Submitted:
04 March 2023
Posted:
06 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
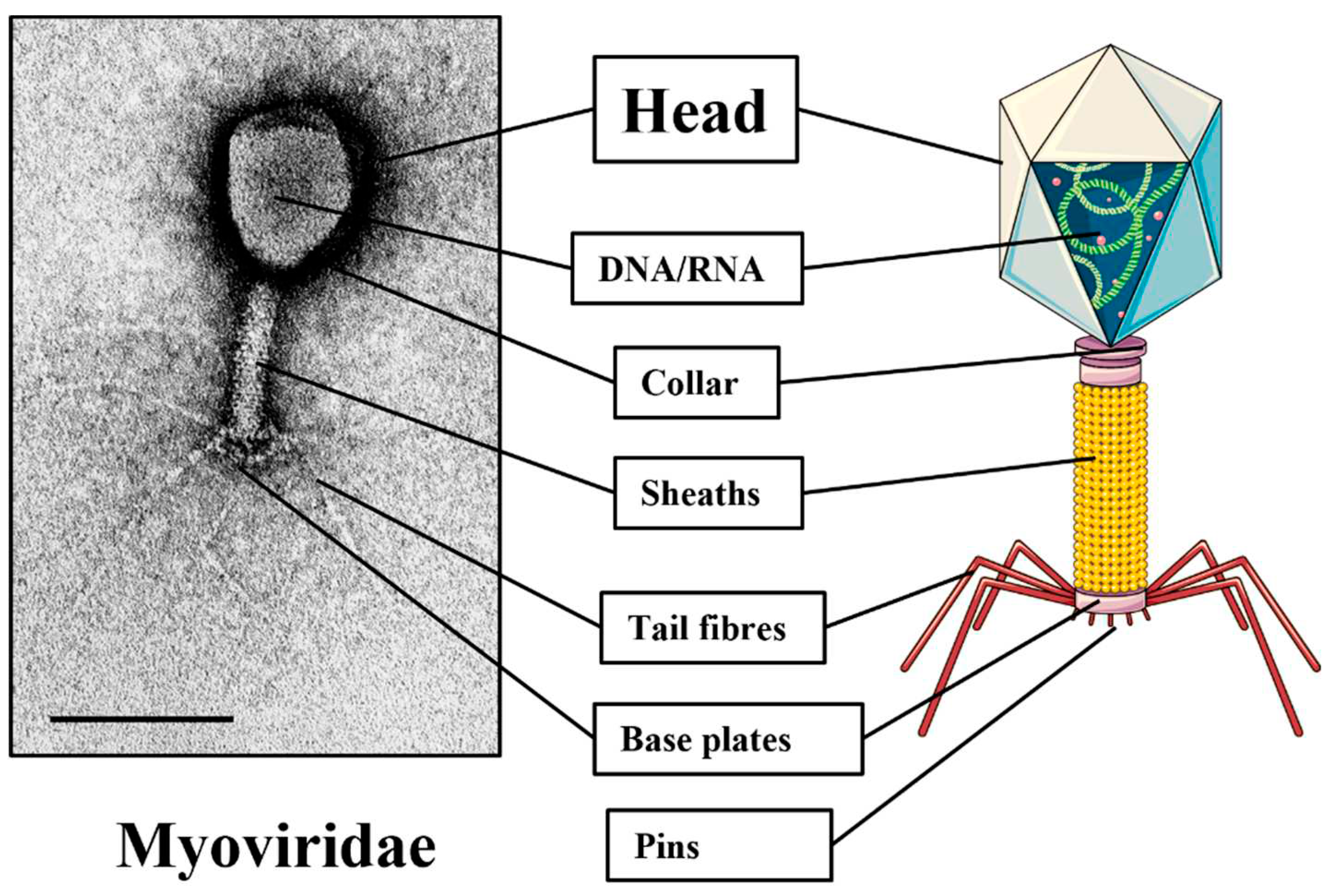
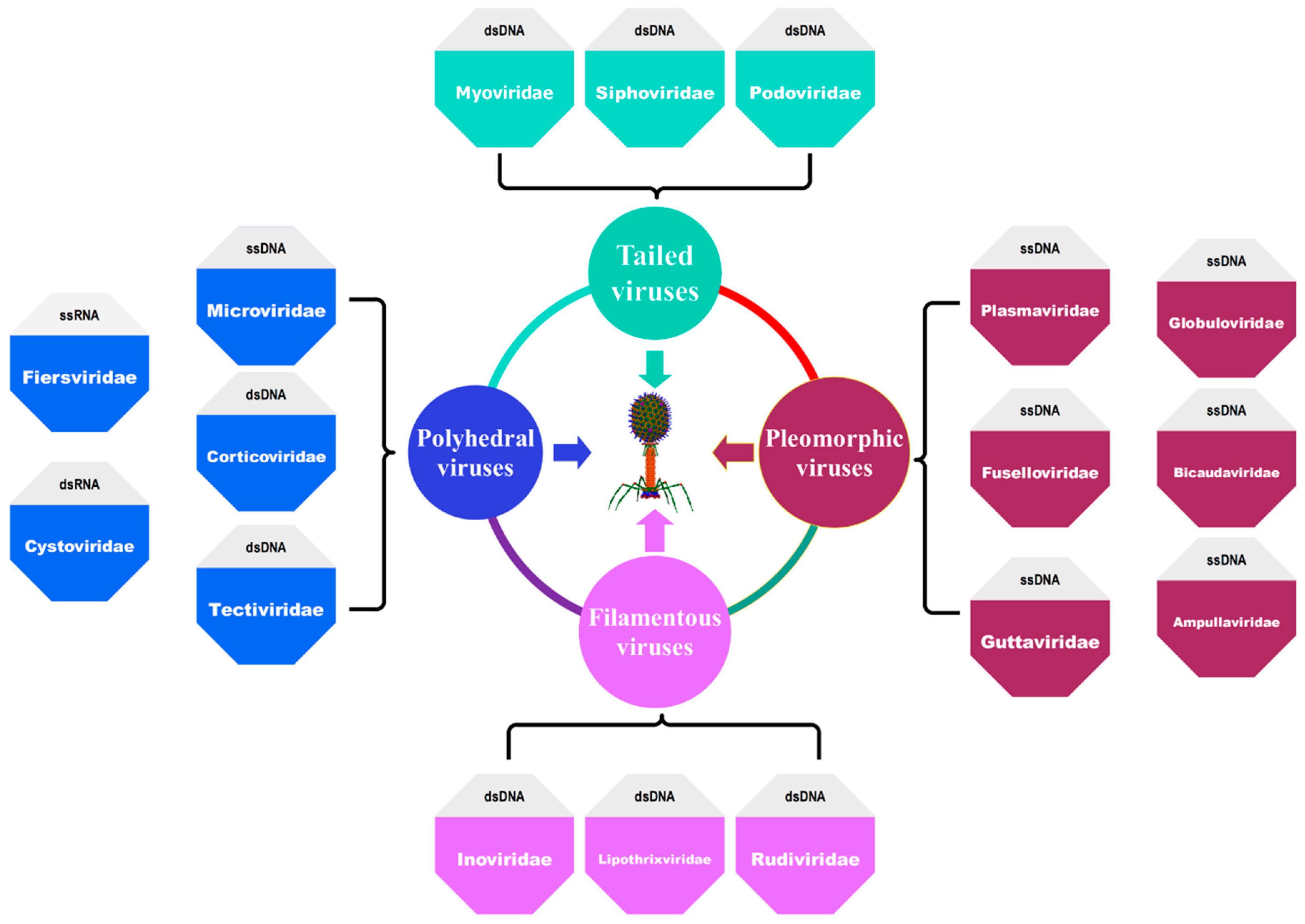
2. Understanding bacteriophages
3. Bacteriophages applications in various sectors:
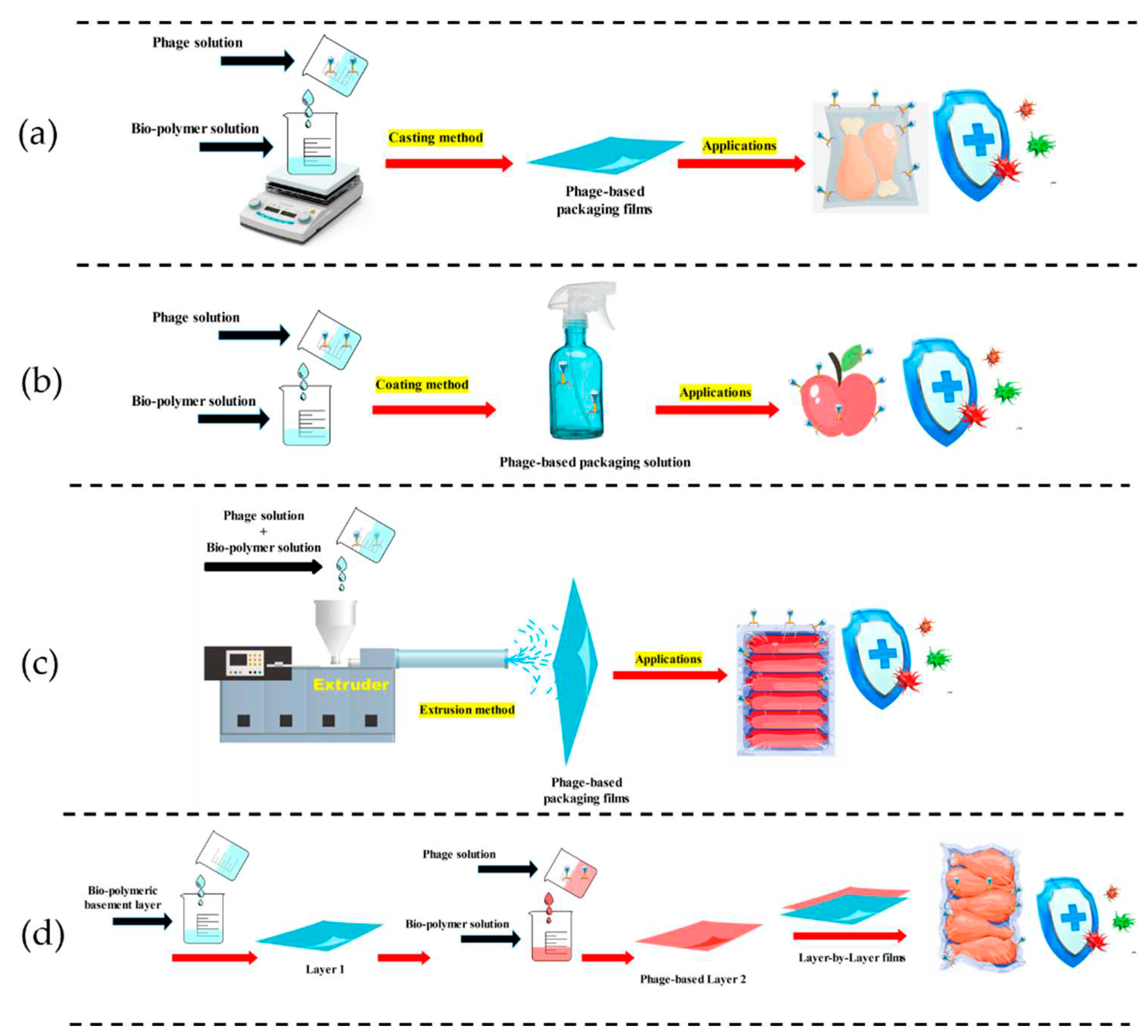
4. Recent updates on bacteriophage-based food packaging:
5. Summary and future research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Priyadarshi, R.; Kim, S.-M.; Rhim, J.-W. Pectin/Pullulan Blend Films for Food Packaging: Effect of Blending Ratio. Food Chem 2021, 347, 129022. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Priyadarshi, R.; Ezati, P.; Rhim, J.W. Curcumin and Its Uses in Active and Smart Food Packaging Applications - a Comprehensive Review. Food Chem 2022, 375, 131885. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Riahi, Z.; Rhim, J.-W.; Han, S.; Lee, S.-G. Sulfur Quantum Dots as Fillers in Gelatin/Agar-Based Functional Food Packaging Films. ACS Appl Nano Mater 2021, 4, 14292–14302. [Google Scholar] [CrossRef]
