Submitted:
22 February 2023
Posted:
03 March 2023
You are already at the latest version
Abstract
Keywords:
Introduction
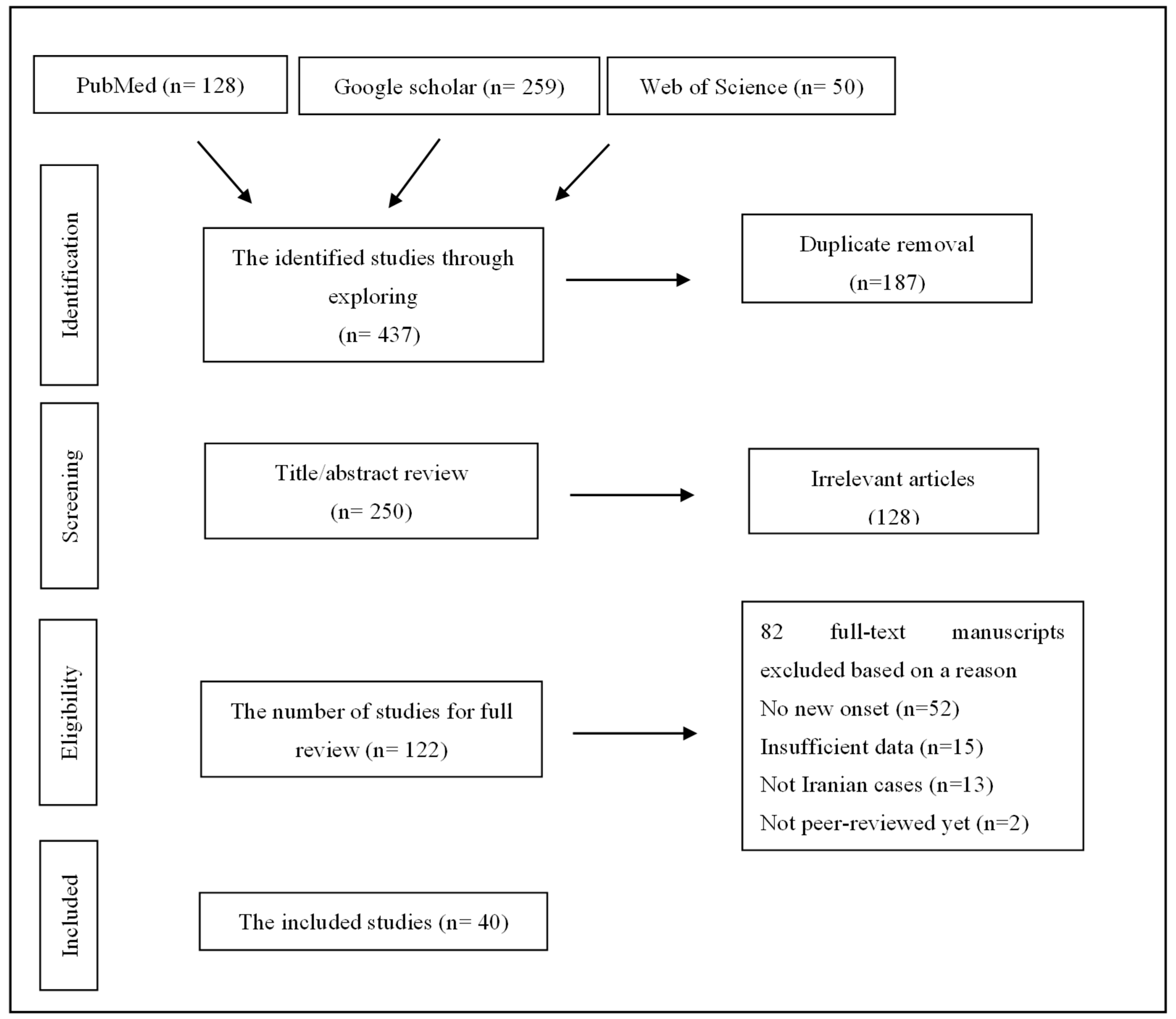
Methodology
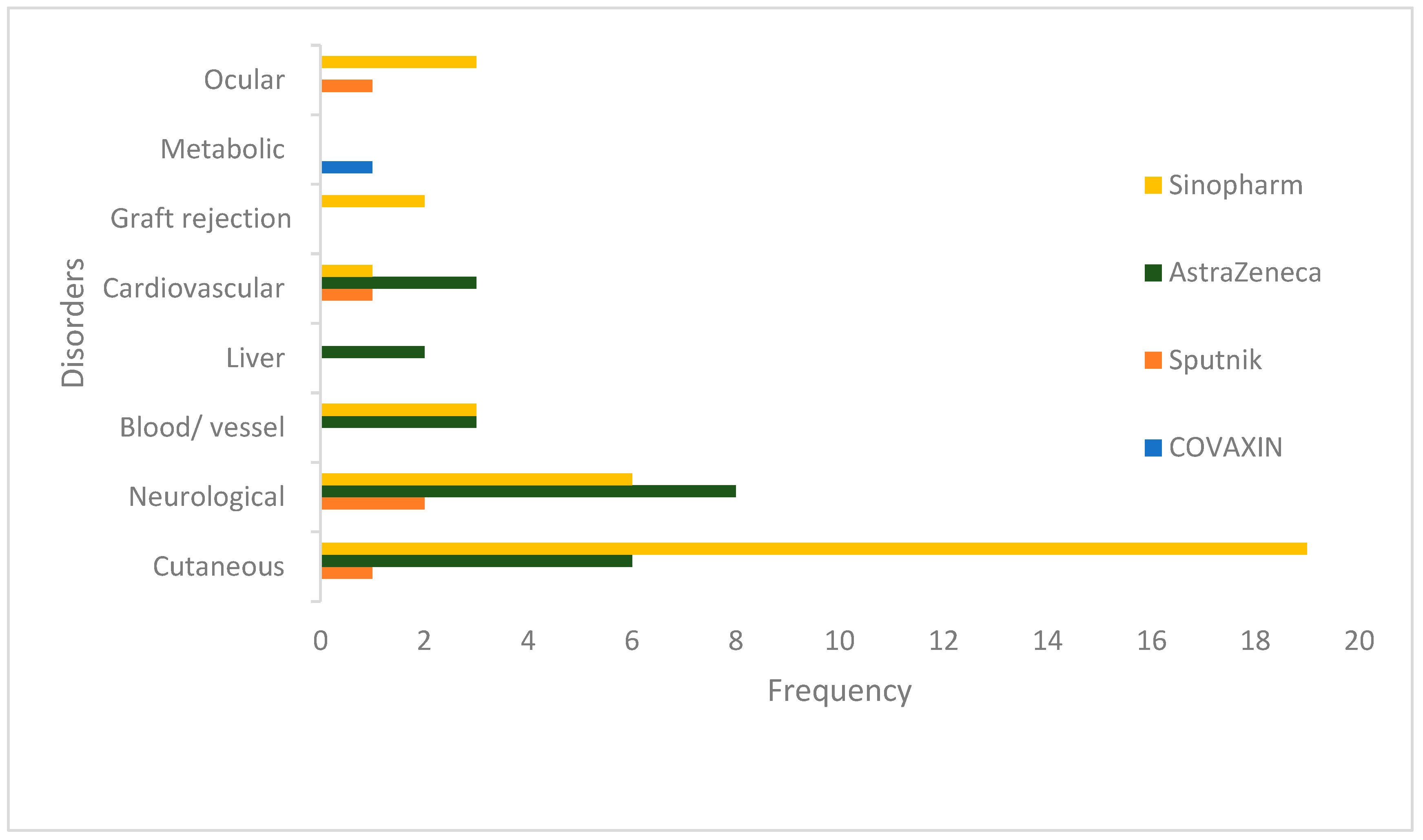
Results
Discussion
Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fotouhi F, Salehi-Vaziri M, Farahmand B, Mostafavi E, Pouriayevali MH, Jalali T, et al. Prolonged Viral Shedding and Antibody Persistence in Patients with COVID-19. Microbes and Infection. 2021 2021/03/17:104810. Pubmed Central PMCID: PMC7963517. [CrossRef]
- Mostafa Salehi-Vaziri TJ, Behrokh Farahmand, Fatemeh Fotouhi, Mohammad Banifazl, Mohammad Hassan Pouriayevali, Mona Sadat Larijani, Neda Afzali and Amitis Ramezani. Clinical Characteristics of SARS-CoV-2 by Re-infection Vs. Reactivation: A Case Series From Iran. European Journal of Clinical Microbiology & Infectious Diseases. 2021 2021/02/05. [CrossRef]
- Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 Vaccines at Pandemic Speed. New England Journal of Medicine. 2020. [CrossRef]
- Menni C, May A, Polidori L, Louca P, Wolf J, Capdevila J, et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. The Lancet Infectious Diseases. 2022;22(7):1002-10. [CrossRef]
- Immunogenicity and safety of pastocovac vaccine as a booster dose in comparison with sinopharm and pastocovac Plus boosters in Iranian adults aged 18 to 80 who received 2 doses of Sinopharm vaccine: a parallel group clinical trial [Internet]. IRCT Iranian Registry of Clinical Trials. 2022 [cited 2022/04/07].
- Chenchula S, Karunakaran P, Sharma S, Chavan M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review. Journal of Medical Virology. 2022;94(7):2969-76. [CrossRef]
- Anjana NKN, Annie TT, Siba S, Meenu MS, Chintha S, Anish TSN. Manifestations and risk factors of post COVID syndrome among COVID-19 patients presented with minimal symptoms – A study from Kerala, India. Journal of Family Medicine and Primary Care. 2021;10(11). [CrossRef]
- Sadat Larijani M, Ashrafian F, Bagheri Amiri F, Banifazl M, Bavand A, Karami A, et al. Characterization of long COVID-19 manifestations and its associated factors: A prospective cohort study from Iran. Microbial Pathogenesis. 2022 2022/08/01/;169:105618. [CrossRef]
- Graña C, Ghosn L, Evrenoglou T, Jarde A, Minozzi S, Bergman H, et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst Rev. 2022 Dec 7;12(12):Cd015477. PubMed PMID: 36473651. Pubmed Central PMCID: PMC9726273 known. Alexander Jarde: none known. Silvia Minozzi: no relevant interests; Joint Co-ordinating Editor and Method editor of the Drugs and Alcohol Group. Hanna Bergman: Cochrane Response – consultant; WHO – grant/contract (Cochrane Response was commissioned by the WHO to perform review tasks that contribute to this publication). Brian Buckley: none known. Katrin Probyn: Cochrane Response – consultant; WHO – consultant (Cochrane Response was commissioned to perform review tasks that contribute to this publication). Gemma Villanueva: Cochrane Response – employment (Cochrane Response has been commissioned by WHO to perform parts of this systematic review). Nicholas Henschke: Cochrane Response – consultant; WHO – consultant (Cochrane Response was commissioned by the WHO to perform review tasks that contributed to this publication). Hillary Bonnet: none known. Rouba Assi: none known. Sonia Menon: P95 – consultant. Melanie Marti: no relevant interests; Medical Officer at WHO. Declan Devane: Health Research Board (HRB) – grant/contract; registered nurse and registered midwife but no longer in clinical practice; Editor, Cochrane Pregnancy and Childbirth Group. Patrick Mallon: AstraZeneca – Advisory Board; spoken of vaccine effectiveness to media (print, online, and live); works as a consultant in a hospital that provides vaccinations; employed by St Vincent’s University Hospital. Jean-Daniel Lelievre: no relevant interests; published numerous interviews in the national press on the subject of COVID vaccination; Head of the Department of Infectious Diseases and Clinical Immunology CHU Henri Mondor APHP, Créteil; WHO (IVRI-AC): expert Vaccelarate (European project on COVID19 Vaccine): head of WP; involved with COVICOMPARE P et M Studies (APHP, INSERM) (public fundings). Lisa Askie: no relevant interests; Co-convenor, Cochrane Prospective Meta-analysis Methods Group. Tamara Kredo: no relevant interests; Medical Officer in an Infectious Diseases Clinic at Tygerberg Hospital, Stellenbosch University. Gabriel Ferrand: none known. Mauricia Davidson: none known. Carolina Riveros: no relevant interests; works as an epidemiologist. David Tovey: no relevant interests; Emeritus Editor in Chief, Feedback Editors for 2 Cochrane review groups. Joerg J Meerpohl: no relevant interests; member of the German Standing Vaccination Committee (STIKO). Giacomo Grasselli: Pfizer – speaking engagement. Gabriel Rada: none known. Asbjørn Hróbjartsson: no relevant interests; Cochrane Methodology Review Group Editor. Philippe Ravaud: no relevant interests; involved with Mariette CORIMUNO-19 Collaborative 2021, the Ministry of Health, Programme Hospitalier de Recherche Clinique, Foundation for Medical Research, and AP-HP Foundation. Anna Chaimani: none known. Isabelle Boutron: no relevant interests; member of Cochrane Editorial Board. Epub 2022/12/07. eng.
