Submitted:
01 March 2023
Posted:
03 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction, PCR Amplification and Sequencing
2.3. Quality Control and Data Standardization
2.4. Estimation of Alpha and Beta Diversity
2.5. Gene Function Predication
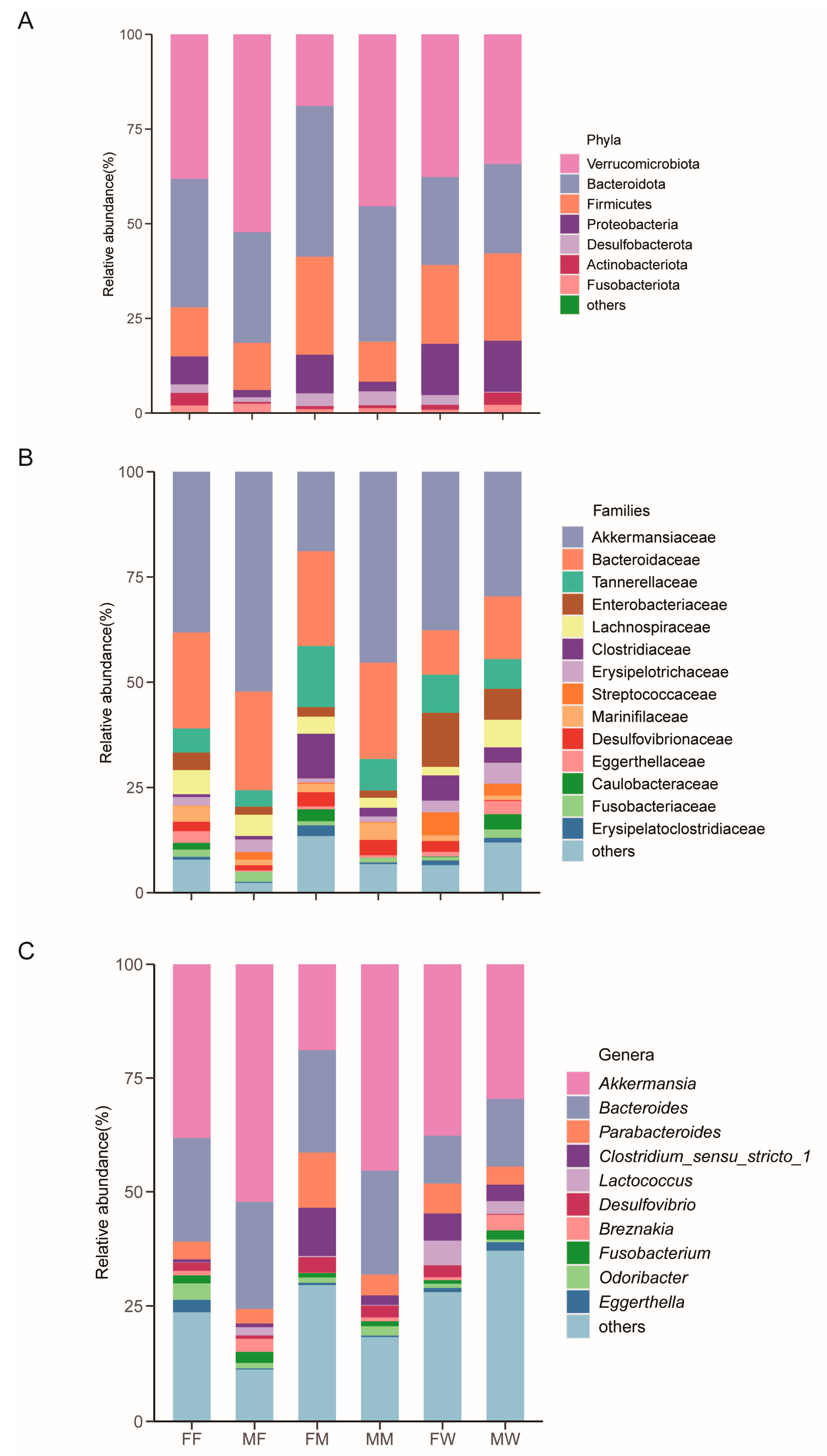
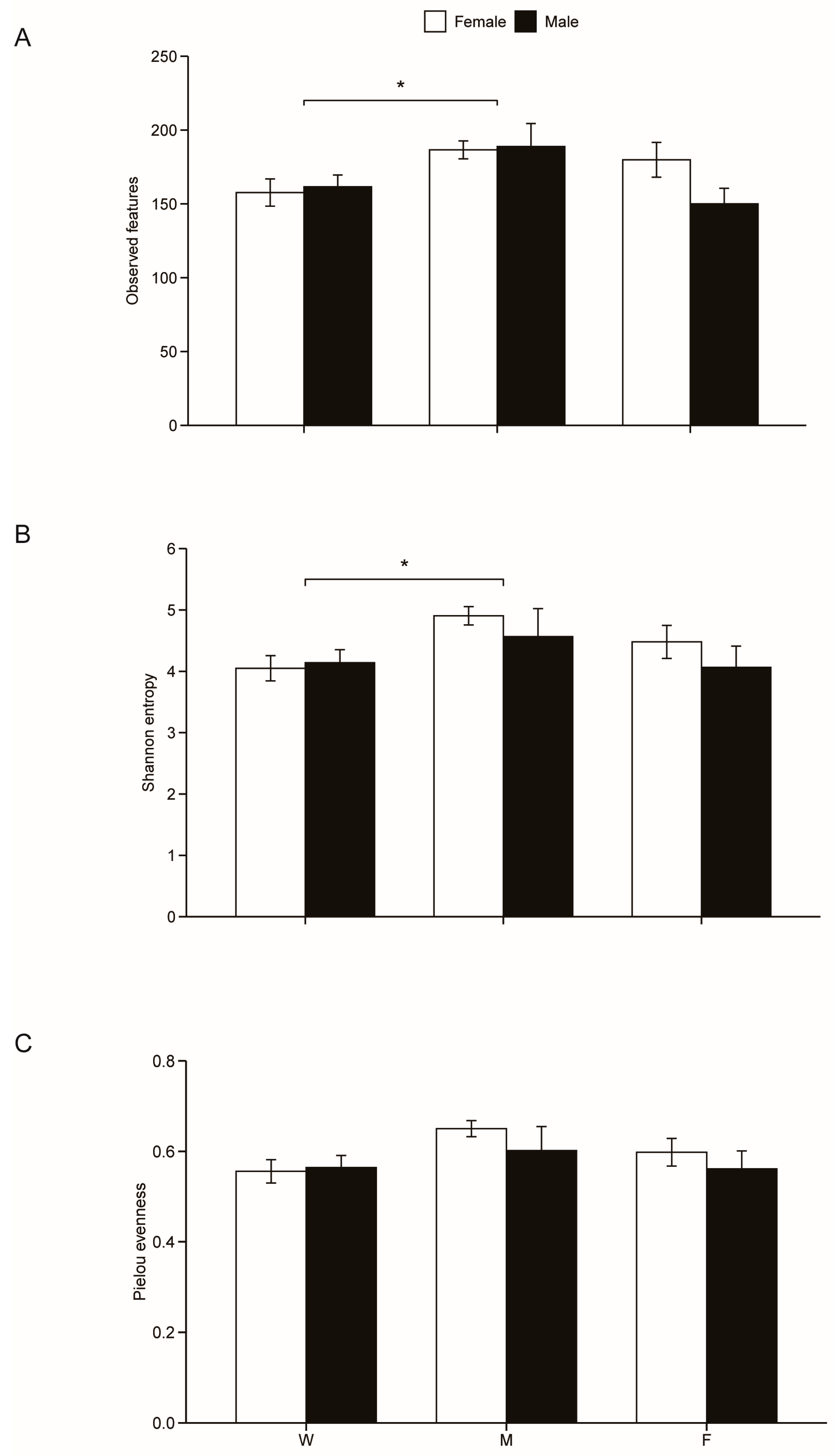
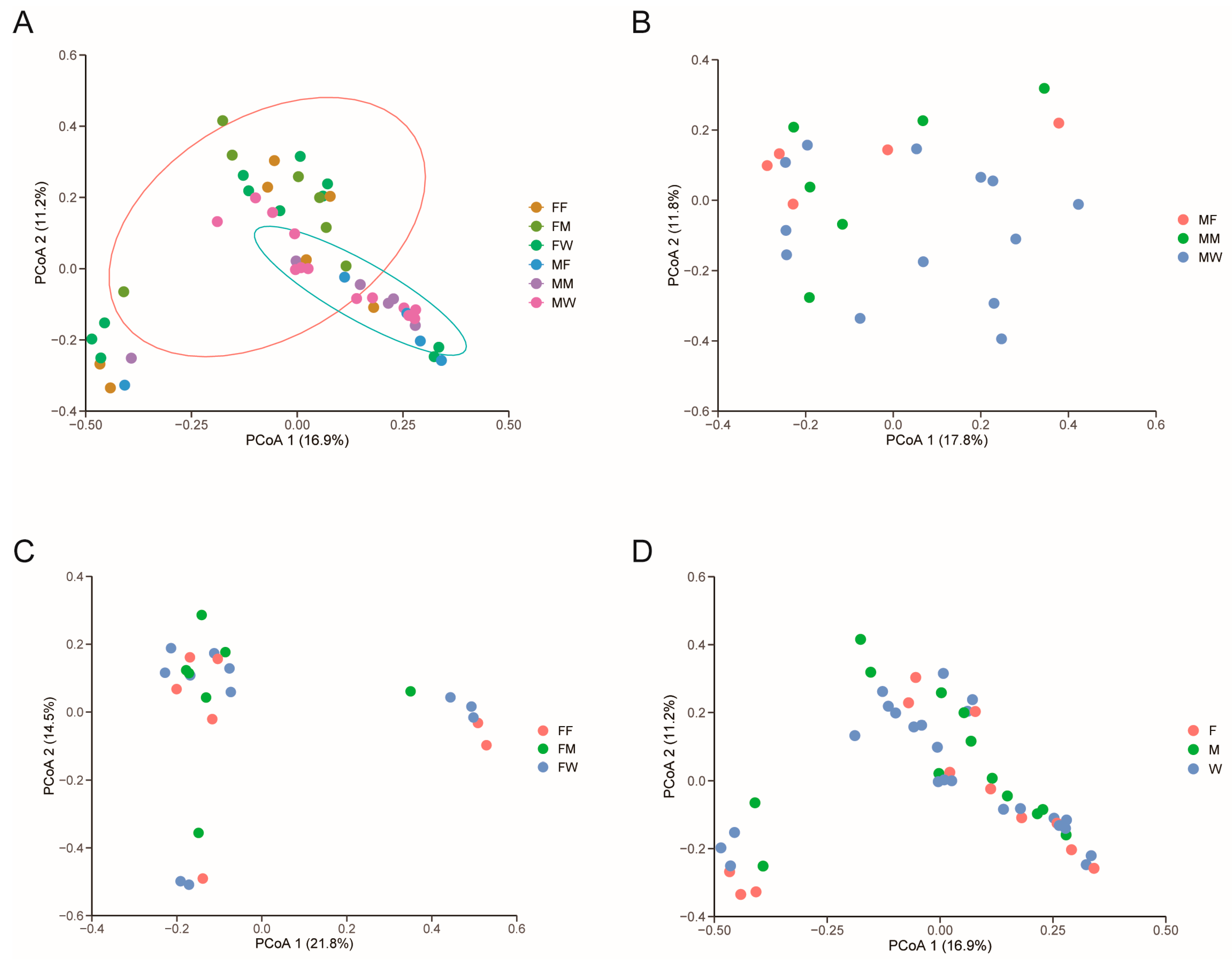
3. Results
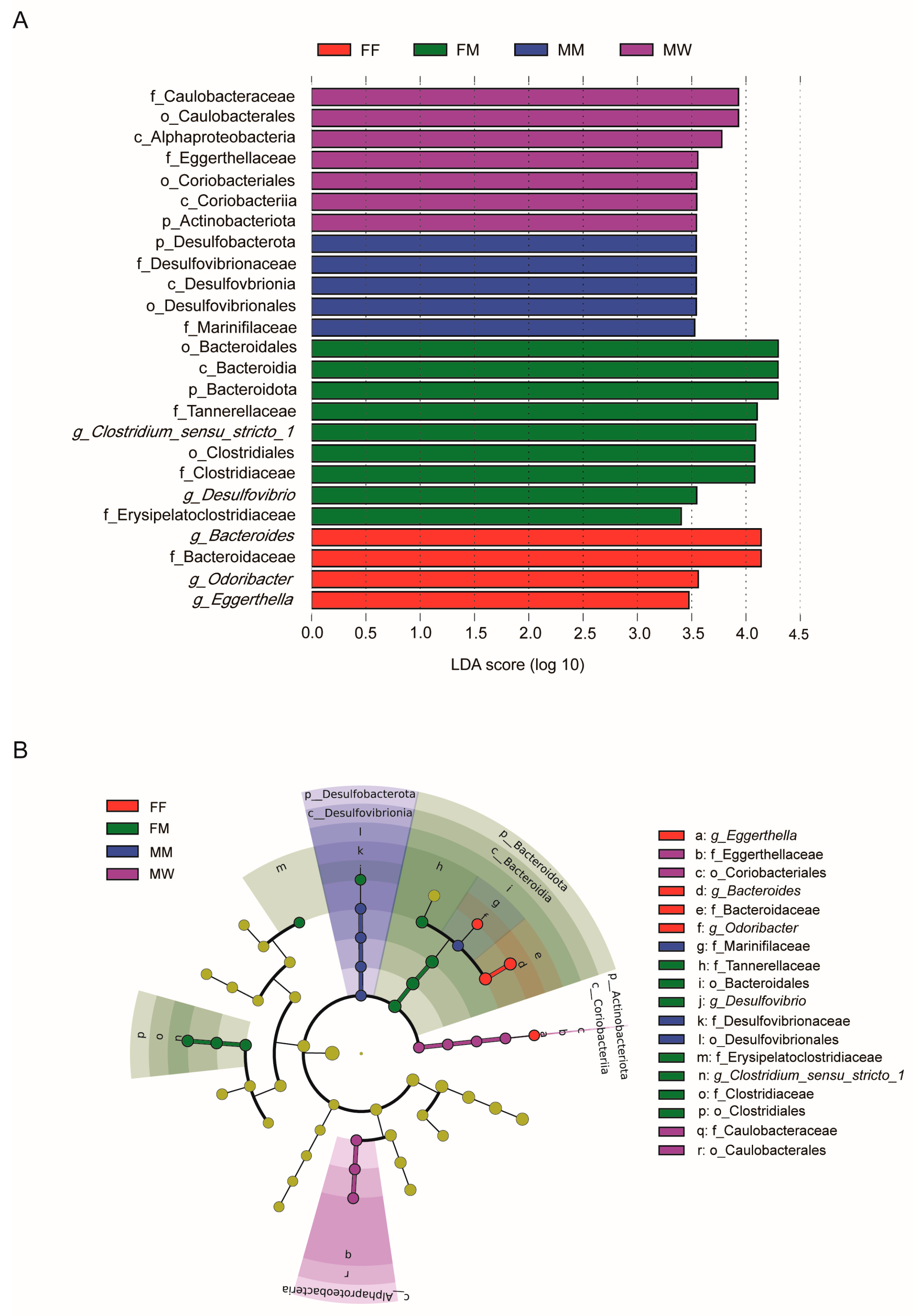
3.2. Dietary and Sexual Correlates of Gut Microbiota
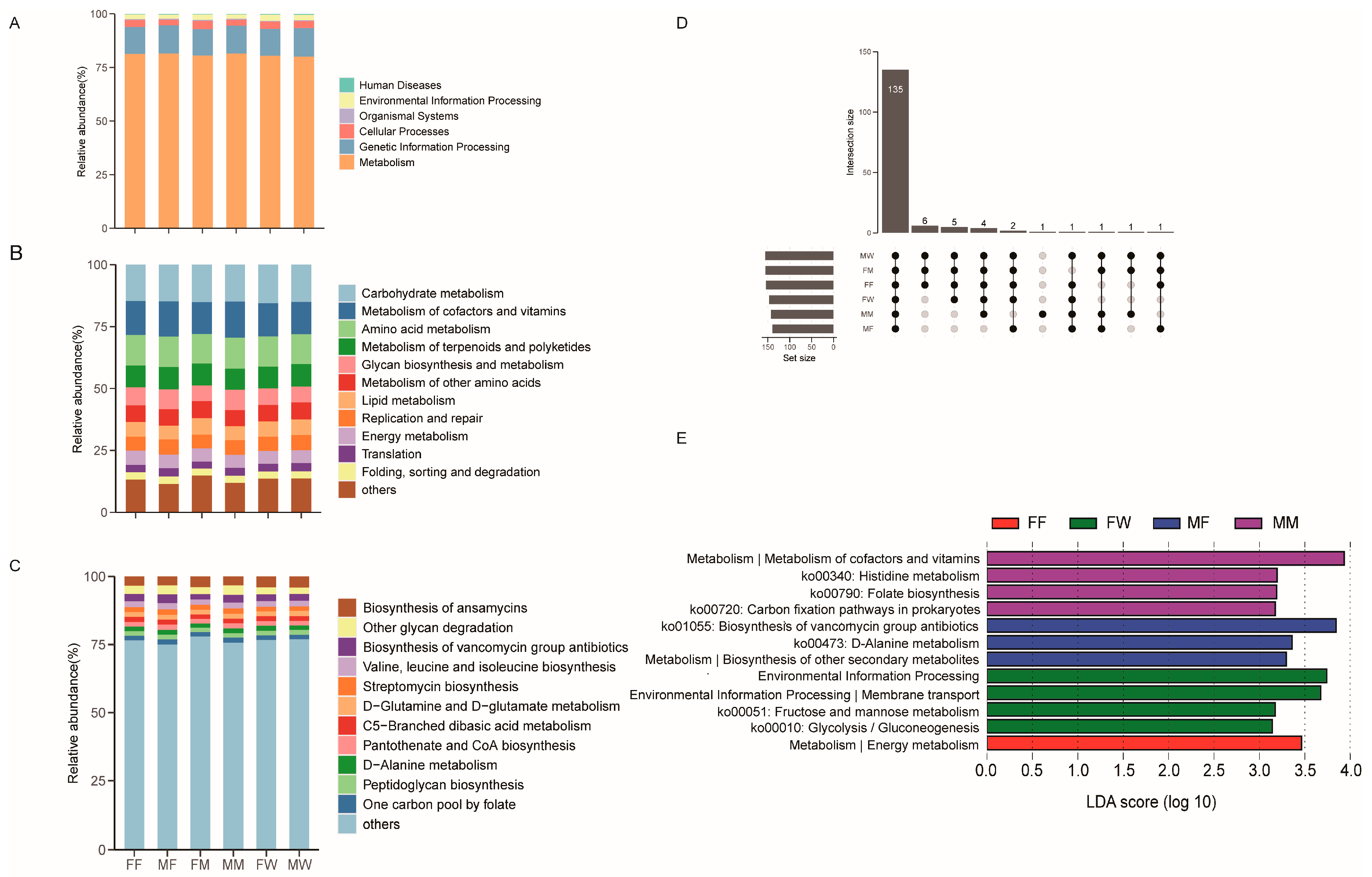
3.2. The Predicted Metagenomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
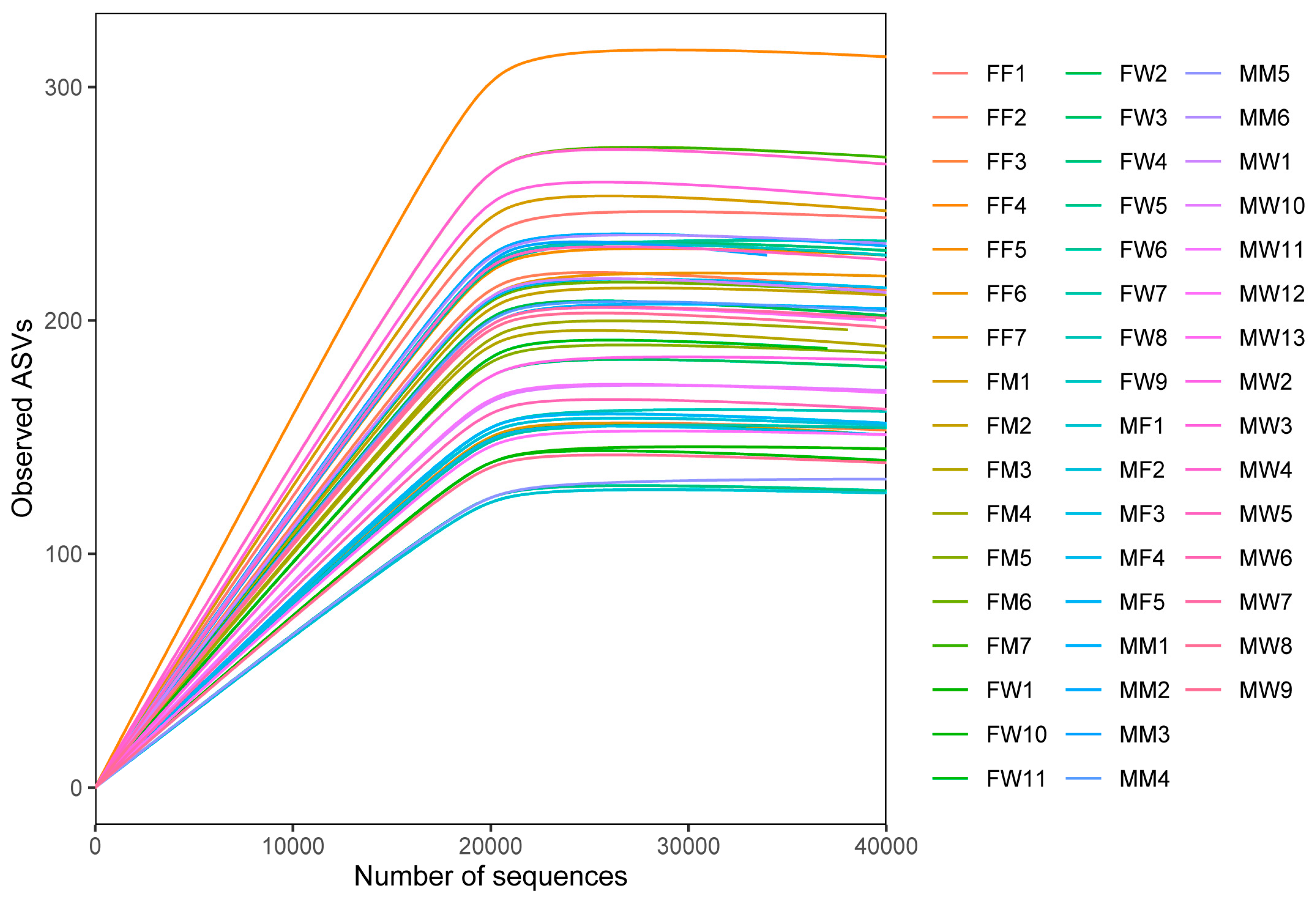
| Sample ID | Group | Raw reads | High-quality reads | Average sequence length | Minimum sequence length | Maximum sequence length | Accession number |
|---|---|---|---|---|---|---|---|
| FF1 | FF | 73684 | 40053 | 406.59 | 258 | 422 | SAMC798099 |
| FF2 | FF | 88949 | 60703 | 406.61 | 257 | 422 | SAMC798104 |
| FF3 | FF | 90123 | 51799 | 408.68 | 395 | 422 | SAMC798108 |
| FF4 | FF | 94885 | 66593 | 407.10 | 395 | 422 | SAMC798112 |
| FF5 | FF | 88354 | 44352 | 406.84 | 259 | 422 | SAMC798130 |
| FF6 | FF | 87440 | 53660 | 407.19 | 260 | 422 | SAMC798131 |
| FF7 | FF | 73619 | 38059 | 411.72 | 395 | 422 | SAMC798132 |
| FM1 | FM | 75766 | 41882 | 405.6 | 258 | 422 | SAMC798096 |
| FM2 | FM | 102760 | 70196 | 408.12 | 395 | 422 | SAMC798098 |
| FM3 | FM | 72769 | 40632 | 409.31 | 395 | 422 | SAMC798101 |
| FM4 | FM | 67705 | 38056 | 408.60 | 260 | 422 | SAMC798103 |
| FM5 | FM | 74409 | 43685 | 403.84 | 261 | 422 | SAMC798105 |
| FM6 | FM | 72310 | 43249 | 409.58 | 395 | 422 | SAMC798107 |
| FM7 | FM | 81193 | 59389 | 407.66 | 259 | 422 | SAMC798110 |
| FW1 | FW | 112652 | 66225 | 406.46 | 259 | 422 | SAMC798095 |
| FW2 | FW | 86098 | 43304 | 409.95 | 395 | 422 | SAMC798097 |
| FW3 | FW | 76989 | 41571 | 411.82 | 395 | 422 | SAMC798100 |
| FW4 | FW | 84867 | 46340 | 404.12 | 261 | 422 | SAMC798102 |
| FW5 | FW | 69268 | 42933 | 409.83 | 259 | 422 | SAMC798106 |
| FW6 | FW | 150444 | 86689 | 401.75 | 260 | 422 | SAMC798109 |
| FW7 | FW | 92941 | 52980 | 408.74 | 395 | 422 | SAMC798111 |
| FW8 | FW | 86275 | 48301 | 409.68 | 395 | 422 | SAMC798137 |
| FW9 | FW | 82425 | 50606 | 407.51 | 395 | 422 | SAMC798138 |
| FW10 | FW | 81409 | 47539 | 411.59 | 260 | 422 | SAMC798139 |
| FW11 | FW | 72672 | 37028 | 408.33 | 257 | 422 | SAMC798140 |
| MF1 | MF | 77935 | 47389 | 408.57 | 395 | 421 | SAMC798115 |
| MF2 | MF | 82673 | 45365 | 410.01 | 307 | 422 | SAMC798122 |
| MF3 | MF | 93453 | 52888 | 406.79 | 257 | 422 | SAMC798123 |
| MF4 | MF | 92104 | 41266 | 401.99 | 259 | 422 | SAMC798124 |
| MF5 | MF | 90387 | 50096 | 411.24 | 395 | 422 | SAMC798129 |
| MM1 | MM | 82136 | 45335 | 408.02 | 395 | 422 | SAMC798114 |
| MM2 | MM | 104528 | 55091 | 410.22 | 395 | 422 | SAMC798117 |
| MM3 | MM | 92997 | 33970 | 408.92 | 258 | 422 | SAMC798119 |
| MM4 | MM | 96831 | 44585 | 411.18 | 395 | 422 | SAMC798121 |
