Submitted:
25 February 2023
Posted:
27 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
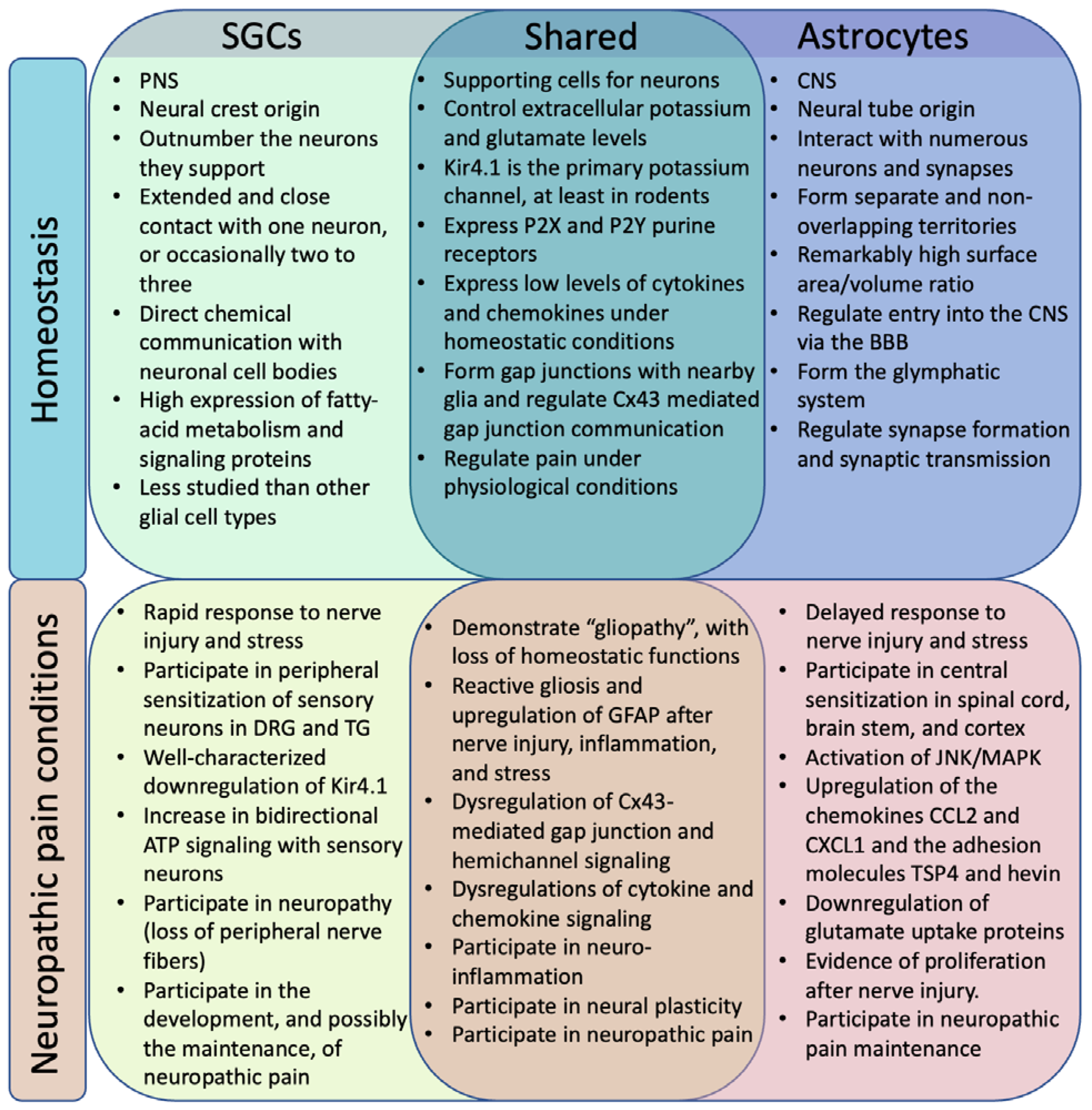
2. SGCs and Astrocytes in Homeostasis
2.1. Location
2.2. Development
2.3. Morphology

2.4. Diversity
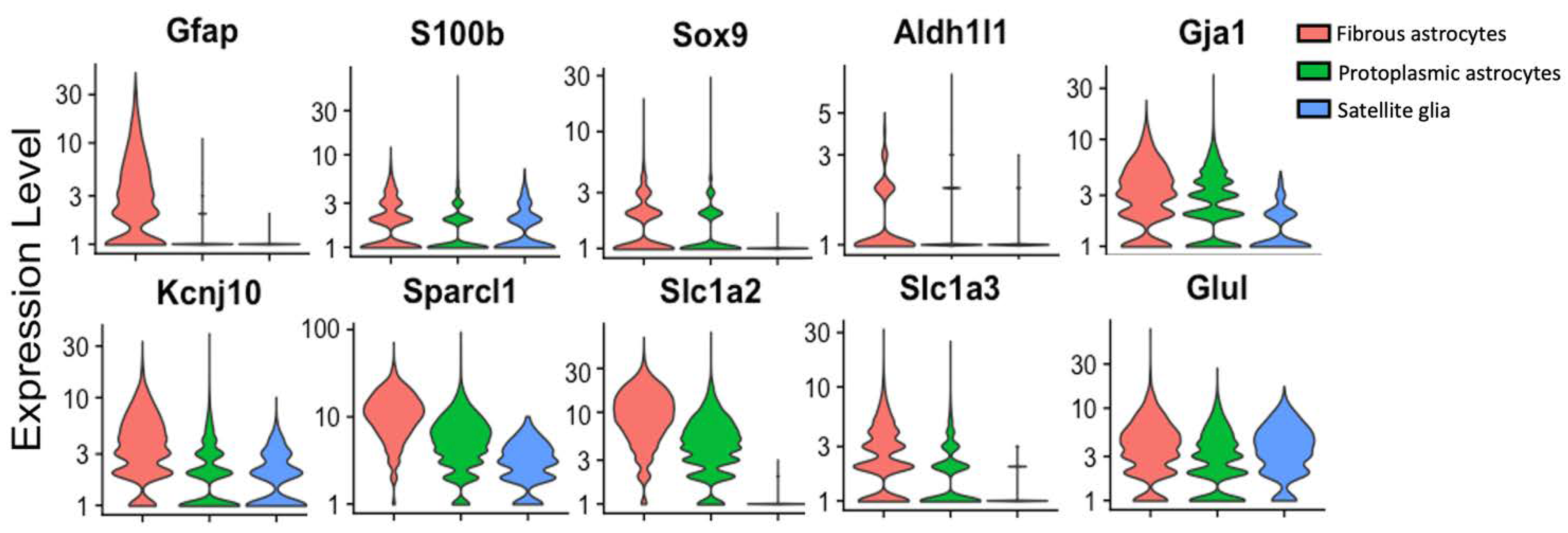
2.5. Common non-GFAP Markers
3. SGCs and Astrocytes in Neuropathic Pain
3.1. GFAP
3.2. Reactive Gliosis: Beyond GFAP
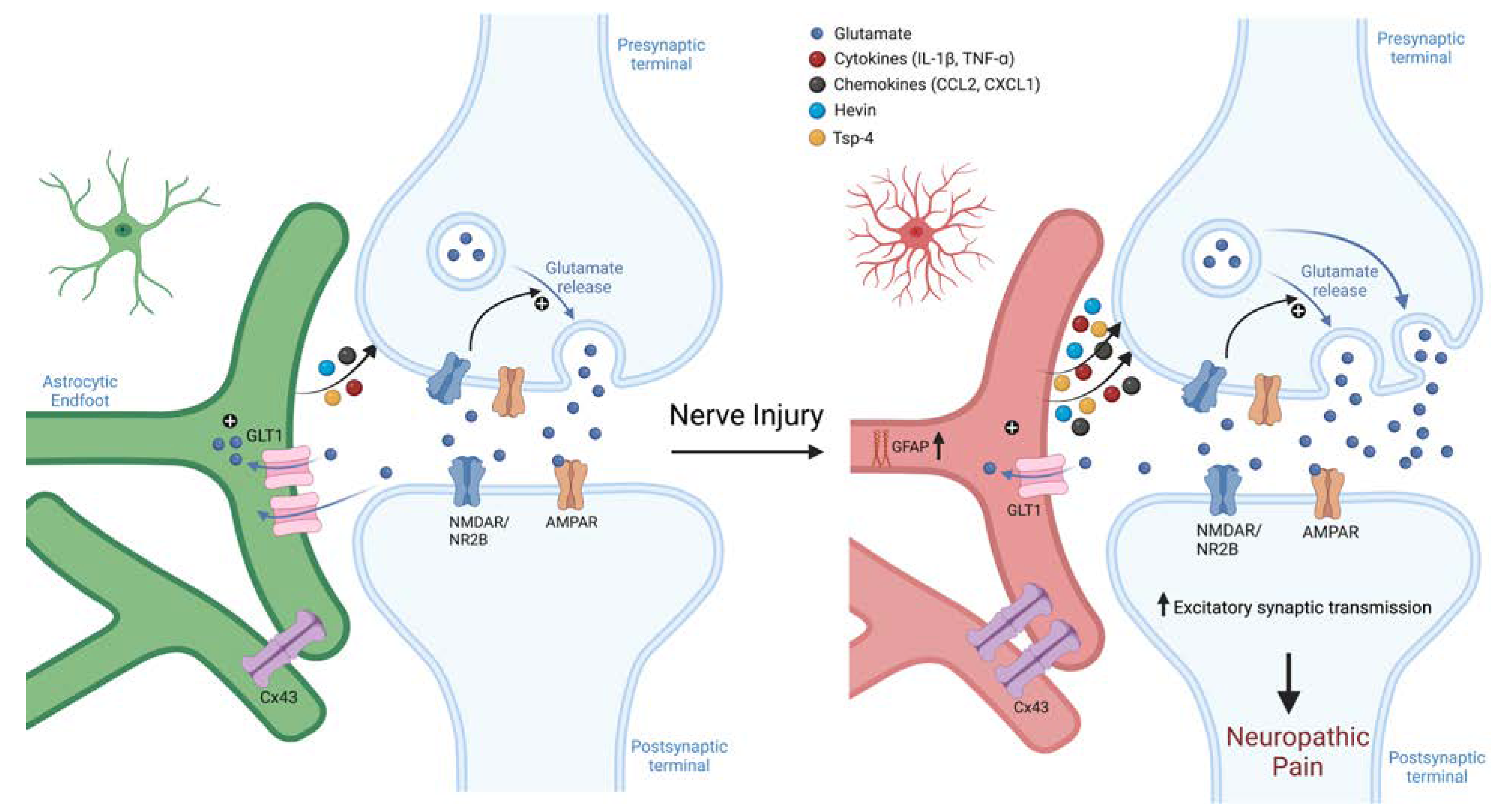
3.3. Gap Junctions
3.4. Pannexins
3.5. ATP Signaling
3.6. Cytokine and Chemokine Mediated Immune Signaling
3.7. Glutamate Transporter Signaling
3.8. Potassium Channels
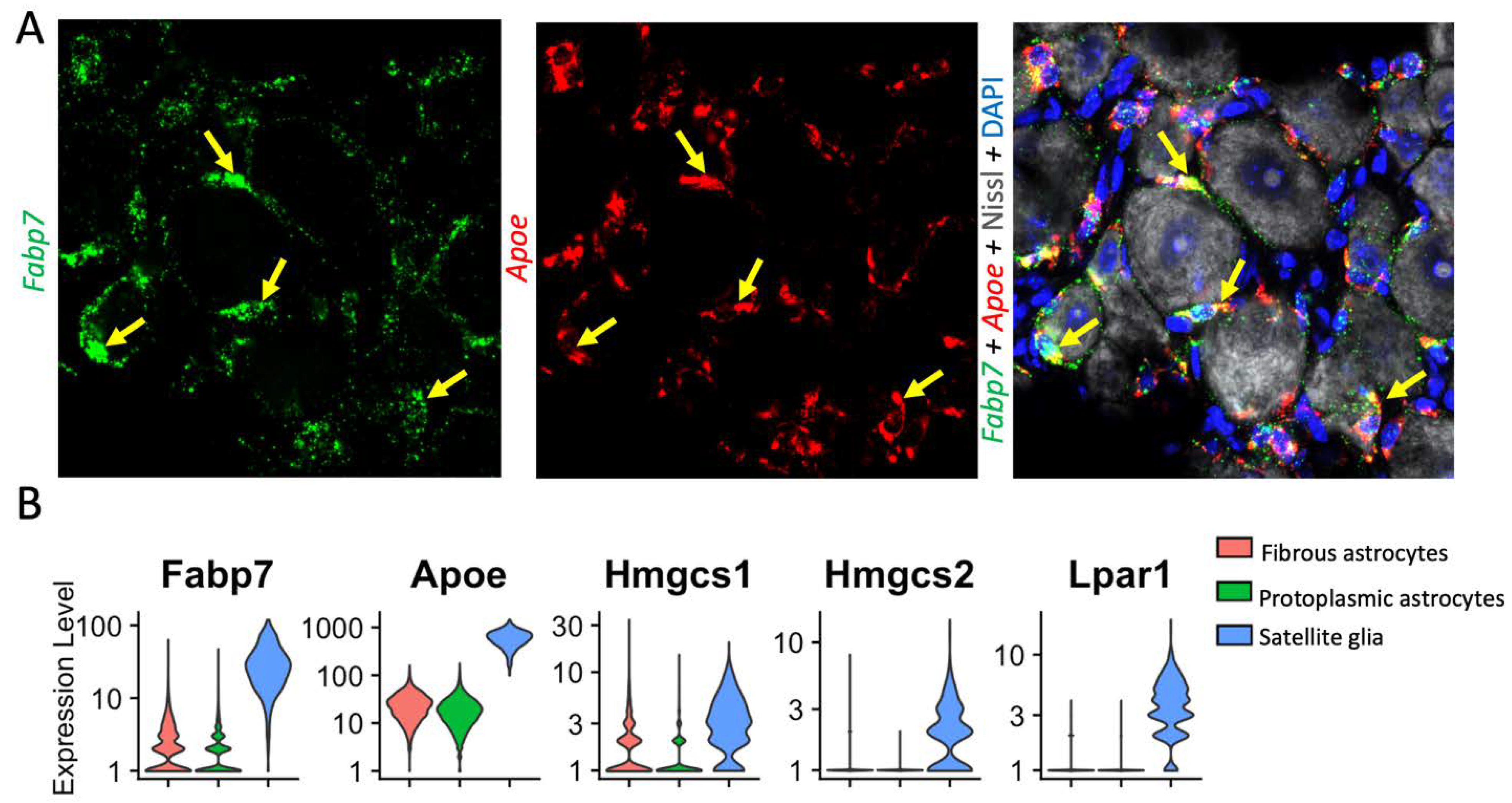
3.9. Fatty Acid Signaling and Metabolism
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Connor, A.B. Neuropathic pain: Quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics 2009, 27, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Schultheis, B.C.; Hanes, M.C.; Jolly, S.M.; Chakravarthy, K.V.; Deer, T.R.; Levy, R.M.; Hunter, C.W. A Comprehensive Algorithm for Management of Neuropathic Pain. Pain Med. 2019, 20, S2–S12. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Collins, F.S. The role of science in addressing the opioid crisis. N. Engl. J. Med. 2017, 377, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Milligan, E.D.; Watkins, L.R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009, 10, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Berta, T.; Nedergaard, M. Glia and pain: Is chronic pain a gliopathy? Pain 2013, 154 (Suppl. 1), S10–S28. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Inoue, K.; Salter, M.W. Neuropathic pain and spinal microglia: A big problem from molecules in ‘small’glia. Trends Neurosci. 2005, 28, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Fei, Y.; Su, W.; Chen, G. Emerging Role of Schwann Cells in Neuropathic Pain: Receptors, Glial Mediators and Myelination. Front. Cell. Neurosci. 2019, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.-R. Central nervous system targets: Glial cell mechanisms in chronic pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef]
- Gritsch, S.; Lu, J.; Thilemann, S.; Wörtge, S.; Möbius, W.; Bruttger, J.; Karram, K.; Ruhwedel, T.; Blanfeld, M.; Vardeh, D. Oligodendrocyte ablation triggers central pain independently of innate or adaptive immune responses in mice. Nat. Commun. 2014, 5, 5472. [Google Scholar] [CrossRef]
- Inoue, K.; Tsuda, M. Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 2018, 19, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.-Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.-R. Microglia in pain: Detrimental and protective roles in pathogenesis and resolution of pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [PubMed]
- Hanani, M.; Verkhratsky, A. Satellite glial cells and astrocytes, a comparative review. Neurochem. Res. 2021, 46, 2525–2537. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mei, X.; Zhang, P.; Ma, C.; White, F.A.; Donnelly, D.F.; Lamotte, R.H. Altered functional properties of satellite glial cells in compressed spinal ganglia. Glia 2009, 57, 1588–1599. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.-Y.; Gerner, P.; Woolf, C.J.; Ji, R.-R. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 2005, 114, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yang, L.; Xu, Y.; Ai, L.; Chen, J.; Xiong, F.; Hu, L.; Chen, H.; Liu, J.; Yan, X. The modulatory effect of motor cortex astrocytes on diabetic neuropathic pain. J. Neurosci. 2021, 41, 5287–5302. [Google Scholar] [CrossRef] [PubMed]
- Takeda, I.; Yoshihara, K.; Cheung, D.L.; Kobayashi, T.; Agetsuma, M.; Tsuda, M.; Eto, K.; Koizumi, S.; Wake, H.; Moorhouse, A.J. Controlled activation of cortical astrocytes modulates neuropathic pain-like behaviour. Nat. Commun. 2022, 13, 4100. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Hayashi, H.; Ishikawa, T.; Shibata, K.; Shigetomi, E.; Shinozaki, Y.; Inada, H.; Roh, S.E.; Kim, S.J.; Lee, G. Cortical astrocytes rewire somatosensory cortical circuits for peripheral neuropathic pain. J. Clin. Investig. 2016, 126, 1983–1997. [Google Scholar] [CrossRef]
- Chen, F.-L.; Dong, Y.-L.; Zhang, Z.-J.; Cao, D.-L.; Xu, J.; Hui, J.; Zhu, L.; Gao, Y.-J. Activation of astrocytes in the anterior cingulate cortex contributes to the affective component of pain in an inflammatory pain model. Brain Res. Bull. 2012, 87, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Danjo, Y.; Shigetomi, E.; Hirayama, Y.J.; Kobayashi, K.; Ishikawa, T.; Fukazawa, Y.; Shibata, K.; Takanashi, K.; Parajuli, B.; Shinozaki, Y. Transient astrocytic mGluR5 expression drives synaptic plasticity and subsequent chronic pain in mice. J. Exp. Med. 2022, 219, e20210989. [Google Scholar] [CrossRef]
