Submitted:
01 March 2023
Posted:
06 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
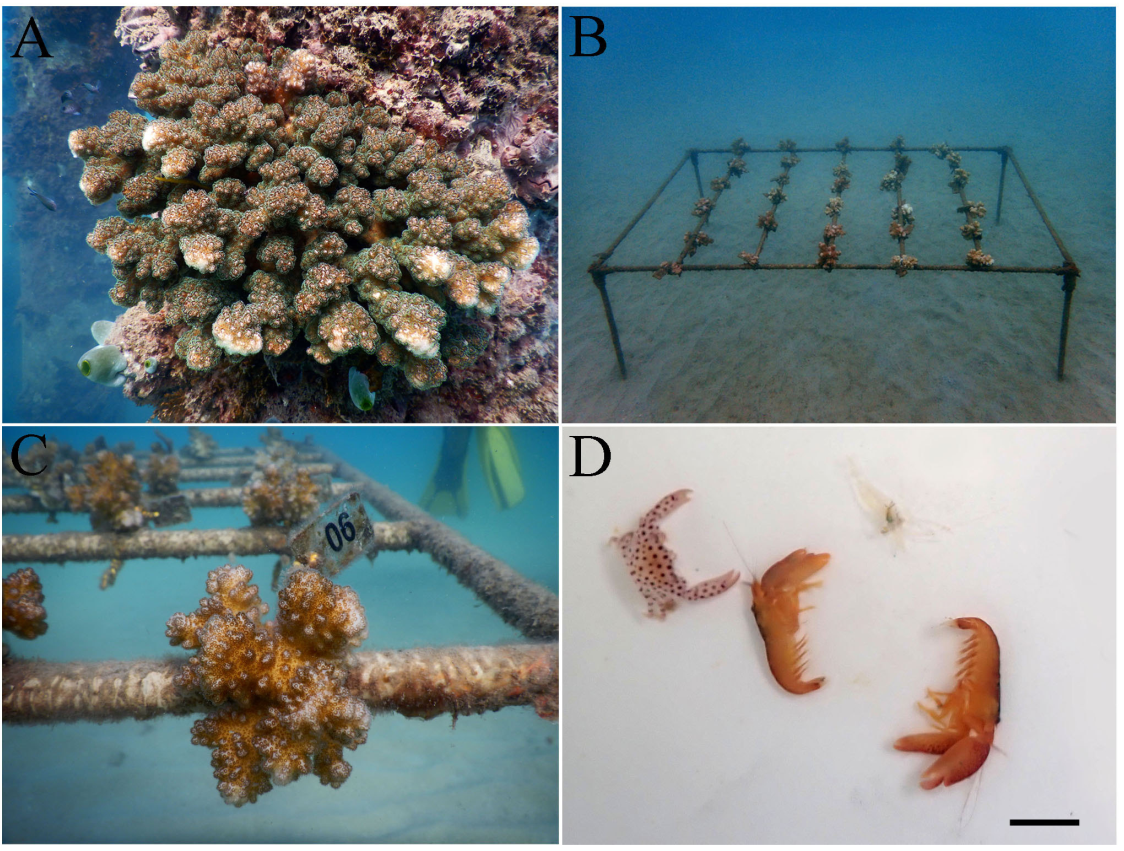
2.1. Host coral and sampling location
2.2. Experimental design
2.3. Identification and categorization of the species
2.4. Statistical analysis.
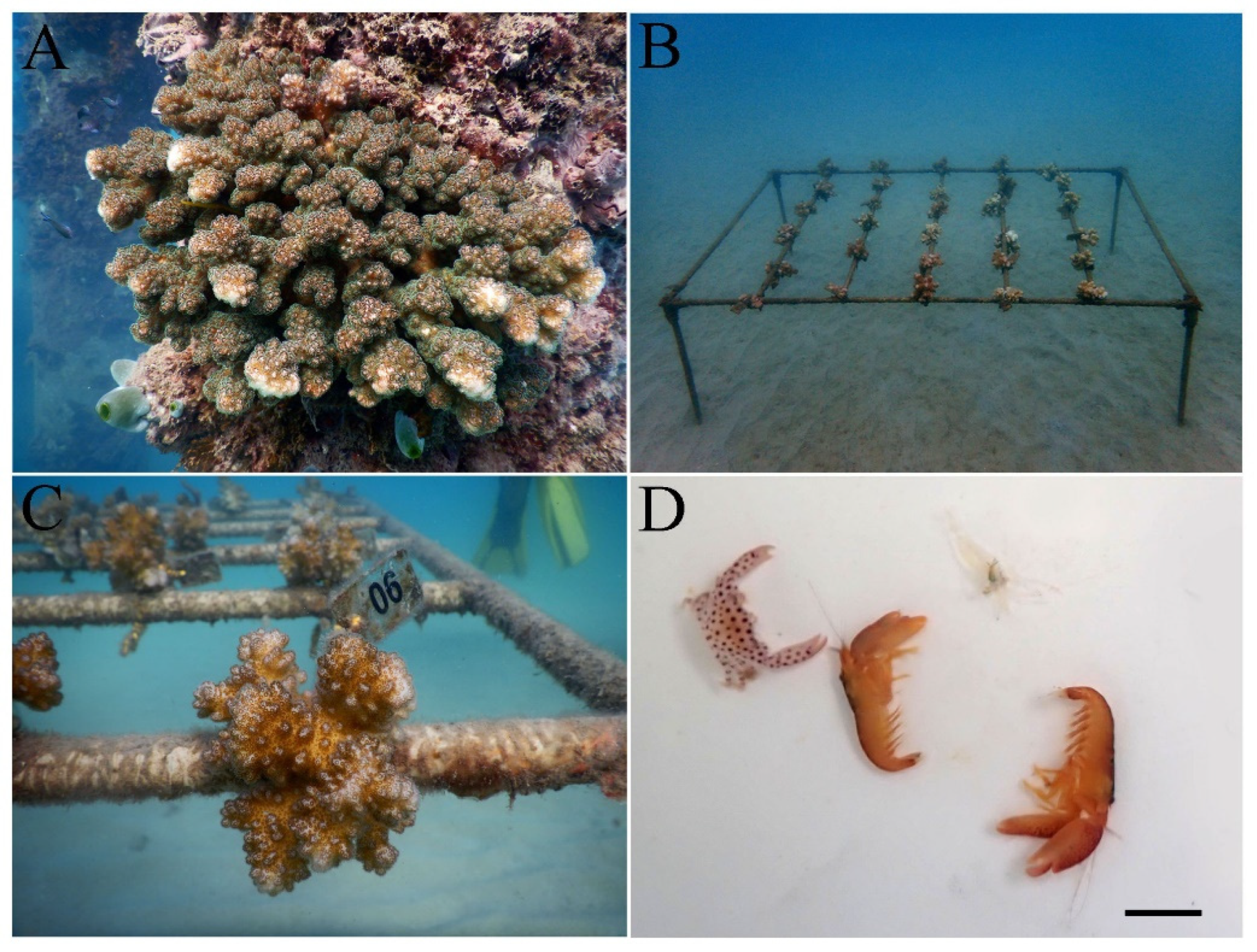
3. Results
3.1. Taxonomic composition
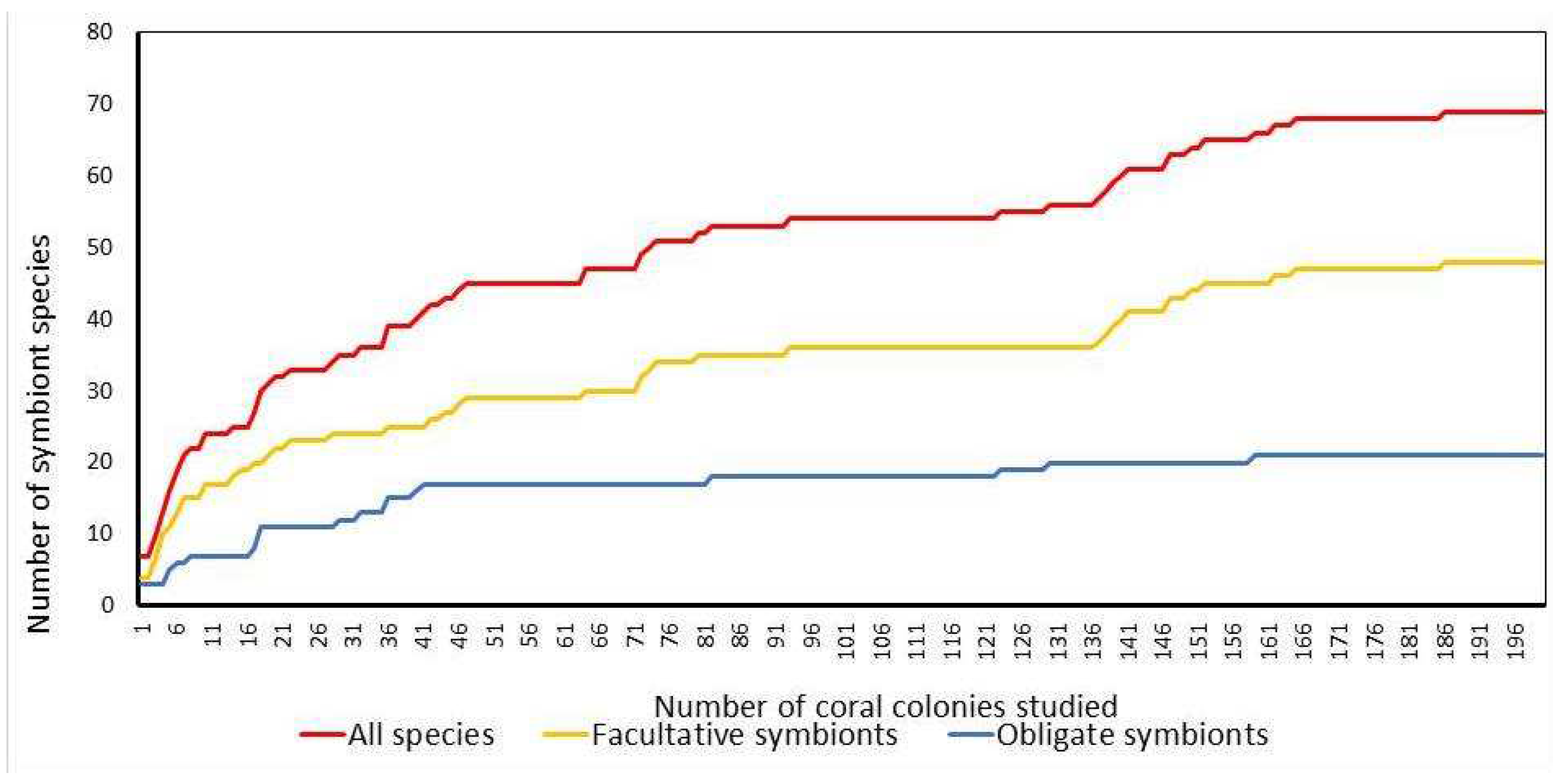
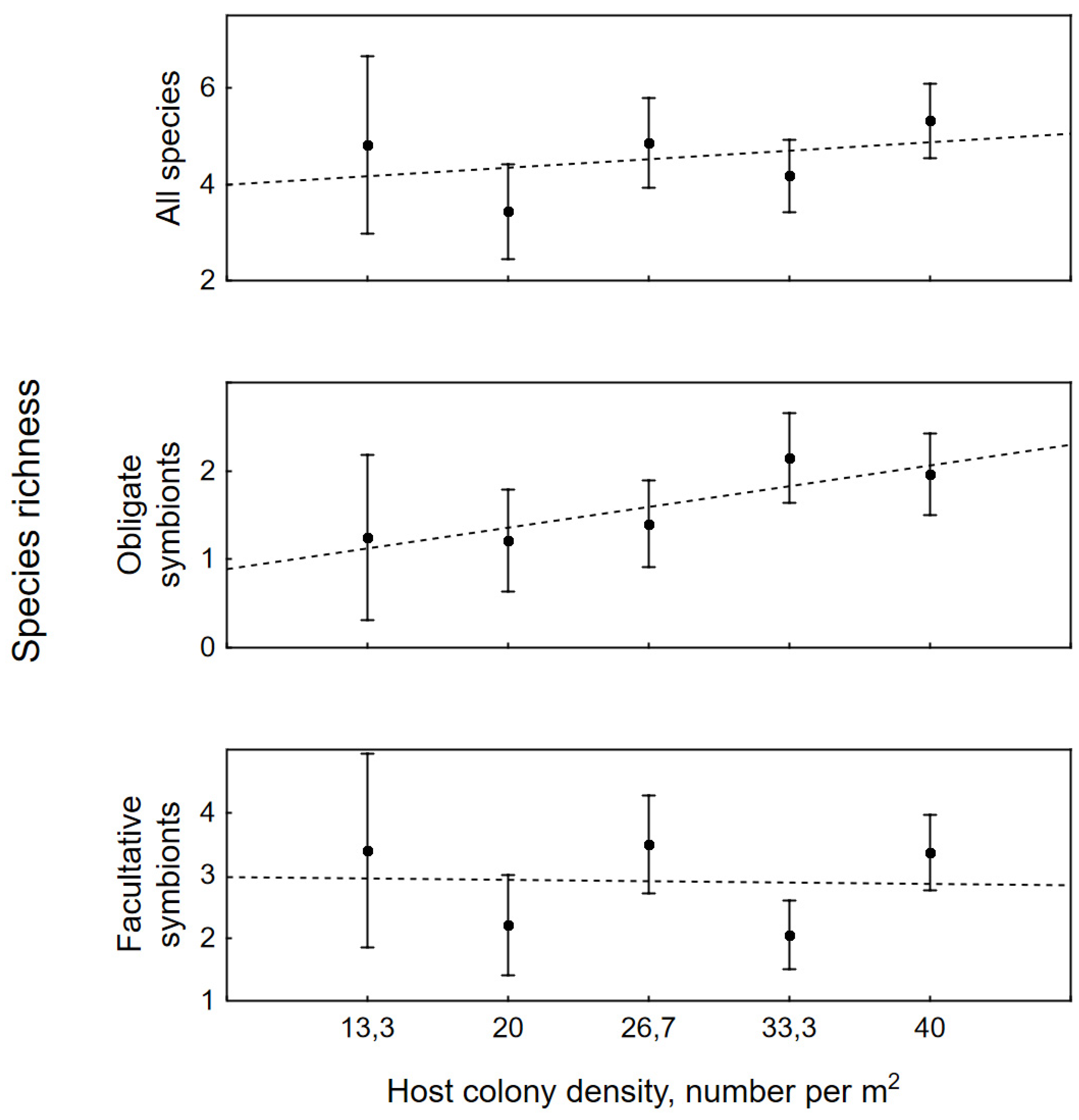
3.2. The impact of colony size, bleaching and host coral density on symbiotic communities.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stella, J.S.; Pratchett, M.S.; Hutchings, P.A.; Jones, G.P. Coral-associated invertebrates: Diversity, ecological importance and vulnerability to disturbance. Oceanogr. Mar. Biol. An Annu. Rev. 2011, 49, 43–104. [Google Scholar] [CrossRef]
- Hoeksema, B.W.; Van Der Meij, S.E.T.; Fransen, C.H.J.M. The mushroom coral as a habitat. J. Mar. Biol. Assoc. United Kingdom 2012, 92, 647–663. [Google Scholar] [CrossRef]
- Enochs, I.C. Motile cryptofauna associated with live and dead coral substrates: implications for coral mortality and framework erosion. Mar. Biol. 2012, 159, 709–722. [Google Scholar] [CrossRef]
- Montano, S. The extraordinary importance of coral associated fauna. Diversity 2020, 12, 357. [Google Scholar] [CrossRef]
- Stimson, J. Stimulation of fat-body production in the polyps of the coral Pocillopora damicornis by the presence of mutualistic crabsof the genus Trapezia. Mar. Biol. 1990, 106, 211–218. [Google Scholar] [CrossRef]
- Stewart, H.L.; Holbrook, S.J.; Schmitt, R.J.; Brooks, A.J. Symbiotic crabs maintain coral health by clearing sediments. Coral Reefs 2006, 25, 609–615. [Google Scholar] [CrossRef]
