Submitted:
27 February 2023
Posted:
28 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Grazing area and animals
2.2. Intake and digestibility
2.3. Nitrogen metabolism
2.4. Rumen fermentation parameters and microbiota population
2.5. Statistical analyses
3. Results
3.1. Intake and digestibility
| Supplements1 | SEM | P-value2 | ||||||
|---|---|---|---|---|---|---|---|---|
| EW | EPHA | MW | MPHA | ST | PHA | ST × PHA | ||
| Intake (% BW) | ||||||||
| Total DM | 2.52 | 2.42 | 1.87 | 1.97 | 0.08 | 0.001 | 0.977 | 0.623 |
| Forage DM | 2.25 | 2.15 | 1.87 | 1.97 | 0.07 | 0.059 | 0.983 | 0.474 |
| apNDF | 1.30 | 1.26 | 1.16 | 1.10 | 0.10 | 0.121 | 0.595 | 0.949 |
| Intake (kg/d) | ||||||||
| Total DM | 11.60 | 10.92 | 8.75 | 9.15 | 0.38 | 0.001 | 0.810 | 0.386 |
| Forage DM | 10.36 | 9.68 | 8.65 | 9.04 | 0.32 | 0.070 | 0.816 | 0.392 |
| Supplement DM | 1.24 | 1.23 | 0.10 | 0.11 | 0.02 | <0.001 | 0.747 | 0.702 |
| OM | 10.27 | 9.63 | 7.88 | 8.24 | 0.33 | 0.003 | 0.803 | 0.417 |
| CP | 1.79 | 1.66 | 1.39 | 1.39 | 0.06 | 0.006 | 0.551 | 0.559 |
| apNDF | 6.02 | 5.66 | 4.76 | 5.04 | 0.19 | 0.017 | 0.911 | 0.381 |
| GE, MJ/d | 201.5 | 189.0 | 156.4 | 162.3 | 6.35 | 0.004 | 0.761 | 0.411 |
| Digestible energy, MJ/d | 138.6 | 125.6 | 102.8 | 98.51 | 5.45 | 0.002 | 0.340 | 0.627 |
| Metabolizable energy, MJ/d | 117.6 | 107.3 | 85.44 | 79.43 | 8.76 | 0.001 | 0.321 | 0.791 |
| g CP/kg DOM | 247.5 | 249.5 | 259.4 | 262.8 | 13.87 | 0.111 | 0.721 | 0.927 |
| Digestibility (%) | ||||||||
| DM | 67.0 | 65.1 | 62.5 | 60.6 | 0.70 | <0.001 | 0.073 | 0.177 |
| OM | 71.0 | 69.5 | 64.0 | 64.1 | 0.90 | <0.001 | 0.555 | 0.364 |
| CP | 70.0 | 67.3 | 58.9 | 59.7 | 1.31 | <0.001 | 0.515 | 0.235 |
| apNDF | 72.5 | 71.9 | 66.2 | 67.2 | 0.91 | <0.001 | 0.877 | 0.560 |
| GE | 68.5 | 66.3 | 60.0 | 60.6 | 1.02 | <0.001 | 0.536 | 0.219 |
3.2. Nitrogen metabolism
| Supplements1 | SEM | P-value2 | ||||||
|---|---|---|---|---|---|---|---|---|
| EW | EPHA | MW | MPHA | ST | PHA | ST × PHA | ||
| N balance | ||||||||
| N intake, g/d | 286.0 | 266.1 | 222.6 | 222.4 | 9.69 | 0.006 | 0.551 | 0.559 |
| Fecal N excreted, g/d | 89.77 | 91.59 | 79.37 | 84.30 | 2.94 | 0.081 | 0.407 | 0.906 |
| Urinary N excreted, g/d | 116.3a | 96.6ab | 89.7b | 96.1b | 3.93 | 0.006 | 0.271 | 0.033 |
| N retained, g/d | 94.51 | 99.73 | 54.46 | 45.82 | 7.59 | <0.001 | 0.611 | 0.992 |
| N retained, % of N intake | 31.10 | 33.99 | 21.97 | 18.96 | 2.04 | 0.050 | 0.987 | 0.409 |
| Ruminal microbial N synthesis | ||||||||
| Nmic, g N/d | 60.15 | 54.43 | 55.92 | 56.63 | 6.48 | 0.848 | 0.636 | 0.544 |
| Pmic, g protein/d | 375.95 | 340.18 | 349.51 | 353.95 | 40.47 | 0.848 | 0.636 | 0.544 |
| ENmic, g Nmic/kg OMFR | 13.10 | 13.36 | 17.39 | 17.00 | 2.29 | 0.055 | 0.974 | 0.868 |