| MM5 | MM | 94575 | 57840 | 407.83 | 395 | 421 | SAMC798126 |
| MM6 | MM | 88806 | 51696 | 408.94 | 395 | 422 | SAMC798128 |
| MW1 | MW | 98013 | 54728 | 407.02 | 395 | 422 | SAMC798113 |
| MW2 | MW | 86628 | 56998 | 403.80 | 283 | 422 | SAMC798116 |
| MW3 | MW | 97957 | 70420 | 405.41 | 260 | 422 | SAMC798118 |
| MW4 | MW | 90098 | 60973 | 406.60 | 260 | 423 | SAMC798120 |
| MW5 | MW | 96383 | 51194 | 405.50 | 258 | 422 | SAMC798125 |
| MW6 | MW | 90030 | 47425 | 413.33 | 395 | 422 | SAMC798127 |
| MW7 | MW | 95586 | 53061 | 408.23 | 258 | 422 | SAMC798133 |
| MW8 | MW | 71594 | 47456 | 408.50 | 395 | 422 | SAMC798134 |
| MW9 | MW | 89601 | 45778 | 406.39 | 258 | 422 | SAMC798135 |
| MW10 | MW | 90782 | 53788 | 406.60 | 257 | 422 | SAMC798136 |
| MW11 | MW | 89660 | 55005 | 407.86 | 260 | 422 | SAMC798141 |
| MW12 | MW | 93227 | 55424 | 406.95 | 281 | 422 | SAMC798142 |
| MW13 | MW | 73681 | 39467 | 410.10 | 395 | 422 | SAMC798143 |
| Sample ID | Group | ASVs | Genus | Family | Class | Order | Phylum |
|---|---|---|---|---|---|---|---|
| FF1 | FF | 211 | 68 | 46 | 14 | 29 | 8 |
| FF2 | FF | 189 | 78 | 52 | 15 | 34 | 10 |
| FF3 | FF | 146 | 43 | 34 | 13 | 25 | 8 |
| FF4 | FF | 197 | 84 | 52 | 15 | 35 | 10 |
| FF5 | FF | 211 | 59 | 35 | 12 | 21 | 9 |
| FF6 | FF | 173 | 61 | 41 | 14 | 29 | 8 |
| FF7 | FF | 132 | 41 | 31 | 12 | 19 | 8 |
| FM1 | FM | 213 | 71 | 43 | 11 | 24 | 7 |
| FM2 | FM | 189 | 75 | 51 | 14 | 32 | 10 |
| FM3 | FM | 179 | 52 | 36 | 12 | 23 | 7 |
| FM4 | FM | 188 | 60 | 40 | 12 | 27 | 8 |
| FM5 | FM | 162 | 49 | 38 | 12 | 23 | 7 |
| FM6 | FM | 178 | 72 | 45 | 12 | 27 | 8 |
| FM7 | FM | 197 | 80 | 49 | 14 | 31 | 10 |
| FW1 | FW | 126 | 55 | 34 | 10 | 21 | 7 |
| FW2 | FW | 179 | 50 | 37 | 12 | 24 | 8 |
| FW3 | FW | 167 | 51 | 37 | 10 | 21 | 7 |
| FW4 | FW | 191 | 59 | 37 | 12 | 25 | 8 |
| FW5 | FW | 115 | 41 | 29 | 10 | 19 | 7 |
| FW6 | FW | 206 | 56 | 38 | 13 | 26 | 8 |
| FW7 | FW | 145 | 42 | 28 | 10 | 19 | 7 |
| FW8 | FW | 147 | 41 | 28 | 10 | 18 | 7 |
| FW9 | FW | 173 | 56 | 40 | 11 | 23 | 6 |
| FW10 | FW | 114 | 35 | 32 | 12 | 22 | 7 |
| FW11 | FW | 171 | 48 | 31 | 12 | 21 | 9 |
| MF1 | MF | 122 | 35 | 30 | 12 | 22 | 8 |
| MF2 | MF | 150 | 43 | 32 | 13 | 21 | 8 |
| MF3 | MF | 142 | 45 | 33 | 12 | 22 | 8 |
| MF4 | MF | 187 | 53 | 34 | 12 | 22 | 7 |
| MF5 | MF | 149 | 43 | 32 | 11 | 19 | 8 |
| MM1 | MM | 197 | 58 | 38 | 11 | 22 | 8 |
| MM2 | MM | 212 | 56 | 37 | 11 | 22 | 6 |
| MM3 | MM | 214 | 60 | 39 | 13 | 25 | 9 |
| MM4 | MM | 186 | 46 | 31 | 13 | 21 | 8 |
| MM5 | MM | 114 | 34 | 33 | 11 | 23 | 6 |
| MM6 | MM | 210 | 58 | 44 | 12 | 27 | 7 |
| MW1 | MW | 204 | 54 | 33 | 13 | 23 | 8 |
| MW2 | MW | 134 | 52 | 40 | 11 | 28 | 7 |
| MW3 | MW | 128 | 59 | 44 | 14 | 28 | 9 |
| MW4 | MW | 183 | 77 | 47 | 13 | 30 | 9 |
| MW5 | MW | 204 | 50 | 33 | 11 | 20 | 7 |
| MW6 | MW | 147 | 36 | 24 | 11 | 16 | 7 |
| MW7 | MW | 190 | 48 | 29 | 12 | 19 | 8 |
| MW8 | MW | 117 | 39 | 28 | 12 | 19 | 8 |
| MW9 | MW | 172 | 53 | 35 | 14 | 23 | 9 |
