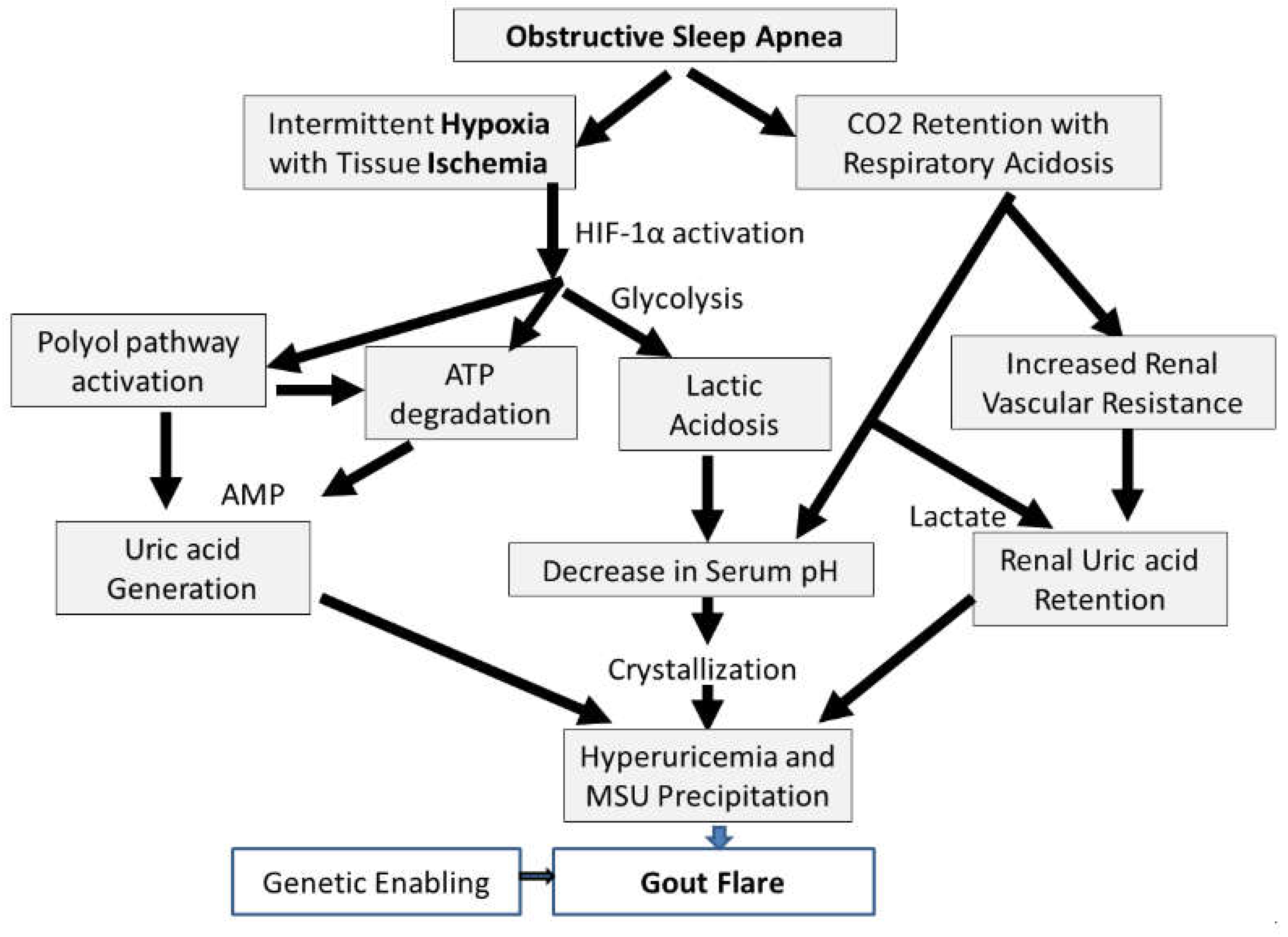
3. How is OSA Connected with Gout?: Understanding the Pathophysiology
4. Does Treating OSA Provide Another Means for Controlling Gout and Its Comorbidities?
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalbeth N, Choi HK, Terkeltaub R. Review: Gout: A Roadmap to Approaches for Improving Global Outcomes. Arthritis Rheumatol 2017;69(1):22-34. [CrossRef]
- Major TJ, Dalbeth N, Stahl EA, Merriman TR. An update on the genetics of hyperuricaemia and gout. Nat Rev Rheumatol 2018;14(6):341-353. [CrossRef]
- Choi HK. Diet, alcohol, and gout: how do we advise patients given recent developments? Curr Rheumatol Rep 2005;7(3):220-6. (In eng) (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15918999). [CrossRef]
- Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004;350(11):1093-103. (In eng) (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15014182). [CrossRef]
- Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ 2008;336(7639):309-12. (In eng) (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18244959). [CrossRef]
- Juraschek SP, Kovell LC, Miller ER, 3rd, Gelber AC. Association of kidney disease with prevalent gout in the United States in 1988-1994 and 2007-2010. Semin Arthritis Rheum 2013 (In Eng). DOI: S0049-0172(12)00266-1 [pii]. [CrossRef]
- Quinones GA, Natali A, Baldi S, et al. Effect of insulin on uric acid excretion in humans. Am J Physiol 1995;268(1 Pt 1):E1-5. (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7840165). [CrossRef]
- Johnson RJ, Nakagawa T, Sanchez-Lozada LG, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013;62(10):3307-15. (In eng). DOI: 62/10/3307 [pii]. [CrossRef]
- Blagojevic-Bucknall M, Mallen C, Muller S, et al. The Risk of Gout Among Patients With Sleep Apnea: A Matched Cohort Study. Arthritis Rheumatol 2019;71(1):154-160. [CrossRef]
- Roddy E, Muller S, Hayward R, Mallen CD. The association of gout with sleep disorders: a cross-sectional study in primary care. BMC Musculoskelet Disord 2013;14:119. [CrossRef]
- Singh JA. Self-reported sleep quality and sleep disorders in people with physician-diagnosed gout: an Internet cross-sectional survey. Arthritis Res Ther 2019;21(1):36. [CrossRef]
- Singh JA, Cleveland JD. Gout and the Risk of Incident Obstructive Sleep Apnea in Adults 65 Years or Older: An Observational Study. J Clin Sleep Med 2018;14(9):1521-1527. [CrossRef]
