Submitted:
26 February 2023
Posted:
28 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
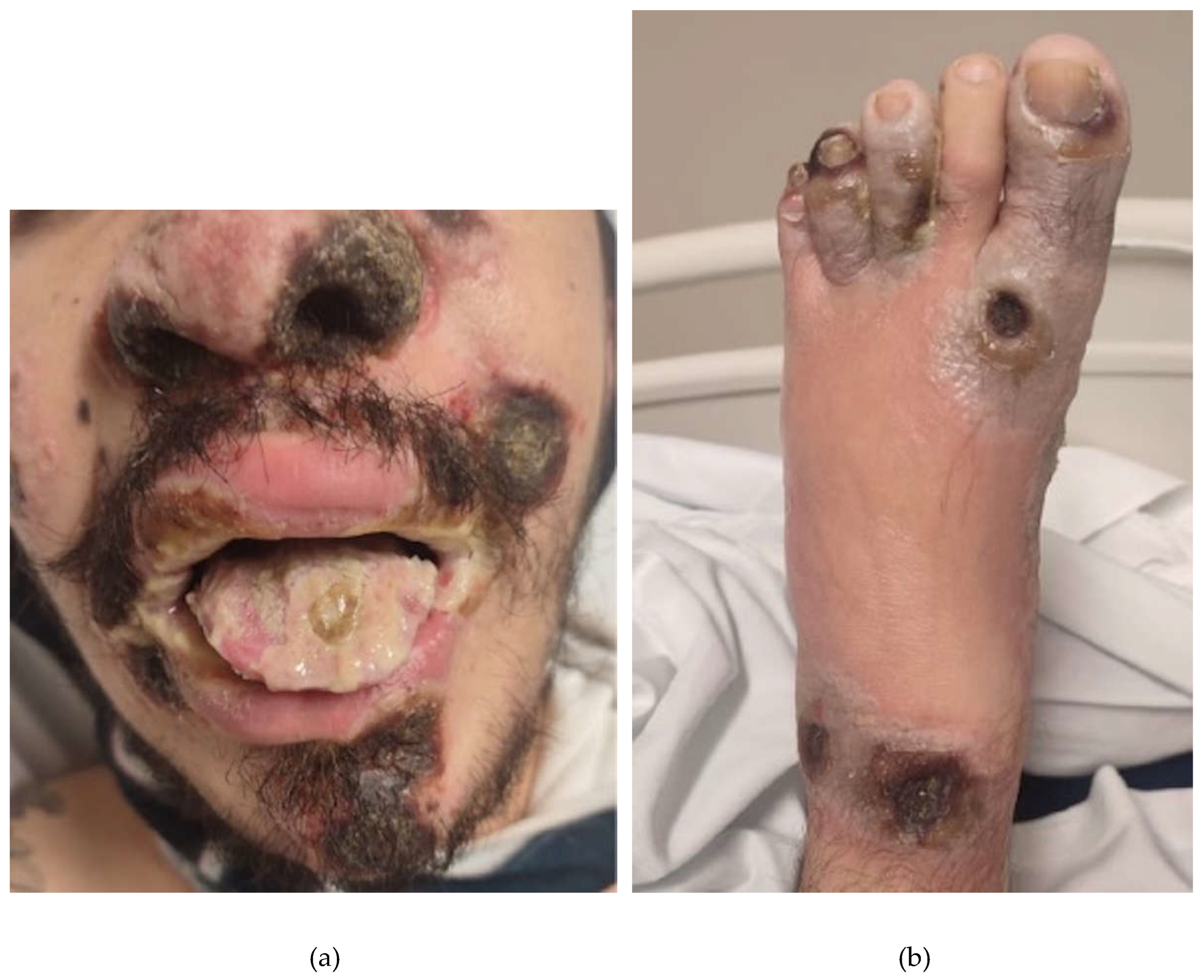
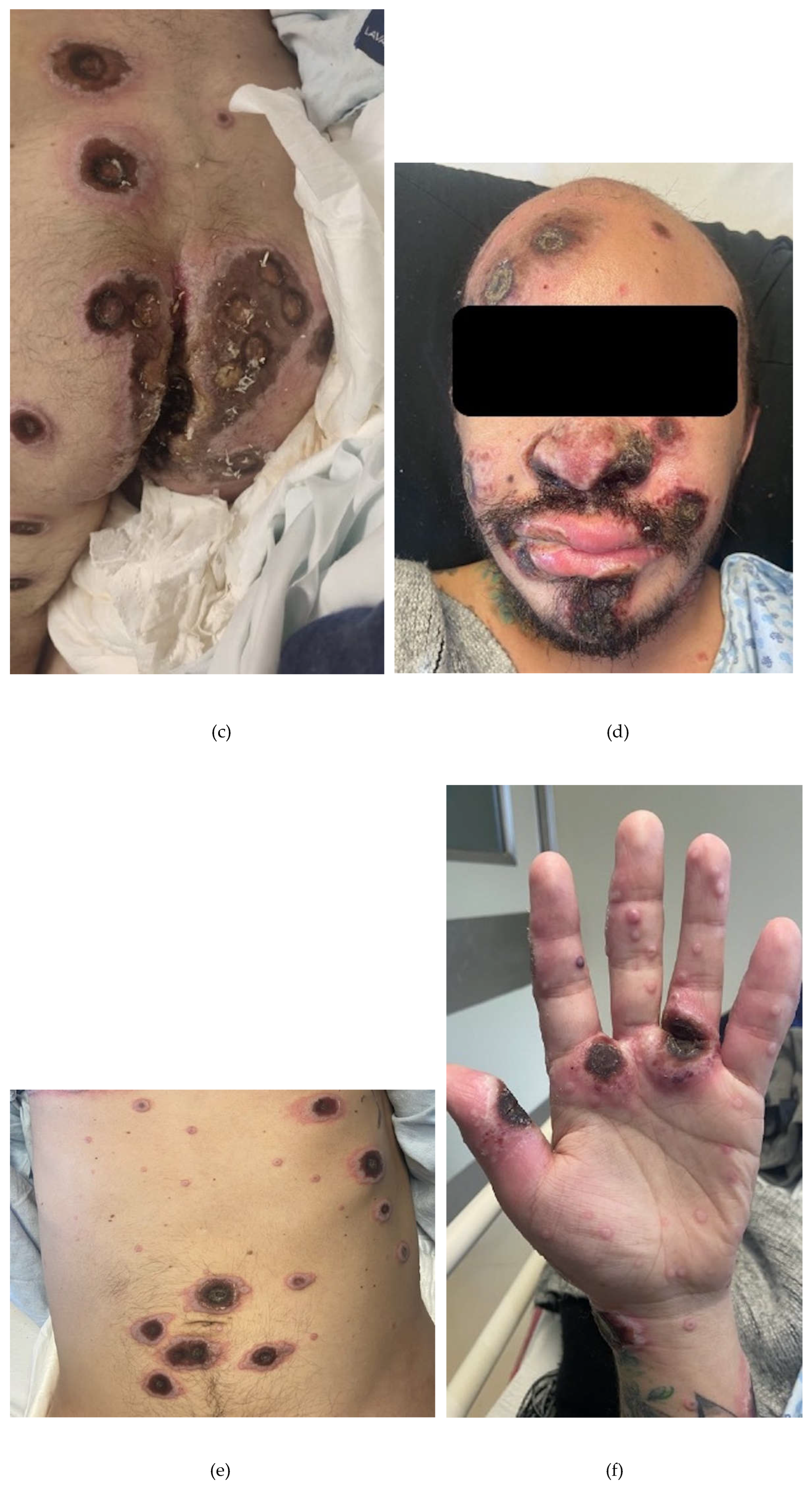
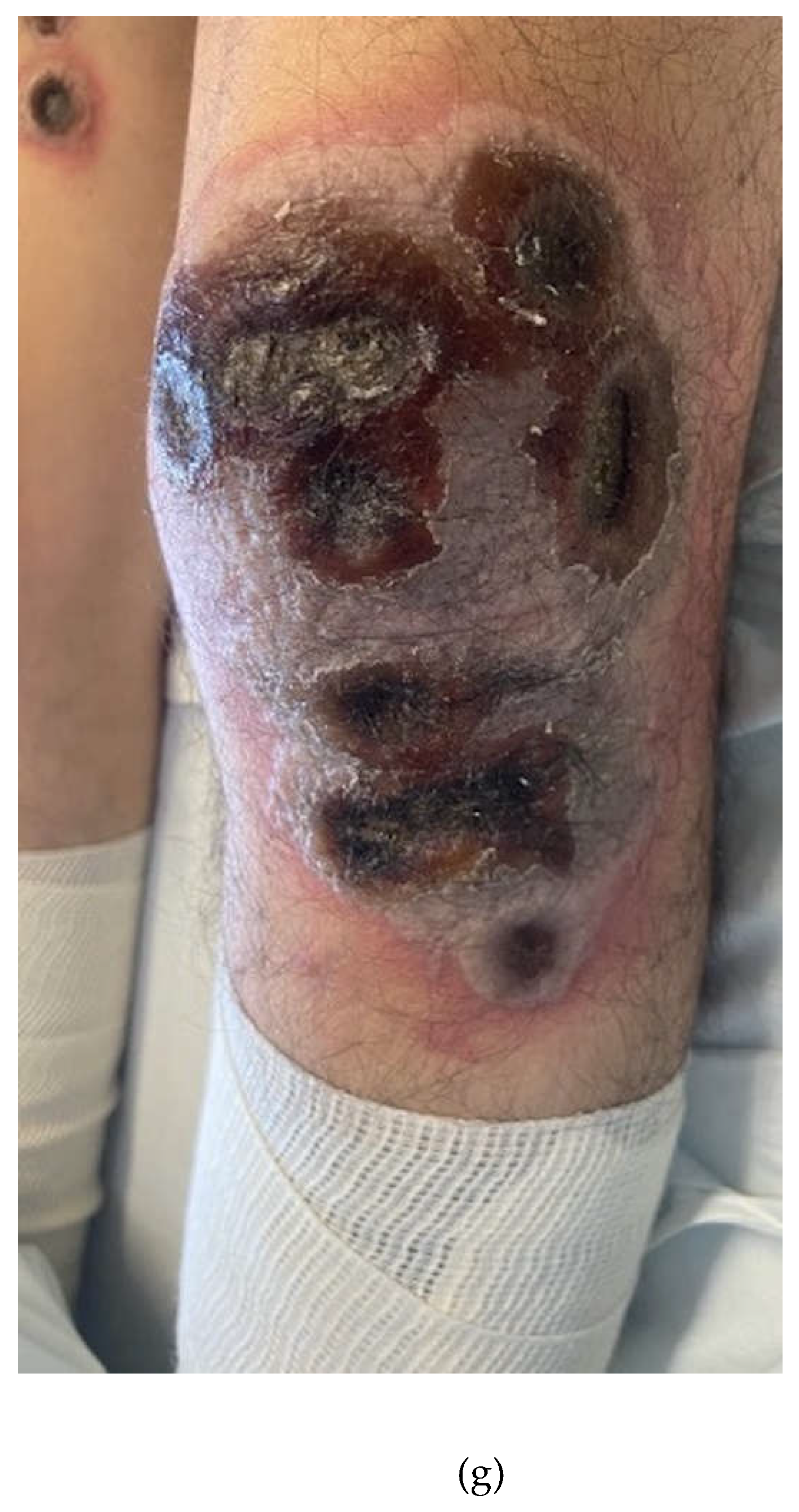
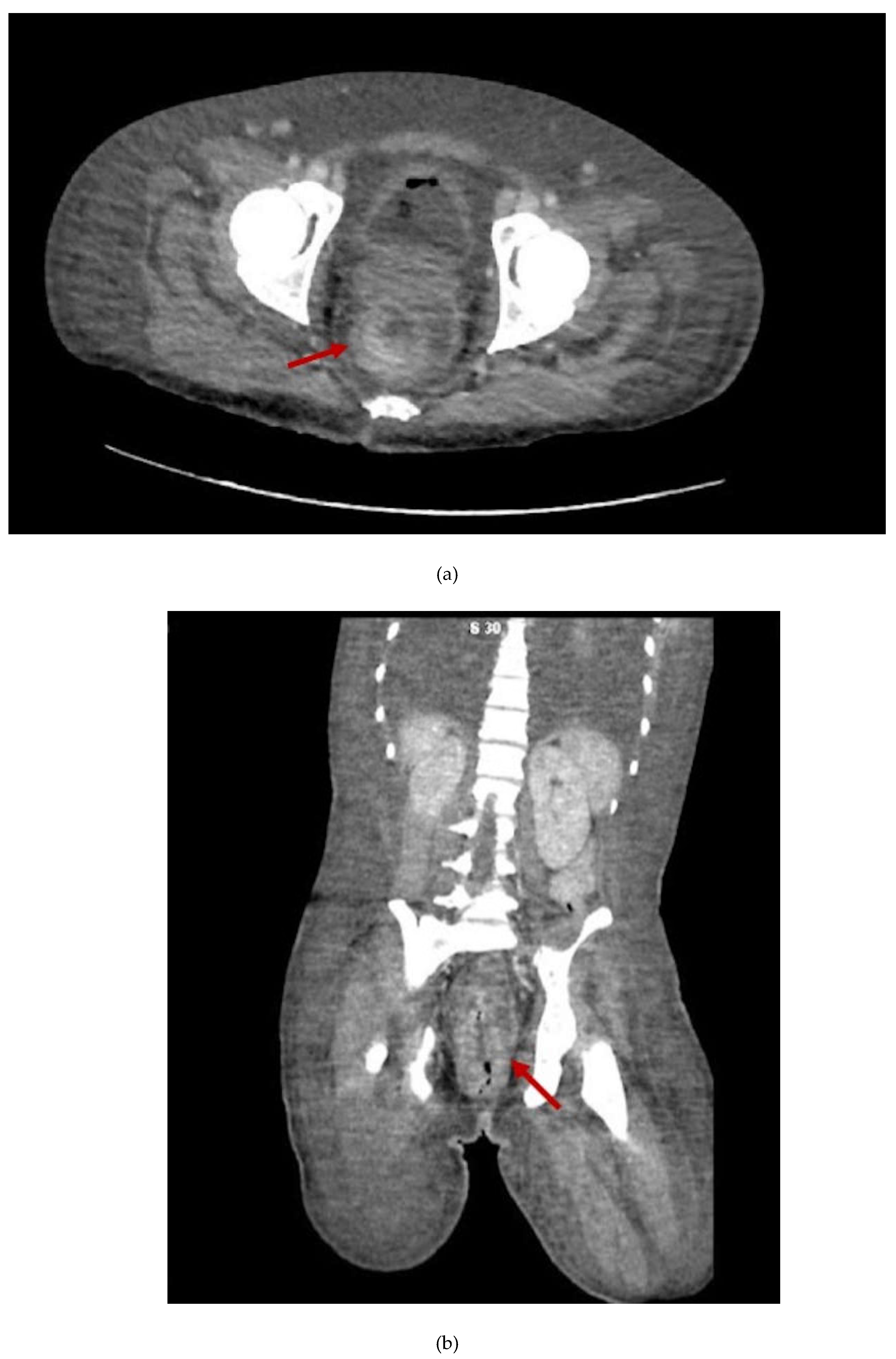
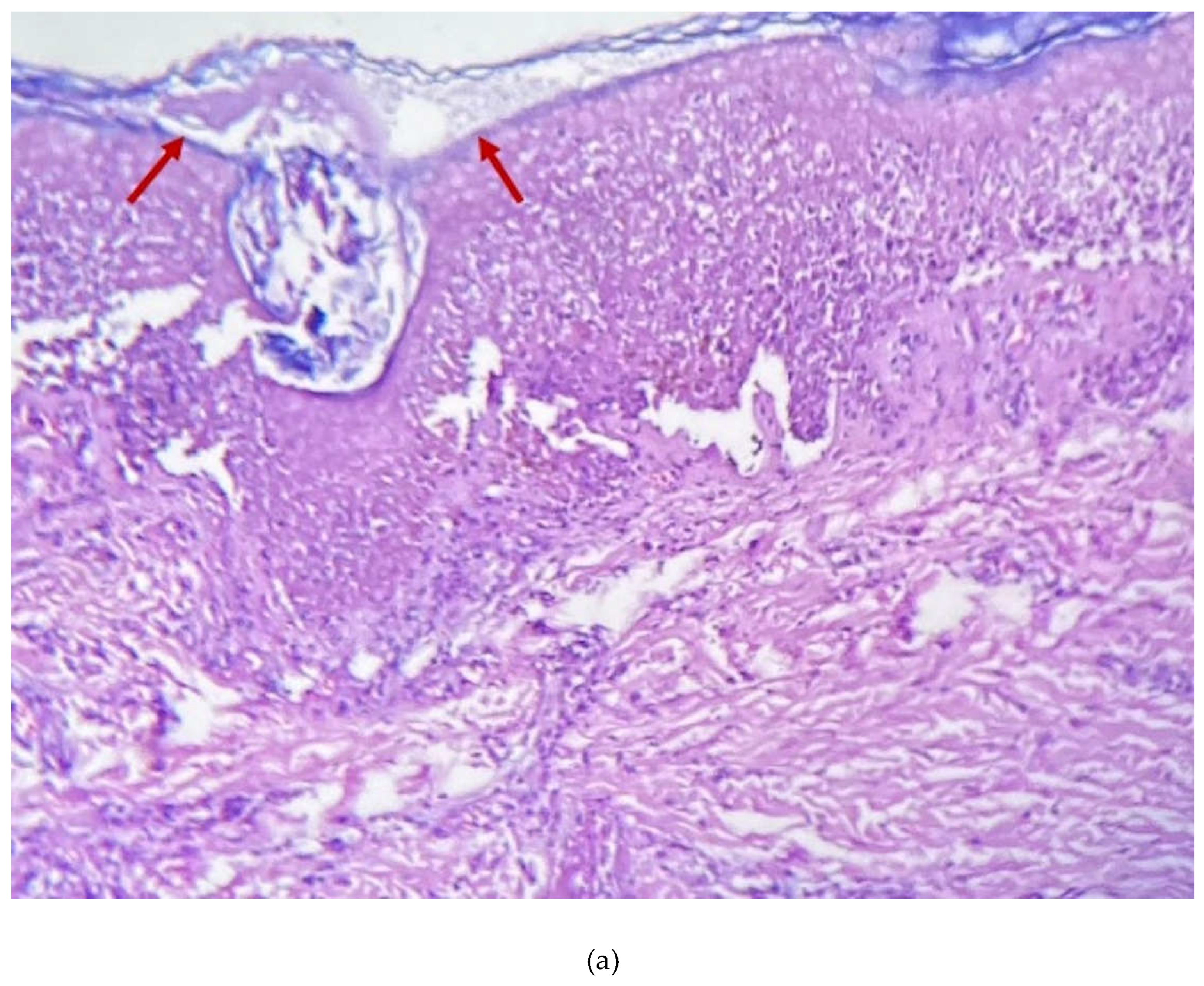
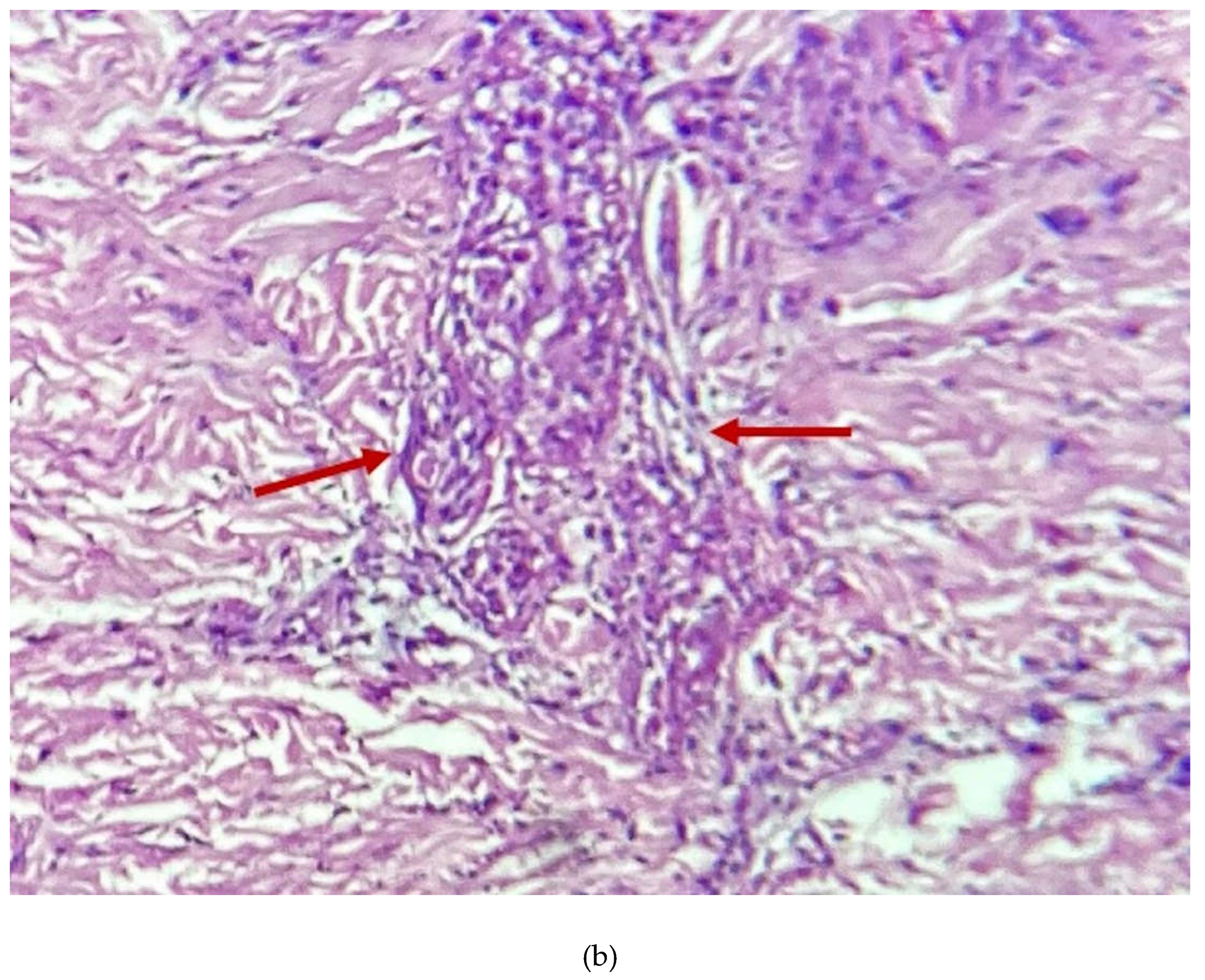
2. Case Presentation Section
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human Monkeypox: Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention. Infect. Dis. Clin. North Am. 2019, 33, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Tarín-Vicente, E.J.; Alemany, A.; Agud-Dios, M.; Ubals, M.; Suñer, C.; Antón, A.; Arando, M.; Arroyo-Andrés, J.; Calderón-Lozano, L.; Casañ, C.; Cabrera, J.M.; Coll, P.; Descalzo, V.; Folgueira, M.D.; García-Pérez, J.N.; Gil-Cruz, E.; González-Rodríguez, B.; Gutiérrez-Collar, C.; Hernández-Rodríguez, Á.; López-Roa, P.; de los Ángeles Meléndez, M.; Montero-Menárguez, J.; Muñoz-Gallego, I.; Palencia-Pérez, S.I.; Paredes, R.; Pérez-Rivilla, A.; Piñana, M.; Prat, N.; Ramirez, A.; Rivero, Á.; Rubio-Muñiz, C.A.; Vall, M.; Acosta-Velásquez, K.S.; Wang, A.; Galván-Casas, C.; Marks, M.; Ortiz-Romero, P.L.; Mitjà, O. Clinical Presentation and Virological Assessment of Confirmed Human Monkeypox Virus Cases in Spain: A Prospective Observational Cohort Study. Lancet (London, England) 2022, 400, 661. [Google Scholar] [CrossRef] [PubMed]
- Minhaj, F.S.; Ogale, Y.P.; Whitehill, F.; Schultz, J.; Foote, M.; Davidson, W.; Hughes, C.M.; Wilkins, K.; Bachmann, L.; Chatelain, R.; Donnelly, M.A.P.; Mendoza, R.; Downes, B.L.; Roskosky, M.; Barnes, M.; Gallagher, G.R.; Basgoz, N.; Ruiz, V.; Kyaw, N.T.T.; Feldpausch, A.; Valderrama, A.; Alvarado-Ramy, F.; Dowell, C.H.; Chow, C.C.; Li, Y.; Quilter, L.; Brooks, J.; Daskalakis, D.C.; McClung, R.P.; Petersen, B.W.; Damon, I.; Hutson, C.; McQuiston, J.; Rao, A.K.; Belay, E.; McCollum, A.M.; Angelo, K.; Arduino, M.; Arthur, R.; Baird, N.; Batross, J.; Beeson, A.; Bhingarde, J.; Bowen, M.; Brown, C.; Brown, C.M.; Burakoff, A.; Charniga, K.; Chen, T.-H.; Chen, S.; Clay, P.; Cope, J.; Cope, J.; Dankwa, M.A.; Delaney, L.; Perio, M. De; Decenteceo, M.; Delea, K.; Doty, J.B.; Duchin, J.; Dunlap, J.; Fagan, R.; Furness, B.; Gearhart, S.; Gigante, C.; Gilliland, A.; Gosdin, L.; Griffin, I.; Groccia, A.; Guagliardo, S.; Hercules, Y.; Jackson, K.; Jarquin, P.; Kachur, R.; Kallen, A.; Kao, R.; Kelly, A.; Khan, M.; Khan, T.; Kofman, A.; Kornylo, K.; Kuhar, D.; LaFlam, M.; Lash, R.; Lashombe, A.; Lowe, D.; MacGurn, A.; Masters, N.; McCaffrey, K.; Mink, J.L.; Monroe, B.; Morgan, C.N.; Nakazawa, Y.; Nash, J.; Navarra, T.; Newton, D.; Osinubi, M.; Osorio, V.; Pearson, C.; Petras, J.; Philpott, D.; Pickrel, A.; Potvin, B.; Priyamvada, L.; Rey, A.; Ricketts, E.; Rodriguez, S.; Rushmore, J.; Satheshkumar, P.S.; Segaloff, H.; Sekkarie, A.; Sharma, A.; Sims, E.; Smith, D.; Smith, T.; Smith, T.; Solanky, D.; Spiknall, I.; Stanek, D.R.; Stenger, M.; Strona, F.; Tardivel, K.; Tyagi, E.; Wortley, P.; Valencia, D.; Waltenburg, M.; Whitehouse, E.; Wong, M. Monkeypox Outbreak — Nine States, May 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; Apea, V.; Boesecke, C.; Vandekerckhove, L.; Yakubovsky, M.; Sendagorta, E.; Blanco, J.L.; Florence, E.; Moschese, D.; Maltez, F.M.; Goorhuis, A.; Pourcher, V.; Migaud, P.; Noe, S.; Pintado, C.; Maggi, F.; Hansen, A.-B. E.; Hoffmann, C.; Lezama, J.I.; Mussini, C.; Cattelan, A.; Makofane, K.; Tan, D.; Nozza, S.; Nemeth, J.; Klein, M.B.; Orkin, C.M. Monkeypox Virus Infection in Humans across 16 Countries — April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Multi-country monkeypox outbreak: situation update. (accessed 2023-01-22). Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON393.
