1. Introduction
Physical activity is a crucial aspect of human life, as suggested by the evolutionary theory linking basal physical activities to survival[
1,
2]. Compelling evidence from recent decades shows that exercise protects against degenerative conditions such as muscle atrophy[
3], osteoporosis[
4], Alzheimer's disease (AD) and Parkinson's disease (PD)[
5], as well as slow their progression in individuals who have already been diagnosed. Exercise has also been linked to improve tissue growth and renewal in the regenerative aspect, such as increased myogenisis[
6] and osteogensis[
7], as well as better neurogensis[
8], which may further contribute to overall health and a mitigated risk of degenerative disease. Molecular effectors involved in exercise-related benefits have been identified through advanced molecular techniques.
In 2012, Fibronectin domain-containing protein 5 (FNDC5), an underappreciated transmembrane protein, was discovered as the precursor of irisin, a myokine primarily expressed in skeletal muscle during exercise[
9]. It promotes the browning of white adipose tissue and activates thermogenesis in response to mechanical stimuli like exercise, following upregulation of peroxisome proliferator-activated receptor gamma coactivator-1-alpha (PGC-1α)[
9]. Since the groundbreaking discovery of irisin, the scientists have been captivated into this “golden rush”, as evidenced by a steady annual increase in the number of publications on this topic, with over thousands of studies published to date. Primarily, Irisin has garnered significant research attention for its role in regulating energy metabolism and metabolic disorders such as obesity and diabetes mellitus, which are closely linked to physical activity levels[
10,
11]. The ability of irisin to induce positive effects of exercise at the molecular level has prompted further investigation into its pathobiological roles, clinical significance, and therapeutic potential in various diseases, encompassing not only metabolic disorders but also degenerative conditions[
12].
Clinical studies have explored the correlation between plasma irisin levels and degenerative disorders, utilizing a range of assays such as antibody-based methods (Western Blot, ELISA, Protein Liquid Chip Assay) and label-free methods (Quantitative Mass Spectrometry) to detect irisin concentration[
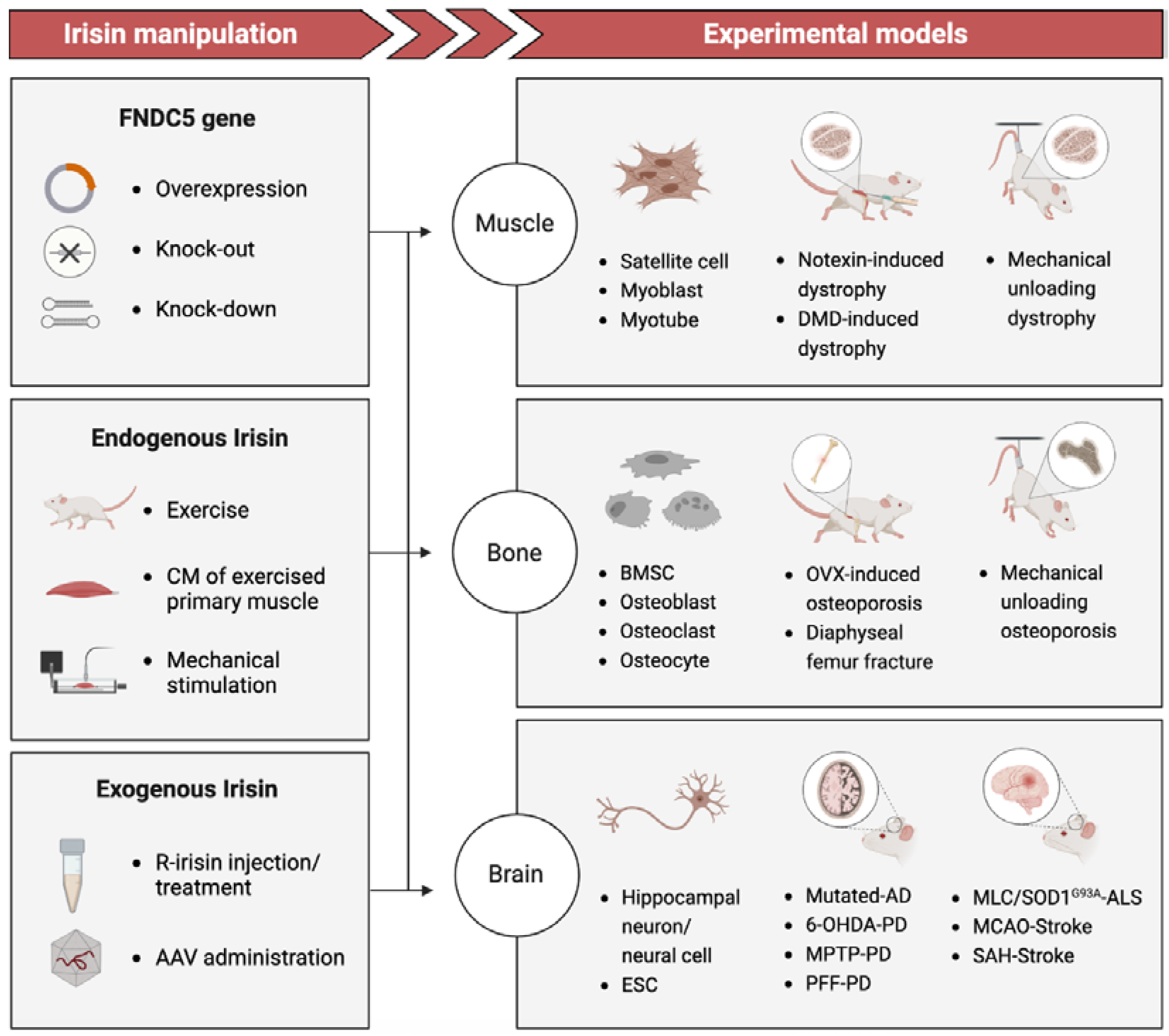
13]. While these investigations have directly provided initial evidence of how serum level of irisin associated with gradually deterioration of tissues and organs over time, the lack of reliable measurements for endogenous irisin has resulted in numerous contradictions and uncertainties. However, Basic studies (
Figure 1) using disease-mimicking animal models and tissue-engineering models to manipulate irisin levels via recombinant irisin (r-irisin) administration or gene gain/loss of function have demonstrated the regenerative potential of irisin treatment in reversing functional impairment and potentially permanent damage[
14,
15,
16]. The underlying mechanism by which irisin promotes tissue regeneration appears to exhibit variability on a case-by-case basis.
This article aims to comprehensively and systematically review the clinical and basic studies of irisin in the context of degenerative conditions. Furthermore, we explore and discuss the physiological processes involved in deteriorated or damaged tissue, wherein irisin is hypothesized to act as a regenerative effector and facilitates tissue regeneration.
2. Current Evidence: Irisin As a Potential Link to Degenerative Disorders
Degenerative diseases are a group of conditions that involve the gradual deterioration of tissues and organs over time, resulting in functional impairment and potentially permanent damage. These conditions typically become more prevalent as individuals age and can affect various parts of the body, such as muscles, bones, the brain, and nervous system. Examples of typical degenerative conditions include muscle atrophy, osteoporosis, amyotrophic lateral sclerosis (ALS), AD and PD. Currently, there is no cure for most degenerative diseases, and treatment options typically focus on managing symptoms and improving quality of life[
30,
31,
32]. Considering the responsiveness of irisin to exercise, researchers are highly motivated to explore the potential of this versatile myokine as a biomarker or preferred treatment option[
33].
2.1. Muscle Atrophy
Skeletal muscle atrophy denotes the loss of muscle mass resulting from an imbalance between protein synthesis and degradation, leading to reduced cross-sectional area of myofibers and diminished muscle strength[
34]. Potential triggers of muscle wasting include long-term immobilization, malnutrition, severe injuries, aging, as well as various serious and often chronic diseases, leading to increased morbidity, mortality, and decreased quality of life[
35]. Thus, addressing muscle wasting and fatigue has become a significant clinical concern. Irisin has emerged as a promising biomarker for predicting the onset of muscle atrophy[
16,
36]. However, to develop this molecule as an effective therapeutic intervention, a comprehensive understanding of both the underlying molecular processes and clinical investigations is required.
2.1.1. Clinical Studies
Back in 2014, An initial cohort investigation suggested that the levels of circulating irisin are correlated with the gains in muscle mass and strength resulting from exercise training[
10]. However, the limited sample size and outbalanced training regimens have weakened the convinces of this correlation between exercise-adaptive irisin and enhancing muscle mass. In another study involving human subjects, a positive correlation was also observed between irisin levels and grip strength (R=0.526, p=0.002) as well as leg strength (R=0.414, p=0.003) in the resistance training exercise group[
37]. This finding suggests that irisin levels may be indicative of muscle status, although the indirect nature of this evidence necessitates further investigation. A cross-sectional analysis of community-dwelling Koreans has provided compelling evidence supporting the potential utility of circulating irisin levels as a novel biomarker for age-related muscle degeneration[
38]. Notably, this study represents the first investigation of irisin in a pathological context and directly implicates irisin as a potential biomarker of muscle dysfunction with important implications for predicting the onset of sarcopenia and offering new avenues for monitoring age-related changes in muscle function. Parallel findings were reported in postmenopausal women with sarcopenia[
39]. Nonetheless, the use of irisin-based early screening as a staging tool for sarcopenia remains controversial in light of varying cohorts of older adults[
40], obesity groups[
41] or dialysis patients[
42].
In order to fully elucidate the potential of irisin as a biomarker and therapeutic target, further studies are needed to address the ambiguities surrounding the sarcopenia syndrome. This will require the standardization of parameters and techniques to more precisely define study cohorts, as well as the expansion of participant numbers and types.