- Stier, A.C.; Gil, M.A.; McKeon, C.S.; Lemer, S.; Leray, M.; Mills, S.C.; Osenberg, C.W. Housekeeping mutualisms: Do more symbionts facilitate host performance? PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Pollock, F.J.; Katz, S.M.; Bourne, D.G.; Willis, B.L. Cymo melanodactylus crabs slow progression of white syndrome lesions on corals. Coral Reefs 2013, 32, 43–48. [Google Scholar] [CrossRef]
- Glynn, P.W. Some physical and biological determinants of coral community structure in the Eastern Pacific. Ecol. Monogr. 1976, 46, 431–456. [Google Scholar] [CrossRef]
- Pratchett, M.S. Influence of coral symbionts on feeding preferences of crown-of-thorns starfish Acanthaster planci in the western Pacific. Mar. Ecol. Prog. Ser. 2001, 214, 111–119. [Google Scholar] [CrossRef]
- McKeon, C.S.; Stier, A.C.; McIlroy, S.E.; Bolker, B.M. Multiple defender effects: synergistic coral defense by mutualist crustaceans. 2012, 169, 1095−1103. [CrossRef]
- Rouzé, H.; Lecellier, G.; Mills, S.C.; Planes, S.; Berteaux-Lecellier, V.; Stewart, H. Juvenile Trapezia spp. crabs can increase juvenile host coral survival by protection from predation. Mar. Ecol. Prog. Ser. 2014, 515, 151–159. [Google Scholar] [CrossRef]
- Montano, S.; Fattorini, S.; Parravicini, V.; Berumen, M.L.; Galli, P.; Maggioni, D.; Arrigoni, R.; Seveso, D.; Strona, G. Corals hosting symbiotic hydrozoans are less susceptible to predation and disease. Proc. R. Soc. B. 2017, 284, 20172405. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.K.; Graham, N.A.J.; Polunin, N.V.C. Appraisal of visual assessments of habitat complexity and benthic composition on coral reefs. Mar. Biol. 2007, 151, 1069–1076. [Google Scholar] [CrossRef]
- Patton, W.K. Decapod Crustacea commensal with Queensland branching corals. Crustaceana 1966, 10, 271–295. [Google Scholar] [CrossRef]
