1. Introduction
Insulin resistance is a metabolic disorder in which cells fail to respond adequately to normal or increased insulin levels, causing impaired glucose uptake and utilization (1). Insulin resistance may occur with hypertriglyceridemia, hypercholesterolemia, glucose intolerance, obesity, Type 2 DM, and cardiovascular diseases. Thyroid hormones are also one of the important determinants involved in glucose homeostasis (2). Contrary to a general opinion that insulin is the main hormone responsible for blood glycemic control, there is reported that the synergistic effects of insulin and triiodothyronine affect glucose and lipid metabolism (3). In studies, it has been shown that patients with overt hypothyroidism and hyperthyroidism are more likely to develop insulin resistance and dysglycemia (2). However, it has also been stated that thyroid hormones are associated with insulin resistance in individuals with subclinical hypothyroidism and hyperthyroidism with mild thyroid dysfunction (4). This shows that abnormal thyroid hormones and thyroid-stimulating hormone (TSH) levels are effective on glucose metabolism and insulin resistance. The seriousness of the illness picture is proportional to the seriousness of the disorders. The development of insulin resistance in hypothyroidism is related to decreased blood flow, gluconeogenesis, lipolysis, and basal insulin secretion in peripheral tissues (2).
Overt hypothyroidism is also identified as a possible risk factor for increased risk of coronary artery disease (CAD) and death, it is often related to hyperlipidemia (5). Similarly, asymptomatic subclinical hypothyroidism, characterized by serum free T4 concentrations at normal levels and slightly elevated serum TSH concentrations are also accompanied by a risk of CAD, increased cholesterol, and LDL cholesterol levels (6). It has been indicated that the total cholesterol and LDL cholesterol levels of these individuals can be reduced by thyroxine treatment (7).
Vitamin D is involved in bone mineral homeostasis. However, high expression of Vitamin D receptors (VDR) has been shown in many tissues, including the thyroid gland in recent years (8). It has been shown that vitamin D is an effective hormone not only in the skeletal system but also in many target tissues and also affects the secretion of pituitary hormones (9). In addition, it has also been stated that vitamin D has anti-proliferative effects on thyrocytes in vitro. In the studies conducted, the correlation between low Vitamin D levels and subclinical hypothyroidism was drawn attention (10).
In the levels of thyroid hormones and TSH are observed differences which are documented with little individual variation within the normal range (11). Similarly, substantial variation in thyroid function can be seen between populations. Such variations are due to a combination of genetics and environmental factors, for which the level of iodine intake is of great importance. Studies have conducted an increased risk of developing thyroid dysfunction in people with high normal TSH levels (12). It has been shown that the TSH value has above 2.5 mIU/L in about 9% of the population and the risk of developing hypothyroidism in the future is high. Therefore, although the optimal level for serum TSH levels is not clear, the general trend in recent years is to narrow the optimal TSH range (13).
Tsh levels harm insulin resistance and cardiovascular risk factors. In addition, in euthyroid individuals, even small differences in thyroid function have a possible contribution to these negative effects. Therefore, the question of whether there is an associatiın between TSH levels in the normal range with metabolic parameters and vitamin D levels will allow the identification of early new markers of cardiovascular risk. This study was conducted to investigate the potential relationship of TSH levels in the reference range with insulin resistance, serum 25(OH)D, and lipid levels in healthy, euthyroid adults.
2. Methods
2.1. Study design and participants
This study was approved by the ethics committee of Istanbul Başakşehir Çam and Sakura City Hospital (No.2021.06.120). Due to the retrospective and observational character of the study design, the requirement for informed consent has been waived.
The records of 561 healthy, euthyroid adult patients who applied to the Internal Medicine Outpatient Clinic of Başakşehir Çam and Sakura City Hospital in Istanbul between September 2020-December 2020 were retrospectively analyzed. Those with diabetes mellitus, thyroid dysfunction, thyroid nodule, cancer, autoimmune thyroiditis, using thyroid medication, pregnancy, infection, malignancy and cardiovascular disease were not included in the study. The normal ranges of TSH are between 0.3-2.5 mIU/mL in 95 percent of the population (13). According to the TSH levels, the cases were divided into 2 groups as group 1 (low normal range (0.27-2.5 mIU/mL), (n=285) and group 2 (high normal range (2.5-4.2 mIU/mL), (n=276)). Fasting glucose, insulin, HOMA-IR, 25(OH)D levels, and lipid levels were compared between individuals with high normal and low normal TSH levels.
2.2. Data collection and analysis
Age, gender, body mass index (BMI), and biochemical data obtained retrospectively from the hospital information system of the patients who had simultaneous measurements of fasting glucose, insulin, TSH, 25(OH)D, and lipid profile were analyzed and compared. All tests were analyzed in Istanbul Başakşehir Çam and Sakura City Hospital Central Laboratory. The analysis of biochemical tests was carried out with serum obtained. Serum glucose concentrations with enzymatic reference hexokinase, urea level with kinetic urease and glutamate dehydrogenase, creatinine level with kinetic colorimetric, cholesterol and triglyceride with the enzymatic colorimetric method, HDL with homogeneous enzymatic colorimetric method were measured on the Roche Cobas 8000 (Roche Indianapolis/America) analyzer. Serum TSH and insulin concentrations quantitatively with sandwich principle, fT4 and 25 (OH) D concentrations with competition principle method were measured on the Roche Diagnostics Cobas 8000 (Roche Indianapolis/America) analyzer. Serum LDL cholesterol levels were calculated. Evaluation of insulin resistance is based on simultaneous measurements of glucose and insulin. HOMA-IR= fasting glucose (mmol/L) x fasting insulin (µu/ml)/22.5 (4).
