1. Introduction
Dry eye disease (DED) is one of the most frequently reported ophthalmologic diseases. Approximately 25% of the patients in ophthalmic clinics report about symptoms of DED [
1]. Andrew de Roetth introduced the term “dry eye” in 1950 [
2].
Dryness of the eyes, caused by a decreased aqueous phase of the tear film, was for a long time suspected to be the main characteristic of DED.
In 2007, the International Dry Eye Workshop (DEWS) announced a new definition of DED, which is as follows: “Dry eye is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage of the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface.” [
3]
DED can be caused by many different factors, e.g. intense screen work, drugs, hormonal changes, air pollution, air-conditioners, as well as intense UV-radiation [
4,
5,
6]. First symptoms of DED occur mostly between 40 to 50 years of age. Foreign body sensation, redness, dryness as well as burning in the eyes are the most often reported symptoms.
In the treatment of DED, hyaluronic acid containing eye drops are well described and established. Hyaluronic acid (HA), a compound found in the human body in greatest amounts in the skin, synovial fluid, the vitreous body, and the umbilical cord, is characterized to be well resorbable and biocompatible [
7,
8,
9,
10]. For instance, it is able to bind large numbers of water molecules. HA is known to play an important role in signal transduction, ovulation, fertilization, wound healing and tumour physiology [
11]. Due to these properties, HA containing treatments can be found in different medical fields. In ophthalmological treatments, HA is used as “lubricant” component as it shows great viscoelastic properties. It reduces the symptoms of DED by stabilising the tear film, reducing friction during blinking, and it prevents harmful substances to bind to the eye [
12].
Earlier studies have shown, that mucilaginous substances from plant extracts can support the lubricating effect of HA [
13,
14,
15]. For this reason, eye drops, containing HA and mallow extract were developed. The main active ingredient of the wild mallow is the abundant plant mucus, which has the ability to bind and store water.
During the development of this medical device, it was detected by physical measurement of the solution, that the combination of HA and mallow extract resulted in a reduced static surface tension compared to HA alone. It can be concluded that the combined product remains longer on the eye and, theoretically, the eye is lubricated and refreshed for a longer period of time. To prove these observations in vivo, we conducted this pilot study, which compared the combined product with eye drops that do only contain HA.
2. Materials and Methods
2.1. Study Design and Patient Population
This open-label, multicentre crossover pilot study was conducted at five ophthalmologic practices in Milan, Italy. Twenty patients were enrolled, four at each practice. All patients signed an informed consent form before study entry. The combination product as well as the comparator product are CE-marked medical devices and were applied according to their intended use.
The study was conducted as a crossover study. Each patient administered each of the two eye drops products. It was up to the investigator to decide which of the two treatments was given first. 9 patients started with the combination of HA and mallow extract, 11 patients received HA only first. Treatment duration was 30 +/- 3 days for one product, followed by a washout phase of 7 days and another 30 +/- 3 days administration of the second product.
The patients (≥ 18 years) to be included had to have a diagnostically confirmed moderate DED defined by TBUT < 10 s, OS > 2, and OSDI between 23 and 32.
Patients underwent a general eye examination at baseline and periodically during the study. A history of eye infections or severe inflammation, as well as an eye surgery, in the 6 months before the study was an exclusion criterion. This was also the case for any uncontrolled serious systemic disease that could affect the eye and other ongoing eye diseases.
2.2. Products Used, Dosage and Administration
Both products used are CE certified medical devices. They were used in this study according to their intended use. The first product, containing HA and mallow extract, was a sterile eye drop product in single doses (Visiodoron Malva®, Weleda AG). 1 ml of these eye drops contain 0.15 % sodium hyaluronate, 0.5 % extract of mallow flowers (Malva sylvestris L.), citrate buffer, sodium chloride and water for injection. The original package contained 20 single dose vials of 0.4 ml.
The second product, containing HA only, was also a sterile eye drop product in single doses (BLUyal® UD, PHARMA STULLN GmbH). 1 ml of this comparator contains: 0.15 % sodium hyaluronate, sodium chloride, phosphate buffer and water for injection. The original package contained 20 single dose vials of 0.35 ml.
The patient instilled twice daily one drop in the lower conjunctival sac of each eye. For the treatment duration of 30 +/- 3 days, the participant used the same product and – after the washout period of 7 days – switched to the other product.
2.3. Efficacy and Safety Endpoints
As primary variables the tear film breakup time (TBUT), the lissamine green staining of the ocular surface (Oxford Scheme, OS), and the efficacy and safety assessment by the ophthalmologist were assessed.
For evaluation of tear film stability, fluorescein is instilled into the eye and the interval between one complete blink and the first appearance of a dry spot in the precorneal tear film is measured in seconds with the slit lamp with cobalt blue filter. Values > 15 s are normal, values < 10 s are pathological.
Ocular surface staining is used as an indicator for the condition of the ocular surface. In this study, color staining is used to detect epithelial defects of the ocular surface, which could be attributed to inflammatory processes. For ocular surface coloring, lissamine green was used. The findings were graded according to the Oxford Scheme (OS)3. For each of the three zones (cornea, nasal bulbar conjunctiva and temporal bulbar conjunctiva,) a score from 1 to 3 was used. Maximum score (the worst case) is 9; the score is proportional to the dry eye clinical picture.
At the end of each treatment period, the ophthalmologist rated efficacy with the categories: unsatisfactory, satisfactory, good, very good. Likewise, safety was rated using the same categories.