- Yang, D.; Jacobson, A.; Meerschaert, K.A.; Sifakis, J.J.; Wu, M.; Chen, X.; Yang, T.; Zhou, Y.; Anekal, P.V.; Rucker, R.A. Nociceptor neurons direct goblet cells via a CGRP-RAMP1 axis to drive mucus production and gut barrier protection. Cell 2022, 185, 4190–4205.e25. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.D.; Yao, M.; Huang, B.; Xu, L.S.; Zheng, Y.; Chu, Y.X.; Wang, H.Q.; Liu, M.J.; Xu, S.J.; Li, H.B. Glial activation in the periaqueductal gray promotes descending facilitation of neuropathic pain through the p38 MAPK signaling pathway. J. Neurosci. Res. 2016, 94, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Loggia, M.L.; Chonde, D.B.; Akeju, O.; Arabasz, G.; Catana, C.; Edwards, R.R.; Hill, E.; Hsu, S.; Izquierdo-Garcia, D.; Ji, R.-R. Evidence for brain glial activation in chronic pain patients. Brain 2015, 138, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C. Transcriptional control of neural crest specification into peripheral glia. Glia 2015, 63, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- George, D.; Ahrens, P.; Lambert, S. S atellite glial cells represent a population of developmentally arrested S chwann cells. Glia 2018, 66, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Jager, S.E.; Pallesen, L.T.; Lin, L.; Izzi, F.; Pinheiro, A.M.; Villa-Hernandez, S.; Cesare, P.; Vaegter, C.B.; Denk, F. Comparative transcriptional analysis of satellite glial cell injury response. Wellcome Open Res. 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Wyburn, G. The capsule of spinal ganglion cells. J. Anat. 1958, 92, 528. [Google Scholar] [PubMed]
- Pannese, E. The Satellite Cells of the Sensory Ganglia; 2013. [Google Scholar]
- Pannese, E. Biology and Pathology of Perineuronal Satellite Cells in Sensory Ganglia; Springer, 2018. [Google Scholar]
- Ledda, M.; De Palo, S.; Pannese, E. Ratios between number of neuroglial cells and number and volume of nerve cells in the spinal ganglia of two species of reptiles and three species of mammals. Tissue Cell 2004, 36, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Pannese, E.; Ledda, M.; Arcidiacono, G.; Rigamonti, L. Clusters of nerve cell bodies enclosed within a common connective tissue envelope in the spinal ganglia of the lizard and rat. Cell Tissue Res. 1991, 264, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Pannese, E.; Ledda, M.; Martinelli, C.; Sartori, P. Age-related decrease of the perineuronal satellite cell number in the rabbit spinal ganglia. JPNS-NEW YORK- 1997, 2, 77–82. [Google Scholar]
- Hanani, M.; Spray, D.C. Emerging importance of satellite glia in nervous system function and dysfunction. Nat. Rev. Neurosci. 2020, 21, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Austin, G. Intracellular potentials of mammalian dorsal root ganglion cells. J. Neurophysiol. 1961, 24, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Hanani, M. Satellite glial cells in sensory ganglia: From form to function. Brain Res. Rev. 2005, 48, 457–476. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Hao, H.; Yang, Y.; Huang, S.; Wang, C.; Gigout, S.; Ramli, R.; Li, X.; Jaworska, E.; Edwards, I. Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission. J. Clin. Investig. 2017, 127, 1741–1756. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Anderson, M.; Park, K.; Zheng, Q.; Agarwal, A.; Gong, C.; Young, L.; He, S.; LaVinka, P.C.; Zhou, F. Coupled activation of primary sensory neurons contributes to chronic pain. Neuron 2016, 91, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Fellin, T.; Takano, H.; Dong, J.H.; Haydon, P.G. Synaptic islands defined by the territory of a single astrocyte. J. Neurosci. 2007, 27, 6473–6477. [Google Scholar] [CrossRef] [PubMed]
- Von Bartheld, C.S.; Bahney, J.; Herculano-Houzel, S. The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J. Comp. Neurol. 2016, 524, 3865–3895. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. From blood–brain barrier to blood–brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Mapps, A.A.; Thomsen, M.B.; Boehm, E.; Zhao, H.; Hattar, S.; Kuruvilla, R. Diversity of satellite glia in sympathetic and sensory ganglia. Cell Rep. 2022, 38, 110328. [Google Scholar] [CrossRef]
- Hochstim, C.; Deneen, B.; Lukaszewicz, A.; Zhou, Q.; Anderson, D.J. Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell 2008, 133, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, A.; Hochgerner, H.; Lönnerberg, P.; Johnsson, A.; Memic, F.; Van Der Zwan, J.; Häring, M.; Braun, E.; Borm, L.E.; La Manno, G. Molecular architecture of the mouse nervous system. Cell 2018, 174, 999–1014.e22. [Google Scholar] [CrossRef] [PubMed]
- Cajal, S.R. Histologie du syste me nerveux de I’Homme et des verte be s. Maloine 1911, 2, 891–942. [Google Scholar]
- Kronschläger, M.T.; Siegert, A.S.; Resch, F.J.; Rajendran, P.S.; Khakh, B.S.; Sandkühler, J. Lamina-specific properties of spinal astrocytes. Glia 2021, 69, 1749–1766. [Google Scholar] [CrossRef]
- Kohro, Y.; Matsuda, T.; Yoshihara, K.; Kohno, K.; Koga, K.; Katsuragi, R.; Oka, T.; Tashima, R.; Muneta, S.; Yamane, T. Spinal astrocytes in superficial laminae gate brainstem descending control of mechanosensory hypersensitivity. Nat. Neurosci. 2020, 23, 1376–1387. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ford, N.C.; He, S.; Huang, Q.; Anderson, M.; Chen, Z.; Yang, F.; Crawford, L.K.; Caterina, M.J.; Guan, Y. Astrocytes contribute to pain gating in the spinal cord. Sci. Adv. 2021, 7, eabi6287. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R.; Donnelly, C.R.; Nedergaard, M. Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 2019, 20, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Cornwell, A.; Li, J.; Peng, S.; Osorio, M.J.; Aalling, N.; Wang, S.; Benraiss, A.; Lou, N.; Goldman, S.A.; et al. SOX9 Is an Astrocyte-Specific Nuclear Marker in the Adult Brain Outside the Neurogenic Regions. J. Neurosci. 2017, 37, 4493–4507. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, S.; Chen, Y.; Wu, D.; Hu, X.; Lu, Y.; Wang, L.; Bao, L.; Li, C.; Zhang, X. Single-cell transcriptomic analysis of somatosensory neurons uncovers temporal development of neuropathic pain. Cell Res. 2021, 31, 904–918. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Kim, J.-H.; Kim, J.-H.; Jha, M.K.; Jung, J.Y.; Lee, M.-G.; Choi, I.-S.; Jang, I.