Submitted:
28 February 2023
Posted:
03 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
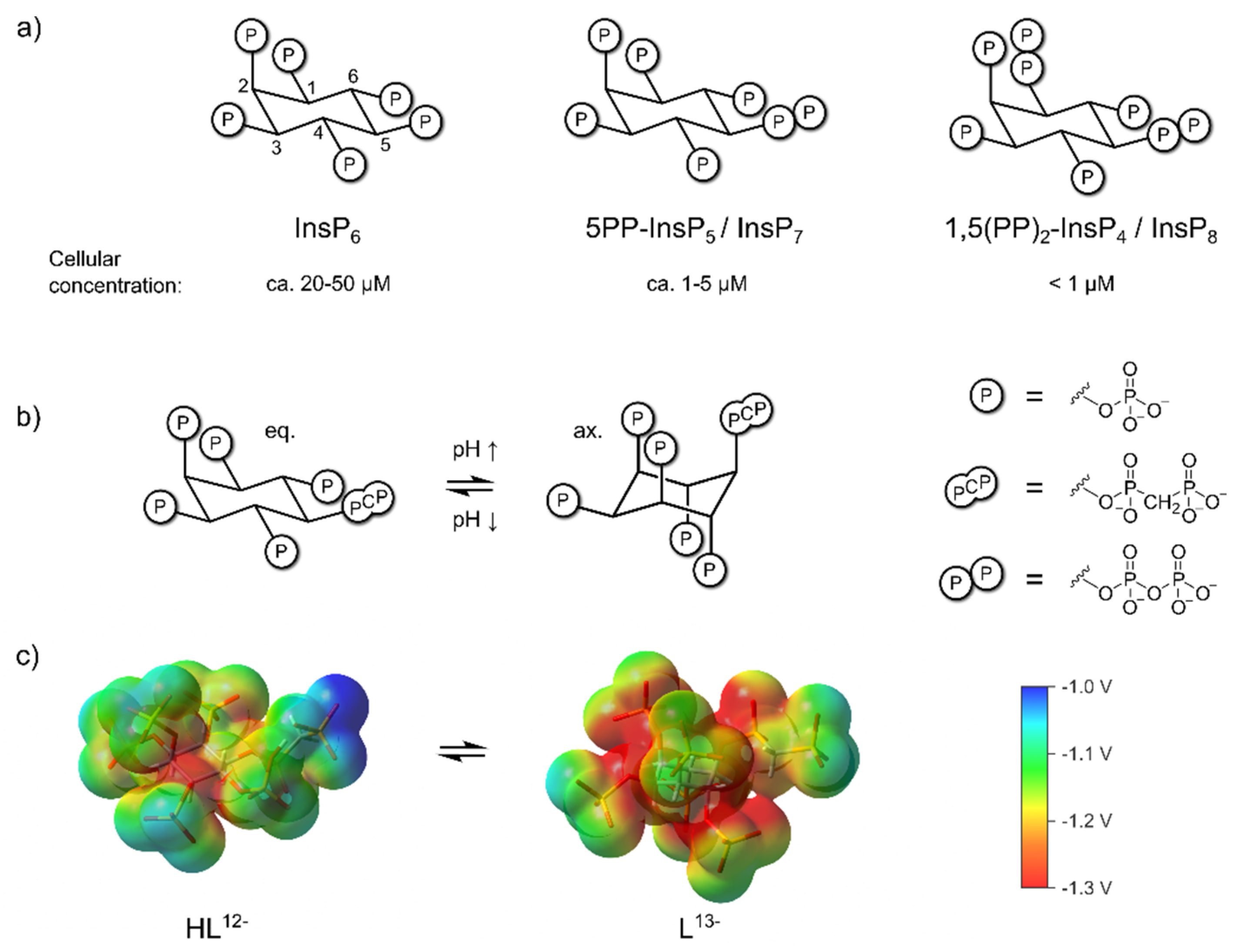
2. Materials and Methods
Synthesis and BIRD-HMQC NMR analysis of InsPs
Assignment of HMQC-NMR spectra of axial conformations using NOESY-HMQC NMR
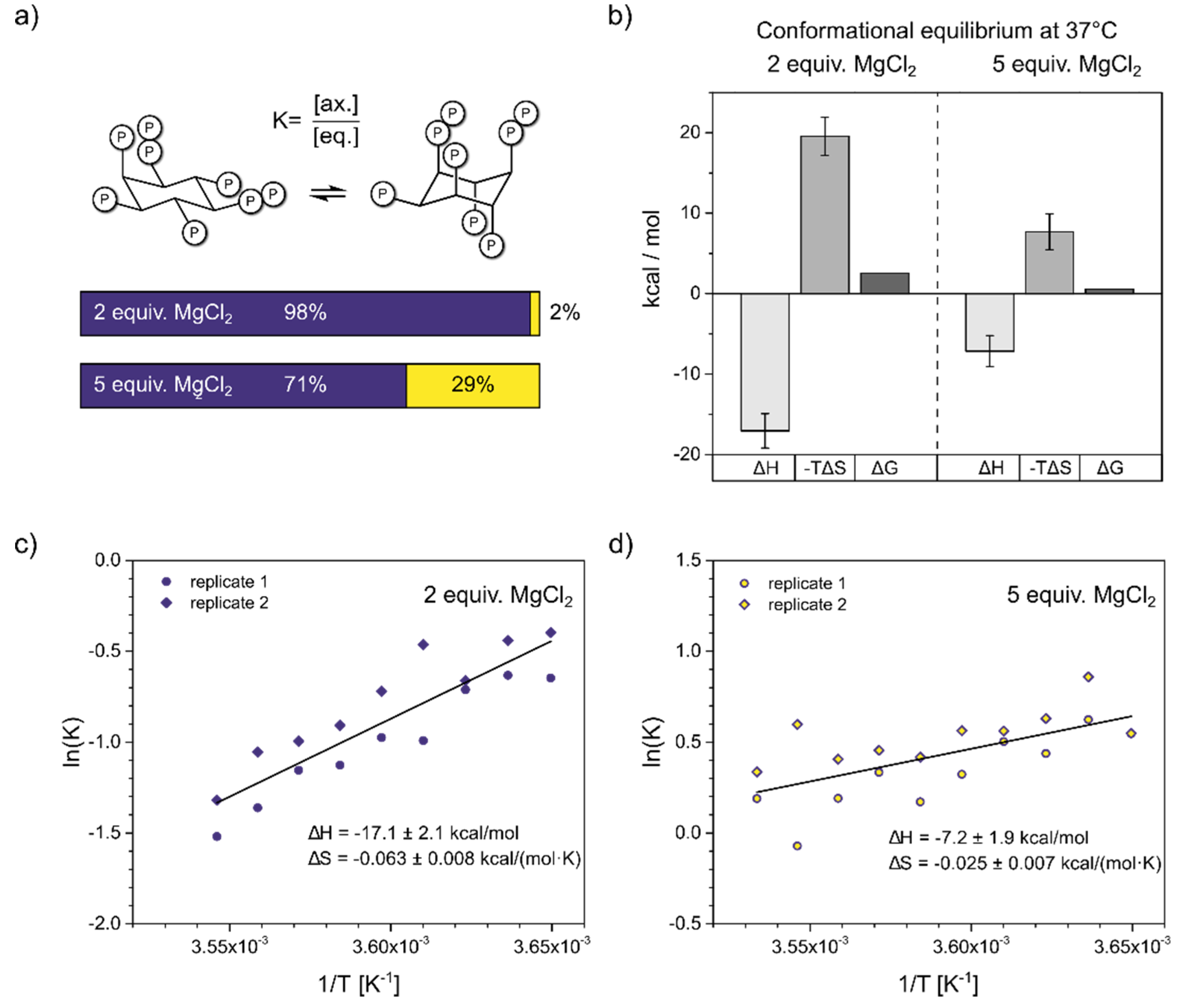
Van’t Hoff thermodynamic analysis of conformational equilibrium:
NMR titrations
Isothermal titration calorimetry
DFT calculations
3. Results
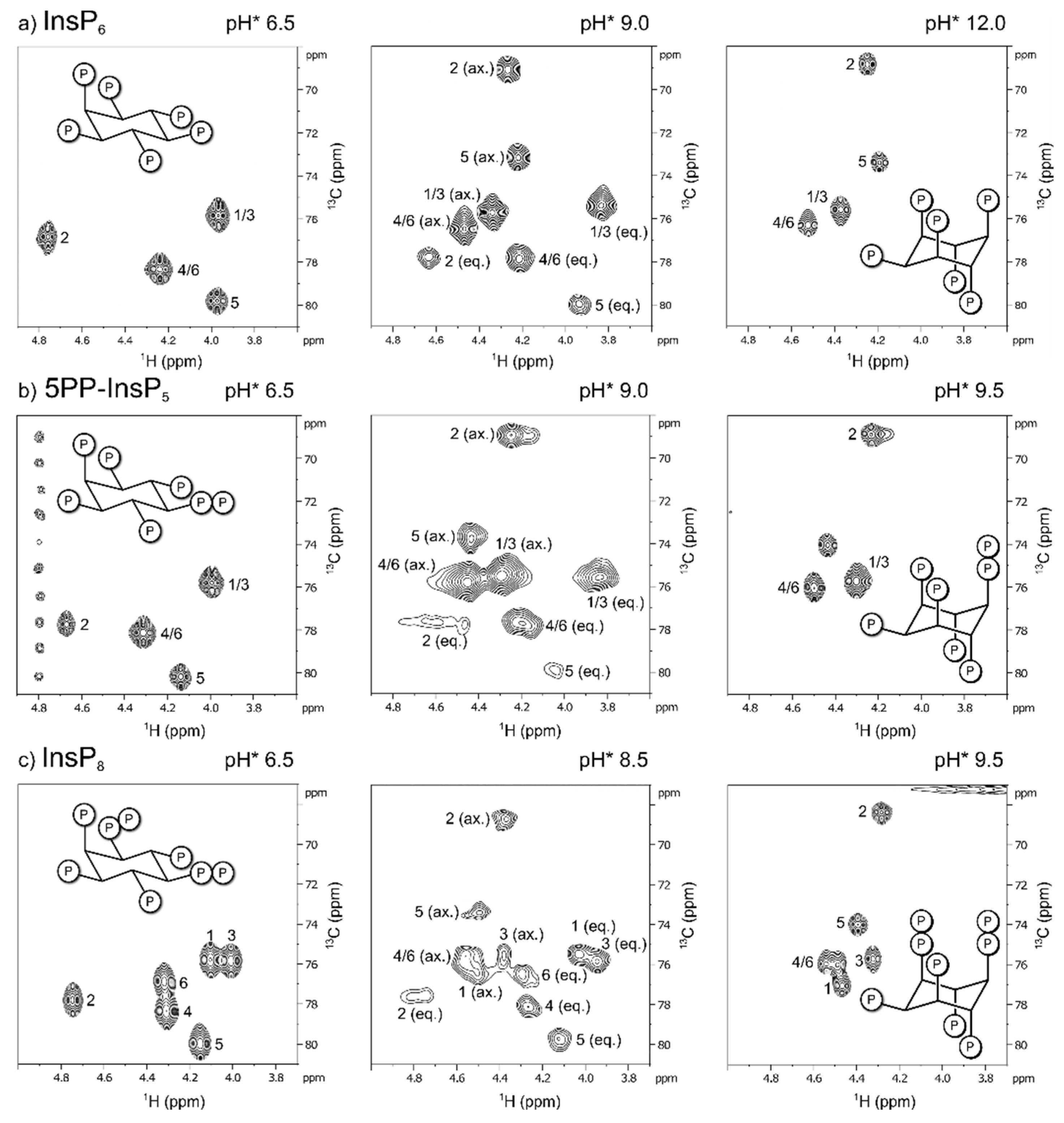
3.1. 1H,13C-HMQC NMR spectra of PP-InsPs show two distinct conformations simultaneously
3.2. Conformational equilibria of InsP6 and PP-InsPs are sensitive to pH and ionic composition
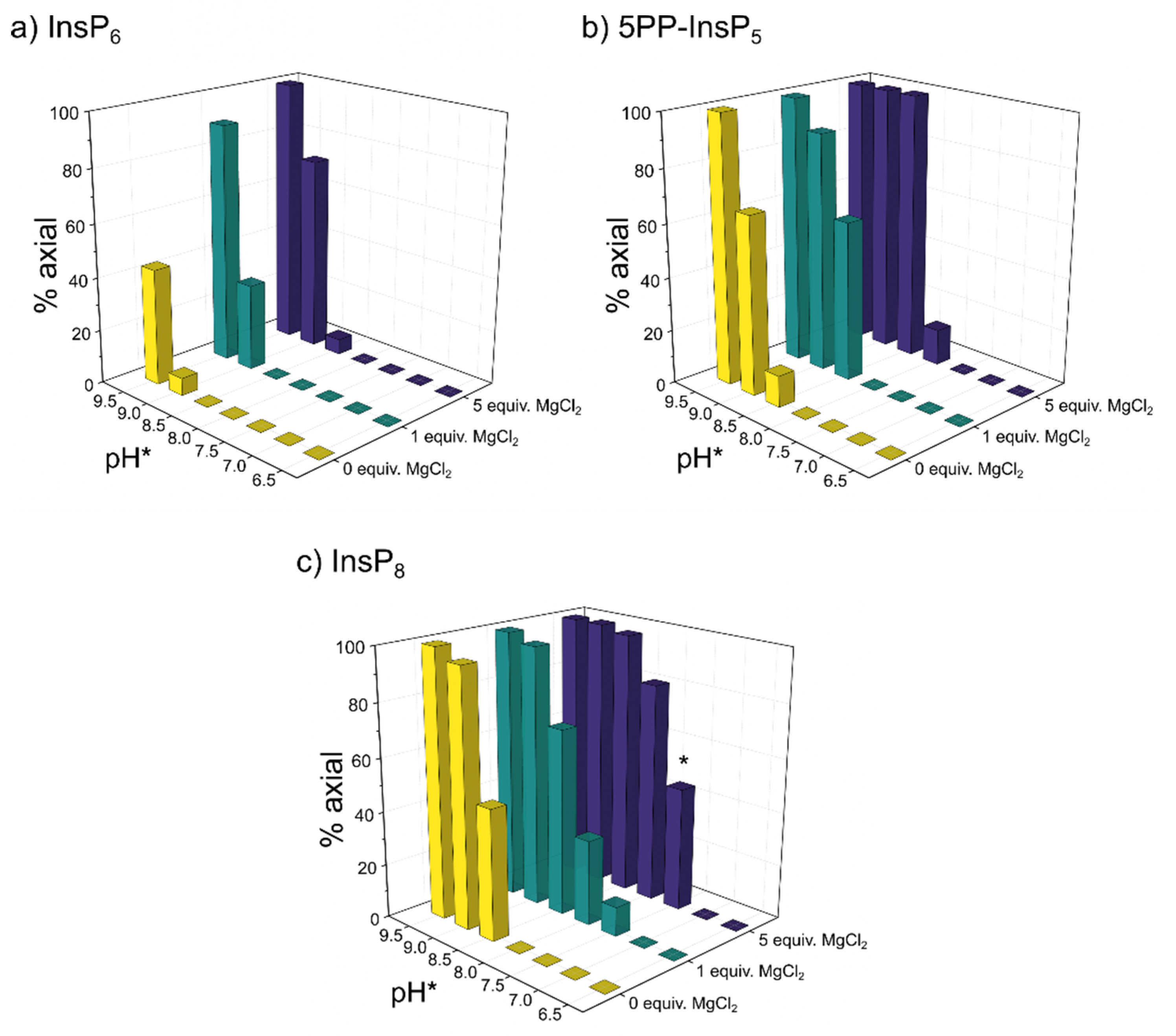
3.3. InsP8 is present in both conformations under near-physiological conditions
3.3. InsP8 forms strong complexes with potassium and magnesium ions
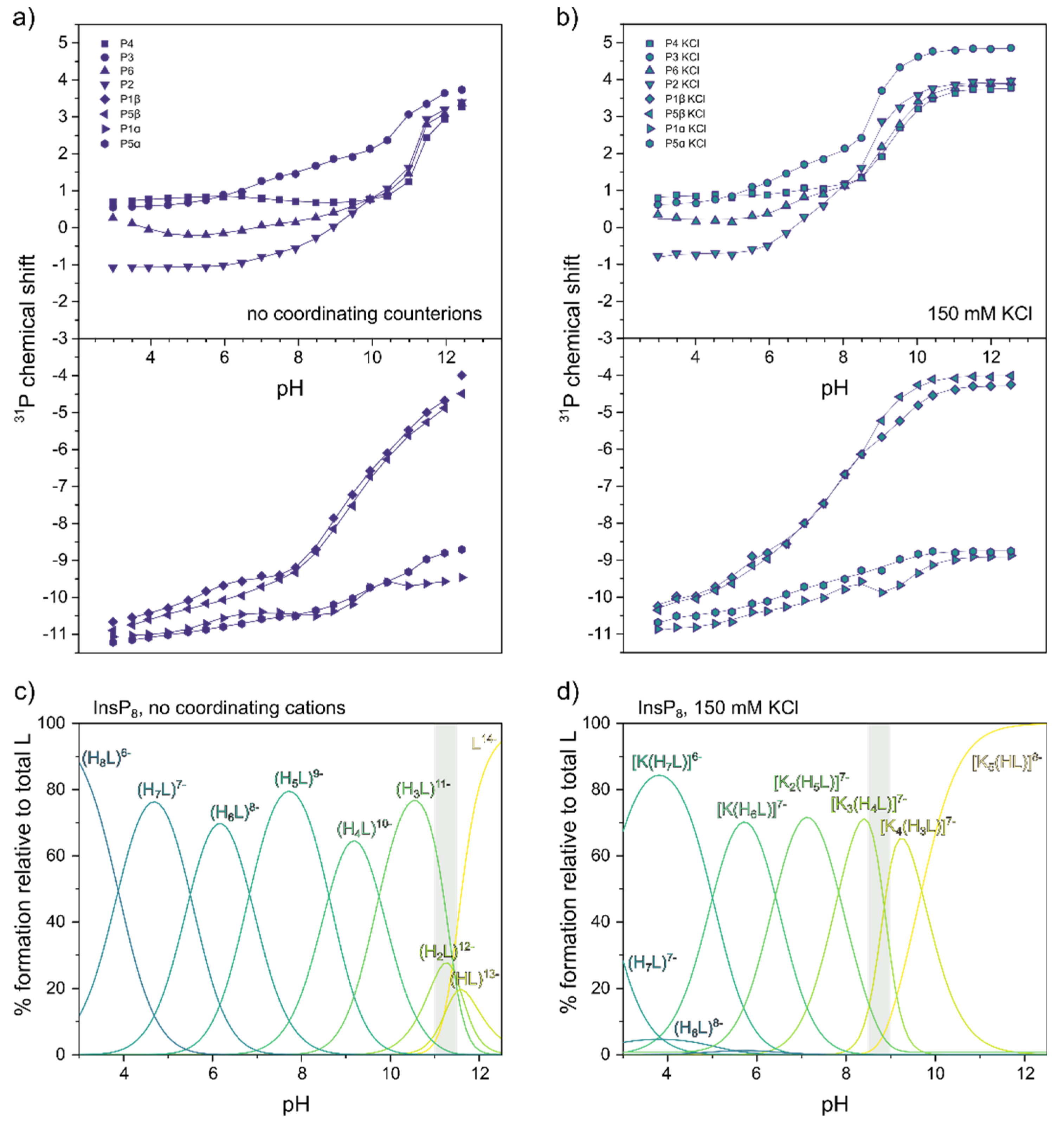
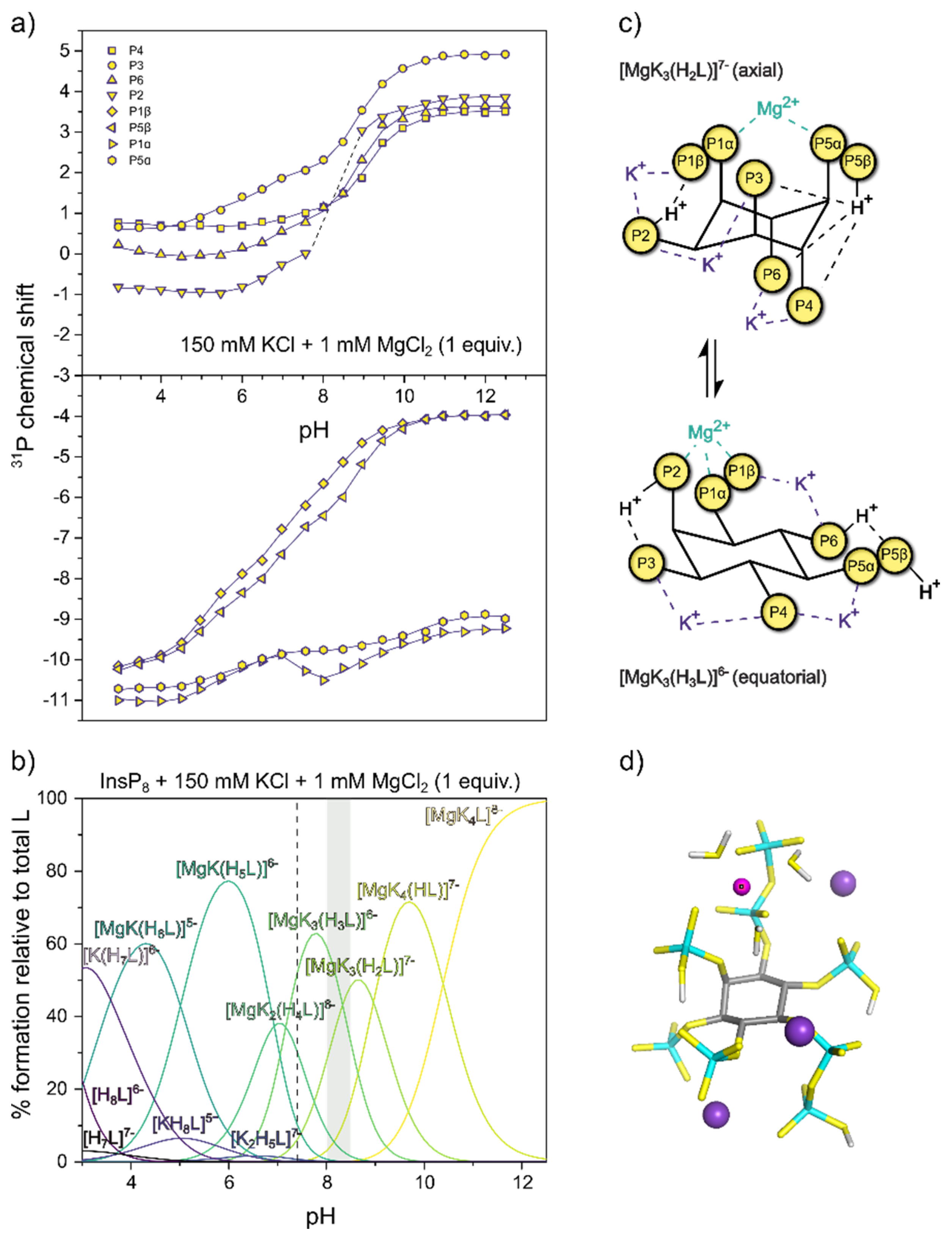
| Equilibrium | log K | ||
|---|---|---|---|
| InsP8 | 5PCP‒InsP5 | InsP6 | |
| 31P NMR (22 °C)a | 31P NMR (22 °C)b | Potentiometry (37 °C)b | |
| L14‒ + H+ ↔ HL13‒ | 11.21(1) | 11.48(1) | 10.8(1) |
| L14‒ + 2 H+ ↔ H2L12‒ | 22.78(2) | 22.42(2) | 21.3(1) |
| L14‒ + 3 H+ ↔ H3L11‒ | 34.22(2) | 32.26(2) | 31.63(6) |
| L14‒ + 4 H+ ↔ H4L10‒ | 43.96(2) | 40.94(2) | 40.42(6) |
| L14‒ + 5 H+ ↔ H5L9‒ | 52.58(3) | 47.67(2) | 47.32(6) |
| L14‒ + 6 H+ ↔ H6L8‒ | 59.41(8) | 51.93(2) | 53.04(7) |
| L14‒ + 7 H+ ↔ H7L7‒ | 64.91(6) | 55.64(2) | 56.14(9) |
