Submitted:
25 February 2023
Posted:
27 February 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Measurement of Human Intelligence and Neurocognition
3. Theories linking brain structure and neurocognitive function
4. Structural MRI to Infer Intelligence and Neurocognition
5. Diffusion MRI to Infer Intelligence and Neurocognition
6. Functional MRI to Infer Intelligence and Neurocognition
7. Opportunities and Challenges
8. Conclusion
References
- Morley, J.E.; Morris, J.C.; Berg-Weger, M.; et al. Brain health: the importance of recognizing cognitive impairment: An IAGG consensus conference. J. Am. Med. Dir. Assoc. 2015, 16, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Latal, B.; Patel, P.; Liamlahi, R.; Knirsch, W.; Tuura, R.O.; von Rhein, M. Hippocampal volume reduction is associated with intellectual functions in adolescents with congenital heart disease. Pediatr. Res. 2016, 80, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Kessler, N.; Feldmann, M.; Schlosser, L.; et al. Structural brain abnormalities in adults with congenital heart disease: prevalence and association with estimated intelligence quotient. Int. J. Cardiol. 2020, 306, 61–66. [Google Scholar] [CrossRef]
- Watson, C.G.; Stopp, C.; Wypij, D.; Bellinger, D.C.; Newburger, J.W.; Rivkin, M.J. Altered white matter microstructure correlates with IQ and processing speed in children and adolescents post-fontan. J. Pediatr. 2018, 200, 140–149e4. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.; Galdi, P.; Paul, L.K.; Adolphs, R. A distributed brain network predicts general intelligence from resting-state human neuroimaging data. Philos. Trans. R Soc. B Biol. Sci. 2018, 373, 20170284. [Google Scholar] [CrossRef]
- Kanai, R.; Rees, G. The structural basis of inter-individual differences in human behaviour and cognition. Nat. Rev. Neurosci. 2011, 12, 231–242. [Google Scholar] [CrossRef]
- Liamlahi, R.; Latal, B. Neurodevelopmental outcome of children with congenital heart disease. Handb. Clin. Neurol. 2019, 162, 329–345. [Google Scholar] [PubMed]
- Urschel, S.; Bond, G.Y.; Dinu, I.A.; et al. Neurocognitive outcomes after heart transplantation in early childhood. J. Heart Lung Transplant. 2018, 37, 740–748. [Google Scholar] [CrossRef]
- Pallas, S.L. Intrinsic and extrinsic factors that shape neocortical specification. Trends Neurosci. 2001, 24, 417–423. [Google Scholar] [CrossRef]
- Spitzka, E.A. Brain-weight, cranial capacity and the form of the head, and their relations to the mental powers of man. Science 1903, 17, 753–754. [Google Scholar] [CrossRef]
- Pol, H.E.H.; Schnack, H.G.; Posthuma, D.; et al. Genetic contributions to human brain morphology and intelligence. J. Neurosci. 2006, 26, 10235–10242. [Google Scholar]
- Rushton, J.P.; Ankney, C.D. Whole brain size and general mental ability: a review. Int. J. Neurosci. 2009, 119, 692–732. [Google Scholar] [CrossRef] [PubMed]
- Deary, I.J.; Bastin, M.E.; Pattie, A.; et al. White matter integrity and cognition in childhood and old age. Neurology. 2006, 66, 505–512. [Google Scholar] [CrossRef]
- Schmithorst, V.J.; Wilke, M.; Dardzinski, B.J.; Holland, S.K. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum. Brain. Mapp. 2005, 26, 139–147. [Google Scholar] [CrossRef]
- Jensen, A.R. Clocking the Mind: Mental Chronometry and Individual Differences; Elsevier, 2006. [Google Scholar]
- Poldrack, R.A.; Gorgolewski, K.J. Making big data open: data sharing in neuroimaging. Nat. Neurosci. 2014, 17, 1510–1517. [Google Scholar] [CrossRef]
- Graham, S.A.; Lee, E.E.; Jeste, D.V.; et al. Artificial intelligence approaches to predicting and detecting cognitive decline in older adults: A conceptual review. Psychiatry Res. 2020, 284, 112732. [Google Scholar] [CrossRef] [PubMed]
- Dizaji, A.S.; Vieira, B.H.; Khodaei, M.R.; et al. Linking Brain Biology to Intellectual Endowment: A Review on the Associations of Human Intelligence With Neuroimaging Data. Basic Clin. Neurosci. 2021, 12, 1. [Google Scholar] [CrossRef]
- Naef, N.; Schlosser, L.; Brugger, P.; et al. Brain volumes in adults with congenital heart disease correlate with executive function abilities. Brain Imaging Behav Published online. 2021, 1–9. [Google Scholar] [CrossRef]
- Fontes, K.; Rohlicek, C.V.; Saint-Martin, C.; et al. Hippocampal alterations and functional correlates in adolescents and young adults with congenital heart disease. Hum. Brain Mapp. 2019, 40, 3548–3560. [Google Scholar] [CrossRef]
- Pike, N.A.; Roy, B.; Moye, S.; et al. Reduced hippocampal volumes and memory deficits in adolescents with single ventricle heart disease. Brain Behav. 2021, 11, e01977. [Google Scholar] [CrossRef] [PubMed]
- Ehrler, M.; Latal, B.; Kretschmar, O.; von Rhein, M.; Tuura, R.O. Altered frontal white matter microstructure is associated with working memory impairments in adolescents with congenital heart disease: a diffusion tensor imaging study. NeuroImage Clin. 2020, 25, 102123. [Google Scholar] [CrossRef]
- McGrew, K.S. CHC Theory and the Human Cognitive Abilities Project: Standing on the Shoulders of the Giants of Psychometric Intelligence Research; Elsevier, 2009. [Google Scholar]
- Carroll, J.B. Human Cognitive Abilities: A Survey of Factor-Analytic Studies; Cambridge University Press, 1993. [Google Scholar]
- Horn, J.L.; Cattell, R.B. Refinement and test of the theory of fluid and crystallized general intelligences. J. Educ. Psychol. 1966, 57, 253. [Google Scholar] [CrossRef]
- Spearmen, C. General intelligence objectively determined and measured. Am. J. Psychol. 1904, 15, 107–197. [Google Scholar] [CrossRef]
- Cattell, R.B. Theory of fluid and crystallized intelligence: A critical experiment. J. Educ. Psychol. 1963, 54, 1. [Google Scholar] [CrossRef]
- Schneider, W.J.; McGrew, K.S. The Cattell–Horn–Carroll theory of cognitive abilities. Published online 2018.
- McGrew, K. Cattell-Horn-Carroll CHC (Gf-Gc) Theory: Past, Present & Future.