- Liu Q, Qin C, Liu M, Liu J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infectious Diseases of Poverty. 2021 2021/11/14;10(1):132. [CrossRef]
- Correction to: Abstract 10712: Mrna COVID Vaccines Dramatically Increase Endothelial Inflammatory Markers and ACS Risk as Measured by the PULS Cardiac Test: a Warning. Circulation. 2021 Dec 21:CIR0000000000001053. PubMed PMID: 34932387. Epub 2021/12/22. eng.
- Lai FTT, Li X, Peng K, Huang L, Ip P, Tong X, et al. Carditis After COVID-19 Vaccination With a Messenger RNA Vaccine and an Inactivated Virus Vaccine. Annals of Internal Medicine. 2022 2022/03/15;175(3):362-70. [CrossRef]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed). 2009 Jul 21;339:b2535. PubMed PMID: 19622551. Pubmed Central PMCID: PMC2714657. Epub 2009/07/23. eng. [CrossRef]
- Khajavirad N, Salehi M, Haji Ghadery A, Khalili H, Arab Ahmadi M, Dehghan Manshadi SA, et al. Serious events following COVID-19 vaccination with ChAdOx1 nCoV-19 vaccine (Vaxzevria): A short case series from Iran. Clin Case Rep. 2022 Feb;10(2):e05390. PubMed PMID: 35145690. Pubmed Central PMCID: PMC8818285. Epub 2022/02/12. eng. [CrossRef]
- Sepaskhah M, Ansari Asl F, Taheri M, Akbarzadeh Jahromi M. COVID-19 vaccine-induced Radiation Recall Dermatitis: Report of a case. Clin Case Rep. 2022 Feb;10(2):e05490. PubMed PMID: 35228886. Pubmed Central PMCID: PMC8864568. Epub 2022/03/02. eng. [CrossRef]
- Aryanian Z, Balighi K, Hatami P, Tootoonchi NM, Goodarzi A, Mohseni Afshar Z. Morphea in two patients after being infected to and being vaccinated against SARS-CoV-2 infection. Clinical case reports. 2022 Apr;10(4):e05667. PubMed PMID: 35449768. Pubmed Central PMCID: PMC9014706. Epub 2022/04/23. eng. [CrossRef]
- Sahraei Z, Abtahi-Naeini B, Saffaei A. Sputnik-V vaccine-induced panniculitis as a local reactions. Clin Case Rep. 2022 Jun;10(6):e05923. PubMed PMID: 35662784. Pubmed Central PMCID: PMC9163468. Epub 2022/06/07. eng. [CrossRef]
- Ganjei Z, Yazdan Panah M, Rahmati R, Zari Meidani F, Mosavi A. COVID-19 vaccination and alopecia areata: a case report and literature review. Clin Case Rep. 2022 Sep;10(9):e6039. PubMed PMID: 36172335. Pubmed Central PMCID: PMC9468559. Epub 2022/09/30. eng. [CrossRef]
- Shakoei S, Kalantari Y, Nasimi M, Tootoonchi N, Ansari MS, Razavi Z, et al. Cutaneous manifestations following COVID-19 vaccination: A report of 25 cases. Dermatologic therapy. 2022 Aug;35(8):e15651. PubMed PMID: 35716105. Pubmed Central PMCID: PMC9349410. Epub 2022/06/19. eng. [CrossRef]
- Mohamadzadeh D, Assar S, Pournazari M, Soufivand P, Danaei S. Disseminated cutaneous herpes simplex infection after COVID-19 vaccination in a rheumatoid arthritis patient: a case report and review. Reumatismo. 2022 Sep 13;74(2). PubMed PMID: 36101991. Epub 2022/09/15. eng. [CrossRef]
- Mardani M, Mardani S, Asadi Kani Z, Hakamifard A. An extremely rare mucocutaneous adverse reaction following COVID-19 vaccination: Toxic epidermal necrolysis. Dermatologic therapy. 2022 May;35(5):e15416. PubMed PMID: 35238119. Pubmed Central PMCID: PMC9111664. Epub 2022/03/04. eng. [CrossRef]
- Saffarian Z, Samii R, Ghanadan A, Vahidnezhad H. De novo severe pemphigus vulgaris following SARS-CoV-2 vaccination with BBIBP-CorV. Dermatologic therapy. 2022 Jun;35(6):e15448. PubMed PMID: 35289040. Pubmed Central PMCID: PMC9111647. Epub 2022/03/16. eng.
