1. Introduction
Recent studies suggest plate tectonics as a fundamental driver of global biodiversity in marine and terrestrial ecosystems (e.g., Descombes et al. 2017). Understanding these processes is critical to ecology and biology. Insects are the world’s diverse group of animals and are essential to the functioning of terrestrial ecosystems. This is especially true for red wood ants (Formica rufa-group; hereafter RWA), a key ecological species (e.g., Frouz and Jílková 2008). Their interactions with the environment are diverse, e.g., contributing to forest habitat biodiversity (e.g., Frouz et al. 2008), controlling of undesirable insects (e.g., Robinson et al. 2016), indicating undetected tectonic activity and geogenic gases (“GeoBio-Interactions”) that play a critical role in their settlement (Berberich et al. 2014, 2016a-c, 2018a,b, 2019, 2022a,b)).
Recent works report declines in insect diversity, species and biomass in Europe due to habitat loss and fragmentation, pollution, climate change, or invasive species (e.g., Hallmann et al. 2017; Wagner et al. 2021). For RWA, some researchers suggest declines (e.g., Crist 2009; Çamlitepe and Aksoy 2019), while others report population increases (e.g., Stoschek and Roch 2006; Wilson 2011). A decline in RWA populations is also postulated for Germany (Sturm and Distler 2003). Therefore, RWA are considered of conservation concern in Germany (e.g., §39 BNAtSchG 2009; § 1 BArtSchV 2013) and are included in the German government’s Insect Conservation Action Program (AP Insektenschutz 2019).
However, a reliable statistical database for Germany on the distribution of RWA nests and species is completely lacking so far, as RWA nests have not been systematically monitored and recorded since the 1980s. Therefore, no statements about a necessary protection status can be made at present. In this re-inventory study, conducted after 12 years in Westeifel Volcanic Field (WEVF), we applied our standardized and integrated mapping approach for RWA, that not only counts the number of RWA nests, but records the coordinates of their location and monitors the entire ecosystem around a RWA nest, e.g., tree species, tree age, herb layer (Berberich et al. 2022a). For a comparative analysis of RWAs population dynamics, 12 study sites (total ≈1281 ha) were selected that had to meet the following criteria: a) more than 20 nests in 2009 (Berberich 2010), (b) well defined study site boundaries and c) location within and on the edges of the WEVF.
We investigated forest-tectonic interactions by asking four interrelated questions: (1) Are RWA nest numbers decreasing or increasing in the study sites compared to the 2009 inventory? (2) What influence do variable factors (e.g., type and composition, herbaceous layer, tree age, clearings) have on potential changes in nest numbers? (3) What influence do quasi-invariant factors, such as ground movement or Radon concentrations have on RWA nest distribution? and (4) Are there other biological influences on RWA nests, such as woodpeckers or specific herb layer plants? We asked these questions specifically with respect to individual RWA nests. These results will further improve the understanding of the “GeoBio-Interactions”, contribute to better protection of RWA in forest management in the WEVF, and contribute to the German government’s Insect Conservation Action Program.
2. Materials and Methods
2.1. Location
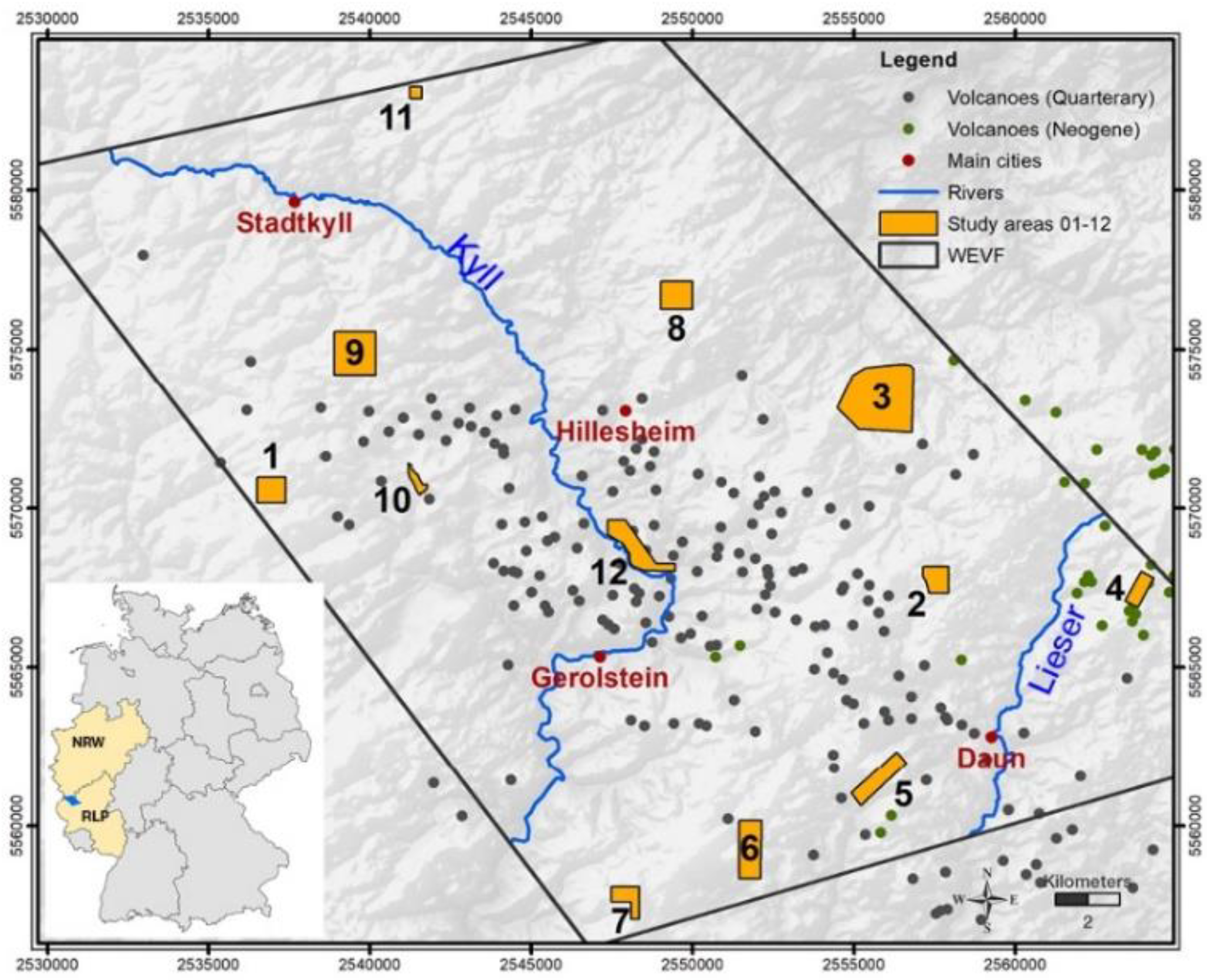
The 12 forested study sites, Duppach (01-Dup); Dockweiler Wald (02-Doc), Oberehe (03-Obe), Samersbach (04-Sam), Neunkirchen/Oberstadtfeld (05-Neu), Marschbachtal (06-Mar), Salm (07-Sal), Berndorf (08-Ber), Lissendorf (09-Lis), Heidberg (10-Hei), Vierherrenstein (11-Vie) and Rockeskyll (12-Roc) were located around and within the volcanic center of the Quaternary WEVF (
Figure 1), which is part of one of the youngest and most active volcanic regions in Europe (Schmincke 2007). The site locations are about ≈100 km away from Cologne, North-Rhine-Westphalia (NRW), and ≈60 km from Koblenz (Rhineland-Palatinate, RLP), West Germany and had been part of the PhD thesis of the first author (Berberich 2010). The hilly landscape (350–650 m a.s.l.) is characterized by individual ridges, cinder cones and basalt domes as well as deep valleys, and cut by rivers such as river Kyll. The forest, with an area share of almost 50%, is the economically most important sector, followed by the agricultural one (42%). The annual average temperatures range from -1.5 °C to 14 °C (kwis-rlp 2021).
2.1. Forest owners
The study sites are part of five forest districts (FD) in RLP and NRW: FD Prüm, FD Gerolstein, FA Daun, FD Hillesheim, and FD Gemeindeforstamt Dahlem. The State (SF), municipal communities in RLP and NRW (MF), and private persons (PF) hold proportions of the forest. In accordance with the five forest districts, the medium tree age class was chosen for all analyses.
2.3. Standardized, integrated mapping approach and data collection
Mapping of RWA nests followed the area-wide, systematic, reproducible, and integrated approach developed by Berberich et al. (Berberich et al. 2022a; Berberich 2010). A total of ≈1281 ha were mapped in two inventory campaigns, in 2009 and 2021. The total number of RWA nests (ntot) was mapped in all 12 study sites using GPS receivers (Garmin 60CSx and 62S). Six nest height classes (NH; start-ups: 0.01–0.10 m, short: 0.11–0.50 m, medium: 0.51–1.00 m, tall: 1.01–1.50 m, very tall: 1.51–2.00 m, extra tall: >2.01 m), five diameter classes (ND; small: 0.01–0.50 m, medium: 0.51–1.00 m, large: 1.01–1.50 m, very large: 1.51–2.00 m, extra-large > 2.01 m), inactive (ninact) and active nests (nact), and the nest location (e.g., within the forest, forest roads, forest edges, open areas), were classified in the field. In addition, qualitative information on tree species (e.g., spruce, pine) and herbaceous layer (e.g., nettles, grass, blackberry) on and around the RWA nest was recorded. Furthermore, cavities created by woodpeckers in each nest (WpC) were counted in the field. Finally, at least two photographs (landscape and normal format) were taken of each nest. These photographs were used to compare and (re)-identify a) nests, b) forest composition, and c) herbaceous layer mapped in 2009 and 2021.
2.4. Definition of variable site factors
In this study, we applied similar variable factors as in a previous study (Berberich et al. 2022a) that are influenced in a short time frame: 1) total number of nests (ntot), 2) numbers of active nests (nact), 3) number of inactive nests (ninact), 4) NH, 5) ND, 6) nest location, 7) primary tree species (TSprime), 8) secondary tree species (TSsec), 9) medium tree age classes, 10) herbaceous layer, 11) spatial distribution of RWA nests, 12) woodpecker cavities (WpC), and 13) clearing plots. Forest information on ownership type (SF; MF; PF) and medium tree age (mTA) was taken from the most recent forest inventories and management plans of the different forest offices, which contain five general tree age classes: newly planted (<20 years), young (21-40 years), early mature (41-60 years), medium mature (61-80 years), mature (≥81–140 years).
2.5. Data analysis
MATLAB R2019b and the geographic information system ArcGIS 10.8.2 were used for analyses. To investigate whether RWA nests were evenly or randomly distributed or clustered, point distribution statistics (X²–test) was applied. Patterns of multiple dependent variable factors, were investigated by applying one-way ANOVA, Kruskal-Wallis-Test and Multivariate ANalysis Of Variance (MANOVA). To identify differences in the qualitative data set of the herbaceous layer and to visualize the results word frequency analysis and font colors were applied.