- Kaufman, A.S. Contemporary Intellectual Assessment: Theories, Tests, and Issues; Guilford Publications, 2018. [Google Scholar]
- Hartman, D.E. Wechsler Adult Intelligence Scale IV (WAIS IV): return of the gold standard. Appl. Neuropsychol. 2009, 16, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Benson, N.; Hulac, D.M.; Kranzler, J.H. Independent examination of the Wechsler Adult Intelligence Scale—Fourth Edition (WAIS-IV): what does the WAIS-IV measure? Psychol. Assess. 2010, 22, 121. [Google Scholar] [CrossRef]
- Watkins, M.W.; Canivez, G.L. Assessing the psychometric utility of IQ scores: A tutorial using the Wechsler intelligence scale for children–fifth edition. Sch. Psychol. Rev. Published online. 2021, 1–15. [Google Scholar] [CrossRef]
- Wechsler, D. WASI-II: Wechsler Abbreviated Scale of Intelligence. PsychCorp 2011. [Google Scholar]
- Woodcock, R.W.; McGrew, K.S.; Mather, N.; Schrank, F.A. Woodcock-Johnson III diagnostic supplement to the tests of cognitive abilities. Itasca IL Riverside. 2003, 10, 003435520104400407. [Google Scholar]
- Bornman, J.; Romski, M.; Tonsing, K.; et al. Adapting and translating the Mullen Scales of Early Learning for the South African context. S. Afr. J. Commun. Disord. 2018, 65, 1–9. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, E.S.L.; de Kieviet, J.F.; Königs, M.; van Elburg, R.M.; Oosterlaan, J. Predictive value of the Bayley scales of infant development on development of very preterm/very low birth weight children: a meta-analysis. Early Hum. Dev. 2013, 89, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Kubinger, K.D. Psychologische Diagnostik: Theorie Und Praxis Psychologischen Diagnostizierens; Hogrefe Verlag, 2006. [Google Scholar]
- Wegenschimmel, B.; Leiss, U.; Veigl, M.; et al. Do we still need IQ-scores? Misleading interpretations of neurocognitive outcome in pediatric patients with medulloblastoma: a retrospective study. J Neurooncol. 2017, 135, 361–369. [Google Scholar] [CrossRef]
- Zgaljardic, D.J.; Temple, R.O. Neuropsychological Assessment Battery (NAB): Performance in a sample of patients with moderate-to-severe traumatic brain injury. Appl. Neuropsychol. 2010, 17, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Akshoomoff, N.; Beaumont, J.L.; Bauer, P.J.; et al. VIII. NIH Toolbox Cognition Battery (CB): composite scores of crystallized, fluid, and overall cognition. Monogr. Soc. Res. Child. Dev. 2013, 78, 119–132. [Google Scholar] [CrossRef]
- Denboer, J.W.; Nicholls, C.; Corte, C.; Chestnut, K. National Institutes of Health Toolbox Cognition Battery; Oxford University Press, 2014. [Google Scholar]
- Barbey, A.K. Network neuroscience theory of human intelligence. Trends Cogn. Sci. 2018, 22, 8–20. [Google Scholar] [CrossRef]
- Duncan, J.; Owen, A.M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000, 23, 475–483. [Google Scholar] [CrossRef]
- Duncan, J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn. Sci. 2010, 14, 172–179. [Google Scholar] [CrossRef]
- Kovacs, K.; Conway, A.R. Process overlap theory: A unified account of the general factor of intelligence. Psychol. Inq. 2016, 27, 151–177. [Google Scholar] [CrossRef]
- Jung, R.E.; Haier, R.J. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav. Brain. Sci. 2007, 30, 135–154. [Google Scholar] [CrossRef]
- Deary, I.J.; Penke, L.; Johnson, W. The neuroscience of human intelligence differences. Nat. Rev. Neurosci. 2010, 11, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Colom, R.; Karama, S.; Jung, R.E.; Haier, R.J. Human intelligence and brain networks. Dialogues Clin. Neurosci. Published online 2022. [CrossRef]
- Lerch, J.P.; Van Der Kouwe, A.J.; Raznahan, A.; et al. Studying neuroanatomy using MRI. Nat. Neurosci. 2017, 20, 314–326. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. Neuroimage. 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.; Woolrich, M.W.; Smith, S.M. Fsl. Neuroimage. 2012, 62, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.W. AFNI: what a long strange trip it’s been. Neuroimage. 2012, 62, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Yeo, B.T.; Genon, S. Imaging-based parcellations of the human brain. Nat. Rev. Neurosci. 2018, 19, 672–686. [Google Scholar] [CrossRef]
- Zhang-James, Y.; Glatt, S.J.; Faraone, S.V. Nu Support Vector Machine in Prediction of Fluid Intelligence Using MRI Data. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 92–98. [Google Scholar]
- Chen, H.; Dou, Q.; Yu, L.; Qin, J.; Heng, P.A. VoxResNet: Deep voxelwise residual networks for brain segmentation from 3D MR images. NeuroImage. 2018, 170, 446–455. [Google Scholar] [CrossRef]