| MW10 | MW | 161 | 48 | 31 | 12 | 20 | 8 |
| MW11 | MW | 152 | 58 | 40 | 14 | 27 | 9 |
| MW12 | MW | 135 | 44 | 26 | 10 | 18 | 7 |
| MW13 | MW | 173 | 50 | 35 | 12 | 22 | 7 |
| Taxonomy | df | H | p |
|---|---|---|---|
| f__Caulobacteraceae | 5 | 11.76 | 0.04 |
| f__Desulfovibrioria | 5 | 13.85 | 0.02 |
| f__Eggerthellaceae | 5 | 17.08 | 0.004 |
| f__Erysipelatoclostridiaceae | 5 | 11.23 | 0.05 |
| f__Marinifilaceae | 5 | 13.23 | 0.02 |
| f__Tannerellaceae | 5 | 10.51 | 0.06 |
| g__Bacteroides | 5 | 17.77 | 0.003 |
| g__Clostridium_sensu_stricto_1 | 5 | 14.60 | 0.01 |
| g__Desulfovibrio | 5 | 16.18 | 0.006 |
| g__Eggerthella | 5 | 15.56 | 0.008 |
| g__Odoribacter | 5 | 13.62 | 0.02 |
| Level name | df | H | p |
|---|---|---|---|
| Metabolism|Energy metabolism | 5 | 15.66 | 0.008 |
| Environmental Information Processing | 5 | 13.22 | 0.02 |
| Environmental Information Processing|Membrane transport | 5 | 13.54 | 0.02 |
| Metabolism|Biosynthesis of other secondary metabolites | 5 | 12.35 | 0.03 |
| Metabolism|Metabolism of cofactors and vitamins | 5 | 12.03 | 0.03 |
| ko00010 | 5 | 11.47 | 0.04 |
| ko00051 | 5 | 11.26 | 0.05 |
| ko00340 | 5 | 12.82 | 0.02 |
| ko00473 | 5 | 11.90 | 0.04 |
| ko00720 | 5 | 14.23 | 0.01 |
| ko00790 | 5 | 12.88 | 0.02 |
| ko01055 | 5 | 13.40 | 0.02 |
References
- Bruls, T.; Weissenbach, J. The human metagenome: our other genome? Hum. Mol. Genet. 2011, 20, R142–R148. [Google Scholar] [CrossRef]
- Qin, J.-J.; Li, R.-Q.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Macke, E.; Tasiemski, A.; Massol, F.; Callens, M.; Decaestecker, E. Life history and eco-evolutionary dynamics in light of the gut microbiota. Oikos 2017, 126, 508–531. [Google Scholar] [CrossRef]
- Sommer, F.; Bäckhed, F. The gut microbiota — masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J. Y.; Groer, M.; Dutra, S.; Sarkar, A.; McSkimming, D. Gut microbiota and immune system interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Videvall, E.; Song, S.J.; Bensch, H.M.; Strandh, M.; Engelbrecht, A.; Serfontein, N.; Hellgren, O.; Olivier, A.; Cloete, S.; Knight, R.; et al. Major shifts in gut microbiota during development and its relationship to growth in ostriches. Mol. Ecol. 2019, 28, 2653–2667. [Google Scholar] [CrossRef]
- Parashar, A.; Udayabanu, M. Gut microbiota regulates key modulators of social behavior. Eur. Neuropsychopharmacol. 2016, 26, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Kohl, K.D.; Brun, A.; Magallanes, M.; Brinkerhoff, J.; Laspiur, A.; Acosta, J.C.; Caviedes-Vidal, E.; Bordenstein, S.R. Gut microbial ecology of lizards: insights into diversity in the wild, effects of captivity, variation across gut regions and transmission. Mol. Ecol. 2017, 26, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J. A.; Liu, H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef]