2.3. Statistical Analysis
In calculating the sample size of this study a Power analysis was determined by taking at least 80% and Type-1 error of 5 % for each variable. Kolmogorov-Smirnov and Skewness-Kurtosis tests were determined to check whether the continuous measurements in the study were normally distributed, and Parametric tests were applied because the measurements were distributed normally. The ”Independent T-test“ was used to compare continuous measurements according to the ”TSH groups". Pearson correlation coefficients were calculated to determine the relationships between continuous measurements. The chi-square test was used to determine the relationships between categorical variables. The SPSS (IBM SPSS for Windows, ver.26) statistical package program was used for analysis, and the statistical significance level was taken as 5 % in the calculations.
3. Results
3.1. Basic and metabolic characteristics of groups formed according to TSH levels
A total of 561 adult participants were included in this study. TSH levels of all individuals were within normal limits (0.27–4.2 ml/mL). 285 patients had low normal TSH levels and 276 patients had high normal TSH levels. The mean age was 37.47±12.43 years in the low normal TSH group and 37.89 ± 12.84 years in the high normal TSH group. 73.7% and 75.7% of the patients were women, while 26.3% and 24.3% were men, respectively. There was no statistically significant relationship between the groups in terms of gender (p<0.05).
In the table below; The biochemical measurements of the patients "according to TSH groups" were compared and the results were given (
Table 1).
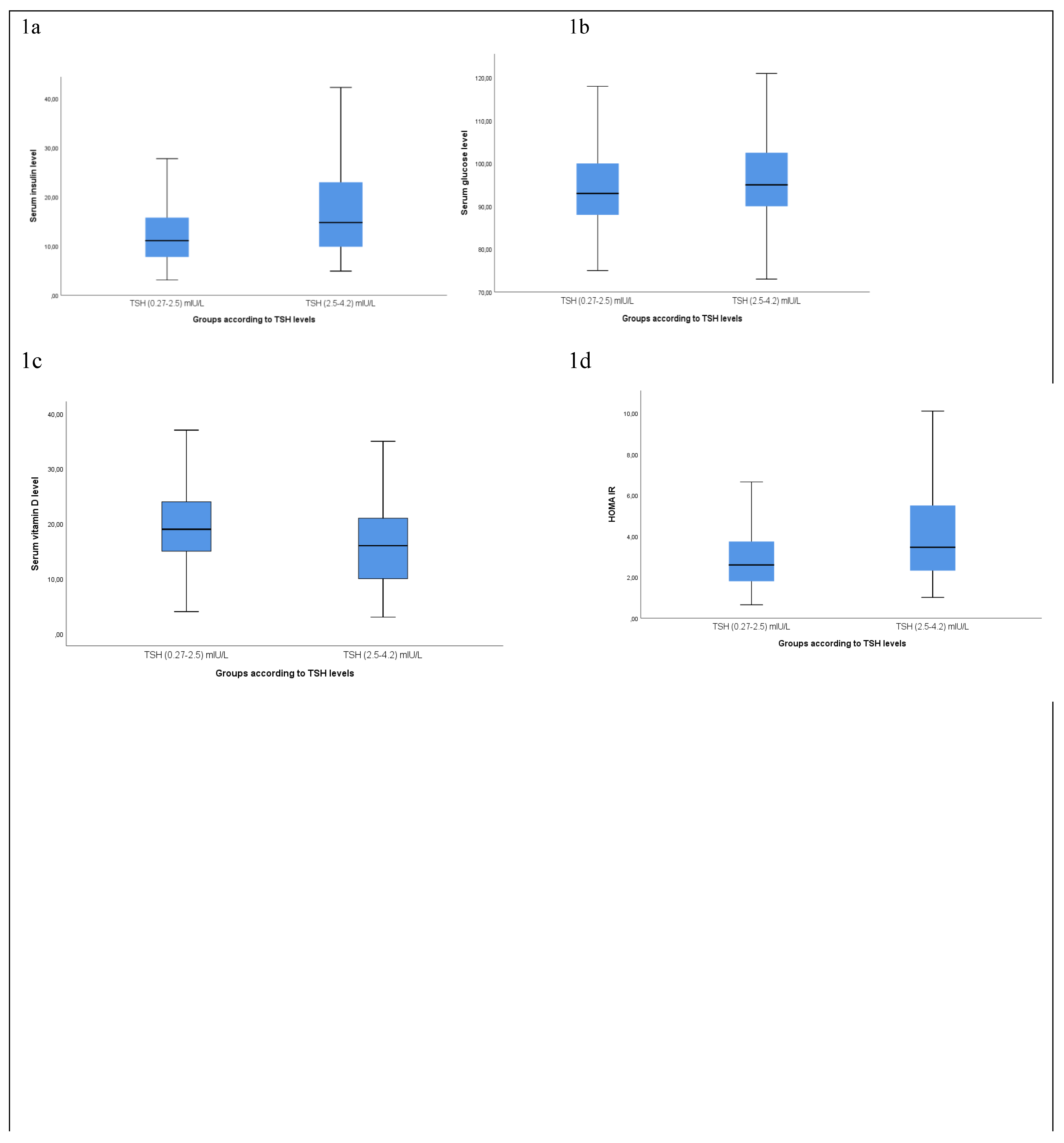
The fasting, insulin, glucose, and HOMA-IR levels of the patients changed according to the groups and were found to be statistically significantly higher in group 2 than in group 1 (p<0.05) (
Figure 1a,b,c). The BMI value of the patients was found to be statistically significantly increased while Vitamin D, and HDL significantly decreased in group 2 (p<0.05) (
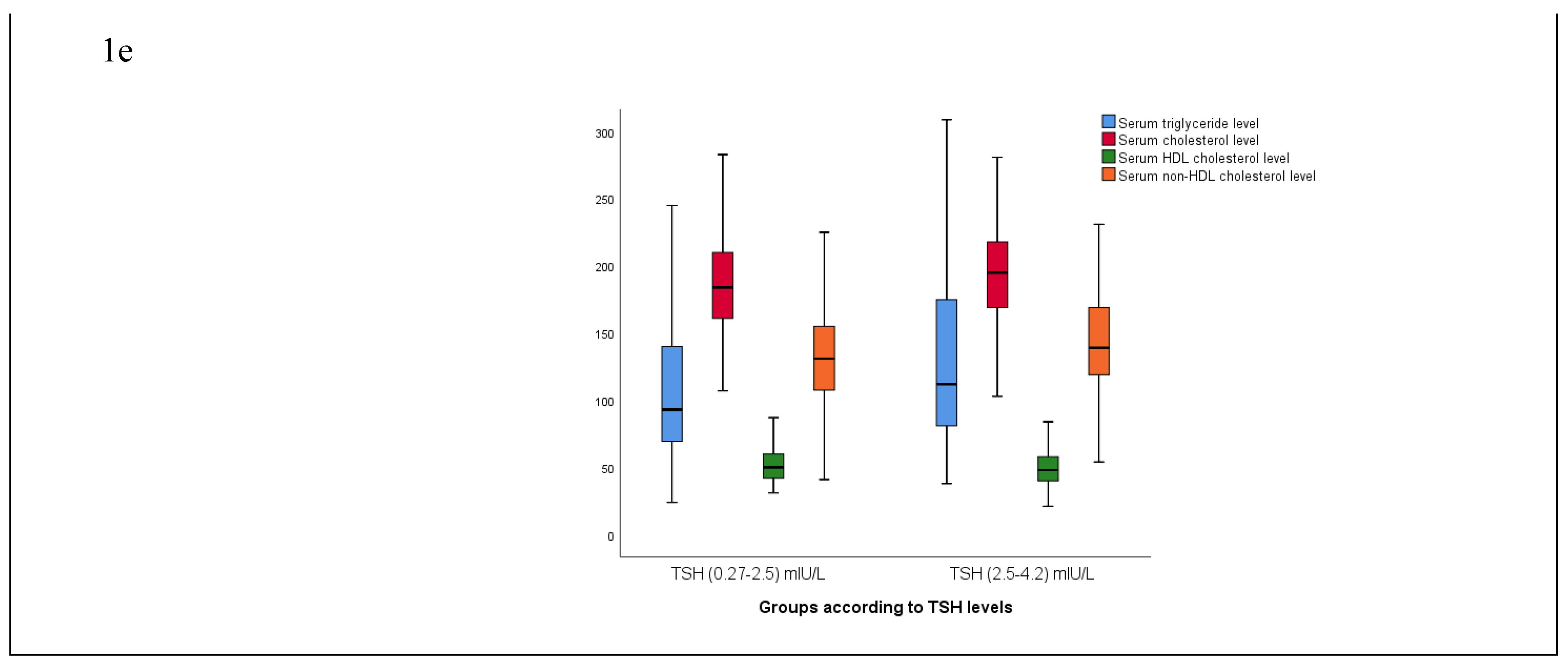
Figure 1d,e). Similarly, the triglyceride, cholesterol, LDL, and non-HDL cholesterol levels of the patients were found to be significantly increased in group 2 compared to group 1 (p<0.05) (
Figure 1e).