| EPmic, g Pmic/kg DOM | 53.21 | 54.28 | 70.64 | 69.06 | 9.29 | 0.055 | 0.974 | 0.868 |
| Plasma urea N3, mg/dL | 15.95 | 16.01 | 14.52 | 14.61 | 0.22 | <0.001 | 0.786 | 0.939 |
3.3. Rumen fermentation parameters
| Supplements1 | SEM | P-value2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EW | EPHA | MW | MPHA | ST | PHA | Time | ST × PHA | ST × Time | ||
| pH | 6.25 | 6.14 | 6.35 | 6.34 | 0.02 | 0.115 | 0.691 | <0.001 | 0.679 | 0.726 |
| NH3-N, mg/dL | 17.56 | 15.46 | 14.24 | 14.23 | 0.46 | 0.003 | 0.228 | <0.001 | 0.233 | 0.149 |
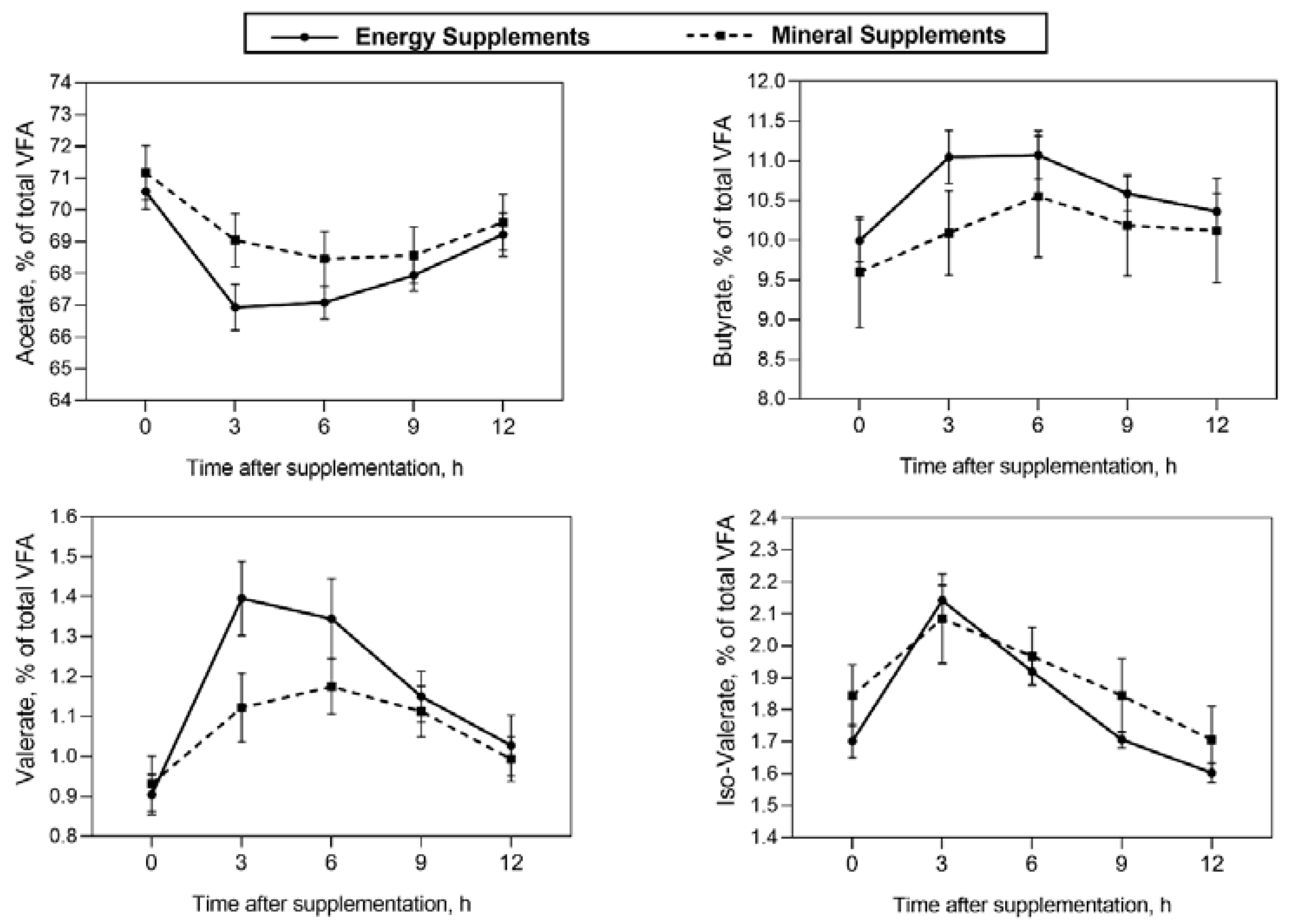
| Total VFA, mmol/L | 117.0 | 112.9 | 115.8 | 118.7 | 1.16 | 0.723 | 0.740 | <0.001 | 0.394 | 0.671 |
| Individual VFA, % of total VFA | ||||||||||
| Acetate | 68.30 | 66.74 | 69.70 | 68.99 | 0.15 | 0.003 | 0.335 | <0.001 | 0.190 | <0.001 |
| Propionate | 17.05 | 16.74 | 16.34 | 16.87 | 0.08 | 0.010 | 0.074 | <0.001 | 0.235 | 0.106 |
| Butyrate | 10.66 | 10.31 | 10.06 | 10.16 | 0.06 | 0.198 | 0.792 | <0.001 | 0.360 | <0.001 |
| iso-Butyrate | 0.95 | 0.93 | 0.93 | 1.13 | 0.01 | 0.406 | 0.635 | <0.001 | 0.978 | 0.458 |
| Valerate | 1.22 | 1.08 | 1.08 | 1.05 | 0.02 | 0.001 | 0.015 | <0.001 | 0.149 | <0.001 |
| iso-Valerate | 1.83 | 1.76 | 1.89 | 1.89 | 0.02 | 0.058 | 0.672 | <0.001 | 0.749 | 0.008 |
| A:P ratio | 4.02 | 3.91 | 4.29 | 4.10 | 0.03 | 0.006 | 0.085 | <0.001 | 0.141 | 0.009 |
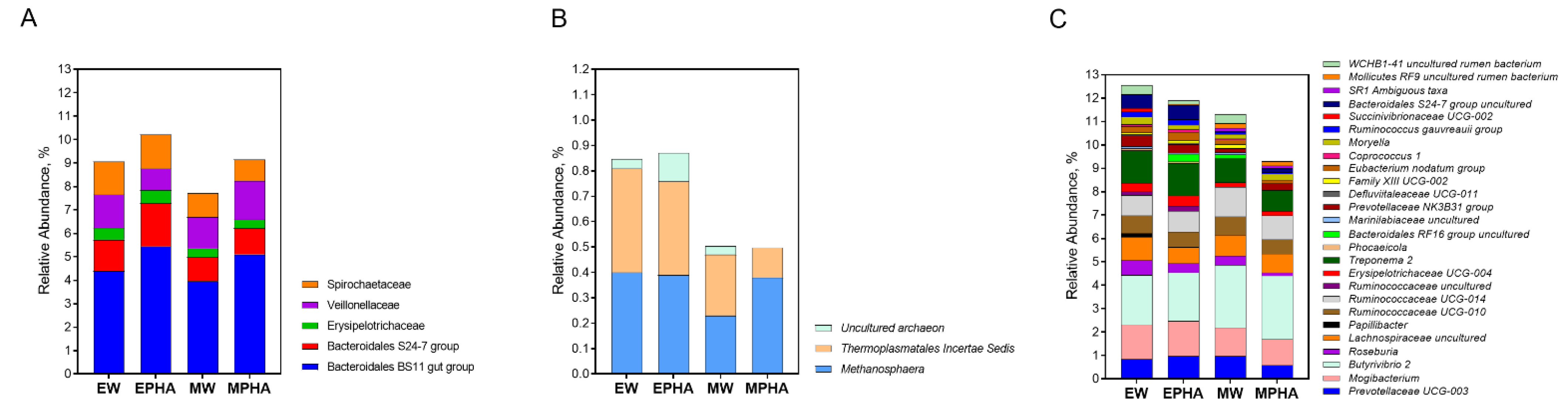
3.4. Ruminal microbiota population
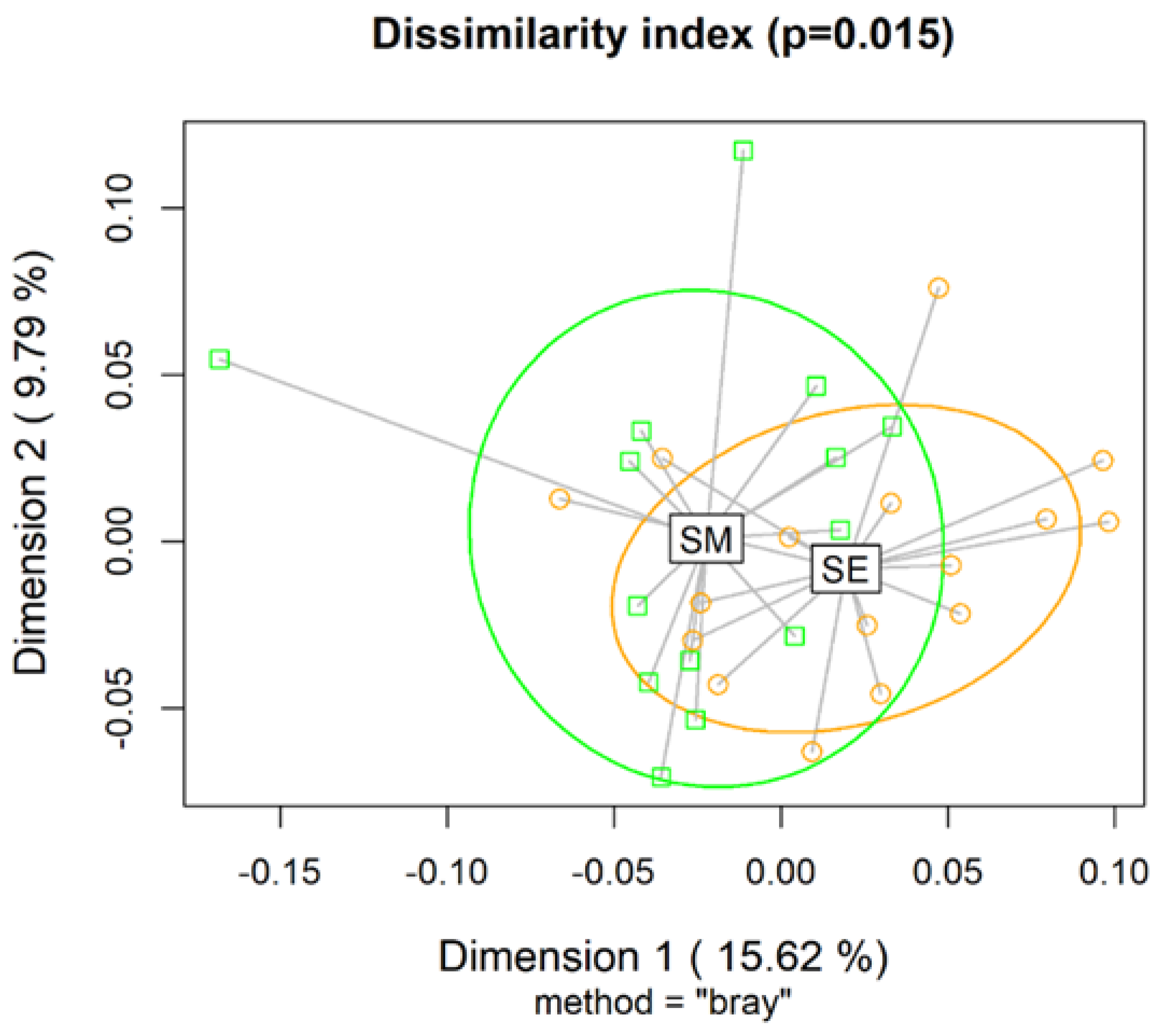
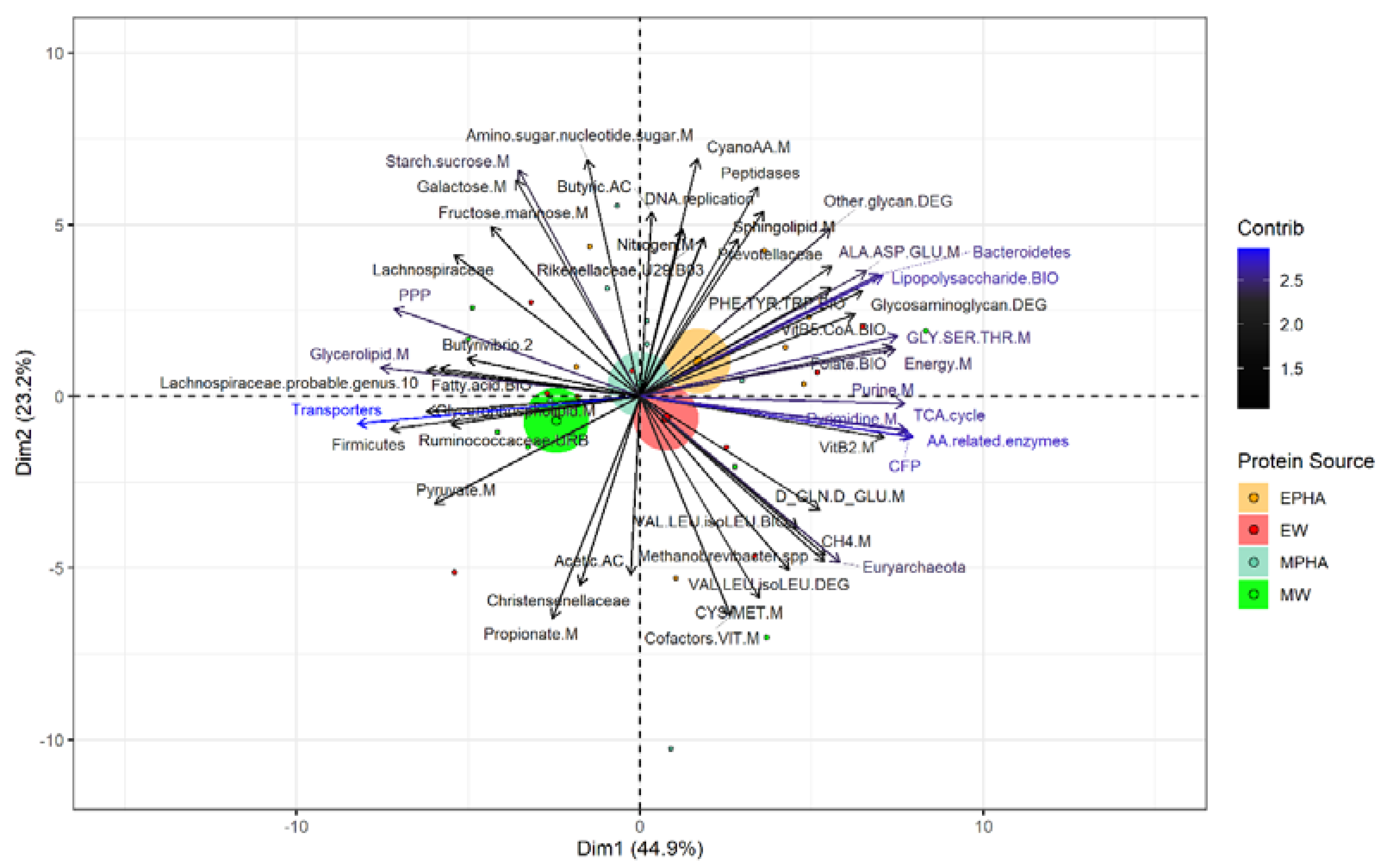
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, K.L.C.A.; Granja-Salcedo, Y.T.; Messana, J.D.; Carneiro de Souza, V.; Generoso Ganga, M.J.; Detogni Colovate, P.H.; Kishi, L.T.; Berchielli, T.T. Rumen bacterial diversity in relation to nitrogen retention in beef cattle. Anaerobe 2021, 67, 102316. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, L.O.; Callaway, T.R.; Muir, J.P.; Anderson, R.C. Potential environmental benefits of feed additives and other strategies for ruminant production. Rev. Bras. Zootec. 2011, 40, 291–309. [Google Scholar]
- Clark, S.; Daly, R.; Jordan, E.; Lee, J.; Mathew, A.; Ebner, P. The future of biosecurity and antimicrobial use in livestock production in the United States and the role of extension. J. Anim. Sci. 2012, 90, 2852–2861. [Google Scholar] [CrossRef] [PubMed]
- Aboagye, I.A.; Oba, M.; Castillo, A.R.; Koenig, K.M.; Iwaasa, A.D.; Beauchemin, K.A. Effects of hydrolyzable tannin with or without condensed tannin on methane emissions, nitrogen use, and performance of beef cattle fed a high-forage diet. J. Anim. Sci. 2018, 96, 5276–5286. [Google Scholar] [CrossRef]