3. Results
In both inventories, RWA nests were spatially clustered, as indicated by a nearest neighbor ratio <1 (2009: 0.4; 2021: 0.3) and Z-statistic < -1.96 (2009: -11.03; 2021: -20.12) at the 95% significance level. The returned value of
p indicate that Kruskal-Wallis and multiple comparison results for both inventories for physical nest parameters (NH, ND) of active nests, medium tree age (mTA) for TS
prime and woodpecker cavities (WpC) in nests confirmed the null hypothesis that the data for NH-ND, NH-mTA, ND-WpC, ND-mTA, WpC-mTA come from the same distribution at the 1% significance level and that there is no significant difference between these groups in both inventories (
Table 1a). A difference is indicated by a slightly elevated
p-value for NH-WpC in the 2021 inventory suggesting a change in continuous distribution (
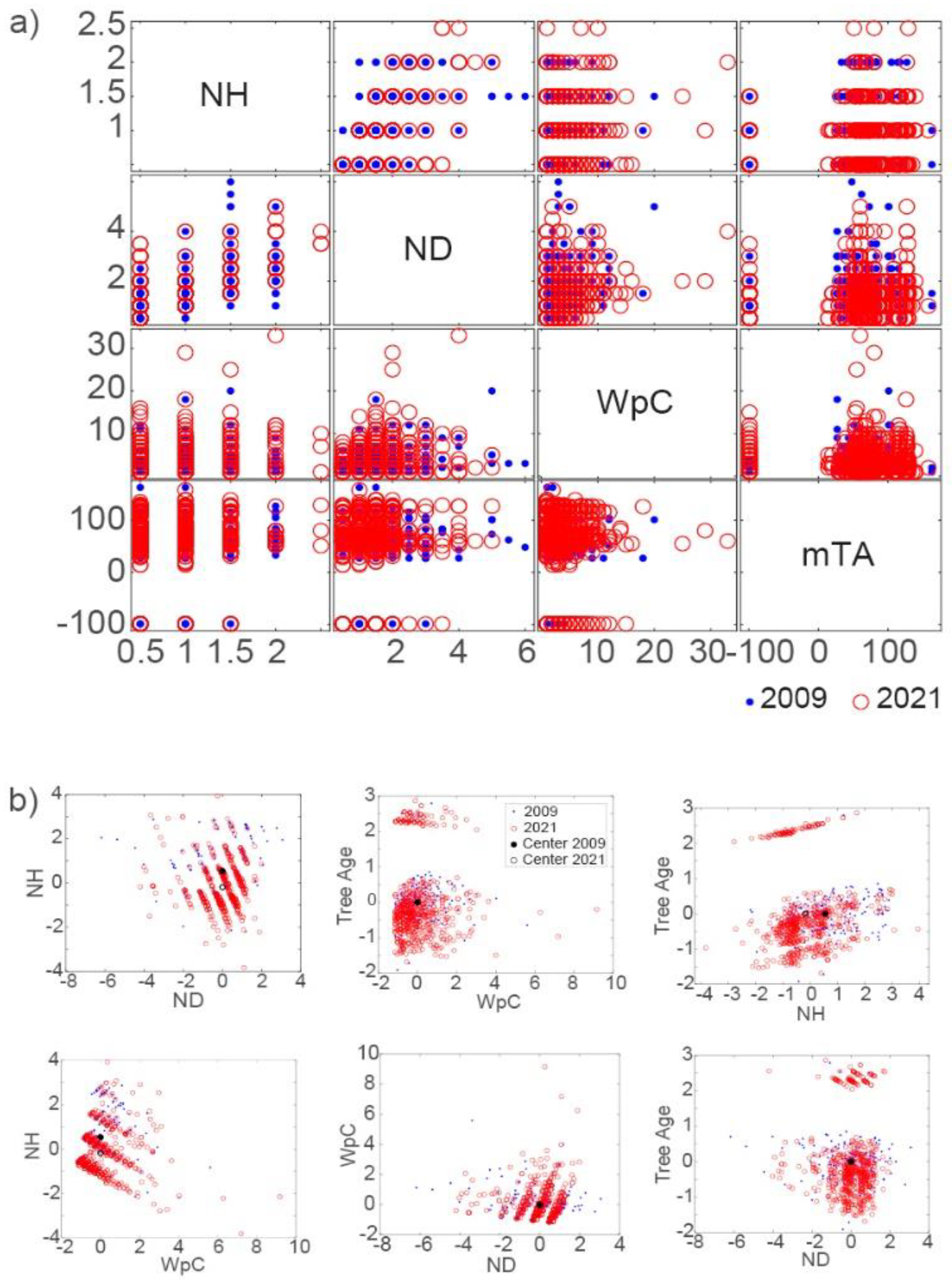
Table 1b). Results of the MANOVA differ from 2009 to 2021 (
Figure 2a) and showed in combination with the grouped scatter plots of the first two canonical variables more separation between groups and a) a shift to smaller NH and ND sizes, b) a strong increase in WpC and c) a shift of mTA to more mature forests in 2021 (
Figure 2b).
3.1. Physical nest parameters
In both inventories, nest counts differed between active nests (n
act) and inactive nests (n
inact). In 2009, a total of 1144 nests (n
tot) were mapped (n
act = 1099; n
inact = 45); in 2021, this number increased to 1252 nests (n
act = 1164; n
inact = 88). This is an overall increase of 108 RWA nests (≈10%) in all sites and an increase in active nests of ≈6 % in 2021. Eight of 12 study sites showed a significant increase in nest numbers compared to 2009, of which four sites 05-Neu (98%), 01-Dup and 10-Hei (both 46%) and 12-Roc (45 %) had the highest increase; four study sites (02-Doc, 03-Obe, 07-Sal, 09-Lis) showed a decrease (
Figure 3;
Table 2). The re-inventory in study site 07-Sal could only be carried out incompletely, because due to the hazard situation (snow breakage) in winter 2020/2021, central areas were not allowed to be entered. Therefore, it cannot be excluded that the total number of nests was higher, so that the decrease of nests in this area must be interpreted with reservation.
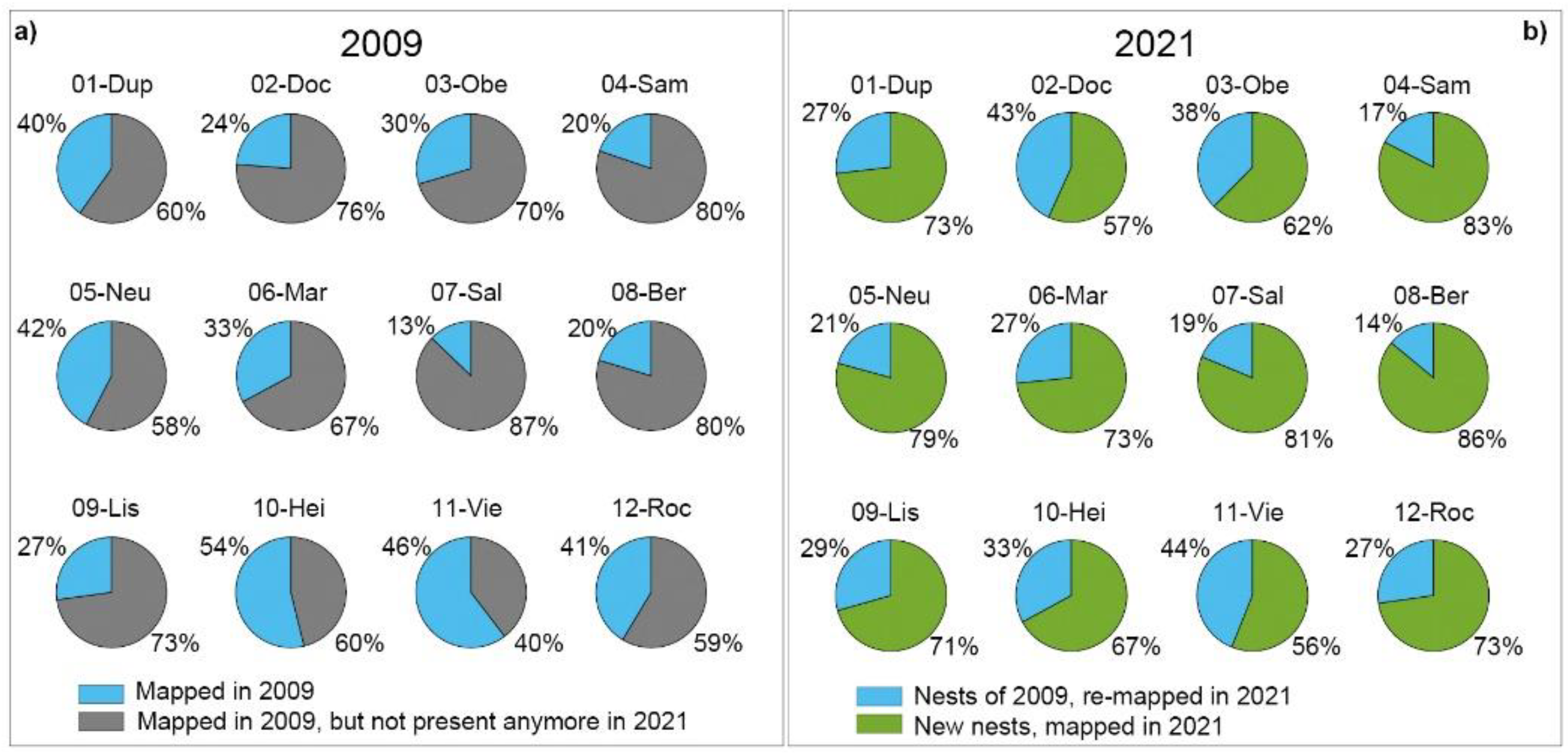
In total, 342 RWA (n
totR) and their forest habitat were re-identified and re-mapped (n
actR=296 nests) using the GPS records and the 2009 photo data base. The number of re-mapped nests varied across the 12 study sites (
Figure 3b;
Table 2): The highest re-mapped nest numbers were found in study sites 11-Vie (≈44 %), 02-Doc (≈43 %), 03-Obe (≈38 %), and 10-Hei (≈33 %). Sites 08-Ber (≈86 %), 04-Sam (≈83 %), 07-Sal (≈81 %), and 05-Neu (≈79 %) had the highest increase in new nests. On the other hand, the highest nest losses were recorded in 07-Sal (≈87 %), 08-Ber and 04-Sam (both ≈80 %), and 02-Doc (≈76 %). Only active nests (2009: 1099 n
act; 2021: 1164 n
act) are discussed below.
In 2021, a strong overall shift toward smaller nests was observed, e.g., start-ups and short nests as ≈65 % of nests were recorded in this two height classes (2009: ≈47 %), suggesting a surge in new nest foundations. In particular, short nests nearly doubled from ≈34 % in 2009 to ≈55 % in 2021. A quarter (≈24 %) of the nests were medium sized in 2021 (2009: ≈30 %). In 2021, ≈11 % (2009: ≈23 %) could be classified as tall and very tall nests (
Table 2). The same trend was observed for nest diameters. In 2021, ≈61 % could be classified as small and medium diameters (2009: ≈54 %). One fifth of all nests showed large diameters in both inventories. In 2021, the largest diameters (≥1.51 m) were also declining (≈17 %; 2009: ≈25 %).
3.2. Forest stands and clearing plots
In 2009, coniferous forest was strongly-dominated by spruce (Picea abies; ≈79%) as the primary tree species (TSprime). Mixed conifer stands were characterized by spruce (TSprime) and larch (Lariyx decidua; ≈5%), Douglas-fir (Pseudotsuga menziesii; ≈4%) and pine (Pinus sylvestris; ≈1%). Deciduous trees such as sessile oak (Quercus petraea) and beech (Fagus silvatica) accounted for ≈2 % as TSprime, whereas beech (Fagus silvatica) accounted for ≈15 % as secondary tree species (TSsec). In 2021, forest compositions changed as the proportion of spruce (≈76 % TSprime) and larch (≈3% TSprime) decreased. The proportion of sessile oak and beech as TSsec (≈2 %) was also reduced.