- Patton, W.K. Distribution and ecology of animals associated with branching corals (Acropora spp.) from the Great Barrier Reef, Australia. Bull. Mar. Sci. 1994, 55, 193–211. [Google Scholar]
- Stella, J.S.; Jones, G.P.; Pratchett, M.S. Variation in the structure of epifaunal invertebrate assemblages among coral hosts. Coral Reefs 2010, 29, 957–973. [Google Scholar] [CrossRef]
- Britayev, T.A.; Spiridonov, V.A.; Deart, Y.V.; El-Sherbiny, M. Biodiversity of the community associated with Pocillopora verrucosa (Scleractinia: Pocilloporidae) in the Red Sea. Mar. Biodivers. 2017, 47, 1093–1109. [Google Scholar] [CrossRef]
- Abele, L.G.; Patton, W.K. The size of coral heads and the community biology of associate decapod crustaceans. Journal of Biogeography 1976, 3, 35–47. [Google Scholar] [CrossRef]
- Lewis, J.B.; Snelgrove, P.V.R. Corallum morphology and composition of crustacean cryptofauna of the hermatypic coral Madracis mirabilis. Mar. Biol. 1990, 106, 267–272. [Google Scholar] [CrossRef]
- Pisapia, C.; Stella, J.; Silbiger, N.J.; Carpenter, R. Epifaunal invertebrate assemblages associated with branching pocilloporids in Moorea, French Polynesia. PeerJ 2020, 8, e9364. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Abele, L.G. Community patterns of coral-associated decapods. Mar. Ecol. Prog. Ser. 1983, 13, 131–139. [Google Scholar] [CrossRef]
- Britayev, T.A.; Mikheev, V.N. Clumped spatial distribution of scleractinian corals influences the structure of their symbiotic associations. Dokl. Biol. Sci. 2013, 448, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Counsell, C.W.; Donahue, M.J.; Edwards, K.F.; Franklin., E.C.; Hixon. Variation in coral-associated cryptofaunal communities across spatial scales and environmental gradients. Coral Reefs 2018, 37, 827–840. [Google Scholar] [CrossRef]