3.2. Correlation analysis
3.2.1. TSH and insulin resistance
In the table below; the results of correlation analysis between “TSH” and “HOMA-IR” measurements were shown separately in the groups (
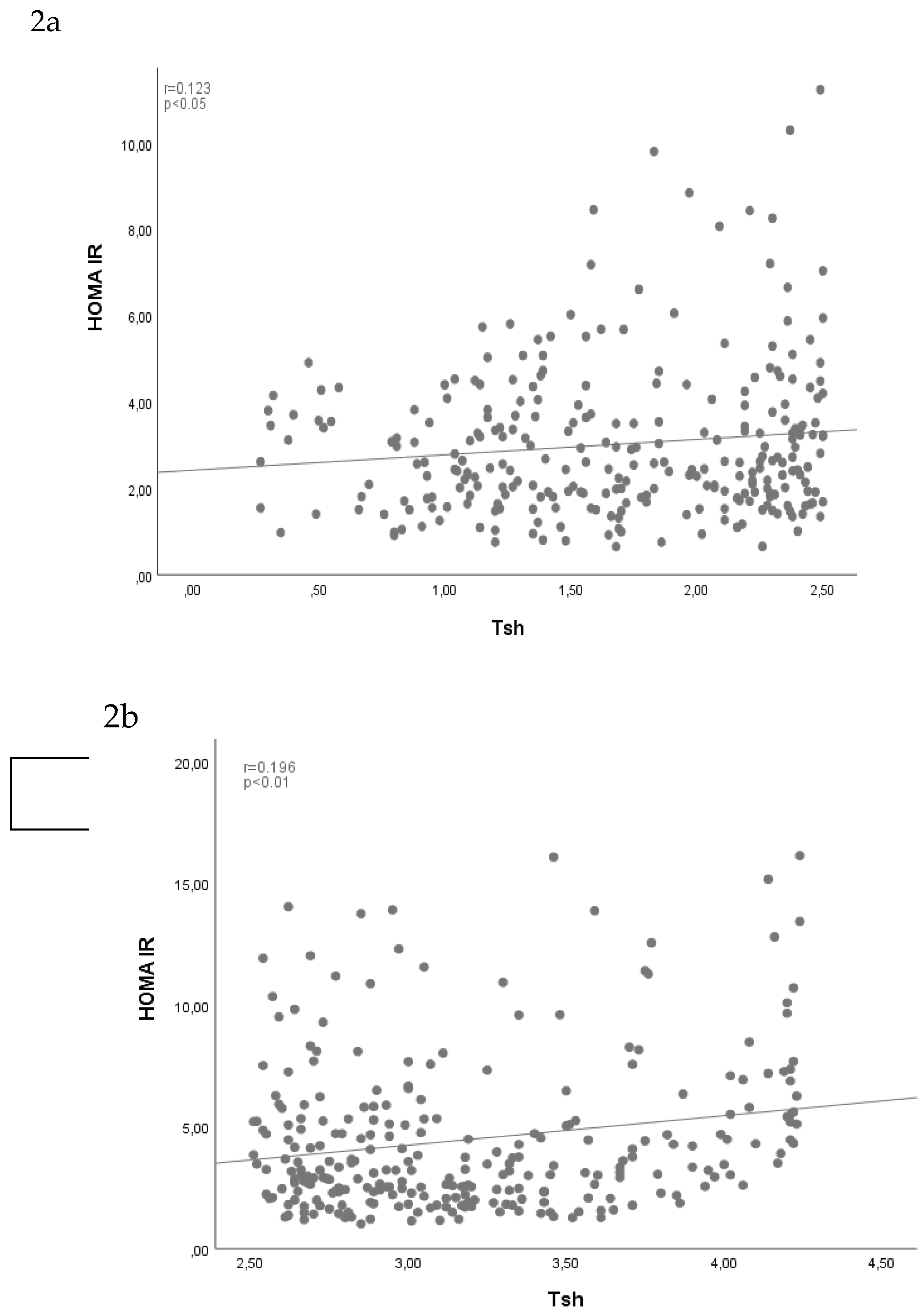
Table 2). Accordingly; a statistically significant positive correlation (12.3%) was found between the “TSH” and “HOMA-IR” measurements in the “low normal range” group (p<0.05) (
Figure 2 a). Similarly; a statistically significant positive correlation (19.6%) was found between “TSH” and “HOMA-IR” measurements in the “high normal range” group (p<0.05) (
Figure 2 b).
3.2.2. TSH and other data
There was a significant positive correlation between TSH and HOMA-IR (r =0.123, p = 0.041), fT4 (r = -0.404, p =0.040) in the low normal range group. The relationship between vitamin D and cholesterol (r =0.141, p =0.045), LDL cholesterol (r =0.162, p =0.025), non-HDL cholesterol (r =0.157, p =0.027), HOMA-IR and glucose (r =0.443, p <0.001), BMI (r =0.329, p <0.001), insulin (r =0.982, p <0.001), HDL cholesterol (r =-0.203, p =0.001), non-HDL cholesterol (r =0.132, p = 0.040), triglyceride (r = 0.405, p <0.001) levels was statistically significant. There was a positive correlation between glucose and insulin (r =0.295, p <0.001), triglyceride (r =0.168, p =0.007), non-HDL cholesterol (r =0.141, p =0.025), BMI (r =0.289, p =0.001) levels was also statistically significant.