In absolute numbers, early mature (41-60 years) spruce-dominated forests (TSprime) were the preferred location for RWA nests (41 %) in all study areas. Here, ≈13 % of short and ≈14 % of medium-sized nest were mapped in 2009. A quarter (≈25 %) of all nests were mapped in mature (≥81–140 years) spruce-dominated forests (TSprime), of which ≈9 % were short and ≈7 % medium nests. In 2021, ≈45 % of the RWA nests (≈25 % short nests) were mapped in early-to medium-mature spruce forests (41-80 years) and one third (≈30 %) in mature (≥81–140 years; ≈14 % short nests). Deciduous trees, e.g., beech, were not relevant TSprimes as given by the low RWA nest counts. In 2009, percentages of ND classes in early mature forest were the similar (≈11 %) for small and large diameters. In mature forests, small and medium diameters accounted for ≈13 % (2009) and≈17 % (2021), respectively. Forest composition observed in the field differed from forest records, showing a shift from conifer stands such as pure spruce stands (2009: ≈53%; 2021: ≈34%) and spruce-larch stands (2009: ≈3.2%; 2021: ≈1.5%) to naturally regenerated mixed stands consisting of e.g., spruce-beech (2009: ≈21%; 2021: ≈30%), spruce-oak (2009: ≈2%; 2021: ≈5%).
Overall, clearing activities increased at each site between the two inventories, e.g., due to bark beetle infestation or snow breakage. The size of clearing plots with active RWA nests doubled from 5 ha in 2009 to ≈11 ha in 2021, but the total number of active nests on these clearing plots quadrupled in 2021 (n
actC = 205; 2009: n
actC = 51;
Table 3), especially for start-ups (≈14%) and short (≈64%) nests, but also for medium nests (≈18%). The greatest clearing activity occurred at four study sites that showed large increases in nest numbers in the clearing plots: 03-Obe (2021: 61; 2009: 26), 04-Sam (2021: 58; 2009: 16), 08-Ber (2021: 29; 2009: 4), and 07-Sal (2021: 17; 2009: 0). Surprisingly, 41 active RWA nests from 2009 were re-identified on current clearing plots: 01-Dup (3), 03-Obe (18), 04-Sam (11); 05-Neu (1), 07-Sal (2), 08-Ber (2), and 09-Lis (4) in 2021. About one third of these nests (n
act = 13) were previously mapped in 2009 (03-Obe: 9), 04-Sam (2), and 08-Ber (2) on clearing plots.
The percentages of nest numbers in SF, MF and PF were almost the same for the three different forest owners in both inventories: SF (≈26 %), MF (≈61 %), and PF (≈13 %;
Table 3). In 2009, one third (≈30 %) of tall and very tall nests (≥1.01 m) were observed in PF, one-fourth (≈25 %) in SF, and one fifth (≈20 %) in MF. In 2021, the numbers were lower (PF ≈21 %, SF ≈12 %, and MF ≈8 %). In exchange, the number of nests doubled for short nests (0.11–0.50 m NH) in SF, MF and PF, with the highest increase (2021 = ≈57 %; 2009 = ≈35 %) in MF, followed by PF (2021 = ≈54 %; 2009 = ≈22 %) and SF (2021 = ≈52 %; 2009 = ≈35 %). At study sites 02-Doc and 09-Lis, losses were highest primarily for short, medium, and tall to very tall nests in MF.
In 2021, ≈71 % (2009: ≈76 %) of active nests were within forest stands (including forest roads and skid trails), approximately one fifth (2021: 19 %; 2009: ≈8 %) were in open space areas (e.g., clearing plots), and ≈11 % (2009: ≈15 %) were at forest edges.
3.3. Herbaceous layer
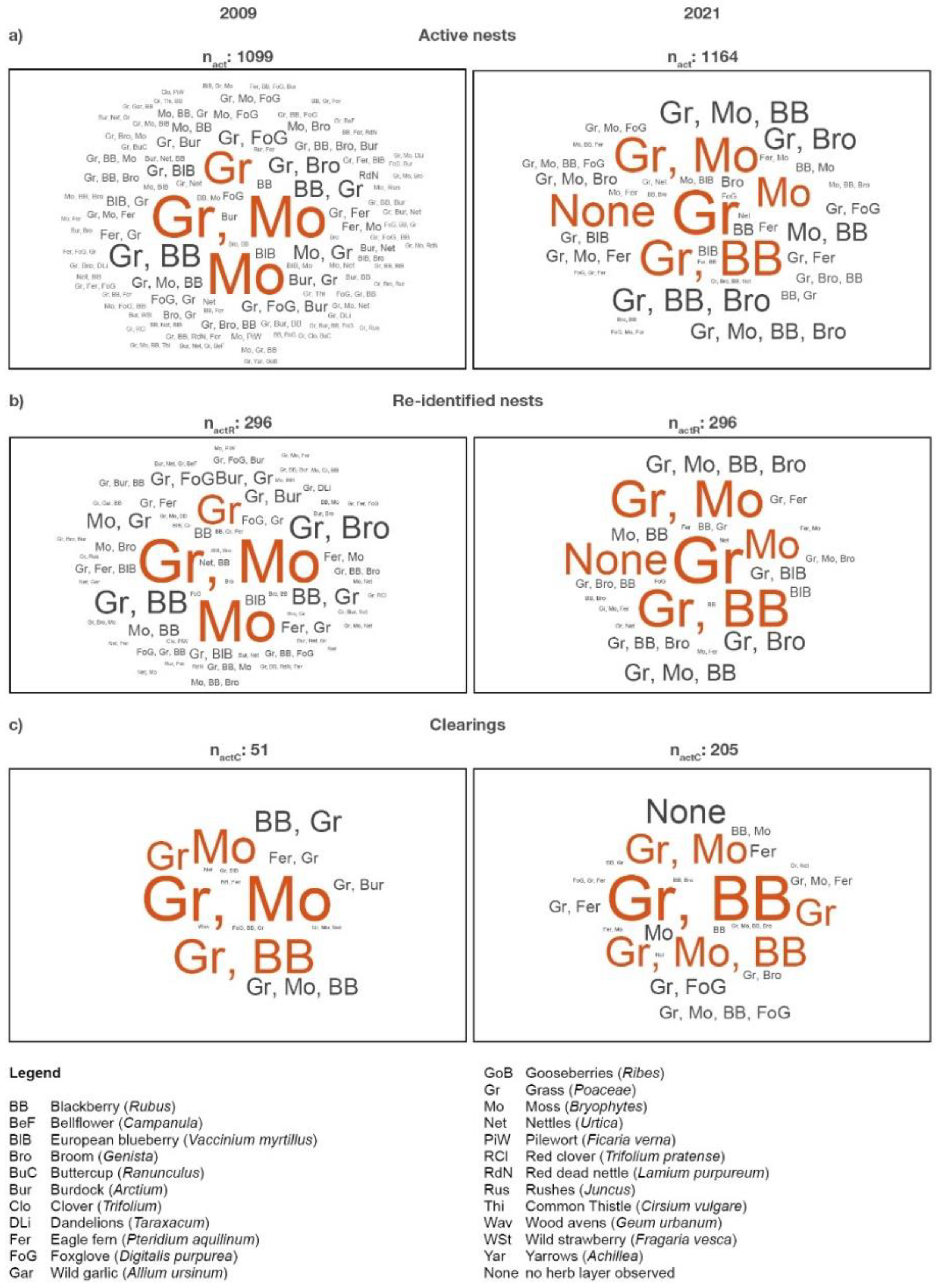
In both inventories, single occurrences of grasses (
Poaceae) and mosses (
Bryophta) or a combination of both herbs were highly abundant as visualized by word clouds in
Figure 4a (2009: ≈54 %; 2021: ≈45 %). The combination of grasses (
Poaceae) and blackberries (
Rubus) increased from ≈9 % (2009) to ≈14 % (2021). In addition, the proportion of blackberries in combination with moss, broom (
Genista) and other plants, e.g., common thistle
(Cirsium vulgare), stinging nettle (
Urtica) increased from ≈20 % in 2009 to ≈31 % in 2021, indicating a greater distribution of blackberries at and on RWA nests in 2021. In 2009, 196 nests of almost all nest heights (29 start-ups, 68 short, 70 medium sized, 18 tall, and 11 very tall nests) were affected by the proliferating blackberry or blackberry in combination with other herbs. In 2021, the number of these nests doubled (380 nests: 31 start-ups, 208 short, 108 medium sized, 31 tall, and 2 very tall nests). Other typical plants of the herb layer, such as burdock (
Arctium; 2009:≈8 %; 2021: 0 %), European blueberry (
Vaccinium myrtillus; 2009: ≈6 %; 2021: ≈1 %), eagle fern (
Pteridium aquilinum; 2009: ≈7 %; 2021: ≈3 %); foxglove (
Digitalis purpurea; 2009: ≈6 %; 2021: ≈1 %
) mostly occurred in combination with grasses and mosses; other plants only played a minor role in 2021 (
Figure 4a). In 2009, other herbs such as buttercup
(Ranunculus), clover (
Trifolium), dandelion
(Taraxacum), bellflower
(Campanula), red dead nettle (
Lamium purpureum), wild garlic
(Allium ursinum), gooseberries
(Ribes), pilewort
(Ficaria verna), rushes
(Juncus), wood avens (
Geum urbanum), wild strawberry (
Fragaria vesca) or yarrows
(Achillea) could be observed, but played a minor role.
The herb layer around and on the 296 re-identified RWA nests showed a similar picture as visualized in
Figure 4b. Single occurrences of grasses (
Poaceae) and mosses (
Bryophta) or a combination of both herbs were highly abundant (2009: ≈46 %; 2021: ≈49 %). The combination of grass, moss and blackberry increased from ≈8 % (2009) to ≈18 % (2021), and the combination of grass, moss and broom doubled from ≈6 % (2009) to ≈11 % (2021). In 2021, more than one third of the re-identified nests (84 nests) were affected by the proliferating blackberry as single plant or in combination with grass, moss or broom (6 start-ups, 41 short, 25 medium sized, 11 tall, and 1 very tall nests). Half of the 84 nests (34 nests) were already infested by blackberry in 2009. Other typical plants of the herb layer still observed in 2009 played a minor to no role. The proportion of herb-free plots was ≈11 % in 2021 (2009: 0 %).
Even in cleared areas (
Figure 4c), the proportion of the most dominant herbs (grass, moss, blackberry) increased from ≈60 % (2009) to ≈79 % (2021). Combinations of these dominant plants with fern and broom accounted for ≈12 % in 2021.
3.5. Woodpecker cavities
Woodpecker cavities (WpC) were observed throughout the nest surface in both inventories. Nest counts (2009: 224 n
act; 2021: 634 n
act) with WpC and counts of WpC in nest (2009: 699; 2021: 2362) tripled in 2021 compared to 2009 (
Table 4). In 2021, less than half of active nests (≈46 %) had no woodpecker cavities (2009: ≈80 %), and ≈39 % (2009: ≈17 %) had cavity counts between 1–4. The WpC to nest ratio nearly tripled from 0.6 in 2009 to 2.0 in 2021. In 2021, one fifth ≈22 % (2009: ≈4 %) of short nests and ≈12 % of medium-sized nests had 1–4 cavities (2009: ≈7 %). In both inventories, larger nests (tall–extra tall) had fewer cavities than smaller nests (
Table 5). In 2009, seven short–medium-sized nests had >10 woodpecker cavities (04-Sam = 3; 06-Mar = 2; 07-Sal = 2); in 2021, there were 21 short– extra tall nests (01-Dup= 1; 03-Obe= 4; 04-Sam= 5; 05-Neu= 6; 11-Vie= 5) with >10 woodpecker cavities.
An increase in woodpecker cavities was observed in SF (2021: ≈32 %; 2009: ≈25 %) and PF (2021: 13 %; 2009: ≈8 %). In MF, the cavities decreased from ≈67 % (2009) to ≈56 % in 2021. In 2021, the cavity-to-NH class ratio was highest for short (0.4), medium (0.3) and tall (0.1) nests in SF; in PF the highest ratio had been observed for extra tall nests (0.1). In 2009, ratios were much lower for all NH classes and forest owners.