As secondary variables, intensity of symptoms (per eye, 7 patient-rated symptoms (each ranging from 0 (absent/none) to 10 (maximum intensity)), resulting in a total symptom score of 0 to 140), Schirmer test, ocular surface disease index (OSDI), efficacy assessment by the patient, patient satisfaction, and individual patient preference were evaluated.
The OSDI is a scoring system consisting of three sub-scales, which cover in total 12 questions. The patient is asked to rate each symptom, condition or situation from 0 (not present/none) to 4 (always). From the ratings, a sum-score is calculated. The resulting score is then classified according to a predefined grading system with the categories normal, mild, moderate, severe [
16].
At the end of each treatment period, the patients rated efficacy using the following categories: 0 = no symptom relief, 1 = low symptom relief, 2 = good symptom relief, 3 = very good symptom relief. After each treatment period, the patients were asked to rate their satisfaction with the applied treatment according to the following categories: very unsatisfied, unsatisfied, satisfied, very satisfied, I don’t know. After completion of both treatment periods, the patient was asked the following questions:
1. If you compare the first treatment period with the second one, have you perceived a difference?
Answer options: Yes (if yes, please specify), no, I don’t know.
2. Which product would you prefer?
Answer options: Product 1, product 2, no preference, I don’t know.
2.4. Statistical Analysis
A descriptive analysis of all data was performed, as well as an exploratory analysis of the target variables. For the analysis, the data were transferred from the Excel spreadsheets to SAS data sets using the SAS software version 9.3 (PROC IMPORT procedure). Five SAS data sets were created (visit 1, visit 2, visit 3, visit 4, and patient diary). The CRF and patient diary entries were checked for completeness and plausibility by the data management. Missing values were not tracked. For the analysis of the pilot study, the following validation groups were defined: The data of all patients, for whom in each of both treatment periods at least the main target variable was documented at least once, were included in the "intent-to-treat" (ITT) analysis. In the safety analysis, all patients were included that received and applied the medical device during the study.
3. Results
Between the 16th December 2016 and 2nd May 2017, 20 patients at five ophthalmologic practices in Italy were included in this study. All patients were included in the ITT-Set and the safety analysis set.
There were no premature terminations nor withdrawals in this study.
3.1. Primary Variables
For both primary variables, TBUT and OS, their dependency on the treatment sequence was analysed in order to detect carry-over effects. The analysis showed that a carry-over effect could be excluded (TBUT: p = 0.5804; OS: p = 0.2844). Consequently, data from both treatment periods was included into the analysis.
3.1.1. Measurement of Tear Film Breakup Time (TBUT)
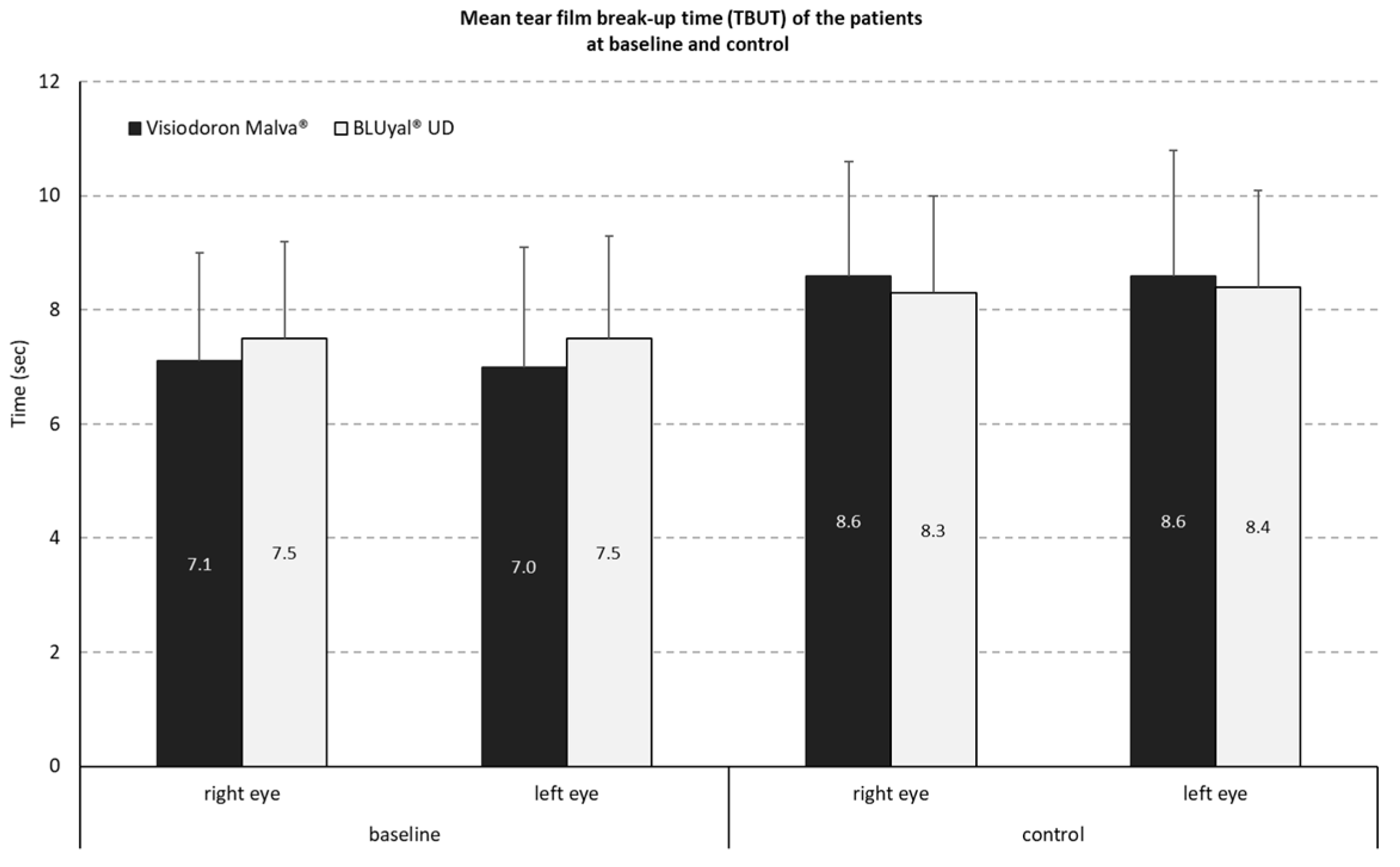
The distribution of TBUT values during the study showed for the combined product (containing HA and mallow extract) a mean difference of -1.4 s (SD 1.3s) on the right and -1.6 s (SD 1.4 s) on the left eye. For the comparator preparation without mallow extract, the mean difference was -0.9 s, with a SD of 0.9 on the right eye and a SD of 1.0s on the left eye (see
Figure 1).