The relationship between TSH and glucose (r =0.231, p <0.001), insulin (r =0.165, p =0.006), HOMA-IR (r =0.196, p <0.001), fT4 (r =-0.477, p =0.025), vitamin D (r =-0.200, p =0.003), cholesterol (r =0.143, p =0.024), LDL cholesterol (r =0.154, p =0.018), non-HDL cholesterol (r = 0.134, p = 0.035) levels was statistically significant in the high normal range group. There was a significant positive correlation between HOMA-IR with glucose (r =0.560, p <0.001), insulin (r =0.979, p <0.001), BMI (r =0.426, p <0.001), triglyceride (r =0.276, p <0.001), HDL cholesterol (r =-0.291, p <0.001), non-HDL cholesterol (r =0.159, p =0.013) levels was statistically significant. The relationship between glucose and insulin (r =0.413, p <0.001), triglyceride (r =0.231, p <0.001), HDL cholesterol (r =-0.224, p <0.001), non-HDL cholesterol (r =0.185, p =0.003) levels was also statistically significant.
4. Discussion
As far as we know, this is the first study aimed at assessing whether TSH levels within the reference range are simultaneously related to insulin resistance, lipid profile, and vitamin D levels. In our study, the mean serum TSH level of all our patients was within the normal range. Our results showed that the increase in serum TSH levels within the reference range was positively associated with insulin resistance and BMI. The correlation between TSH and HOMA-IR was better in the high normal TSH group than in the low normal TSH group. In addition, cholesterol, LDL, and non-HDL cholesterol levels were found to be positively correlated with TSH levels, and fT4 and vitamin D were negatively associated. These data suggest that thyroid hormones are associated with metabolic status and may lead to increased insulin resistance and related diseases. Our data are similar to several studies showing that TSH levels in adults may be associated with insulin resistance (12,14).
Serum TSH concentrations were found to be positively correlated with fasting and post-loading insulin levels and negatively correlated with insulin sensitivity in euthyroid individuals (15). Ping Zhu et al. reported that TSH levels in 447 euthyroid individuals were positively and linearly related to HOMA-IR in both diabetic and nondiabetic groups (16). Mueller et al. also in a study, found a correlation between insulin resistance with TSH in polycystic ovary syndrome, independent of age and BMI (14). In our study, we found that increases in TSH levels in the reference range were positively associated with HOMA-IR, as reported by Vaia Lambadiari et al. (17). Studies suggest that even small deviations in thyroid hormone levels in the physiological range can lead to insulin resistance (2). Thyroid hormones control metabolic rate, core body temperature, appetite, and sympathetic activity as well as regulate insulin secretion and destruction (17). Studies conducted showed that thyroid hormones may affect insulin sensitivity by affecting the activation or expression of β adrenergic, and gamma receptors (18). Brenta et al. have reported that patients with hypothyroidism showed much lower glucose utilization during intravenous insulin tolerance tests (19). Thyroid hormone therapy has been reported to increase insulin sensitivity in obese diabetic rodents (20). In tissue cultures from rats, pose to the thyroid hormone has been shown to cause raised GLUT 4 expression and glucose transport rate in the precursor cells of brown adipocytes. As a result, even mild hypothyroidism can trigger insulin resistance by causing reduced transcription of glucose transporters such as GLUT4 (18).
It has been stated that thyroid function is positively related to insulin resistance in obese individuals (21). Insulin resistance is determined by adipose tissue and associated inflammatory changes. As a possible key factor in the development of IR, it has also been suggested that leptin, which causes obesity, may be related to TSH (14). Studies conducted on euthyroid individuals have shown a positive relationship between serum TSH and BMI (12). Even minor changes in thyroid function have possible implications for the risk of developing obesity. Our results are consistent with the results of Mueller et al., in which BMI is positively correlated with TSH levels in the reference range (14). TSH receptors are found in a variety of body cells, including adipocytes. TSH mediates leptin secretion by binding to receptors on adipocytes (16). Leptin also has a possible role in the regulation of thyroid function through the stimulation of TRH. Moreover, thyroid hormones, and their metabolites also cause adiposity and weight gain. Mechanisms are related to adenosine triphosphate utilization, synthesis, direct impact on mitochondrial biogenesis, and its inotropic and chronotropic effects (22). This condition the idea that changes in the thyroid function are the main ones, and changes in BMI through changes in energy expenditure are secondarily effective. The increase in BMI and fat mass can also subsequently cause an increase in serum leptin (12). Since ectopic fat has a critical role in the formation of insulin resistance, fat cells may be an important factor in the relationship between TSH and insulin resistance (16). Our data confirm the results of some studies showing a close relationship between BMI and HOMA-IR and that development of obesity is a risk factor for the formation of IR (23,24).