2.1.2. Basic Studies
A complex interplay of pathophysiological factors orchestrates molecular mechanisms leading to muscle-specific protein degradation[
34]. Among these factors, maladapted anabolic proteins, such as myogenin[
43], and insulin-like growth factor 1(IGF-1)[
44], and catabolic proteins, including myostatin[
45], act in concert to disrupt the delicate equilibrium between muscle synthesis and breakdown. Circulating irisin levels have been reported as positively correlated with IGF-1 concentrations in humans[
46]. Similarly, increased FNDC5 mRNA and irisin secretion have been observed during myogenic differentiation in human myocytes, with upregulated PGC1α and myogenin expression [
47]. In a mouse model featuring myostatin-knockout, elevated irisin levels were observed in conjunction with increased musculature[
48]. These findings initially implied that irisin may play an important role in the regulation of muscle growth.
Irisin-treatment experiment and showed its ability of stimulating IGF-1 and inhibiting myostanin via extracellular signal-regulated kinases (Erk) pathway, which is involved in the synthesis of anabolic proteins, resulting in muscle growth[
47]. R-irisin therapy prevents and ameliorates disuse-induced muscle atrophy in a hind-limb suspended mice model by increasing muscle mass[
36]. Subsequent research has disposed the underlying mechanisms through which murine irisin protein injection can increase myogenic differentiation and myoblast fusion, leading to significant hypertrophy in mice. Specifically, these effects are mediated through the activation of interleukin-6 (IL-6) signaling[
16]. In the same study, they have also found the regenerative role of irisin as it triggered the satellite cell activation and reduced protein degradation to resist the injury-induced muscle loss. In addition, muscle atrophy is characterized by a shift in the fiber-type composition, for instance, the loss of contractile proteins can predominantly affect the type II fast fibers during aging[
49]. Correspondingly, a recent investigation showed a transition of fiber types, as an adaptive response to mechanical stimulation, is positively associated with the secretion level of irisin[
50].
Taken together, these findings suggest that irisin/FNDC5 may play a pleiotropic role in inducing an exercise phenotype in muscle mass and musculature, providing valuable insights into the potential mechanisms underlying the effects of irisin on muscle physiology, as well as highlighting the potential for irisin-based interventions aimed at enhancing muscle regeneration. With additional research on human skeletal muscle, irisin may hold the promise to pave the way for new interventions aimed at enhancing skeletal muscle function and improving overall health.
2.2. Osteoporosis
Osteoporosis is a skeletal disorder characterized by compromised bone strength resulting from the deterioration of bone mass, bone mineral density (BMD), and bone microarchitecture, which predisposes a person to an increased risk of fractures and disability[
51]. Osteoporosis is a prevalent degenerative disorder that primarily affects the elderly population[
52]. However, it can also manifest in young individuals with metabolic disorders[
53], neurodegenerative diseases[
54], cancer[
55], and astronauts during long-time space exploration, as a result of microgravity[
56]. While pharmacological intervention such as bisphosphonates, is an important treatment option for osteoporosis, there is growing concern about their long-term safety[
57]. As an alternative, exercise has emerged as a potent non-pharmacological intervention for both bone prevention and recovery[
58]. With the ability to replicate the effects of exercise, irisin has been extensively studied as a potential non-invasive treatment for bone tissue, with promising prospects for future development.
2.2.1. Clinical Studies
Early studies on irisin primarily are composed of epidemiological analyses that examined the relationship between irisin levels and fracture rates. Anastasilakis et al. reported that circulating irisin levels are associated with previous osteoporotic fractures postmenopausal women[
59]. Although this conclusion has been further validated in another 2 groups of postmenopausal women with either osteoporosis[
60] or hip fracture[
61]. However, it is still unclear whether this association is independent or if it is due to confounding factors, such as lower muscle mass. Singhal et al. provided important insights into the role of irisin in bone health, particularly in the context of physical activity, as the levels of irisin in this study were compared between adolescent female athletes and non-athletes, with results demonstrating that irisin levels serve as a determinant of areal and volumetric BMD, as well as bone strength estimates, in athletes but not in non-athletes[
62]. An inverse correlation has been observed between irisin levels and vertebral fragility fractures in overweight cohorts, while no significant correlation is found with BMD or lean mass[
63]. A noteworthy study has highlighted the therapeutic potential of irisin by revealing elevated levels of irisin in the blood during the bone healing process in patients, as well as by detecting the presence of irisin receptors in their bone cells[
64].
Collectively, numerous clinical evidences have disclosed a positive link between circulating levels of irisin and bone formation/repair. Nonetheless, it is acknowledged that the group sizes in these studies are relatively limited, meanwhile, the reported reference values of irisin levels are quantitatively inconsistent, varying widely from pg/mL to μg/mL in serum. These variations may be attributed to factors such as age, gender, body mass, pathological conditions, and most likely, differences in methodology and study design.
2.2.2. Basic Studies
The bone possesses an exceptional endogenous regenerative capacity that allows for scarless healing and the restoration of its previous mechanical function, even in challenging conditions such as fracture, advanced age or metabolic and degenerative disorders[
65]. The four most crucial cells involved in bone regeneration are osteogenic cells, osteoblasts, osteocytes, and osteoclasts. Together, they constitute the basic multicellular unit (BMU) responsible for the dynamic maintenance of bone formation and bone resorption[
66]. Irisin has been proven with the ability to regulate these four types of cells, tipping the balance of catabolic and anabolic responses[
18,
20,
67,
68,
69,
70].
Irisin exerts a potent anabolic impact on osteogenesis in vitro and in vivo. Specifically, both r-irisin and culture-media induced irisin (CM-irisin) enhances osteoblast differentiation and mineralization in bone marrow stromal cells (BMSCs)[
68,
70] and MC3T3 cells[
71] at a concentration of 100ng/ml, with accumulated collagen type 1 alpha-1 (Col1α 1) and alkaline phosphatase (Alp) in vitro. In vivo study conducted on young healthy mice found that r-irisin improved cortical bone mass and mechanical properties. In an osteoporotic model induced by mechanical unloading, treatment with r-irisin prevented bone density reduction and reversed cortical and trabecular BMD loss, indicating its potential to treat osteoporosis via bone formation. However, in another osteoporotic model induced by ovariectomy (OVX), Kim et al. disclosed that ablation of FNDC5 gene can prevent OVX-induced osteoporosis via inhibition of bone resorption, as osteoblast number or bone formation rate remains the same[
20]. Further evidence in the same study pointed out 6 daily injections of 1 mg/kg of irisin unleashed the expression of sclerostin[
20], an inhibitor of bone formation, while this inhibitor was suppressed with 4 weekly dose of 100μg/ml irisin[
69]. Whether irisin exerts catabolic or anabolic effects on the skeleton is likely depends on the dosage and administration regimen. It is hypothesized that intermittent pulses of irisin at lower doses, as seen during exercise, may provide anabolic benefits on bone, while high doses could lead to bone catabolism. Interestingly, both dosing patterns can prevent osteocyte osteolysis in the context of osteoporotic mice, demonstrating the ability of irisin in regulating bone modeling[
20,
72]. Additional studies validated the effects of irisin on osteoclastogenesis in vitro at a concentration of 2-10ng/ml and increased bone resorption in vivo in Fndc5-overexpressed mice model [
18].
Together, these findings suggest the intricate coordination mechanisms of irisin in bone metabolism and highlight the role of the exercise-mimetic myokine irisin in bone tissue as a multi-play of different types of bone cells. However, exploring the effects of irisin on the BMU, optimizing the in vivo dosage of irisin, and developing authentic bone injury model to directly examine the regenerative function of irisin in bone healing need further exploration.
2.3. Neurodegenerative Disease and Injury
Neurodegenerative disorders are characterized by progressive loss of specific groups of neurons, resulting in a deterioration in the intellectual and cognitive abilities[
73]. Examples of such diseases include but not limited in AD, PD, Huntington's disease, amyotrophic lateral sclerosis (ALS), which vary in their pathophysiology and can cause life-threatening impairments in memory, movement, speech, and breathing[
74]. Neurodegenerative diseases have become an increasingly prevalent issue, and despite extensive attempts into their mechanisms and potential solutions, finding an effective cure remains challenging[
75]. Numerous health research agencies, including the US National Institute on Aging, have affirmed the significant impact of appropriate physical exercise on enhancing cognition, across various age groups[
76]. Scientific literatures also report the positive effects of consistent, prolonged physical activity and exercise interventions on cognitive function[
77,
78]. As few treatments are available to approach cognitive impairment, exercise-mimicking irisin holds great promise as a non-pharmaceutical intervention for neurodegenerative disease or damages[
79].
2.3.1. Clinical Studies
A noteworthy investigation has revealed a decline in FNDC5/irisin levels within the brain and cerebrospinal fluid (CSF) of AD patients, but not in plasma, suggesting a distinct decrease within the central nervous system (CNS)[
21]. This study also identified a positive correlation between age and CSF irisin in non-demented individuals, but not in MCI and AD patients, implying that the age-related increase in brain FNDC5/irisin might be an endogenous mechanism to tackle the challenges confronted by the aging brain. Likewise, other studies have indicated that the cognitive benefits of circulating irisin may be weakened by pathological conditions induced by AD in the brain. The association between plasma levels of irisin and cognition is significant in participants without AD, but lost significance in those with AD[
80]. In addition, individuals with obesity and low serum irisin levels exhibits neurocognitive deficits during a visuospatial working memory task, indicating a potential influence of irisin on cognitive function in this population[
81]. However, this conclusion can be misleading due to the confounding effect of obesity.
Due to its anabolic role in skeletal muscle, FNDC5/irisin is believed to be linked to the pathophysiological process of ALS[
82,
83]. ALS patients present about four times higher levels of circulating irisin compared to healthy controls, and irisin is significantly associated with disease severity, respiratory function decline, and free fat mass level in ALS patients, particularly those with a hyper-metabolic status[
84]. In addition, irisin serves as an independent prognostic marker that enhances current risk stratification for stroke patients[
85], who undergo complex pathophysiological processes that lead to cerebral injury after stroke include excitotoxicity, oxidative stress, inflammation, and apoptosis. The CSF irisin concentration derived from stroke patients is lower than those derived from controls[
86]. Furthermore, Irisin appears to be a promising therapeutic approach for PD by evidence that exercise increased serum irisin levels in PD patients, positively linked to improved balance function (BBS scores) and reduced risk of falls and postural instability Irisin exhibits neuroprotection by preventing mitochondrial damage in PD patients.
2.3.2. Basic Studies
While cellular senescence can be a helpful response to injury, it can worsen age-related brain dysfunction when tissue regeneration is depleted or saturated in older individuals. FNDC5/irisin appears to be expressed robustly not only in skeletal muscle but also in various regions of brain tissue[
87,
88].
The roles of irisin in improving cognitive functions in clinical AD have already been described in previous clinical studies. Elevated circulating irisin by peripheral delivery could pass through the blood–brain barrier and result in enrichment of central irisin. Emerging experimental models have been conducted and established its role in ameliorating both the cognitive deficit and neuropathology in AD animals. Loss- and gain-of-function studies demonstrate irisin's protective role in restoring memory and cognitive function impairment[
21,
89]. This could be attributed to the modulation of β-cleavage of amyloid precursor protein, resulting in reduced Aβ production, by FNDC5 binding with the first 1-16 amino acids of amyloid precursor protein[
90]. In addition, the benefits of exercised on cognition or memory function can be ascribed to the activation of the PGC-1a/FNDC5/ brain-derived neurotrophic factor (BDNF) signaling via adenosine 50-monophosphate–activated protein kinase (AMPK) pathway[
21,
91,
92,
93].
In a rat model of PD induced by 6-hydroxydopamine (OHDA), levels of PGC1a, FNDC5, and BDNF in the striatum and hippocampus were negatively affected, leading to increased neurodegeneration. However, this decline was prevented by a 16-week treadmill training prior to the onset of PD[
22]. Upon another toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced PD rat model, Zarbakhsh et al. revealed that Irisin as a neurotrophic factor protects against dopaminergic neurons in the injured brain, suggesting its potential not only in the prevention of PD but also the prevention of neural damage in the brain[
23,
24]. The translational promise of irisin has been highlighted in a recent study, in which irisin prevents the formation of pathologic α-synuclein (α-Syn) and protects neurons against α-Syn preformed fibril (PFF) induced neurotoxicity in rats. Besides, elevating blood irisin levels in mice prevents neurodegeneration and physiological deficits induced by α-syn preformed fibrils injection[
25]. Camerino et al. proposed that irisin may restore muscle-nerve communication as a critical link between muscle and CNS in ALS and a likely pharmacological target in an ALS mouse model[
26].
After traumatic brain injury or stroke, multiple molecular mechanisms such as excitotoxicity, oxidative stress, inflammation, and apoptosis are involved in the pathophysiology[
94]. Several animal models have been used to assess the therapeutic potential of irisin in brain injury. Irisin has been shown to attenuate brain damage by reducing apoptosis and increasing BDNF protein in the cerebral cortex of mice in an experimental cerebral edema stroke model[
27]. Irisin reduces apoptotic cells in the ischemic cerebral cortex and enhances the immune reactivity of BDNF, resulting in a significant reduction in infarct size and cerebral edema[
27]. Cerebral Ischemic stroke, as the most common form of stoke, accounts for 80 % of the stroke cases[
94]. Exercise-induced hormone irisin also protects against neuronal injury via activation of the protein kinase B (Pkb, or Akt) and Erk1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia[
28]. Lastly, as another destructive form of stroke, Subarachnoid hemorrhage is relieved through the administration of exogenous irisin, which provides neuroprotective effects including maintaining mitochondrial morphology and promoting mitochondrial biogenesis[
29].
Current evidence shed light on more about the neuroprotective role of irisin as a transmitter of exercise defending neurodegeneration and destroyed brain function. Optimization of irisin delivery as a biologic therapy, such as adeno-associated virus (AAV) administration, holds promise for the treatment of PD and other neurodegenerative disorders[
25]. To better understand the potential therapeutic use of irisin in addressing neurodegenerative diseases and relevant injuries more investigations including more experimental models are to be conducted.
3. Is Irisin an Oracle to Tissue Repair/Regeneration?
The regenerative potential of exercise has been a focus of research in recent decades, as many adult human organs have limited regenerative capacity. Enhancing tissue regeneration is a major challenge in regenerative medicine. As previously discussed, irisin, an exercise-mimetic with therapeutic potential for various degenerative conditions, may offer a pharmaceutical alternative for individuals who are unable to exercise as well as provide a new approach to combat injury or senescence. The following section explores more particular roles of Irisin in tissue repair/regeneration.
3.1. Role of Irisin in Regulating Inflammatory Responses
Inflammation plays a crucial role in both chronic and degenerative diseases, but it also facilitates regeneration in injured tissues by clearing damaged cells and promoting tissue regrowth[
95,
96]. Insufficient inflammation can lead to damage of tissues by harmful stimuli, while persistent unresolved inflammation can result in various pathologies, such as fibrosis[
97] and cancer[
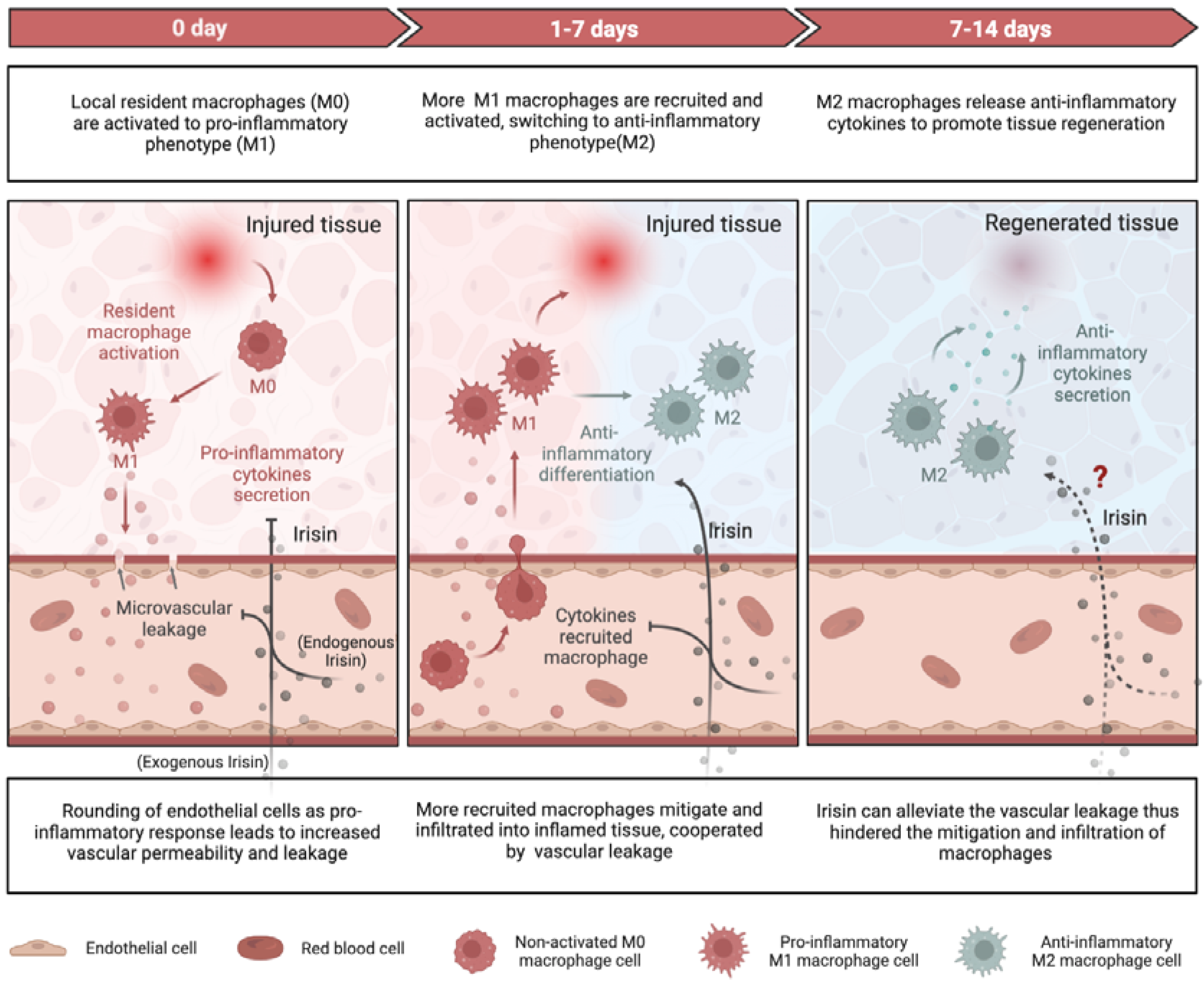
98]. Recent researches have elucidated the mechanisms by which irisin, an exercise resemble mediator, alleviate inflammatory responses during tissue repair/regeneration at the molecular and cellular levels (
Figure 2).
3.1.1. Pro-Inflammation and Anti-Inflammation
After exercise, which is the primary trigger for irisin production, the levels of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 β (IL-1β), IL-6, and macrophage inflammatory protein 1α and 1β (MIP1α, MIP1α) decline in the bloodstream, while the levels of anti-inflammatory cytokines including interleukin-4 (IL-4), interleukin-10 (IL-10), interleukin-1RA (IL-1RA), and interleukin-13 (IL-13) rise[
99,
100,
101,
102]. Mazur et al. reported an anti-inflammatory action of irisin downregulating IL-6 and TNF-α expression and secretion via inhibition of nuclear factor kappa B (NF-κB) in in adipocytes[
103]. Similarly, they also observed inhibited production of pro-inflammatory cytokines including IL-1β, TNFα, IL-6, and MCP-1 in RAW 264.7 macrophages via mitogen-activated protein kinases (MAPK) pathway with irisin treatment[
104].