-S.; Lim, D.G.; Hwang, S.-H. Reversible induction of pain hypersensitivity following optogenetic stimulation of spinal astrocytes. Cell Rep. 2016, 17, 3049–3061. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-J.; Ji, R.-R. Chemokines, neuronal–glial interactions, and central processing of neuropathic pain. Pharmacol. Ther. 2010, 126, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.-J.; Weng, H.-R.; Dougherty, P.M. Plasticity in Expression of the Glutamate Transporters GLT-1 and GLAST in Spinal Dorsal Horn Glial Cells following Partial Sciatic Nerve Ligation. Mol. Pain 2009, 5, 1744–8069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; De Koninck, Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J. Neurochem. 2006, 97, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, T.; Chen, X.; Li, L.; Feng, M.; Zhang, Y.; Wan, L.; Zhang, C.; Yao, W. Microglia induce the transformation of A1/A2 reactive astrocytes via the CXCR7/PI3K/Akt pathway in chronic post-surgical pain. J. Neuroinflammation 2020, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Middeldorp, J.; Hol, E. GFAP in health and disease. Prog. Neurobiol. 2011, 93, 421–443. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Thorpe, R.; Mirsky, R. Molecular identity, distribution and heterogeneity of glial fibrillary acidic protein: An immunoblotting and immunohistochemical study of Schwann cells, satellite cells, enteric glia and astrocytes. J. Neurocytol. 1984, 13, 187–200. [Google Scholar] [CrossRef]
- Hanani, M. How is peripheral injury signaled to satellite glial cells in sensory ganglia? Cells 2022, 11, 512. [Google Scholar] [CrossRef]
- Chen, S.; Rio, C.; Ji, R.-R.; Dikkes, P.; Coggeshall, R.E.; Woolf, C.J.; Corfas, G. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat. Neurosci. 2003, 6, 1186–1193. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature 1980, 286, 736–737. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Yen, S.-H.; Fields, K.L. Antibodies to neurofilament, glial filament, and fibroblast intermediate filament proteins bind to different cell types of the nervous system. J. Cell Biol. 1981, 88, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Figueroa, K.W.; Li, K.-W.; Boroujerdi, A.; Yolo, T.; Luo, Z.D. Profiling of dynamically changed gene expression in dorsal root ganglia post peripheral nerve injury and a critical role of injury-induced glial fibrillary acetic protein in maintenance of pain behaviors. Pain 2009, 143, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Woodham, P.; Anderson, P.; Nadim, W.; Turmaine, M. Satellite cells surrounding axotomised rat dorsal root ganglion cells increase expression of a GFAP-like protein. Neurosci. Lett. 1989, 98, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-Y.; Sun, Y.-N.; Wang, F.-T.; Li, Q.; Su, L.; Zhao, Z.-F.; Meng, X.-L.; Zhao, H.; Wu, X.; Sun, Q. Activation of satellite glial cells in lumbar dorsal root ganglia contributes to neuropathic pain after spinal nerve ligation. Brain Res. 2012, 1427, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Hanani, M.; Blum, E.; Liu, S.; Peng, L.; Liang, S. Satellite glial cells in dorsal root ganglia are activated in streptozotocin-treated rodents. J. Cell. Mol. Med. 2014, 18, 2367–2371. [Google Scholar] [CrossRef] [PubMed]
- Warwick, R.; Hanani, M. The contribution of satellite glial cells to chemotherapy-induced neuropathic pain. Eur. J. Pain 2013, 17, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.; Schmitt, L.-I.; Kutritz, A.; Kleinschnitz, C.; Hagenacker, T. Cisplatin-induced activation and functional modulation of satellite glial cells lead to cytokine-mediated modulation of sensory neuron excitability. Exp. Neurol. 2021, 341, 113695. [Google Scholar] [CrossRef] [PubMed]
- Mohr, K.M.; Pallesen, L.T.; Richner, M.; Vaegter, C.B. Discrepancy in the usage of GFAP as a marker of satellite glial cell reactivity. Biomedicines 2021, 9, 1022. [Google Scholar] [CrossRef]
- Avraham, O.; Deng, P.-Y.; Jones, S.; Kuruvilla, R.; Semenkovich, C.F.; Klyachko, V.A.; Cavalli, V. Satellite glial cells promote regenerative growth in sensory neurons. Nat. Commun. 2020, 11, 4891. [Google Scholar] [CrossRef] [PubMed]
- Kurabe, M.; Sasaki, M.; Furutani, K.; Furue, H.; Kamiya, Y.; Baba, H. Structural and functional properties of spinal dorsal horn neurons after peripheral nerve injury change overtime via astrocyte activation. Iscience 2022, 25, 105555. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Hu, L.; Wang, X.; Sun, Q.; Hu, T.; Liu, J.; Shen, D.; Zhang, Y.; Chen, W.; Wei, C. The contribution of spinal dorsal horn astrocytes in neuropathic pain at the early stage of EAE. Neurobiol. Dis. 2022, 175, 105914. [Google Scholar] [CrossRef] [PubMed]
- Garrison, C.; Dougherty, P.; Kajander, K.; Carlton, S. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res. 1991, 565, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nesic, O.; Lee, J.; Johnson, K.M.; Ye, Z.; Xu, G.Y.; Unabia, G.C.; Wood, T.G.; McAdoo, D.J.; Westlund, K.N.; Hulsebosch, C.E. Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J. Neurochem. 2005, 95, 998–1014. [Google Scholar] [CrossRef] [PubMed]