- Sun, J.; Kato, I. Gut microbiota, inflammation and colorectal cancer. Genes Dis. 2016, 3, 130–143. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Kartzinel, T.R.; Hsing, J.C.; Musili, P.M.; Brown, B.R.P.; Pringle, R.M. Covariation of diet and gut microbiome in African megafauna. Proc. Natl. Acad. Sci. USA. 2019, 116, 23588–23593. [Google Scholar] [CrossRef]
- Org, E.; Mehrabian, M.; Parks, B. W.; Shipkova, P.; Liu, X.-Q.; Drake, T.A.; Lusis, A.J. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 2016, 7, 313–322. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Godneva, A.; Bar, N.; Kurilshikov, A.; Lotan-Pompan, M.; Weinberger, A.; Fu, J.-Y.; Wijmenga, C.; Zhernakova, A.; et al. Structural variation in the gut microbiome associates with host health. Nature 2019, 568, 43–48. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef]
- Zhang, W.-Y.; Li, N.; Tang, X.-L.; Liu, N.-F.; Zhao, W. Changes in intestinal microbiota across an altitudinal gradient in the lizard Phrynocephalus vlangalii. Ecol. Evol. 2018, 8, 4695–4703. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.-S.; Liang, X.-X.; Yang, M.-Y.; Wang, T.-T.; Chen, J.-P.; Du, W.-G.; Li, H.; Sun, B.-J. Captivity influences gut microbiota in crocodile lizards (Shinisaurus crocodilurus). Front. Microbiol. 2020, 11, 550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, Y.-T.; Dai, Y.-Y.; Jiang, Y.-J.; Lin, L.-H.; Li, H.; Li, P.; Qu, Y.-F.; Ji, X. Captivity affects diversity, abundance and functional pathways of gut microbiota in the northern grass lizard Takydromus septentrionalis. MicrobiologyOpen 2020, 9, e1095. [Google Scholar] [CrossRef]
- Borbón-García, A.; Reyes, A.; Vives-Flórez, M.; Caballero, S. Captivity shapes the gut microbiota of Andean bears: insights into health surveillance. Front. Microbiol. 2017, 8, 1316. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qu, J.-P.; Li, T.-T.; Yao, M.-J.; Li, J.-Y.; Li, X.-Z. Gut microbiota may predict host divergence time during Glires evolution. FEMS Microbiol. Ecol. 2017, 93, fix009. [Google Scholar] [CrossRef]
- Hu, Z.-H.; Chen, X.; Chang, J.; Yu, J.-H.; Tong, Q.; Li, S.-G.; Niu, H.-X. Compositional and predicted functional analysis of the gut microbiota of Radix auricularia (Linnaeus) via high-throughput Illumina sequencing. PeerJ 2018, 6, e5537. [Google Scholar] [CrossRef]
- Chen, L.; Li, S.-X.; Xiao, Q.; Lin, Y.; Li, X.-X.; Qu, Y.-F.; Wu, G.-G.; Li, H. Composition and diversity of gut microbiota in Pomacea canaliculata in sexes and between developmental stages. BMC Microbiol. 2021, 21, 200. [Google Scholar] [CrossRef]
- Colman, D.R.; Toolson, E.C.; Takacs-Vesbach, C.D. Do diet and taxonomy influence insect gut bacterial communities? Mol. Ecol. 2012, 21, 5124–5137. [Google Scholar] [CrossRef]
- Vences, M.; Lyra, M.L.; Kueneman, J.G.; Bletz, M.C.; Archer, H.M.; Canitz, J.; Handreck, S.; Randrianiaina, R.-D.; Struck, U.; Bhuju, S.; et al. Gut bacterial communities across tadpole ecomorphs in two diverse tropical anuran faunas. Sci. Nat. 2016, 103, 25. [Google Scholar] [CrossRef]