- Mitjà, O.; Ogoina, D.; Titanji, B.K.; Galvan, C.; Muyembe, J.-J.; Marks, M.; Orkin, C.M. Monkeypox. Lancet 2023, 401, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Núñez, I.; García-Grimshaw, M.; Ceballos-Liceaga, S.E.; Toledo-Salinas, C.; Carbajal-Sandoval, G.; Sosa-Laso, L.; García-Rodríguez, G.; Cortés-Alcalá, R.; Torre, A. de la; Fragoso-Saavedra, S.; Quintero-Villegas, A.; López-Gatell, H.; Reyes-Terán, G.; Valdés-Ferrer, S.I. Epidemiological and Clinical Characteristics of Patients with Human Monkeypox Infection in Mexico: A Nationwide Observational Study. Lancet Reg. Heal. - Am. 2023, 17, 100392. [Google Scholar] [CrossRef]
- Fink, D.L.; Callaby, H.; Luintel, A.; Beynon, W.; Bond, H.; Lim, E.Y.; Gkrania-Klotsas, E.; Heskin, J.; Bracchi, M.; Rathish, B.; Milligan, I.; O’Hara, G.; Rimmer, S.; Peters, J.R.; Payne, L.; Mody, N.; Hodgson, B.; Lewthwaite, P.; Lester, R.; Woolley, S.D.; Sturdy, A.; Whittington, A.; Johnson, L.; Jacobs, N.; Quartey, J.; Ai Payne, B.; Crowe, S.; Elliott, I.A.; Harrison, T.; Cole, J.; Beard, K.; Cusack, T.-P.; Jones, I.; Banerjee, R.; Rampling, T.; Specialist and High Consequence Infectious Diseases Centres Network for Monkeypox; Dunning, J. Clinical Features and Management of Individuals Admitted to Hospital with Monkeypox and Associated Complications across the UK: A Retrospective Cohort Study. Lancet. Infect. Dis. 2022, 0. [Google Scholar] [CrossRef]
- Miller, M.J.; Cash-Goldwasser, S.; Marx, G.E.; Schrodt, C.A.; Kimball, A.; Padgett, K.; Noe, R.S.; McCormick, D.W.; Wong, J.M.; Labuda, S.M.; Borah, B.F.; Zulu, I.; Asif, A.; Kaur, G.; McNicholl, J.M.; Kourtis, A.; Tadros, A.; Reagan-Steiner, S.; Ritter, J.M.; Yu, Y.; Yu, P.; Clinton, R.; Parker, C.; Click, E.S.; Salzer, J.S.; McCollum, A.M.; Petersen, B.; Minhaj, F.S.; Brown, E.; Fischer, M.P.; Atmar, R.L.; DiNardo, A.R.; Xu, Y.; Brown, C.; Goodman, J.C.; Holloman, A.; Gallardo, J.; Siatecka, H.; Huffman, G.; Powell, J.; Alapat, P.; Sarkar, P.; Hanania, N.A.; Bruck, O.; Brass, S.D.; Mehta, A.; Dretler, A.W.; Feldpausch, A.; Pavlick, J.; Spencer, H.; Ghinai, I.; Black, S.R.; Hernandez-Guarin, L.N.; Won, S.Y.; Shankaran, S.; Simms, A.T.; Alarcón, J.; O’Shea, J.G.; Brooks, J.T.; McQuiston, J.; Honein, M.A.; O’Connor, S.M.; Chatham-Stephens, K.; O’Laughlin, K.; Rao, A.K.; Raizes, E.; Gold, J.A.W.; Morris, S.B.; Duessel, S.; Danaie, D.; Hickman, A.; Griffith, B.; Sanneh, H.; Hutchins, H.; Phyathep, C.; Carpenter, A.; Shelus, V.; Petras, J.; Hennessee, I.; Davis, M.; McArdle, C.; Dawson, P.; Gutelius, B.; Bisgard, K.; Wong, K.; Galang, R.R.; Perkins, K.M.; Filardo, T.D.; Davidson, W.; Hutson, C.; Lowe, D.; Zucker, J.E.; Wheeler, D.A.; He, L.; Jain, A.K.; Semeniuk, O.; Chatterji, D.; McClure, M.; Li, L.X.; Mata, J.; Beselman, S.; Cross, S.L.; Menzies, B.; Keller, M.; York, N.; Chaturvedi, V.; York, N.; Thet, A.; Carroll, R.; Hebert, C.; Patel, G.; Gandhi, V.; Abrams-Downey, A.; Nawab, M.; Landon, E.; Lee, G.; Kaplan-Lewis, E.; Miranda, C.; Carmack, A.E.; Traver, E.C.; Lazarte, S.; Perl, T.M.; Chow, J.; Kitchell, E.; Nijhawan, A.; Habib, O.; Bernus, A.; Andujar, G.; Davar, K.; Holtom, P.; Wald-Dickler, N.; Lorio, M.A.; Gaviria, J.; Chu, V.; Wolfe, C.R.; McKellar, M.S.; Farran, S.; Diaz