Submitted:
28 February 2023
Posted:
01 March 2023
You are already at the latest version
Abstract
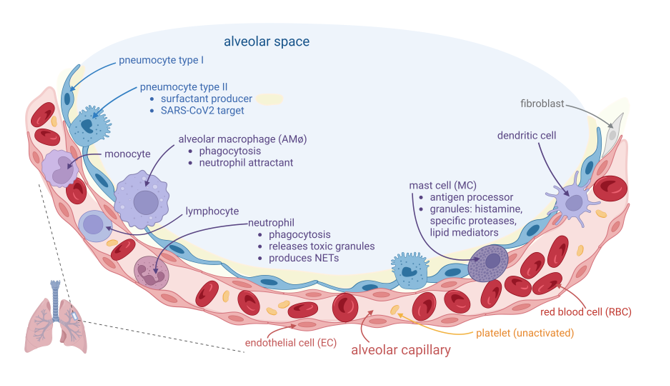
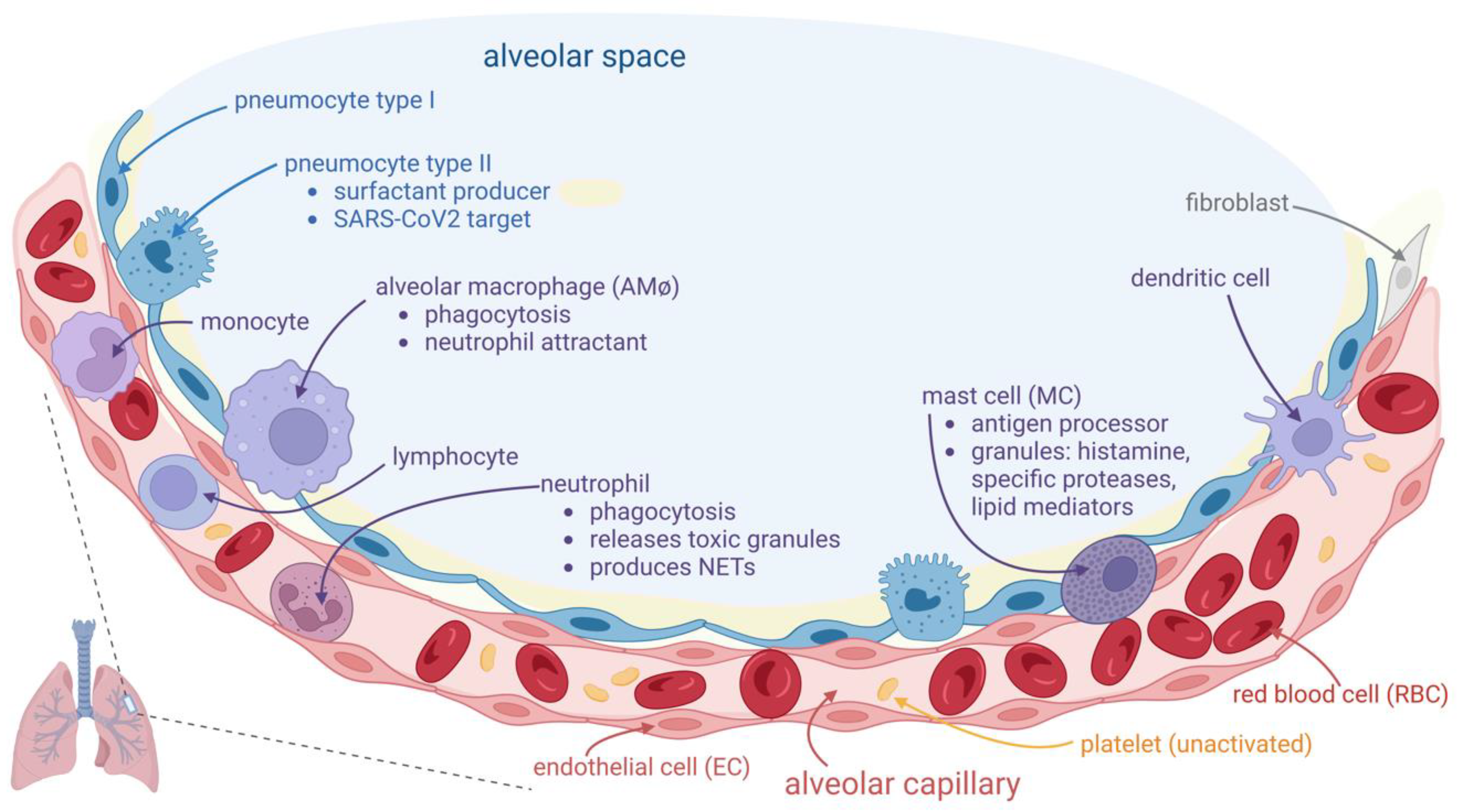
Keywords:
1. Introduction
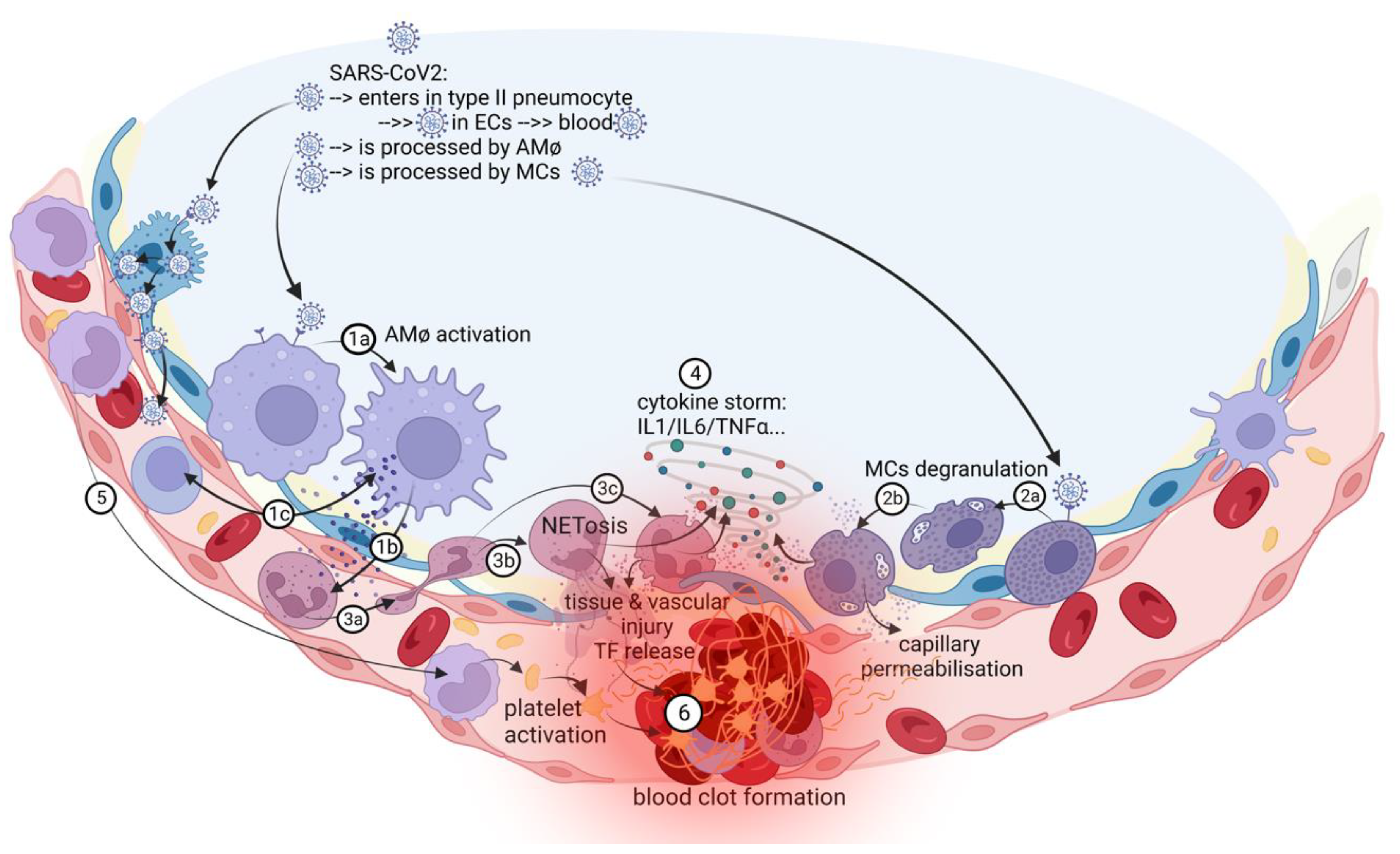
2. COVID-19 associated pulmonary thrombosis is an in situ immunothrombosis
3. Biological mechanisms for in situ pulmonary immunothrombosis
3.1. Inflammatory pathways
3.1.1. Macrophages (AMφs), monocytes, and T cells
3.1.2. The NETs-thrombosis axis in COVID-19
3.1.3. Mast cells (MCs), cytokines and chemokines
3.1.4. Complement pathways
3.2. Coagulation pathways
3.2.1. ECs and platelets
3.2.2. vWF
3.2.3. Thrombomodulin and P-selectin
3.2.4. Intrinsic and extrinsic coagulation pathways
3.2.5. Fibrinolytic disbalance and the central role of PAI-1
3.2.6. SARS-CoV-2-RBCs axis
4. Chest CT imaging data supporting the role of pulmonary immunothrombosis
5. The role of venous thromboembolism
6. The failure of anticoagulation treatment in immunothrombosis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manolis, A.S., et al., COVID-19 Infection: Viral Macro- and Micro-Vascular Coagulopathy and Thromboembolism/Prophylactic and Therapeutic Management. J Cardiovasc Pharmacol Ther, 2021. 26(1): p. 12-24. [CrossRef]
- Loo, J., D.A. Spittle, and M. Newnham, COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax, 2021. 76(4): p. 412-420. [CrossRef]
- Tang, N., et al., Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost, 2020. 18(4): p. 844-847. [CrossRef]
- Won, T., et al., Endothelial thrombomodulin downregulation caused by hypoxia contributes to severe infiltration and coagulopathy in COVID-19 patient lungs. eBioMedicine, 2022. 75: p. 103812. [CrossRef]
- Mueller-Peltzer, K., et al., Pulmonary artery thrombi are co-located with opacifications in SARS-CoV2 induced ARDS. Respir Med, 2020. 172: p. 106135.