- Qu, Y.-F.; Wu, Y.-Q.; Zhao, Y.-T.; Lin, L.-H.; Du, Y.; Li, P.; Li, H.; Ji, X. The invasive red-eared slider turtle is more successful than the native Chinese three-keeled pond turtle: evidence from the gut microbiota. PeerJ 2020, 8, e10271. [Google Scholar] [CrossRef]
- Dewar, M.L.; Arnould, J.P.Y.; Krause, L.; Dann, P.; Smith, S.C. Interspecific variations in the faecal microbiota of Procellariiform seabirds. FEMS Microbiol. Ecol. 2014, 89, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Gibiino, G.; Lopetuso, L.R.; Scaldaferri, F.; Rizzatti, G.; Binda, C.; Gasbarrini, A. Exploring Bacteroidetes: metabolic key points and immunological tricks of our gut commensals. Digest. Liver Dis. 2018, 50, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Smidt, H.; de Vos, W.M.; Belzer, C. The function of our microbiota: who is out there and what do they do? Front. Cell. Infect. Microbiol. 2012, 2, 104. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.; McAllister, T.A. Antimicrobial usage and resistance in beef production. J. Anim. Sci. Biotechnol. 2016, 7, 68. [Google Scholar] [CrossRef]
- Du, Y.; Chen, J.-Q.; Liu, Q.; Fu, J.-C.; Lin, C.-X.; Lin, L.-H.; L, H.; Qu, Y.-F.; Ji, X. Dietary correlates of oral and gut microbiota in the water monitor lizard, Varanus salvator (Laurenti, 1768). Front. Microbiol. 2022, 12, 771527. [Google Scholar] [CrossRef] [PubMed]
- Kohl, K.D.; Amaya, J.; Passement, C.A.; Dearing, M.D.; McCue, M.D. Unique and shared responses of the gut microbiota to prolonged fasting: a comparative study across five classes of vertebrate hosts. FEMS Microbiol. Ecol. 2014, 90, 883–894. [Google Scholar] [CrossRef] [PubMed]
- McCue, M.D.; Passement, C.A.; Meyerholz, D.K. Maintenance of distal intestinal structure in the face of prolonged fasting: a comparative examination of species from five vertebrate classes. Anat. Rec. 2017, 300, 2208–2219. [Google Scholar] [CrossRef]
- Naher, K.; Alam, A.S.; Rahman, S.; Kabir, M.M. Gut contents of common house gecko, Hemidactylus frenatus (Schlegel, 1836) in Jahangirnagar university campus, Savar, Bangladesh. Bangl. J. Zool. 2015, 41, 229–232. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Wang, Y.-J.; Luo, L.-H.; Yang, J.; Yang, L.-F.; Liu, M.; Li, Y.-R.; Qian, T.-M.; Zhng, Y.; et al. Gekko japonicus genome reveals evolution of adhesive toe pads and tail regeneration. Nat. Commun. 2015, 6, 10033. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.-H.; Yang, J.; Wang, J.; Ji, X. Offspring sex in a TSD gecko correlates with an interaction between incubation temperature and yolk steroid hormones. Naturwissenschaften 2012, 99, 999–1006. [Google Scholar] [CrossRef]
- Li, S.-R.; Xu, Z.-W.; Luo, L.-G.; Ping, J.; Zhou, H.-B.; Xie, L.; Zhang, Y.-P. Latitudinal variation in the pattern of temperature-dependent sex determination in the Japanese gecko, Gekko japonicus. Animals 2022, 12, 942. [Google Scholar] [CrossRef]
- Wang, F.-F.; Chen, M.-Y.; Cai, F.-N.; Li, P.; Yan, J.; Zhou, K.-Y. Expression of specific corneous beta proteins in the developing digits of the Japanese gecko (Gekko japonicus) reveals their role in the growth of adhesive setae. Comp. Biochem. Physiol. B. 2020, 240, 110370. [Google Scholar] [CrossRef]