Thyroid hormones are associated with dyslipidemia as they act as stimulators in the synthesis and destruction of lipids (25). Asvold et al. in a study conducted with people without known thyroid disease; reported that TSH increases in the normal range were positively, and significantly correlated with serum cholesterol, triglyceride, LDL, and non-HDL cholesterol levels (26). In our study, as in some studies conducted in individuals without significant thyroid dysfunction, increases in TSH showed an association with total cholesterol (27, 28) LDL cholesterol (27, 28) non-HDL cholesterol (27), and TG (28) levels. Thyroid hormones induce lipolysis in adipocytes and thus rise fatty acid levels in vivo. It also stimulates the re-esterification of free fatty acids to triacylglycerol, fatty acid oxidation in the liver, and de novo lipogenesis from glucose metabolism (22). The higher total cholesterol and LDL cholesterol levels we detected may be due to less cell surface receptor expression for LDL leading to a reduction in LDL catabolism (26). Decreased lipoprotein lipase activity and impaired clearance of lipoproteins due to less LDL receptor function may also outcome in high triglycerides in patients with high TSH (18). Increased accumulation of triglyceride in muscle tissue of type 2 diabetics and obese has been related to IR (29). It is also known that insulin resistance is accompanied by high hepatic cholesterol, very low-density lipoprotein production, precursor LDL particles, and increased HDL cholesterol clearance. Since LDL particles have a lower affinity than the LDL receptor, there is a retardation in their clearance (30). Studies have shown that thyroid hormone replacement reduces total serum cholesterol and LDL cholesterol levels in persons with TSH in the upper limit of the reference range (31).
Data on the direct interactions among circulating TSH and vitamin D levels are still insufficient. Tamer et al. found the frequency of vitamin D deficiency to be higher in those with obvious hypothyroidism (94%) or subclinical hypothyroidism (98%) than in those with euthyroidism (86%) (32). Our results showed that TSH levels at the upper limits of the normal range were related to lower vitamin D. Barchetta et al. in his study, which examined the relationship of TSH levels with the seasons in euthyroid adults, revealed for the first time that vitamin D deficiency was related with higher TSH levels, and showed that the relationship between serum TSH levels with vitamin D status was independent of the season (33). Liu et al. found that mice that received intraperitoneal injection of calcitriol before sensitization with porcine thyroglobulin did not show signs of inflammation of the thyroid, and reported that vitamin D had a protective role against the formation of thyroiditis (34). Gelbard et al. VDRs have been detected in the pituitary gland and it has been shown that vitamin D regulates the secretion of pituitary hormones in experimental studies (35). Vitamin D levels can affect the hypothalamus-pituitary-thyroid axis exerting a direct effect on thyrocytes as well as VDR expression in hypothalamic and thyrotropic pituitary cells. Vitamin D insufficiency may result in decreased sensitivity of thyrocytes to TSH stimuli and increased serum TSH levels (33).
There is concurrence that many persons with high normal TSH are probably to have early signs of thyroid dysfunction. Some authors state that the reference range for TSH must keep as 0.4 -4.0 mIU/l, as there is not sufficient cause for lowering the upper limit of the reference range for TSH (36). Mueller et al. in their study, associated the 2 mIU/l TSH threshold for IR detection with the best sensitivity and specificity in women with PCOS (14). Some studies suggest that individuals with a TSH value above 2.5 mIU/L are at risk in terms of thyroid disorders (13). Studies in euthyroid individuals have detected that increased thyroid antibodies correlate with TSH levels (31). In addition, the results of the 20-year follow-up of study also showed that TSH elevations greater than 2 mU/l were related to an increased hypothyroidism risk (37). The American Society of Clinical Endocrinologists stated that it would be appropriate to change the upper limit of the TSH reference range for adults to 3.0 mIU/L (38).
Study Limitations
Our study has some limitations. The sample size of the study was relatively small, retrospective, and cross-sectional. Our findings indicate that multicenter, larger prospective studies are needed for the relationship between insulin resistance, hyperlipidemia, and vitamin D in people with TSH levels within the normal range.
5. Conclusion
In conclusion, our study showed that increased TSH levels within the reference range were positively correlated with insulin resistance and cholesterol, LDL cholesterol, and non-HDL cholesterol levels, and negatively correlated with vitamin D and fT4 levels. These data suggest that even TSH levels showing clinically normal thyroid function may be associated with low vitamin D levels and may conduce to the development of IR and dyslipidemia, which are effective in the formation of Type 2 DM and CAD. Therefore, we believe that determining the optimal TSH target and regularly reviewing serum TSH levels with thyroid status can reduce cardiometabolic risk and related morbidity and mortality.
Funding
This work was not funded by any institution.
Institutional Review Board Statement
This study was approved by the ethics committee of Istanbul Başakşehir Çam and Sakura City Hospital (No.2021.06.120). Due to the retrospective and observational character of the study design, the requirement for informed consent has been waived.
Data Availability Statement
In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Please refer to suggested Data Availability Statements in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics. If the study did not report any data, you might add “Not applicable” here.
Acknowledgments
I would like to thank Prof Dr Yüksel Altuntaş and Doç Dr Alper Gümüş, who read and evaluated my article and supported it with their ideas.
Conflicts of Interest
The author declares that they have no conflict of interest.