| L14‒ + 8 H+ ↔ H8L6‒ | 68.79(7) | ‒‒‒ | ‒‒‒ |
| 5 K+ + HL13‒ ↔ [K5(HL)]8‒ | 12.47(3) | 6.57(3) | ‒‒‒ |
| 4 K+ + H2L12‒ ↔ [K4(H2L)]8‒ | 9.76(3) | 4.61(3) | ‒‒‒ |
| 4 K+ + H3L11‒ ↔ [K4(H3L)]7‒ | ‒‒‒ | 4.50(5) | 5.42(5) |
| 3 K+ + H4L10‒ ↔ [K3(H4L)]7‒ | 5.446(5) | 3.94(4) | 3.36(5) |
| 2 K+ + H5L9‒ ↔ [K2(H5L)]7‒ | 3.820(7) | 2.79(7) | ‒‒‒ |
| K+ + H6L8‒ ↔ [K(H6L)]7‒ | 2.58(5) | ‒‒‒ | ‒‒‒ |
| K+ + H7L7‒ ↔ [K(H7L)]6‒ | 2.08(3) | ‒‒‒ | ‒‒‒ |
| Mg2+ + L14‒ + 4 K+ ↔ [MgK4L]8‒ | 22.4(1) | ‒‒‒ | ‒‒‒ |
| Mg2+ + HL13‒ + 4 K+ ↔ [MgK4(HL)]7‒ | 21.6(1) | 11.64(5) | ‒‒‒ |
| Mg2+ + H2L12‒ + 3 K+ ↔ [MgK3(H2L)]7‒ | 18.1(1) | 9.75(7) | ‒‒‒ |
| Mg2+ + H3L11‒ + 3 K+ ↔ [MgK3(H3L)]6‒ | 15.0(1) | 8.79(7) | ‒‒‒ |
| Mg2+ + H4L10‒ + 2 K+ ↔ [MgK2(H4L)]6‒ | 11.6(1) | 6.96(7) | ‒‒‒ |
| Mg2+ + H5L9‒ + K+ ↔ [MgK(H5L)]6‒ | 9.1(1) | 5.26(7) | ‒‒‒ |
| Mg2+ + H6L8‒ + K+ ↔ [MgK(H6L)]5‒ | 7.3(1) | ‒‒‒ | ‒‒‒ |
| Mg2+ + H6L8‒ ↔ [Mg(H6L)]6‒ | ‒‒‒ | 4.71(7) | ‒‒‒ |
3.4. Complex speciation of InsP8 affects protein binding
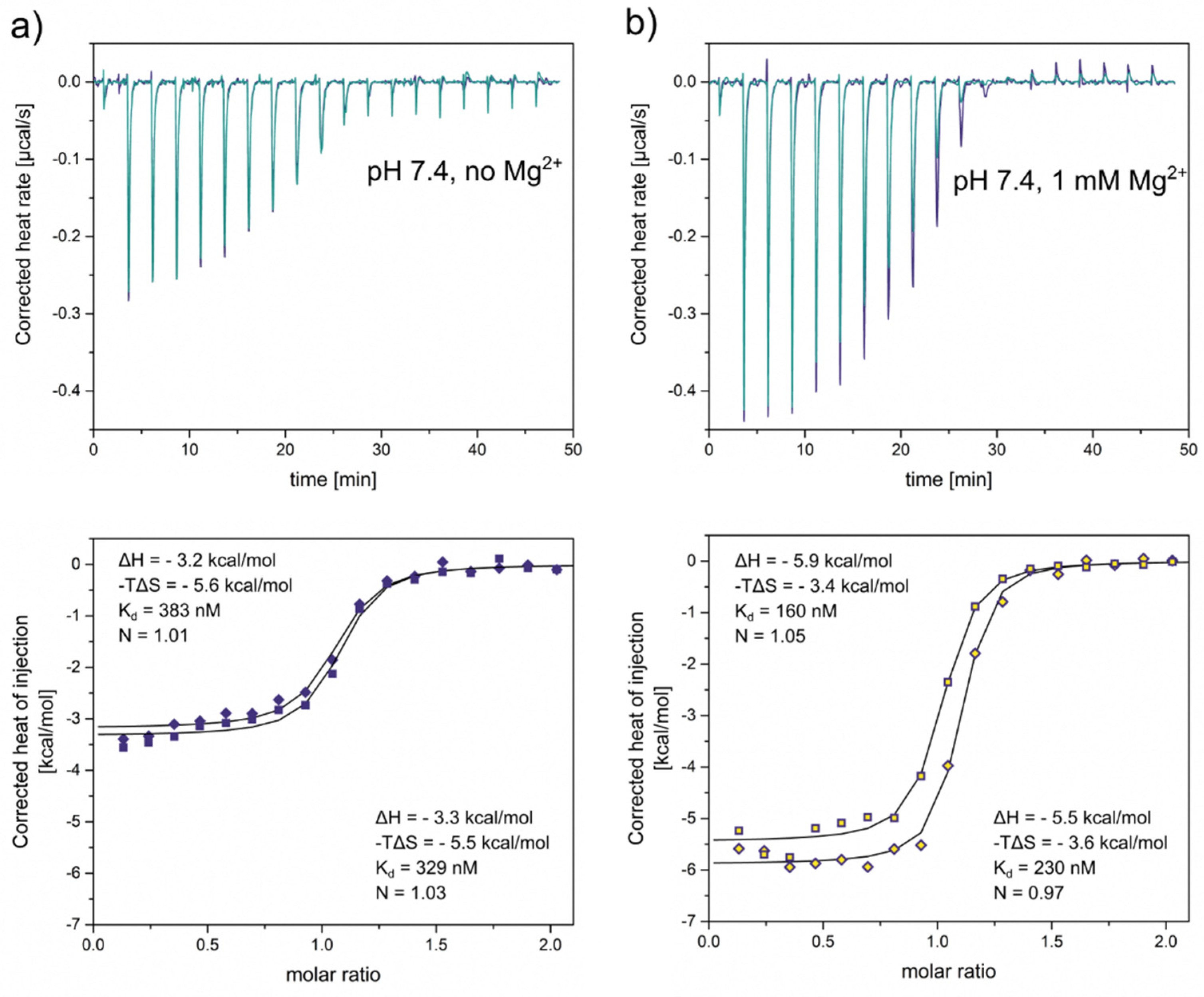
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatree, S.; Thongmaen, N.; Tantivejkul, K.; Sitticharoon, C.; Vucenik, I. Role of Inositols and Inositol Phosphates in Energy Metabolism. Molecules 2020, 25, 1–18. [CrossRef]
- Benjamin, B.; Garg, A.; Jork, N.; Jessen, H.J.; Schwer, B. Activities and Structure-Function Analysis of Fission Yeast Inositol Pyrophosphate (IPP) Kinase-Pyrophosphatase Asp1 and Its Impact on Regulation of Pho1 Gene Expression. Epub 2022, 13. [CrossRef]