- Tsukahara, J.S.; Harrison, T.L.; Draheim, C.; Martin, J.D.; Engle, R.W. Attention control: The missing link between sensory discrimination and intelligence. Atten. Percept Psychophys. 2020, 82, 3445–3478. [Google Scholar] [CrossRef]
- Cabeza, R.; Nyberg, L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 2000, 12, 1–47. [Google Scholar] [CrossRef]
- MacDonald, A.W.; Cohen, J.D.; Stenger, V.A.; Carter, C.S. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000, 288, 1835–1838. [Google Scholar] [CrossRef]
- Chiang, J.N.; Reggente, N.; Dell’Italia, J.; Zheng, Z.S.; Lutkenhoff, E.S. Predicting Fluid Intelligence Using Anatomical Measures Within Functionally Defined Brain Networks. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 143–149. [Google Scholar]
- Srivastava, S.; Eitel, F.; Ritter, K. Predicting fluid intelligence in adolescent brain mri data: An ensemble approach. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 74–82. [Google Scholar]
- Ren, H.; Wang, X.; Wang, S.; Zhang, Z. Predict Fluid Intelligence of Adolescent Using Ensemble Learning. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 66–73. [Google Scholar]
- Tamez-Pena, J.; Orozco, J.; Sosa, P.; Valdes, A.; Nezhadmoghadam, F. Ensemble of svm, random-forest and the bswims method to predict and describe structural associations with fluid intelligence scores from t1-weighed mri. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 47–56. [Google Scholar]
- Brueggeman, L.; Koomar, T.; Huang, Y.; et al. Ensemble Modeling of Neurocognitive Performance Using MRI-Derived Brain Structure Volumes. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 124–132. [Google Scholar]
- Mihalik, A.; Brudfors, M.; Robu, M.; et al. ABCD Neurocognitive Prediction Challenge 2019: predicting individual fluid intelligence scores from structural MRI using probabilistic segmentation and kernel ridge regression. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 133–142. [Google Scholar]
- Ranjbar, S.; Singleton, K.W.; Curtin, L.; et al. Sex differences in predicting fluid intelligence of adolescent brain from T1-weighted MRIs. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 150–157. [Google Scholar]
- Wlaszczyk, A.; Kaminska, A.; Pietraszek, A.; Dabrowski, J.; Pawlak, M.A.; Nowicka, H. Predicting Fluid Intelligence from Structural MRI Using Random Forest regression. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 83–91. [Google Scholar]
- Kao, P.Y.; Zhang, A.; Goebel, M.; Chen, J.W.; Manjunath, B.S. Predicting Fluid Intelligence of Children using T1-weighted MR Images and a StackNet. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 9–16. [Google Scholar]
- Li, T.; Wang, X.; Luo, T.; et al. Adolescent Fluid Intelligence Prediction from Regional Brain Volumes and Cortical Curvatures Using BlockPC-XGBoost. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 167–175. [Google Scholar]
- Saha, S.; Pagnozzi, A.; Bradford, D.; Fripp, J. Predicting fluid intelligence in adolescence from structural MRI with deep learning methods. Intelligence. 2021, 88, 101568. [Google Scholar] [CrossRef]
- Kane, M.J.; Engle, R.W. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychon. Bull. Rev. 2002, 9, 637–671. [Google Scholar] [CrossRef]
- Conway, A.R.; Cowan, N.; Bunting, M.F.; Therriault, D.J.; Minkoff, S.R. A latent variable analysis of working memory capacity, short-term memory capacity, processing speed, and general fluid intelligence. Intelligence. 2002, 30, 163–183. [Google Scholar] [CrossRef]
- Paul, E.J.; Larsen, R.J.; Nikolaidis, A.; et al. Dissociable brain biomarkers of fluid intelligence. Neuroimage. 2016, 137, 201–211. [Google Scholar] [CrossRef]
- Morsing, E.; Malova, M.; Kahn, A.; et al. Brain Volumes and Developmental Outcome in Childhood Following Fetal Growth Restriction Leading to Very Preterm Birth. Front. Physiol. 2018, 9, 1583. [Google Scholar] [CrossRef]
- Ogawa, T.; Aihara, T.; Shimokawa, T.; Yamashita, O. Large-scale brain network associated with creative insight: combined voxel-based morphometry and resting-state functional connectivity analyses. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hilger, K.; Winter, N.R.; Leenings, R.; et al. Predicting intelligence from brain gray matter volume. Brain Struct. Funct. 2020, 225, 2111–2129. [Google Scholar] [CrossRef]
- Packard, M.G.; Knowlton, B.J. Learning and memory functions of the basal ganglia. Annu. Rev. Neurosci. 2002, 25, 563–593. [Google Scholar] [CrossRef]