- Amin, U.; Khan, M.K.I.; Maan, A.A.; Nazir, A.; Riaz, S.; Khan, M.U.; Sultan, M.; Munekata, P.E.S.; Lorenzo, J.M. Biodegradable Active, Intelligent, and Smart Packaging Materials for Food Applications. Food Packag Shelf Life 2022, 33, 100903. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, H.; Julian McClements, D.; Chen, L.; Jiao, A.; Tian, Y.; Miao, M.; Jin, Z. Recent Advances in Intelligent Food Packaging Materials: Principles, Preparation and Applications. Food Chem 2022, 375, 131738. [Google Scholar] [CrossRef] [PubMed]
- Kuai, L.; Liu, F.; Chiou, B. sen; Avena-Bustillos, R.J.; McHugh, T.H.; Zhong, F. Controlled Release of Antioxidants from Active Food Packaging: A Review. Food Hydrocoll 2021, 120, 106992. [Google Scholar] [CrossRef]
- Perera, K.Y.; Jaiswal, S.; Jaiswal, A.K. A Review on Nanomaterials and Nanohybrids Based Bio-Nanocomposites for Food Packaging. Food Chem 2022, 376, 131912. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, S.; Wang, Q.; Lin, G.; Niu, B.; Guo, R.; Yan, H.; Wang, H. Sodium Alginate/Chitosan-Based Intelligent Bilayer Film with Antimicrobial Activity for Pork Preservation and Freshness Monitoring. Food Control 2023, 148, 109615. [Google Scholar] [CrossRef]
- Huang, K.; Nitin, N. Edible Bacteriophage Based Antimicrobial Coating on Fish Feed for Enhanced Treatment of Bacterial Infections in Aquaculture Industry. Aquaculture 2019, 502, 18–25. [Google Scholar] [CrossRef]
- Azman, N.H.; Khairul, W.M.; Sarbon, N.M. A Comprehensive Review on Biocompatible Film Sensor Containing Natural Extract: Active/Intelligent Food Packaging. Food Control 2022, 141, 109189. [Google Scholar] [CrossRef]
- CDC Four Steps to Food Safety-CDC. Available online: https://www.cdc.gov/foodsafety/keep-food-safe.html (accessed on 29 January 2023).