- Pascual-Ortiz, M.; Walla, E.; Fleig, U.; Saiardi, A. The PPIP5K Family Member Asp1 Controls Inorganic Polyphosphate Metabolism in S. Pombe. J. Fungi 2021, 7, 1–12. [CrossRef]
- Zhu, J.; Lau, K.; Puschmann, R.; Harmel, R.K.; Zhang, Y.; Pries, V.; Gaugler, P.; Broger, L.; Dutta, A.K.; Jessen, H.J.; et al. Two Bifunctional Inositol Pyrophosphate Kinases/Phosphatases Control Plant Phosphate Homeostasis. Elife 2019, 8, 1–25. [CrossRef]
- Dong, J.; Ma, G.; Sui, L.; Wei, M.; Satheesh, V.; Zhang, R.; Ge, S.; Li, J.; Zhang, T.E.; Wittwer, C.; et al. Inositol Pyrophosphate InsP8 Acts as an Intracellular Phosphate Signal in Arabidopsis. Mol. Plant 2019, 12, 1463–1473. [CrossRef]
- Li, X.; Gu, C.; Hostachy, S.; Sahu, S.; Wittwer, C.; Jessen, H.J.; Fiedler, D.; Wang, H.; Shears, S.B. Control of XPR1-Dependent Cellular Phosphate Efflux by InsP8 Is an Exemplar for Functionally-Exclusive Inositol Pyrophosphate Signaling. Proc. Natl. Acad. Sci. 2020, 117, 3568 LP – 3574. [CrossRef]
- Wilson, M.S.; Jessen, H.J.; Saiardi, A. The Inositol Hexakisphosphate Kinases IP6K1 and -2 Regulate Human Cellular Phosphate Homeostasis, Including XPR1-Mediated Phosphate Export. J. Biol. Chem. 2019, 294, 11597–11608. [CrossRef]
- Legati, A.; Giovannini, D.; Nicolas, G.; López-Sánchez, U.; Quintáns, B.; Oliveira, J.R.M.; Sears, R.L.; Ramos, E.M.; Spiteri, E.; Sobrido, M.-J.; et al. Mutations in XPR1 Cause Primary Familial Brain Calcification Associated with Altered Phosphate Export. Nat. Genet. 2015, 47, 579–581. [CrossRef]