- Cidrini, I.A.; Granja-Salcedo, Y.T.; Prados, L.F.; Kishi, L.T.; Siqueira, G.R.; Resende, F.D. Effect of tannin extract associated with two levels of non-protein nitrogen in the supplement on performance, ruminal parameters, and microbial diversity of grazing Nellore cattle during the growing phase at dry season. Anim. Feed Sci. Technol. 2022, 286, 115269. [Google Scholar] [CrossRef]
- Jayanegara, A.; Goel, G.; Makkar, H.P.S.; Becker, K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Witzig, M.; Zeder, M.; Rodehutscord, M. Effect of the ionophore monensin and tannin extracts supplemented to grass silage on populations of ruminal cellulolytics and methanogens in vitro. Anaerobe 2018, 50, 44–54. [Google Scholar] [CrossRef]
- Krueger, W.K.; Gutierrez-Banuelos, H.; Carstens, G.E.; Min, B.R.; Pinchak, W.E.; Gomez, R.R.; Anderson, R.C.; Krueger, N.A.; Forbes, T.D.A. Effects of dietary tannin source on performance, feed efficiency, ruminal fermentation, and carcass and non-carcass traits in steers fed a high-grain diet. Anim. Feed Sci. Technol. 2010, 159, 1–9. [Google Scholar] [CrossRef]
- Toral, P.G.; Hervas, G.; Bichi, E.; Belenguer, A.; Frutos, P. Tannins as feed additives to modulate ruminal biohydrogenation: Effects on animal performance, milk fatty acid composition and ruminal fermentation in dairy ewes fed a diet containing sunflower oil. Anim. Feed Sci. Technol. 2011, 164, 199–206. [Google Scholar] [CrossRef]
- Stewart, E.K.; Beauchemin, K.A.; Dai, X.; MacAdam, J.W.; Christensen, R.G.; Villalba, J.J. Effect of tannin-containing hays on enteric methane emissions and nitrogen partitioning in beef cattle. J. Anim. Sci. 2019, 97, 3286–3299. [Google Scholar] [CrossRef]
- Nelson, K.E.; Pell, A.N.; Schofield, P.; Zinder, S. Isolation and Characterization of an Anaerobic Ruminal Bacterium Capable of Degrading Hydrolyzable Tannins. Appl. Environ. Microbiol. 1995, 61, 3293–3298. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Alves, S.P.; Cappucci, A.; Cook, S.R.; Duarte, A.; Caldeira, R.M.; McAllister, T.A.; Bessa, R.J.B. Effects of condensed and hydrolysable tannins on rumen metabolism with emphasis on the biohydrogenation of unsaturated fatty acids. J. Agric. Food Chem. 2018, 66, 3367–3377. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wei, C.; Zhao, G.Y.; Xu, Z.W.; Lin, S.X. Effects of dietary supplementing tannic acid in the ration of beef cattle on rumen fermentation, methane emission, microbial flora and nutrient digestibility. J. Anim. Physiol. Anim. Nutr. 2016, 101, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Aboagye, I.A.; Oba, M.; Koenig, K.M.; Zhao, G.Y.; Beauchemin, K.A. Use of gallic acid and hydrolyzable tannins to reduce methane emission and nitrogen excretion in beef cattle fed a diet containing alfalfa silage. J. Anim. Sci. 2019, 97, 2230–2244. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Effects of essential oils on methane production and fermentation by and abundance and diversity of, rumen microbial populations. Appl. Environ. Microbiol. 2012, 78, 4271–4280. [Google Scholar] [CrossRef]
