Introduction
Polycythemia vera (PV) is a clonal proliferation of the erythroid lineage due to a mutation of hematopoietic stem cells [
1]. Secondary polycythemia is a complication of the altered hematopoietic cells environment, often related to the hyperproduction of erythropoietin (EPO) [
2]. The main risk of polycythemia is related to thromboembolic events.
Observations have shown that patients with polycythemia are iron deficient at the time of diagnosis [
3,
4,
5]. The paradoxical association between polycythemia and iron deficiency is mostly reported during PV. Therapeutic phlebotomy is one of the causes of iron deficiency. Other more complex hypotheses have been described, some of which are still debated [
6].
Studies of iron deficiency during polycythemia are limited in the literature. Some authors have suggested that iron deficiency is specific to PV. Others have reported a high risk of thrombosis [
7]. In order to provide new insights into the understanding of this association, we conducted the present study. Our main objective was to report the prevalence of iron deficiency during polycythemia. In a second step, we evaluated the diagnostic performance of serum ferritin in PV and describe the pathophysiological mechanisms.
Setting and type of study
This is a retrospective descriptive and analytical study, carried out in the internal medicine department of the Henri Mondor Hospital, Aurillac, France.
Population and study period
The study concerned patients over 18 years of age, registered and followed in this department during a 12-year period, from January 1, 2010 to December 31, 2021.
Inclusion/exclusion criteria
We included patients diagnosed with polycythemia. We excluded patients who did not have a biological assessment of iron deficiency before therapeutic phlebotomy.
Studied parameters
We collected demographic characteristics, body mass index (BMI), blood count with mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC), C-reactive protein (CRP), transaminases, lactate dehydrogenase (LDH) and EPO levels, isotopic measure of red blood cell volume, and janus kinase 2 (JAK2) mutation testing. Serum ferritin level and transferrin saturation coefficient (TSC) performed before therapeutic phlebotomy were collected. In patients with PV, we collected the result of bone marrow cytology, treatment, iron supplementation, and the latest available serum ferritin values.
Definitions
Polycythemia was defined as elevated hemoglobin greater than 16.0 g/dL in women and 16.5 g/dL in men, or hematocrit greater than 48% and 49%, respectively, after eliminating dehydration and hypovolemia. PV was defined according to the World Health Organization (WHO) criteria revised in 2016 [
8]. The diagnosis requires meeting either the three major criteria or the first two major criteria and the minor criterion. The absence of JAK2 mutation associated with serum EPO level above the normal value was suggestive of secondary polycythemia.
Iron deficiency was defined as a serum ferritin level less than 30 ng/mL or TSC less than 20% [
9].
Statistical analysis
Data were analyzed with Epi Info 7 software. Frequencies and means were calculated by descriptive statistics. Continuous variables were presented as means and categorical variables were expressed as frequencies and percentages. The Chi-square test was used in the univariate analysis. Values with p <0.05 were accepted as statistically significant.
We considered the JAK2 mutation as the gold standard for the diagnosis of PV. We calculated the sensitivity, specificity, and positive and negative predictive values of serum ferritin and JAK2 mutation.
Polycythemia
One hundred and fourteen patients were included. PV was found in 33.33% of cases and secondary polycythemia in 66.67% of cases.
Demographic characteristics
The mean age of patients was 61.79 years (± 15.44) with extremes of 19 and 89 years. The sex ratio was 4.43. In the PV group, the mean age was 65.13 years (± 16.5) and the sex ratio was 1.71. In the secondary polycythemia group, the mean age was 60.11 (± 14.7) and the sex ratio was 9.86. The demographic characteristics of the patients are represented in
Table 1.
Biological characteristics
Mean hemoglobin and hematocrit levels were 17.89 g/dL and 53.68%, respectively. The mean serum ferritin level was 223.72 ng/mL with extremes of 1.6 and 977 µg/L and the mean TSC was 32.78% with extremes of 5.8 and 56.5%. Isotopic measure of red blood cell volume was performed in 11 patients and the mean level was 125.64%. The JAK2 mutation was found in 84.21% of patients in the PV group and was VF617F in all cases. Seven patients had a bone marrow cytology result: five cases of erythroid hyperplasia and myelofibrosis, one case of global hyperplasia and one case of myelodysplasia.