4. Discussion
4.1. Pre-requisites for (re-)inventories of RWA nests
Quantifying species distribution and abundance is essential for ecology and conservation. Rapid changes in spatial distribution of species and their abundance have consequences for a wide range of ecosystems, including the loss of potentially important features and functions (Keil et al. 2015). Effective approaches to species monitoring, management, and conservation necessarily rely on timely, science-based, standardized, and integrated information and data. Such information is particularly important for the sessile RWA, a keystone species that is abundant in in Northern Hemisphere forests. A decline in RWA occurrences is generalized postulated for Germany (e.g., Bretz 2020) leading to the endangerment status of RWA and their inclusion in regulatory documents such as the Federal Species Protection Ordinance (BArtSchV 2013) and German government’s Insect Conservation Action Program (AP Insektenschutz 2019). However, there is no long-term, area-wide RWA monitoring based on reliable, statistically valid GPS-data for Germany to substantiate the postulated threats and declines, as standardized and systematic monitoring of RWA has been discontinued in Germany since the 1980s. A recently published study showed the poor data situation for the different RWA species and their occurrence in most European countries (Balzani et al. 2022).
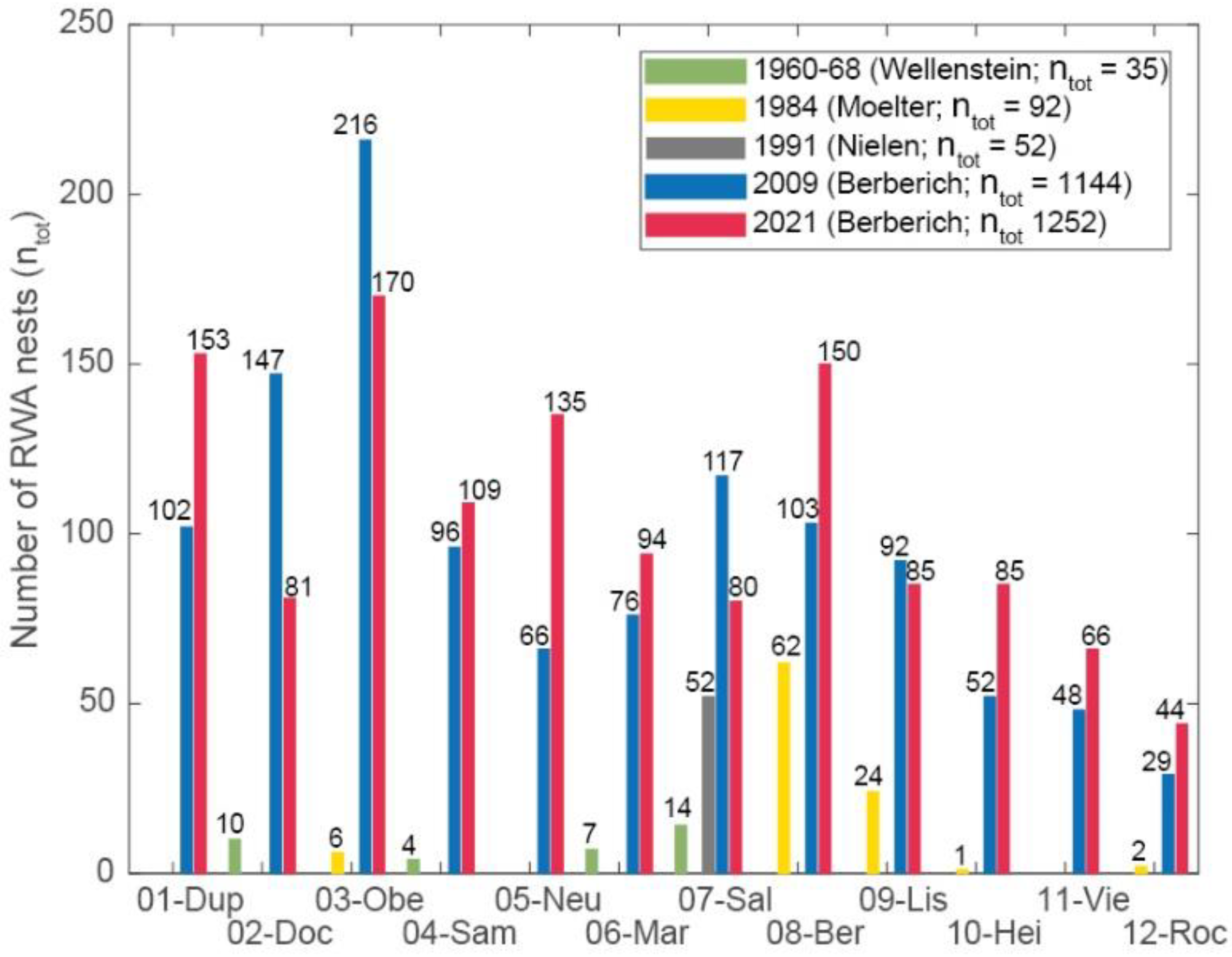
For effective protection of RWA, a reliable data base providing scientific evidence of the status quo of RWA occurrences, their increase or decrease, is absolutely necessary. Six factors are important: 1) A scientifically reliable data base of RWA occurrences based on a standardized and comparable mapping approach: The area-wide, standardized, systematic and integrated mapping approach for (re)inventories that we have developed, which also includes a photo database in which each RWA nest and its habitat is documented with at least two photos (e.g., Berberich et al. 2022a; Berberich 2010), has shown that a general statement about a RWA decline in Germany, as suggested e.g., by Sturm and Distler (2003), is not tenable for the WEVF. The opposite is the case. The total number of RWA nests increased by 10 %, and at eight of 12 study sites there was an increase in active RWA nests by an average of ≈40 % and at one site (05-Neu) by up to 98%; only four study sites showed a decrease. 2) Observers experience to document all nest sizes from start-ups to very tall nests: Working with lay observers protecting RWA in Germany revealed that lay people overlooked not only these small nests but even medium-sized nests in the field (Berberich et al. 2016c; Reimann 2021). This is a possible explanation for the large differences among studies on RWA population dynamics. In our mapping approach it is these small nests that are mapped because of their importance for understanding population dynamics in a study area and for evaluating spatial distribution and tectonic patterns (Berberich et al. 2022a,b; 2019; 2016a,c). 3) Inappropriate selection of a mapping method: our approach produces more accurate results in contrast to imperfect detection and underdetection of small NH classes by using random sampling, transects (at 20-50 m intervals), or the use of satellite imagery with a 30 m threshold spacing without the ability to identify start-ups and short nests (e.g., Sondeij et al. 2018; Klimetzek et al. 2021; Reimann 2021; Véle and Frouz 2023). 4) Incorrect documentation of mapping results: We have learned from working with lay observers that they rarely record nests digitally with GPS and only vaguely record the data collected from memory on analog maps and not using geographical information systems (GIS). Published results based on such unusable data are not comparable and lead to misinterpretations. 5) Statistically inadequate sampling sizes: Re-inventories by e.g., members of the “Ameisenschutzwarte” who postulate general declines in RWA occurrences, draw their conclusions sometimes of e.g., no more than one nest (Bär 2021 pers. comm;) or 14 nests for the entire study (Reimann, 2021). Drawing conclusions from such an insignificant database leads to misinterpretations. The authors’ experience has shown that a minimum number of 1000+ nests per study to be mapped will provide a more complete and statistically adequate database (Berberich et al. 2022a; 2016a; 2014). 6) Short time intervals for re-inventories: This is the most critical aspect of re-inventories. Recent studies conducted after several decades show a mixed picture: stable RWA nest counts in England and Romania (two decades), a pressure situation in Belgium (three decades), and both decreases and increases in RWA nest counts in The Netherlands (six decades (Robinson and Robinson 2008; Dekoninck et al. 2010; Mabelis and Korczyńska 2016; Van Buggenum 2021; Klimetzek et al. 2021). The results of re-inventories after several decades should be viewed critically, because a time interval of more than 20 years seems much too long to document population dynamics. Compared to previous inventories conducted 18, 16, and 7 years ago at the 12 sites studied (
Figure 5; Berberich 2010), the 2009 and 2021 inventories already showed an increase in RWA nest counts at all sites, with a maximum increase e.g., for 10-Hei by 52-fold in 2009 and 85-fold in 2021. In addition, the photo database of RWA nests and habitats was found to be a very useful tool for re identifying ≈340 RWA nests and their forest habitat. Therefore, it is suggested that re-inventories should be conducted earlier than after 20 years because climate change leading to hot summers and a lack of precipitation is affecting forest vitality in Germany much faster than expected and could also have an impact on RWA population dynamics.
4.2. Relation of tree species, tree age, RWA nests and woodpecker populations
The age structure of the German forest is characterized by the large-scale reforestation after World War II, with trees now between 40 and 60 years old and an average forest age of 77 years (BMEL 2023). In the WEVF, the preferred tree age classes by RWA nests correspond to the reforested and averaged tree age: early to medium mature (41-80 years) and mature (≥81–140 years) spruce forests (2009: ≈66%; 2021: ≈75%), confirming our findings in the Oberpfalz region that
F. polyctena are more abundant in mature forests (
Figure 2; (Berberich et al. 2022a). The distribution of nest heights was also consistent with the findings in the Oberpfalz region (Berberich et al. 2022a): One-fifth (2009) and one third (2021) of start-ups to short nests and ≈10 % (2009; 2021: ≈6 %) of tall–very tall nests were observed in medium mature (61-80 years) and mature (≥81–140 years) spruce dominated stands. Young (21-40 years) spruce-dominated stands comprised only ≈9% (2009) and ≈4% (2021) of all NH classes from start-ups to very tall nests in the WEVF. This finding contrasts with the results by Sondeij et al. (2008), who found that very young open canopy forests (<20 years) promote preferred habitats for nest settlements, and Domisch et al. (2005), who found no RWA occurrences in 20-years old Scots pine stands. Positive effects on species and habitat diversity are also expected from further development toward deciduous and mixed forests. Natural beech regeneration is increasingly gaining dominance in RLP forests (MUFV 2010). This could be confirmed by our findings. The forest composition observed in the field shows a shift from coniferous stands to naturally regenerated mixed stands, consisting of e.g., spruce-beech (2009: ≈21%; 2021: ≈30%) and spruce-oak (2009: ≈2%; 2021: ≈5%) at RWA nest sites. This is a positive development for beech and oak, as their vitality was affected by drought stress, oak powdery mildew (
Microsphaera alphitoides), and insect infestation (MKUEM 2021) from 2018 to 2020.
