Submitted:
28 February 2023
Posted:
06 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Technical equipment
2.3. Manual Muscle Tests
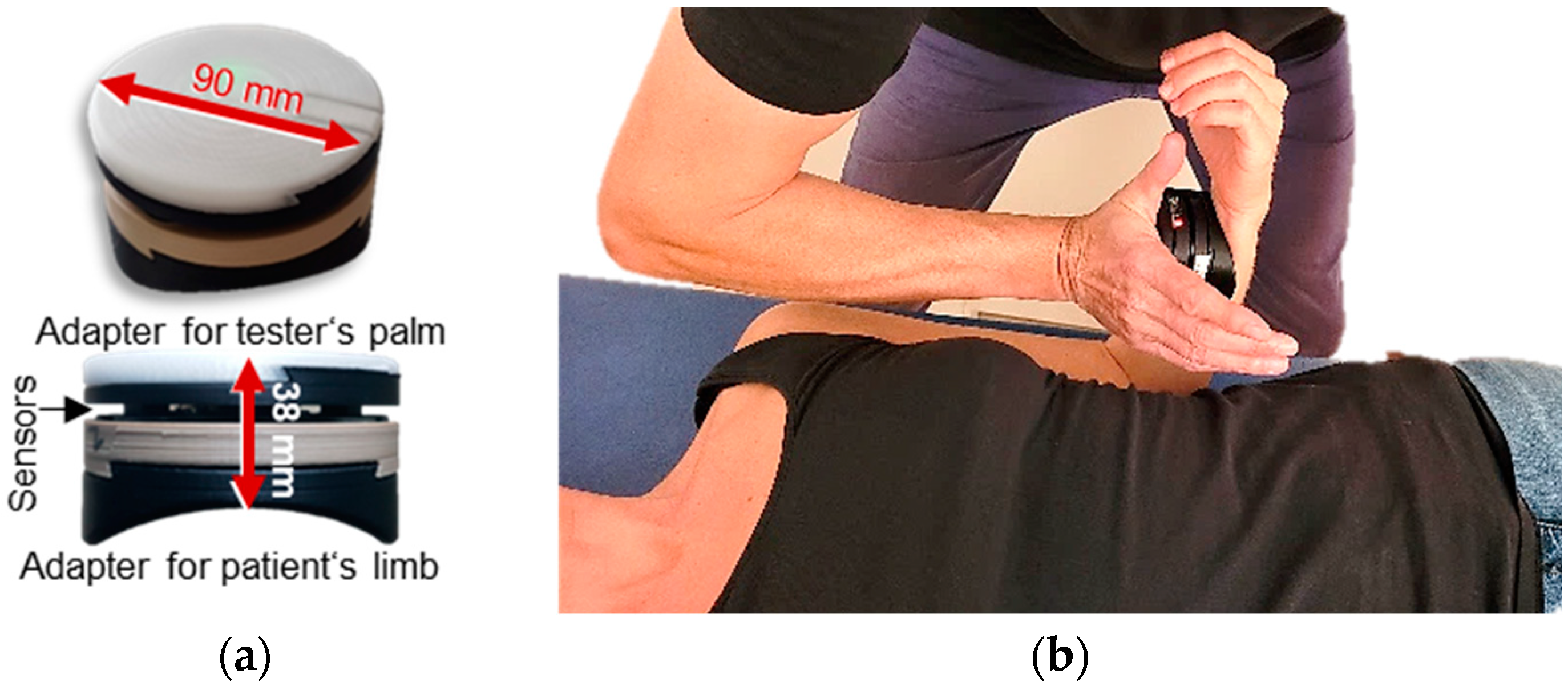
2.4. Setting and Measurement Procedure
-
Procedure CL: pre-contraction in lengthened position with passive returnFrom the test position, the elbow joint was brought passively into maximal extension by the tester (neutral zero position with maximal supination of the forearm). In that position, the participant was instructed to push shortly (~1 s) with self-estimated 20% of the MVIC against a stable resistance which was provided by the tester. The handheld device recorded the force of pre-contraction between tester’s palm and participant’s forearm. Afterwards the tester guided the limb back to the test position of the MMT. To ensure that the elbow flexors stayed passively thereby and did not support the flexion actively the participant should push slightly against the tester (activation of elbow extensors) during the return. Due to the passive shortening after pre-contraction in lengthening position, this procedure was assumed to produce a slack in muscle fibres. Back in test position after the CL procedure, the tester started the MMT after ~2 s to achieve a temporal sequence similar to the second procedure.