References
- Liu, X.; Tang, H.Y.; Luo, Z.C. Insulin Resistance and Skin Diseases. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2020, 42, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Gierach, M.; Gierach, J.; Junik, R. Insulin resistance and thyroid disorders. Endokrynol. Pol. 2014, 65, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Tull, E.S.; Talbott, E.O.; Vogt, M.T.; Kuller, L.H. A hypothesis of synergism: the interrelationship of T3 and insulin to disturbances in metabolic homeostasis. Med. Hypotheses 2002, 59, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Maratou, E.; Hadjidakis, D.J.; Kollias, A.; Tsegka, K.; Peppa, M.; Alevizaki, M.; et al. Studies of insulin resistance in patients with clinical and subclinical hypothyroidism. Eur. J. Endocrinol. 2009, 160, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Cappola, A.R.; Ladenson, P.W. Hypothyroidism and atherosclerosis. J. Clin. Endocr. Metab. 2003, 88, 2438–2444. [Google Scholar] [CrossRef]
- Walsh, J.P.; Bremner, A.P.; Bulsara, M.K.; O’Leary, P.; Leedman, P.J.; Feddema, P.; et al. Thyroid dysfunction and serum lipids: a community-based study. Clin. Endocrinol. 2005, 63, 670–675. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, L.; Wang, F.; Yuan, Z.; Zhang, X.; Xu, C.; et al. A Worthy Finding: Decrease in Total Cholesterol and Low-Density Lipoprotein Cholesterol in Treated Mild Subclinical Hypothyroidism. Thyroid 2016, 26, 1019–1029. [Google Scholar] [CrossRef]
- Stumpf, W.E.; Sar, M.; O’Brien, L.P. Vitamin D sites of action in the pituitary studied by combined autoradiography-immunohistochemistry. Histochemistry 1987, 88, 11–16. [Google Scholar] [CrossRef]
- Nettore, I.C.; Albano, L.; Ungaro, P.; Colao, A.; Macchia, P.E. Sunshine vitamin and thyroid. Rev. Endocr. Metab. Disord. 2017, 18, 347–354. [Google Scholar] [CrossRef]
- Verrusio, W.; Magro, V.M.; Renzi, A.; Casciaro, B.; Andreozzi, P.; Cacciafesta, M. Thyroid hormones, metabolic syndrome and Vitamin D in middle-aged and older euthyroid subjects: a preliminary study. Aging Clin. Exp. Res. 2019, 31, 1337–1341. [Google Scholar] [CrossRef]
- Andersen, S.; Pedersen, K.M.; Bruun, N.H.; Laurberg, P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J. Clin. Endocrinol. Metab. 2002, 87, 1068–1072. [Google Scholar] [CrossRef]
- Knudsen, N.; Laurberg, P.; Rasmussen, L.B.; Bülow, I.; Perrild, H.; Ovesen, L.; et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J. Clin. Endocrinol. Metab. 2005, 90, 4019–4024. [Google Scholar] [CrossRef]
- Çevlik, T.; Haklar, G.; Şirikçi, Ö.; Emerk, K. TSH değerleri 2,5-4,2 mIU/L aralığında bulunan bireylerin subklinik hipotroidi geliştirme riski. Nobel Med. 2016, 12, 20–25. [Google Scholar]
- Mueller, A.; Schöfl, C.; Dittrich, R.; Cupisti, S.; Oppelt, P.G.; Schild, R.L.; et al. Thyroid-stimulating hormone is associated with insulin resistance independently of body mass index and age in women with polycystic ovary syndrome. Hum. Reprod. 2009, 24, 2924–2930. [Google Scholar] [CrossRef]
- Fernandez-Real, J.-M.; Lopez-Bermejo, A.; Castro, A.; Casamitjana, R.; Ricart, W. Thyroid function is intrinsically linked to insulin sensitivity and endothelium-dependent vasodilation in healthy euthyroid subjects. J. Clin. Endocr. Metab. 2006, 91, 3337–3343. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, X.; Mao, X. Thyroid-Stimulating Hormone Levels Are Positively Associated with Insulin Resistance. Med. Sci. Monit. 2018, 24, 342–347. [Google Scholar] [CrossRef]
- Lambadiari, V.; Mitrou, P.; Maratou, E.; Raptis, A.E.; Tountas, N.; Raptis, S.A.; et al. Thyroid hormones are positively associated with insulin resistance early in the development of type 2 diabetes. Endocrine 2011, 39, 28–32. [Google Scholar] [CrossRef]
- Nader, N.S.; Bahn, R.S.; Johnson, M.D.; Weaver, A.L.; Singh, R.; Kumar, S. Relationships between thyroid function and lipid status or insulin resistance in a pediatric population. Thyroid 2010, 20, 1333–1339. [Google Scholar] [CrossRef]
- Brenta, G.; Celi, F.S.; Pisarev, M.; Schnitman, M.; Sinay, I.; Arias, P. Acute thyroid hormone withdrawal in athyreotic patients results in a state of insulin resistance. Thyroid 2009, 19, 665–669. [Google Scholar] [CrossRef]