- Okada-Ogawa, A.; Suzuki, I.; Sessle, B.J.; Chiang, C.-Y.; Salter, M.W.; Dostrovsky, J.O.; Tsuboi, Y.; Kondo, M.; Kitagawa, J.; Kobayashi, A. Astroglia in medullary dorsal horn (trigeminal spinal subnucleus caudalis) are involved in trigeminal neuropathic pain mechanisms. J. Neurosci. 2009, 29, 11161–11171. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.-Y.; Wen, Y.-R.; Zhang, D.-R.; Borsello, T.; Bonny, C.; Strichartz, G.R.; Decosterd, I.; Ji, R.-R. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: Respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J. Neurosci. 2006, 26, 3551–3560. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.R.; Zhang, H.; Dougherty, P.M. Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib-induced peripheral neuropathy in the rat. Neuroscience 2014, 274, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Luo, X.; Qadri, M.Y.; Berta, T.; Ji, R.-R. Sex-dependent glial signaling in pathological pain: Distinct roles of spinal microglia and astrocytes. Neurosci. Bull. 2018, 34, 98–108. [Google Scholar] [CrossRef]
- Del Valle, L.; Schwartzman, R.J.; Alexander, G. Spinal cord histopathological alterations in a patient with longstanding complex regional pain syndrome. Brain Behav. Immun. 2009, 23, 85–91. [Google Scholar] [CrossRef]
- Shi, Y.; Gelman, B.B.; Lisinicchia, J.G.; Tang, S.-J. Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J. Neurosci. 2012, 32, 10833–10840. [Google Scholar] [CrossRef] [PubMed]
- Vit, J.P.; Jasmin, L.; Bhargava, A.; Ohara, P.T. Satellite glial cells in the trigeminal ganglion as a determinant of orofacial neuropathic pain. Neuron Glia Biol. 2006, 2, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Donegan, M.; Kernisant, M.; Cua, C.; Jasmin, L.; Ohara, P.T. Satellite glial cell proliferation in the trigeminal ganglia after chronic constriction injury of the infraorbital nerve. Glia 2013, 61, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, D.S.M.; Castro-Lopes, J.M.; Neto, F.L.M. Satellite glial cells surrounding primary afferent neurons are activated and proliferate during monoarthritis in rats: Is there a role for ATF3? PLoS ONE 2014, 9, e108152. [Google Scholar] [CrossRef] [PubMed]
- Jager, S.E.; Pallesen, L.T.; Richner, M.; Harley, P.; Hore, Z.; McMahon, S.; Denk, F.; Vægter, C.B. Changes in the transcriptional fingerprint of satellite glial cells following peripheral nerve injury. Glia 2020, 68, 1375–1395. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; McLachlan, E. Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience 2002, 112, 23–38. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, R.; Yang, J.; Zhao, Y.; Qi, C.; Bian, G.; Wang, M.; Shan, J.; Wang, C.; Wang, D. Chronic pain induces nociceptive neurogenesis in dorsal root ganglia from Sox2-positive satellite cells. Glia 2019, 67, 1062–1075. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, D.; Xu, J.; Zhang, H.; Yu, W. New Insights on the Role of Satellite Glial Cells. Stem Cell Rev. Rep. 2022, 1–10. [Google Scholar] [CrossRef]
- Dyachuk, V.; Furlan, A.; Shahidi, M.K.; Giovenco, M.; Kaukua, N.; Konstantinidou, C.; Pachnis, V.; Memic, F.; Marklund, U.; Müller, T. Parasympathetic neurons originate from nerve-associated peripheral glial progenitors. Science 2014, 345, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Kohro, Y.; Yano, T.; Tsujikawa, T.; Kitano, J.; Tozaki-Saitoh, H.; Koyanagi, S.; Ohdo, S.; Ji, R.-R.; Salter, M.W. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain 2011, 134, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Katsura, H.; Obata, K.; Miyoshi, K.; Kondo, T.; Yamanaka, H.; Kobayashi, K.; Dai, Y.; Fukuoka, T.; Sakagami, M.; Noguchi, K. Transforming growth factor-activated kinase 1 induced in spinal astrocytes contributes to mechanical hypersensitivity after nerve injury. Glia 2008, 56, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Asano, S.; Hayashi, Y.; Iwata, K.; Okada-Ogawa, A.; Hitomi, S.; Shibuta, I.; Imamura, Y.; Shinoda, M. Microglia–Astrocyte Communication via C1q Contributes to Orofacial Neuropathic Pain Associated with Infraorbital Nerve Injury. Int. J. Mol. Sci. 2020, 21, 6834. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shen, L.; Xu, L.; Zhu, A.; Huang, Y. Ror2 mediates chronic post-thoracotomy pain by inducing the transformation of A1/A2 reactive astrocytes in rats. Cell. Signal. 2022, 89, 110183. [Google Scholar] [CrossRef] [PubMed]
- Kronschläger, M.; Drdla-Schutting, R.; Gassner, M.; Honsek, S.; Teuchmann, H.; Sandkühler, J. Gliogenic LTP spreads widely in nociceptive pathways. Science 2016, 354, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Schoffnegger, D.; Drdla-Schutting, R.; Hönigsperger, C.; Wunderbaldinger, G.; Gassner, M.; Sandkühler, J. Induction of thermal hyperalgesia and synaptic long-term potentiation in the spinal cord lamina I by TNF-α and IL-1β is mediated by glial cells. J. Neurosci. 2013, 33, 6540–6551. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Stogsdill, J.A.; Pulimood, N.S.; Dingsdale, H.; Kim, Y.H.; Pilaz, L.-J.; Kim, I.H.; Manhaes, A.C.; Rodrigues, W.S.; Pamukcu, A. Astrocytes assemble thalamocortical synapses by bridging NRX1α and NL1 via hevin. Cell 2016, 164, 183–196. [Google Scholar] [CrossRef]
- Chen, G.; Xu, J.; Luo, H.; Luo, X.; Singh, S.K.; Ramirez, J.J.; James, M.L.; Mathew, J.P.; Berger, M.; Eroglu, C. Hevin/Sparcl1 drives pathological pain through spinal cord astrocyte and NMDA receptor signaling. JCI Insight 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Li, K.-W.; Boroujerdi, A.; Yu, Y.P.; Zhou, C.-Y.; Deng, P.; Park, J.; Zhang, X.; Lee, J.; Corpe, M. Thrombospondin-4 contributes to spinal sensitization and neuropathic pain states. J. Neurosci. 2012, 32, 8977–8987. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Acosta-Gutierrez, S.; Lavriha, P.; Othman, A.; Lopez-Pigozzi, D.; Bayraktar, E.; Schuster, D.; Picotti, P.; Zamboni, N.; Bortolozzi, M. Structure of the connexin-43 gap junction channel reveals a closed sieve-like molecular gate. bioRxiv 2022. [Google Scholar]