-
Procedure CL-CT: CL with subsequent second pre-contraction in test positionFor that, procedure CL was extended by a second pre-contraction immediately after the forearm was returned to test position. The second contraction should also amount self-estimated ~20% of the MVIC and should last ~1 s. Immediately after this second pre-contraction the MMT was performed to assess the AF. It was assumed that procedure CL-CT eliminates the slack in muscle fibres. A minimal intensity of 10% of the MVIC was regarded as necessary to resolve the reflex activity [18]. Hence, 20% of the MVIC was chosen to ensure that this minimal level would be certainly achieved (considering that the self-estimation would show some variance).
2.5. Data processing and statistical analyses
- MVIC: the peak value of each MVIC test was determined. The peak value of first MVIC test refers to the individual’s MVIC. The second MVIC test was analysed to investigate possible fatiguing effects in comparison to the initial MVIC.
- Maximal Adaptive Force (AFmax): the peak value of each MMT trial was selected and referred to AFmax of a single MMT. This was either reached during isometric actions (stable MMT) or during eccentric ones (unstable MMTs). For the former, AFmax = AFisomax. For the latter, AFmax > AFisomax (Figure 2).
- Maximal isometric Adaptive Force (AFisomax): this refers to the highest force value under isometric conditions during the MMT. The gyrometer signal was used to determine if the forearm moved in direction of elbow extension during the force increase (breaking point) – indicating muscle lengthening. If muscle lengthening occurred, the force value at the breaking point referred to AFisomax (Figure 2a). In case the isometric position was maintained up to the peak value, AFisomax = AFmax (Figure 2b). For a detailed description see [30,31,32,34].
- Slope: the difference quotient was used to determine the slope before the breaking point. Reference points were time and force of 70% and 100% of the averaged AFisomax of all as unstable assessed MMTs of one muscle. The decadic logarithm was taken from slope values since the slope rise was exponential [lg(N/s)].
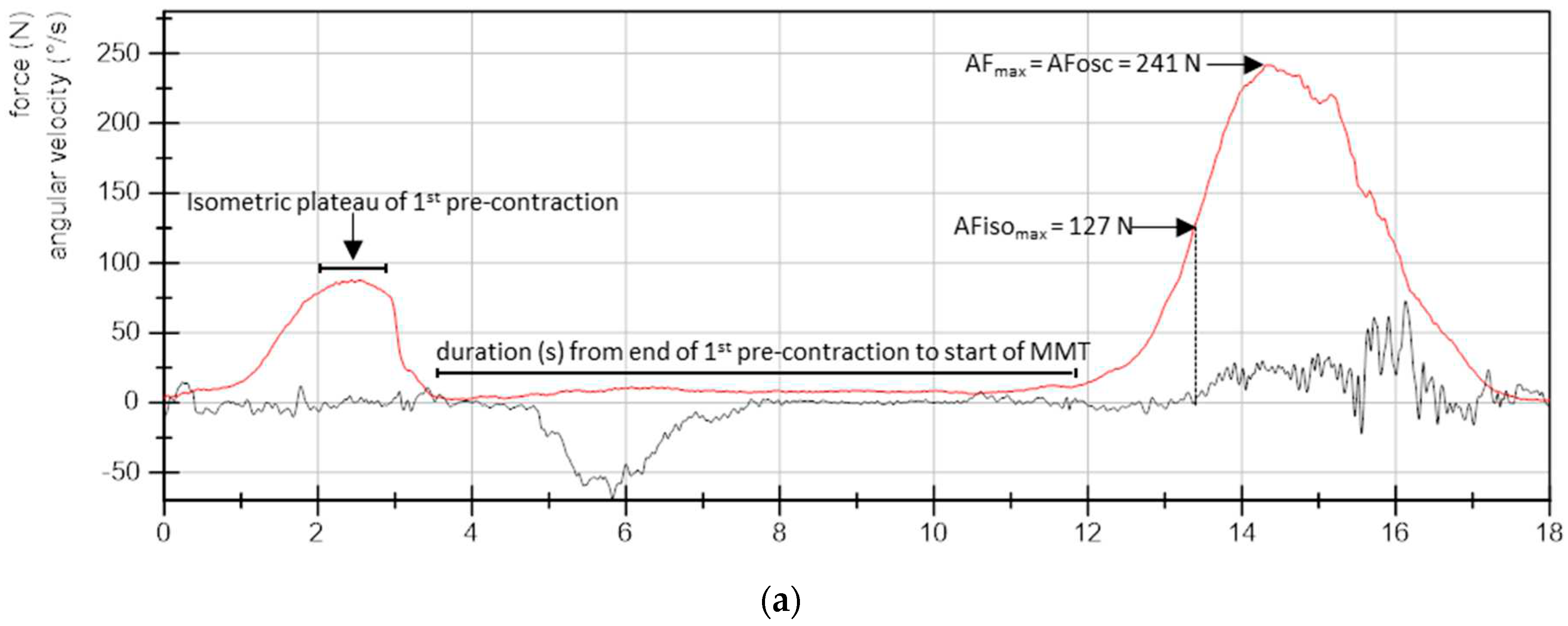
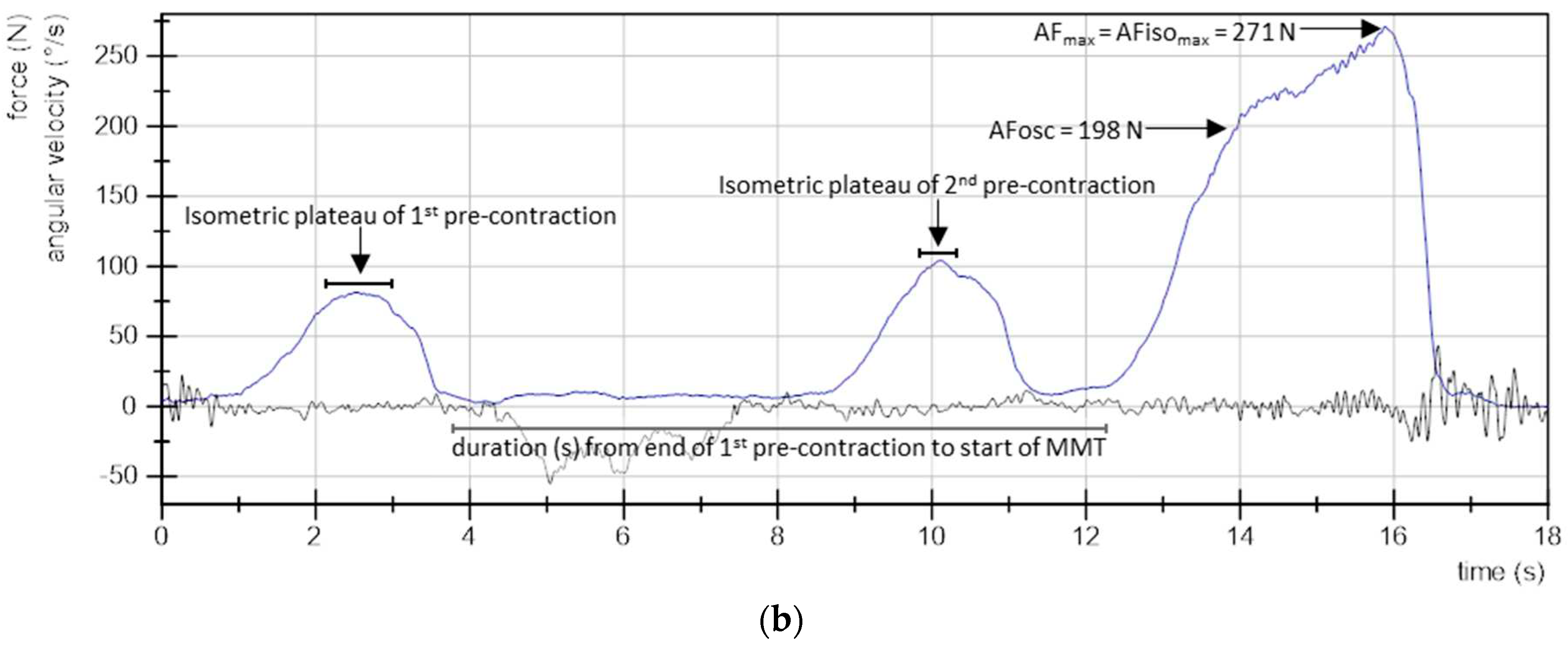
3. Results
3.1. Pre-contractions: duration and force
3.2. Parameters of Adaptive Force in comparison of the different procedures
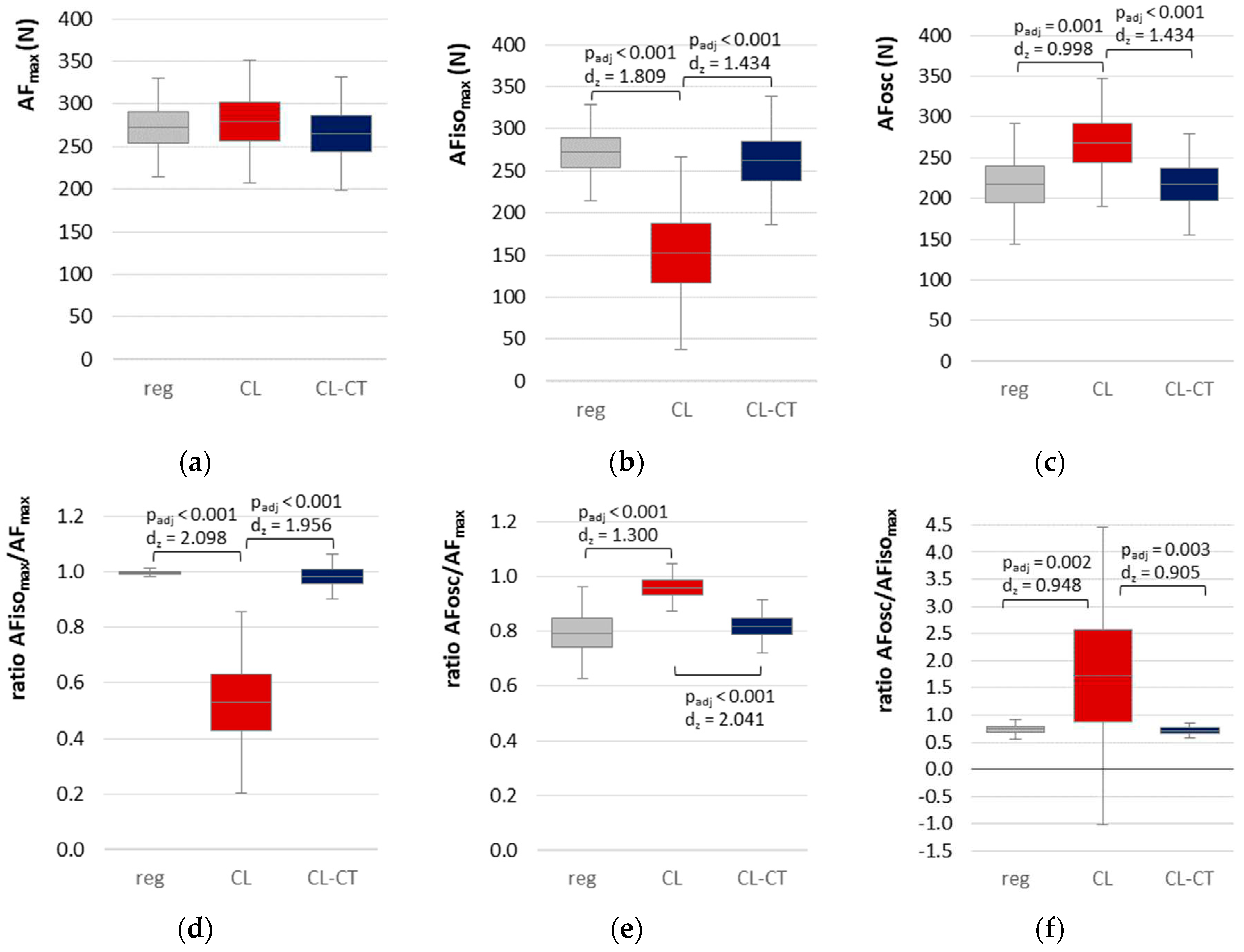