- Hofer, U. The Cost of Antimicrobial Resistance. Nature Reviews Microbiology 2018, 17, 3–3. [Google Scholar] [CrossRef] [PubMed]
- C Reygaert, W. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter Baumannii Infection. Antimicrob Agents Chemother 2017, 61. [Google Scholar] [CrossRef]
- Orlova, E.V. Bacteriophages and Their Structural Organisation; IntechOpen, 2012; ISBN 978-953-51-0272-4. [Google Scholar]
- Batinovic; Wassef; Knowler; Rice; Stanton; Rose; Tucci; Nittami; Vinh; Drummond; et al. Bacteriophages in Natural and Artificial Environments. Pathogens 2019, 8, 100. [Google Scholar] [CrossRef]
- Abedon, S.T.; Thomas-Abedon, C.; Thomas, A.; Mazure, H. Bacteriophage Prehistory. Bacteriophage 2011, 1, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A. The Future of Bacteriophage Biology. Nat Rev Genet 2003, 4, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; López, A.; Sáez-Orviz, S.; Marcet, I.; García, P.; Rendueles, M.; Díaz, M. Effectiveness of Bacteriophages Incorporated in Gelatine Films against Staphylococcus Aureus. Food Control 2021, 121, 107666. [Google Scholar] [CrossRef]
- Hendrix, R.W. Bacteriophage Genomics. Curr Opin Microbiol 2003, 6, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S.; Streisinger, G.; Horne, R.W.; Champe, S.P.; Barnett, L.; Benzer, S.; Rees, M.W. Structural Components of Bacteriophage. J Mol Biol 1959, 1, 281–292. [Google Scholar] [CrossRef]
- Ackermann, H.-W. Phage Classification and Characterization. 2009; pp. 127–140.
- Ackermann, H.W. 5500 Phages Examined in the Electron Microscope. Arch Virol 2007, 152, 227–243. [Google Scholar] [CrossRef]
- Ackermann, H.W. Frequency of Morphological Phage Descriptions in 1995. Arch Virol 1996, 141, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.W.; Prangishvili, D. Prokaryote Viruses Studied by Electron Microscopy. Arch Virol 2012, 157, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Hyman, P. Phages for Phage Therapy: Isolation, Characterization, and Host Range Breadth. Pharmaceuticals 2019, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Hay, I.D.; Lithgow, T. Filamentous Phages: Masters of a Microbial Sharing Economy. EMBO Rep 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, H.M. ICTV Virus Taxonomy Profile: Corticoviridae. Journal of General Virology 2017, 98, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Prangishvili, D.; Mochizuki, T.; Krupovic, M. ICTV Virus Taxonomy Profile: Guttaviridae. Journal of General Virology 2018, 99, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Krupovic, M. ICTV Virus Taxonomy Profile: Plasmaviridae. Journal of General Virology 2018, 99, 617–618. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, H.M. ICTV Virus Taxonomy Profile: Corticoviridae. Journal of General Virology 2017, 98, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Poranen, M.M.; Mäntynen, S. ICTV Virus Taxonomy Profile: Cystoviridae. Journal of General Virology 2017, 98, 2423–2424. [Google Scholar] [CrossRef]
- Prangishvili, D.; Krupovic, M. ICTV Virus Taxonomy Profile: Bicaudaviridae. Journal of General Virology 2018, 99, 864–865. [Google Scholar] [CrossRef]
- Ackermann, H.-W. Tailed Bacteriophages: The Order Caudovirales. 1998; pp. 135–201.