- Xing, L.; Yang, T.; Cui, S.; Chen, G. Connexin hemichannels in astrocytes: Role in CNS disorders. Front. Mol. Neurosci. 2019, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.E.; Sáez, J.C.; Bukauskas, F.F.; Bennett, M.V. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. USA 2003, 100, 11388–11393. [Google Scholar] [CrossRef] [PubMed]
- Pannese, E.; Ledda, M.; Cherkas, P.; Huang, T.; Hanani, M. Satellite cell reactions to axon injury of sensory ganglion neurons: Increase in number of gap junctions and formation of bridges connecting previously separate perineuronal sheaths. Anat. Embryol. 2003, 206, 337–347. [Google Scholar] [CrossRef]
- Hanani, M.; Huang, T.; Cherkas, P.; Ledda, M.; Pannese, E. Glial cell plasticity in sensory ganglia induced by nerve damage. Neuroscience 2002, 114, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Cherkas, P.S.; Huang, T.-Y.; Pannicke, T.; Tal, M.; Reichenbach, A.; Hanani, M. The effects of axotomy on neurons and satellite glial cells in mouse trigeminal ganglion. Pain 2004, 110, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, J.N.; Warwick, R.; Duroux, M.; Hanani, M.; Gazerani, P. Oxaliplatin enhances gap junction-mediated coupling in cell cultures of mouse trigeminal ganglia. Exp. Cell Res. 2015, 336, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Ohara, P.T.; Vit, J.-P.; Bhargava, A.; Jasmin, L. Evidence for a role of connexin 43 in trigeminal pain using RNA interference in vivo. J. Neurophysiol. 2008, 100, 3064–3073. [Google Scholar] [CrossRef] [PubMed]
- Procacci, P.; Magnaghi, V.; Pannese, E. Perineuronal satellite cells in mouse spinal ganglia express the gap junction protein connexin43 throughout life with decline in old age. Brain Res. Bull. 2008, 75, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Hanani, M.; Ledda, M.; De Palo, S.; Pannese, E. Aging is associated with an increase in dye coupling and in gap junction number in satellite glial cells of murine dorsal root ganglia. Neuroscience 2006, 137, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Hanani, M.; Spray, D.C.; Huang, T.-Y. Age-Related Changes in Neurons and Satellite Glial Cells in Mouse Dorsal Root Ganglia. Int. J. Mol. Sci. 2023, 24, 2677. [Google Scholar] [CrossRef]
- Vicario, N.; Denaro, S.; Turnaturi, R.; Longhitano, L.; Spitale, F.M.; Spoto, S.; Marrazzo, A.; Zappalà, A.; Tibullo, D.; Li Volti, G. Mu and Delta Opioid Receptor Targeting Reduces Connexin 43-Based Heterocellular Coupling during Neuropathic Pain. Int. J. Mol. Sci. 2022, 23, 5864. [Google Scholar] [CrossRef]
- Morioka, N.; Fujii, S.; Kondo, S.; Zhang, F.F.; Miyauchi, K.; Nakamura, Y.; Hisaoka-Nakashima, K.; Nakata, Y. Downregulation of spinal astrocytic connexin43 leads to upregulation of interleukin-6 and cyclooxygenase-2 and mechanical hypersensitivity in mice. Glia 2018, 66, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Spataro, L.E.; Sloane, E.M.; Milligan, E.D.; Wieseler-Frank, J.; Schoeniger, D.; Jekich, B.M.; Barrientos, R.M.; Maier, S.F.; Watkins, L.R. Spinal gap junctions: Potential involvement in pain facilitation. J. Pain 2004, 5, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Park, C.-K.; Xie, R.-G.; Berta, T.; Nedergaard, M.; Ji, R.-R. Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain 2014, 137, 2193–2209. [Google Scholar] [CrossRef] [PubMed]
- Tonkin, R.S.; Bowles, C.; Perera, C.J.; Keating, B.A.; Makker, P.G.; Duffy, S.S.; Lees, J.G.; Tran, C.; Don, A.S.; Fath, T. Attenuation of mechanical pain hypersensitivity by treatment with Peptide5, a connexin-43 mimetic peptide, involves inhibition of NLRP3 inflammasome in nerve-injured mice. Exp. Neurol. 2018, 300, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Kress, B.; Han, X.; Moll, K.; Peng, W.; Ji, R.R.; Nedergaard, M. Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia 2012, 60, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Locovei, S.; Dahl, G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004, 572, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.K.; Patil, C.S.; Jackson, M.F. Pannexin-1 in the CNS: Emerging concepts in health and disease. J. Neurochem. 2020, 154, 468–485. [Google Scholar] [CrossRef] [PubMed]
- Mousseau, M.; Burma, N.E.; Lee, K.Y.; Leduc-Pessah, H.; Kwok, C.H.; Reid, A.R.; O’Brien, M.; Sagalajev, B.; Stratton, J.A.; Patrick, N. Microglial pannexin-1 channel activation is a spinal determinant of joint pain. Sci. Adv. 2018, 4, eaas9846. [Google Scholar] [CrossRef]
- Weaver, J.L.; Arandjelovic, S.; Brown, G.; K Mendu, S.; S Schappe, M.; Buckley, M.W.; Chiu, Y.-H.; Shu, S.; Kim, J.K.; Chung, J. Hematopoietic pannexin 1 function is critical for neuropathic pain. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Hanstein, R.; Hanani, M.; Scemes, E.; Spray, D.C. Glial pannexin1 contributes to tactile hypersensitivity in a mouse model of orofacial pain. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, Q.; Song, X.; Ford, N.C.; Zhang, C.; Xu, Q.; Lay, M.; He, S.-Q.; Dong, X.; Hanani, M. Purinergic signaling between neurons and satellite glial cells of mouse dorsal root ganglia modulates neuronal excitability in vivo. Pain 2022, 163, 1636–1647. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, C.; Song, X.; Cui, X.; Liu, J.; Ford, N.C.; He, S.; Zhu, G.; Dong, X.; Hanani, M. BzATP Activates Satellite Glial Cells and Increases the Excitability of Dorsal Root Ganglia Neurons In Vivo. Cells 2022, 11, 2280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Laumet, G.; Chen, S.-R.; Hittelman, W.N.; Pan, H.-L. Pannexin-1 up-regulation in the dorsal root ganglion contributes to neuropathic pain development. J. Biol. Chem. 2015, 290, 14647–14655. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Liu, J.; Chu, Y.; Liu, Q.; Mai, L.; Fan, W. Single-cell RNA sequencing reveals distinct transcriptional features of the purinergic signaling in mouse trigeminal ganglion. Front. Mol. Neurosci. 2022, 15, 1038539. [Google Scholar] [CrossRef] [PubMed]