The pro-inflammatory favored nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is also suppressed by irisin. Another pro-inflammatory oriented process, the activation of NLR family pyrin domain containing 3 (NLRP3) inflammasome when recognizing damaged proteins is inhibited by irisin[
105]. Irisin has been shown to mitigate lipopolysaccharide (LPS) induced liver or cardiac injury via suppressing NF-κB activities. Specifically, through inhibiting the activation of toll-like receptor 4 (TLR4) and downstream signaling molecules, which induce inflammatory response as sensing tissue injuries, irisin deceases the level of pro-inflammatory cytokines including IL-1β, TNF-α, and IL-6 and mitigate inflammation in a LPS-stressed cardiac injury in vivo[
106] and LPS-induced H9c2 cardiomyocyte injury in vitro[
107]. Furthermore, coordinated with melatonin, irisin could protect heart against sepsis-induced myocardial depression via impeding the macrophage stimulating 1 (Mst1) and hence c-Jun N-terminal kinase (JNK) pathway[
108]. Similar role of irisin was observed in brain injury, as irisin alleviates neuroinflammation, at least partially, via the integrin αVβ5/AMPK signaling pathway.[
109]
3.1.2. Macrophage Function
Irisin has also been shown to regulate macrophage function by mitigating the excessive production of reactive oxygen species (ROS), indicating its potential anti-inflammatory properties[
110]. M1-type macrophages secrete pro-inflammatory cytokines such as TNF-α and IL-1β, whereas M2-type macrophages produce anti-inflammatory cytokines such as IL-10[
111]. In the context of obesity-induced chronic inflammation, the administration of exogenous FNDC5 was found to inhibit LPS-induced differentiation of M1-type macrophages, while the deficiency of FNDC5 promoted such differentiation.[
112,
113]. The impact of irisin on M1 macrophages have been clarified, but it remains to be determined if irisin directly mediates the M2 macrophages.
3.1.3. Vascular Permeability
Irisin has the capacity of enhancing the vascular permeability via AMPK phosphorylation, as documented in several research publications [
47,
71]. Through AMPK signaling, both cell division cycle 42 (Cdc42) and rac family small GTPase 1(Rac1) are activated, which in turn reinforce the endothelial barrier function and prevents microvascular leakage during inflammation[
114]. The src family kinases (SFKs) mediates the vascular leakage through another mechanism-rounding of endothelial cells in response to various stimuli including LPS[
115]. Irisin has been found to suppress the tyrosine kinase activity of SFKs, thereby curtailing the downstream increased vascular permeability when disposing to inflammatory responses[
114]. By virtue of its interactions with either SFKs or AMPK signaling, irisin can attenuate vascular permeability and impede the infiltration and recruitment of macrophages or leukocytes into inflamed tissues, culminating in a dampened inflammatory response[
116].
Overall, irisin plays a protective role in reducing severe inflammation by decreasing pro-inflammatory cytokines, increasing anti-inflammatory cytokines, promoting M2-type macrophage polarization, and inhibiting vascular permeability to prevent immune cells infiltration into damaged tissues (
Figure 2). The essentiality of irisin's anti-inflammatory role in repairing and regenerating adipose, cardiovascular, liver, and brain tissues is well-established. However, for other types of tissue damage, such as muscle injury, further research is required.
3.2. Role of Irisin in Coordinating Proliferation, Differentiation and Apoptosis
In various types of stem cells and precursor cells, FNDC5/Irisin has been shown to promote proliferation, differentiation, and maturation, facilitating myogenesis, osteogenesis, and neurogenesis in both physiological and pathological conditions. In this chapter, we particularly focus on the regenerative role of irisin in the context of tissue self-renewal/repair with disease or damage.
3.2.1. Myogenesis
Regeneration of adult skeletal muscle is an asynchronous process that involves the activation, proliferation, and fusion of satellite cells to form new muscle fibers[
117]. Irisin has been found to play a role in this process by participating in myogenesis, including the activation of satellite cells, myogenic differentiation, and hypertrophic protein synthesis during the recovery or healing of atrophic muscle.
Satellite cells comprise a heterogeneous population of muscle stem cells that are typically activated by traumatic stimuli, exercise, or growth signals[
118]. Following activation, these cells undergo either symmetric or asymmetric divisions, resulting in an increased pool size or committed satellite cell progenitors, respectively, which are responsible for myogenesis[
119]. Subsequently, myogenic progenitors proliferate before differentiating, either by fusing with each other or with damaged fibers, leading to the restoration of fiber function and integrity, thereby protecting against degeneration or injury in adult muscle[
120]. A dosage of 2.5 μg/g irisin intraperitoneally is able to awaken quiescent satellite cells with upregulated MyoD and Pax7 expression in notexin-induced muscle injury in mice[
16]. Similar upregulation is observed in primary satellite cells derived from mouse hindlimb muscle in vitro[
16]. By contrast, treatment with 100 µg/kg irisin fails to affect satellite cells within vastus lateralis muscle in a hindlimb-suspended mice model[
69]. This contradiction can be attributed to by the dosage of irisin or the injection pattern of it.
Irisin has been recognized as a pro-myogenic effector that promotes the differentiation and fusion of myogenic myoblasts through IL-6 signaling both in vitro and in vivo[
16]. This pro-myogenic effect leads to significant hypertrophy in injured muscle with increased numbers of myofibers[
16] and greater cross-sectional area (CSA)[
16,
69], as well as an enhancement of grip strength of uninjured muscle[
16]. Irisin has also been shown to increase myotube number and fusion index in both C2C12 myoblast-induced and primary myotubes in vitro[
16]. Moreover, irisin promotes skeletal muscle hypertrophy by boosting protein synthesis and reducing protein breakdown[
16,
69]. Irisin also enhances mitochondrial density and size, and promotes the transition of fast-type fibers towards the slow phenotype to counteract the reduction of slow fibers caused by unloaded-induced muscular atrophy[
69]. Additionally, irisin has been demonstrated to protect against fibrosis, myofiber necrosis, and sarcolemma instability in mice with dystrophic myofiber damage[
19].
While irisin has demonstrated potential in promoting muscle regeneration, further research is necessary to clarify conflicting findings on its effect on satellite cell activation, and optimize therapeutic dosages with consideration of irisin's half-life. Intriguingly, combining irisin with biomaterials capable of sustained release may offer a promising approach for delivering irisin to soft tissues like muscle.
3.2.2. Osteogenesis
Regeneration of adult skeletal muscle is an asynchronous process that involves the activation, proliferation, and fusion of satellite cells to form new muscle fibers[
117]. Irisin has been found to play a role in this process by participating in myogenesis, including the activation of satellite cells, myogenic differentiation, and hypertrophic protein synthesis during the recovery or healing of atrophic muscle.
While Irisin has been found to activate the p38 and Erk signaling pathways, thereby promoting proliferation and differentiation of osteoblasts in vitro, its role in regulating bone modeling and whether it can tip the balance towards bone formation under conditions such as osteoporosis or fracture remains a topic of debate.
Colaianni et al. were the first to demonstrate that Irisin has the potential to restore bone mass in osteoporotic mice induced by hind-limb suspension, which is consistent with a previous study showing that upon a same dose of 100 μg/kg of r-irisin administered weekly for four weeks promotes healthy cortical bone formation[
69]. The absence of mechanical loading was found to decrease the gene expression of osteoprotegerin (Opg) without affecting the expression of receptor activator of nuclear factor kappa-Β ligand (Rankl), resulting in an increased Rankl/ Osteoprotegerin (Opg) ratio, which usually a signal of osteoclast activation[
121]. Interestingly, treatment with r-Irisin was shown to compromise the decrease in Opg expression without altering Rankl expression, hence leading to a similar Rankl/Opg ratio to that observed in mice under normal mechanical loading conditions[
69]. This indicates a negative correlation between irisin and osteoclastogenesis. A recent study showed irisin can increase the number of osteoclasts within the callus during fracture healing in mice[
122]. However, no evidence directly showed the osteoclast function was inhibited by irisin with respect to bone remodeling/repair in vivo. In contrary, in the OXV-induced osteoporotic mice, the ablation of FNDC5/irisin can inhibit bone resorption as osteocytic osteolysis is eliminated by inactivation of osteoclasts, suggesting a positive correlation between irisin and osteoclastogensis[
20].
Administering 100 μg/kg of r-irisin once a week for four weeks in a mouse model of disuse-induced osteoporosis resulted in decreased empty lacunae and prevented osteocyte apoptosis. Irisin inhibited caspase activation in cortical bone and activated an Erk-dependent pathway including MAPK, Erk1 and Erk2, as well as upregulated the transcription factor 4 (Atf4) in osteocytes. B-cell lymphoma-2 (Bcl-2) proteins regulate apoptosis, and their relative expression with the Bcl-2 Antagonist X (Bax) protein determines cell survival. In vitro experiments on Mlo-y4 osteocyte-like cells showed that irisin increased osteocyte viability and prevented caspase activation induced by dexamethasone and hydrogen peroxide via upregulation of the pro-survival Blc2/Bax ratio.
Collectively, the regulation of osteoblasts, osteoclasts, and osteocytes by irisin likely depends on the physiological state of the bone tissue, and the effects of irisin may vary depending on the concentration or dosage administered. Further investigation is necessary to gain a comprehensive understanding of the therapeutic potential of irisin for osteoporosis and bone healing by focusing on the bone multicellular unit.
3.2.3. Neurogenesis
As mentioned in chapter 2.3, irisin is more likely thought to play a neuroprotective role rather than a regenerative one in brain disorders such as AD, PD, ALS and stroke-related injury.