- Chipman, P.R.; Agbandje-McKenna, M.; Renaudin, J.; Baker, T.S.; McKenna, R. Structural Analysis of the Spiroplasma Virus, SpV4: Implications for Evolutionary Variation to Obtain Host Diversity among the Microviridae. Structure 1998, 6, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Prangishvili, D.; Arnold, H.P.; Götz, D.; Ziese, U.; Holz, I.; Kristjansson, J.K.; Zillig, W. A Novel Virus Family, the Rudiviridae: Structure, Virus-Host Interactions and Genome Variability of the Sulfolobus Viruses SIRV1 and SIRV2. Genetics 1999, 152, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, R. The Virus in the Rivers: Histories and Antibiotic Afterlives of the Bacteriophage at the Sangam in Allahabad. Notes and Records: the Royal Society Journal of the History of Science 2020, 74, 625–651. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, V.; Nair, A.; Mallya, R.; Khan, T.; Omri, A. Antimicrobial Nanomaterials for Food Packaging. Antibiotics 2022, 11. [Google Scholar] [CrossRef]
- Gordillo Altamirano, F.L.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin Microbiol Rev 2019, 32. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Settier-Ramírez, L.; Gavara, R.; Hernández-Muñoz, P.; López Carballo, G. Development of Biodegradable Films Loaded with Phages with Antilisterial Properties. Polymers (Basel) 2021, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.W.; Skinner, J.; Sulakvelidze, A.; Mathis, G.F.; Hofacre, C.L. Bacteriophage Therapy for Control of Necrotic Enteritis of Broiler Chickens Experimentally Infected with Clostridium Perfringens. Avian Dis 2010, 54, 33–40. [Google Scholar] [CrossRef]
- Keerqin, C.; McGlashan, K.; Van, T.T.H.; Chinivasagam, H.N.; Moore, R.J.; Choct, M.; Wu, S.-B. A Lytic Bacteriophage Isolate Reduced Clostridium Perfringens Induced Lesions in Necrotic Enteritis Challenged Broilers. Front Vet Sci 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Plaza, N.; Castillo, D.; Pérez-Reytor, D.; Higuera, G.; García, K.; Bastías, R. Bacteriophages in the Control of Pathogenic Vibrios. Electronic Journal of Biotechnology 2018, 31, 24–33. [Google Scholar] [CrossRef]
- Silva, Y.J.; Costa, L.; Pereira, C.; Mateus, C.; Cunha, Â.; Calado, R.; Gomes, N.C.M.; Pardo, M.A.; Hernandez, I.; Almeida, A. Phage Therapy as an Approach to Prevent Vibrio Anguillarum Infections in Fish Larvae Production. PLoS One 2014, 9, e114197. [Google Scholar] [CrossRef]
- Holtappels, D.; Fortuna, K.; Lavigne, R.; Wagemans, J. The Future of Phage Biocontrol in Integrated Plant Protection for Sustainable Crop Production. Curr Opin Biotechnol 2021, 68, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Svircev, A.; Roach, D.; Castle, A. Framing the Future with Bacteriophages in Agriculture. Viruses 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Kutateladze, M.; Adamia, R. Bacteriophages as Potential New Therapeutics to Replace or Supplement Antibiotics. Trends Biotechnol 2010, 28, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Kutateladze, M. Experience of the Eliava Institute in Bacteriophage Therapy. Virol Sin 2015, 30, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Zolnikov, T.R. Global Health in Action Against a Superbug. Am J Public Health 2019, 109, 523–524. [Google Scholar] [CrossRef]
- Azam, A.H.; Tan, X.-E.; Veeranarayanan, S.; Kiga, K.; Cui, L. Bacteriophage Technology and Modern Medicine. Antibiotics 2021, 10, 999. [Google Scholar] [CrossRef]
- Raza, A.; jamil, M.; Tahir Aleem, M.; Aamir Aslam, M.; Muhammad Ali, H.; khan, S.; Kareem, N.; Asghar, T.; Gul, K.; Nadeem, H.; et al. Bacteriophage Therapy: Recent Development and Applications. Scholars Bulletin 2021, 7, 27–37. [Google Scholar] [CrossRef]
- Singh, S.; Shalini, R. Effect of Hurdle Technology in Food Preservation: A Review. Crit Rev Food Sci Nutr 2016, 56, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Chiozzi, V.; Agriopoulou, S.; Varzakas, T. Advances, Applications, and Comparison of Thermal (Pasteurization, Sterilization, and Aseptic Packaging) against Non-Thermal (Ultrasounds, UV Radiation, Ozonation, High Hydrostatic Pressure) Technologies in Food Processing. Applied Sciences 2022, 12, 2202. [Google Scholar] [CrossRef]