- Tricomi, E.; Delgado, M.R.; McCandliss, B.D.; McClelland, J.L.; Fiez, J.A. Performance feedback drives caudate activation in a phonological learning task. J. Cogn. Neurosci. 2006, 18, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Grazioplene, R.G.; GRyman, S.; Gray, J.R.; Rustichini, A.; Jung, R.E.; DeYoung, C.G. Subcortical intelligence: Caudate volume predicts IQ in healthy adults. Hum. Brain Mapp. 2015, 36, 1407–1416. [Google Scholar] [CrossRef]
- Westlye, L.T.; Walhovd, K.B.; Bjørnerud, A.; Due-Tønnessen, P.; Fjell, A.M. Error-related negativity is mediated by fractional anisotropy in the posterior cingulate gyrus—a study combining diffusion tensor imaging and electrophysiology in healthy adults. Cereb. Cortex. 2009, 19, 293–304. [Google Scholar] [CrossRef]
- Bar, M.; Tootell, R.B.; Schacter, D.L.; et al. Cortical mechanisms specific to explicit visual object recognition. Neuron. 2001, 29, 529–535. [Google Scholar] [CrossRef]
- McCandliss, B.D.; Cohen, L.; Dehaene, S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn. Sci. 2003, 7, 293–299. [Google Scholar] [CrossRef]
- McClelland, J.L.; Rogers, T.T. The parallel distributed processing approach to semantic cognition. Nat. Rev. Neurosci. 2003, 4, 310–322. [Google Scholar] [CrossRef]
- Oxtoby, N.P.; Ferreira, F.S.; Mihalik, A.; et al. ABCD Neurocognitive Prediction Challenge 2019: Predicting individual residual fluid intelligence scores from cortical grey matter morphology. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 114–123. [Google Scholar]
- Rebsamen, M.; Rummel, C.; Mürner-Lavanchy, I.; Reyes, M.; Wiest, R.; McKinley, R. Surface-Based Brain Morphometry for the Prediction of Fluid Intelligence in the Neurocognitive Prediction Challenge 2019. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 26–34. [Google Scholar]
- Valverde, J.M.; Imani, V.; Lewis, J.D.; Tohka, J. Predicting intelligence based on cortical WM/GM contrast, cortical thickness and volumetry. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 57–65. [Google Scholar]
- Pölsterl, S.; Gutiérrez-Becker, B.; Sarasua, I.; Roy, A.G.; Wachinger, C. Prediction of Fluid Intelligence from T1-Weighted Magnetic Resonance Images. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 35–46. [Google Scholar]
- Pölsterl, S.; Gutiérrez-Becker, B.; Sarasua, I.; Roy, A.G.; Wachinger, C. An AutoML Approach for the Prediction of Fluid Intelligence from MRI-Derived Features. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 99–107. [Google Scholar]
- Guerdan, L.; Sun, P.; Rowland, C.; et al. Deep learning vs. classical machine learning: A comparison of methods for fluid intelligence prediction. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 17–25. [Google Scholar]
- Li, T.; McCorkle, G.S.; Williams, D.K.; Badger, T.M.; Ou, X. Cortical Morphometry is Associated with Neuropsychological Function in Healthy 8-Year-Old Children. J. Neuroimaging. 2020, 30, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Tadayon, E.; Pascual-Leone, A.; Santarnecchi, E. Differential contribution of cortical thickness, surface area, and gyrification to fluid and crystallized intelligence. Cereb. Cortex. 2020, 30, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Raikes, A.; Smith, R.; et al. The relationship between general intelligence and cortical structure in healthy individuals. Neuroscience. 2018, 388, 36–44. [Google Scholar] [CrossRef]
- Girault, J.B.; Cornea, E.; Goldman, B.D.; et al. Cortical structure and cognition in infants and toddlers. Cereb. Cortex. 2020, 30, 786–800. [Google Scholar] [CrossRef] [PubMed]
- Adeli, E.; Meng, Y.; Li, G.; Lin, W.; Shen, D. Multi-task prediction of infant cognitive scores from longitudinal incomplete neuroimaging data. NeuroImage. 2019, 185, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Adeli, E.; Wu, Z.; Li, G.; Lin, W.; Shen, D. Infant brain development prediction with latent partial multi-view representation learning. IEEE Trans. Med. Imaging. 2018, 38, 909–918. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, J.; Ni, H.; et al. Infant Cognitive Scores Prediction with Multi-stream Attention-Based Temporal Path Signature Features. In International Conference on Medical Image Computing and Computer-Assisted Intervention; Springer, 2020; pp. 134–144. [Google Scholar]
- Squeglia, L.M.; Jacobus, J.; Sorg, S.F.; Jernigan, T.L.; Tapert, S.F. Early adolescent cortical thinning is related to better neuropsychological performance. J. Int. Neuropsychol. Soc. 2013, 19, 962–970. [Google Scholar] [CrossRef]