Nevertheless, FNDC5/irisin has been demonstrated to have a crucial role in neuronal differentiation and maturation during neurogenesis when viewed through the lens of neuron development, through loss or gain of gene function. Studies have demonstrated that FNDC5 knockdown significantly decreases the neural differentiation rate of mouse embryonic stem cells, highlighting the importance of FNDC5 in the generation and development of the nervous system[
123,
124]. Similarly, FNDC5 has been shown to enhance the proliferation of primary mouse neural cells and increase the expression of neurotrophins such as BDNF[
125]. In humans, the role of FNDC5 in neural differentiation has also been determined, with sequential increases in FNDC5 expression observed during human neural differentiation, reaching its highest levels in neural cells. Additionally, retinoic acid (RA)-induced FNDC5 overexpression was found in human neural tissues including the forebrain, hindbrain, myelencephalon, midbrain, and cervical spinal cord, but not in other tissues such as the heart, lungs, and spleen[
17]. Furthermore, the pharmacological role of irisin was evaluated as it directly regulates hippocampal neurogenesis by increasing the proliferation of mouse hippocampal neuronal cells.[
126]. Additionally, irisin may play a role in synaptic plasticity, memory preservation, and cognitive ability, beyond its known role in neural differentiation[
127].
Studies investigating the therapeutic potential of irisin after brain damage have primarily focused on its ability to alleviate apoptosis rather than promote neurogenesis. Specifically, increasing the levels of PGC1a, FNDC5, and BDNF has been shown to enhance neuronal cell survival and counteract the apoptotic effects on neurons[
128]. It has been shown to reduce apoptosis and increase BDNF protein, resulting in a significant reduction in infarct size and cerebral edema in animal models of stroke[
27]. Irisin also protects against neuronal injury via activation of the Akt and Erk1/2 signaling pathways[
28] and promotes mitochondrial biogenesis in subarachnoid hemorrhage[
29].Furthermore, A recent study demonstrated the protective effect of irisin on the peripheral nervous system (PNS) by ameliorating neuroinflammation-induced neuronal apoptosis in burn-related neuropathy, using recombinant adenovirus containing the irisin sequence[
129].
4. Conclusions and Further Remarks
The positive impacts of physical exercise on degenerative disorders are widely acknowledged but poorly understood. Irisin, a molecule that imitates exercise, has been recognized as a crucial element in deciphering the link between exercise and the body's adaptive reactions, such as protecting tissues against deteriorating process. A precise understanding of the effects of exercise-mimicking molecules at the cellular, tissue, and systemic levels is vital to explore their potential as prognostic or diagnostic biomarkers for degenerative conditions, as well as therapeutic agents for tissue repair and healing. This is especially important for individuals who are unable to engage in physical exercise.
This review highlights the correlation between irisin levels and various degenerative conditions such as muscle atrophy, osteoporosis, and neurodegenerative diseases. Clinical evidence suggests a negative association between circulating irisin levels and these conditions, although there are some ambiguities to be addressed. First, the small group sizes in these studies and the potential influence of confounding factors such as age, gender, and disease severity need to be considered. Furthermore, the absence of standardized quantitative assays for irisin prevents its designation as a biomarker, as reported reference values in serum show a wide variation, ranging from pg/mL to μg/mL.
In addition, this review shifts our focus from clinical settings to laboratory investigations, highlighting the research progress made over the past decade regarding the therapeutic application of irisin through loss or gain of FNDC5 function and administration of r-irisin via injection or AAV vector delivery. While these studies have provided insights into the underlying mechanisms of irisin's therapeutic effects on degenerative diseases, the clinical translation of these findings is challenged by the supraphysiological levels and the pattern of administration of irisin typically used in cell culture and animal studies.
Together, we summarize the current knowledge on the protective impacts of irisin on tissue inflammation, as well as its ability to coordinate a set of cell types with respect to their proliferation, differentiation, and apoptosis in damaged tissues, including adipose, liver, cardiovascular, muscle, bone, and brain. These findings highlight the promise of irisin as a bio-link to degenerative disorders and a therapeutic target for tissue repair/ healing. However, given the complexity of tissue regeneration, including multiple cell types and structures involved, further multidisciplinary approaches are required to comprehensively interrogate the roles of irisin in tissue regeneration.
Author Contributions
Conceptualization, L.W., H.K., Y.Z.; writing—original draft preparation, Y.Z.; writing—review and editing, L.W.; Supervision, C.L. and Y.F.; project administration, Y.Z.; funding acquisition, L.W., Y.F.; All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by National Natural Science Foundation of China (No. T2288101, 12172034, 11827803, U20A20390). Beijing Municipal Natural Science Foundation (7212205), the 111 project (B13003) and the Fundamental Research Funds for the Central Universities.
Data Availability Statement
The information that supports the findings of this study is available in this article.
Acknowledgments
We thank National Natural Science Foundation of China, Beijing Municipal Natural Science Foundation, the 111 project, and the Fundamental Research Funds for the Central Universities for their support on this program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Silverman, M.N.; Deuster, P.A. Biological Mechanisms Underlying the Role of Physical Fitness in Health and Resilience. Interface Focus 2014, 4. [Google Scholar] [CrossRef]
- Raichlen, D.A.; Polk, J.D. Linking Brains and Brawn: Exercise and the Evolution of Human Neurobiology. Proc. R. Soc. B Biol. Sci. 2013, 280. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.Z.; No, M.H.; Heo, J.W.; Park, D.H.; Kang, J.H.; Kim, S.H.; Kwak, H.B. Role of Exercise in Age-Related Sarcopenia. J. Exerc. Rehabil. 2018, 14, 551. [Google Scholar] [CrossRef]
- Howe, T.E.; Shea, B.; Dawson, L.J.; Downie, F.; Murray, A.; Ross, C.; Harbour, R.T.; Caldwell, L.M.; Creed, G. Exercise for Preventing and Treating Osteoporosis in Postmenopausal Women. Cochrane database Syst. Rev. 2011. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, T.; Chu, J.M.T.; Chen, Y.; Dunnett, S.; Ho, Y.S.; Wong, G.T.C.; Chang, R.C.C. The Beneficial Effects of Physical Exercise in the Brain and Related Pathophysiological Mechanisms in Neurodegenerative Diseases. Lab. Investig. 2019 997 2019, 99, 943–957. [Google Scholar] [CrossRef]
- Takada, S.; Okita, K.; Suga, T.; Omokawa, M.; Kadoguchi, T.; Sato, T.; Takahashi, M.; Yokota, T.; Hirabayashi, K.; Morita, N.; et al. Low-Intensity Exercise Can Increase Muscle Mass and Strength Proportionally to Enhanced Metabolic Stress under Ischemic Conditions. J. Appl. Physiol. 2012, 113, 199–205. [Google Scholar]
- Vicente-Rodríguez, G. How Does Exercise Affect Bone Development during Growth? Sport. Med. 2006, 36, 561–569. [Google Scholar]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Kurdiova, T.; Balaz, M.; Vician, M.; Maderova, D.; Vlcek, M.; Valkovic, L.; Srbecky, M.; Imrich, R.; Kyselovicova, O.; Belan, V.; et al. Effects of Obesity, Diabetes and Exercise on Fndc5 Gene Expression and Irisin Release in Human Skeletal Muscle and Adipose Tissue: In Vivo and in Vitro Studies. J. Physiol. 2014, 592, 1091–1107. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Delezie, J.; Handschin, C. Endocrine Crosstalk Between Skeletal Muscle and the Brain. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Maak, S.; Norheim, F.; Drevon, C.A.; Erickson, H.P. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 2021, 42, 436. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Peng, J.; Jiang, Y. FNDC5/Irisin: A New Protagonist in Acute Brain Injury. Stem Cells Dev. 2020, 29, 533–543. [Google Scholar] [PubMed]