- Jagtap, N.S.; Wagh, R. v.; Chatli, M.K.; Malav, O.P.; Kumar, P.; Mehta, N. Functional Goat Meat Nuggets Fortified with Novel Bioactive Carica Papaya L. and Origanum Vulgare Extracts and Storage Stability Thereof. Nutr Food Sci 2019, 50, 402–414. [Google Scholar] [CrossRef]
- Abera, G. Review on High-Pressure Processing of Foods. Cogent Food Agric 2019, 5, 1568725. [Google Scholar] [CrossRef]
- Wagh, R. v.; Chatli, M.K. Response Surface Optimization of Extraction Protocols to Obtain Phenolic Rich Antioxidant from Sea Buckthorn and Their Potential Application into Model Meat System. J Food Sci Technol 2017, 54, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J. Irradiation as a Method for Decontaminating Food. Int J Food Microbiol 1998, 44, 189–204. [Google Scholar] [CrossRef]
- Kumar, V.; Chatli, M.K.; Wagh, R. v.; Mehta, N.; Kumar, P. Effect of the Combination of Natural Antioxidants and Packaging Methods on Quality of Pork Patties during Storage. J Food Sci Technol 2015, 52, 6230–6241. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, N.S.; Wagh, R. v.; Chatli, M.K.; Malav, O.P.; Kumar, P.; Mehta, N. Chevon Meat Storage Stability Infused with Response Surface Methodology Optimized Origanum Vulgare Leaf Extracts. Agricultural Research 2020, 9, 663–674. [Google Scholar] [CrossRef]
- Jagtap, N.S.; Wagh, R. v.; Chatli, M.K.; Kumar, P.; Malav, O.P.; Mehta, N. Optimisation of Extraction Protocol for Carica Papaya L. to Obtain Phenolic Rich Phyto-Extract with Prospective Application in Chevon Emulsion System. J Food Sci Technol 2019, 56, 71–82. [Google Scholar] [CrossRef]
- Wagh, R.V.; Chatli, M.K.; Ruusunen, M.; Puolanne, E.; Ertbjerg, P. Effect of Various Phyto-Extracts on Physico-Chemical, Colour, and Oxidative Stability of Pork Frankfurters. Asian-Australas J Anim Sci 2015, 28, 1178–1186. [Google Scholar] [CrossRef]
- Tauxe, R. v. Emerging Foodborne Pathogens. Int J Food Microbiol 2002, 78, 31–41. [Google Scholar] [CrossRef]
- Hameed, F.; Bandral, J.D.; Gupta, N.; Nayik, G.A.; Sood, M.; Rahman, R. Use of Bacteriophages as a Target Specific Therapy against Food-Borne Pathogens in Food Industry-a Review: Bacteriophage. Journal of microbiology, biotechnology and food sciences 2022, 11, e2949–e2949. [Google Scholar] [CrossRef]
- Sulakvelidze, A. Using Lytic Bacteriophages to Eliminate or Significantly Reduce Contamination of Food by Foodborne Bacterial Pathogens. J Sci Food Agric 2013, 93, 3137–3146. [Google Scholar] [CrossRef]
- Ackermann, H.W. Frequency of Morphological Phage Descriptions in the Year 2000. Arch Virol 2001, 146, 843–857. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial Food Packaging: Potential and Pitfalls. Front Microbiol 2015, 6. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Cristian Oprea, O.; Durmu¸s, D.; Kaya, A.; Andronescu, E. Biodegradable Antimicrobial Food Packaging: Trends and Perspectives. Foods. [CrossRef] [PubMed]
- López de Dicastillo, C.; Settier-Ramírez, L.; Gavara, R.; Hernández-Muñoz, P.; López Carballo, G. Development of Biodegradable Films Loaded with Phages with Antilisterial Properties. Polymers (Basel) 2021, 13, 327. [Google Scholar] [CrossRef]
- Radford, D.; Guild, B.; Strange, P.; Ahmed, R.; Lim, L.-T.; Balamurugan, S. Characterization of Antimicrobial Properties of Salmonella Phage Felix O1 and Listeria Phage A511 Embedded in Xanthan Coatings on Poly(Lactic Acid) Films. Food Microbiol 2017, 66, 117–128. [Google Scholar] [CrossRef]
- Korehei, R.; Kadla, J. Incorporation of T4 Bacteriophage in Electrospun Fibres. J Appl Microbiol 2013, 114, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Pacan, J.C.; Wang, Q.; Sabour, P.M.; Huang, X.; Xu, Y. Enhanced Alginate Microspheres as Means of Oral Delivery of Bacteriophage for Reducing Staphylococcus Aureus Intestinal Carriage. Food Hydrocoll 2012, 26, 434–440. [Google Scholar] [CrossRef]
- Choi, I.; Yoo, D.S.; Chang, Y.; Kim, S.Y.; Han, J. Polycaprolactone Film Functionalized with Bacteriophage T4 Promotes Antibacterial Activity of Food Packaging toward Escherichia Coli. Food Chem 2021, 346, 128883. [Google Scholar] [CrossRef] [PubMed]
- Gouvêa, D.M.; Mendonça, R.C.S.; Soto, M.L.; Cruz, R.S. Acetate Cellulose Film with Bacteriophages for Potential Antimicrobial Use in Food Packaging. LWT - Food Science and Technology 2015, 63, 85–91. [Google Scholar] [CrossRef]