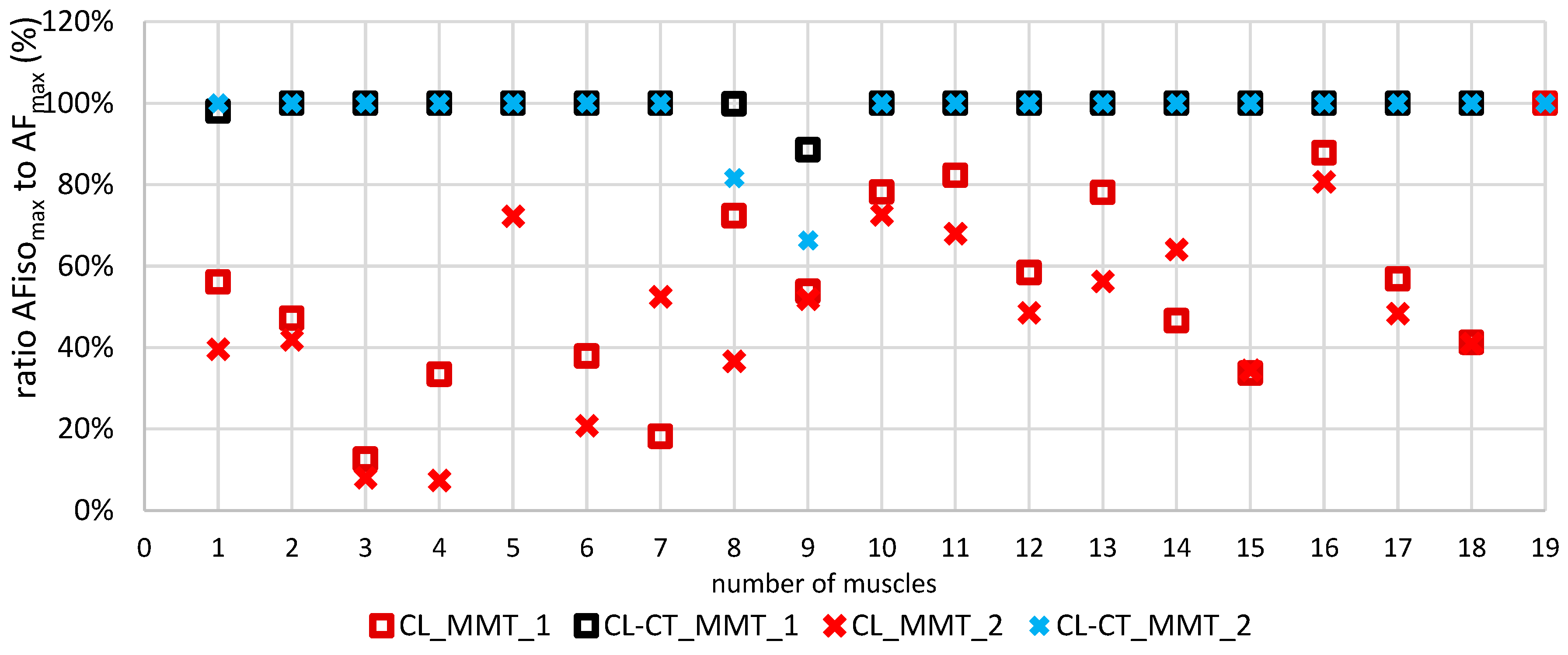
3.3. Onset of Oscillations in the course of Adaptive Force comparing the different procedures
3.4. Maximal voluntary isometric contraction
4. Discussion
4.1. Methodological considerations regarding the comparison of the procedures
4.2. Neurophysiological considerations
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ardigo, A. Histoire Du Concept de Force. Revue Philosophique de la France Et de l’Etranger 1882, 14, 117. [Google Scholar]
- Proske, U.; Gandevia, S.C. The Proprioceptive Senses: Their Roles in Signaling Body Shape, Body Position and Movement, and Muscle Force. Physiological Reviews 2012, 92, 1651–1697. [Google Scholar] [CrossRef] [PubMed]
- Proske, U.; Gandevia, S.C. The Kinaesthetic Senses: The Kinaesthetic Senses. The Journal of Physiology 2009, 587, 4139–4146. [Google Scholar] [CrossRef] [PubMed]
- Giuriati, W.; Ravara, B.; Porzionato, A.; Albertin, G.; Stecco, C.; Macchi, V.; De Caro, R.; Martinello, T.; Gomiero, C.; Patruno, M.; et al. Muscle Spindles of the Rat Sternomastoid Muscle. Eur J Transl Myol 2018, 28. [Google Scholar] [CrossRef] [PubMed]
- Macefield, V.G.; Knellwolf, T.P. Functional Properties of Human Muscle Spindles. Journal of Neurophysiology 2018, 120, 452–467. [Google Scholar] [CrossRef] [PubMed]
- Behm, D.G.; Chaouachi, A. A Review of the Acute Effects of Static and Dynamic Stretching on Performance. Eur J Appl Physiol 2011, 111, 2633–2651. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.K. Tension Due to Interaction between the Sliding Filaments in Resting Striated Muscle. the Effect of Stimulation. The Journal of Physiology 1968, 199, 637–684. [Google Scholar] [CrossRef] [PubMed]
- Hagbarth, K.-E.; Nordin, M. Postural After-Contractions in Man Attributed to Muscle Spindle Thixotropy. The Journal of Physiology 1998, 506, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Avela, J.; Kyröläinen, H.; Komi, P.V. Altered Reflex Sensitivity after Repeated and Prolonged Passive Muscle Stretching. Journal of Applied Physiology 1999, 86, 1283–1291. [Google Scholar] [CrossRef]
- Padilha, U.C.; Vieira, A.; Vieira, D.C.L.; De Lima, F.D.; Rocha Junior, V.A.; Tufano, J.J.; Bottaro, M. Could Inter-Set Stretching Increase Acute Neuromuscular and Metabolic Responses during Resistance Exercise? Eur J Transl Myol 2019, 29. [Google Scholar] [CrossRef]