- Oba, S., et al., Arterial and Venous Thrombosis Complicated in COVID-19: A Retrospective Single Center Analysis in Japan. Front Cardiovasc Med, 2021. 8: p. 767074. [CrossRef]
- Suh, Y.J., et al., Pulmonary Embolism and Deep Vein Thrombosis in COVID-19: A Systematic Review and Meta-Analysis. Radiology, 2021. 298(2): p. E70-e80. [CrossRef]
- Portier, I., R.A. Campbell, and F. Denorme, Mechanisms of immunothrombosis in COVID-19. Curr Opin Hematol, 2021. 28(6): p. 445-453. [CrossRef]
- Conway, E.M., et al., Understanding COVID-19-associated coagulopathy. Nat Rev Immunol, 2022. 22(10): p. 639-649. [CrossRef]
- Payus, A.O., C.L.S. Lin, and A. Ibrahim, The poorly understood yet potent risk of pulmonary artery thrombosis in-situ in Post-Acute COVID-19 syndrome. J Cardiothorac Surg, 2023. 18(1): p. 42. [CrossRef]
- Cuevas Vilaplana, A., I. Roldan Torres, and J. Vizuete Del Rio, [Myocarditis and in situ thrombosis in the right ventricle in a COVID-19 patient]. Hipertens Riesgo Vasc, 2021. 38(3): p. 148-150. [CrossRef]
- Khismatullin, R.R., et al., Pathology of lung-specific thrombosis and inflammation in COVID-19. J Thromb Haemost, 2021. 19(12): p. 3062-3072. [CrossRef]
- Quartuccio, L., et al., Clinical, laboratory and immunohistochemical characterization of in situ pulmonary arterial thrombosis in fatal COVID-19. Thromb Res, 2022. 219: p. 95-101. [CrossRef]
- Agrati, C., et al., The Role of P-Selectin in COVID-19 Coagulopathy: An Updated Review. Int J Mol Sci, 2021. 22(15). [CrossRef]
- Lamers, M.M. and B.L. Haagmans, SARS-CoV-2 pathogenesis. Nature Reviews Microbiology, 2022. 20(5): p. 270-284. [CrossRef]
- Lim, M.S. and S. McRae, COVID-19 and immunothrombosis: Pathophysiology and therapeutic implications. Crit Rev Oncol Hematol, 2021. 168: p. 103529. [CrossRef]
- Thachil, J. and A. Srivastava, SARS-2 Coronavirus-Associated Hemostatic Lung Abnormality in COVID-19: Is It Pulmonary Thrombosis or Pulmonary Embolism? Semin Thromb Hemost, 2020. 46(7): p. 777-780. [CrossRef]
- Morrell, C.N., et al., Emerging roles for platelets as immune and inflammatory cells. Blood, 2014. 123(18): p. 2759-67. [CrossRef]
- Wang, L., et al., In situ pulmonary thrombosis in patients with COVID-19 pneumonia: different phenotypes may exist. Thromb Res, 2020. 196: p. 541-542. [CrossRef]
- Martín Giménez, V.M., et al., Lungs as target of COVID-19 infection: Protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment. Life Sci, 2020. 254: p. 117808. [CrossRef]
- Calabrese, F., et al., Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Arch, 2020. 477(3): p. 359-372. [CrossRef]
- Ackermann, M., et al., Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med, 2020. 383(2): p. 120-128. [CrossRef]
- Menter, T., et al., Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology, 2020. 77(2): p. 198-209. [CrossRef]
- Carsana, L., et al., Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis, 2020. 20(10): p. 1135-1140. [CrossRef]
- Duarte-Neto, A.N., et al., Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology, 2020. 77(2): p. 186-197. [CrossRef]
- Cacciola, R., et al., Cellular and molecular mechanisms in COVID-19 coagulopathy: role of inflammation and endotheliopathy. J Thromb Thrombolysis, 2022. 53(2): p. 282-290. [CrossRef]
- Golubeva, M.G., Role of P-Selectin in the Development of Hemostasis Disorders in COVID-19. Biology Bulletin Reviews, 2022. 12(4): p. 406-413. [CrossRef]
- Vivan, M.A., et al., Pulmonary embolism in patients with COVID-19 and D-dimer diagnostic value: A retrospective study. Braz J Infect Dis, 2022. 26(6): p. 102702. [CrossRef]
- Močibob, L., et al., COVID-19 and Pulmonary Thrombosis-An Unresolved Clinical Puzzle: A Single-Center Cohort Study. J Clin Med, 2022. 11(23). [CrossRef]
- Jalde, F.C., et al., Widespread Parenchymal Abnormalities and Pulmonary Embolism on Contrast-Enhanced CT Predict Disease Severity and Mortality in Hospitalized COVID-19 Patients. Front Med (Lausanne), 2021. 8: p. 666723. [CrossRef]
- Masselli, G., et al., Role of CT angiography in detecting acute pulmonary embolism associated with COVID-19 pneumonia. Radiol Med, 2021. 126(12): p. 1553-1560. [CrossRef]
- Smilowitz, N.R., et al., C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J, 2021. 42(23): p. 2270-2279. [CrossRef]
- Sultana, G.N.N., et al., Studying C-reactive protein and D-dimer levels in blood may prevent severe complications: A study in Bangladeshi COVID-19 patients. Front Genet, 2022. 13: p. 966595. [CrossRef]