Table 2 shows the biological characteristics of the patients.
Prevalence of iron deficiency
Twenty-four patients had iron deficiency, a prevalence of 21.05%. The prevalence was 60.53% in the PV group and 1.32% in the secondary polycythemia group (
Table 3). The risk of iron deficiency was significantly elevated in the PV group with 52.63% sensitivity and 100% specificity (
Table 4).
Iron deficiency and thrombosis
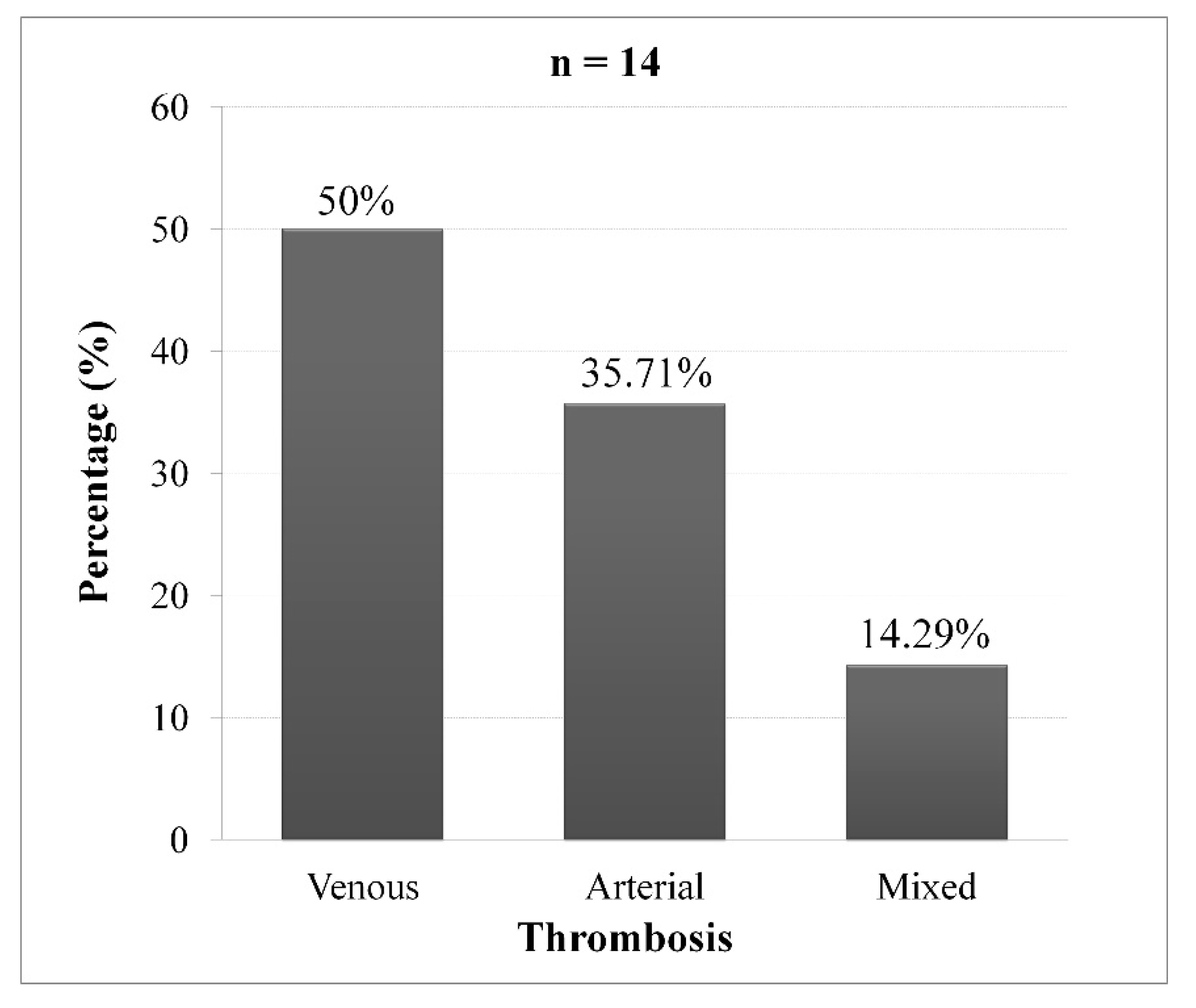
Among the 38 patients diagnosed with PV, 14 patients (36.84%) had thrombosis. The proportion of thrombosis was 43.48% in patients with iron deficiency (p = 0.241 and OR = 2.11; CI95% [0.51 - 8.66]). Arterial thrombosis was found in 50% of cases (
Figure 1).