The analysis of the mean intra-individual differences of the mean TBUT reduction by a two-sided one sample t-test at an alpha level of 5 % (with normal distribution assumption) resulted in a p-value of p = 0.1497. That means there is no significant (5 % level) difference in TBUT reduction between both treatments.
3.1.2. Reduction of Lissamine Green Staining of Ocular Surface (OS)
The mean OS reduction of both eyes was 1.13 for the eye drops containing HA in combination with mallow extract and 0.83 for HA alone. With respect to the condition of the ocular surface as graded by the OS, the positive reduction of mean OS values represents improvement for both treatments.
The analysis of the mean intra-individual differences of the mean OS reduction by a two-sided one sample Wilcoxon signed-rank test at an alpha level of 5 % (no normal distribution assumption) resulted in a p-value of p = 0.3149. That means there is no significant (5 % level) difference in OS reduction between the two products.
3.1.3. Safety and Efficacy Assessments by the Ophthalmologists
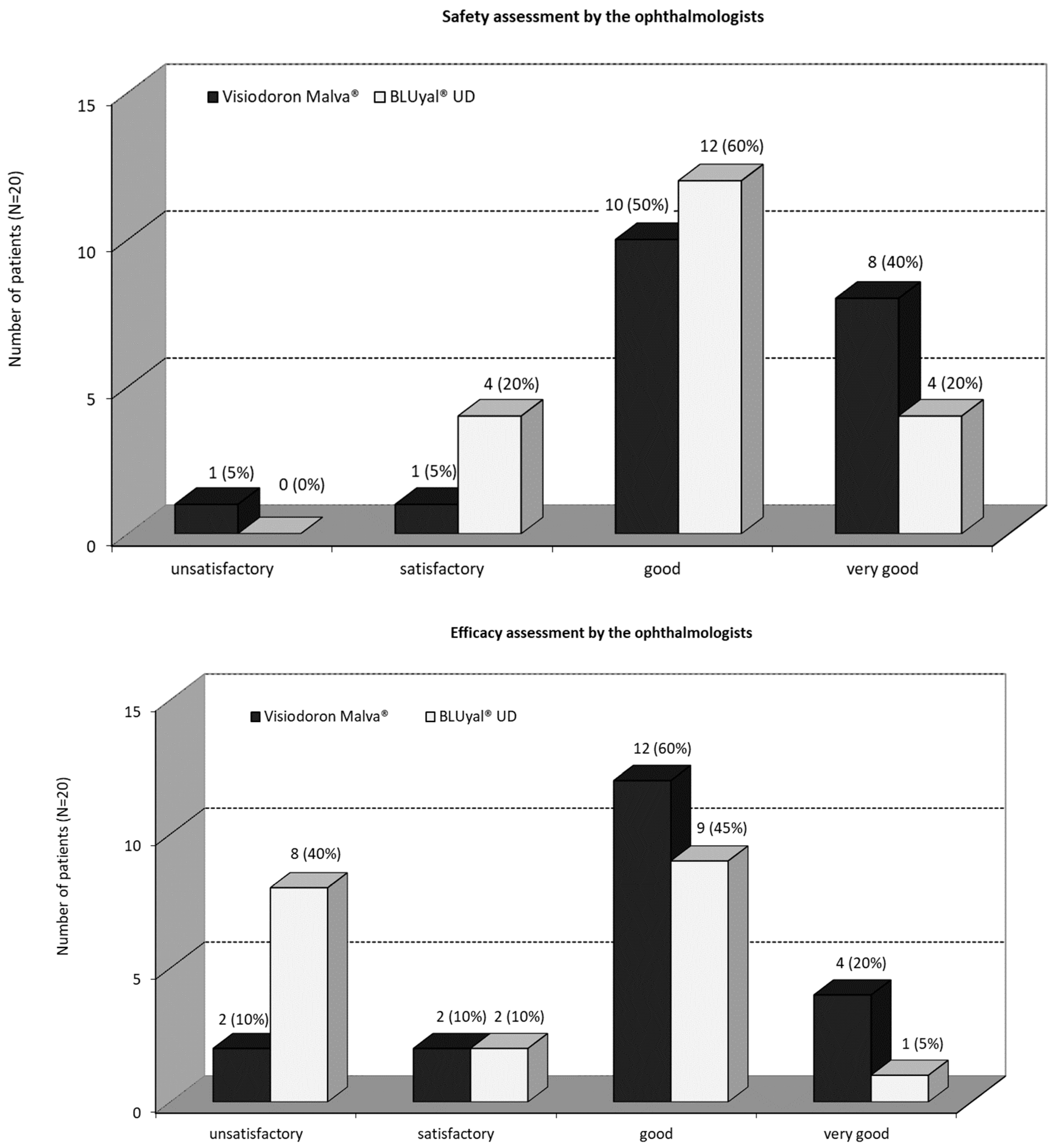
As one can see in
Figure 2a), in 10 patients (50 %) the ophthalmologists rated the overall safety of the mallow extract containing medical device “good”, in 8 patients (40 %) “very good” and in one patient (5 %) “satisfactory”, respectively “unsatisfactory”. In 12 (60 %) patients, the ophthalmologists rated the overall safety of the comparator as “good” and in 4 (20 %) patients as “very good” respectively “satisfactory”. There was no significant difference (5 % level) between the ratings (p = 0.2057, Fisher’s exact test). Anyway, the safety assessment by the ophthalmologists showed a better rating for the mallow extract containing medication and the assessment “very good” was twice as high as for the comparator product.