- Kan, T.; He, Z.; Du, J.; Xu, M.; Cui, J.; Han, X.; Tong, D.; Li, H.; Yan, M.; Yu, Z. Irisin Promotes Fracture Healing by Improving Osteogenesis and Angiogenesis. J. Orthop. Transl. 2022, 37, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin Is a Pro-Myogenic Factor That Induces Skeletal Muscle Hypertrophy and Rescues Denervation-Induced Atrophy. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Ghahrizjani, F.; Ghaedi, K.; Salamian, A.; Tanhaei, S.; Shoaraye Nejati, A.; Salehi, H.; Nabiuni, M.; Baharvand, H.; Nasr-Esfahani, M.H. Enhanced Expression of FNDC5 in Human Embryonic Stem Cell-Derived Neural Cells along with Relevant Embryonic Neural Tissues. Gene 2015, 557, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Estell, E.G.; Le, P.T.; Vegting, Y.; Kim, H.; Wrann, C.; Bouxsein, M.L.; Nagano, K.; Baron, R.; Spiegelman, B.M.; Rosen, C.J. Irisin Directly Stimulates Osteoclastogenesis and Bone Resorption in Vitro and in Vivo. Elife 2020, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.M.; Sim, C.M.; Subramaniyam, N.; Ge, X.; Sharma, M.; Kambadur, R.; McFarlane, C. Irisin Treatment Improves Healing of Dystrophic Skeletal Muscle. Oncotarget 2017, 8, 98553. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via AV Integrin. Cell 2019, 178, 507. [Google Scholar] [CrossRef]
- Lourenco, M. V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-Linked FNDC5/Irisin Rescues Synaptic Plasticity and Memory Defects in Alzheimer’s Models. Nat. Med. 2019 251 2019, 25, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, Z.; Marandi, S.M.; Alaei, H.; Esfarjani, F. The Effect of Preventive Exercise on the Neuroprotection in 6-Hydroxydopamine-Lesioned Rat Brain. Appl. Physiol. Nutr. Metab. 2019, 44, 1267–1275. [Google Scholar] [PubMed]
- Zarbakhsh, S.; Safari, M.; Aldaghi, M.R.; Sameni, H.R.; Ghahari, L.; Khaleghi Lagmouj, Y.; Rahimi Jaberi, K.; Parsaie, H. Irisin Protects the Substantia Nigra Dopaminergic Neurons in the Rat Model of Parkinson’s Disease. Iran. J. Basic Med. Sci. 2019, 22, 722. [Google Scholar] [CrossRef] [PubMed]
- Mudò, G.; Mäkelä, J.; Di Liberto, V.; Tselykh, T. V.; Olivieri, M.; Piepponen, P.; Eriksson, O.; Mälkiä, A.; Bonomo, A.; Kairisalo, M.; et al. Transgenic Expression and Activation of PGC-1α Protect Dopaminergic Neurons in the MPTP Mouse Model of Parkinson’s Disease. Cell. Mol. Life Sci. 2012, 69, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Kam, T.I.; Park, H.; Chou, S.C.; Van Vranken, J.G.; Mittenbuhler, M.J.; Kim, H.; Mu, A.; Choi, Y.R.; Biswas, D.; Wang, J.; et al. Amelioration of Pathologic α-Synuclein-Induced Parkinson’s Disease by Irisin. Proc. Natl. Acad. Sci. U. S. A. 2022, 119, e2204835119. [Google Scholar]
- Camerino, G.M.; Fonzino, A.; Conte, E.; De Bellis, M.; Mele, A.; Liantonio, A.; Tricarico, D.; Tarantino, N.; Dobrowolny, G.; Musarò, A.; et al. Elucidating the Contribution of Skeletal Muscle Ion Channels to Amyotrophic Lateral Sclerosis in Search of New Therapeutic Options. Sci. Reports 2019 91 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Asadi, Y.; Gorjipour, F.; Behrouzifar, S.; Vakili, A. Irisin Peptide Protects Brain Against Ischemic Injury Through Reducing Apoptosis and Enhancing BDNF in a Rodent Model of Stroke. Neurochem. Res. 2018, 43, 1549–1560. [Google Scholar]
- Li, D.J.; Li, Y.H.; Yuan, H. Bin; Qu, L.F.; Wang, P. The Novel Exercise-Induced Hormone Irisin Protects against Neuronal Injury via Activation of the Akt and ERK1/2 Signaling Pathways and Contributes to the Neuroprotection of Physical Exercise in Cerebral Ischemia. Metabolism 2017, 68, 31–42. [Google Scholar] [CrossRef]
- Tu, T.; Yin, S.; Pang, J.; Zhang, X.; Zhang, L.; Zhang, Y.; Xie, Y.; Guo, K.; Chen, L.; Peng, J.; et al. Irisin Contributes to Neuroprotection by Promoting Mitochondrial Biogenesis After Experimental Subarachnoid Hemorrhage. Front. Aging Neurosci. 2021, 13, 640215. [Google Scholar] [PubMed]
- LeBoff, M.S.; Greenspan, S.L.; Insogna, K.L.; Lewiecki, E.M.; Saag, K.G.; Singer, A.J.; Siris, E.S. The Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2022, 33, 2049. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Kwon, K.S. Pharmacological Interventions for Treatment of Sarcopenia: Current Status of Drug Development for Sarcopenia. Ann. Geriatr. Med. Res. 2019, 23, 98. [Google Scholar] [CrossRef]
- Kumar, S. Overcoming Gaps in the Treatment of Neurodegenerative Disease. EBioMedicine 2020, 60, 103088. [Google Scholar] [CrossRef]
- Korta, P.; Pocheć, E.; Mazur-Biały, A. Irisin as a Multifunctional Protein: Implications for Health and Certain Diseases. Medicina (B. Aires). 2019, 55. [Google Scholar] [CrossRef]
- Fanzani, A.; Conraads, V.M.; Penna, F.; Martinet, W. Molecular and Cellular Mechanisms of Skeletal Muscle Atrophy: An Update. J. Cachexia. Sarcopenia Muscle 2012, 3, 163. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of Muscle Atrophy and Hypertrophy: Implications in Health and Disease. Nat. Commun. 2021 121 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin Prevents and Restores Bone Loss and Muscle Atrophy in Hind-Limb Suspended Mice. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Kim, H.J.; So, B.; Choi, M.; Kang, D.; Song, W. Resistance Exercise Training Increases the Expression of Irisin Concomitant with Improvement of Muscle Function in Aging Mice and Humans. Exp. Gerontol. 2015, 70, 11–17. [Google Scholar] [CrossRef]
- Chang, J.S.; Kim, T.H.; Nguyen, T.T.; Park, K.S.; Kim, N.; Kong, I.D. Circulating Irisin Levels as a Predictive Biomarker for Sarcopenia: A Cross-Sectional Community-Based Study. Geriatr. Gerontol. Int. 2017, 17, 2266–2273. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, H.C.; Zhang, D.; Yeom, H.; Lim, S.K. The Novel Myokine Irisin: Clinical Implications and Potential Role as a Biomarker for Sarcopenia in Postmenopausal Women. Endocrine 2019, 64, 341–348. [Google Scholar]
- Lee, M.J.; Lee, S.A.; Nam, B.Y.; Park, S.; Lee, S.H.; Ryu, H.J.; Kwon, Y.E.; Kim, Y.L.; Park, K.S.; Oh, H.J.; et al. Irisin, a Novel Myokine Is an Independent Predictor for Sarcopenia and Carotid Atherosclerosis in Dialysis Patients. Atherosclerosis 2015, 242, 476–482. [Google Scholar] [CrossRef]
- Choi, H.Y.; Kim, S.; Park, J.W.; Lee, N.S.; Hwang, S.Y.; Huh, J.Y.; Hong, H.C.; Yoo, H.J.; Baik, S.H.; Youn, B.S.; et al. Implication of Circulating Irisin Levels with Brown Adipose Tissue and Sarcopenia in Humans. J. Clin. Endocrinol. Metab. 2014, 99, 2778–2785. [Google Scholar] [CrossRef]
- Baek, J.Y.; Jang, I.Y.; Jung, H.W.; Park, S.J.; Lee, J.Y.; Choi, E.; Lee, Y.S.; Lee, E.; Kim, B.J. Serum Irisin Level Is Independent of Sarcopenia and Related Muscle Parameters in Older Adults. Exp. Gerontol. 2022, 162, 111744. [Google Scholar] [CrossRef] [PubMed]
- Ganassi, M.; Badodi, S.; Wanders, K.; Zammit, P.S. Myogenin Is an Essential Regulator of Adult Myofibre Growth and Muscle Stem Cell Homeostasis. Elife 2020, 9, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, F.; Barberi, L.; Dobrowolny, G.; Villa Nova Bacurau, A.; Nicoletti, C.; Rizzuto, E.; Rosenthal, N.; Scicchitano, B.M.; Musarò, A. Effects of IGF-1 Isoforms on Muscle Growth and Sarcopenia. Aging Cell 2019, 18, e12954. [Google Scholar] [CrossRef] [PubMed]
- Ríos, R.; Carneiro, I.; Arce, V.M.; Devesa, J. Myostatin Is an Inhibitor of Myogenic Differentiation. Am. J. Physiol. Cell Physiol. 2002, 282. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and Irisin in Humans: I. Predictors of Circulating Concentrations in Serum and Plasma and II. MRNA Expression and Circulating Concentrations in Response to Weight Loss and Exercise. Metabolism. 2012, 61, 1725–1738. [Google Scholar] [CrossRef]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin Stimulates Muscle Growth-Related Genes and Regulates Adipocyte Differentiation and Metabolism in Humans. Int. J. Obes. (Lond). 2014, 38, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Liang, X.; Bi, P.; Kuang, S. Myostatin Knockout Drives Browning of White Adipose Tissue through Activating the AMPK-PGC1α-Fndc5 Pathway in Muscle. FASEB J. 2013, 27, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Vandervoort, A.A. Aging of the Human Neuromuscular System. Muscle Nerve 2002, 25, 17–25. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Kang, H.; Lin, C.Y.; Fan, Y. Applying Exercise-Mimetic Engineered Skeletal Muscle Model to Interrogate the Adaptive Response of Irisin to Mechanical Force. iScience 2022, 25. [Google Scholar]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An Overview and Management of Osteoporosis. Eur. J. Rheumatol. 2017, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Reppe, S.; Lien, T.G.; Hsu, Y.-H.; Gautvik, V.T.; Olstad, O.K.; Yu, R.; Bakke, H.G.; Lyle, R.; Kringen, M.K.; Glad, I.K.; et al. Skeletal Aging and Osteoporosis: Mechanisms and Therapeutics. Int. J. Mol. Sci. 2021, Vol. 22, Page 3553 2021, 22, 3553. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, S.; Bruyère, O.; Bergmann, P.; Cavalier, E.; Gielen, E.; Goemaere, S.; Kaufman, J.M.; Lapauw, B.; Laurent, M.R.; De Schepper, J.; et al. How to Manage Osteoporosis before the Age of 50. Maturitas 2020, 138, 14–25. [Google Scholar] [PubMed]