- Kim, D.-I.; Choi, W.-J.; Park, I.-K.; Kim, J.-S.; Kim, I.-H.; Park, D. Comparisons of microhabitat use of Schlegel’s Japanese gecko (Gekko japonicus) among three populations and four land cover types. J. Ecol. Environ. 2018, 42, 24. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J; Arumugan, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- R Development Core Team. R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. 2022. Available at: http://www.R-project.org.
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Ma, J.-E.; Li, J.; Zhang, X.-J.; Li, L.-M.; He, N.; Liu, H.-Y.; Luo, S.-Y.; Wu, Z.-J.; Han, R.-C.; et al. Diets alter the gut microbiome of crocodile lizards. Front. Microbiol. 2017, 8, 2073. [Google Scholar] [CrossRef]
- Zhang, B.; Ren, J.; Yang, D.-D.; Liu, S.-R.; Gong, X.-G. Comparative analysis and characterization of the gut microbiota of four farmed snakes from southern China. PeerJ 2019, 7, e6658. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.-F.; Wu, Y.-Q.; Jiang, Y.-J.; Ji, X. Diet rather than genetic status shapes the gut microbiota in two congeneric snakes. In Review 2022. [CrossRef]
- Campos, P.; Guivernau, M.; Prenafeta-Boldú, F.X.; Cardona, L. Fast acquisition of a polysaccharide fermenting gut microbiome by juvenile green turtles Chelonia mydas after settlement in coastal habitats. Microbiome 2018, 6, 69. [Google Scholar] [CrossRef]
- Keenan, S.W.; Elsey, R.M. The good, the bad, and the unknown: Microbial symbioses of the American alligator. Integr. Comp. Biol. 2015, 55, 972–985. [Google Scholar] [CrossRef]
- Tang, K.-Y.; Wang, Z.-W.; Wan, Q.-H.; Fang, S.-G. Metagenomics reveals seasonal functional adaptation of the gut microbiome to host feeding and fasting in the Chinese alligator. Front. Microbiol. 2019, 10, 2409. [Google Scholar] [CrossRef] [PubMed]
- Gryaznova, M.; Dvoretskaya, Y.; Burakova, I.; Syromyatnikov, M.; Popov, E.; Kokina, A.; Mikhaylov, E.; Popov, V. Dynamics of changes in the gut microbiota of healthy mice fed with lactic acid bacteria and bifidobacteria. Microorganisms 2022, 10, 1020. [Google Scholar] [CrossRef]
- Ulker, İ.; Yildiran, H. The effects of bariatric surgery on gut microbiota in patients with obesity: a review of the literature. Biosci. Microb. Food Health 2019, 38, 3–9. [Google Scholar] [CrossRef]
- Thomas, F.; Hehemann, J.-H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and gut Bacteroidetes: The food connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef] [PubMed]
- Blaut, M. Composition and function of the gut microbiome. In The Gut Microbiome in Health and Disease; Haller, D., Ed.; Springer International Publishing: Cham, 2018; pp. 5–30. [Google Scholar]
- Colston, T.J.; Jackson, C.R. Microbiome evolution along divergent branches of the vertebrate tree of life: what is known and unknown. Mol. Ecol. 2016, 25, 3776–3800. [Google Scholar] [CrossRef] [PubMed]