- Gorog, D.A., et al., Current and novel biomarkers of thrombotic risk in COVID-19: a Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nature Reviews Cardiology, 2022. 19(7): p. 475-495. [CrossRef]
- Lucijanić, M., et al., Clinical and prognostic significance of C-reactive protein to albumin ratio in hospitalized coronavirus disease 2019 (COVID-19) patients. Wiener klinische Wochenschrift, 2022. 134(9): p. 377-384. [CrossRef]
- Fay, W.P., Linking inflammation and thrombosis: Role of C-reactive protein. World J Cardiol, 2010. 2(11): p. 365-9. [CrossRef]
- Galland, J., et al., White blood count, D-dimers, and ferritin levels as predictive factors of pulmonary embolism suspected upon admission in noncritically ill COVID-19 patients: The French multicenter CLOTVID retrospective study. Eur J Haematol, 2021. 107(2): p. 190-201. [CrossRef]
- Kaushal, K., et al., Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. J Crit Care, 2022. 67: p. 172-181. [CrossRef]
- Wu, R., et al., Inflammasome-Dependent Coagulation Activation in Sepsis. Front Immunol, 2021. 12: p. 641750. [CrossRef]
- Tuculeanu, G., et al., Coagulation Disorders in Sepsis and COVID-19—Two Sides of the Same Coin? A Review of Inflammation–Coagulation Crosstalk in Bacterial Sepsis and COVID-19. Journal of Clinical Medicine, 2023. 12(2): p. 601. [CrossRef]
- Delorey, T.M., et al., COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature, 2021. 595(7865): p. 107-113. [CrossRef]
- Savla, S.R., K.S. Prabhavalkar, and L.K. Bhatt, Cytokine storm associated coagulation complications in COVID-19 patients: Pathogenesis and Management. Expert Rev Anti Infect Ther, 2021. 19(11): p. 1397-1413. [CrossRef]
- Bain, C.C., C.D. Lucas, and A.G. Rossi, Pulmonary macrophages and SARS-Cov2 infection. Int Rev Cell Mol Biol, 2022. 367: p. 1-28. [CrossRef]
- Faggioli, P.M., N. Mumoli, and A. Mazzone, Iloprost in COVID-19: The Rationale of Therapeutic Benefit. Front Cardiovasc Med, 2021. 8: p. 649499. [CrossRef]
- Hottz, E.D., et al., Platelet-monocyte interaction amplifies thromboinflammation through tissue factor signaling in COVID-19. Blood Adv, 2022. 6(17): p. 5085-5099. [CrossRef]
- Szturmowicz, M. and U. Demkow, Neutrophil Extracellular Traps (NETs) in Severe SARS-CoV-2 Lung Disease. Int J Mol Sci, 2021. 22(16). [CrossRef]
- Zhou, Y., et al., The Emerging Role of Neutrophil Extracellular Traps in Arterial, Venous and Cancer-Associated Thrombosis. Front Cardiovasc Med, 2021. 8: p. 786387. [CrossRef]
- Al-Kuraishy, H.M., et al., Neutrophil Extracellular Traps (NETs) and Covid-19: A new frontiers for therapeutic modality. Int Immunopharmacol, 2022. 104: p. 108516. [CrossRef]
- Middleton, E.A., et al., Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood, 2020. 136(10): p. 1169-1179. [CrossRef]
- Ackermann, M., et al., Patients with COVID-19: in the dark-NETs of neutrophils. Cell Death & Differentiation, 2021. 28(11): p. 3125-3139. [CrossRef]
- Pastorek, M., M. Dúbrava, and P. Celec, On the Origin of Neutrophil Extracellular Traps in COVID-19. Front Immunol, 2022. 13: p. 821007. [CrossRef]
- Teluguakula, N., Neutrophils Set Extracellular Traps to Injure Lungs in Coronavirus Disease 2019. J Infect Dis, 2021. 223(9): p. 1503-1505. [CrossRef]
- Ouwendijk, W.J.D., et al., High Levels of Neutrophil Extracellular Traps Persist in the Lower Respiratory Tract of Critically Ill Patients With Coronavirus Disease 2019. J Infect Dis, 2021. 223(9): p. 1512-1521. [CrossRef]
- Sung, P.-S., et al., CLEC5A and TLR2 are critical in SARS-CoV-2-induced NET formation and lung inflammation. Journal of Biomedical Science, 2022. 29(1): p. 52. [CrossRef]
- Lam, H.Y., et al., Mast cells: Therapeutic targets for COVID-19 and beyond. IUBMB Life, 2021. 73(11): p. 1278-1292. [CrossRef]
- Galli, S.J., N. Gaudenzio, and M. Tsai, Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu Rev Immunol, 2020. 38: p. 49-77. [CrossRef]
- Cildir, G., et al., The transcriptional program, functional heterogeneity, and clinical targeting of mast cells. J Exp Med, 2017. 214(9): p. 2491-2506. [CrossRef]
- Theoharides, T.C., P. Valent, and C. Akin, Mast Cells, Mastocytosis, and Related Disorders. New England Journal of Medicine, 2015. 373(2): p. 163-172. [CrossRef]