Treatment
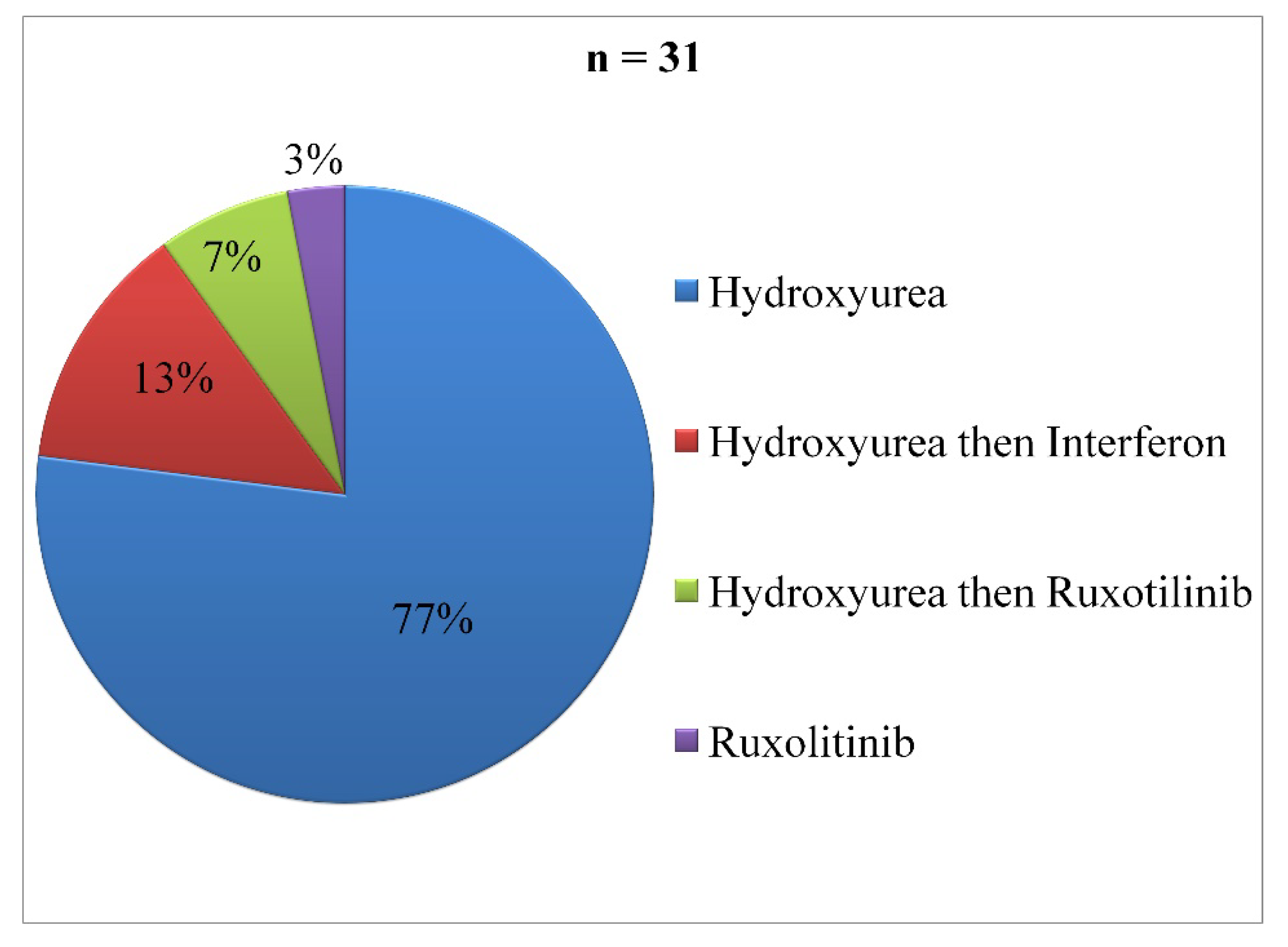
In the PV group, 81.58% of cases had received pharmacological treatment. Hydroxyurea was used in 77% of cases (
Figure 2). The mean duration of treatment was 36.36 months (± 23.43) with a minimum of five months and a maximum of 108 months. Therapeutic phlebotomy was performed in 55.26% of cases. Two patients (5.26%) had received iron supplementation. During follow-up, only four patients had a serum ferritin control, represented in
Table 5.