- Yang, J.J.; Yoon, U.; Yun, H.J.; et al. Prediction for human intelligence using morphometric characteristics of cortical surface: partial least square analysis. Neuroscience. 2013, 246, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wee, C.Y.; Suk, H.I.; Tang, X.; Shen, D. MRI-based intelligence quotient (IQ) estimation with sparse learning. PLoS ONE 2015, 10, e0117295. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Shamosh, N.A.; Cho, S.H.; et al. Multiple bases of human intelligence revealed by cortical thickness and neural activation. J. Neurosci. 2008, 28, 10323–10329. [Google Scholar] [CrossRef]
- Wright, I.C.; McGuire, P.K.; Poline, J.B.; et al. A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neuroimage. 1995, 2, 244–252. [Google Scholar] [CrossRef]
- Kim, H.; Kim J hoon Possin, K.L.; et al. Surface-based morphometry reveals caudate subnuclear structural damage in patients with premotor Huntington disease. Brain Imaging Behav. 2017, 11, 1365–1372. [Google Scholar] [CrossRef]
- Whitwell, J.L. Voxel-based morphometry: an automated technique for assessing structural changes in the brain. J. Neurosci. 2009, 29, 9661–9664. [Google Scholar] [CrossRef] [PubMed]
- Hidese, S.; Ota, M.; Matsuo, J.; et al. Correlation Between the Wechsler Adult Intelligence Scale-3 (rd) Edition Metrics and Brain Structure in Healthy Individuals: A Whole-Brain Magnetic Resonance Imaging Study. Front. Hum. Neurosci 2020, 14. [Google Scholar]
- McDermott, C.L.; Seidlitz, J.; Nadig, A.; et al. Longitudinally mapping childhood socioeconomic status associations with cortical and subcortical morphology. J. Neurosci. 2019, 39, 1365–1373. [Google Scholar] [CrossRef]
- Ramsden, S.; Richardson, F.M.; Josse, G.; et al. Verbal and non-verbal intelligence changes in the teenage brain. Nature. 2011, 479, 113–116. [Google Scholar] [CrossRef]
- Vang, Y.S.; Cao, Y.; Xie, X. A Combined Deep Learning-Gradient Boosting Machine Framework for Fluid Intelligence Prediction. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 1–8. [Google Scholar]
- Pominova, M.; Kuzina, A.; Kondrateva, E.; et al. Ensemble of 3D CNN regressors with data fusion for fluid intelligence prediction. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 158–166. [Google Scholar]
- Zou, Y.; Jang, I.; Reese, T.G.; Yao, J.; Zhu, W.; Rispoli, J.V. Cortical and Subcortical Contributions to Predicting Intelligence Using 3D ConvNets. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 176–185. [Google Scholar]
- Liu, L.; Yu, L.; Wang, S.; Heng, P.A. Predicting Fluid Intelligence from MRI Images with Encoder-Decoder Regularization. In Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction; Springer, 2019; pp. 108–113. [Google Scholar]
- Arrieta, A.B.; Díaz-Rodríguez, N.; Del Ser, J.; et al. Explainable Artificial Intelligence (XAI): Concepts, taxonomies, opportunities and challenges toward responsible AI. Inf. Fusion. 2020, 58, 82–115. [Google Scholar] [CrossRef]
- Gunning, D.; Stefik, M.; Choi, J.; Miller, T.; Stumpf, S.; Yang, G.Z. XAI—Explainable artificial intelligence. Sci. Robot. 2019, 4, eaay7120. [Google Scholar] [CrossRef] [PubMed]
- Speith, T. A review of taxonomies of explainable artificial intelligence (XAI) methods. In 2022 ACM Conference on Fairness, Accountability, and Transparency; 2022; pp. 2239–2250. [Google Scholar]
- Malpas, C.B.; Genc, S.; Saling, M.M.; Velakoulis, D.; Desmond, P.M.; O’Brien, T.J. MRI correlates of general intelligence in neurotypical adults. J. Clin. Neurosci. 2016, 24, 128–134. [Google Scholar] [CrossRef]
- Feng, K.; Rowell, A.C.; Andres, A.; et al. Diffusion tensor MRI of white matter of healthy full-term newborns: relationship to neurodevelopmental outcomes. Radiology. 2019, 292, 179–187. [Google Scholar] [CrossRef]
- Konrad, A.; Vucurevic, G.; Musso, F.; Winterer, G. VBM–DTI correlates of verbal intelligence: a potential link to Broca’s Area. J. Cogn. Neurosci. 2012, 24, 888–895. [Google Scholar] [CrossRef]
- Casson, I.R.; Viano, D.C.; Haacke, E.M.; Kou, Z.; LeStrange, D.G. Is there chronic brain damage in retired NFL players? Neuroradiology, neuropsychology, and neurology examinations of 45 retired players. Sports Health. 2014, 6, 384–395. [Google Scholar] [CrossRef]
- Lee, S.J.; Steiner, R.J.; Yu, Y.; et al. Common and heritable components of white matter microstructure predict cognitive function at 1 and 2 y. Proc. Natl. Acad. Sci. 2017, 114, 148–153. [Google Scholar] [CrossRef]
- Zhang, Z.; Allen, G.I.; Zhu, H.; Dunson, D. Tensor network factorizations: Relationships between brain structural connectomes and traits. Neuroimage. 2019, 197, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Gore, J.C.; Li, M.; Gao, Y.; et al. Functional MRI and resting state connectivity in white matter-a mini-review. Magn. Reson. Imaging. 2019, 63, 1–11. [Google Scholar] [CrossRef]