There was a recognizable better efficacy assessment by the ophthalmologist for the combination product compared to the eye drops only containing HA (
Figure 2b). In 4 (20 %) patients the ophthalmologists assessed the overall efficacy of the mallow extract containing preparation as “very good”, for 12 (60 %) patients as “good”, and for 2 (10 %) patients “satisfactory”, respectively “unsatisfactory”. The ophthalmologists rated the overall efficacy of the comparator treatment in one (5 %) patient as “very good”, in 9 (45 %) patients as “good”, in 2 (10 %) patients “satisfactory” and in 8 (40 %) patients “unsatisfactory”. The Fisher’s exact test (5 % level) showed no significant difference (p = 0.1233).
3.2. Secondary Variables
3.2.1. Total Symptom Score Per Patient
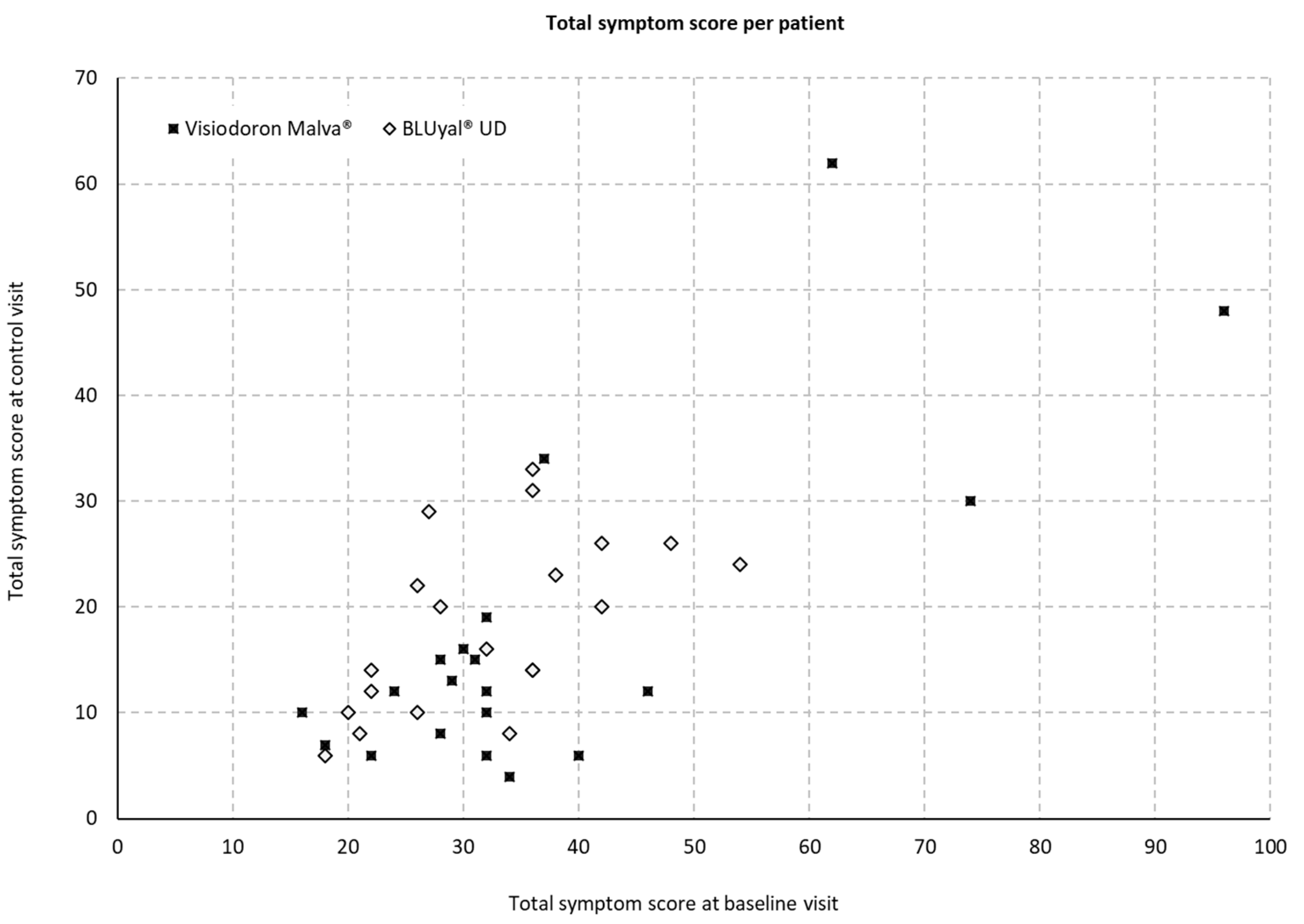
At the baseline and the control visit, the following symptoms were assessed for both eyes: burning, itching, foreign body sensation, blurred vision, sensation of dryness, photophobia, and pain.