Polycythemia
Epidemiologically, polycythemia is often difficult to report because of its primary or secondary origin. For PV, the annual incidence ranges from 0.4 to 2.8 cases per 100,000 population [
10]. The incidence rate increases in patients older than 50 years and men are the most affected [
11].
PV is one of the BCR-ABL negative myeloproliferative neoplasia including essential thrombocythemia and primary myelofibrosis [
1]. It is characterized by a mutation of hematopoietic stem cells. The JAK2 mutation in exon 14 or JAK2V617F is encountered in 95-99% of cases. The rest (1-5%) are mutations in exon 12 [
12,
13]. In our study, the JAK2V617F mutation was found in 84.21% of patients in the PV group.
Secondary polycythemia is characterized by the elevation of erythrocyte mass during certain situations: hypoxia, malignancies (hepatocellular carcinoma, renal cancer, parathyroid carcinoma, cerebral hemangioblastoma) and benign tumors (renal cyst, uterine leiomyoma, meningioma) [
14]. The two main causes of secondary polycythemia are smoking and obstructive sleep apnea [
2].
The need for diagnostic criteria to distinguish PV from secondary polycythemia has been studied repeatedly [
15,
16,
17]. The diagnostic criteria established by the Polycythemia Vera Study Group emphasize the importance of bone marrow examination.
17 These criteria have been criticized by several authors [
16,
18]. Nowadays, bone marrow examination, clinical exclusion of secondary polycythemia, JAK2 mutation and EPO testing are the most accepted criteria.
Iron deficiency
Globally, iron deficiency is the leading cause of anemia making iron deficiency anemia a major health problem. Iron deficiency anemia is most prevalent in children, pregnant women and women of childbearing age. In the elderly, it accounts for 30% of anemia cases and is often difficult to treat [
19].
There are two types of iron deficiency: absolute and functional [
9]. Absolute iron deficiency is the reduction of iron stores in macrophages and hepatocytes. It can occur in cases of increased requirement, decreased intake, malabsorption or chronic bleeding. Functional iron deficiency is secondary to the mobilization of iron stores into the circulation following chronic inflammation or infection.
Serum ferritin is the most reliable test commonly used to diagnose iron deficiency. A ferritin threshold of 30 ng/mL has a sensitivity of 92% and specificity of 98% [
9]. In the presence of inflammation, a low TSC (<20%) can be used [
20]. Hypochromia and microcytosis are not specific. Other second-line tests have been proposed, such as cross-linked hemoglobin, soluble transferrin receptor and hepcidin level [
9].
Iron homeostasis is mainly controlled by hepcidin, a peptide produced by hepatocytes, which plays a role in intestinal absorption and transport of iron to tissues [
21]. Hepcidin reduces the utilization of iron in cells and prevents its accumulation in tissues. Hepcidin expression is elevated during iron overload, inflammation and infection. It is decreased during iron deficiency, hypoxia or high erythropoiesis activity.