Wong, R.A.; Schliep, T.; Shaw, R.; Tebas, P.; Richterman, A.; Aurelius, M.; Peterson, L.; Trible, R.; Rehman, T.; Sabzwari, R.; Hines, E.; Birkey, T.; King, J.; Farabi, A.; Jenny-Avital, E.; Touleyrou, L.; Sandhu, A.; Newman, G.; Bhamidipati, D.; Bhamidipati, D.; Vigil, K.; Caro, M.; Banowski, K.; Chinyadza, T.W.; Rosenzweig, J.; Jones, M.S.; Camargo, J.F.; Marsh, K.J.; Liu, E.W.; Guerrero-Wooley, R.; Pottinger, P. Severe Monkeypox in Hospitalized Patients — United States, August 10–October 10, 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Bilinska, J.; Tam, J.C.H.; Da Silva Fontoura, D.; Mason, C.Y.; Daunt, A.; Snell, L.B.; Murphy, J.; Potter, J.; Tuudah, C.; Sundramoorthi, R.; Abeywickrema, M.; Pley, C.; Naidu, V.; Nebbia, G.; Aarons, E.; Botgros, A.; Douthwaite, S.T.; Van Nispen Tot Pannerden, C.; Winslow, H.; Brown, A.; Chilton, D.; Nori, A. Clinical Features and Novel Presentations of Human Monkeypox in a Central London Centre during the 2022 Outbreak: Descriptive Case Series. BMJ 2022, 378. [Google Scholar] [CrossRef]
- Agrati, C.; Cossarizza, A.; Mazzotta, V.; Grassi, G.; Casetti, R.; De Biasi, S.; Pinnetti, C.; Gili, S.; Mondi, A.; Cristofanelli, F.; Lo Tartaro, D.; Notari, S.; Maffongelli, G.; Gagliardini, R.; Gibellini, L.; Aguglia, C.; Lanini, S.; D’Abramo, A.; Matusali, G.; Fontana, C.; Nicastri, E.; Maggi, F.; Girardi, E.; Vaia, F.; Antinori, A. Immunological Signature in Human Cases of Monkeypox Infection in 2022 Outbreak: An Observational Study. Lancet Infect. Dis. 2022, 0. [Google Scholar] [CrossRef]
- Afrashteh, S.; Fararouei, M.; Ghaem, H.; Aryaie, M. Factors Associated with Baseline CD4 Cell Counts and Advanced HIV Disease among Male and Female HIV-Positive Patients in Iran: A Retrospective Cohort Study. J. Trop. Med. 2022, 2022. [Google Scholar] [CrossRef]
- Chiesa, A.; Ochola, E.; Oreni, L.; Vassalini, P.; Rizzardini, G.; Galli, M. Hepatitis B and HIV Coinfection in Northern Uganda: Is a Decline in HBV Prevalence on the Horizon? PLoS One 2020, 15, e0242278. [Google Scholar] [CrossRef]
- Anderson, M.; Gaseitsiwe, S.; Moyo, S.; Thami, K.P.; Mohammed, T.; Setlhare, D.; Sebunya, T.K.; Powell, E.A.; Makhema, J.; Blackard, J.T.; Marlink, R.; Essex, M.; Musonda, R.M. Slow CD4+ T-Cell Recovery in Human Immunodeficiency Virus/Hepatitis B Virus-Coinfected Patients Initiating Truvada-Based Combination Antiretroviral Therapy in Botswana. Open Forum Infect. Dis. 2016, 3, 1–8. [Google Scholar] [CrossRef]
- Menezes, Y.R.; de Miranda, A.B. Severe Disseminated Clinical Presentation of Monkeypox Virus Infection in an Immunosuppressed Patient: First Death Report in Brazil. Rev. Soc. Bras. Med. Trop. 2022, 55, 2022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