Old forests with several development phases are an important factor for biodiversity. They provide a rich supply of deadwood and biotope trees, which more often than young trees offer special microhabitats such as coarse bark, crown deadwood or woodpecker cavities (BMEL 2023). Deadwood is part of the natural cycle in the forest and has reached a share of 6 % of the living wood stock in Germany. Many species are specialized in this which serves, for example, as a food source, shelter, breeding ground, and drumming ground for various species, such as woodpeckers, bats, insects, fungi, and lichens (BMEL 2023; Zimmerer 2021). Woodpecker species are considered indicators of forest biodiversity because they adapted to old-growth forest habitat structures, use large breeding territories and are active year round (Wübbenhorst and Sübeck 2001; Zimmerer 2021). The observation of a tripling of woodpecker cavities in RWA nests in 2021 compared to 2009, especially in short and medium sized nests (
Table 5), may indicate a substantial increase in the number of foraging woodpeckers. This is especially true for the Black
(Dryocopus martius), Green
(Picus viridis) and the Gray woodpecker
(Picus canus) in the studies areas, as these three species feed on ants and forage not only on tree trunks, but mostly on the ground in RWA nests. Foraging in smaller nests could be more effective and beneficial than in larger nests because prey is captured more quickly. Woodpeckers that forage in large nests must dig small tunnels into the nest and remain for up to 12 minutes, such as the Green woodpecker
(Picus viridis. Such burrows in RWA nests also provide access to an additional food source for smaller birds, such as blackbirds (
Turdus merula; up to 12 minutes foraging in such burrows), as recorded by one of our four AntCams (AntCam data by Berberich & Berberich, unpublished). Our findings of woodpecker cavities in nests are consistent with up to fivefold population increases of various woodpecker species, e.g., the black
(Dryocopus martius), middle
(Dendrocopos medius) and lesser spotted woodpecker
(Dryobates minor), in the largest contiguous riparian woodland area of Rhineland-Palatinate (Froehlich-Schmitt 2018). Furthermore, our results showed that woodpeckers were more abundant in state forests and private forest, suggesting a different forest managing than in municipal forests. Although biotope trees and deadwood are actively preserved, especially in SF, for insect conservation reasons (BMEL 2021), these key structures are ephemeral and require permanent replenishment (Zimmerer 2021). Therefore, it is also conceivable that at the time of the two inventories, the number of these habitats had decreased and woodpeckers were therefore focusing more on RWA nests. In this case, woodpecker populations in the study areas would not increase, but habitat trees would decrease. Integrated monitoring woodpecker cavities in RWA nests is therefore another valuable, albeit indirect, indicator tool for monitoring sustainable forest management and assessing woodpecker populations and their habitats in the forest. Our monitoring contributes to the findings by Wübbenhorst and Südbeck (2001) that other indicator species besides woodpeckers are needed as part of a monitoring system for sustainability in forests.
German forests are currently facing outbreaks of bark beetles, the most devastating tree-killers in coniferous forests, especially in spruce stands. European spruce bark beetle species (
Ips typographus, Pitogenes chalcographus) benefit from climate change and higher temperatures by developing more generations per year, resulting in bark beetle-induced tree mortality (KHVO Eifel 2019; MKUEM 2021). Bark beetle introduce a wood-decay fungi that lead to enzymatic degradation of lignin and significantly alter the physical and mechanical properties of wood (Hysek et al. 2021). Rapid salvage clearing of infested standing spruce trees are preferred combat measures. RWA live in trophobiosis with many plant-sucking insects (
Aphidae, Coccidae, Psyllidae) especially on spruce. They protect these insects from predators and parasites (Adlung 1966). Degradation of lignin or holocellulose is hypothesized to have a negative effects on plant-sucking insects that feed RWA. The overall large shift toward smaller nest sizes in all tree age classes (
Figure 2) suggests that a) there is an increase in new nest settlements establishments, which is contrary to the general statement of a decline in RWA nests (Sturm and Distler 2003; Reimann 2021), b) there is increasing pressure on RWA food resources due to a change in nutrient cycling in spruce stands as a result of a substantial reduction in wood quality (Hysek et al. 2021), and c) smaller colonies in smaller RWA nests could be an advantage for the survival of the whole colony after clearing bark beetle-infested trees and creating clearing areas. This could be confirmed by our results: Although clearing plots doubled in size due to bark beetle infestations in 2021, the total number of active nests on these clearing plots quadrupled in 2021, a result that contrasts with Véle and Frouz (2023), who suggest that bark beetle outbreaks reduce RWA nest survival, particularly in clearings. However, another positive factor favoring nest settlements could be positive ground movement rates due to Eifel plume-induced crustal deformation (Kreemer et al. 2020), as observed at nearly all study sites, because rising ground opens pathways for geogenic gases that support RWA nest settlements (Berberich et al. 2016a,b; 2018a,6; 2022b).
The herbaceous layer with its high species diversity has an important role in maintaining biodiversity in the forest. This is because the herbaceous layer community is sensitive to spatial and temporal disturbances. Changes and increasing loss of species diversity provide important information about forest site characteristics (Gilliam 2007). In general, the composition of the herbaceous layer around a nest site and on RWA nests changed dramatically from 2009 to 2021, even though both surveys were conducted in the same months. Compared to 2009, typical herb layer plants observed in 2009 played little to no role in 2021, indicating lower species diversity. The most important herbs were grass, moss or a combination of both, and broom. However, it is noticeable that the highly proliferating blackberry as a single plant or in combination with e.g., grasses or mosses is becoming more dominant on the study sites (
Figure 4). Blackberries are considered pests and competitors for trees and are controlled by foresters to prevent re-emergence (oral comm. from forester managers). Extreme weather events such as extreme drought and prolonged heat waves from 2018 to 2020, have affected forest vitality in RLP and contributed to the emergence of blackberry (MKUEM 2021; BMEL 2021). However, this study does not confirm that RWA nests are affected by blackberries as claimed by Véle and Frouz (2023). In 2009, a quarter of the nests that were re-inventoried in 2021 were affected by blackberries. In 2021, the proportion increased to ≈26 %. Although this plant is highly sprawling and sometimes covers the entire nest area, RWAs do not appear to be disturbed in their daily routine.
4.3. GeoBio-Interactions
The Eifel with its WEVF is one of the most active volcanic areas in Germany (Schmincke 2007; Kreemer et al. 2020;). A maximum uplift of ≈1 mm/year combined with a significant horizontal extension surrounded by a radial shortening pattern suggests that uplift forces induced by the magma reservoir (Eifel plume) at the bottom of the lithosphere can explain this remarkable surface deformation (Kreemer et al. 2020). As already shown for e.g., the Black Forest, Lake of Constance, Oberpfalz, and Romania (Berberich et al. 206a; 2019; 2022a), the combination of tectonic-volcanic processes and geochemical composition of bedrock is also responsible for the distribution patterns for RWA nests and their high nest numbers (Berberich et al. 2022a). Medium to high soil Radon concentrations at all study sites had a significant influence on spatial distribution of nests because RWA nests are in direct contact with the overall natural Rn potential of the bedrock (Lower Devonian Klerfer Schichten and Gladbach Schichten and Triassic Buntsandstein), their structural dispersal pathways (e.g., Rn degassing faults) and the high U-content in the small grain fractions (< 0.125 mm) of the soils (Kemski et al. 2012). Study sites with maximum concentration of 114 kBq/m³ (11-Vie) and 101 kBq/m³ (12-Roc) showed an increase in RWA nests of ≈31 % (11-Vie) and ≈45 % (12-Roc). This is thought to be caused by micro-fracturing due to a high stress condition caused by buoyancy forces induced by the Eifel plume leading to the formation of new emanation surfaces and substantial increase of the radon signal, although the applied stress remains constant over time (Tuccemei et al. 2015). Although Radon concentrations were high at the 09-Lis (114 kBq/m³), 03-Obe (90 kBq/m³), 02-Doc (82 kBq/m³), and 07-Sal (83 kBq/m³) study sites (BfS 2020), there was a decrease in nests ranging from -8 % (09-Lis) to -60 % (02-Doc). Tectonic movements could be an additional factor determining the number of nests. Local negative displacement rates are thought to slowly close pathways for geogenic gases, as observed at the four sites (Wolf, 2016; Berberich 2018b, 2022b). Since local negative displacement rates are nearly equal positive ascending rates in 09-Lis, this could explain the only slight loss of RWA nests in this area (Wolf 2016).
5. Conclusions
For effective protection of RWA, a reliable database that provides scientific evidence of the status quo of RWA occurrence, its increase or decrease, is absolutely necessary. Our standardized, area-wide, reproducible and integrated re-inventory approach not only monitors the entire habitat at a RWA nest, but is capable of detecting and identifying particularly small nest sizes. In combination with presence/absence data and through re-identification of formerly mapped nests, the application of such a comprehensive approach leads to more exact and realistic RWA nest counts in (re)-inventories, as shown for the WEVF (12 study sites with a total of 1281 ha). An increase in the total number of nests from 1144 in 2009 to 1252 in 2021 and a dramatic increase for the study site 10-Hei up to 52-fold (2009) and 85-fold (2021) compared to an earlier inventory in 1984 were found. These results are in contrast to previous postulations of a decline in RWA. Early to medium mature (41-80 years) and mature (≥81–140 years) spruce forests were the preferred habitat. Grass, moss or a combination of both, and broom were strongly represented in the herbaceous layer, and other plants only played a minor role in 2021 compared to 2009, suggesting a decline in species diversity. Such realistic results from RWA nests in forest habitats provide an useful database for further improving knowledge of ant habitats. Monitoring of woodpecker cavities in RWA nests is suggested as an indirect indicator tool for assessing woodpecker populations in forests. Tectonic processes, such as positive ground movement rates due to crustal deformation caused by the Eifel plume, as observed in the WEVF, are suggested as another positive factor thriving nest settlements as rising ground opens pathways for geogenic gases that support nest settlements. This study contributes to the urgent need for updating statistically valid data needed to a) effectively prove the status-quo of RWA occurrences, b) protect RWA as ecosystem engineers, c) further understanding of GeoBio-Interactions in the wake of climate change, and c) contribute to the German government’s Insect Conservation Action Program. Two further planned inventories in two other study sites, which will be carried out after 8 and 4 years, will show whether the development observed here in terms of RWA nests and WpC is also confirmed in these areas.
Author Contributions
G.M.B. conceived the idea, designed the study, performed the field work, carried out the statistical analysis and wrote the manuscript. M.B.B. performed the field work, analyzed the data and contributed to the manuscript. Both authors edited the manuscript and approved the final version.
Data Availability Statement
The data sets generated and analyzed during the current study were uploaded to PANGEA (
www.pangea.de; [PANGAEA-ISSUES] (PDI-34174) Data submission 2023-02-21T16:52:21Z (Gabriele M. Berberich). Currently, a doi is being created by PANGEA.
Acknowledgments
–We greatly acknowledge the support of the district foresters of the forest districts FD Prüm (Peter Wind), FD Gerolstein (Michael Schimper), FA Daun (Horst Womelsdorf), FD Hillesheim (Johannes Pinn; all RLP), and FD Gemeindeforstamt Dahlem (Ditmar Krumpen, NRW) that provided data from the 10-year forest inventory and management plan of 2009 and their forest rangers (in alphabetical order; FD Daun: Jürgen Beck, Harald Fell, Gerhard Herzog, Michael Hoppe, Dana Justen, Ingrid Lamour, Jürgen Sohns; FD Gerolstein: Norbert Bischof, Thorsten Thelen; FD Hillesheim: Tim Dürselen, Markus Schüller) for their support in the field. None of them had a role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Conflicts of Interest Statement
G.M.B and M.B.B. declare no potential conflict of interest with respect to the research, authorship, and publication of this article.
References
- Adlung KG (1966) A Critical Evaluation of the European Research on Use of Red Wood Ants (Formica rufa Group) for the Protection of Forests against Harmful Insects. Z Angw Entomol. [CrossRef]
- AP Insektenschutz (2019) Aktionsprogramm Insektenschutz der Bundesregierung – Gemeinsam wirksam gegen das Insektensterben. Drucksache 19/13031. 09.09.2019.
- Balzani P, Dekoninck W, Feldhaar H, Freitag A, Frizzi F, Frouz J, Masoni A, Robinson E, Sorvari J, Santini G (2022) Challenges and a call to action for protecting European red wood ants. Conserv Biol. [CrossRef]
- BArtSchV (2013) Verordnung zum Schutz wild lebender Tier- und Pflanzenarten. Bundesartenschutzverordnung (BArtSchV) vom 16. Februar 2005 (BGBl. I S. 258, 896), die zuletzt durch Artikel 10 des Gesetzes vom 21. Januar 2013 (BGBl. I S. 95) geändert worden ist.