Iron deficiency in polycythemia
In recent decades, the association between polycythemia and iron deficiency has become a much discussed topic. Iron deficiency has been mostly observed in patients with JAK2 mutation [
6]. In the present study, the prevalence of iron deficiency was high (60.53%) in patients with PV with increased risk (OR = 115; CI95% [14.4 - 918.2]). Hypothesizing that iron deficiency could be a diagnostic criterion for PV, the diagnostic performance evaluation showed a specificity of 100%.
Iron deficiency may have an impact on the diagnostic management of polycythemia. The clinical signs of iron deficiency may overlap with those of polycythemia. Polycythemia may be masked due to microcytosis or anemia. This problem has been reported in several cases [
22,
23]. The diagnosis may be delayed, increasing morbidity and mortality.
Excluding therapeutic phlebotomy, iron deficiency during PV results from several concomitant factors. First, hematopoietic activity results in the extensive use of iron in hemoglobin synthesis. Secondly, the role of hepcidin has been suggested by some authors, reporting an increase in levels during PV [
6]. Hepcidin production could be a defense mechanism against erythroid activity. This hypothesis remains inconclusive as other authors have observed normal hepcidin levels [
24]. Third, the physiologically hypoxic intestinal lumen promotes iron absorption independently of hepcidin. During PV, reduced hypoxia prevents iron absorption in duodenal enterocytes [
25]. Fourth, iron deficiency can be explained by an aberrant iron restriction response. This response diverts iron intended for hemoglobin synthesis away from the needs of other cellular functions [
26]. Finally, the usual causes of iron deficiency can occur, including digestive bleeding.
Complications of PV are related to thrombosis (41%), progression to myelofibrosis (10-20%) and blast phase (3-10%). Authors have shown that the presence of iron deficiency is associated with an increased risk of thromboembolic events [
7]. In our study, this association was not significant.
Treatment
The primary goal of PV treatment is to maintain the hematocrit level below 45% to reduce the risk of thrombosis [
27]. Patients younger than 60 years with low risk of thrombosis are often maintained on therapeutic phlebotomy. In case of repeated phlebotomy frequencies or a high risk of thrombosis, cytoreductive therapy is indicated. Hydroxyurea is the first choice because of its good tolerance and lower toxicity. Interferon alpha, Ruxolitinib (JAK2 inhibitor) and Busulfan are second-line agents, indicated in case of failure or intolerance to Hydroxyurea [
28].
Treatment of iron deficiency during PV can be complex. Although suggested by some authors, iron supplementation is not recommended because of the progressive increase in red blood cell mass [
29]. In our study, only 5.26% of cases had received iron supplementation.
According to recent studies, Ruxolitinib is able to manage PV by maintaining iron homeostasis and normalizing in case of initial iron deficiency [
28]. Currently, hepcidin mimetics are undergoing clinical trials [
30]. They could induce erythropoiesis without contributing to iron deficiency and reduce the use of cytoreductive agents.
Study strengths and limitations
The study has several limitations. Clinical signs were not studied. It would be interesting to evaluate the signs of polycythemia associated with iron deficiency. There were only four patients with control ferritin, which is not appropriate to conclude the evolution.
However, the study showed that the prevalence of iron deficiency was high in PV and the risk was statistically high. Iron deficiency is less sensitive but very specific in the diagnosis of PV. To our knowledge, this is the first study to determine the prevalence of iron deficiency in both types of polycythemia and to evaluate its diagnostic performance. Contrary to the literature, iron deficiency was not associated with thrombosis.
Our approach emphasizes the routine performance of serum ferritin and CST in the initial evaluation of polycythemia. Further, larger studies are needed to strengthen the specificity of iron deficiency in PV. The mechanisms of iron deficiency are complex and multifactorial. Iron deficiency may mask the diagnosis of polycythemia due to microcytosis and anemia. Cytoreductive agents have been shown to be effective in maintaining iron homeostasis. Iron supplementation is not recommended.