- Babazadeh A, Miladi R, Barary M, Shirvani M, Ebrahimpour S, Aryanian Z, et al. COVID-19 vaccine-related new-onset lichen planus. Clinical case reports. 2022 Feb;10(2):e05323. PubMed PMID: 35140945. Pubmed Central PMCID: PMC8810943. Epub 2022/02/11. eng. [CrossRef]
- Mansouri P, Farshi S. A case of Steven-Johnson syndrome after COVID-19 vaccination. Journal of cosmetic dermatology. 2022 Apr;21(4):1358-60. PubMed PMID: 35020263. Epub 2022/01/13. eng. [CrossRef]
- Mahmoudi Hamidabad N, Mafi AR, Abolmaali M. Mild Facial Paresis in a Recipient of Gam-COVID-Vac Vaccine: A Case Report. Clinical medicine insights Case reports. 2022;15:11795476221129120. PubMed PMID: 36225861. Pubmed Central PMCID: PMC9548508. Epub 2022/10/14. eng. [CrossRef]
- Moslemi M, Ardalan M, Haramshahi M, Mirzaei H, Sani SK, Dastgir R, et al. Herpes simplex encephalitis following ChAdOx1 nCoV-19 vaccination: a case report and review of the literature. BMC infectious diseases. 2022 Mar 3;22(1):217. PubMed PMID: 35241013. Pubmed Central PMCID: PMC8892827. Epub 2022/03/05. eng. [CrossRef]
- Maroufi SF, Naderi Behdani F, Rezania F, Tanhapour Khotbehsara S, Mirzaasgari Z. Longitudinally extensive transverse myelitis after Covid-19 vaccination: case report and review of literature. Human vaccines & immunotherapeutics. 2022 Dec 31;18(1):2040239. PubMed PMID: 35240927. Pubmed Central PMCID: PMC9009891. Epub 2022/03/05. eng. [CrossRef]
- Shahali H, Hamidi Farahani R, Hazrati P, Hazrati E. Acute vestibular neuritis: A rare complication after the adenoviral vector-based COVID-19 vaccine. Journal of neurovirology. 2022 Dec;28(4-6):609-15. PubMed PMID: 35877063. Pubmed Central PMCID: PMC9310685. Epub 2022/07/26. eng. [CrossRef]
- Mirmosayyeb O, Barzegar M, Rezaei M, Baharlouie N, Shaygannejad V. Bell’s palsy after Sputnik V COVID-19 (Gam-COVID-Vac) vaccination. Clinical case reports. 2022 Feb;10(2):e05468. PubMed PMID: 35228880. Pubmed Central PMCID: PMC8867017. Epub 2022/03/02. eng. [CrossRef]
- Shahali H, Farahani RH, Asgari A, Hazrati E. Thalamic hemi-chorea: a rare complication after receiving the adenoviral vector-based COVID-19 vaccine: a case report. Clinical and experimental vaccine research. 2022 May;11(2):217-21. PubMed PMID: 35799877. Pubmed Central PMCID: PMC9200646. Epub 2022/07/09. eng. [CrossRef]
- Fakhari MS, Poorsaadat L, Mahmoodiyeh B. Guillain-Barré syndrome following COVID-19 vaccine: A case report. Clin Case Rep. 2022 Oct;10(10):e6451. PubMed PMID: 36254149. Pubmed Central PMCID: PMC9558586. Epub 2022/10/19. eng. [CrossRef]
- Tabatabaee S, Rezania F, Alwedaie SMJ, Malekdar E, Badi Z, Tabatabaei SM, et al. Post COVID-19 vaccination Guillain-Barre syndrome: three cases. Human vaccines & immunotherapeutics. 2022 Nov 30;18(5):2045153. PubMed PMID: 35240922. Pubmed Central PMCID: PMC9196795. Epub 2022/03/05. eng. [CrossRef]
- Bazrafshan H, Mohamadi Jahromi LS, Parvin R, Ashraf A. A case of Guillain-Barre syndrome after the second dose of AstraZeneca COVID-19 vaccination. Turkish journal of physical medicine and rehabilitation. 2022 Jun;68(2):295-9. PubMed PMID: 35989967. Pubmed Central PMCID: PMC9366477. Epub 2022/08/23. eng. [CrossRef]
- Zavari A, Hamidabad NM, Hassanzadeh M. Aseptic meningitis following AZD1222 COVID-19 vaccination. The American journal of emergency medicine. 2022 May;55:225.e5-.e6. PubMed PMID: 34955313. Pubmed Central PMCID: PMC8684093. Epub 2021/12/28. eng. [CrossRef]
- Sepahvand M, Yazdi N, Rohani M, Emamikhah M. Cervical longitudinally extensive myelitis after vaccination with inactivated virus-based COVID-19 vaccine. Radiology case reports. 2022 Feb;17(2):303-5. PubMed PMID: 34849183. Pubmed Central PMCID: PMC8614237. Epub 2021/12/02. eng. [CrossRef]
- Ahmad HR, Timmermans VM, Dakakni T. Acute Disseminated Encephalomyelitis After SARS-CoV-2 Vaccination. The American journal of case reports. 2022 Jun 19;23:e936574. PubMed PMID: 35717556. Pubmed Central PMCID: PMC9218399. Epub 2022/06/20. eng. [CrossRef]
- Rahmanian E, Alikhani M, Loghman M, Beikmohamadi Hezaveh S, Zangeneh S, Shahriarirad R, et al. COVID-19 vaccine-induced vasculitis in a patient with sarcoidosis: A case report. Clinical Case Reports. 2022 2022/12/01;10(12):e6501. [CrossRef]
- Haj Mohamad Ebrahim Ketabforoush A, Molaverdi G, Nirouei M, Abbasi Khoshsirat N. Cerebral venous sinus thrombosis following intracerebral hemorrhage after COVID-19 AstraZeneca vaccination: A case report. Clinical case reports. 2022 Nov;10(11):e6505. PubMed PMID: 36397844. Pubmed Central PMCID: PMC9664546. Epub 2022/11/19. eng.