- Pelegrin, P.; Surprenant, A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 2006, 25, 5071–5082. [Google Scholar] [CrossRef] [PubMed]
- Silverman, W.R.; de Rivero Vaccari, J.P.; Locovei, S.; Qiu, F.; Carlsson, S.K.; Scemes, E.; Keane, R.W.; Dahl, G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J. Biol. Chem. 2009, 284, 18143–18151. [Google Scholar] [CrossRef]
- Suadicani, S.O.; Iglesias, R.; Wang, J.; Dahl, G.; Spray, D.C.; Scemes, E. ATP signaling is deficient in cultured Pannexin1-null mouse astrocytes. Glia 2012, 60, 1106–1116. [Google Scholar] [CrossRef]
- Kennedy, C. The P2Y/P2X divide: How it began. Biochem. Pharmacol. 2021, 187, 114408. [Google Scholar] [CrossRef]
- Chessell, I.P.; Hatcher, J.P.; Bountra, C.; Michel, A.D.; Hughes, J.P.; Green, P.; Egerton, J.; Murfin, M.; Richardson, J.; Peck, W.L. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 2005, 114, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Honore, P.; Donnelly-Roberts, D.; Namovic, M.; Zhong, C.; Wade, C.; Chandran, P.; Zhu, C.; Carroll, W.; Perez-Medrano, A.; Iwakura, Y. The antihyperalgesic activity of a selective P2X7 receptor antagonist, A-839977, is lost in IL-1αβ knockout mice. Behav. Brain Res. 2009, 204, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Honore, P.; Donnelly-Roberts, D.; Namovic, M.T.; Hsieh, G.; Zhu, C.Z.; Mikusa, J.P.; Hernandez, G.; Zhong, C.; Gauvin, D.M.; Chandran, P.; et al. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J. Pharmacol. Exp. Ther. 2006, 319, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Andó, R.D.; Méhész, B.; Gyires, K.; Illes, P.; Sperlágh, B. A comparative analysis of the activity of ligands acting at P2X and P2Y receptor subtypes in models of neuropathic, acute and inflammatory pain. Br. J. Pharmacol. 2010, 159, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Suadicani, S.O.; Cherkas, P.S.; Zuckerman, J.; Smith, D.N.; Spray, D.C.; Hanani, M. Bidirectional calcium signaling between satellite glial cells and neurons in cultured mouse trigeminal ganglia. Neuron Glia Biol. 2010, 6, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, G.-W.; Wang, C.; Gu, Y.; Huang, L.-Y.M. Mechanisms underlying enhanced P2X receptor-mediated responses in the neuropathic pain state. Pain 2005, 119, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Matsuka, Y.; Neubert, J.K.; Maidment, N.T.; Spigelman, I. Concurrent release of ATP and substance P within guinea pig trigeminal ganglia in vivo. Brain Res. 2001, 915, 248–255. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Han, P.; Faltynek, C.R.; Jarvis, M.F.; Shieh, C.-C. Functional expression of P2X7 receptors in non-neuronal cells of rat dorsal root ganglia. Brain Res. 2005, 1052, 63–70. [Google Scholar] [CrossRef]
- Xiang, Z.; Bo, X.; Burnstock, G. Localization of ATP-gated P2X receptor immunoreactivity in rat sensory and sympathetic ganglia. Neurosci. Lett. 1998, 256, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, R.; Cherkas, P.S.; Hanani, M. Peripheral inflammation upregulates P2X receptor expression in satellite glial cells of mouse trigeminal ganglia: A calcium imaging study. Neuropharmacology 2011, 61, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zou, L.; Xie, J.; Xie, W.; Wen, S.; Xie, Q.; Gao, Y.; Li, G.; Zhang, C.; Xu, C. LncRNA NONRATT021972 siRNA regulates neuropathic pain behaviors in type 2 diabetic rats through the P2X7 receptor in dorsal root ganglia. Mol. Brain 2016, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Wang, C.; Huang, L.-Y. Neuronal somatic ATP release triggers neuron–satellite glial cell communication in dorsal root ganglia. Proc. Natl. Acad. Sci. USA 2007, 104, 9864–9869. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ying, Y.; Wang, W.; Liu, X.; Xu, X.; Wei, X.; Ruan, X. The role of P2X7R/ERK signaling in dorsal root ganglia satellite glial cells in the development of chronic postsurgical pain induced by skin/muscle incision and retraction (SMIR). Brain Behav. Immun. 2018, 69, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, X.; Wang, C.; Li, G.; Gu, Y.; Huang, L.-Y.M. Activation of P2X7 receptors in glial satellite cells reduces pain through downregulation of P2X3 receptors in nociceptive neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 16773–16778. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, A.; Shinoda, M.; Honda, K.; Toyofuku, A.; Sessle, B.J.; Iwata, K. Satellite glial cell P2Y12 receptor in the trigeminal ganglion is involved in lingual neuropathic pain mechanisms in rats. Mol. Pain 2012, 8, 1744–8069. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-Y.; Huang, L.-Y.M. Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J. Neurosci. 2002, 22, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-Y.; Huang, L.-Y.M. Ca2+/calmodulin-dependent protein kinase II potentiates ATP responses by promoting trafficking of P2X receptors. Proc. Natl. Acad. Sci. USA 2004, 101, 11868–11873. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chung, K.; Chung, J.M. Development of purinergic sensitivity in sensory neurons after peripheral nerve injury in the rat. Brain Res. 2001, 915, 161–169. [Google Scholar] [CrossRef]