- Ricaud, K.; Even, M.; Lavigne, F.; Davail, S.; Arroyo, J. Evolution of intestinal microbiota and body compartments during spontaneous hyperphagia in the Greylag goose. Poultry Sci. 2019, 98, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, T.-T.; Beasley, D.E.; Heděnec, P.; Xiao, Z.-S.; Zhang, S.-H.; Li, J.-B.; Lim, Q.; Li, X.-Z. Diet diversity is associated with beta but not alpha diversity of pika gut microbiota. Front. Microbiol. 2016, 7, 1169. [Google Scholar] [CrossRef] [PubMed]
- Bolnick, D.I.; Snowberg, L.K.; Hirsch, P.E.; Lauber, C.L.; Knight, R.; Caporaso, J.G.; Svanbäck, R. Individuals’ diet diversity influences gut microbial diversity in two freshwater fish (threespine stickleback and Eurasian perch). Ecol. Lett. 2014, 17, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Liao, W.-B. Seasonal variation in gut microbiota related to diet in Fejervarya limnocharis. Animals 2021, 11, 1393. [Google Scholar] [CrossRef]
- Jiang, M.; Xu, M.-Y.; Ying, C.-P.; Yin, D.-H.; Dai, P.; Yang, Y.-P.; Ye, K.; Liu, K. The intestinal microbiota of lake anchovy varies according to sex, body size, and local habitat in Taihu Lake, China. MicrobiologyOpen 9, 2020, e00955. [CrossRef]
- Shu, Y.-L.; Hong, P.; Tang, D.; Qing, H.; Donde, O.O.; Wang, H.; Xiao, B.-D.; Wu, H.-L. Comparison of intestinal microbes in female and male Chinese concave-eared frogs (Odorrana tormota) and effect of nematode infection on gut bacterial communities. MicrobiologyOpen 2019, 8, e749. [Google Scholar] [CrossRef]
- Zhu, L.-F.; Clayton, J.B.; Suhr Van Haute, M. J.; Yang, Q.-N.; Hassenstab, H.R.; Mustoe, A.C.; Knights, D.; Benson, A.K.; French, J.A. Sex bias in gut microbiome transmission in newly paired marmosets (Callithrix jacchus). mSystems 2020, 5, e00910-19. [Google Scholar] [CrossRef]
- Méndez-Pérez, R.; García-López, R.; Bautista-López, J.S.B.-L.; Vázquez-Castellanos, J.; Alvarez-González, C.; Peña-Marín, E.; Rodríguez, V.I.D.; Melgar-Valdés, C.; Moya, A.; Alvarez-González, C.A.; et al. High-throughput sequencing of the 16S rRNA gene to analyze the gut microbiome in juvenile and adult tropical gar (Atractosteus tropicus). Lat. Am. J. Aquat. Res. 2020, 48, 456–479. [Google Scholar] [CrossRef]
- Zhu, C.-H.; Xu, W.-J.; Tao, Z.-Y.; Song, W.-T.; Liu, H.-X.; Zhang, S.-J.; Li, H.-F. Effects of rearing conditions and sex on cecal microbiota in ducks. Front. Microbiol. 2020, 11, 565367. [Google Scholar] [CrossRef]
- Pafčo, B.; Sharma, A.K.; Petrželková, K.J.; Vlčková, K.; Todd, A.; Yeoman, C.J.; Wilson, B.A.; Stumpf, R.; White, B.A.; Nelson, K.; et al. Gut microbiome composition of wild western lowland gorillas is associated with individual age and sex factors. Am. J. Phys. Anthropol. 2019, 169, 575–585. [Google Scholar] [CrossRef]
- Matthies, A.; Blaut, M.; Braune, A. Isolation of a human intestinal bacterium capable of daidzein and genistein conversion. Appl. Environ. Microbiol. 2009, 75, 1740–1744. [Google Scholar] [CrossRef]