- Gebremeskel, S., et al., Mast Cell and Eosinophil Activation Are Associated With COVID-19 and TLR-Mediated Viral Inflammation: Implications for an Anti-Siglec-8 Antibody. Front Immunol, 2021. 12: p. 650331. [CrossRef]
- Marshall, J.S., L. Portales-Cervantes, and E. Leong, Mast Cell Responses to Viruses and Pathogen Products. Int J Mol Sci, 2019. 20(17). [CrossRef]
- Budnevsky, A.V., et al., Role of mast cells in the pathogenesis of severe lung damage in COVID-19 patients. Respir Res, 2022. 23(1): p. 371. [CrossRef]
- Conti, P., et al., Mast cells activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19. J Biol Regul Homeost Agents, 2020. 34(5): p. 1629-1632. [CrossRef]
- Conti, P., et al., IL-1 induces throboxane-A2 (TxA2) in COVID-19 causing inflammation and micro-thrombi: inhibitory effect of the IL-1 receptor antagonist (IL-1Ra). J Biol Regul Homeost Agents, 2020. 34(5): p. 1623-1627. [CrossRef]
- Hoffmann, M., et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell, 2020. 181(2): p. 271-280.e8. [CrossRef]
- Jackson, C.B., et al., Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol, 2022. 23(1): p. 3-20. [CrossRef]
- Wismans, L.V., et al., Increase of mast cells in COVID-19 pneumonia may contribute to pulmonary fibrosis and thrombosis. Histopathology, 2023. 82(3): p. 407-419. [CrossRef]
- Wu, M.L., et al., SARS-CoV-2-triggered mast cell rapid degranulation induces alveolar epithelial inflammation and lung injury. Signal Transduct Target Ther, 2021. 6(1): p. 428. [CrossRef]
- Liu, S., et al., Mast cells promote viral entry of SARS-CoV-2 via formation of chymase/spike protein complex. Eur J Pharmacol, 2022. 930: p. 175169. [CrossRef]
- Krysko, O., et al., Severity of SARS-CoV-2 infection is associated with high numbers of alveolar mast cells and their degranulation. Front Immunol, 2022. 13: p. 968981. [CrossRef]
- Motta Junior, J.D.S., et al., Mast Cells in Alveolar Septa of COVID-19 Patients: A Pathogenic Pathway That May Link Interstitial Edema to Immunothrombosis. Front Immunol, 2020. 11: p. 574862. [CrossRef]
- Gasparello, J., et al., In vitro induction of interleukin-8 by SARS-CoV-2 Spike protein is inhibited in bronchial epithelial IB3-1 cells by a miR-93-5p agomiR. Int Immunopharmacol, 2021. 101(Pt B): p. 108201. [CrossRef]
- Halova, I., L. Draberova, and P. Draber, Mast cell chemotaxis - chemoattractants and signaling pathways. Front Immunol, 2012. 3: p. 119. [CrossRef]
- Costela-Ruiz, V.J., et al., SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev, 2020. 54: p. 62-75. [CrossRef]
- Hafezi, B., et al., Cytokine Storm Syndrome in SARS-CoV-2 Infections: A Functional Role of Mast Cells. Cells, 2021. 10(7). [CrossRef]
- Theoharides, T.C., Potential association of mast cells with coronavirus disease 2019. Ann Allergy Asthma Immunol, 2021. 126(3): p. 217-218. [CrossRef]
- Setyo Nugroho, G.M., et al., Interleukin-6 (IL-6) expression of lung tissue in COVID-19 patient severity through core biopsy post mortem. Ann Med Surg (Lond), 2022. 82: p. 104648. [CrossRef]
- Harapan, H., et al., The prevalence, predictors and outcomes of acute liver injury among patients with COVID-19: A systematic review and meta-analysis. Rev Med Virol, 2022. 32(3): p. e2304. [CrossRef]
- Colafrancesco, S., et al., Targeting the Immune System for Pulmonary Inflammation and Cardiovascular Complications in COVID-19 Patients. Front Immunol, 2020. 11: p. 1439. [CrossRef]
- Rad, F., et al., The Relationship between Inflammatory Cytokines and Coagulopathy in Patients with COVID-19. J Clin Med, 2021. 10(9). [CrossRef]
- Abd El-Ghani, S.E.-S., et al., Serum interleukin 1β and sP-selectin as biomarkers of inflammation and thrombosis, could they be predictors of disease severity in COVID 19 Egyptian patients? (a cross-sectional study). Thrombosis Journal, 2022. 20(1): p. 77. [CrossRef]
- Gianni, P., et al., Complement-mediated microvascular injury and thrombosis in the pathogenesis of severe COVID-19: A review. World J Exp Med, 2022. 12(4): p. 53-67. [CrossRef]
- Ali, Y.M., et al., Lectin Pathway Mediates Complement Activation by SARS-CoV-2 Proteins. Front Immunol, 2021. 12: p. 714511. [CrossRef]
- Boussier, J., et al., Severe COVID-19 is associated with hyperactivation of the alternative complement pathway. J Allergy Clin Immunol, 2022. 149(2): p. 550-556.e2. [CrossRef]
- Niederreiter, J., et al., Complement Activation via the Lectin and Alternative Pathway in Patients With Severe COVID-19. Frontiers in Immunology, 2022. 13. [CrossRef]