- Roos, P.M. Osteoporosis in Neurodegeneration. J. Trace Elem. Med. Biol. 2014, 28, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T. Osteoporosis and Cancer. Curr. Osteoporos. Rep. 2013, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Stavnichuk, M.; Mikolajewicz, N.; Corlett, T.; Morris, M.; Komarova, S. V. A Systematic Review and Meta-Analysis of Bone Loss in Space Travelers. npj Microgravity 2020 61 2020, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. Pharm. Ther. 2018, 43, 92. [Google Scholar]
- Sibonga, J.; Matsumoto, T.; Jones, J.; Shapiro, J.; Lang, T.; Shackelford, L.; Smith, S.M.; Young, M.; Keyak, J.; Kohri, K.; et al. Resistive Exercise in Astronauts on Prolonged Spaceflights Provides Partial Protection against Spaceflight-Induced Bone Loss. Bone 2019, 128, 112037. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Gkiomisi, A.; Bisbinas, I.; Katsarou, A.; Filippaios, A.; Mantzoros, C.S. Circulating Irisin Is Associated with Osteoporotic Fractures in Postmenopausal Women with Low Bone Mass but Is Not Affected by Either Teriparatide or Denosumab Treatment for 3 Months. Osteoporos. Int. 2014, 25, 1633–1642. [Google Scholar] [CrossRef]
- Roomi, A.B.; Nori, W.; Hamed, R.M. Lower Serum Irisin Levels Are Associated with Increased Osteoporosis and Oxidative Stress in Postmenopausal. Reports Biochem. Mol. Biol. 2021, 10, 13. [Google Scholar] [CrossRef]
- Yan, J.; Liu, H.J.; Guo, W.C.; Yang, J. Low Serum Concentrations of Irisin Are Associated with Increased Risk of Hip Fracture in Chinese Older Women. Jt. Bone Spine 2018, 85, 353–358. [Google Scholar] [CrossRef]
- Singhal, V.; Lawson, E.A.; Ackerman, K.E.; Fazeli, P.K.; Clarke, H.; Lee, H.; Eddy, K.; Marengi, D.A.; Derrico, N.P.; Bouxsein, M.L.; et al. Irisin Levels Are Lower in Young Amenorrheic Athletes Compared with Eumenorrheic Athletes and Non-Athletes and Are Associated with Bone Density and Strength Estimates. PLoS One 2014, 9, e100218. [Google Scholar] [CrossRef]
- Palermo, A.; Strollo, R.; Maddaloni, E.; Tuccinardi, D.; D’Onofrio, L.; Briganti, S.I.; Defeudis, G.; De Pascalis, M.; Lazzaro, M.C.; Colleluori, G.; et al. Irisin Is Associated with Osteoporotic Fractures Independently of Bone Mineral Density, Body Composition or Daily Physical Activity. Clin. Endocrinol. (Oxf). 2015, 82, 615–619. [Google Scholar] [CrossRef]
- Serbest, S.; Tiftikçi, U.; Tosun, H.B.; Kısa, Ü. The Irisin Hormone Profile and Expression in Human Bone Tissue in the Bone Healing Process in Patients. Med. Sci. Monit. 2017, 23, 4278. [Google Scholar] [CrossRef] [PubMed]
- Duda, G.N.; Geissler, S.; Checa, S.; Tsitsilonis, S.; Petersen, A.; Schmidt-Bleek, K. The Decisive Early Phase of Bone Regeneration. Nat. Rev. Rheumatol. 2023 192 2023, 19, 78–95. [Google Scholar] [CrossRef]
- Kular, J.; Tickner, J.; Chim, S.M.; Xu, J. An Overview of the Regulation of Bone Remodelling at the Cellular Level. Clin. Biochem. 2012, 45, 863–873. [Google Scholar] [CrossRef]
- Buccoliero, C.; Oranger, A.; Colaianni, G.; Pignataro, P.; Zerlotin, R.; Lovero, R.; Errede, M.; Grano, M. The Effect of Irisin on Bone Cells in Vivo and in Vitro. Biochem. Soc. Trans. 2021, 49, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Oranger, A.; Mori, G.; Brunetti, G.; Colucci, S.; Cinti, S.; Grano, M. Irisin Enhances Osteoblast Differentiation In Vitro. Int. J. Endocrinol. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin Prevents and Restores Bone Loss and Muscle Atrophy in Hind-Limb Suspended Mice. Sci. Reports 2017 71 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Comite, M.D.; Mori, G.; et al. The Myokine Irisin Increases Cortical Bone Mass. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 12157–12162. [Google Scholar] [CrossRef]
- Qiao, X.Y.; Nie, Y.; Ma, Y.X.; Chen, Y.; Cheng, R.; Yinrg, W.Y.; Hu, Y.; Xu, W.M.; Xu, L.Z. Irisin Promotes Osteoblast Proliferation and Differentiation via Activating the MAP Kinase Signaling Pathways. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Storlino, G.; Colaianni, G.; Sanesi, L.; Lippo, L.; Brunetti, G.; Errede, M.; Colucci, S.; Passeri, G.; Grano, M. Irisin Prevents Disuse-Induced Osteocyte Apoptosis. J. Bone Miner. Res. 2020, 35, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative Disease: Models, Mechanisms, and a New Hope. Dis. Model. Mech. 2017, 10, 499. [Google Scholar] [CrossRef]
- Miller, J.H.; Das, V. Potential for Treatment of Neurodegenerative Diseases with Natural Products or Synthetic Compounds That Stabilize Microtubules. Curr. Pharm. Des. 2020, 26, 4362–4372. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhu, J.Y.; Liu, X.D.; Luo, M.Y.; Xu, N.J. Roles of Physical Exercise in Neurodegeneration: Reversal of Epigenetic Clock. Transl. Neurodegener. 2021 101 2021, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Whitty, E.; Mansour, H.; Aguirre, E.; Palomo, M.; Charlesworth, G.; Ramjee, S.; Poppe, M.; Brodaty, H.; Kales, H.C.; Morgan-Trimmer, S.; et al. Efficacy of Lifestyle and Psychosocial Interventions in Reducing Cognitive Decline in Older People: Systematic Review. Ageing Res. Rev. 2020, 62. [Google Scholar] [CrossRef] [PubMed]
- Küster, O.C.; Fissler, P.; Laptinskaya, D.; Thurm, F.; Scharpf, A.; Woll, A.; Kolassa, S.; Kramer, A.F.; Elbert, T.; von Arnim, C.A.F.; et al. Cognitive Change Is More Positively Associated with an Active Lifestyle than with Training Interventions in Older Adults at Risk of Dementia: A Controlled Interventional Clinical Trial. BMC Psychiatry 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.F.; Erickson, K.I. Capitalizing on Cortical Plasticity: Influence of Physical Activity on Cognition and Brain Function. Trends Cogn. Sci. 2007, 11, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Kwak, S.; Ha, J.; Oh, D.J.; Kim, M.; Cho, S.Y.; Kim, H.; Lee, J.Y.; Kim, E. Loss of Association between Plasma Irisin Levels and Cognition in Alzheimer’s Disease. Psychoneuroendocrinology 2022, 136. [Google Scholar] [CrossRef]
- Tsai, C.L.; Pai, M.C. Circulating Levels of Irisin in Obese Individuals at Genetic Risk for Alzheimer’s Disease: Correlations with Amyloid-β, Metabolic, and Neurocognitive Indices. Behav. Brain Res. 2021, 400, 113013. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, S.; Parone, P.A.; Lopes, V.S.; Lillo, C.; McAlonis-Downes, M.; Lee, S.K.; Vetto, A.P.; Petrosyan, S.; Marsala, M.; Murphy, A.N.; et al. Elevated PGC-1α Activity Sustains Mitochondrial Biogenesis and Muscle Function without Extending Survival in a Mouse Model of Inherited ALS. Cell Metab. 2012, 15, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Bayer, H.; Lang, K.; Buck, E.; Higelin, J.; Barteczko, L.; Pasquarelli, N.; Sprissler, J.; Lucas, T.; Holzmann, K.; Demestre, M.; et al. ALS-Causing Mutations Differentially Affect PGC-1α Expression and Function in the Brain vs. Peripheral Tissues. Neurobiol. Dis. 2017, 97, 36–45. [Google Scholar] [CrossRef]
- Lunetta, C.; Lizio, A.; Tremolizzo, L.; Ruscica, M.; Macchi, C.; Riva, N.; Weydt, P.; Corradi, E.; Magni, P.; Sansone, V. Serum Irisin Is Upregulated in Patients Affected by Amyotrophic Lateral Sclerosis and Correlates with Functional and Metabolic Status. J. Neurol. 2018, 265, 3001–3008. [Google Scholar] [PubMed]
- Tu, W.J.; Qiu, H.C.; Cao, J.L.; Liu, Q.; Zeng, X.W.; Zhao, J.Z. Decreased Concentration of Irisin Is Associated with Poor Functional Outcome in Ischemic Stroke. Neurotherapeutics 2018, 15, 1158–1167. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, Z.; Ke, J.; Wang, Y.; Wu, H. Exercise-Linked Irisin Prevents Mortality and Enhances Cognition in a Mice Model of Cerebral Ischemia by Regulating Klotho Expression. Oxid. Med. Cell. Longev. 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Dun, S.L.; Lyu, R.M.; Chen, Y.H.; Chang, J.K.; Luo, J.J.; Dun, N.J. Irisin-Immunoreactivity in Neural and Non-Neural Cells of the Rodent. Neuroscience 2013, 240, 155. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Valaris, S.; Young, M.F.; Haley, E.B.; Luo, R.; Bond, S.F.; Mazuera, S.; Kitchen, R.R.; Caldarone, B.J.; Bettio, L.E.B.; et al. Exercise Hormone Irisin Is a Critical Regulator of Cognitive Function. Nat. Metab. 2021 38 2021, 3, 1058–1070. [Google Scholar] [CrossRef]