- Yousaf, R.; Gu, C.; Ahmed, Z.M.; Khan, S.N.; Friedman, T.B.; Riazuddin, S.; Shears, S.B.; Riazuddin, S. Mutations in Diphosphoinositol-Pentakisphosphate Kinase PPIP5K2 Are Associated with Hearing Loss in Human and Mouse. PLoS Genet. 2018, 14, 1–20. [CrossRef]
- Khaled, M.L.; Bykhovskaya, Y.; Gu, C.; Liu, A.; Drewry, M.D.; Chen, Z.; Mysona, B.A.; Parker, E.; McNabb, R.P.; Yu, H.; et al. PPIP5K2 and PCSK1 Are Candidate Genetic Contributors to Familial Keratoconus. Sci. Rep. 2019, 9, 1–16. [CrossRef]
- Szijgyarto, Z.; Garedew, A.; Azevedo, C.; Saiardi, A. Influence of Inositol Pyrophosphates on Cellular Energy Dynamics. Science 2011, 334, 802–805. [CrossRef]
- Qin, N.; Li, L.; Ji, X.; Pereira, R.; Chen, Y.; Yin, S.; Li, C.; Wan, X.; Luo, H.; Zhang, Y.; et al. Flux Regulation Through Glycolysis and Respiration Is Balanced by Inositol Pyrophosphates. SSRN Electron. J. 2022, 1–16. [CrossRef]
- Socie, G.; Rossi, D.J.; Bryder, D.; Weissman, I.L.; Steen, R.; Veiby, O.P.; Friedrich, W.; Egeland, T.; Kohn, T.; Schulz, a S.; et al. Requirement of Inositol Pyrophosphates for Full Exocytotic Capacity in Pancreatic b Cells. Science 2007, 318, 1299–1302. [CrossRef]
- Zhang, X.; Li, N.; Zhang, J.; Zhang, Y.; Yang, X.; Luo, Y.; Zhang, B.; Xu, Z.; Zhu, Z.; Yang, X.; et al. 5-IP7 Is a GPCR Messenger Mediating Neural Control of Synaptotagmin-Dependent Insulin Exocytosis and Glucose Homeostasis. Nat. Metab. 2021. [CrossRef]