- Berberich GM, Berberich MB (2022b) Comparison of Geogases in Two Cenozoic Sedimentary Basins. Geosciences. [CrossRef]
- Berberich GM, Berberich MB, Gibhardt M (2022a) Red wood Ants (Formica rufa-group) prefer mature pine forests in Variscan granite environments(Hymenoptera: Formicidae) Fragm Entomol. [CrossRef]
- Berberich GM, Klimetzek D, Paraschiv M, Stancioiu PT, Grumpe A (2019) Biogeostatistics confirm: Even a low total number of red wood ant nests provide new information on tectonics in the East Carpathian Orogen (Romania) Ecol Indic. [CrossRef]
- Berberich GM, Berberich MB, Ellison AM, Wöhler C (2018b) Degassing Rhythms and Fluctuations of Geogenic Gases in A Red Wood-Ant Nest and in Soil in The Neuwied Basin (East Eifel Volcanic Field, Germany. Insects. [CrossRef]
- Berberich GM, Ellison AM, Berberich MB, Grumpe A, Becker A, Wöhler C (2018a) Can a red wood-ant nest be a trap for fault-related CH4 micro-seepage? A case study from continuous short-term in-situ sampling. Animals. [CrossRef]
- Berberich GM, Dormann CF, Klimetzek D, Berberich MB, Sanders NJ, Ellison AM (2016c) Detection probabilities for sessile organisms. Ecosphere. 7(11):e01546. 10.1002/ecs2.1546.
- Berberich GM, Sattler T, Klimetzek D, Benk SA, Berberich MB, Polag D, Schöler HF, Atlas E (2016b) Halogenation processes linked to red wood ant nests (Formica spp.) and tectonics. J Atm Chem. [CrossRef]
- Berberich GM, Grumpe A, Berberich MB, Klimetzek D, Wöhler C (2016a) Are red wood ants (Formica rufa-group) tectonic indicators? A statistical approach. Ecol Indic. [CrossRef]
- Berberich G, Klimetzek D, Wöhler C, Grumpe A (2014) Statistical Correlation between Red Wood Ant Sites and Tectonically Active Fault Structures. Mitt Dtsch Ges allg angew Ent. 19(2014):45-50.
- Berberich, G (2010) Identifikation junger gasführender Störungszonen in der West- und Hocheifel mit Hilfe von Bioindikatoren. Dissertation. University of Duisburg-Essen, Essen, Germany.
- BfS (2020) GEOPORTAL. Bundesamt für Strahlenschutz (BfS), Salzgitter. www.imis.bfs.de/geoportal/. Available online (accessed on 4 July 2021).
- BMEL (2023) Der Wald in Deutschland. Ausgewählte Ergebnisse der dritten Bundeswaldinventur. Bundesministerium für Ernährung und Landwirtschaft (BMEL). www.bundeswaldinventur.de Available online (accessed on 1 February 2023).
- BMEL (2021) Ergebnisse der Waldzustandserhebung 2020. Bundesministerium für Ernährung und Landwirtschaft (BMEL). p 72. www.bmel.de. Available online (accessed on 1 February 2023).
- BNatSchG (2009) Gesetz über Naturschutz und Landschaftspflege (Bundesnaturschutzgesetz - BNatSchG) vom 29. Juli 2009 (BGBl. I S.2542 / FNA 791- 9).
- Bretz D (2020) Gewaltiger Waldameisenrückgang im Forstamt Weilburg: Dramatische Bilanz nach 25 Jahren Waldameisen-Kartierung. Ameisenschutz aktuell. pp: 92-110.
- Çamlitepe Y, Aksoy V (2019) Distribution and conservation status of the European red wood ant species Formica pratensis. J. Entomol. Res. Soc. 21(2): 199–211.
- Crist TO (2009) Biodiversity, species interactions and functional role of ants (Hymenoptera: Formicidae) in fragmented landscapes: a review. Myrmec. News, 12:3-13.
- Dekoninck W, Hendrickx F, Grootaert P, Maelfait JP (2010) Present conservation status of red wood ants in northwestern Belgium: Worse than previously, but not a lost cause. Eur. J. Entomol. [CrossRef]
- Descombes P, Leprieur F, Albouy C, Heine C, Pellissier L (2017) Spatial imprints of plate tectonics on extant richness of terrestrial vertebrates. J. Biogeography. [CrossRef]
- Domisch T, Finér L, Jurgensen MF (2005) Red wood ant mound densities in managed boreal forests. Ann. Zool. Fenn. 42: 277–282.
- EU-DEM (2021) Digital Elevation Model über Europa aus dem GMES RDA-Projekt (EU-DEM) ist ein Digital SurfaceModel (DSM), The official portal for European data 2021. Available online: https://land.copernicus.eu/imagery-in-situ/eu-dem/eu-dem-v1-0-and-derived-products/slope (accessed on 20 June 2021).
- Froehlich-Schmitt B (2018) Spechte in der Hördter Rheinaue nach 40 Jahren. 28. Jahrestagung der Fachgruppe Spechte. Ornithol. Anz. 57.
- Frouz J, Jilkova V (2008) The effect of ants on soil properties and processes (Hymenoptera: Formicidae). Myrmec. News, 11, 191–199.
- Frouz J, Rybnicek M, Cudlin P, Chmelikova E (2008) Influence of the wood ant Formica polyctena on soil nutrient and the spruce tree growth. J. Appl. Entom. [CrossRef]
- Gilliam FS (2007) The Ecological Significance of the Herbaceous Layer in Temperate Forest Ecosystems. BioScience. https://academic.oup.com/bioscience/article/57/10/845/232416.
- Hallmann CA, Sorg M, Jongejans E et al. (2017) More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE, 12(10): e0185809, 21 pp.
- Hýsek Š, Löwe R, Turcáni M (2021) What Happens to Wood after a Tree Is Attacked by a Bark Beetle? Forests . [CrossRef]
- Keil P, Storch D, Jetz W (2015) On the decline of biodiversity due to area loss. Nat. Com. [CrossRef]
- Kemski J, Klingel R, Siehl A, Neznal M, Matolin M (2021) Erarbeitung fachlicher Grundlagen zum Beurteilung der Vergleichbarkeit unterschiedlicher Messmethoden zur Bestimmung der Radonbodenluftkonzentration. Vorhaben 3609S10003. Bd. 2 Sachstandsbericht „Radonmessungen in der Bodenluft. Einflussfaktoren, Messverfahren, Bewertung“. BfS-RESFOR-63/12-Bd.2. urn:nbn:de:0221-201203237830.
- KHVO Eifel (2019) Newsletter Nr. 2. Dezember 2019. www.holzvermarktung-eifel.de Available online (accessed on 1 February 2023).
- Klimetzek D, Stancioiu PT, Paraschiv M, Nita MD (2021) Ecological Monitoring with Spy Satellite Images—The Case of Red Wood Ants in Romania. Remote Sens. [CrossRef]
- Kreemer C, Blewitt G, Davis PM (2020) Geodetic evidence for a buoyant mantle plume beneath the Eifel volcanic area, NW Europe. Geophys J Int. [CrossRef]
- KWIS-RLP (2021). Klimawandelinformationssystem Rheinland-Pfalz. Available online: www.kwis-rlp.de/anpassungsportal/regionale-informationen/osteifel/ (accessed on 22 October 2021).
- Mabelis AA, Korczyńska J (2016) Long term impact of agriculture on the survival of wood ants of the Formica rufa group (Formicidae). J Insect Conserv. [CrossRef]
- MKUEM (2021) Waldzustandsbericht 2021. (Ministerium für Klimaschutz, Umwelt, Energie und Mobilität). pp. 82. www.mkuem.rlp.de; www.wald.rlp.de. Available online (accessed on 1 February 2023).
- MUFV (2010) Waldzustandsbericht 2010. Ministerium für Umwelt, Forsten und Verbraucherschutz (MUFV). www.mufv.rlp.de; www.wald-rlp.de. Available online (accessed on 1 February 2023).
- Reimann H (2021) Zustand eines Waldameisenvorkommens 2010 und 2021. Ameisenschutz aktuell 2021, 35, 4/21. Pp.74-78. ISSN 0941-7958.
- Robinson EJH, Stockan JA, Iason GR (2016) Wood Ants and their Interaction with Other Organisms Wood ants and their interaction with other organisms. in J Stockan & EJH Robinson (eds), Wood Ant Ecology and Conservation., 8, Cambridge University Press, Cambridge. pp. 177–206.
- Robinson NA, Robinson EJH (2008) The population of the red wood ant Formica rufa L. (Hymenoptera: Formicidae) at Gait Barrows National Nature Reserve, Lancashire, England over the 20 year period 1986–2006: nest longevity, reproduction and the effect of management. Br J Entomol Nat Hist. 21: 225–241.
- Schmincke HU (2007) The Quaternary Volcanic Fields of the East and West Eifel (Germany). In: Ritter R, Christensen U (eds) Mantle Plumes – a Multidisciplinary Approach. Springer Heidelberg, Germany.
- Sondeij I, Domisch T, Finér L, Czechowski W (2018) Wood ants in the Białowieża Forest and factors affecting their distribution. Ann Zool Fen. 55: 103–114.
- Stoschek N, Roch T (2006) Zentrale Erfassung von Waldameisen im Freistaat Sachsen. AFZ-Der Wald, 61:186-188.
- Sturm P, Distler H (2003) Rote Liste gefährdeter Ameisen (Hymenoptera: Formicoidea) Bayerns. BayLfU 2003, 166: 208–212.
- Tuccimei P, Mollo S, Soligo M, Scarlato P, Castelluccio M (2015) Real-time setup to measure radon emission during rock deformation: implications for geochemical surveillance. Geosci Instrum Method Data Syst. [CrossRef]
- Van Buggenum HJM (2022) Presence after three decades of red wood ants (Formica rufa group; Hymenoptera: Formicidae) in forests in an agricultural landscape. Eur. J. Entomol. [CrossRef]
- Véle A, Frouz J (2023) Bark Beetle Attacks Reduce Survival of Wood Ant Nests. Forests. [CrossRef]
- Wagner DL, Grames EM, Forister MR, Berenbaum MR, Stopakd D (2021) Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Natn. Acad. Sci. U.S.A. 118: e2023989118, 10 pp.
- Wilson P (2011) Wood Ants of Wyre. Wyre Forest Study Group Review, 2011:17-22.
- Wolf C (2016) Copernicus-Dienst Rhein-Mosel. Copernicus-Dienst zur Unterstützung von Gefährdungsanalysen und Regionalplanung im Rhein-Mosel-Gebiet. Bundesanstalt für Geowissenschaften und Rohstoffe (BGR). BGR-Nr. 05-3039-01.
- Wübbenhorst J, Südbeck P (2001) Woodpeckers as Indicators for Sustainable Forestry? First Results of a study in the EU/LIFE – demonstration areas Lüneburger Heide und Solling. Demonstration of methods to monitor sustainable forestry.EU/LIFE project 1998 – 2001 (LIFE98ENV/S/000478).
- Zimmerer V (2021) Erfolgsmelder im Waldnaturschutz. Digitale Ausgabe Bayerisches Landwirtschaftliches Wochenblatt BLW 25-2021. www.digitalmagazin.de/marken/blw/hauptheft/2021-25/wald/026_erfolgsmelder-im-waldnaturschutz. Available online (accessed on 1 February 2023).
Figure 1.
Schematic depiction of the position of the 12 study sites (orange areas) within the West Eifel Volcanic Field (WEVF; black border), main rivers (blue lines), Quaternary (gray dots) and Neogene volcanoes (green dots) underlaid by a digital surface model (DSM). Inlet shows location of the WEVF within Germany in Rhineland-Palatinate (RLP) and North-Rhine-Westphalia (NRW; Berberich 2010; EU-DEM 2021).