- Koritschoner, N.P.; Alvarez-Dolado, M.; Kurz, S.M.; Heikenwälder, M.F.; Hacker, C.; Vogel, F.; et al. Thyroid hormone regulates the obesity gene tub. EMBO Rep. 2001, 2, 499–504. [Google Scholar] [CrossRef]
- Ferrannini, E.; Iervasi, G.; Cobb, J.; Ndreu, R.; Nannipieri, M. Insulin resistance and normal thyroid hormone levels: prospective study and metabolomic analysis. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E429–E436. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.F.D.S.; Dos Santos, P.B.; Pazos-Moura, C.C. The role of thyroid hormone in metabolism and metabolic syndrome. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820917869. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; McCarthy, M.I.; Wass, J.A.; Franks, S. Obesity and polycystic ovary syndrome. Clin. Endocrinol. 2006, 65, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Gambineri, A.; Pelusi, C.; Vicennati, V.; Pagotto, U.; Pasquali, R. Obesity and the polycystic ovary syndrome. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Senese, R.; Lasala, P.; Leanza, C.; Lange, P. New avenues for regulation of lipid metabolism by thyroid hormones and analogs. Front. Physiol. 2014, 5, 475. [Google Scholar] [CrossRef]
- Asvold, B.O.; Vatten, L.J.; Nilsen, T.I.L.; Bjøro, T. The association between TSH within the reference range and serum lipid concentrations in a population-based study, The HUNT Study. Eur. J. Endocrinol. 2007, 156, 181–186. [Google Scholar] [CrossRef]
- Chubb, S.A.P.; Davis, W.A.; Davis, T.M.E. Interactions among thyroid function, insulin sensitivity, and serum lipid concentrations: the Fremantle diabetes study. J. Clin. Endocrinol. Metab. 2005, 90, 5317–5320. [Google Scholar] [CrossRef]
- Skoczyńska, A.; Wojakowska, A.; Turczyn, B.; Zatońska, K.; Wolyniec, M.; Rogala, N.; et al. Serum lipid transfer proteins in hypothyroid patients are inversely correlated with Thyroid-Stimulating Hormone (TSH) levels. Med. Sci. Monit. 2016, 22, 4661–4669. [Google Scholar] [CrossRef]
- Lou, M.; Luo, P.; Tang, R.; Peng, Y.; Yu, S.; Huang, W.; et al. Relationship between neutrophil-lymphocyte ratio and insulin resistance in newly diagnosed type 2 diabetes mellitus patients. BMC Endocr. Disord. 2015, 2, 15, 9. [Google Scholar] [CrossRef]
- Bakker, S.J.; Maaten, J.C.; Popp-Snijders, C.; Slaets, J.P.; Heine, R.J.; Gans, R.O. The relationship between thyrotropin and low-density lipoprotein cholesterol is modified by insulin sensitivity in healthy euthyroid subjects. J. Clin. Endocrinol. Metab.. 2001, 86, 1206–1211. [Google Scholar] [CrossRef]
- Michalopoulou, G.; Alevizaki, M.; Piperingos, G.; Mitsibounas, D.; Mantzos, E.; Adamopoulos, P.; et al. High serum cholesterol levels in persons with ‘high-normal’ TSH levels: should one extend the definition of subclinical hypothyroidism? Eur. J. Endocrinol. 1998, 138, 141–145. [Google Scholar] [CrossRef]
- Tamer, G.; Arik, S.; Tamer, I.; Coksert, D. Relative vitamin D insufficiency in Hashimoto’s thyroiditis. Thyroid. Off. J. Am. Thyroid. Assoc. 2011, 21, 891–896. [Google Scholar] [CrossRef]
- Barchetta, I.; Baroni, M.G.; Leonetti, F.; Bernardinis, M.D.; Bertoccini, L.; Fontana, M.; et al. TSH levels are associated with vitamin D status and seasonality in an adult population of euthyroid adults. Clin. Exp. Med. 2015, 15, 389–396. [Google Scholar] [CrossRef]
- Liu, S.; Xiong, F.; Liu, E.M.; Zhu, M.; Lei, P.Y. Effects of 1,25-dihydroxy vitamin D3 in rats with experimental autoimmune thyroiditis. J. S. Med. Univ. 2010, 30, 1573–1576. [Google Scholar]
- Gelbard, H.A.; Stern, P.H.; U’Prichard, D.C. 1 alpha, 25-dihydroxyvitamin D3 nuclear receptors in the pituitary. Science 1980, 209, 1247–1249. [Google Scholar] [CrossRef]
- Brabant, G.; Beck-Peccoz, P.; Jarzab, B.; Laurberg, P.; Orgiazzi, J.; Szabolcs, I.; et al. Is there a need to redefine the upper normal limit of TSH? Eur. J. Endocrinol. 2006, 154, 633–637. [Google Scholar] [CrossRef]
- Vanderpump, M.P.J.; Tunbridge, W.M.G.; French, J.M.; Appleton, D.; Bates, D.; Clark, F.; et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin. Endocrinol. 1995, 43, 55–68. [Google Scholar] [CrossRef]
- Baskin, H.J.; Cobin, R.H.; Duick, D.S.; Gharib, H.; Guttler, R.B.; Kaplan, M.M.; et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr. Pract. 2002, 8, 457–469. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).