- Chakraborty, A.; Koldobskiy, M.A.; Bello, N.T.; Maxwell, M.; Potter, J.J.; Juluri, K.R.; Maag, D.; Kim, S.; Huang, A.S.; Dailey, M.J.; et al. Inositol Pyrophosphates Inhibit Akt Signaling, Thereby Regulating Insulin Sensitivity and Weight Gain. Cell 2010, 143, 897–910. [CrossRef]
- Pavlovic, I.; Thakor, D.T.; Vargas, J.R.; McKinlay, C.J.; Hauke, S.; Anstaett, P.; Camunã, R.C.; Bigler, L.; Gasser, G.; Schultz, C.; et al. Cellular Delivery and Photochemical Release of a Caged Inositol-Pyrophosphate Induces PH-Domain Translocation in Cellulo. Nat. Commun. 2016, 7, 1–8. [CrossRef]
- Zhang, Z.; Zhao, C.; Liu, B.; Liang, D.; Qin, X.; Li, X.; Zhang, R.; Li, C.; Wang, H.; Sun, D.; et al. Inositol Pyrophosphates Mediate the Effects of Aging on Bone Marrow Mesenchymal Stem Cells by Inhibiting Akt Signaling. Stem Cell Res. Ther. 2014, 5, 1–12. [CrossRef]
- Zhu, Q.; Ghoshal, S.; Rodrigues, A.; Gao, S.; Asterian, A.; Kamenecka, T.M.; Barrow, J.C.; Chakraborty, A. Adipocyte-Specific Deletion of Ip6k1 Reduces Diet-Induced Obesity by Enhancing AMPK-Mediated Thermogenesis. J. Clin. Invest. 2016, 126, 4273–4288. [CrossRef]
- Moritoh, Y.; Abe, S. ichi; Akiyama, H.; Kobayashi, A.; Koyama, R.; Hara, R.; Kasai, S.; Watanabe, M. The Enzymatic Activity of Inositol Hexakisphosphate Kinase Controls Circulating Phosphate in Mammals. Nat. Commun. 2021, 12. [CrossRef]
- Gerasimaite, R.; Pavlovic, I.; Capolicchio, S.; Hofer, A.; Schmidt, A.; Jessen, H.J.; Mayer, A. Inositol Pyrophosphate Specificity of the SPX-Dependent Polyphosphate Polymerase VTC. ACS Chem. Biol. 2017, 12, 648–653. [CrossRef]