Figure 3 displays the total symptom scores on both visits and for both treatments. The scoring can range from 0 to 140.
The evaluation of the sum-score of all symptoms showed a mean difference of 9.95 with an SD of 6.39 (minimum 0, maximum 24, median 8) for the eye drops containing HA and mallow extract and 6.95 with an SD of 4.19 (minimum -1, maximum 15, median 7) for the comparator. However, a two-sample t-test with a sufficient normal approximation at alpha level of 5 % showed no significant difference for the alleviation of symptoms between both medical devices (p = 0.0873).
3.2.2. Ocular Surface Disease Index (OSDI)
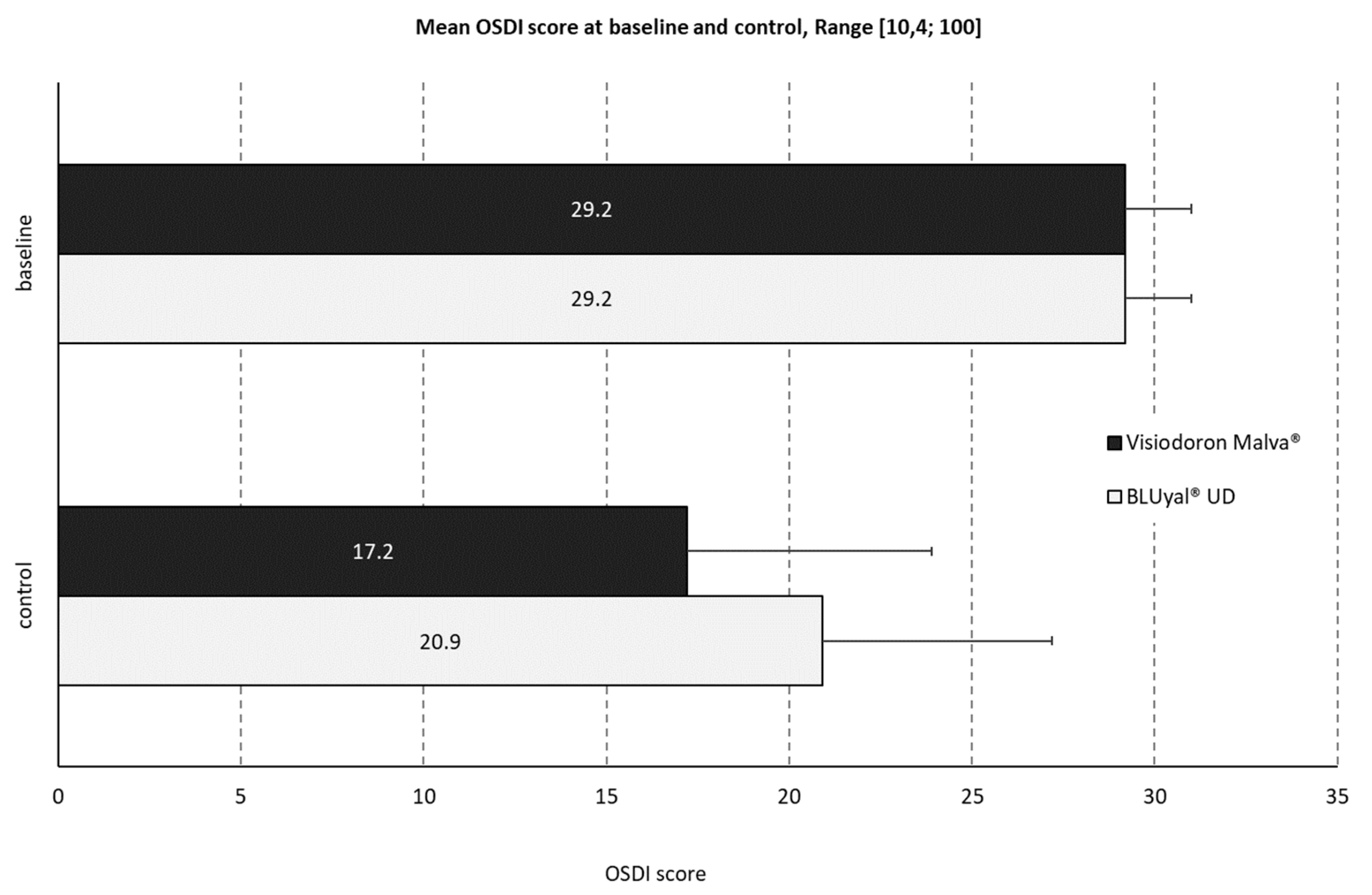
The mean baseline OSDI score (SD1.8) was identical for both products (see
Figure 4). The control score of the mallow extract containing preparation was 17.2 (SD 6.7, minimum 8.3, maximum 35.0, median 16.7). For the comparator, the mean OSDI control score was slightly higher: 20.9 (SD 6.3, minimum 10.4, maximum 33.3, median 19.8).