- Lakie, M.; Campbell, K.S. Muscle Thixotropy—Where Are We Now? Journal of Applied Physiology 2019, 126, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, P.W.; Walsh, L.D.; D’Souza, A.; Héroux, M.E.; Bolsterlee, B.; Gandevia, S.C.; Herbert, R.D. History-Dependence of Muscle Slack Length Following Contraction and Stretch in the Human Vastus Lateralis: History-Dependence of Muscle Slack Length. J Physiol 2018, 596, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.L.; Prochazka, A.; Proske, U. The After-Effects of Stretch and Fusimotor Stimulation on the Responses of Primary Endings of Cat Muscle Spindles. The Journal of Physiology 1984, 356, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.P.; Campbell, K.S.; Horslen, B.C.; Nardelli, P.; Housley, S.N.; Cope, T.C.; Ting, L.H. Diverse and Complex Muscle Spindle Afferent Firing Properties Emerge from Multiscale Muscle Mechanics. eLife 2020, 9, e55177. [Google Scholar] [CrossRef]
- Proske, U.; Tsay, A.; Allen, T. Muscle Thixotropy as a Tool in the Study of Proprioception. Exp Brain Res 2014, 232, 3397–3412. [Google Scholar] [CrossRef] [PubMed]
- Proske, U.; Morgan, D.L.; Gregory, J.E. Thixotropy in Skeletal Muscle and in Muscle Spindles: A Review. Progress in Neurobiology 1993, 41, 705–721. [Google Scholar] [CrossRef]
- Banks, R.W.; Ellaway, P.H.; Prochazka, A.; Proske, U. Secondary Endings of Muscle Spindles: Structure, Reflex Action, Role in Motor Control and Proprioception. Experimental Physiology 2021, 106, 2339–2366. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.E.; Wise, A.K.; Wood, S.A.; Prochazka, A.; Proske, U. Muscle History, Fusimotor Activity and the Human Stretch Reflex. The Journal of Physiology 1998, 513, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Budini, F.; Tilp, M. Changes in H-Reflex Amplitude to Muscle Stretch and Lengthening in Humans. Reviews in the Neurosciences 2016, 27, 511–522. [Google Scholar] [CrossRef]
- Héroux, M.E.; Anderman, I.; Nykvist Vouis, S.; Diong, J.; Stubbs, P.W.; Herbert, R.D. History-Dependence of Muscle Slack Length in Humans: Effects of Contraction Intensity, Stretch Amplitude, and Time. Journal of Applied Physiology 2020, 129, 957–966. [Google Scholar] [CrossRef]
- Wood, S.A.; Gregory, J.E.; Proske, U. The Influence of Muscle Spindle Discharge on the Human H Reflex and the Monosynaptic Reflex in the Cat. The Journal of Physiology 1996, 497, 279–290. [Google Scholar] [CrossRef]