- Cortés-Lorenzo, C.; Sánchez-Peinado, M. del M.; Oliver-Rodríguez, B.; Vílchez, J.L.; González-López, J.J.; Rodríguez-Díaz, M. Two novel strains within the family Caulobacteraceae capable of degradation of linear alkylbenzene sulfonates as pure cultures. Int. Biodeter. Biodegr. 2013, 85, 62–65. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Kaoutari, A.E.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.-X.; Liu, P.; Song, P.-X.; Chen, X.-Y.; Ma, X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 2017, 7, 43412. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Wang, R.; Ji, G.-C.; Elmassry, M.M.; Zabet-Moghaddam, M.; Vellers, H.; Hamood, A.N.; Gong, X.X.; Mirzaei, P.; Sang, S.-M.; et al. Dietary supplementation of gingerols- and shogaols-enriched ginger root extract attenuate pain-associated behaviors while modulating gut microbiota and metabolites in rats with spinal nerve ligation. J. Nutr. Biochem. 2022, 100, 108904. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, J.-Q.; Liu, Y.; Zhang, J.; Chen, X.-H.; Qu, Y.-F. Comparative study on gut microbiota in three anura frogs from a mountain stream. Ecol. Evol. 2022, 12, e8854. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.-L.; Zhao, X.-Z.; Li, Q.; He, C.; Zhao, W.-J.; Liu, S.-Y.; Ding, J.-M.; Ye, W.-X.; Wang, J.; Chen, Y.; et al. Genome and metagenome analyses reveal adaptive evolution of the host and interaction with the gut microbiota in the goose. Sci. Rep. 2016, 6, 32961. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.; Pillai, V.V.; Goddard, J.M.; Park, H.G.; Kothapalli, K.S.; Ross, D.A.; Ketterings, Q.M.; Brenna, J.T.; Milstein, M.B.; Marquiset, H.; et al. Sustainable production of housefly (Musca domestica) larvae as a protein-rich feed ingredient by utilizing cattle manure. PLoS ONE 2017, 12, e0171708. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, L.; Coretti, L.; Dipineto, L.; Bovera, F.; Menna, F.; Chiariotti, L.; Nizza, A.; Lembo, F.; Fioretti, A. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 2017, 7, 16269. [Google Scholar] [CrossRef]
- Waśko, A.; Bulak, P.; Polak-Berecka, M.; Nowak, K.; Polakowski, C.; Bieganowski, C. The first report of the physicochemical structure of chitin isolated from Hermetia illucens. Int. J. Biol. Macromol. 2016, 92, 316–320. [Google Scholar] [CrossRef]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of edible cricket consumption on gut microbiota in healthy adults, a double-blind, randomized crossover trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef]
| Abundance of prey items | Order |
| Numerous (> 500) | Lepidoptera, Diptera |
| More (between 100 and 500) | Coleoptera, Hemiptera |
| Medium (between 50 and 100) | Hymenoptera, Ephemeroptera, Trichoptera |
| Fewer (between 10 and 50) | Orthoptera, Mantodea, Neuroptera, Megaloptera, Thysanoptera, Plecoptera, Blattodea |
| Least (< 10) | Dermaptera, Odonata, Corrodentia, Rhaphidioptera |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