- Geraci, J.I.; Garciarena, A.D.; Gagliostro, G.A.; Beauchemin, K.A.; Colombatto, D. Plant extracts containing cinnamaldehyde, eugenol and capsicum oleoresin added to feedlot cattle diets Ruminal environment, short term intake pattern and animal performance. Anim. Feed Sci. Technol. 2012, 176, 123–130. [Google Scholar] [CrossRef]
- Busquet, M.; Calsamiglia, S.; Ferret, A.; Kamel, C. Plant extracts effect in vitro rumen microbial fermentation. J. Dairy Sci. 2006, 89, 761–771. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.W.; Castillejos, L.; Ferret, A. Essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef]
- García, V.; Catalá-Gregori, P.; Madrid, J.; Hernández, F.; Megías, M.D.; Andrade-Montemayor, H.M. Potential of carvacrol to modify in vitro rumen fermentation as compared with monensin. Animal 2007, 1, 675–680. [Google Scholar] [CrossRef]
- Durmic, Z.; Moate, P.J.; Eckard, R.; Revell, D.K.; Williams, R.; Vercoe, P.E. In vitro screening of selected feed additives, plant essential oils and plant extracts for rumen methane mitigation. J. Sci. Food Agric. 2014, 94, 1191–1196. [Google Scholar] [CrossRef]
- Rossi, C.A.S.; Grossi, S.; Dell’Anno, M.; Compiani, R.; Rossi, L. Effect of a Blend of Essential Oils, Bioflavonoids and Tannins on In Vitro Methane Production and In Vivo Production Efficiency in Dairy Cows. Animals 2022, 12, 728. [Google Scholar] [CrossRef]
- Teobaldo, R.W.; Cardoso, A.d.S.; Brito, T.R.; Leite, R.G.; Romanzini, E.P.; Granja-Salcedo, Y.T.; Reis, R.A. Response of Phytogenic Additives on Enteric Methane Emissions and Animal Performance of Nellore Bulls Raised in Grassland. Sustainability 2022, 14, 9395. [Google Scholar] [CrossRef]
- Detmann, E.; Valente, É.E.L.; Batista, E.D.; Huhtanen, P. An evaluation of the performance and efficiency of nitrogen utilization in cattle fed tropical grass pastures with supplementation. Livest. Sci. 2014, 162, 141–153. [Google Scholar] [CrossRef]
- Mott, G.O.; Lucas, H.L. The design conduct and interpretation of grazing trials on cultivated and improved pastures. In Proceedings of the 6th International Grassland Congress, State College, PA, USA, 17–23 August 1952. [Google Scholar]