- Vonasek, E.; Le, P.; Nitin, N. Encapsulation of Bacteriophages in Whey Protein Films for Extended Storage and Release. Food Hydrocoll 2014, 37, 7–13. [Google Scholar] [CrossRef]
- Salalha, W.; Kuhn, J.; Dror, Y.; Zussman, E. Encapsulation of Bacteria and Viruses in Electrospun Nanofibres. Nanotechnology 2006, 17, 4675–4681. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Han, P.; Dong, S.; Li, H. Preparation and Application of Bacteriophage-Loaded Chitosan Microspheres for Controlling Lactobacillus Plantarum Contamination in Bioethanol Fermentation. RSC Adv 2015, 5, 69886–69893. [Google Scholar] [CrossRef]
- Boggione, D.M.G.; Batalha, L.S.; Gontijo, M.T.P.; Lopez, M.E.S.; Teixeira, A.V.N.C.; Santos, I.J.B.; Mendonça, R.C.S. Evaluation of Microencapsulation of the UFV-AREG1 Bacteriophage in Alginate-Ca Microcapsules Using Microfluidic Devices. Colloids Surf B Biointerfaces 2017, 158, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Radford, D.; Guild, B.; Strange, P.; Ahmed, R.; Lim, L.-T.; Balamurugan, S. Characterization of Antimicrobial Properties of Salmonella Phage Felix O1 and Listeria Phage A511 Embedded in Xanthan Coatings on Poly(Lactic Acid) Films. Food Microbiol 2017, 66, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Amarillas, L.; Lightbourn-Rojas, L.; Angulo-Gaxiola, A.K.; Basilio Heredia, J.; González-Robles, A.; León-Félix, J. The Antibacterial Effect of Chitosan-based Edible Coating Incorporated with a Lytic Bacteriophage against Escherichia Coli O157:H7 on the Surface of Tomatoes. J Food Saf 2018, 38. [Google Scholar] [CrossRef]
- Alves, D.; Marques, A.; Milho, C.; Costa, M.J.; Pastrana, L.M.; Cerqueira, M.A.; Sillankorva, S.M. Bacteriophage ΦIBB-PF7A Loaded on Sodium Alginate-Based Films to Prevent Microbial Meat Spoilage. Int J Food Microbiol 2019, 291, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kimmelshue, C.; Goggi, A.S.; Cademartiri, R. The Use of Biological Seed Coatings Based on Bacteriophages and Polymers against Clavibacter Michiganensis Subsp Nebraskensis in Maize Seeds. Sci Rep 2019, 9, 17950. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Nitin, N. Edible Bacteriophage Based Antimicrobial Coating on Fish Feed for Enhanced Treatment of Bacterial Infections in Aquaculture Industry. Aquaculture 2019, 502, 18–25. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.; Cui, H. Inactivation of Escherichia Coli O157:H7 Treated by Poly-L-lysine-coated Bacteriophages Liposomes in Pork. J Food Saf 2018, 38. [Google Scholar] [CrossRef]
- Novello, J.; Sillankorva, S.; Pires, P.; Azeredo, J.; Wanke, C.H.; Tondo, E.C.; Bianchi, O. Inactivation of Pseudomonas Aeruginosa in Mineral Water by DP1 Bacteriophage Immobilized on Ethylene-vinyl Acetate Copolymer Used as Seal Caps of Plastic Bottles. J Appl Polym Sci 2020, 137, 49009. [Google Scholar] [CrossRef]
- Choi, I.; Yoo, D.S.; Chang, Y.; Kim, S.Y.; Han, J. Polycaprolactone Film Functionalized with Bacteriophage T4 Promotes Antibacterial Activity of Food Packaging toward Escherichia Coli. Food Chem 2021, 346, 128883. [Google Scholar] [CrossRef]
- Kim, S.; Chang, Y. Anti-Salmonella Polyvinyl Alcohol Coating Containing a Virulent Phage PBSE191 and Its Application on Chicken Eggshell. Food Research International 2022, 162, 111971. [Google Scholar] [CrossRef]
- Weng, S.; López, A.; Sáez-Orviz, S.; Marcet, I.; García, P.; Rendueles, M.; Díaz, M. Effectiveness of Bacteriophages Incorporated in Gelatine Films against Staphylococcus Aureus. Food Control 2021, 121, 107666. [Google Scholar] [CrossRef]
- Stipniece, L.; Rezevska, D.; Kroica, J.; Racenis, K. Effect of the Biopolymer Carrier on Staphylococcus Aureus Bacteriophage Lytic Activity. Biomolecules 2022, 12, 1875. [Google Scholar] [CrossRef]
- Cui, H.; Yang, X.; Li, C.; Ye, Y.; Chen, X.; Lin, L. Enhancing Anti-E. Coli O157:H7 Activity of Composite Phage Nanofiber Film by D-Phenylalanine for Food Packaging. Int J Food Microbiol 2022, 376, 109762. [Google Scholar] [CrossRef] [PubMed]
- Kamali, S.; Yavarmanesh, M.; Habibi Najafi, M.B.; Koocheki, A. Development of Whey Protein Concentrate/Pullulan Composite Films Containing Bacteriophage A511: Functional Properties and Anti-Listerial Effects during Storage. Food Packag Shelf Life 2022, 33, 100902. [Google Scholar] [CrossRef]