Figure 1.
Schematic depiction of the position of the 12 study sites (orange areas) within the West Eifel Volcanic Field (WEVF; black border), main rivers (blue lines), Quaternary (gray dots) and Neogene volcanoes (green dots) underlaid by a digital surface model (DSM). Inlet shows location of the WEVF within Germany in Rhineland-Palatinate (RLP) and North-Rhine-Westphalia (NRW; Berberich 2010; EU-DEM 2021).
Figure 2.
Results of MANOVA for NH, ND, medium tree age (TSprime) and woodpecker cavities (WpC) showing a) a grouped plot matrix and b) grouped scatter plots of the first two canonical variables and their centers for the 2009 and 2021 inventories. Tree age with a -100 signature represent no trees or clearings in the study sites.
Figure 2.
Results of MANOVA for NH, ND, medium tree age (TSprime) and woodpecker cavities (WpC) showing a) a grouped plot matrix and b) grouped scatter plots of the first two canonical variables and their centers for the 2009 and 2021 inventories. Tree age with a -100 signature represent no trees or clearings in the study sites.
Figure 3.
Gain and loss of RWA nests (total) in 12 study sites for the a) 2009 and b) 2021 inventory.
Figure 3.
Gain and loss of RWA nests (total) in 12 study sites for the a) 2009 and b) 2021 inventory.
Figure 4.
Visual representation of the qualitative composition of main herbs around and on a) active RWA nests (nact), b) re-identified nests (nactR), and c) nests on clearings (nactC) for the 2009 and 2021 inventory. The larger and bolder a term for a herb, the more frequently it appears and the more important are the herbs: Dominant herbs are shown in red, bold and large letters. Herbs that hold the same share are listed in the order of occurrence and are separated by commas.
Figure 4.
Visual representation of the qualitative composition of main herbs around and on a) active RWA nests (nact), b) re-identified nests (nactR), and c) nests on clearings (nactC) for the 2009 and 2021 inventory. The larger and bolder a term for a herb, the more frequently it appears and the more important are the herbs: Dominant herbs are shown in red, bold and large letters. Herbs that hold the same share are listed in the order of occurrence and are separated by commas.
Figure 5.
Comparison of total RWA nest numbers (ntot) in different mapping campaigns conducted by Wellenstein (1960 and 1968), Moelter (1984), Nielen (1991) with the 2009 and 2021 inventories. Data 1960-2009 taken from Berberich (2010).
Figure 5.
Comparison of total RWA nest numbers (ntot) in different mapping campaigns conducted by Wellenstein (1960 and 1968), Moelter (1984), Nielen (1991) with the 2009 and 2021 inventories. Data 1960-2009 taken from Berberich (2010).
Table 1.
Results of a) Kruskal-Wallis ANOVA test and b) multiple comparison results for the 2009 and 2021 inventory for physical nest parameters (NH, ND) of active nests and medium tree age (mTA) for TSprime and woodpecker cavities (WpC) at the 1% significance level.
Table 1.
Results of a) Kruskal-Wallis ANOVA test and b) multiple comparison results for the 2009 and 2021 inventory for physical nest parameters (NH, ND) of active nests and medium tree age (mTA) for TSprime and woodpecker cavities (WpC) at the 1% significance level.
| a) 2009 |
a) 2021 |
| Source |
SS |
df |
MS |
Chi-sq |
Prob>
Chi-sq
|
Source |
SS |
df |
MS |
Chi-sq |
Prob>
Chi-sq
|
| Groups |
4503029613 |
3 |
1501009871 |
2974.7 |
0.00 |
Groups |
3916176557 |
3 |
1305392186 |
2418.48 |
0.00 |
| Error |
2003097694 |
4295 |
466379.0 |
|
|
Error |
3287961357 |
4446 |
739532.47 |
|
|
| Total |
6506127307 |
4298 |
|
|
|
Total |
7204137914 |
4449 |
|
|
|
| b) 2009 |
b) 2021 |
| Group A |
Group B |
Lower
Limit
|
A-B |
Upper
Limit
|
p-Value |
Group A |
Group B |
Lower
Limit
|
A-B |
Upper
Limit
|
p-Value |
| NH |
ND |
-525.5 |
-390.7 |
-255.8 |
0.0000 |
NH |
ND |
-711.1 |
-575.5 |
-440.0 |
0.0000 |
| NH |
WpC |
758.8 |
893.6 |
1028.5 |
0.0000 |
NH |
WpC |
-362.1 |
-226.6 |
-91.1 |
0.0001 |
| NH |
mTA |
-2119.8 |
-1981.8 |
-1843.7 |
0.0000 |
NH |
mTA |
-2634.6 |
-2492.0 |
-2349.4 |
0.0000 |
| ND |
WpC |
1149.5 |
1284.3 |
1419.1 |
0.0000 |
ND |
WpC |
213.4 |
348.9 |
484.5 |
0.0000 |
| ND |
mTA |
-1729.2 |
-1591.1 |
-1453.1 |
0.0000 |
ND |
mTA |
-2059.1 |
-1916.5 |
-1773.9 |
0.0000 |
| WpC |
mTA |
-3013.5 |
-2875.4 |
-2737.4 |
0.0000 |
WpC |
mTA |
-2408.0 |
-2265.4 |
-2122.8 |
0.0000 |
Table 2.
Descriptive statistics of total nest numbers (ntot), active nest numbers (nact), nest height (NH) and diameter classes (ND) of RWA nests in the 12 study sites for the 2009 and 2021 inventories. Increase in active nest numbers (Δ nact) and percentages are set in bold. – = not present.
Table 2.
Descriptive statistics of total nest numbers (ntot), active nest numbers (nact), nest height (NH) and diameter classes (ND) of RWA nests in the 12 study sites for the 2009 and 2021 inventories. Increase in active nest numbers (Δ nact) and percentages are set in bold. – = not present.
| |
2009 |
2021 |
2009–2021 |
| Nest height (NH) classes of active nests (nact) |
| No |
Study site |
Numbers |
m |
Numbers |
m |
nact
|
% |
| ntot |
nact |
start-ups |
short |
medium |
tall |
very tall |
extra tall |
ntot |
nact |
start-ups |
short |
medium |
tall |
very tall |
extra tall |
0.01
–
0.10
|
0.11
–
0.50
|
0.51
–
1.00
|
1.01
–
1.50
|
1.51
–
2.00
|
>2.01 |
0.01
–
0.10
|
0.11
–
0.50
|
0.51
–
1.00
|
1.01
–
1.50
|
1.51
–
2.00
|
>2.01 |
| 01 |
Dup |
102 |
97 |
1 |
26 |
26 |
18 |
26 |
– |
153 |
142 |
14 |
44 |
40 |
32 |
12 |
– |
45 |
46 |
| 02 |
Doc |
147 |
142 |
10 |
40 |
74 |
5 |
13 |
– |
81 |
57 |
10 |
24 |
16 |
5 |
1 |
1 |
-85 |
-60 |
| 03 |
Obe |
216 |
210 |
44 |
74 |
37 |
3 |
52 |
– |
170 |
163 |
23 |
77 |
46 |
14 |
2 |
1 |
-47 |
-22 |
| 04 |
Sam |
96 |
93 |
7 |
31 |
42 |
9 |
4 |
– |
109 |
103 |
2 |
59 |
30 |
11 |
1 |
– |
10 |
11 |
| 05 |
Neu |
66 |
64 |
10 |
19 |
27 |
7 |
1 |
– |
135 |
127 |
11 |
74 |
37 |
5 |
– |
– |
63 |
98 |
| 06 |
Mar |
76 |
71 |
18 |
17 |
17 |
19 |
– |
– |
94 |
93 |
4 |
56 |
22 |
11 |
– |
– |
22 |
31 |
| 07 |
Sal |
117 |
108 |
17 |
57 |
21 |
13 |
– |
– |
80 |
78 |
2 |
53 |
22 |
1 |
– |
– |
-30 |
-28 |
| 08 |
Ber |
103 |
100 |
22 |
56 |
22 |
– |
– |
– |
150 |
142 |
31 |
96 |
15 |
– |
– |
– |
42 |
42 |
| 09 |
Lis |
92 |
85 |
9 |
34 |
24 |
18 |
– |
– |
85 |
78 |
5 |
54 |
11 |
8 |
– |
– |
-7 |
-8 |
| 10 |
Hei |
52 |
52 |
5 |
5 |
28 |
14 |
– |
– |
85 |
76 |
3 |
62 |
10 |
1 |
– |
– |
24 |
46 |
| 11 |
Vie |
48 |
48 |
– |
11 |
– |
14 |
23 |
– |
66 |
63 |
2 |
17 |
21 |
12 |
10 |
1 |
15 |
31 |
| 12 |
Roc |
29 |
29 |
– |
3 |
10 |
16 |
– |
– |
44 |
42 |
1 |
29 |
9 |
2 |
1 |
– |
13 |
45 |
| |
Total |
1144 |
1099 |
143 |
373 |
328 |
136 |
119 |
0 |
1252 |
1164 |
108 |
645 |
279 |
102 |
27 |
3 |
65 |
6 |
| Nest diameter (ND) classes of active nests (nact) |
| No |
Study site |
Numbers |
m |
Numbers |
m |
nact
|
% |
| ntot |
nact |
small |
medium |
large |
very large |
extra-large |
– |
ntot |
nact |
small |
medium |
large |
very large |
extra-large |
– |
0.01
–
0.50
|
0.51
–
1.00
|
1.01
–
1.50
|
1.51
–
2.00
|
>2.01 |
– |
0.01
–
0.50
|
0.51
–
1.00
|
1.01
–
1.50
|
1.51
–
2.00
|
>2.01 |
– |
| 01 |
Dup |
102 |
97 |
28 |
19 |
16 |
16 |
18 |
– |
153 |
142 |
28 |
29 |
29 |
22 |
34 |
– |
45 |
46 |
| 02 |
Doc |
147 |
142 |
16 |
44 |
49 |
24 |
9 |
– |
81 |
57 |
15 |
16 |
12 |
6 |
8 |
– |
-85 |
-60 |
| 03 |
Obe |
216 |
210 |
76 |
58 |
35 |
19 |
22 |
– |
170 |
163 |
62 |
45 |
32 |
16 |
8 |
– |
-47 |
-22 |
| 04 |
Sam |
96 |
93 |
8 |
21 |
28 |
14 |
22 |
– |
109 |
103 |
17 |
32 |
37 |
13 |
4 |
– |
10 |
11 |
| 05 |
Neu |
66 |
64 |
15 |
12 |
13 |
15 |
9 |
– |
135 |
127 |
38 |
44 |
31 |
13 |
1 |
– |
63 |
98 |
| 06 |
Mar |
76 |
71 |
25 |
13 |
10 |
6 |
17 |
– |
94 |
93 |
33 |
31 |
26 |
3 |
– |
– |
22 |
31 |
| 07 |
Sal |
117 |
108 |
50 |
23 |
15 |
7 |
13 |
– |
80 |
78 |
28 |
32 |
12 |
3 |
3 |
– |
-30 |
-28 |
| 08 |
Ber |
103 |
100 |
36 |
39 |
19 |
4 |
2 |
– |
150 |
142 |
81 |
33 |
17 |
7 |
4 |
– |
42 |
42 |
| 09 |
Lis |
92 |
85 |
29 |
20 |
15 |
10 |
11 |
– |
85 |
78 |
22 |
32 |
15 |
6 |
3 |
– |
-7 |
-8 |
| 10 |
Hei |
52 |
52 |
14 |
18 |
13 |
3 |
4 |
– |
85 |
76 |
16 |
32 |
15 |
8 |
5 |
– |
24 |
46 |
| 11 |
Vie |
48 |
48 |
3 |
12 |
9 |
11 |
13 |
– |
66 |
63 |
7 |
11 |
23 |
10 |
12 |
– |
15 |
31 |
| 12 |
Roc |
29 |
29 |
5 |
2 |
12 |
5 |
5 |
– |
44 |
42 |
5 |
18 |
11 |
8 |
– |
– |
13 |
45 |
| |
Total |
1144 |
1099 |
305 |
281 |
234 |
134 |
145 |
– |
1252 |
1164 |
352 |
355 |
260 |
115 |
82 |
– |
65 |
6 |
Table 3.