- Shirer, W.R.; Ryali, S.; Rykhlevskaia, E.; Menon, V.; Greicius, M.D. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb. Cortex. 2012, 22, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Zhou, Y.; Li, J.; et al. Brain spontaneous functional connectivity and intelligence. Neuroimage. 2008, 41, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.; Kim, H.; Kim, H.; Youm, Y.; Chey, J. Distributed functional connectivity predicts neuropsychological test performance among older adults. Hum. Brain Mapp. Published online. 2021. [CrossRef]
- Finn, E.S.; Shen, X.; Scheinost, D.; et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat. Neurosci. 2015, 18, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.A.; Garcia, J.O.; Yeh, F.C.; Vettel, J.M.; Verstynen, T. Local connectome phenotypes predict social, health, and cognitive factors. Netw. Neurosci. 2018, 2, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Sripada, C.; Rutherford, S.; Angstadt, M.; et al. Prediction of neurocognition in youth from resting state fMRI. Mol. Psychiatry. 2020, 25, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Qi, S.; Du, Y.; et al. Predicting individualized intelligence quotient scores using brainnetome-atlas based functional connectivity. 2017 IEEE 27th International Workshop on Machine Learning for Signal Processing (MLSP); IEEE, 2017; pp. 1–6. [Google Scholar]
- Hart, S.J.; Davenport, M.L.; Hooper, S.R.; Belger, A. Visuospatial executive function in Turner syndrome: functional MRI and neurocognitive findings. Brain. 2006, 129, 1125–1136. [Google Scholar] [CrossRef]
- Greene, A.S.; Gao, S.; Scheinost, D.; Constable, R.T. Task-induced brain state manipulation improves prediction of individual traits. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Elliott, M.L.; Knodt, A.R.; Cooke, M.; et al. General functional connectivity: Shared features of resting-state and task fMRI drive reliable and heritable individual differences in functional brain networks. Neuroimage. 2019, 189, 516–532. [Google Scholar] [CrossRef]
- He, T.; Kong, R.; Holmes, A.; et al. Do deep neural networks outperform kernel regression for functional connectivity prediction of behavior. BioRxiv Published online. 2018, 473603. [Google Scholar]
- Li, C.; Yang, G.; Li, M.; Li, B. Fluid intelligence relates to the resting state amplitude of low-frequency fluctuation and functional connectivity: a multivariate pattern analysis. NeuroReport. 2018, 29, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.; Galdi, P.; Han, Y.; Paul, L.K.; Adolphs, R. Resting-state functional brain connectivity best predicts the personality dimension of openness to experience. Personal Neurosci. 2018, 1. [Google Scholar] [CrossRef]
- Yoo, K.; Rosenberg, M.D.; Noble, S.; Scheinost, D.; Constable, R.T.; Chun, M.M. Multivariate approaches improve the reliability and validity of functional connectivity and prediction of individual behaviors. Neuroimage. 2019, 197, 212–223. [Google Scholar] [CrossRef]
- Noble, S.; Spann, M.N.; Tokoglu, F.; Shen, X.; Constable, R.T.; Scheinost, D. Influences on the test–retest reliability of functional connectivity MRI and its relationship with behavioral utility. Cereb. Cortex. 2017, 27, 5415–5429. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, A.M.; Grady, D.; Barrett-Connor, E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. Arch. Intern. Med. 2002, 162, 1737–1745. [Google Scholar] [CrossRef]
- Cosgrove, K.P.; Mazure, C.M.; Staley, J.K. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol. Psychiatry. 2007, 62, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Sowell, E.R.; Thompson, P.M.; Leonard, C.M.; Welcome, S.E.; Kan, E.; Toga, A.W. Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. 2004, 24, 8223–8231. [Google Scholar] [CrossRef]
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999, 2, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Herting, M.M.; Johnson, C.; Mills, K.L.; et al. Development of subcortical volumes across adolescence in males and females: A multisample study of longitudinal changes. NeuroImage. 2018, 172, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Nagel, B.J.; Herting, M.M.; Maxwell, E.C.; Bruno, R.; Fair, D. Hemispheric lateralization of verbal and spatial working memory during adolescence. Brain Cogn. 2013, 82, 58–68. [Google Scholar] [CrossRef]