- Veiga, N.; Torres, J.; Cerdá, F.; González, G.; Gómez, K.; Mansell, D.; Freeman, S.; Domínguez, S.; Kremer, C. Redox and Structural Aspects on Iron Inositol 1,2,3-Trisphosphate Interaction: An Experimental and Computational Approach. J. Mol. Struct. 2011, 994, 343–349. [CrossRef]
- Veiga, N.; Torres, J.; MacHo, I.; Gómez, K.; González, G.; Kremer, C. Coordination, Microprotonation Equilibria and Conformational Changes of Myo-Inositol Hexakisphosphate with Pertinence to Its Biological Function. Dalt. Trans. 2014, 43, 16238–16251. [CrossRef]
- Hager, A.; Wu, M.; Wang, H.; Brown, N.W.J.; Shears, S.B.; Veiga, N.; Fiedler, D. Cellular Cations Control Conformational Switching of Inositol Pyrophosphate Analogues. Chemistry 2016, 22, 12406–12414. [CrossRef]
- Barker, C.J.; Wright, J.; Hughes, P.J.; Kirk, C.J.; Michell, R.H. Complex Changes in Cellular Inositol Phosphate Complement Accompany Transit through the Cell Cycle. Biochem. J. 2004, 380, 465–473. [CrossRef]
- Harmel, R.K.; Puschmann, R.; Nguyen Trung, M.; Saiardi, A.; Schmieder, P.; Fiedler, D. Harnessing (13)C-Labeled Myo-Inositol to Interrogate Inositol Phosphate Messengers by NMR. Chem. Sci. 2019, 10, 5267–5274. [CrossRef]
- Qiu, D.; Wilson, M.S.; Eisenbeis, V.B.; Harmel, R.K.; Riemer, E.; Haas, T.M.; Wittwer, C.; Jork, N.; Gu, C.; Shears, S.B.; et al. Analysis of Inositol Phosphate Metabolism by Capillary Electrophoresis Electrospray Ionization Mass Spectrometry. Nat. Commun. 2020, 1–12. [CrossRef]