- Yaghoubi F, Dalil D. Acquired thrombotic thrombocytopenic purpura after AstraZeneca vaccine: A case report. Caspian journal of internal medicine. 2022;13(Suppl 3):299-302. PubMed PMID: 35872667. Pubmed Central PMCID: PMC9272963. Epub 2022/07/26. eng. [CrossRef]
- Saffarian Z, Samii R, Hadizadeh A, Ghanadan A, Vahidnezhad H. Purpuric dermatosis and lymphocytic vasculopathy following SARS-CoV-2 vaccination: Report of two patients. Dermatologic therapy. 2022 Nov;35(11):e15898. PubMed PMID: 36196579. Pubmed Central PMCID: PMC9874540. Epub 2022/10/06. eng. [CrossRef]
- Naghashzadeh F, Shafaghi S, Dorudinia A, Naji SA, Marjani M, Amin A, et al. Myocarditis following rAd26 and rAd5 vector-based COVID-19 vaccine: case report. ESC heart failure. 2022 Apr;9(2):1483-6. PubMed PMID: 35106967. Pubmed Central PMCID: PMC8934948. Epub 2022/02/03. eng. [CrossRef]
- Servatyari K, Hassani A. The first report of myocarditis followed by AstraZeneca vaccination in Iran. Chronic Diseases Journal. 2022 06/20;10(2):117-20.
- Hassanzadeh S, Sadeghi S, Mirdamadi A, Nematollahi A. Myocarditis following AstraZeneca (an adenovirus vector vaccine) COVID-19 vaccination: A case report. Clinical Case Reports. 2022 2022/04/01;10(4):e05744. [CrossRef]
- Mehrabi Nasab E, Athari SS. The first report of 2:1 atrioventricular block following COVID-19 vaccination. Clin Case Rep. 2022 May;10(5):e05797. PubMed PMID: 35540716. Pubmed Central PMCID: PMC9066801. Epub 2022/05/12. eng. [CrossRef]
- Azdaki N, Farzad M. Long QT interval and syncope after a single dose of COVID-19 vaccination: a case report. The Pan African medical journal. 2021;40:67. PubMed PMID: 34804335. Pubmed Central PMCID: PMC8590254. Epub 2021/11/23. eng. [CrossRef]
- Dehghani A, Ghanbari H, Houshang-Jahromi M-h, Pourazizi M. Paracentral acute middle maculopathy and COVID-19 vaccination: Causation versus coincidence finding. Clinical Case Reports. 2022 2022/03/01;10(3):e05578. [CrossRef]
- Mohammadpour M, Farrokhpour H, Sadeghi R. Herpetic endotheliitis and stromal keratitis following inactivated COVID-19 vaccination. Clin Case Rep. 2022 Oct;10(10):e6397. PubMed PMID: 36237947. Pubmed Central PMCID: PMC9536498. Epub 2022/10/15. eng. [CrossRef]
- Farahani AA, Shahali H. Intracranial Hypertension and Papilledema: An Unusual Complication After the Adenoviral DNA Vector-Based Coronavirus Disease 2019 Vaccination in an Air Medical Transportation Pilot. Air medical journal. 2022 Nov-Dec;41(6):560-5. PubMed PMID: 36494173. Pubmed Central PMCID: PMC9350672. Epub 2022/12/10. eng. [CrossRef]
- Fekri S, Khorshidifar M, Dehghani MS, Nouri H, Abtahi SH. Acute macular neuroretinopathy and COVID-19 vaccination: Case report and literature review. Journal francais d’ophtalmologie. 2023 Jan;46(1):72-82. PubMed PMID: 36496293. Pubmed Central PMCID: PMC9684098. Epub 2022/12/11. eng. [CrossRef]
- Barary M, Sharifi-Razavi A, Rakhshani N, Sio TT, Ebrahimpour S, Baziboroun M. Fulminant hepatitis following COVID-19 vaccination: A case report. Clinical case reports. 2022 Jul;10(7):e6066. PubMed PMID: 35865787. Pubmed Central PMCID: PMC9295676. Epub 2022/07/23. eng. [CrossRef]
- Sohrabi M, SobheRakhshankhah E, Ziaei H, AtaeeKachuee M, Zamani F. Acute liver failure after vaccination against of COVID-19; a case report and review literature. Respiratory medicine case reports. 2022;35:101568. PubMed PMID: 34926142. Pubmed Central PMCID: PMC8668601. Epub 2021/12/21. eng. [CrossRef]
- Bennet WM, Elamin A, Newell-Price JD. Subacute thyroiditis following COVID-19 vaccination: Case report and Society for Endocrinology survey. Clinical endocrinology. 2023 Mar;98(3):452-3. PubMed PMID: 35261054. Pubmed Central PMCID: PMC9111779. Epub 2022/03/10. eng. [CrossRef]
- Mohammadzadeh M, Hooshmandi S, Jafari M, Hassanpour K. Presumably Corneal Graft Rejection after COVID-19 Vaccination. Case reports in ophthalmology. 2022 May-Aug;13(2):562-9. PubMed PMID: 36160489. Pubmed Central PMCID: PMC9386427. Epub 2022/09/27. eng. [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard [Internet]. World Health Organization. [cited 2023/6/2]. Available from: https://covid19.who.int/.
- Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. The Lancet Infectious Diseases. 2021 2021/07/01/;21(7):939-49. [CrossRef]
- Sah R, Shrestha S, Mehta R, Sah SK, Rabaan AA, Dhama K, et al. AZD1222 (Covishield) vaccination for COVID-19: Experiences, challenges, and solutions in Nepal. Travel Medicine and Infectious Disease. 2021 2021/03/01/;40:101989. [CrossRef]
- Shrestha S, Devbhandari RP, Shrestha A, Aryal S, Rajbhandari P, Shakya B, et al. Adverse events following the first dose of ChAdOx1 nCoV-19 (COVISHIELD) vaccine in the first phase of vaccine roll out in Nepal. Journal of Patan Academy of Health Sciences. 2021;8(1):9-17. [CrossRef]
- Pagotto V, Ferloni A, Soriano MM, Díaz M, Braguinsky Golde N, González MI, et al. Active monitoring of early safety of Sputnik V vaccine in Buenos Aires, Argentina. MEDICINA (Buenos Aires). 2021;81(3):408-14.
- Montalti M, Soldà G, Di Valerio Z, Salussolia A, Lenzi J, Forcellini M, et al. ROCCA observational study: Early results on safety of Sputnik V vaccine (Gam-COVID-Vac) in the Republic of San Marino using active surveillance. EClinicalMedicine. 2021;38:101027. [CrossRef]
- Das AS, Regenhardt RW, Feske SK, Gurol ME. Treatment Approaches to Lacunar Stroke. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2019 Aug;28(8):2055-78. PubMed PMID: 31151838. Pubmed Central PMCID: PMC7456600. Epub 2019/06/04. eng. [CrossRef]
- Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas AM. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. Journal of thrombosis and haemostasis : JTH. 2021 Jul;19(7):1771-5. PubMed PMID: 33877737. Pubmed Central PMCID: PMC8250306. Epub 2021/04/21. eng. [CrossRef]
- Roy A, Verma N, Singh S, Pradhan P, Taneja S, Singh M. Immune-mediated liver injury following COVID-19 vaccination: A systematic review. Hepatology Communications. 2022 2022/09/01;6(9):2513-22. [CrossRef]
- Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. The Lancet Infectious Diseases. 2021 2021/05/01/;21(5):637-46. [CrossRef]
- Zhang M-X, Zhang T-T, Shi G-F, Cheng F-M, Zheng Y-M, Tung T-H, et al. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Review of Vaccines. 2021 2021/07/03;20(7):891-8. [CrossRef]
- Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA. 2021;326(1):35-45. [CrossRef]
- Bhandari B, Rayamajhi G, Lamichhane P, Shenoy AK. Adverse Events following Immunization with COVID-19 Vaccines: A Narrative Review. BioMed Research International. 2022 2022/08/16;2022:2911333. [CrossRef]
- Shah AP, Dzhaber D, Kenyon KR, Riaz KM, Ouano DP, Koo EH. Acute Corneal Transplant Rejection After COVID-19 Vaccination. Cornea. 2022 Jan 1;41(1):121-4. PubMed PMID: 34620770. Epub 2021/10/09. eng. [CrossRef]
- Molero-Senosiain M, Houben I, Savant S, Savant V. Five Cases of Corneal Graft Rejection After Recent COVID-19 Vaccinations and a Review of the Literature. Cornea. 2022;41(5):669-72. PubMed PMID: 00003226-202205000-00028. [CrossRef]
- Alhumaid S, Rabaan AA, Dhama K, Yong SJ, Nainu F, Hajissa K, et al. Solid Organ Rejection following SARS-CoV-2 Vaccination or COVID-19 Infection: A Systematic Review and Meta-Analysis. Vaccines. 2022 Aug 10;10(8). PubMed PMID: 36016180. Pubmed Central PMCID: PMC9412452. Epub 2022/08/27. eng. [CrossRef]
- Pourani MR, Shahidi Dadras M, Salari M, Diab R, Namazi N, Abdollahimajd F. Cutaneous adverse events related to COVID-19 vaccines: A cross-sectional questionnaire-based study of 867 patients. Dermatologic therapy. 2022 Feb;35(2):e15223. PubMed PMID: 34820975. Epub 2021/11/26. eng. [CrossRef]
- Gambichler T, Boms S, Susok L, Dickel H, Finis C, Abu Rached N, et al. Cutaneous findings following COVID-19 vaccination: review of world literature and own experience. Journal of the European Academy of Dermatology and Venereology. 2022 2022/02/01;36(2):172-80. [CrossRef]
- Essam R, Ehab R, Al-Razzaz R, Khater MW, Moustafa EA. Alopecia areata after ChAdOx1 nCoV-19 vaccine (Oxford/AstraZeneca): a potential triggering factor? Journal of cosmetic dermatology. 2021 Dec;20(12):3727-9. PubMed PMID: 34559937. Pubmed Central PMCID: PMC8661988. Epub 2021/09/25. eng. [CrossRef]
- Fakhari MS, Poorsaadat L, Mahmoodiyeh B. Guillain–Barré syndrome following COVID-19 vaccine: A case report. Clinical case reports. 2022 2022/10/01;10(10):e6451. [CrossRef]
- Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, et al. Use of the Inactivated Intranasal Influenza Vaccine and the Risk of Bell’s Palsy in Switzerland. New England Journal of Medicine. 2004 2004/02/26;350(9):896-903. [CrossRef]
- Zhou W, Pool V, DeStefano F, Iskander JK, Haber P, Chen RT. A potential signal of Bell’s palsy after parenteral inactivated influenza vaccines: reports to the Vaccine Adverse Event Reporting System (VAERS)—United States, 1991–2001. Pharmacoepidemiology and Drug Safety. 2004 2004/08/01;13(8):505-10. [CrossRef]