- Vendetti, M.S.; Johnson, E.L.; Lemos, C.J.; Bunge, S.A. Hemispheric differences in relational reasoning: novel insights based on an old technique. Front. Hum. Neurosci. 2015, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Steffener, J.; Habeck, C.; O’Shea, D.; Razlighi, Q.; Bherer, L.; Stern, Y. Differences between chronological and brain age are related to education and self-reported physical activity. Neurobiol. Aging. 2016, 40, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Hackman, D.A.; Cserbik, D.; Chen, J.C.; et al. Association of Local Variation in Neighborhood Disadvantage in Metropolitan Areas With Youth Neurocognition and Brain Structure. JAMA Pediatr. Published online. 2021, e210426–e210426. [Google Scholar] [CrossRef]
- Skotting, M.B.; Eskildsen, S.F.; Ovesen, A.S.; et al. Infants with congenital heart defects have reduced brain volumes. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, M.E.; Lambert, H.; Ganeshamoorthy, S.; Brossard-Racine, M. Structural brain abnormalities in adolescents and young adults with congenital heart defect: a systematic review. Dev. Med. Child. Neurol. 2018, 60, 1209–1224. [Google Scholar] [CrossRef]
- Asschenfeldt, B.; Evald, L.; Heiberg, J.; et al. Neuropsychological status and structural brain imaging in adults with simple congenital heart defects closed in childhood. J. Am. Heart. Assoc. 2020, 9, e015843. [Google Scholar] [CrossRef]
- Oster, M.E.; Watkins, S.; Hill, K.D.; Knight, J.H.; Meyer, R.E. Academic outcomes in children with congenital heart defects: a population-based cohort study. Circ. Cardiovasc. Qual. Outcomes. 2017, 10, e003074. [Google Scholar] [CrossRef]
- Savory, K.; Manivannan, S.; Zaben, M.; Uzun, O.; Syed, Y.A. Impact of copy number variation on human neurocognitive deficits and congenital heart defects: a systematic review. Neurosci. Biobehav. Rev. 2020, 108, 83–93. [Google Scholar] [CrossRef]
- Derridj, N.; Guedj, R.; Calderon, J.; et al. Long-term Neurodevelopmental Outcomes of Children with Congenital Heart Defects. J. Pediatr. Published online. 2021. [CrossRef]
- Huang, S.C.; Pareek, A.; Seyyedi, S.; Banerjee, I.; Lungren, M.P. Fusion of medical imaging and electronic health records using deep learning: a systematic review and implementation guidelines. NPJ Digit Med. 2020, 3, 1–9. [Google Scholar] [CrossRef]
- Guyon, I.; Elisseeff, A. An introduction to variable and feature selection. J. Mach. Learn Res. 2003, 3, 1157–1182. [Google Scholar]
- Li, J.; Cheng, K.; Wang, S.; et al. Feature selection: A data perspective. ACM Comput. Surv. CSUR. 2017, 50, 1–45. [Google Scholar] [CrossRef]
- Smith, S.M.; Nichols, T.E. Statistical challenges in “big data” human neuroimaging. Neuron. 2018, 97, 263–268. [Google Scholar] [CrossRef]
- He, S.; Pereira, D.; Perez, J.D.; et al. Multi-channel attention-fusion neural network for brain age estimation: Accuracy, generality, and interpretation with 16,705 healthy MRIs across lifespan. Med. Image Anal. 2021, 72, 102091. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Grant, P.E.; Ou, Y. Global-Local Transformer for Brain Age Estimation. IEEE Trans. Med. Imaging. Published online. 2021. [CrossRef] [PubMed]
- Brookes, A.J.; Robinson, P.N. Human genotype–phenotype databases: aims, challenges and opportunities. Nat. Rev. Genet. 2015, 16, 702–715. [Google Scholar] [CrossRef]
- Sterling, L.H.; Liu, A.; Ganni, E.; et al. Neurocognitive disorders amongst patients with congenital heart disease undergoing procedures in childhood. Int. J. Cardiol. Published online. 2021. [CrossRef]
- Calderon, J.; Bellinger, D.C. Executive function deficits in congenital heart disease: why is intervention important? Cardiol. Young. 2015, 25, 1238–1246. [Google Scholar] [CrossRef]
- Cole, J.H.; Leech, R.; Sharp, D.J.; Initiative, A.D.N. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann. Neurol. 2015, 77, 571–581. [Google Scholar] [CrossRef]
- Cole, J.H.; Ritchie, S.J.; Bastin, M.E.; et al. Brain age predicts mortality. Mol. Psychiatry 2018, 23, 1385. [Google Scholar] [CrossRef]
- Franke, K.; Ziegler, G.; Klöppel, S.; Gaser, C.; Initiative, A.D.N. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage. 2010, 50, 883–892. [Google Scholar] [CrossRef]
- Franke, K.; Gaser, C.; Manor, B.; Novak, V. Advanced BrainAGE in older adults with type 2 diabetes mellitus. Front. Aging Neurosci. 2013, 5, 90. [Google Scholar] [CrossRef] [PubMed]
- Antonova, E.; Sharma, T.; Morris, R.; Kumari, V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr. Res. 2004, 70, 117–145. [Google Scholar] [CrossRef] [PubMed]