- Yousuf, A.; Klinger, F.; Schicker, K.; Boehm, S. Nucleotides control the excitability of sensory neurons via two P2Y receptors and a bifurcated signaling cascade. PAIN® 2011, 152, 1899–1908. [Google Scholar] [CrossRef]
- Malin, S.A.; Molliver, D.C. Gi-and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol. Pain 2010, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Abbracchio, M.P.; Ceruti, S. Roles of P2 receptors in glial cells: Focus on astrocytes. Purinergic Signal 2006, 2, 595–604. [Google Scholar] [CrossRef]
- Kaczmarek-Hajek, K.; Zhang, J.; Kopp, R.; Grosche, A.; Rissiek, B.; Saul, A.; Bruzzone, S.; Engel, T.; Jooss, T.; Krautloher, A.; et al. Re-evaluation of neuronal P2X7 expression using novel mouse models and a P2X7-specific nanobody. Elife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.K.; Staniland, A.A.; Marchand, F.; Kaan, T.K.; McMahon, S.B.; Malcangio, M. P2X7-dependent release of interleukin-1β and nociception in the spinal cord following lipopolysaccharide. J. Neurosci. 2010, 30, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Takahashi, E.; Miyagawa, Y.; Yamanaka, H.; Noguchi, K. Induction of the P2X7 receptor in spinal microglia in a neuropathic pain model. Neurosci. Lett. 2011, 504, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Sorge, R.E.; Trang, T.; Dorfman, R.; Smith, S.B.; Beggs, S.; Ritchie, J.; Austin, J.-S.; Zaykin, D.V.; Meulen, H.V.; Costigan, M. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat. Med. 2012, 18, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Chamessian, A.; Zhang, Y.Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef]
- Takeda, M.; Tanimoto, T.; Kadoi, J.; Nasu, M.; Takahashi, M.; Kitagawa, J.; Matsumoto, S. Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain 2007, 129, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-T.; Xin, W.-J.; Zang, Y.; Wu, C.-Y.; Liu, X.-G. The role of tumor necrosis factor-alpha in the neuropathic pain induced by Lumbar 5 ventral root transection in rat. Pain 2006, 123, 306–321. [Google Scholar] [CrossRef]
- Dubový, P.; Klusáková, I.; Svíženská, I.; Brázda, V. Satellite glial cells express IL-6 and corresponding signal-transducing receptors in the dorsal root ganglia of rat neuropathic pain model. Neuron Glia Biol. 2010, 6, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Kitagawa, J.; Takahashi, M.; Matsumoto, S. Activation of interleukin-1β receptor suppresses the voltage-gated potassium currents in the small-diameter trigeminal ganglion neurons following peripheral inflammation. Pain 2008, 139, 594–602. [Google Scholar] [CrossRef]
- Binshtok, A.M.; Wang, H.; Zimmermann, K.; Amaya, F.; Vardeh, D.; Shi, L.; Brenner, G.J.; Ji, R.-R.; Bean, B.P.; Woolf, C.J. Nociceptors are interleukin-1β sensors. J. Neurosci. 2008, 28, 14062–14073. [Google Scholar] [CrossRef]
- Ohtori, S.; Takahashi, K.; Moriya, H.; Myers, R.R. TNF-α and TNF-α receptor type 1 upregulation in glia and neurons after peripheral nerve injury: Studies in murine DRG and spinal cord. Spine 2004, 29, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Nicol, G.D.; Lopshire, J.C.; Pafford, C.M. Tumor necrosis factor enhances the capsaicin sensitivity of rat sensory neurons. J. Neurosci. 1997, 17, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Ceruti, S.; Fumagalli, M.; Villa, G.; Verderio, C.; Abbracchio, M.P. Purinoceptor-mediated calcium signaling in primary neuron-glia trigeminal cultures. Cell Calcium 2008, 43, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Ceruti, S.; Villa, G.; Fumagalli, M.; Colombo, L.; Magni, G.; Zanardelli, M.; Fabbretti, E.; Verderio, C.; Van Den Maagdenberg, A.M.; Nistri, A. Calcitonin gene-related peptide-mediated enhancement of purinergic neuron/glia communication by the algogenic factor bradykinin in mouse trigeminal ganglia from wild-type and R192Q Cav2. 1 Knock-in mice: Implications for basic mechanisms of migraine pain. J. Neurosci. 2011, 31, 3638–3649. [Google Scholar] [CrossRef]
- Afroz, S.; Arakaki, R.; Iwasa, T.; Oshima, M.; Hosoki, M.; Inoue, M.; Baba, O.; Okayama, Y.; Matsuka, Y. CGRP induces differential regulation of cytokines from satellite glial cells in trigeminal ganglia and orofacial nociception. Int. J. Mol. Sci. 2019, 20, 711. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, J.; Monaco, S.; Kreutzberg, G.W. Spinal cord microglial cells and DRG satellite cells rapidly respond to transection of the rat sciatic nerve. Restor. Neurol. Neurosci. 1991, 2, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Xu, Z.-Z.; Wang, X.; Park, J.Y.; Zhuang, Z.-Y.; Tan, P.-H.; Gao, Y.-J.; Roy, K.; Corfas, G.; Lo, E.H. Distinct roles of matrix metalloproteases in the early-and late-phase development of neuropathic pain. Nat. Med. 2008, 14, 331–336. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, J.A.; Rutkowski, M.D.; Stalder, A.K.; Campbell, I.L. Transgenic expression of TNF by astrocytes increases mechanical allodynia in a mouse neuropathy model. Neuroreport 2000, 11, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-J.; Zhang, L.; Samad, O.A.; Suter, M.R.; Yasuhiko, K.; Xu, Z.-Z.; Park, J.-Y.; Lind, A.-L.; Ma, Q.; Ji, R.-R. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J. Neurosci. 2009, 29, 4096–4108. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Zhang, L.; Ji, R.R. Spinal injection of TNF-α-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1. Glia 2010, 58, 1871–1880. [Google Scholar] [CrossRef]