- Halls, L.K. The approximation of cattle diet through herbage sampling. Rang. Ecol. Manag. 1954, 7, 269–270. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Agricultural Chemists: Rockville, MD, USA, 1990; pp. 1–771. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, and no starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Goering, H.K.; Van Soest, P.J. Forage Fibre Analyses (Apparatus. Reagents. Procedures. and Some Applications); Agriculture Handbook No 379; Agricultural Research Service: Washington, DC, USA, 1970.
- Valente, T.N.P.; Detmann, E.; Queiroz, A.C.D.; Valadares Filho, S.D.C.; Gomes, D.I.; Figueiras, J.F. Evaluation of ruminal degradation profiles of forages using bags made from different textiles. Rev. Bras. Zootec. 2011, 40, 2565–2573. [Google Scholar] [CrossRef]
- Souza, N.K.P.; Detmann, E.; Pina, D.S.; Valadares Filho, S.C.; Sampaio, C.B.; Queiroz, A.C.; Veloso, C.M. Evaluation of chromium concentration in cattle feces using different acid digestion and spectrophotometric quantification techniques. Arq. Bras. Med. Vet. Zoote. 2013, 65, 1472–1482. [Google Scholar] [CrossRef]
- Chen, X.B.; Gomes, M.J. Estimation of Microbial Protein Supply to Sheep and Cattle Based on Urinary Excretion of Purine Derivatives—An Overview of the Technical Details; Occasional Publication; Rowett Research Institute: Bucksburn Aberdeen, Scotland, 1995. [Google Scholar]
- Costa e Silva, L.F.; Valadares Filho, S.C.; Chizzotti, M.L.; Rotta, P.P.; Prados, L.F.; Valadares, R.F.D.; Zanetti, D.; Braga, J.M.S. Creatinine excretion and relationship with body weight of Nellore cattle. Rev. Bras. Zootec. 2012, 41, 807–810. [Google Scholar] [CrossRef]
- Verbic, J.; Chen, X.; MacLeod, N.; Ørskov, E. Excretion of purine derivatives by ruminants. Effect of microbial nucleic acid infusion on purine derivative excretion by steers. J. Agric. Sci. 1990, 114, 243–248. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Valadares, R.F.D.; Valadares Filho, S.C.; Pina, D.S.; Detmann, E.; Leão, M.I. Endogenous fraction and urinary recovery of purine derivatives obtained by different methods in Nellore cattle. J. Anim. Sci. 2011, 89, 510–519. [Google Scholar] [CrossRef]
- ARC. The Nutrient Requirements of Ruminant Livestock; Technical review by an Agricultural Research Council Working Party; Commonwealth Agricultural Bureau: Farnham Royal, UK, 1984. [Google Scholar]
- Famme, P.; Knudsen, J. Total heat balance study of anaerobiosis in Tubiflex (Muller). J. Comp. Physiol. 1984, 154, 587–591. [Google Scholar] [CrossRef]