At baseline, all patients had a ‘moderate’ OSDI classification. After treatment with the product containing HA and mallow extract, 90 % of the patients improved to a ‘mild’ or ‘normal’ classification. For the comparator product, only 60 % of the patients showed an improvement in classification.
The distribution of the Schirmer test values showed almost the same results after both treatments.
3.2.3. Assessment by the Patients
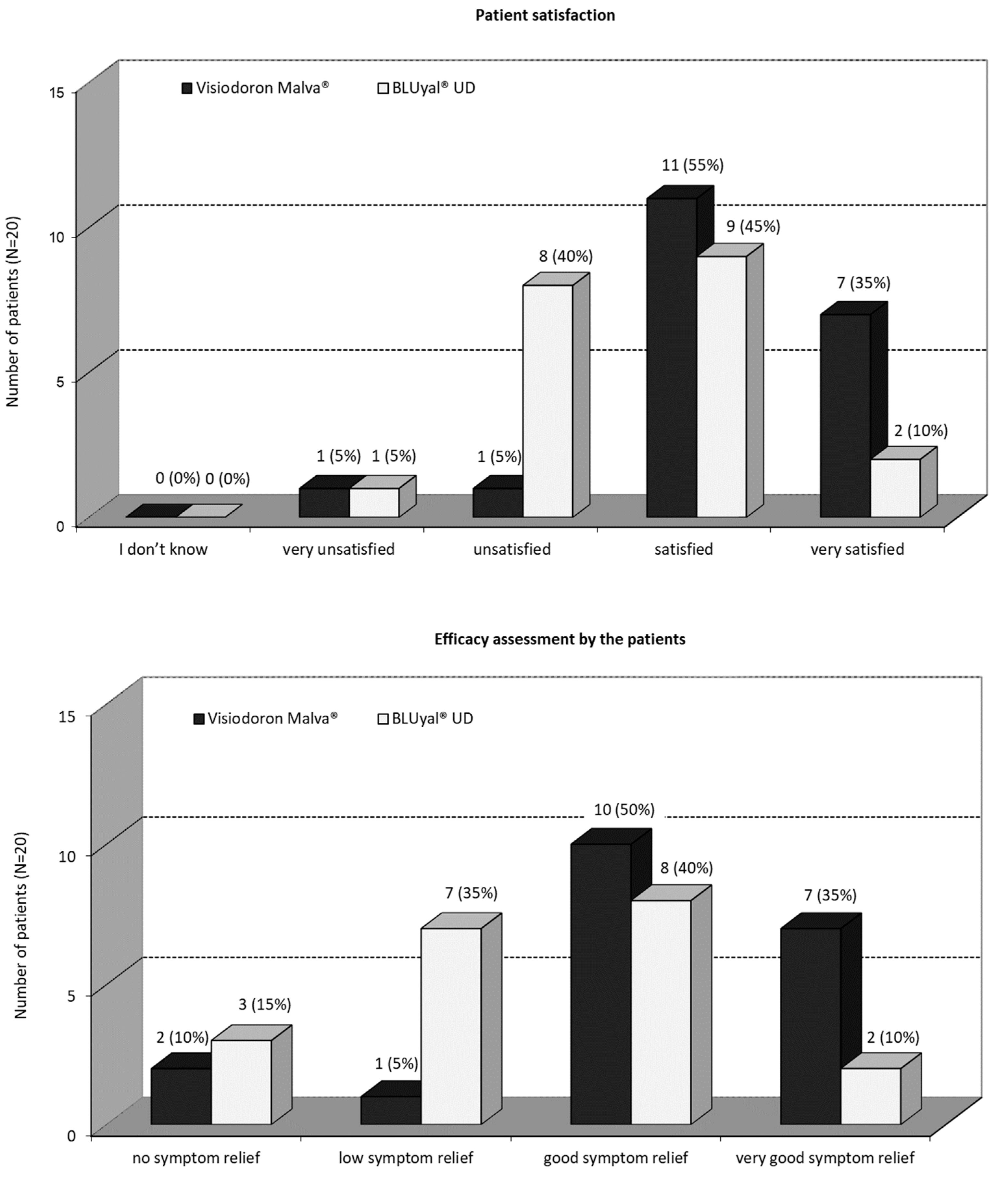
As one can see from
Figure 5a, a great majority of the patients were satisfied or very satisfied, with the mallow extract containing eye drops. With the treatment of the comparator product, 45 % of the patients were satisfied, 10 % were very satisfied, 40 % were unsatisfied and 5 % were very unsatisfied. There was a significant difference (5 % level) in favour of the mallow extract containing eye drops (p = 0.0226; Fisher’s exact test).
Figure 5b shows, that 85 % of the patients assessed the symptom relief, as good or even very good after treatment with the HA and mallow extract containing preparation. For the HA eye drops without mallow extract, 40 % of the patients rated a good symptom relief, 10 % a very good symptom relief, 35 % a low symptom relief and 15 % had no symptom relief. There was a significant difference (5 % level) in favour of the combination product (p = 0.0503; Fisher’s exact test).
In total, the efficacy assessment by the patients as well as the patient satisfaction shows a trend in favour of the mallow extract containing eye drops.