- Foley, J.H., et al., Complement Activation in Arterial and Venous Thrombosis is Mediated by Plasmin. EBioMedicine, 2016. 5: p. 175-82. [CrossRef]
- Tomo, S., et al., Complement activation and coagulopathy - an ominous duo in COVID19. Expert Rev Hematol, 2021. 14(2): p. 155-173 . [CrossRef]
- Magro, C., et al., Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res, 2020. 220: p. 1-13. [CrossRef]
- Holter, J.C., et al., Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc Natl Acad Sci U S A, 2020. 117(40): p. 25018-25025. [CrossRef]
- Peffault de Latour, R., et al., Complement C5 inhibition in patients with COVID-19 - a promising target? Haematologica, 2020. 105(12): p. 2847-2850. [CrossRef]
- Soma, P. and J. Bester, Pathophysiological Changes in Erythrocytes Contributing to Complications of Inflammation and Coagulation in COVID-19. Front Physiol, 2022. 13: p. 899629. [CrossRef]
- de Andrade, S.A., et al., Pathophysiology of COVID-19: Critical Role of Hemostasis. Front Cell Infect Microbiol, 2022. 12: p. 896972. [CrossRef]
- Goshua, G., et al., Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol, 2020. 7(8): p. e575-e582. [CrossRef]
- Varga, Z., et al., Endothelial cell infection and endotheliitis in COVID-19. Lancet, 2020. 395(10234): p. 1417-1418. [CrossRef]
- Tafazoli, A., S. Anil Kumar, and M. Othman, Thrombocytopathy vs Platelet hyper-reactivity in COVID-19: diverse pathologies, disease outcomes and therapeutic implications. Platelets, 2022. 33(1): p. 48-53. [CrossRef]
- Guo, L. and M.T. Rondina, The Era of Thromboinflammation: Platelets Are Dynamic Sensors and Effector Cells During Infectious Diseases. Front Immunol, 2019. 10: p. 2204. [CrossRef]
- Sharma, S., T. Tyagi, and S. Antoniak, Platelet in thrombo-inflammation: Unraveling new therapeutic targets. Front Immunol, 2022. 13: p. 1039843. [CrossRef]
- Comer, S.P., et al., COVID-19 induces a hyperactive phenotype in circulating platelets. PLoS Biol, 2021. 19(2): p. e3001109. [CrossRef]
- Tunjungputri, R.N., et al., The Inter-Relationship of Platelets with Interleukin-1β-Mediated Inflammation in Humans. Thromb Haemost, 2018. 118(12): p. 2112-2125. [CrossRef]
- Fard, M.B., et al., Thrombosis in COVID-19 infection: Role of platelet activation-mediated immunity. Thrombosis Journal, 2021. 19(1): p. 59. [CrossRef]
- Le, H.T., et al., Platelet factor 4 (CXCL4/PF4) upregulates matrix metalloproteinase-2 (MMP-2) in gingival fibroblasts. Scientific Reports, 2022. 12(1): p. 18636. [CrossRef]
- Liu, Q., et al., Anti-PF4 antibodies associated with disease severity in COVID-19. Proc Natl Acad Sci U S A, 2022. 119(47): p. e2213361119. [CrossRef]
- Bongiovanni, D., et al., SARS-CoV-2 infection is associated with a pro-thrombotic platelet phenotype. Cell Death Dis, 2021. 12(1): p. 50. [CrossRef]
- Aloui, C., et al., The signaling role of CD40 ligand in platelet biology and in platelet component transfusion. Int J Mol Sci, 2014. 15(12): p. 22342-64. [CrossRef]
- Tian, X., et al., CXCR4 knockdown prevents inflammatory cytokine expression in macrophages by suppressing activation of MAPK and NF-κB signaling pathways. Cell & Bioscience, 2019. 9(1): p. 55. [CrossRef]
- Gavins, F.N., et al., Microvascular thrombosis and CD40/CD40L signaling. J Thromb Haemost, 2011. 9(3): p. 574-81. [CrossRef]
- Kremer Hovinga, J.A. and J.N. George, Hereditary Thrombotic Thrombocytopenic Purpura. New England Journal of Medicine, 2019. 381(17): p. 1653-1662. [CrossRef]
- Naß, J., J. Terglane, and V. Gerke, Weibel Palade Bodies: Unique Secretory Organelles of Endothelial Cells that Control Blood Vessel Homeostasis. Front Cell Dev Biol, 2021. 9: p. 813995. [CrossRef]
- Mei, Z.W., et al., Role of von Willebrand Factor in COVID-19 Associated Coagulopathy. J Appl Lab Med, 2021. 6(5): p. 1305-1315. [CrossRef]
- Manz, X.D., et al., Epigenetic Modification of the von Willebrand Factor Promoter Drives Platelet Aggregation on the Pulmonary Endothelium in Chronic Thromboembolic Pulmonary Hypertension. Am J Respir Crit Care Med, 2022. 205(7): p. 806-818. [CrossRef]
- Manz, X.D., H.J. Bogaard, and J. Aman, Regulation of VWF (Von Willebrand Factor) in Inflammatory Thrombosis. Arterioscler Thromb Vasc Biol, 2022. 42(11): p. 1307-1320. [CrossRef]
- Khan, S., et al., SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. Elife, 2021. 10.