- Zhang Y, Peloquin CE, Dubreuil M, et al. Sleep Apnea and the Risk of Incident Gout: A Population-Based, Body Mass Index-Matched Cohort Study. Arthritis Rheumatol 2015;67(12):3298-302. [CrossRef]
- Kanbay A, Inonu H, Solak Y, et al. Uric acid as a potential mediator of cardiovascular morbidity in obstructive sleep apnea syndrome. Eur J Intern Med 2014;25(5):471-6. [CrossRef]
- Cantalejo Moreira M, Veiga Cabello RM, Garcia Diaz V, Racionero Casero MA, Zapatero Gaviria A. Gout, hyperuricaemia, sleep apnoea-hypopnoea syndrome and vascular risk. Rheumatology (Oxford) 2013;52(9):1619-22. [CrossRef]
- Edinger JD, Ulmer CS, Means MK. Sensitivity and specificity of polysomnographic criteria for defining insomnia. J Clin Sleep Med 2013;9(5):481-91. [CrossRef]
- Hasday JD, Grum CM. Nocturnal increase of urinary uric acid:creatinine ratio. A biochemical correlate of sleep-associated hypoxemia. Am Rev Respir Dis 1987;135(3):534-8. [CrossRef]
- Gabryelska A, Szmyd B, Szemraj J, Stawski R, Sochal M, Bialasiewicz P. Patients with obstructive sleep apnea present with chronic upregulation of serum HIF-1alpha protein. J Clin Sleep Med 2020;16(10):1761-1768. [CrossRef]
- Kanbay M, Altintas A, Yavuz F, et al. Responses to Hypoxia: How Fructose Metabolism and Hypoxia-Inducible Factor-1a Pathways Converge in Health and Disease. Curr Nutr Rep 2023. [CrossRef]
- Mirtschink P, Krek W. Hypoxia-driven glycolytic and fructolytic metabolic programs: Pivotal to hypertrophic heart disease. Biochim Biophys Acta 2016;1863(7 Pt B):1822-8. [CrossRef]
- Mirtschink P, Krishnan J, Grimm F, et al. HIF-driven SF3B1 induces KHK-C to enforce fructolysis and heart disease. Nature 2015;522(7557):444-9. [CrossRef]
- Nakagawa T, Lanaspa MA, Millan IS, et al. Fructose contributes to the Warburg effect for cancer growth. Cancer Metab 2020;8:16. [CrossRef]
- Nakagawa T, Sanchez-Lozada LG, Andres-Hernando A, et al. Endogenous Fructose Metabolism Could Explain the Warburg Effect and the Protection of SGLT2 Inhibitors in Chronic Kidney Disease. Front Immunol 2021;12:694457. [CrossRef]
- Zheng L, Zhang Z, Sheng P, Mobasheri A. The role of metabolism in chondrocyte dysfunction and progression of osteoarthritis. Ageing Research Reviews 2020; 66: 101249. [CrossRef]
- Wilcox WR, Khalaf AA. Nucleation of monosodium urate crystals. Ann. Rheum Dis. 1975; 34: 332-339. [CrossRef]