- Noda, Y.; Kuzuya, A.; Tanigawa, K.; Araki, M.; Kawai, R.; Ma, B.; Sasakura, Y.; Maesako, M.; Tashiro, Y.; Miyamoto, M.; et al. Fibronectin Type III Domain-Containing Protein 5 Interacts with APP and Decreases Amyloid β Production in Alzheimer’s Disease. Mol. Brain 2018, 11. [Google Scholar] [CrossRef]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The Effects of Acute and Chronic Exercise on PGC-1α, Irisin and Browning of Subcutaneous Adipose Tissue in Humans. FEBS J. 2014, 281, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Belviranlı, M.; Okudan, N. Exercise Training Protects Against Aging-Induced Cognitive Dysfunction via Activation of the Hippocampal PGC-1α/FNDC5/BDNF Pathway. NeuroMolecular Med. 2018, 20, 386–400. [Google Scholar] [PubMed]
- Turner, G.M.; McMullan, C.; Aiyegbusi, O.L.; Bem, D.; Marshall, T.; Calvert, M.; Mant, J.; Belli, A. Stroke Risk Following Traumatic Brain Injury: Systematic Review and Meta-Analysis. Int. J. Stroke 2021, 16, 370. [Google Scholar] [PubMed]
- Frangogiannis, N.G. The Inflammatory Response in Tissue Repair. Inflamm. - From Mol. Cell. Mech. to Clin. 2017, 1517–1538. [Google Scholar] [CrossRef]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and Metabolism in Tissue Repair and Regeneration. Science (80-. ). 2017, 356, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Stramer, B.M.; Mori, R.; Martin, P. The Inflammation-Fibrosis Link? A Jekyll and Hyde Role for Blood Cells during Wound Repair. J. Invest. Dermatol. 2007, 127, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Pro- and Anti-Inflammatory Cytokine Balance in Strenuous Exercise in Humans. J. Physiol. 1999, 515, 287. [Google Scholar] [CrossRef]
- Petersen, A.M.W.; Pedersen, B.K. The Anti-Inflammatory Effect of Exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [PubMed]
- Moldoveanu, A.I.; Shephard, R.J.; Shek, P.N. The Cytokine Response to Physical Activity and Training. Sports Med. 2001, 31, 115. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.; Bilski, J.; Pochec, E.; Brzozowski, T. New Insight Into the Direct Anti-Inflammatory Activity of a Myokine:Irisin against pro-Inflammatory Activation of Adipocytes. J. Physiol. Pharmacol. 2017, 5, 243–251. [Google Scholar]
- Mazur-Bialy, A.I.; Pocheć, E.; Zarawski, M. Anti-Inflammatory Properties of Irisin, Mediator of Physical Activity, Are Connected with TLR4/MyD88 Signaling Pathway Activation. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K. V.; Deng, M.; Ting, J.P.Y. The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019 198 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, M.; Zhao, Y.; Dong, M. Irisin Protects Against LPS-Stressed Cardiac Damage Through Inhibiting Inflammation, Apoptosis, and Pyroptosis. Shock 2021, 56, 1009–1018. [Google Scholar] [CrossRef]
- Li, Q.; Tan, Y.; Chen, S.; Xiao, X.; Zhang, M.; Wu, Q.; Dong, M. Irisin Alleviates LPS-Induced Liver Injury and Inflammation through Inhibition of NLRP3 Inflammasome and NF-ΚB Signaling. J. Recept. Signal Transduct. 2020, 41, 294–303. [Google Scholar] [CrossRef]
- Ouyang, H.; Li, Q.; Zhong, J.; Xia, F.; Zheng, S.; Lu, J.; Deng, Y.; Hu, Y. Combination of Melatonin and Irisin Ameliorates Lipopolysaccharide-Induced Cardiac Dysfunction through Suppressing the Mst1-JNK Pathways. J. Cell. Physiol. 2020, 235, 6647–6659. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, M.; Tan, J.; Pei, X.; Lu, C.; Xin, Y.; Deng, S.; Zhao, F.; Gao, Y.; Gong, Y. Irisin Ameliorates Neuroinflammation and Neuronal Apoptosis through Integrin AVβ5/AMPK Signaling Pathway after Intracerebral Hemorrhage in Mice. J. Neuroinflammation 2022, 19, 1–20. [Google Scholar]
- Mazur-Bialy, A.I. Irisin Acts as a Regulator of Macrophages Host Defense. Life Sci. 2017, 176, 21–25. [Google Scholar] [CrossRef]
- Mills, C.D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Dong, J.; Dong, Y.; Dong, Y.; Chen, F.; Mitch, W.E.; Zhang, L. Inhibition of Myostatin in Mice Improves Insulin Sensitivity via Irisin-Mediated Cross Talk between Muscle and Adipose Tissues. Int. J. Obes. (Lond). 2016, 40, 434. [Google Scholar] [CrossRef]
- Xiong, X.Q.; Geng, Z.; Zhou, B.; Zhang, F.; Han, Y.; Zhou, Y.B.; Wang, J.J.; Gao, X.Y.; Chen, Q.; Li, Y.H.; et al. FNDC5 Attenuates Adipose Tissue Inflammation and Insulin Resistance via AMPK-Mediated Macrophage Polarization in Obesity. Metabolism. 2018, 83, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Zhang, Y.; Liu, C.; Wang, Y.; Zhang, L.; Shi, Z.; Wu, Z.; et al. Exercise Hormone Irisin Mitigates Endothelial Barrier Dysfunction and Microvascular Leakage–Related Diseases. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, Q.; Wu, J.; Zhou, X.; Weng, J.; Xu, J.; Wang, W.; Huang, Q.; Guo, X. Role of Src in Vascular Hyperpermeability Induced by Advanced Glycation End Products. Sci. Reports 2015 51 2015, 5, 1–13. [Google Scholar] [CrossRef]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Li, T.; Wang, T.; Zhang, L.; Wang, M.; Wu, Z.; Lv, Y.; et al. Irisin Reverses Intestinal Epithelial Barrier Dysfunction during Intestinal Injury via Binding to the Integrin AVβ5 Receptor. J. Cell. Mol. Med. 2020, 24, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.N.; Tajbakhsh, S.; Mouly, V.; Cossu, G.; Buckingham, M.; Butler-Browne, G.S. In Vivo Satellite Cell Activation via Myf5 and MyoD in Regenerating Mouse Skeletal Muscle. J. Cell Sci. 1999, 112 Pt 17, 2895–2901. [Google Scholar] [CrossRef]
- Sousa-Victor, P.; García-Prat, L.; Muñoz-Cánoves, P. Control of Satellite Cell Function in Muscle Regeneration and Its Disruption in Ageing. Nat. Rev. Mol. Cell Biol. 2021 233 2021, 23, 204–226. [Google Scholar] [CrossRef]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar] [CrossRef]
- Hill, M.; Wernig, A.; Goldspink, G. Muscle Satellite (Stem) Cell Activation during Local Tissue Injury and Repair. J. Anat. 2003, 203, 89. [Google Scholar] [CrossRef]
- Park, K.; Ju, W.C.; Yeo, J.H.; Kim, J.Y.; Seo, H.S.; Uchida, Y.; Cho, Y. Increased OPG/RANKL Ratio in the Conditioned Medium of Soybean-Treated Osteoblasts Suppresses RANKL-Induced Osteoclast Differentiation. Int. J. Mol. Med. 2014, 33, 178–184. [Google Scholar] [CrossRef]
- Colucci, S.C.; Buccoliero, C.; Sanesi, L.; Errede, M.; Colaianni, G.; Annese, T.; Khan, M.P.; Zerlotin, R.; Dicarlo, M.; Schipani, E.; et al. Systemic Administration of Recombinant Irisin Accelerates Fracture Healing in Mice. Int. J. Mol. Sci. 2021, Vol. 22, Page 10863 2021, 22, 10863. [Google Scholar] [CrossRef]
- Hashemi, M.S.; Ghaedi, K.; Salamian, A.; Karbalaie, K.; Emadi-Baygi, M.; Tanhaei, S.; Nasr-Esfahani, M.H.; Baharvand, H. Fndc5 Knockdown Significantly Decreased Neural Differentiation Rate of Mouse Embryonic Stem Cells. Neuroscience 2013, 231, 296–304. [Google Scholar] [CrossRef]
- Ostadsharif, M.; Ghaedi, K.; Hossein Nasr-Esfahani, M.; Mojbafan, M.; Tanhaie, S.; Karbalaie, K.; Baharvand, H. The Expression of Peroxisomal Protein Transcripts Increased by Retinoic Acid during Neural Differentiation. Differentiation. 2011, 81, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, M.; Rabiee, F.; Ghaedi, K.; Beheshti, S.; Tanhaei, S.; Shoaraye Nejati, A.; Jodeiri Farshbaf, M.; Baharvand, H.; Nasr-Esfahani, M.H. Fndc5 Overexpression Facilitated Neural Differentiation of Mouse Embryonic Stem Cells. Cell Biol. Int. 2015, 39, 629–637. [Google Scholar] [CrossRef]
- Moon, H.S.; Dincer, F.; Mantzoros, C.S. Pharmacological Concentrations of Irisin Increase Cell Proliferation without Influencing Markers of Neurite Outgrowth and Synaptogenesis in Mouse H19-7 Hippocampal Cell Lines. Metabolism. 2013, 62, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, F.; Lachinani, L.; Ghaedi, S.; Nasr-Esfahani, M.H.; Megraw, T.L.; Ghaedi, K. New Insights into the Cellular Activities of Fndc5/Irisin and Its Signaling Pathways. Cell Biosci. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.Y.; Huang, X.; Bi, C.F.; Mao, L.L.; Peng, L.J.; Qian, H.R. PGC-1α or FNDC5 Is Involved in Modulating the Effects of Aβ1-42 Oligomers on Suppressing the Expression of BDNF, a Beneficial Factor for Inhibiting Neuronal Apoptosis, Aβ Deposition and Cognitive Decline of APP/PS1 Tg Mice. Front. Aging Neurosci. 2017, 9. [Google Scholar] [CrossRef]
- Huang, S.H.; Yang, S.M.; Lo, J.J.; Wu, S.H.; Tai, M.H. Irisin Gene Delivery Ameliorates Burn-Induced Sensory and Motor Neuropathy. Int. J. Mol. Sci. 2020, 21, 1–17. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).