- Monjo, F.; Forestier, N. Muscle Spindle Thixotropy Affects Force Perception through Afferent-Induced Facilitation of the Motor Pathways as Revealed by the Kohnstamm Effect. Exp Brain Res 2018, 236, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Chaabene, H.; Behm, D.G.; Negra, Y.; Granacher, U. Acute Effects of Static Stretching on Muscle Strength and Power: An Attempt to Clarify Previous Caveats. Front. Physiol. 2019, 10, 1468. [Google Scholar] [CrossRef] [PubMed]
- Dech, S.; Bittmann, F.N.; Schaefer, L.V. Assessment of the Adaptive Force of Elbow Extensors in Healthy Subjects Quantified by a Novel Pneumatically Driven Measurement System with Considerations of Its Quality Criteria. Diagnostics 2021, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Myer, G.D.; Brent, J.L.; Ford, K.R.; Hewett, T.E. Real-Time Assessment and Neuromuscular Training Feedback Techniques to Prevent Anterior Cruciate Ligament Injury in Female Athletes. Strength & Conditioning Journal 2011, 33, 21–35. [Google Scholar] [CrossRef]
- Garrett, W.E. Muscle Strain Injuries: Clinical and Basic Aspects. Med Sci Sports Exerc 1990, 22, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Read, P.J.; Oliver, J.L.; De Ste Croix, M.B.A.; Myer, G.D.; Lloyd, R.S. Neuromuscular Risk Factors for Knee and Ankle Ligament Injuries in Male Youth Soccer Players. Sports Med 2016, 46, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Beynnon, B.D.; Fleming, B.C. Anterior Cruciate Ligament Strain In-Vivo: A Review of Previous Work. Journal of Biomechanics 1998, 31, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.W.; Barber-Foss, K.D. Sex-Related Injury Patterns among Selected High School Sports. Am J Sports Med 2000, 28, 385–391. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Dech, S.; Wolff, L.L.; Bittmann, F.N. Emotional Imagery Influences the Adaptive Force in Young Women: Unpleasant Imagery Reduces Instantaneously the Muscular Holding Capacity. Brain Sciences 2022, 12, 1318. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Dech, S.; Bittmann, F.N. Adaptive Force and Emotionally Related Imaginations – Preliminary Results Suggest a Reduction of the Maximal Holding Capacity as Reaction to Disgusting Food Imagination. Heliyon 2021, 7, e07827. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.V.; Dech, S.; Aehle, M.; Bittmann, F.N. Disgusting Odours Affect the Characteristics of the Adaptive Force in Contrast to Neutral and Pleasant Odours. Sci. Rep. 2021, 11, 16410. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.V.; Bittmann, F.N. Case Report: Individualized Pulsed Electromagnetic Field Therapy in a Long COVID Patient Using the Adaptive Force as Biomarker. Frontiers in Medicine 2022. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.V.; Bittmann, F.N. The Adaptive Force as Potential Biomechanical Parameter in the Recovery Process of Patients with Long COVID; 2022. [Google Scholar] [CrossRef]
- Bittmann, F.N.; Dech, S.; Aehle, M.; Schaefer, L.V. Manual Muscle Testing - Force Profiles and Their Reproducibility. Diagnostics 2020, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Conable, K.M.; Rosner, A.L. A Narrative Review of Manual Muscle Testing and Implications for Muscle Testing Research. J Chiropr Med 2011, 10, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.V.; Bittmann, F.N. Are There Two Forms of Isometric Muscle Action? Results of the Experimental Study Support a Distinction between a Holding and a Pushing Isometric Muscle Function. BMC Sports Sci. Med. Rehabil. 2017, 9, 11. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Bittmann, F.N. Paired Personal Interaction Reveals Objective Differences between Pushing and Holding Isometric Muscle Action. PLoS ONE 2021, 16, e0238331. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Bittmann, F.N. Case Study: Intra- and Interpersonal Coherence of Muscle and Brain Activity of Two Coupled Persons during Pushing and Holding Isometric Muscle Action. Brain Sciences 2022, 12, 703. [Google Scholar] [CrossRef]
- Khan, A.; Rayner, G.D. Robustness to Non-Normality of Common Tests for the Many-Sample Location Problem. Journal of Applied Mathematics and Decision Sciences 2003, 7, 187–206. [Google Scholar] [CrossRef]
- Blanca, M.J.; Alarcón, R.; Arnau, J. Non-Normal Data: Is ANOVA Still a Valid Option? Psicothema 2017, 552–557. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size—or Why the P Value Is Not Enough. J Grad Med Educ 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Proske, U.; Morgan, D.L.; Gregory, J.E. Thixotropy in Skeletal Muscle and in Muscle Spindles: A Review. Progress in Neurobiology 1993, 41, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.E.; Morgan, D.L.; Proske, U. Two Kinds of Resting Discharge in Cat Muscle Spindles. Journal of Neurophysiology 1991, 66, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Proske, U. Exercise, Fatigue and Proprioception: A Retrospective. Exp Brain Res 2019, 237, 2447–2459. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.J.; Ansems, G.E.; Proske, U. Evidence from Proprioception of Fusimotor Coactivation during Voluntary Contractions in Humans: Fusimotor Coactivation during Voluntary Contractions. Experimental Physiology 2008, 93, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, M.T.; Proske, U.; Struppler, A. Measurements of Muscle Stiffness, the Electromyogram and Activity in Single Muscle Spindles of Human Flexor Muscles Following Conditioning by Passive Stretch or Contraction. Brain Research 1989, 493, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Van Hooren, B.; Bosch, F. Influence of Muscle Slack on High-Intensity Sport Performance: A Review. Strength & Conditioning Journal 2016, 38, 75–87. [Google Scholar] [CrossRef]
- Proske, U.; Morgan, D.L.; Gregory, J.E. Muscle History Dependence of Responses to Stretch of Primary and Secondary Endings of Cat Soleus Muscle Spindles. The Journal of Physiology 1992, 445, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Garrett, W.E. Muscle Strain Injuries. Am J Sports Med 1996, 24, S2-8. [Google Scholar] [CrossRef]
- Kieb, M.; Lorbach, O.; Engelhardt, M. Muskelverletzungen: Diagnostik und Behandlungen. Orthopäde 2010, 39, 1098–1107. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Bittmann, F.N. Coherent Behavior of Neuromuscular Oscillations between Isometrically Interacting Subjects: Experimental Study Utilizing Wavelet Coherence Analysis of Mechanomyographic and Mechanotendographic Signals. Sci Rep 2018, 8, 15456. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Torick, A.H.; Matuschek, H.; Holschneider, M.; Bittmann, F.N. Synchronization of Muscular Oscillations Between Two Subjects During Isometric Interaction. Eur J Transl Myol 2014, 24, 2237. [Google Scholar] [CrossRef]
- Schaefer, L.V. Synchronisationsphänomene myotendinöser Oszillationen interagierender neuromuskulärer Systeme – mit Betrachtung einer Hypothese bezüglich unterschiedlicher Qualitäten isometrischer Muskelaktion. Dissertation, Universität Potsdam, Potsdam, 2014. [Google Scholar]
- McAuley, J.H. Physiological and Pathological Tremors and Rhythmic Central Motor Control. Brain 2000, 123, 1545–1567. [Google Scholar] [CrossRef] [PubMed]