- Jiang, B.-C.; Cao, D.-L.; Zhang, X.; Zhang, Z.-J.; He, L.-N.; Li, C.-H.; Zhang, W.-W.; Wu, X.-B.; Berta, T.; Ji, R.-R. CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5. J. Clin. Investig. 2016, 126, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Berger, U.V.; Hediger, M.A. Distribution of the glutamate transporters GLAST and GLT-1 in rat circumventricular organs, meninges, and dorsal root ganglia. J. Comp. Neurol. 2000, 421, 385–399. [Google Scholar] [CrossRef]
- Carozzi, V.A.; Canta, A.; Oggioni, N.; Ceresa, C.; Marmiroli, P.; Konvalinka, J.; Zoia, C.; Bossi, M.; Ferrarese, C.; Tredici, G. Expression and distribution of ‘high affinity’glutamate transporters GLT1, GLAST, EAAC1 and of GCPII in the rat peripheral nervous system. J. Anat. 2008, 213, 539–546. [Google Scholar] [PubMed]
- Sung, B.; Lim, G.; Mao, J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J. Neurosci. 2003, 23, 2899–2910. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, W.; Wang, Y.; Huang, J.; Wu, S.; Li, Y.Q. Temporal changes of astrocyte activation and glutamate transporter-1 expression in the spinal cord after spinal nerve ligation-induced neuropathic pain. Anat. Rec. 2008, 291, 513–518. [Google Scholar] [CrossRef]
- Liaw, W.J.; Stephens, R.L., Jr.; Binns, B.C.; Chu, Y.; Sepkuty, J.P.; Johns, R.A.; Rothstein, J.D.; Tao, Y.X. Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord. Pain 2005, 115, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhang, L.; Xia, F.; Jin, L.; Liu, S.; Ren, H.; Zhu, C.; Ji, Q.; Tang, J. Astrocytic NDRG2 is critical in the maintenance of neuropathic pain. Brain Behav. Immun. 2020, 89, 300–313. [Google Scholar] [CrossRef]
- Feldman-Goriachnik, R.; Hanani, M. How do neurons in sensory ganglia communicate with satellite glial cells? Brain Res. 2021, 1760, 147384. [Google Scholar] [CrossRef] [PubMed]
- Tsantoulas, C.; McMahon, S.B. Opening paths to novel analgesics: The role of potassium channels in chronic pain. Trends Neurosci. 2014, 37, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef] [PubMed]
- Vit, J.-P.; Ohara, P.T.; Bhargava, A.; Kelley, K.; Jasmin, L. Silencing the Kir4. 1 potassium channel subunit in satellite glial cells of the rat trigeminal ganglion results in pain-like behavior in the absence of nerve injury. J. Neurosci. 2008, 28, 4161–4171. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Takahashi, M.; Nasu, M.; Matsumoto, S. Peripheral inflammation suppresses inward rectifying potassium currents of satellite glial cells in the trigeminal ganglia. Pain 2011, 152, 2147–2156. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Schmidt, T.M.; Perez-Leighton, C.E.; Kofuji, P. Inwardly rectifying potassium channel Kir4. 1 is responsible for the native inward potassium conductance of satellite glial cells in sensory ganglia. Neuroscience 2010, 166, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Neusch, C.; Papadopoulos, N.; Müller, M.; Maletzki, I.; Winter, S.M.; Hirrlinger, J.; Handschuh, M.; Bahr, M.; Richter, D.W.; Kirchhoff, F. Lack of the Kir4. 1 channel subunit abolishes K+ buffering properties of astrocytes in the ventral respiratory group: Impact on extracellular K+ regulation. J. Neurophysiol. 2006, 95, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Djukic, B.; Casper, K.B.; Philpot, B.D.; Chin, L.-S.; McCarthy, K.D. Conditional knock-out of Kir4. 1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J. Neurosci. 2007, 27, 11354–11365. [Google Scholar] [CrossRef] [PubMed]
- Renthal, W.; Tochitsky, I.; Yang, L.; Cheng, Y.C.; Li, E.; Kawaguchi, R.; Geschwind, D.H.; Woolf, C.J. Transcriptional Reprogramming of Distinct Peripheral Sensory Neuron Subtypes after Axonal Injury. Neuron 2020, 108, 128–144.e9. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, K.; Morihiro, Y.; Maekawa, M.; Yasumoto, Y.; Hoshi, H.; Adachi, Y.; Sawada, T.; Tokuda, N.; Kondo, H.; Yoshikawa, T.; et al. FABP7 expression in normal and stab-injured brain cortex and its role in astrocyte proliferation. Histochem. Cell Biol. 2011, 136, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.-C.; Bu, G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Tansley, S.; Uttam, S.; Ureña Guzmán, A.; Yaqubi, M.; Pacis, A.; Parisien, M.; Deamond, H.; Wong, C.; Rabau, O.; Brown, N. Single-cell RNA sequencing reveals time-and sex-specific responses of mouse spinal cord microglia to peripheral nerve injury and links ApoE to chronic pain. Nat. Commun. 2022, 13, 843. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Krock, E.; Barde, S.; Delaney, A.; Ribeiro, J.; Kato, J.; Agalave, N.; Wigerblad, G.; Matteo, R.; Sabbadini, R. Pain-like behavior in the collagen antibody-induced arthritis model is regulated by lysophosphatidic acid and activation of satellite glia cells. Brain Behav. Immun. 2022, 101, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xiang, H.; Fischer, G.; Liu, Z.; Dupont, M.J.; Hogan, Q.H.; Yu, H. HMG-CoA synthase isoenzymes 1 and 2 localize to satellite glial cells in dorsal root ganglia and are differentially regulated by peripheral nerve injury. Brain Res. 2016, 1652, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M.; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M. Integrated analysis of multimodal single-cell data. Cell 2021, 184, 3573–3587.e29. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