Conclusions
The paradoxical association between polycythemia and iron deficiency represents a diagnostic and therapeutic impact. Iron deficiency is rather specific to PV and may mask the diagnosis of polycythemia. Assessment of iron deficiency should be part of the initial evaluation of polycythemia.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
This study respected the Helsinki principles for research and the ethical guidelines of our institution.
Author contributions
RMF Randrianarisoa: conceptualisation, methodology, data collection, original drafting, writing-reviewing and editing. H Ramanandafy: visualisation. A Mania: conceptualisation, methodology, writing-reviewing and editing. H Monjanel, S Trouillier: visualisation, validation.
Funding
The authors declare that they do not have a grant from any specific organization.
Acknowledgements
Thanks to all the staff of the Internal Medicine Department of the Henri Mondor Aurillac Hospital. Thanks to P. Vernet, F. Mondillon, M. Barrière and M. Turot for their support.
References
- Barbui, T.; Thiele, J.; Gisslinger, H.; Kvasnicka, H.M.; Vannucchi, A.M.; Guglielmelli, P.; Orazi, A.; Tefferi, A. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J. 2018, 8(2), 15. [Google Scholar] [CrossRef] [PubMed]
- Kremyanskaya, M.; Mascarenhas, J.; Hoffman, R. Why does my patient have erythrocytosis? Hematol. Oncol. Clin. North Am. 2012, 26(2), 267–283. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, I.; Kahn, S.B.; Brady, L.W. Polycythemia vera: differencial diagnosis by ferrokinetics studies and treatment with Busulfan (Myleran). Brit. J. Haemat. 1968, 14(4), 351–361. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.; Kvasnicka, H.M.; Muehlhausen, K.; Walter, S.; Zankovich, R.; Diehl, V. Polycythemia rubra vera versus secondary polycythemias. A clinicopathological evaluation of distinctive features in 199 patients. Pathol. Res. Pract. 2001, 197(2), 77–84. [Google Scholar] [CrossRef] [PubMed]
- Gianelli, U.; Iurlo, A.; Vener, C.; Moro, A.; Fermo, E.; Bianchi, P.; Graziani, D.; Radaelli, F.; Coggi, G.; Bosari, S.; et al. The significance of bone marrow biopsy and JAK2V617F mutation in the differential diagnosis between the “early” prepolycythemic phase of polycythemia vera and essential thrombocythemia. Am. J. Clin. Pathol. 2008, 130(3), 336–342. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, Y.Z.; Feola, M.; Zimran, E.; Varkonyi, J.; Ganz, T.; Hoffman, R. Dysregulated iron metabolism in polycythemia vera: etiology and consequences. Leukemia 2018, 32(10), 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.H.; Lin, H.C.; Chung, S.D. Association between venous thromboembolism and iron-deficiency anemia: a population-based study. Blood Coagul. Fibrinolysis 2015, 26(4), 368–372. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127(20), 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Zeller, M.P. Management of iron deficiency. Hematology Am. Soc. Hematol. Educ. Program 2019, 2019(1), 315–322. [Google Scholar] [CrossRef] [PubMed]
- Moulard, O.; Mehta, J.; Fryzek, J.; Olivares, R.; Iqbal, U.; Mesa, R.A. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur. J. Haematol. 2014, 92(4), 289–297. [Google Scholar] [CrossRef]
- Deadmond, M.A.; Smith-Gagen, J.A. Changing incidence of myeloproliferative neoplasms: trends and subgroup risk profiles in the USA, 1973-2011. J. Cancer Res. Clin. Oncol. 2015, 141(12), 2131–2138. [Google Scholar] [CrossRef]