- Munday, P.L. Interactions between habitat use and patterns of abundance in coral-dwelling fishes of the genus Gobiodon. Environ. Biol. Fishes 2000 58, 355–369. [CrossRef]
- Depczynski, M.; Bellwood, D.R. Wave energy and spatial variability in community structure of small cryptic coral reef fishes. Mar. Ecol. Prog. Ser. 2005, 303, 283–293. [Google Scholar] [CrossRef]
- Black, R.; Prince, J. Fauna associated with the coral Pocillopora damicornis at the Southern Limit of its distribution in Western Australia. Journal of Biogeography 1983, 10, 135–152. [Google Scholar] [CrossRef]
- Edwards, A. ; Emberton. H. Crustacea associated with the scleractinian coral, Stylophora pistillata (Esper.) in the Sudanese Red Sea. J. Exp. Mar. Biol. Ecol. 1980, 42, 225–240. [Google Scholar] [CrossRef]
- Brown, B.E. Coral bleaching: causes and consequences. Coral Reefs 1997, 16 (Suppl. S1), S129–S138. [Google Scholar] [CrossRef]
- Douglas, A.E. Coral bleaching—how and why? Mar. Pollut. Bull. 2003, 46, 385–392. [Google Scholar] [CrossRef]
- Stella, J.S.; Munday, P.L.; Jones, G.P. Effects of coral bleaching on the obligate coral-dwelling crab Trapezia cymodoce. Coral Reefs 2011, 30, 719–727. [Google Scholar] [CrossRef]
- Tsuchiya, M. Effect of mass coral bleaching on the community structure of small animals associated with the hermatypic coral Pocillopora damicornis. Galaxea, JCRS 1999, 1, 65–72. [Google Scholar] [CrossRef]
- Munday, P.L. Habitat loss, resource specialization, and extinction on coral reefs. Global Change Biol. 2004, 10, 1642–1647. [Google Scholar] [CrossRef]
- Stella, J.S.; Munday, P.L.; Walker, S.P.W.; Pratchett, M.S.; Jones, G.P. From cooperation to combat: adverse effect of thermal stress in a symbiotic coral-crustacean community. Oecologia 2014, 174, 1187–1195. [Google Scholar] [CrossRef]