- Granja-Salcedo, Y.T.; Ramirez-Uscategui, R.A.; Machado, E.G.; Messana, J.D.; Kishi, L.T.; Dias, A.V.L.; Berchielli, T.T. Studies on bacterial community composition are affected by the time and storage method of the rumen content. PLoS ONE 2017, 12, e0176701. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.; Walters, W.A.; Lyons, D.B.; Luzupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2010, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible interactive scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.; McMurdie, P.; Rosen, M.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Opens external link in new window. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 8, 1–10. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef]
- Moore, J.E. Forage crops. In Crop Quality, Storage, and Utilization; Hoveland, C.S., Ed.; Crop Science Society of America: Madison, WI, USA, 1980; pp. 61–91. [Google Scholar]
- Simioni, T.A.; Messana, J.D.; Silva, L.G.; Granja-Salcedo, Y.T.; Torrecilhas, J.A.; San Vito, E.; Lage, J.F.; Reis, R.A.; Berchielli, T.T. Effects of mineral or protein-energy supplementation and genetic group on metabolism parameters of young beef bulls grazing tropical grass during the rainy season. Livest. Sci. 2022, 255, 104805. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Ferret, A.; Reynolds, C.K.; Kristensen, N.B.; Van Vuuren, A.M. Strategies for optimizing nitrogen use by ruminants. Animal 2010, 4, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Cardoso, A.S.; Fonseca, N.V.B.; Romanzini, E.P.; Siniscalchi, D.; Berndt, A.; Ruggieri, A.C.; Reis, R.A. Effects of supplementation with corn distillers’ dried grains on animal performance, nitrogen balance, and enteric CH4 emissions of young Nellore bulls fed a high-tropical forage diet. Animal 2021, 15, 100155. [Google Scholar] [CrossRef]
- Min, B.R.; Gurung, N.; Shange, R.; Solaiman, S. Potential role of rumen microbiota in altering average daily gain and feed efficiency in meat goats fed simple and mixed pastures using bacterial tag-encoded FLX amplicon pyrosequencing. J. Anim. Sci. 2019, 97, 3523–3534. [Google Scholar] [CrossRef]
- Firkins, J.L.; Berger, L.L.; Fahey, G.C. Evaluation of wet and dry distillers grains and dry corn gluten feeds for ruminants. J. Anim. Sci. 1985, 60, 847–860. [Google Scholar] [CrossRef]
- Camargo, K.D.V.; Messana, J.D.; Silva, L.G.; Granja-Salcedo, Y.T.; Dias, A.V.L.; Alves, K.L.G.C.; Gonçalves, P.H.; Souza, W.A.; Reis, R.A.; Berchielli, T.T. Intake, metabolism parameters, and performance of growing beef cattle on pasture supplemented with different rumen undegradable protein with different amino acid profile. Anim. Feed Sci. Technol. 2022, 286, 115258. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Merchen, N.R.; Titgemeyer, E.C. Manipulation of amino acid supply to the growing ruminant. J. Anim. Sci. 1992, 70, 3238–3247. [Google Scholar] [CrossRef] [PubMed]