3.2.4. Individual Patient Preference
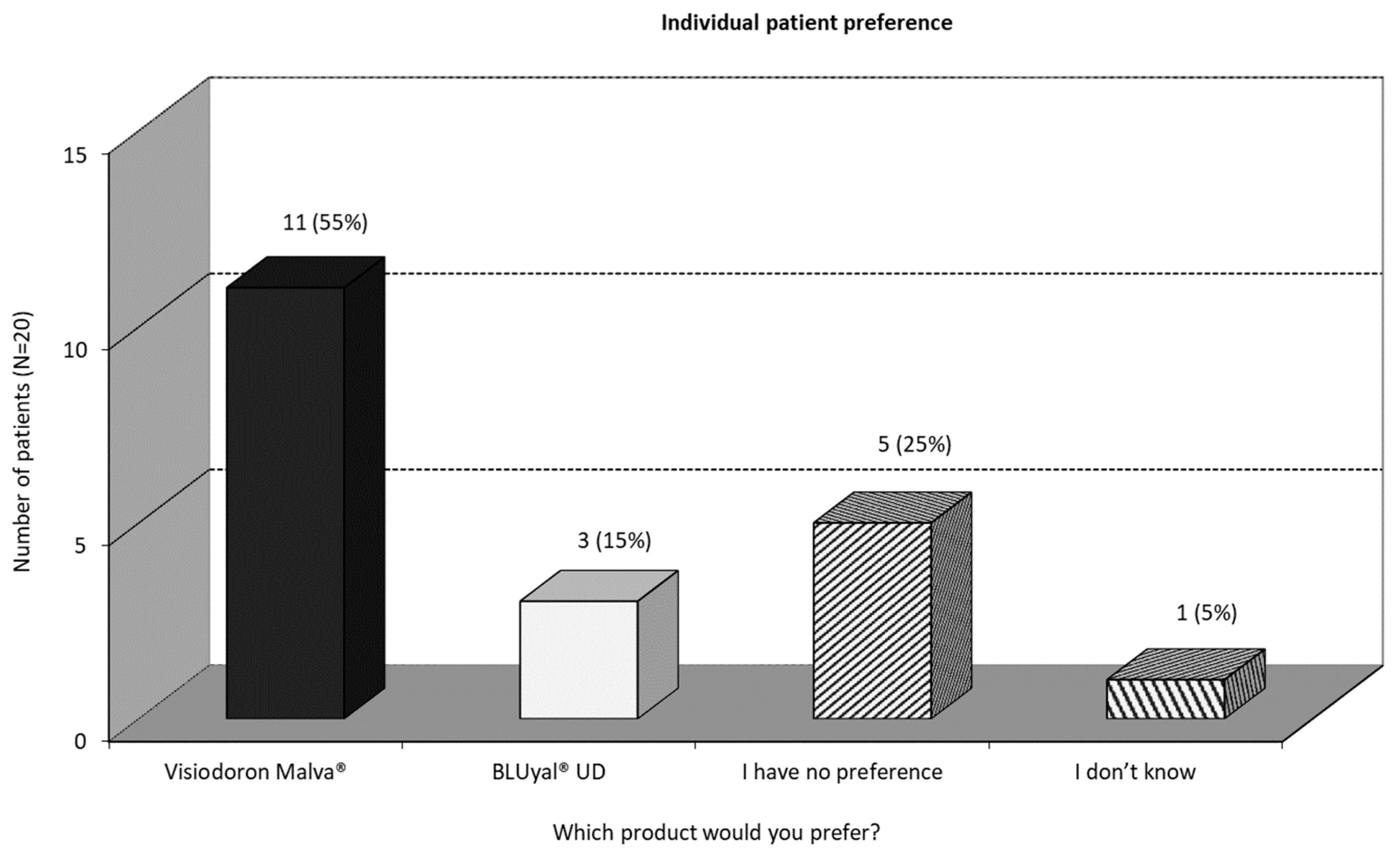
At the end of the study, all patients were asked for their preferred treatment. As one can see in
Figure 6, more than half of the patients would prefer the mallow extract containing preparation, 25 % have no preference and 15 % would prefer the eye drops containing HA only.
One can summarize, that, in terms of subjective measurement, the mallow extract containing eye drops are the preferred treatment in this pilot study.
3.2.5. Safety Assessment
There were no incidents. Consequently, no adverse events (AEs) or serious adverse events (SAEs) were reported or documented during the study.
4. Discussion
The motivation for this exploratory pilot study was to generate preliminary data that allows assessment of the combined lubricating effect of HA and mallow extract.
As demonstrated from the results, both treatments showed improvements in patients with moderate DED. This is in line with expectations as both products contain HA, which effectively lubricates the ocular surface and hereby alleviates symptoms.
For the measured efficacy parameters TBUT, OS-grading, OSDI, and also for the symptoms assessed during the visits at the ophthalmologic practices, no significant differences between the mallow extract containing preparation and its comparator were observed.
On the other hand, there were recognizable and in parts statistically significant differences between the treatments regarding patient satisfaction, individual patient preference and assessments of efficacy by patients and ophthalmologists. There was a difference in alleviation of symptoms as well as patient satisfaction and individual patient preference in favor of the eye drops containing HA in combination with mallow extract. As this is a pilot study, the question why the patients prefer the combined product remains open.
Both products were well tolerated, and no incidents were reported. Both preparations can be assessed as safe.
To what extent the observed differences can be attributed to the mallow extract remains unclear as the explorative design of this pilot study by its nature can only provide indications and deliver qualitative answers to this question. Anyway, earlier studies have shown that medicinal plant extracts improve tear film stability by decreasing osmolality and increasing tear production13-15. Another mechanism, that is already described in the literature is antioxidant activity, the modulation of inflammatory factors, the prevention of cell apoptosis and the regulation of androgens. All these processes can affect lacrimal glands and membrane cells, thereby delivering treatment options in DED.