- O’Sullivan, J.M., et al., Endothelial cells orchestrate COVID-19 coagulopathy. Lancet Haematol, 2020. 7(8): p. e553-e555. [CrossRef]
- Seibert, F.S., et al., Effect of plasma exchange on COVID-19 associated excess of von Willebrand factor and inflammation in critically ill patients. Sci Rep, 2022. 12(1): p. 4801. [CrossRef]
- Doevelaar, A.A.N., et al., von Willebrand Factor Multimer Formation Contributes to Immunothrombosis in Coronavirus Disease 2019. Crit Care Med, 2021. 49(5): p. e512-e520. [CrossRef]
- Mancini, I., et al., The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J Thromb Haemost, 2021. 19(2): p. 513-521. [CrossRef]
- Fenyves, B.G., et al., Plasma P-selectin is an early marker of thromboembolism in COVID-19. Am J Hematol, 2021. 96(12): p. E468-E471. [CrossRef]
- Ito, T., et al., Thrombomodulin in disseminated intravascular coagulation and other critical conditions—a multi-faceted anticoagulant protein with therapeutic potential. Critical Care, 2019. 23(1): p. 280. [CrossRef]
- Yamakawa, K., S. Murao, and M. Aihara, Recombinant Human Soluble Thrombomodulin in Sepsis-Induced Coagulopathy: An Updated Systematic Review and Meta-Analysis. Thromb Haemost, 2019. 119(1): p. 56-65. [CrossRef]
- Leucker, T.M., et al., Effect of Crizanlizumab, a P-Selectin Inhibitor, in COVID-19: A Placebo-Controlled, Randomized Trial. JACC Basic Transl Sci, 2021. 6(12): p. 935-945. [CrossRef]
- Neri, T., D. Nieri, and A. Celi, P-selectin blockade in COVID-19-related ARDS. Am J Physiol Lung Cell Mol Physiol, 2020. 318(6): p. L1237-L1238. [CrossRef]
- Karsli, E., et al., Soluble P-selectin as a potential diagnostic and prognostic biomarker for COVID-19 disease: A case-control study. Life Sci, 2021. 277: p. 119634. [CrossRef]
- Etulain, J., et al., P-selectin promotes neutrophil extracellular trap formation in mice. Blood, 2015. 126(2): p. 242-6. [CrossRef]
- Muller, R., et al., Increased plasma level of soluble P-selectin in non-hospitalized COVID-19 convalescent donors. Thromb Res, 2022. 216: p. 120-124. [CrossRef]
- Wang, S.S.Y., et al., Increased Platelet Activation demonstrated by Elevated CD36 and P-Selectin Expression in 1-Year Post-Recovered COVID-19 Patients. Semin Thromb Hemost, 2023. [CrossRef]
- FitzGerald, E.S., et al., Lung Epithelial Cell Transcriptional Regulation as a Factor in COVID-19-associated Coagulopathies. Am J Respir Cell Mol Biol, 2021. 64(6): p. 687-697. [CrossRef]
- Cañas, C.A., et al., Role of Tissue Factor in the Pathogenesis of COVID-19 and the Possible Ways to Inhibit It. Clin Appl Thromb Hemost, 2021. 27: p. 10760296211003983. [CrossRef]
- Stefely, J.A., et al., Marked factor V activity elevation in severe COVID-19 is associated with venous thromboembolism. Am J Hematol, 2020. 95(12): p. 1522-1530. [CrossRef]
- Wang, J., et al., Coagulation factor V is a T-cell inhibitor expressed by leukocytes in COVID-19. iScience, 2022. 25(3): p. 103971. [CrossRef]
- Antoniak, S., The coagulation system in host defense. Res Pract Thromb Haemost, 2018. 2(3): p. 549-557. [CrossRef]
- Han, M. and D. Pandey, ZMPSTE24 Regulates SARS-CoV-2 Spike Protein-enhanced Expression of Endothelial PAI-1. Am J Respir Cell Mol Biol, 2021. 65(3): p. 300-308. [CrossRef]
- Whyte, C.S., et al., The suboptimal fibrinolytic response in COVID-19 is dictated by high PAI-1. J Thromb Haemost, 2022. 20(10): p. 2394-2406. [CrossRef]
- Bielosludtseva, K., The diagnostic and prognostic role of plasminogen activator inhibitor-1 (PAI-1) in hospitalized patients with pneumonias of different etiologies. European Respiratory Journal, 2022. 60(suppl 66): p. 2495.
- Matsuyama, T., et al., An aberrant STAT pathway is central to COVID-19. Cell Death Differ, 2020. 27(12): p. 3209-3225. [CrossRef]
- Morrow, G.B., C.S. Whyte, and N.J. Mutch, A Serpin With a Finger in Many PAIs: PAI-1’s Central Function in Thromboinflammation and Cardiovascular Disease. Front Cardiovasc Med, 2021. 8: p. 653655. [CrossRef]
- Kwaan, H.C. and P.F. Lindholm, The Central Role of Fibrinolytic Response in COVID-19-A Hematologist’s Perspective. Int J Mol Sci, 2021. 22(3). [CrossRef]
- Kang, S., et al., IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc Natl Acad Sci U S A, 2020. 117(36): p. 22351-22356. [CrossRef]
- Al-Tamimi, A.O., et al., SARS-CoV-2 infection induces soluble platelet activation markers and PAI-1 in the early moderate stage of COVID-19. Int J Lab Hematol, 2022. 44(4): p. 712-721. [CrossRef]
- Poole, L.G., et al., Plasminogen Activator Inhibitor-1 Is Critical in Alcohol-Enhanced Acute Lung Injury in Mice. Am J Respir Cell Mol Biol, 2017. 57(3): p. 315-323. [CrossRef]
- Zuo, Y., et al., Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Scientific Reports, 2021. 11(1): p. 1580. [CrossRef]
- Bouchla, A., et al., Red Blood Cell Abnormalities as the Mirror of SARS-CoV-2 Disease Severity: A Pilot Study. Front Physiol, 2021. 12: p. 825055. [CrossRef]
- Espallargas, I., et al., CT imaging of pulmonary embolism in patients with COVID-19 pneumonia: a retrospective analysis. Eur Radiol, 2021. 31(4): p. 1915-1922. [CrossRef]
- Longchamp, G., et al., Proximal deep vein thrombosis and pulmonary embolism in COVID-19 patients: a systematic review and meta-analysis. Thrombosis Journal, 2021. 19(1): p. 15. [CrossRef]
- Valle, C., et al., Association between pulmonary embolism and COVID-19 severe pneumonia: Experience from two centers in the core of the infection Italian peak. Eur J Radiol, 2021. 137: p. 109613. [CrossRef]
- Niculae, C.M., et al., Acute Pulmonary Artery Thrombosis despite Anticoagulation in Patients with COVID-19 Pneumonia: A Single-Center Retrospective Cohort Study. J Clin Med, 2022. 11(9). [CrossRef]
- Trunz, L.M., et al., Imaging approach to COVID-19 associated pulmonary embolism. Int J Clin Pract, 2021. 75(10): p. e14340. [CrossRef]
- Barnett, N., et al., Prevalence of pulmonary embolism and deep venous thrombosis during the COVID-19 pandemic in an intensive care unit cohort: a service evaluation. Br J Anaesth, 2022. 129(5): p. e124-e126. [CrossRef]
- Cau, R., et al., Complications in COVID-19 patients: Characteristics of pulmonary embolism. Clin Imaging, 2021. 77: p. 244-249. [CrossRef]
- Scialpi, M., et al., Pulmonary embolism in COVID-19: Ancillary findings on chest CT angiography. Lung India, 2021. 38(Supplement): p. S123-S125. [CrossRef]
- Mandal, A.K.J., et al., Covid-19 and in situ pulmonary artery thrombosis. Respir Med, 2021. 176: p. 106176. [CrossRef]
- De Cobelli, F., et al., Pulmonary Vascular Thrombosis in COVID-19 Pneumonia. J Cardiothorac Vasc Anesth, 2021. 35(12): p. 3631-3641. [CrossRef]
- Bompard, F., et al., Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J, 2020. 56(1). [CrossRef]
- Lazar, M., et al., Mortality Predictors in Severe SARS-CoV-2 Infection. Medicina (Kaunas), 2022. 58(7).