- Vonasek, E.L.; Choi, A.H.; Sanchez, J.; Nitin, N. Incorporating Phage Therapy into WPI Dip Coatings for Applications on Fresh Whole and Cut Fruit and Vegetable Surfaces. J Food Sci 2018, 83, 1871–1879. [Google Scholar] [CrossRef]
- Kalkan, S. Vibrio Parahaemolyticus ATCC 17802 Inactivation by Using Methylcellulose Films Containing Encapsulated Bacteriophages. Turk J Vet Anim Sci 2018, 42, 480–485. [Google Scholar] [CrossRef]
- Tomat, D.; Soazo, M.; Verdini, R.; Casabonne, C.; Aquili, V.; Balagué, C.; Quiberoni, A. Evaluation of an WPC Edible Film Added with a Cocktail of Six Lytic Phages against Foodborne Pathogens Such as Enteropathogenic and Shigatoxigenic Escherichia Coli. LWT 2019, 113, 108316. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.-S.; Han, J. Maltodextrin-Trehalose Miscible System-Based Bacteriophage Encapsulation: Studies of Plasticizing Effect on Encapsulated Phage Activity and Food Application as an Antimicrobial Agent. Food Control 2023, 146, 109550. [Google Scholar] [CrossRef]
- Kim, S.; Chang, Y. Anti-Salmonella Polyvinyl Alcohol Coating Containing a Virulent Phage PBSE191 and Its Application on Chicken Eggshell. Food Research International 2022, 162, 111971. [Google Scholar] [CrossRef] [PubMed]
- Lone, A.; Anany, H.; Hakeem, M.; Aguis, L.; Avdjian, A.-C.; Bouget, M.; Atashi, A.; Brovko, L.; Rochefort, D.; Griffiths, M.W. Development of Prototypes of Bioactive Packaging Materials Based on Immobilized Bacteriophages for Control of Growth of Bacterial Pathogens in Foods. Int J Food Microbiol 2016, 217, 49–58. [Google Scholar] [CrossRef] [PubMed]
| Bacteriophage/ Cocktails |
Targeted pathogens |
Bio/ Polymer matrix |
Application | References |
|---|---|---|---|---|
| BFSE16, BFSE18, PaDTA1, PaDTA9, PaDTA10 and PaDTA11 | Salmonella enterica subsp. enterica serovar Typhimurium ATCC 14028 | Acetate cellulose film | Active | [73] |
| T4 bacteriophage | E. coli BL21 | Whey protein films | Active | [74] |
| T7, T4, λ | Escherichia coli, Staphylococcus albus | Poly (vinyl alcohol) | Active | [75] |
| Lactobacillus plantarum bacteriophage | L. plantarum | Chitosan microspheres | Active | [76] |
| LinM-AG8, LmoM-AG13, and LmoM-AG20 | L. monocytogenes and E. coli O104:H4 | Cellulose membranes/ alginate beads | RTE food | [76] |
| UFV-AREG1 | Escherichia coli O157:H7 | Calcium alginate matrix | - | [77] |
| Salmonella phage Felix/Listeria phage A511 | S. Typhimurium and L. monocytogenes cultures. | Poly(lactic acid) | Precooked sliced turkey breast | [78] |
| vB_EcoM34X, vB_EcoSH2Q and vB_EcoMH2W | E. coli O157:H7 CECT 4076 | Chitosan | Tomato | [79] |
| ϕIBB-PF7A | Pseudomonas fluorescens | Sodium alginate | Skinless chicken breast fillets | [80] |
| CN8 bacteriophages | Clavibacter michiganensis subsp. nebraskensis | Polyvinyl polymers with alcohol | Zea mays L. seeds. | [81] |
| T7 phages (#BAA-1025-B2) | E. coli BL21 | Whey protein isolate | Fish feed | [82] |
| E. coli O157:H7 bacteriophages | Escherichia coli O157:H7 | Poly-L-lysine | Pork suspension | [83] |
| vB_PaeM_CEB_DP1 | Pseudomonas aeruginosa | Ethylene-vinyl acetate | Mineral water bottles | [84] |
| Phage T4 | E. coli O157:H7 | Polycaprolactone film | Beef | [85] |
| FO1 | S. Enteritidis | Electrospun PHBV/nanofiber/coating films | ||
| PBSE191 | S. Enteritidis | Polyvinyl alcohol | Active | [86] |
| PhiIPLA-RODI | Staphylococcus aureus | Gelatine | Cheese | [87] |
| Pyo bacteriophages/ Staph bacteriophages | S. aureus | Chitosan and alginate | - | [88] |
| E. coli O157 | Escherichia coli O157:H7 | Sodium alginate /polyethylene oxide (PEO) nanofibers | Beef, cucumber, and cherry tomato | [89] |
| Listeria phage A511 | Listeria monocytogenes 19113 | Whey protein concentrate/pullulan | - | [90] |
| T7 bacteriophages | Escherichia coli BL21 | Whey protein isolate (WPI) | Coating | [91] |
| V. parahaemolyticus-derived phages | Vibrio parahaemolyticus ATCC 17802 | Methylcellulose | films | [92] |
| T-even type, DT1 to DT6 | E. coli DH5α | Whey protein concentrate | Fish fillets | [93] |
| Phage T4 | Escherichia coli K12 | Maltodextrin and trehalose as encapsulating agents | Nutrient broth, skimmed milk, and beef juices | [94] |
| PBSE191 | Salmonella Enteritidis | Polyvinyl alcohol (PVA) film | Chicken eggshell | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