Descriptive statistics of mapped area (ha), forest owners (SF, MF, PF) that hold share of the mapped area, numbers of active nests (nact), size of clearing plots with active RWA nests for the 2009 and 2021 inventory; – = not present.
Table 3.
Descriptive statistics of mapped area (ha), forest owners (SF, MF, PF) that hold share of the mapped area, numbers of active nests (nact), size of clearing plots with active RWA nests for the 2009 and 2021 inventory; – = not present.
| No |
Study site |
Mapped area |
State forest
(SF)
|
Municipal forest (MF) |
Private
forest (PF)
|
Number of nests (nact)
2009
|
Number of nests (nact)
2021
|
Clearing plots with
RWA nests
|
| 2009 |
2021 |
| ha |
ha |
% |
ha |
% |
ha |
% |
SF |
MF |
PF |
SF |
MF |
PF |
ha* |
nact |
ha* |
nact |
| 01 |
Dup |
72.4 |
38.2 |
52.8 |
4.9 |
6.8 |
29.3 |
40.5 |
66 |
– |
31 |
104 |
– |
38 |
2.13 |
2 |
0.38 |
16 |
| 02 |
Doc |
59.3 |
– |
– |
59.3 |
100.0 |
– |
– |
– |
142 |
– |
– |
57 |
– |
– |
– |
0.28 |
– |
| 03 |
Obe |
408.5 |
– |
35.9 |
261.7 |
64.1 |
– |
– |
118 |
92 |
– |
108 |
55 |
– |
1.38 |
26 |
2.69 |
61 |
| 04 |
Sam |
46.7 |
– |
– |
33.9 |
72.6 |
12.8 |
27.4 |
– |
85 |
8 |
– |
98 |
5 |
0.87 |
16 |
3.4 |
58 |
| 05 |
Neu |
92.9 |
– |
– |
60.6 |
65.2 |
32.3 |
34.8 |
– |
34 |
30 |
– |
95 |
32 |
– |
– |
0.03 |
1 |
| 06 |
Mar |
124.7 |
0.8 |
0.6 |
82.8 |
66.4 |
41.1 |
33.0 |
– |
63 |
8 |
– |
77 |
16 |
– |
– |
0.38 |
1 |
| 07 |
Sal |
56.5 |
50.7 |
89.7 |
– |
– |
5.8 |
10.3 |
108 |
– |
– |
78 |
– |
– |
– |
– |
1.36 |
17 |
| 08 |
Ber |
85.2 |
– |
– |
– |
100.0 |
– |
– |
– |
100 |
– |
– |
142 |
– |
0.25 |
4 |
1.24 |
29 |
| 09 |
Lis |
176.6 |
– |
– |
171.2 |
96.9 |
– |
3.1 |
– |
77 |
8 |
– |
73 |
5 |
– |
– |
0.64 |
10 |
| 10 |
Hei |
21.4 |
– |
– |
3.7 |
17.3 |
17.7 |
82.7 |
– |
28 |
24 |
– |
37 |
39 |
– |
– |
0.43 |
12 |
| 11 |
Vie |
14.1 |
– |
– |
6.3 |
44.7 |
7.8 |
55.3 |
– |
25 |
23 |
– |
33 |
30 |
0.15 |
3 |
– |
– |
| 12 |
Roc |
122.9 |
– |
– |
105.1 |
85.5 |
17.8 |
14.5 |
– |
29 |
– |
– |
42 |
|
– |
– |
– |
– |
| Total |
1281.2 |
236.5 |
18.5 |
874.7 |
68.3 |
170.0 |
13.3 |
292 |
675 |
132 |
290 |
709 |
165 |
4.78 |
51 |
10.83 |
205 |
| *ha sizes estimated from satellite imageries (Google Earth) for 2009 and 2020/2021 |
Table 4.
Descriptive statistics of total number (ntot) of woodpecker cavities (WpC) in active nests (nact), number of active nests and WpC/nest ratio per study site, numbers of nest with woodpecker classes and percentage numbers of nest with woodpecker classes for the 12 study sites for the a) 2009 and b) 2021 inventory.
Table 4.
Descriptive statistics of total number (ntot) of woodpecker cavities (WpC) in active nests (nact), number of active nests and WpC/nest ratio per study site, numbers of nest with woodpecker classes and percentage numbers of nest with woodpecker classes for the 12 study sites for the a) 2009 and b) 2021 inventory.
| No |
Study site |
Mapped nests (nact)
|
Numbers of WpC (n) in nact |
Ratio
WpC/
Nest
|
Nests (n) with WPC classes |
Nests (n) with WPC classes (%) |
| 0 |
1–4 |
5–10 |
>10 |
0 |
1–4 |
5–10 |
>10 |
| a) 2009: 224 nests with WpC |
| 01 |
Dup |
97 |
33 |
0.3 |
84 |
13 |
0 |
0 |
86.6 |
13.4 |
0.0 |
0.0 |
| 02 |
Doc |
142 |
159 |
1.1 |
80 |
52 |
10 |
0 |
56.3 |
36.6 |
7.0 |
0.0 |
| 03 |
Obe |
210 |
34 |
0.2 |
195 |
13 |
2 |
0 |
92.9 |
6.2 |
1.0 |
0.0 |
| 04 |
Sam |
93 |
128 |
1.4 |
62 |
25 |
3 |
3 |
66.7 |
26.9 |
3.2 |
3.2 |
| 05 |
Neu |
64 |
55 |
0.8 |
46 |
15 |
3 |
0 |
71.9 |
23.4 |
4.7 |
0.0 |
| 06 |
Mar |
71 |
116 |
1.6 |
47 |
14 |
8 |
2 |
66.2 |
19.7 |
11.3 |
2.8 |
| 07 |
Sal |
108 |
113 |
0.9 |
79 |
24 |
3 |
2 |
72.2 |
22.2 |
3.7 |
1.9 |
| 08 |
Ber |
100 |
0 |
0.0 |
100 |
0 |
0 |
0 |
100.0 |
0.0 |
0.0 |
0.0 |
| 09 |
Lis |
85 |
43 |
0.5 |
69 |
14 |
2 |
0 |
81.2 |
16.5 |
2.4 |
0.0 |
| 10 |
Hei |
52 |
14 |
0.3 |
48 |
4 |
0 |
0 |
92.3 |
7.7 |
0.0 |
0.0 |
| 11 |
Vie |
48 |
12 |
0.3 |
42 |
5 |
1 |
0 |
87.5 |
10.4 |
2.1 |
0.0 |
| 12 |
Roc |
29 |
12 |
0.4 |
23 |
5 |
1 |
0 |
79.3 |
17.2 |
3.4 |
0.0 |
| Sum |
1099 |
699 |
0.6 |
875 |
184 |
33 |
7 |
79.6 |
16.7 |
3.0 |
0.6 |
| b) 2021: 624 nests with WpC |
| 01 |
Dup |
142 |
313 |
2.2 |
60 |
57 |
24 |
1 |
42.3 |
40.1 |
16.9 |
0.7 |
| 02 |
Doc |
57 |
50 |
0.9 |
39 |
15 |
3 |
0 |
68.4 |
26.3 |
5.3 |
0.0 |
| 03 |
Obe |
163 |
339 |
2.1 |
73 |
64 |
22 |
4 |
44.8 |
39.3 |
13.5 |
2.5 |
| 04 |
Sam |
103 |
343 |
3.3 |
26 |
49 |
23 |
5 |
25.2 |
47.6 |
22.3 |
4.9 |
| 05 |
Neu |
127 |
429 |
3.4 |
43 |
49 |
29 |
6 |
33.9 |
38.6 |
22.8 |
4.7 |
| 06 |
Mar |
93 |
150 |
1.6 |
38 |
48 |
7 |
0 |
40.9 |
51.6 |
7.5 |
0.0 |
| 07 |
Sal |
78 |
121 |
1.6 |
38 |
32 |
8 |
0 |
48.7 |
41.0 |
10.3 |
0.0 |
| 08 |
Ber |
142 |
75 |
0.5 |
114 |
24 |
4 |
0 |
80.3 |
16.9 |
2.8 |
0.0 |
| 09 |
Lis |
78 |
156 |
2.0 |
32 |
35 |
11 |
0 |
41.0 |
44.9 |
14.1 |
0.0 |
| 10 |
Hei |
76 |
85 |
1.1 |
41 |
33 |
2 |
0 |
53.9 |
43.4 |
2.6 |
0.0 |
| 11 |
Vie |
63 |
203 |
3.2 |
21 |
27 |
10 |
5 |
33.3 |
42.9 |
15.9 |
7.9 |
| 12 |
Roc |
42 |
98 |
2.3 |
15 |
18 |
9 |
0 |
35.7 |
42.9 |
21.4 |
0.0 |
| Sum |
1164 |
2362 |
2.0 |
540 |
451 |
152 |
21 |
46.4 |
38.7 |
13.1 |
1.8 |
Table 5.
Descriptive statistics of nest height (NH) classes versus woodpecker cavities (WpC) for the a) 2009 and b) 2021 inventory.
Table 5.
Descriptive statistics of nest height (NH) classes versus woodpecker cavities (WpC) for the a) 2009 and b) 2021 inventory.
| Nest height (NH) classes (m) |
Woodpecker cavities (WpC) in nests |
| n |
% |
| 0 |
1–4 |
5–10 |
>10 |
0 |
1–4 |
5–10 |
>10 |
| a) 2009 |
| 0.01–0.10 |
143 |
0 |
0 |
0 |
13.0 |
0.0 |
0.0 |
0.0 |
| 0.11–0.50 |
322 |
45 |
2 |
2 |
29.2 |
4.1 |
0.2 |
0.2 |
| 0.51–1.00 |
246 |
79 |
15 |
2 |
22.4 |
7.2 |
1.4 |
0.2 |
| 1.01–1.50 |
64 |
31 |
11 |
3 |
5.8 |
2.8 |
1.0 |
0.3 |
| 1.51–2.00 |
100 |
29 |
5 |
0 |
9.1 |
2.6 |
0.5 |
0.0 |
| >2.00 |
143 |
0 |
0 |
0 |
0.0 |
0.0 |
0.0 |
0.0 |
| Sum |
875 |
184 |
33 |
7 |
79.6 |
16.7 |
3.0 |
0.6 |
| b) 2021 |
| 0.01–0.10 |
108 |
0 |
0 |
0 |
9.3 |
0.0 |
0.0 |
0.0 |
| 0.11–0.50 |
333 |
253 |
54 |
5 |
28.6 |
21.7 |
4.6 |
0.4 |
| 0.51–1.00 |
73 |
137 |
62 |
6 |
6.3 |
11.8 |
5.3 |
0.5 |
| 1.01–1.50 |
21 |
47 |
29 |
6 |
1.8 |
4.0 |
2.5 |
0.5 |
| 1.51–2.00 |
5 |
14 |
7 |
4 |
0.4 |
1.2 |
0.6 |
0.3 |
| >2.00 |
0 |
0 |
0 |
0 |
0.0 |
0.0 |
0.0 |
0.0 |
| Sum |
540 |
451 |
152 |
21 |
46.4 |
38.7 |
13.1 |
1.8 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).