- Birocchi, S., et al., High rates of pulmonary artery occlusions in COVID-19. A meta-analysis. Eur J Clin Invest, 2021. 51(1): p. e13433. [CrossRef]
- Fauvel, C., et al., Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J, 2020. 41(32): p. 3058-3068. [CrossRef]
- Farouk, N., et al., Admission Levels of Serum P-Selectin and IL-6 Can Predict Development of Deep Venous Thrombosis in Hospitalized Covid-19 Patients. Int J Gen Med, 2022. 15: p. 5599-5607. [CrossRef]
- Marone, E.M., et al., Characteristics of Venous Thromboembolism in COVID-19 Patients: A Multicenter Experience from Northern Italy. Ann Vasc Surg, 2020. 68: p. 83-87. [CrossRef]
- Alonso Martinez, J.L., et al., Central Versus Peripheral Pulmonary Embolism: Analysis of the Impact on the Physiological Parameters and Long-term Survival. N Am J Med Sci, 2016. 8(3): p. 134-42. [CrossRef]
- Cha, S.-I., et al., Pulmonary embolism concurrent with lung cancer and central emboli predict mortality in patients with lung cancer and pulmonary embolism. Journal of Thoracic Disease, 2017. 10(1): p. 262-272. [CrossRef]
- Loffredo, L., et al., Asymptomatic and symptomatic deep venous thrombosis in hospitalized acutely ill medical patients: risk factors and therapeutic implications. Thrombosis Journal, 2022. 20(1): p. 72. [CrossRef]
- Katsoularis, I., et al., Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: nationwide self-controlled cases series and matched cohort study. Bmj, 2022. 377: p. e069590. [CrossRef]
- Poor, H.D., Pulmonary Thrombosis and Thromboembolism in COVID-19. Chest, 2021. 160(4): p. 1471-1480. [CrossRef]
- Cui, S., et al., Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost, 2020. 18(6): p. 1421-1424. [CrossRef]
- Beretta, S., et al., Case Report: Concomitant Massive Cerebral Venous Thrombosis and Internal Iliac Vein Thrombosis Related to Paucisymptomatic COVID-19 Infection. Front Neurol, 2021. 12: p. 622130. [CrossRef]
- Malentacchi, M., et al., Concomitant brain arterial and venous thrombosis in a COVID-19 patient. Eur J Neurol, 2020. 27(9): p. e38-e39. [CrossRef]
- Lucijanic, M., et al., Asymptomatic deep vein thromboses in prolonged hospitalized COVID-19 patients. Wien Klin Wochenschr, 2021. 133(23-24): p. 1281-1288. [CrossRef]
- Horne, M.K., III, The dark side of deep venous thrombosis: the failure of anticoagulation. The American Journal of Medicine, 2001. 110(7): p. 589-590. [CrossRef]
- Rodger, M.A., et al., Management of suspected and confirmed recurrent venous thrombosis while on anticoagulant therapy. What next? Thrombosis Research, 2019. 180: p. 105-109. [CrossRef]
- Mosarla, R.C., et al., Anticoagulation Strategies in Patients With Cancer: JACC Review Topic of the Week. J Am Coll Cardiol, 2019. 73(11): p. 1336-1349. [CrossRef]
- Paez Vargas, J., et al., ANTICOAGULATION, BLEEDING, AND IMMUNOTHROMBOSIS IN CRITICALLY ILL PATIENTS WITH COVID-19. CHEST, 2021. 160(4): p. A994-A995. [CrossRef]
- Tan, B.K., et al., Arterial and venous thromboembolism in COVID-19: a study-level meta-analysis. Thorax, 2021. 76(10): p. 970-979. [CrossRef]
- Bikdeli, B., et al., COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol, 2020. 75(23): p. 2950-2973. [CrossRef]
- Klok, F.A., et al., Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res, 2020. 191: p. 145-147. [CrossRef]
- Llitjos, J.F., et al., High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost, 2020. 18(7): p. 1743-1746. [CrossRef]
- Porembskaya, O., et al., Thrombosis of pulmonary vasculature despite anticoagulation and thrombolysis: The findings from seven autopsies. Thrombosis Update, 2020. 1: p. 100017. [CrossRef]
- Sadeghipour, P., et al., Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. Jama, 2021. 325(16): p. 1620-1630. [CrossRef]
- Lawler, P.R., et al., Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N Engl J Med, 2021. 385(9): p. 790-802. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