- Gu, C.; Wilson, M.S.C.; Jessen, H.J.; Saiardi, A.; Shears, S.B. Inositol Pyrophosphate Profiling of Two HCT116 Cell Lines Uncovers Variation in InsP8 Levels. PLoS One 2016, 11, 1–16. [CrossRef]
- Puschmann, R.; Harmel, R.K.; Fiedler, D. Scalable Chemoenzymatic Synthesis of Inositol Pyrophosphates. Biochemistry 2019, 58, 3927–3932. [CrossRef]
- Findeisen, M.; Berger, T.B. and S. A 1H-NMR Thermometer Suitable for Cryoprobes.Pdf. Magn. Reson. Chem. 2007, 45, 175–178. [CrossRef]
- Krȩzel, A.; Bal, W. A Formula for Correlating PKa Values Determined in D 2O and H2O. J. Inorg. Biochem. 2004, 98, 161–166. [CrossRef]
- Frassineti, Chiara; Ghelli, S. NMR as a Tool for Determining Protonation Constants of Nutral Polyprotic Bases in Solution. Anal. Biochem. 1995, 231, 374–382. [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of Equilibria in Solution. Determination of Equilibrium Constants with the HYPERQUAD Suite of Programs. Talanta 1996, 43, 1739–1753. [CrossRef]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad Simulation and Speciation (HySS): A Utility Program for the Investigation of Equilibria Involving Soluble and Partially Soluble Species. Coord. Chem. Rev. 1999, 184, 311–318. [CrossRef]
- Couto, D.; Richter, A.; Walter, H.; Furkert, D.; Hothorn, M.; Fiedler, D. Using Biotinylated Myo -Inositol Hexakisphosphate to Investigate Inositol Pyrophosphate − Protein Interactions with Surface-Based Biosensors. 2021. [CrossRef]
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ort, and D.J.F. Gaussian 09 2016, Gaussian 09, Revision A.02, Gaussian, Inc., Wallin.