- Renaudin F, Orliaquet L, Castelli F, et al. Gout and pseudo-gout-related crystals promote GLUT1-mediated glycolysis that governs NLRP3 and interleukin-1beta activation on macrophages. Ann. Rheum Dis 2020; 0: 1-9. [CrossRef]
- Shivaram U, Cash ME, Beal A. Nasal continuous positive airway pressure in decompensated hypercapnic respiratory failure as a complication of sleep apnea. Chest 1993;104(3):770-4. [CrossRef]
- Lin T, Huang JF, Lin QC, et al. The effect of CPAP treatment on venous lactate and arterial blood gas among obstructive sleep apnea syndrome patients. Sleep Breath 2017;21(2):303-309. [CrossRef]
- Nakanishi T, Ohya K, Shimada S, Anzai N, Tamai I. Functional cooperation of URAT1 (SLC22A12) and URATv1 (SLC2A9) in renal reabsorption of urate. Nephrol Dial Transplant 2013;28(3):603-11. [CrossRef]
- Chapman CL, Schlader ZJ, Reed EL, Worley ML, Johnson BD. Renal and segmental artery hemodynamic response to acute, mild hypercapnia. Am J Physiol Regul Integr Comp Physiol 2020;318(4):R822-R827. [CrossRef]
- Choi HK, Niu J, Neogi T, et al. Nocturnal risk of gout attacks. Arthritis Rheumatol 2015;67(2):555-62. [CrossRef]
- Clifford AJ, Riumallo JA, Youn VR, Scrimshaw NS. Effect of Oral Purines on Serum and Urinary Uric Acid of Normal, Hyperuricemic and Gouty Humans. J Nutr 1976;106:428-450. [CrossRef]
- Huang CF, Liu JC, Huang HC, et al. Longitudinal transition trajectory of gouty arthritis and its comorbidities: a population-based study. Rheumatol Int. 2017 Feb; 37(2):313-22. [CrossRef]
- Chiang CL, Chen YT, Wang KL, et al. Comorbidities and risk of mortality in patients with sleep apnea. Ann Med. 2017 Aug; 49(5): 377-83. [CrossRef]
- Abrams B. Update on reversibility of obstructive sleep apnea consequences. Med Res Arch. 2020 Apr 8(4). [CrossRef]
- Kinebuchi S, Kazama JJ, Satoh M, et al. Short-term use of continuous positive airway pressure ameliorates glomerular hyperfiltration in patients with obstructive sleep apneoa syndrome. Clin Sci (Lond). 2002 Sep; 107(3):317-22. [CrossRef]
- Koga S, Ikeda S, Yasunaga T, et al. Effects of nasal continuous positive airway pressure on the glomerular filtration rate in patients with obstructive sleep apnea syndrome. Intern Med. 2013 Mar; 52(3):345-9. [CrossRef]
- Yoshihisa A, Takeishi Y. Sleep disordered breathing and cardiovascular disease. J Atheroscler Thromb. 2019 Apr; 26(4): 315-27. [CrossRef]
- Mandal S, Kent BD. Obstructive sleep apnea and coronary artery disease. J Thorac Dis. 2018 Dec; 10(Suppl 34):S4212-20. [CrossRef]