- Froehlich, C.Y. M.; Klanten, O.S.; Hing, M.L.; Dowton, M.; Wong, M.Y.L. Uneven declines between corals and cryptobenthic fish symbionts from multiple disturbances. Sci. Rep. 2021, 11, 16420. [Google Scholar] [CrossRef] [PubMed]
- Combillet, L.; Fabregat-Malé, S.; Mena, S.; Marín-Moraga, J.A.; Gutierrez, M.; Alvarado, J.J. Pocillopora spp. growth analysis on restoration structures in an Eastern Tropical Pacific upwelling area. PeerJ 2022, 10, e13248. [Google Scholar] [CrossRef] [PubMed]
- Coles, S. Species diversity of decapods associated with living and dead reef coral Pocillopora meandrina. Mar. Ecol. Prog. Ser. 1980, 2, 281–291. [Google Scholar] [CrossRef]
- Patton, W.K. Community structure among the animals inhabiting the coral Pocillopora damiconis at Heron Island, Australia. In Symbiosis in the sea; Vernberg, W.B., Ed.; University of South Carolina Press: Columbia, USA, 1974; pp. 219–243. [Google Scholar]
- Tkachenko, K.S.; Soong, K.; Deart, Y.V.; Britayev, T.A. Coral symbiotic communities from different environments of an isolated atoll: reef lagoon versus forereef. Invert. Zool. 2022, 19, 78–90. [Google Scholar] [CrossRef]
- Marin, I.N.; Britayev, T.A.; Anker, A. Pontoniine shrimps associated with cnidarians: new records and list of species from coastal waters of Viet Nam. Arthropoda Selecta 2004, 13, 199–218. [Google Scholar]
- Marin, I.N.; Spiridonov, V.A. Coral-associated crabs (Decapoda: Domecidae, Trapeziidae, Tetraliidae, Xanthidae: Cymoinae) from Nha Trang Bay. In Benthic Fauna of the Bay of Nha Trang; Britayev, T.A., Pavlov, D.S., Eds.; KMK Scientific Press: Moscow, Russia, 2007; pp. 209–234. [Google Scholar]