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for K to Au Including the Outermost Core Orbitale. J. Chem. Phys. 1985, 82, 299–310. [CrossRef]
- Marenich, A. V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [CrossRef]
- Frost, E.B. Conformational States of Myo-Inositol Hexakisphosphate in Aqueous Solution. A 13C NMR, 31P NMR, and Raman Spectroscopic Investigation. Science 1979, 47, 416–417. [CrossRef]
- Barrientos, L.G.; Murthy, P.P.N. Conformational Studies of Myo-Inositol Phosphates. Carbohydr. Res. 1996, 296, 39–54. [CrossRef]
- Veiga, N.; Torres, J.; Macho, I.; Gómez, K.; Godage, H.Y.; Riley, A.M.; Potter, B.V.L.; González, G.; Kremer, C. Inframolecular Acid–Base and Coordination Properties towards Na+ and Mg2+ of Myo-Inositol 1,3,4,5,6-Pentakisphosphate: A Structural Approach to Biologically Relevant Species. J. Chem. Soc. Dalt. Trans. 2013, 42, 6021–6032. [CrossRef]
- Torres, J.; Domínguez, S.; Cerdá, M.F.; Obal, G.; Mederos, A.; Irvine, R.F.; Díaz, A.; Kremer, C. Solution Behaviour of Myo-Inositol Hexakisphosphate in the Presence of Multivalent Cations. Prediction of a Neutral Pentamagnesium Species under Cytosolic/Nuclear Conditions. J. Inorg. Biochem. 2005, 99, 828–840. [CrossRef]
- Torres, J.; Veiga, N.; Gancheff, J.S.; Domínguez, S.; Mederos, A.; Sundberg, M.; Sánchez, A.; Castiglioni, J.; Díaz, A.; Kremer, C. Interaction of Myo-Inositol Hexakisphosphate with Alkali and Alkaline Earth Metal Ions: Spectroscopic, Potentiometric and Theoretical Studies. J. Mol. Struct. 2008, 874, 77–88. [CrossRef]
- Wild, R.; Gerasimaite, R.; Jung, J.-Y.; Truffault, V.; Pavlovic, I.; Schmidt, A.; Saiardi, A.; Jessen, H.J.; Poirier, Y.; Hothorn, M.; et al. Control of Eukaryotic Phosphate Homeostasis by Inositol Polyphosphate Sensor Domains. Science 2016, 352, 986 LP – 990. [CrossRef]
- Reynolds, C.H.; Holloway, M.K. Thermodynamics of Ligand Binding and Efficiency. ACS Med. Chem. Lett. 2011, 2, 433–437. [CrossRef]
- Šala, M.; Makuc, D.; Kolar, J.; Plavec, J.; Pihlar, B. Potentiometric and 31P NMR Studies on Inositol Phosphates and Their Interaction with Iron(III) Ions. Carbohydr. Res. 2011, 346, 488–494. [CrossRef]
- Veiga, N.; Macho, I.; Gómez, K.; González, G.; Kremer, C.; Torres, J. Potentiometric and Spectroscopic Study of the Interaction of 3d Transition Metal Ions with Inositol Hexakisphosphate. J. Mol. Struct. 2015, 1098, 55–65. [CrossRef]
- Asensio G., Hernández-Arriaga A. M., Martín del Campo M., Prieto A. M., Rojo L., Vázquez-Lasa B. A Study on Sr/Zn Phytate Complexes: Structural Properties and Antimicrobial Synergistic Effects against Streptococcus Mutans (Submitted). Sci. Rep. 2022, 1–11. [CrossRef]
- Romani, A.M.P. Intracellular Magnesium Homeostasis. Arch. Biochem. Biophys. 2011, 512, 13–58. [CrossRef]
- Veiga, N.; Torres, J.; Godage, H.Y.; Riley, A.M.; Domínguez, S.; Potter, B.V.L.; Díaz, A.; Kremer, C. The Behaviour of Inositol 1,3,4,5,6-Pentakisphosphate in the Presence of the Major Biological Metal Cations. J. Biol. Inorg. Chem. 2009, 14, 1001–1013. [CrossRef]
- Smith, R.M.; Martell, A.E.; Chen, Y. Critical Evaluation of Stability Constants for Nucleotide Complexes with Protons and Metal Ions and the Accompanying Enthalpy Changes. Pure Appl. Chem. 1991, 63, 1015–1080. [CrossRef]
- Young, B.P.; Shin, J.J.H.; Orij, R.; Chao, J.T.; Li, S.C.; Guan, X.L.; Loewen, C.J.R. Phosphatidic Acid Is a PH Biosensor That Links Membrane Biogenesis to Metabolism. Science 2010, 329, 1085–1088. [CrossRef]
- Orij, R.; Urbanus, M.L.; Vizeacoumar, F.J.; Giaever, G.; Boone, C.; Nislow, C.; Brul, S.; Smits, G.J. Genome-Wide Analysis of Intracellular PH Reveals Quantitative Control of Cell Division Rate by PH(c) in Saccharomyces Cerevisiae. Genome Biol. 2012, 13, R80. [CrossRef]
- Shears, S.B. Assessing the Omnipotence of Inositol Hexakisphosphate. Cell. Signal. 2001, 13, 151–158. [CrossRef]
- Moedritzer, K. PH Dependence of Phosphorus-31 Chemical Shifts and Coupling Constants of Some Oxyacids of Phosphorus. Inorg. Chem. 1967, 6, 936–939. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