- Principi N, Esposito S. Do Vaccines Have a Role as a Cause of Autoimmune Neurological Syndromes? Frontiers in public health. 2020;8:361. PubMed PMID: 32850592. Pubmed Central PMCID: PMC7399175. Epub 2020/08/28. eng. [CrossRef]
- Rahmanian E, Alikhani M, Loghman M, Beikmohamadi Hezaveh S, Zangeneh S, Shahriarirad R, et al. COVID-19 vaccine-induced vasculitis in a patient with sarcoidosis: A case report. Clinical case reports. 2022 Dec;10(12):e6501. PubMed PMID: 36478972. Pubmed Central PMCID: PMC9718919. Epub 2022/12/09. eng. [CrossRef]
- Khajavirad N, Salehi M, Haji ghadery A, Khalili H, Arab Ahmadi M, Dehghan Manshadi SA, et al. Serious events following COVID-19 vaccination with ChAdOx1 nCoV-19 vaccine (Vaxzevria): A short case series from Iran. Clinical case reports. 2022 2022/02/01;10(2):e05390. [CrossRef]
- Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature. 2021 2021/08/01;596(7873):565-9. [CrossRef]
- Bonetto C, Trotta F, Felicetti P, Alarcón GS, Santuccio C, Bachtiar NS, et al. Vasculitis as an adverse event following immunization - Systematic literature review. Vaccine. 2016 Dec 12;34(51):6641-51. PubMed PMID: 26398442. Epub 2015/09/24. eng. [CrossRef]
- Arthur JM, Forrest JC, Boehme KW, Kennedy JL, Owens S, Herzog C, et al. Development of ACE2 autoantibodies after SARS-CoV-2 infection. PloS one. 2021;16(9):e0257016. PubMed PMID: 34478478. Pubmed Central PMCID: PMC8415618. Epub 2021/09/04. eng. [CrossRef]
- Dutta D, Nagappa M, Sreekumaran Nair BV, Das SK, Wahatule R, Sinha S, et al. Variations within Toll-like receptor (TLR) and TLR signaling pathway-related genes and their synergistic effects on the risk of Guillain-Barré syndrome. Journal of the Peripheral Nervous System. 2022 2022/06/01;27(2):131-43. [CrossRef]
| Case no. | Type of disorder | Age | Gender | Comorbidity | Covid-19 test/history | Vaccine type | Time of incidence | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cutaneous involvement | ||||||||
| 1 | Extensive rash and edema | 77 | Female | Hypertension | Negative | AstraZeneca | 2 days after the 1st dose | (14) |
| 2 | Radiation Recall Dermatitis | 50 | Female | History of breast cancer and radical mastectomy | Not stated | Sinopharm | 1week after the 2nd dose | (15) |
| 3 | Erythemato-violaceous and sclerotic lesions | 70 | Female | - | Negative | AstraZeneca | 2 days after the 1st dose | (16) |
| 4 | Panniculitis | 40 | Female | - | Not stated | Sputnik | 13 days after the 1st dose | (17) |
| 5 | Alopecia areata | 23 | Female | - | Not stated | AstraZeneca | 1 week after the 1st dose | (18) |
| 6 | 74 | Male | Fatty liver | Not stated | Sinopharm | 2 days after the 2nd dose | (19) | |
| 7 | 37 | Male | - | Not stated | Sinopharm | 6 days after the both doses | (19) | |
| 8 | Herpes simplex | 63 | Female | Rheumatoid arthritis | Not stated | Sinopharm | 7 days after the 2nd dose | (20) |
| 9 | Toxic Epidermal Necrolysis (TEN) | 76 | Male | Atorvastatin 10 mg/day taken for several years | Not stated | Sinopharm | 1 day after vaccination | (21) |
| 10 | 71 | Male | - | Not stated | Sinopharm | 10 days after the 1st dose | (19) | |
| 11 | Pemphigus vulgaris (PV) | 76 | Female | Diabetes mellitus, hyperlipidemia, and ischemic heart disease | Not stated | Sinopharm | 1 month after the 2nd dose | (22) |
| 12 | 30 | Female | - | Not stated | Sinopharm | 16 days after 1st dose | (19) | |
| 13 | New-onset lichen planus (LP) | 52 | Female | - | Positive | Sinopharm | 1 week after the 2nd dose | (23) |
| 14 | 45 | Female | Hypertension | Not stated | Sinopharm | 14 days after the 1st dose | (19) | |
| 15 | 40 | Male | - | Not stated | Sinopharm | 10 days after the both | (19) | |
| 16 | 45 | Male | - | Not stated | Sinopharm | 7 days after the both | (19) | |
| 17 | 45 | Male | - | Not stated | AstraZeneca | 7 days after the 1st dose | (19) | |
| 18 | 49 | Female | - | Not stated | Sinopharm | 10days after the 1st dose | (19) | |
| 19 | Psoriasis exacerbation | 50 | Male | Arthritis | Not stated | Sinopharm | 4 days after the first dose, 6 days after the 2nd dose | (19) |
| 20 | Bullous pemphigoid | 85 | Female | - | Not stated | Sinopharm | 20days after the 1st dose | (19) |
| 21 | 91 | Male | - | Not stated | Sinopharm | 19 days after the 1st dose | (19) | |
| 22 | Cutaneous vasculitis | 45 | Male | - | Not stated | Sinopharm | 2 days after the 1st dose | (19) |
| 23 | Pytriasis rosea | 26 | Male | Hypertension, diabetes mellitus | Not stated | Sinopharm | 14 days after the booster | (19) |
| 24 | Herpes zoster | 60 | Female | - | Not stated | Sinopharm | 6 days after the 1st dose | (19) |
| 25 | Urticaria and erythema multiform | 31 | Male | - | Not stated | Sinopharm | 11 days after the 2nd dose | (19) |
| 26 | 32 | Female | - | Not stated | AstraZeneca | 20 days after the 1st | (19) | |