- Tkachenko, K.S.; Britayev, T.A.; Huan, N.; Pereladov, M.V.; Latypov, Y. Influence of anthropogenic pressure and seasonal upwelling on coral reefs in Nha Trang Bay (Central Vietnam). Mar. Ecol 2016, 37, 1131–1146. [Google Scholar] [CrossRef]
- Austin, A.D.; Austin, S.A.; Sale, P.E. Community structure of the fauna associated with the coral Pocillopora damicornis (L.) on the Great Barrier Reef. Mar. Freshwater Res. 1980, 31, 163–174. [CrossRef]
- Siebeck, U.E.; Marshall, N.J.; Klüter, A.; Hoegh-Guldberg, O. Monitoring coral bleaching using a colour reference card. Coral Reefs 2006, 25, 453–460. [Google Scholar] [CrossRef]
- Bruce, A.J. New keys for the identification of Indo-West Pacific coral associated pontoniine shrimps, with observations on their ecology: (Crustacea: Decapoda: Palaemonidae). Ophelia, 1998, 49, 29–46. [Google Scholar] [CrossRef]
- Castro, P.; Ng, P.K.L.; Ahyong, S.T. Phylogeny and systematics of the Trapeziidae Miers, 1886 (Crustacea: Brachyura), with the description of a new family. Zootaxa 2004, 643, 1–70. [Google Scholar] [CrossRef]
- Banner, D.M.; Banner, A.B. The alpheid shrimp of Australia Part III: The remaining alpheids, principally the genus Alpheus, and the family Ogyrididae. Rec. Aust. Mus. 1982, 34, 1–357. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef] [PubMed]
- O'Hara, R.; Kotze, J. Do not log-transform count data. Methods Ecol. Evol. 2010, 1, 118–122. [Google Scholar] [CrossRef]
- Britayev, T.A.; Mekhova, E.S. Assessment of hidden diversity of crinoids and their symbionts in the bay of Nhatrang, Vietnam. Org. Divers. Evol. 2011, 11, 275–285. [Google Scholar] [CrossRef]