- Pruszynski, J.A.; Scott, S.H. Optimal Feedback Control and the Long-Latency Stretch Response. Exp Brain Res 2012, 218, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Manning, C.D.; Tolhurst, S.A.; Bawa, P. Proprioceptive Reaction Times and Long-Latency Reflexes in Humans. Exp Brain Res 2012, 221, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, F.; Ekerot, C.-F.; Jörntell, H. In Vivo Analysis of Inhibitory Synaptic Inputs and Rebounds in Deep Cerebellar Nuclear Neurons. PLoS ONE 2011, 6, e18822. [Google Scholar] [CrossRef]
- Lang, E.J.; Apps, R.; Bengtsson, F.; Cerminara, N.L.; De Zeeuw, C.I.; Ebner, T.J.; Heck, D.H.; Jaeger, D.; Jörntell, H.; Kawato, M.; et al. The Roles of the Olivocerebellar Pathway in Motor Learning and Motor Control. A Consensus Paper. Cerebellum 2017, 16, 230–252. [Google Scholar] [CrossRef]
- Vitek, J.L.; Ashe, J.; DeLong, M.R.; Alexander, G.E. Physiologic Properties and Somatotopic Organization of the Primate Motor Thalamus. Journal of Neurophysiology 1994, 71, 1498–1513. [Google Scholar] [CrossRef]
| parameters | Procedure CL) | Procedure CL-CT |
|---|---|---|
| force 1st pre-contraction / MVIC (%) | 25.04 ± 9.44 | 26.44 ± 9.11 |
| force 2nd pre-contraction / MVIC (%) | - | 28.47 ± 8.81 |
| duration of 1st pre-contraction (s) | 1.09 ± 0.33 | 1.12 ± 0.33 |
| duration of 2nd pre-contraction (s) | - | 0.72 ± 0.12 |
| duration 1st pre- contraction to MMT (s) | 7.51 s ± 0.96 | 7.33 ± 1.76 |
| parameter | procedure | M | SD | F | df | p | η² |
|---|---|---|---|---|---|---|---|
| AFmax | regular | 272.574 | 38.846 | 3.193 | 2, 36 | 0.053 | - |
| CL | 279.512 | 49.427 | |||||
| CL-CT | 265.259 | 46.158 | |||||
| AFisomax | regular | 271.774 | 39.367 | 44.946* | 1.24, 22.36 | < 0.0011 | 0.714 |
| CL | 152.146 | 78.607 | |||||
| CL-CT | 262.033 | 52.597 | |||||
| AFosc | regular | 217.580 | 51.058 | 18.992 | 2, 36 | < 0.0011 | 0.512 |
| CL | 268.585 | 53.937 | |||||
| CL-CT | 217.255 | 43.088 | |||||
| ratio AFisomax/AFmax | regular | 0.997 | 0.100 | 75.660 | 1.09, 19.64 | < 0.0011 | 0.808 |
| CL | 0.530 | 0.225 | |||||
| CL-CT | 0.983 | 0.055 | |||||
| ratio AFosc/ AFmax | regular | 0.795 | 0.115 | 26.644* | 1.47, 26.54 | < 0.0011 | 0.597 |
| CL | 0.959 | 0.059 | |||||
| CL-CT | 0.818 | 0.066 | |||||
| ratio AFosc/AFisomax | regular | 0.797 | 0.119 | 16.264* | 1.01, 18.12 | 0.0011 | 0.475 |
| CL | 2.566 | 1.885 | |||||
| CL-CT | 0.836 | 0.089 | |||||
| slope | regular | 2.017 | 0.140 | 19.686 | 2,34 | < 0.0012 | |
| CL | 2.210 | 0.201 | 0.537 | ||||
| CL-CT | 2.137 | 0.121 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