- Marneth, A.E.; Mullally, A. The molecular genetics of myeloproliferative neoplasms. Cold Spring Harb. Perspect. Med. 2020, 10(2), a034876. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Guglielmelli, P.; Lasho, T.L.; Coltro, G.; Finke, C.M.; Loscocco, G.G.; Sordi, B.; Szuber, N.; Rotunno, G.; Pacilli, A.; et al. Mutation-enhanced international prognostic systems for essential thrombocythaemia and polycythaemia vera. Br. J. Haematol. 2020, 189(2), 291–302. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A. Polycythemia vera: a comprehensive review and clinical recommendations. Mayo Clin. Proc. 2003, 78(2), 174–194. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, J.I.; Vardiman, J.W. Hematopathologic findings in the myeloproliferative disorders. Semin. Oncol. 1995, 22(4), 355–373. [Google Scholar] [PubMed]
- Pearson, T.C. Diagnosis and classification of erythrocytoses and thrombocytoses. Baillieres Clin. Haematol. 1998, 11(4), 695–720. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S. Diagnostic criteria and prognosis in polycythemia vera and essential thrombocythemia. Semin. Hematol. 1999, 36(1Suppl 2), 9–13. [Google Scholar]
- Pearson, T.C.; Messinezy, M. The diagnostic criteria of polycythaemia rubra vera. Leuk. Lymphoma 1996, 22 (Suppl 1), 87–93. [Google Scholar] [PubMed]
- Kassebaum, N.J.; GBD 2013 Anemia Collaborators. The global burden of anemia. Hematol. Oncol. Clin. North Am. 2016, 30(2), 247–308. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352(10), 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- De Domenico, I.; Lo, E.; Ward, D.M.; Kaplan, J. Hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Jak2. Proc. Natl. Acad. Sci. USA 2009, 106(10), 3800–3805. [Google Scholar] [CrossRef] [PubMed]
- Kambali, S.; Taj, A. Polycythemia vera masked due to severe iron deficiency anemia. Hematol. Oncol. Stem Cell Ther. 2018, 11(1), 38–40. [Google Scholar] [CrossRef] [PubMed]
- Aladağ, E.; Sağlam, E.A.; Aksu, S.; Demiroğlu, H.; Sayınalp, N.; Göker, H.; Haznedaroğlu, İ.C.; Özcebe, O.İ.; Büyükaşık, Y. Unclassifiable non-CML classical myeloproliferative diseases with microcytosis: findings indicating diagnosis of polycythemia vera masked by iron deficiency. Turk. J. Med. Sci. 2019, 49(5), 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, C.; Tarkun, P.; Birtaş Ateşoğlu, E.; Eraldemir, C.; Özsoy, Ö.D.; Terzi Demirsoy, E.; Mehtap, Ö.; Gedük, A.; Hacıhanefioğlu, A. The role of hepcidin, GDF15, and mitoferrin-1 in iron metabolism of polycythemia vera and essential thrombocytosis patients. Turk. J. Med. Sci. 2019, 49(1), 74–80. [Google Scholar] [CrossRef] [PubMed]
- Schächner, E.; Ronen, M.; Pinkhas, J.; Djaldetti, M. Iron absorption in patients with polycythemia vera: a comparative study using the whole-body counter and the ferrous sulfate absorption test. Eur. J. Nucl. Med. 1978, 3(2), 125–127. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.; Delehanty, L.; Grado, S.; Holy, M.; White, Z. 3rd; Freeman, K.; Kurita, R.; Nakamura, Y.; Bullock, G.; Goldfarb, A. Iron modulation of erythropoiesis is associated with Scribble-mediated control of the erythropoietin receptor. J. Exp. Med. 2018, 215(2), 661–679. [Google Scholar] [CrossRef] [PubMed]
- Marchioli, R.; Vannucchi, A.M.; Barbui, T. Treatment target in polycythemia vera. N. Engl. J. Med. 2013, 368(16), 1556. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, A.M.; Kiladjian, J.J.; Griesshammer, M.; Masszi, T.; Durrant, S.; Passamonti, F.; Harrison, C.N.; Pane, F.; Zachee, P.; Mesa, R.; et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N. Engl. J. Med. 2015, 372(5), 426–435. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.H.; Van der Weyden, M.B.; Young, I.F.; Wiley, J.S. Pruritus and severe iron deficiency in polycythaemia vera. Br. Med. J. (Clin. Res. Ed.), 1982, 285(6335), 91-92. [CrossRef]
- Casu, C.; Nemeth, E.; Rivella, S. Hepcidin agonists as therapeutic tools. Blood 2018, 131(16), 1790–1794. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).