| 27 | Morphea | 35 | Female | Hyperlipidemia, diabetes | Not stated | AstraZeneca | 10 days after the 1st | (19) |
| 28 | Steven-Johnson syndrome | 63 | Female | Mild plaque-type psoriasis type II diabetes mellitus |
Not stated | Sinopharm | 24h after vaccination | (24) |
| Neurologic involvement | ||||||||
| 29 | Facial Paresis | 34 | Female | Migraine attacks (under treatment) | Not stated | Sputnik V | 1 day after the 1st dose | (25) |
| 30 | Encephalopathy | 27 | male | - | Not stated | AstraZeneca | 8 days after the 1st dose | (26) |
| 31 | 56 | Female | - | Negative | AstraZeneca | 2 days after the 1st dose | (14) | |
| 32 | Transverse myelitis | 31 | Female | - | Negative | AstraZeneca | 3 weeks after the 1st dose | (27) |
| 33 | Acute vestibular neuritis | 51 | Male | - | Negative | AstraZeneca | 11 days after the 1st dose | (28) |
| 34 | Bell’s palsy | 27 | Female | - | Negative | Sputnik V | 3-5 days after the 1st dose | (29) |
| 35 | 58 | Male | Controlled diabetes mellitus | Not stated | Sputnik | 10 days after the 1st dose | (29) | |
| 36 | Thalamic hemi-chorea | 72 | Male | History of laparoscopic cholecystectomy | Negative | AstraZeneca | 9 days after the 1st dose | (30) |
| 37 | Guillain-Barre syndrome | 60 | Male | Controlled hypertension and hypothyroidism | Negative | Sinopharm | 20 days after the booster | (31) |
| 38 | 46 | Male | - | Negative | AstraZeneca | 3 days after the 2nd dose | (32) | |
| 39 | 36 | Male | - | Negative | Sinopharm | 5 days after the 1st dose | (32) | |
| 40 | 32 | Male | - | Negative | Sinopharm | 14 days after the 1st dose | (32) | |
| 41 | 68 | female | - | Negative | AstraZeneca | 4 days post the 2nd | (33) | |
| 42 | Aseptic meningitis | 26 | Female | - | Negative | AstraZeneca | A few hours the 1st dose | (34) |
| 43 | extensive myelitis | 71 | Male | Diabetes mellitus, hypertension and Ischemic Heart Disease | Not stated | Sinopharm | 5 days after the 1st dose | (35) |
| 44 | Acute disseminated encephalomyelitis | 37 | Male | - | Negative | Sinopharm | few days to one month after the 1st dose | (36) |
| Vessel/Blood involvement | ||||||||
| 45 | Thrombotic thrombocytopenia | 70 | Female | Diabetes mellitus type 2, hypertension, and coronary artery disease | Not stated | AstraZeneca | 1day after the 1st dose | (14) |
| 46 | Vasculitis | 55 | Female | controlled sarcoidosis | Not stated | Sinopharm | 3 days after the 1st dose | (37) |
| 47 | Cerebral venous sinus thrombosis | 55 | Female | Hypertension/ a surgery history of hysterectomy 10 years ago | Negative | AstraZeneca | After the 1st dose | (38) |
| 48 | Acquired thrombotic thrombocytopenic purpura (aTTP) | 22 | Female | - | Negative | AstraZeneca | 3 weeks after the 1st dose | (39) |
| 49 | Purpuric dermatosis &lymphocytic vasculopathy | 53 | Female | History of treated breast cancer | Not stated | Sinopharm | 9 days after the 1st dose | (40) |
| 50 | 50 | Male | - | Not stated | Sinopharm | 2 months after vaccination | (40) | |
| Cardiac involvement | ||||||||
| 51 | Myocarditis | 29 | Male | - | Negative | Sputnik V | 2days after the 2nd dose | (41) |
| 52 | 26 | Male | - | Negative | AstraZeneca | 4 days after the 2nd dose | (42) | |
| 53 | 32 | Female | - | Negative | AstraZeneca | 3 days after the 1st dose | (43) | |
| 54 | Atrioventricular block | 65 | Male | - | Not stated | Sinopharm | A few days after vaccination | (44) |
| 55 | Long QT interval and syncope | 70 | Male | Hypertension (HTN) and diabetes mellitus under medical treatment | Negative | AstraZeneca | 3days after the 1st | (45) |
| Ocular involvement | ||||||||
| 56 | Paracentral acute middle maculopathy | 38 | Male | - | Negative | Sinopharm | 2 weeks after vaccination | (46) |
| 57 | Herpetic endotheliitis and stromal keratitis | 30 | Female | Hypothyroidism | Not stated | Sinopharm | 2weeks after vaccination | (47) |
| 58 | Intracranial hypertension and papilledema | 32 | Male | - | Not stated | Sputnik V | 3 days after the 1st dose | (48) |
| 59 | Acute macular neuroretinopathy | 18 | Female | - | Negative | Sinopharm | 5 days after the 1st dose | (49) |
| Liver involvement | ||||||||
| 60 | Fulminant hepatitis | 35 | Male | Controlled psychological problems | Not stated | AstraZeneca | 8 days after the 1st dose | (50) |
| 61 | Acute liver failure | 34 | Male | - | Not stated | AstraZeneca | 2 days after the 1st dose | (51) |
| Thyroid disorder | ||||||||
| 62 | Subacute thyroiditis | 34 | Female | - | Negative | COVAXIN | 11 days after the 1st dose | (52) |
| Graft rejection | ||||||||
| 63 | Corneal Graft Rejection | 36 | Female | Penetrating keratoplasty (PKP) secondary to herpes simplex keratitis (HSK) | Not stated | Sinopharm | 7 days after the 1st dose | (53) |
| 64 | 54 | Female | Not stated | Sinopharm | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