- Berkelhammer, L.D.; Williamson, A.L.; Sanford, S.D.; et al. Neurocognitive sequelae of pediatric sickle cell disease: a review of the literature. Child. Neuropsychol. 2007, 13, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Shields, L.B.; Choucair, A.K. Management of low-grade gliomas: a review of patient-perceived quality of life and neurocognitive outcome. World Neurosurg. 2014, 82, e299–e309. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, S.; Terrando, N. Narrative review article: neuroinflammation and perioperative neurocognitive disorders. Anesth. Analg. 2019, 128, 781. [Google Scholar] [CrossRef] [PubMed]
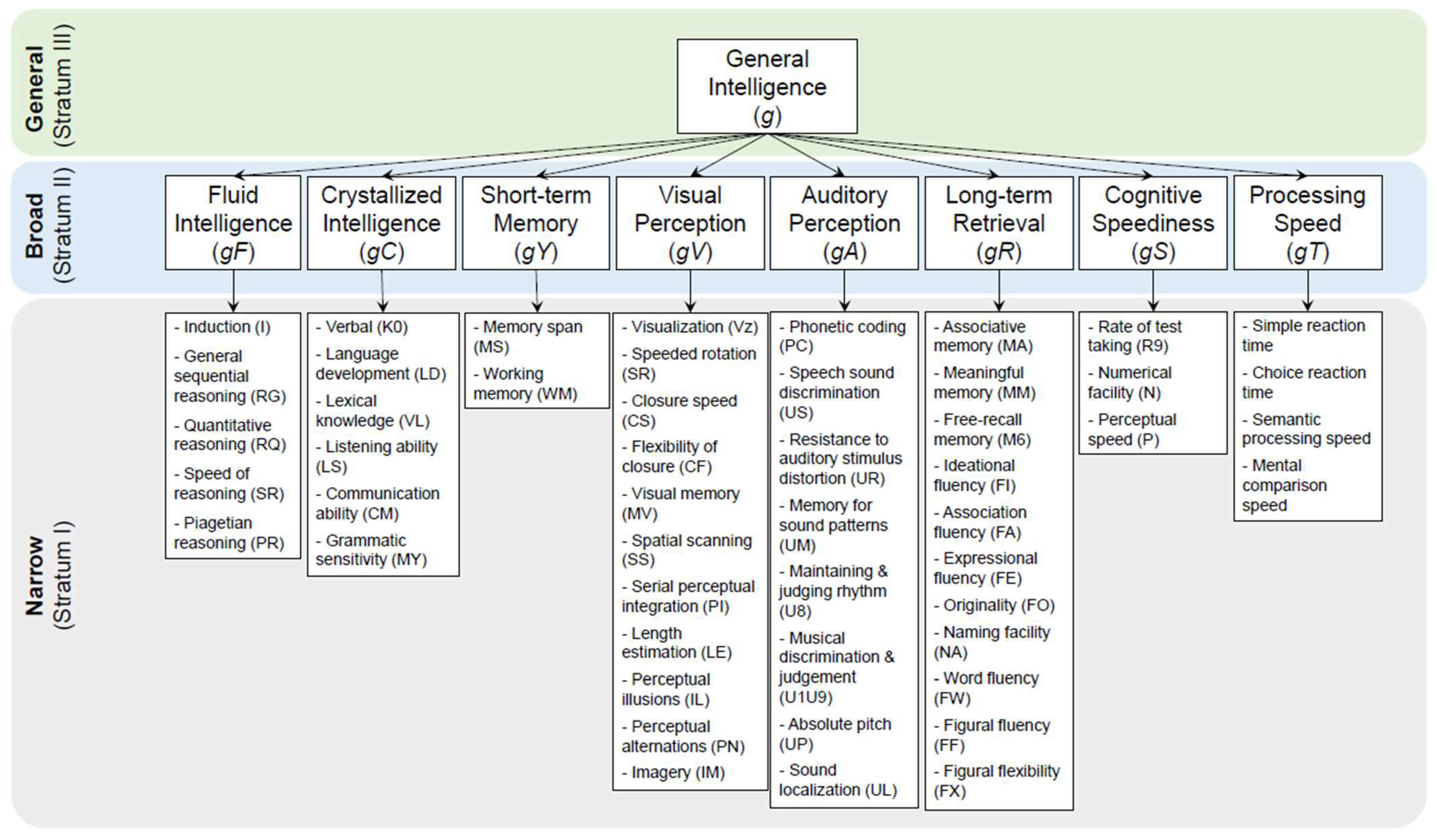
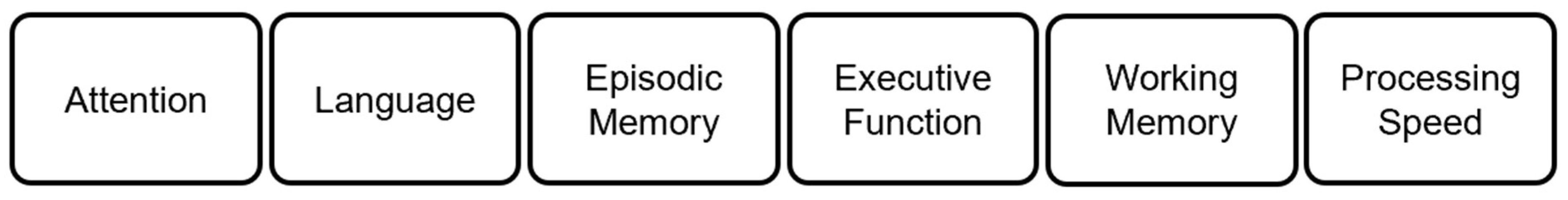
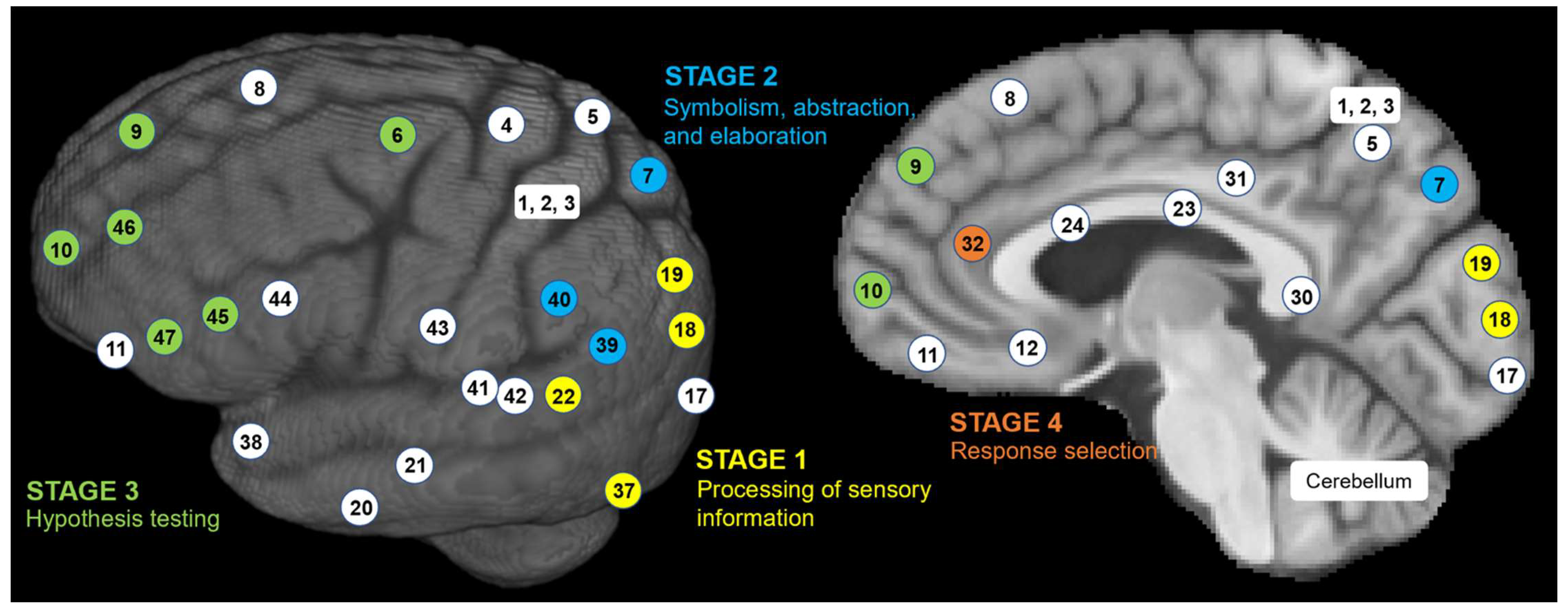
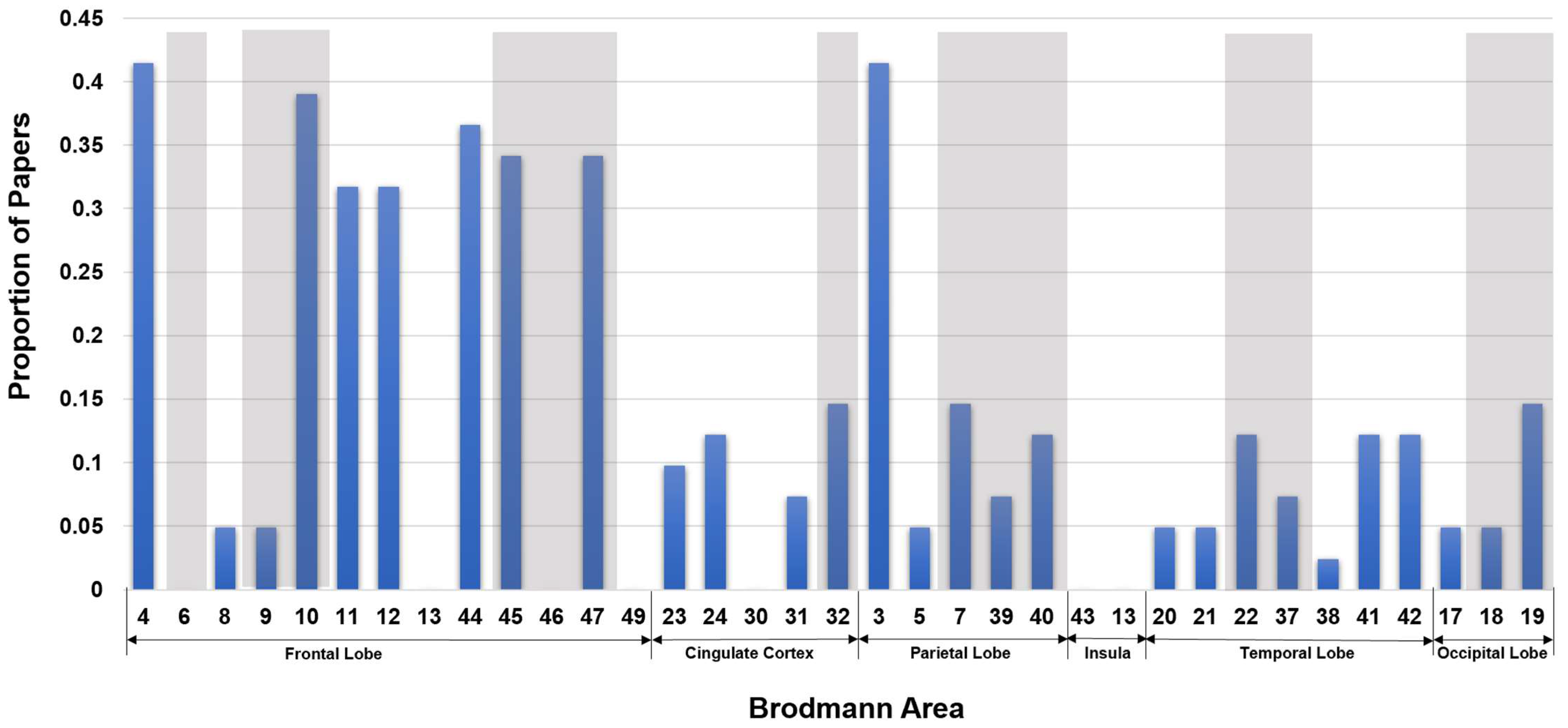
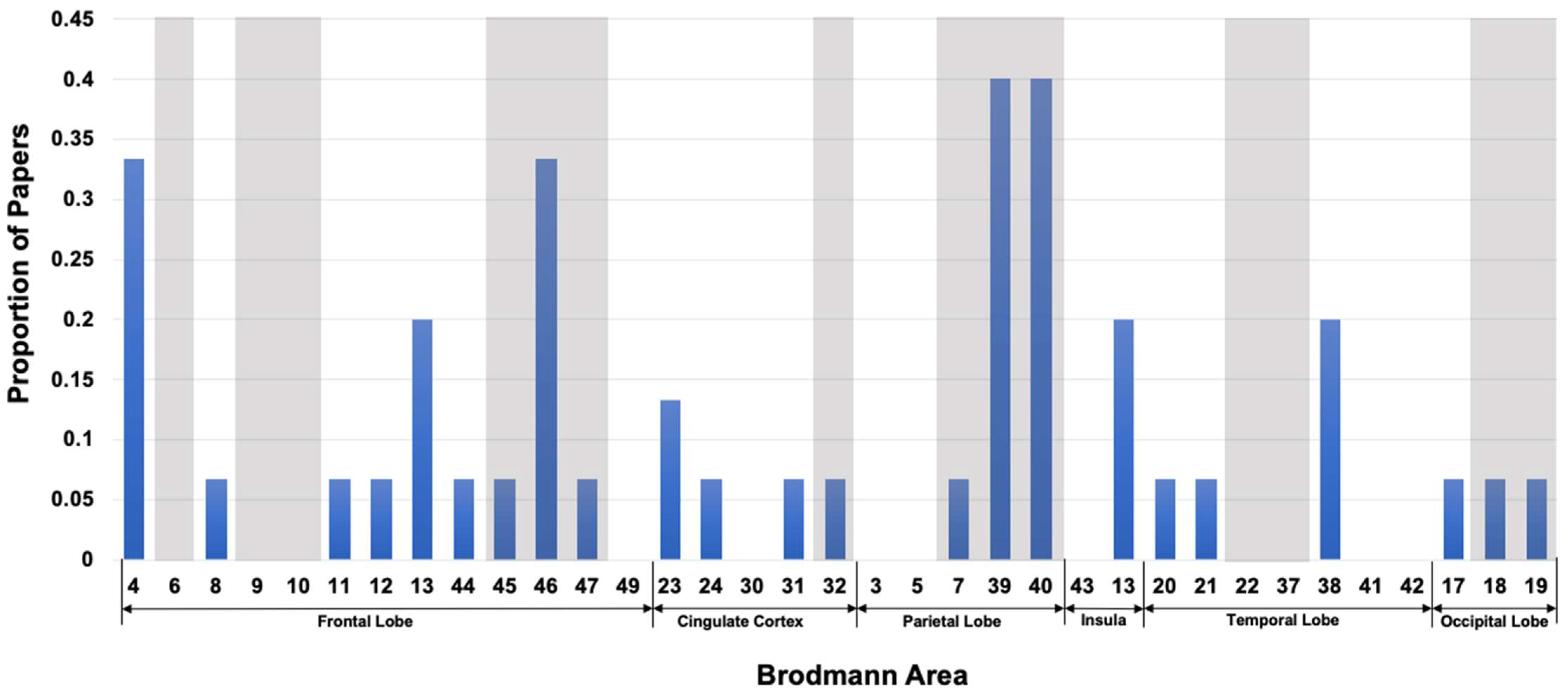
| FrontalLobe | CingulateCortex | ParietalLobe | Insula | TemporalLobe | OccipitalLobe | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BA Name | Primary motor cortex | Premotor & Suppl. Motor cortex | Frontal eye field | Dorsolateral prefrontal cortex | Anterior prefrontal cortex | Orbital and rectus gyri | Orbitofrontal area | Insular cortex | Pars opercularis | Pars opercularis | Dorsolateral prefrontal cortex | Pars orbitalis | Parasubicular area | Ventral posterior cingulate cortex | Ventral anterior cingulate cortex | Part of cingulate cortex | Dorsal Posterior cingulate cortex | Dorsal anterior cingulate cortex | Primary Somatosensory Cortex | Somatosensory Assoc. Cortex | Somatosensory Assoc. Cortex | Angular gyrus | Supramarginal gyrus | Primary gustatory cortex | Insular cortex | Inferior temporal gyrus | Middle temporal gyrus | Superior temporal gyrus | Fusiform gyrus | Temporopolar area | Auditory cortex | Auditory cortex | Primary visual cortex (V1) | Secondary visual cortex (V2) | Associative visual cortex (V3-5) | Cerebellum |
| BA # | 4 | 6 | 8 | 9 | 10 | 11 | 12 | 13 | 44 | 45 | 46 | 47 | 49 | 23 | 24 | 30 | 31 | 32 | 3 | 5 | 7 | 39 | 40 | 43 | 13 | 20 | 21 | 22 | 37 | 38 | 41 | 42 | 17 | 18 | 19 | |
| LH | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||
| RH | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Data Sources | ABCD | ABIDE-I | ABIDE-II | ADNI-1 | ADNIGO/2 | ADNI-3 | AIBL | BeijingEn | BeijingEOEC | BeijingEOEC-II | Berilin | BGSP | CamCAN | CMI | CoRR | DLBS | NIH-PD | NKI-Rockland | Enhan. NKI-RS | HCP-Dev. | HCP-Y.A. | HCP-Aging | Huaxi | IXI-600 | MCIC | NIFD | NYU | OASIS-3 | PING | PNC | PPMI | QValencia | SALD | SLIM | 1000FC | COBRE | NMorphCH | UKBB | ||
| N | 11,873 | 567 | 593 | 229 | 188 | 106 | 610 | 180 | 48 | 20 | 50 | 1,570 | 653 | 2,694 | 1,532 | 315 | 548 | 207 | 1,335 | 654 | 1,206 | 689 | 58 | 595 | 95 | 140 | 49 | 604 | 1,493 | 1,445 | 74 | 45 | 494 | 580 | 1,181 | 74 | 44 | 2,201 | ||
| MRI | Structural MRI | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Diffusion MRI | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Functional MRI | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| IQ | General Intelligence | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||||||||||
| Crystalized Intelligence | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||||||
| Fluid Intelligence | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||
| Academic Achievement | X | X | X | X | X | |||||||||||||||||||||||||||||||||||
| Cognition Battery | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||||||||
| Reaction Time | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||
| Neurocognition | Language | Expressive Vocabulary | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||||||||
| Receptive Language | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||||||
| Verbal Fluency | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||||||
| Reading | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||||||||||
| Memory | Short-term Memory | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||||
| Episodic Memory | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||
| Semantic Memory | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||||||
| Executive Function | General | X | X | X | X | X | X | |||||||||||||||||||||||||||||||||
| Attention | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||||
| Strategy | X | X | X | X | X | X | X | X | X | |||||||||||||||||||||||||||||||
| Cognitive Flexibility | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||||||||||
| Visuospatial | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||||||
| Neurodevelopment | X | X | X | |||||||||||||||||||||||||||||||||||||
| Global Cognition | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||||||||
| Everyday Functionality | X | X | X | X | X | X | X | X | ||||||||||||||||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