- Menci, R.; Coppa, M.; Torrent, A.; Natalello, A.; Valenti, B.; Luciano, G.; Priolo, A.; Niderkorn, V. Effects of two tannin extracts at different doses in interaction with a green or dry forage substrate on in vitro rumen fermentation and biohydrogenation. Anim. Feed Sci. Technol. 2021, 278, 114977. [Google Scholar] [CrossRef]
- Cobellis, G.; Trabalza-Marinucci, M.; Marcotullio, M.C.; Yu, Z. Evaluation of different essential oils in modulating methane and ammonia production, rumen fermentation, and rumen bacteria in vitro. Anim. Feed Sci. Technol. 2016, 215, 25–36. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition—Review. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Poppi, D.P.; McLennan, S.R. Protein and energy utilization by ruminants at pasture. J. Anim. Sci. 1995, 73, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Vendramini, J.M.B.; Sanchez, J.M.D.; Cooke, R.F.; Aguiar, A.D.; Moriel, P.; da Silva, W.L.; Cunha, O.F.R.; Ferreira, P.D.S.; Pereira, A.C. Stocking rate and monensin supplemental level effects on growth performance of beef cattle consuming warm-season grasses. J. Anim. Sci. 2015, 93, 3682–3689. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.M.; Milligan, L.P. The degradation and utilization of endogenous urea the gastrointestinal tract of ruminants: A review. Can. J. Anim. Sci. 1980, 60, 205–221. [Google Scholar] [CrossRef]
- Kawamoto, H.; Nakatsubo, F.; Murakami, K. Quantitative determination of tannin and protein in the precipitates by high-performance liquid chromatography. Phytochemistry 1995, 40, 1503–1505. [Google Scholar] [CrossRef]
- Rabee, A.E.; Kewan, K. Z.; Lamara, M. Identification of Micro-Organisms that Tolerant to Anti-Nutritional Factors in the Rumen of Camel. J. Anim. Poult. Prod. 2022, 13, 7–13. [Google Scholar] [CrossRef]
- Auffret, M.D.; Dewhurst, R.J.; Duthie, C.A.; Rooke, J.A.; Wallace, R.J.; Freeman, T.C.; Stewart, R.; Watson, M.; Roehe, R. The rumen microbiome as a reservoir of antimicrobial resistance and pathogenicity genes is directly affected by diet in beef cattle. Microbiome 2017, 5, 159. [Google Scholar] [CrossRef]
- Min, B.R.; Wright, C.; Ho, P.; Eun, J.S.; Gurung, N.; Shange, R. The effect of phytochemical tannins-containing diet on rumen fermentation characteristics and microbial diversity dynamics in goats using 16S rDNA amplicon pyrosequencing. Agric. Food Anal. Bacteriol. 2014, 4, 195–211. [Google Scholar]
- Solden, L.M.; Hoyt, D.W.; Collins, W.B.; Plank, J.E.; Daly, R.A.; Hildebrand, E.; Beavers, T.J.; Wolfe, R.; Nicora, C.D.; Purvine, S.O.; et al. New roles in hemicellulosic sugar fermentation for the uncultivated Bacteroidetes family BS11. ISME J. 2017, 11, 691–703. [Google Scholar] [CrossRef]
- Wu, K.; Cheng, L. Ruminiclostridium. In Bergey’s Manual of Systematics of Archaea and Bacteria; Trujillo, M.E., Dedysh, S., DeVos, P., Hedlund, B., Kämpfer, P., Rainey, F.A., Whitman, W.B., Eds.; 2022. [Google Scholar]