- Geovanini GR, Lorenzi-Filko G. Cardiac rhythm disorders in obstructive sleep apnea. J Thorac Dis. 2018 Dec; 10(Suppl 34):S4221-30. [CrossRef]
- Kim D, Shim CY, Cho YJ, et al. Continuous positive airway pressure restores cardiac mechanical function in patients with severe obstructive sleep apnea: A randomized sham-controlled study. J An Soc Endocardiogr. 2019 Jul; 32(7):826-35. [CrossRef]
- Li X, Liu C, Zhang H, et al. Effect of 12-month nasal continuous positive airway pressure therapy for obstructive sleep apnea on progression of chronic kidney disease. Medicine (Baltimore). 2019 Feb; 98(8):e14545. [CrossRef]
| Ref # |
Description of Study | Number of Participants | Results | Risk Ratio |
|---|---|---|---|---|
| 9 | Data from UK Clinical Practice Research Datalink. Comparison of gout incidence rate in cohort diagnosed with OSA vs. cohort (matched by age, sex, practice) never diagnosed with OSA. 5.8 yrs. median follow-up. |
15,878 diagnosed with OSA. 63,296 never diagnosed with OSA* |
OSA: 4.9% with gout. Incidence rate 7.83/KPyr No OSA: 2.6% with gout. Incidence rate 4.03/KPyr |
1.9 |
| 10 | Data from UK general practice data base. Comparison of gout prevalence in those diagnosed with OSA vs. those never diagnosed with OSA. |
1,689 diagnosed with OSA. 6,756 never diagnosed with OSA* |
OSA: 0.7% No OSA: 0.3% |
2.3 |
| 11 | Internet questioning about sleep disorders of people with physician-diagnosed gout who visited a gout education website. |
454 | 320 with gout. 77 with diagnosed OSA. |
N/A |
| 12 | Data from 5% US Medicare beneficiary sample 2006-2012. Selected entries with new diagnosis of OSA. |
1.74 M, follow-up of 10,448,472 person-years* |
Incidence rates with OSA: 14.3/KPyr No OSA: 3.9/KPyr |
3.7 |
| 13 | Data from UK Health Improvement Network.Comparison of gout incidence rate with first SA diagnosis vs. cohort (matched by age, sex, BMI) never diagnosed with SA. |
9,865 with first SA diagnosis. 43,598 never diagnosed with SA* |
Incidence rates with SA: 8.4/KPyr No SA: 4.8/KPyr |
1.75 |
| 14 | All participants tested for OSA, and questioned about history of cardiovascular disease. |
72 controls (AHI<5) 47 mild (5<AHI<15) 75 moderate OSA (15<AHI<30) 192 severe (AHI>30) |
Serum uric acid data show monotonic increase with AHI in those who had a cardiovascular event. |
N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