Further investigations are needed to assess the impact of mallow extract on treatment of DED, for example measurements of the osmolarity of the tear film or with markers for inflammatory cytokines.
Author Contributions
Conceptualization, Andrea Attilio Basile; Data curation, Andrea Attilio Basile; Formal analysis, Rebecca Hufnagel; Funding acquisition, Andrea Attilio Basile; Investigation, Andrea Attilio Basile, Giulia Mandelli and Magda Cendali; Methodology, Andrea Attilio Basile, Giulia Mandelli and Magda Cendali; Project administration, Andrea Attilio Basile and Rebecca Hufnagel; Resources, Andrea Attilio Basile, Giulia Mandelli, Magda Cendali and Rebecca Hufnagel; Software, Rebecca Hufnagel; Supervision, Andrea Attilio Basile; Validation, Rebecca Hufnagel; Visualization, Andrea Attilio Basile, Giulia Mandelli and Rebecca Hufnagel; Writing – original draft, Rebecca Hufnagel; Writing – review & editing, Andrea Attilio Basile, Giulia Mandelli and Magda Cendali. Andrea Attilio Basile, Giulia Mandelli, and Magda Cendali are ophthalmologists and have contributed patients to the study as investigators. Andrea Attilio Basile supervised the research team. All authors participated in the revision of the manuscript drafts and all approved the final version of the manuscript.
Funding
This study was funded by Weleda Italy and Weleda Germany.
Statement of Ethics
Prior to study entry, informed consent was obtained from all patients involved in the pilot study. Ethical review and approval were waived for this study, due to the fact that at the time of study conduct, neither a favourable opinion from an ethics committee nor approval from the authorities was required in Italy for studies involving medical devices, provided they were conducted within the scope of the intended use, as in the present case. There was also no legal obligation to register such a study in a publicly accessible database, as the study had not been imposed by the authorities.
Data Availability Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants. Disclosure of data would not be in accordance with the European General Data Protection Regulation.
Acknowledgements
The authors would like to thank the members of the study group, Dott. Luca Boerci, Dott. Danilo Mazzacane and all patients for their contribution and participation in this study. Furthermore, the authors are very grateful for the support by Gabriella Sterrantino and Dr. Emilio Zavattaro ((former) employee of Weleda Italia), and Cristina Semaca (Weleda Germany) for preparation and evaluation of this study and the support of Dr. Nicola Liefold (Scientific Writing) in writing the paper. The funding of the medical writing assistance was done by Weleda Germany.
References
- O'Brien PD and Collum, LM. Dry eye: diagnosis and current treatment strategies. Curr. Allergy Asthma Rep. 2004, 4, 314–319. [Google Scholar] [CrossRef]
- Murube, J. Andrew de Roetth (1893-1981): dacryologist who introduced the term dry eye. Ocul. Surf. 2004, 2, 225–227. [Google Scholar] [CrossRef] [PubMed]
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 75–92. [CrossRef]
- Wolkoff P, Nojgaard JK, Troiano P, et al. Eye complaints in the office environment: precorneal tear film integrity influenced by eye blinking efficiency. Occup. Environ. Med. 2005, 62, 4–12. [Google Scholar] [CrossRef]
- Wolkoff P, Nojgaard JK, Franck C, et al. The modern office environment desiccates the eyes? Indoor Air 2006, 16, 258–265. [Google Scholar] [CrossRef]
- Khurana AK, Choudhary R, Ahluwalia BK, et al. Hospital epidemiology of dry eye. Indian. J. Ophthalmol. 1991, 39, 55–58. [Google Scholar]
- Juhlin, L. Hyaluronan in skin. J. Intern. Med. 1997, 242, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Hamerman D and Schuster, H. Hyaluronate in normal human synovial fluid. J. Clin. Invest. 1958, 37, 57–64. [Google Scholar] [CrossRef]
- Meyer K and Palmer, JW. The polysaccharide of the vitreous humor. J. Biol. Chem. 1934, 107, 629–634. [Google Scholar] [CrossRef]
- Weissmann B and Meyer, K. The structure of hyalobiuronic acid and of hyaluronic acid from umbilical Cord1, 2. J. Am. Chem. Soc. 1954, 76, 1753–1757. [Google Scholar] [CrossRef]
- Toole, BP. Hyaluronan: from extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Salwowska NM, Bebenek KA, Zadlo DA, et al. Physiochemical properties and application of hyaluronic acid: a systematic review. J. Cosmet. Dermatol. 2016, 15, 520–526. [Google Scholar] [CrossRef]
- Hitoe S, Tanaka J and Shimoda H. MaquiBright standardized maqui berry extract significantly increases tear fluid production and ameliorates dry eye-related symptoms in a clinical pilot trial. Panminerva Med. 2014, 56, 1–6. [Google Scholar]
- 14. Kim CS, Jo K, Lee IS, et al. Topical Application of Apricot Kernel Extract Improves Dry Eye Symptoms in a Unilateral Exorbital Lacrimal Gland Excision Mouse. Nutrients. [CrossRef]
- 15. Kimura Y, Mori D, Imada T, et al. Restoration of Tear Secretion in a Murine Dry Eye Model by Oral Administration of Palmitoleic Acid. Nutrients. [CrossRef]
- Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).