- Kittelmann, S.; Pinares-Patino, C.S.; Seedorf, H.; Kirk, M.R.; Ganesh, S.; McEwan, J.C.; Janssen, P. Two different bacterial community types are linked with the low-methane emission trait in sheep. PLoS ONE. 2014, 9, e103171. [Google Scholar] [CrossRef]
- Kletzin, A.; Urich, T.; Müller, F.; Bandeiras, T.M.; Gomes, C.M. Dissimilatory oxidation and reduction of elemental sulfur in thermophilic archaea. J. Bioenerg. Biomembr. 2004, 36, 77–91. [Google Scholar] [CrossRef] [PubMed]
| Chemical composition, % DM | Supplements1 | Forage2 | |||
|---|---|---|---|---|---|
| EW | EPHA | MW | MPHA | ||
| Mineral matter, % | 34.02 | 33.12 | - | - | 8.91±0.06 |
| Organic matter, % | 65.98 | 66.88 | - | - | 91.08±0.07 |
| apNDF3, % | 30.32 | 30.31 | - | - | 54.85±0.38 |
| iNDF4, % | 15.75 | 15.61 | - | - | 17.75±0.26 |
| Ether extract, % | 1.81 | 2.11 | - | - | 2.47±0.05 |
| Gross energy, MJ/kg DM | 12.58 | 13.33 | - | - | 17.95±0.04 |
| Crude protein, % | 14.87 | 15.02 | - | - | 15.28±0.22 |
| Fraction5, as % of crude protein | |||||
| A | 29.01 | 36.98 | - | - | 26.64 |
| B1 | 6.33 | 6.48 | - | - | 7.40 |
| B2 | 50.92 | 45.01 | - | - | 45.70 |
| B3 | 10.89 | 8.76 | - | - | 14.20 |
| C | 2.85 | 2.77 | - | - | 6.06 |
| Supplements1 | P-value2 | ||||||
|---|---|---|---|---|---|---|---|
| Domain; Phylum (%) | EW | EPHA | MW | MPHA | ST | PHA | ST × PHA |
| Archaea; Euryarchaeota | 4.84 ± 1.55 | 4.37 ± 1.32 | 3.07 ± 3.20 | 3.80 ± 1.52 | 0.343 | 0.782 | 0.626 |
| Bacteria; Firmicutes | 48.88 ± 5.80 | 46.24 ± 5.12 | 51.22 ± 2.91 | 47.99 ± 4.84 | 0.069 | 0.418 | 0.254 |
| Bacteria; Bacteroidetes | 27.71 ± 3.53 | 30.93 ± 4.62 | 25.87 ± 1.58 | 27.88 ± 2.90 | 0.089 | 0.244 | 0.248 |
| Bacteria; Proteobacteria | 1.42 ± 0.63 | 1.40 ± 0.39 | 1.02 ± 0.57 | 1.05 ± 0.64 | 0.046 | 0.828 | 0.259 |
| Bacteria; Spirochaetae | 1.42 ± 0.50 | 1.45 ± 0.51 | 1.02 ± 0.44 | 0.91 ± 0.40 | 0.022 | 0.953 | 0.152 |
| Bacteria; Chloroflexi | 1.09 ± 0.40 | 0.99 ± 0.39 | 0.83 ± 0.90 | 0.98 ± 0.63 | 0.812 | 0.953 | 0.953 |
| Bacteria; Actinobacteria | 0.98 ± 0.43 | 0.95 ± 0.33 | 0.91 ± 0.52 | 0.88 ± 1.15 | 0.782 | 0.984 | 0.978 |
| Bacteria; Tenericutes | 0.74 ± 0.24 | 0.65 ± 0.11 | 0.94 ± 0.44 | 1.03 ± 0.38 | 0.114 | 0.664 | 0.360 |
| Bacteria; SR1 (Absconditabacteria) | 0.45 ± 0.36 | 0.65 ± 0.45 | 0.77 ± 0.21 | 0.52 ± 0.45 | 0.332 | 0.722 | 0.584 |
| Bacteria; Verrucomicrobia | 0.44 ± 0.30 | 0.32 ± 0.22 | 0.51 ± 0.23 | 0.15 ± 0.43 | 0.635 | 0.022 | 0.133 |
| Bacteria; Fibrobacteres | 0.20 ± 0.14 | 0.29 ± 0.08 | 0.21 ± 0.21 | 0.15 ± 0.18 | 0.275 | 0.551 | 0.404 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