- Hoeksema, B.W. Delineation of the Indo-Malayan Centre of maximum marine biodiversity: the Coral Triangle. In Biogeography, time and place: distributions, barriers and islands; Renema, W., Ed.; Springer: Dordrecht, 2007; pp. 117–178. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Yamauchi, Y.; Moretzsohn, F.; Tsukiji, M. Species composition and some population traits of obligate symbiotic xanthid crabs, Trapezia and Tetralia, associated with bleached corals. In Proc 7th Intl Coral Reef Symp. 1992, 1, 56–63. [Google Scholar]
- Castro, P. Movements between corals colonies in Trapezia ferruginea (Crustacea: Brachyura), an obligate symbiont of scleractinian corals. Mar. Biol. 1978, 46, 237–245. [Google Scholar] [CrossRef]
- Arneberg, P.; Skorping, A.; Grenfell, B.; Read, A.F. Host densities as determinants of abundance in parasite communities. Proc. R. Soc. Lond B 1998, 265, 1283–1289. [Google Scholar] [CrossRef]
- Anderson, M.R.; May, R.M. Infectious diseases of humans: dynamics and control. Oxford University Press, New York, 1991.
- Marsden, J.R. Coral preference behaviour by planktotrophic larvae of Spirobranchus giganteus corniculatus (Serpulidae: Polychaeta). Coral Reefs 1987, 6, 71–74. [Google Scholar] [CrossRef]
- Thiel, M.; Zander, A.; Baeza, J.A. Movements of the symbiotic crab Liopetrolisthes mitra between its host sea urchin Tetrapygus niger. Bull. Mar. Sci. 2003, 72, 89–101. [Google Scholar]
- Dgebuadze, P. Yu.; Mehova, E.S.; Britayev, T.A. Recolonization of the Himerometra robustipinna (Himerometridae, Crinoidea) by macrosymbionts: an in situ experiment. Symbiosis 2012, 58, 253–258. [Google Scholar] [CrossRef]
- Britayev, T.A.; Mekhova, E.S. Do symbiotic polychaetes migrate from host to host? Memoirs of Museum Victoria 2014, 71, 21–25. [Google Scholar] [CrossRef]
- Preston, E.M. A computer simulation of competition among five sympatric congeneric species of xanthid crabs. Ecology 1973, 54, 469–483. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




