Submitted:
05 March 2023
Posted:
07 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Problem Formulation
2.1. Fluid Model
2.2. Structure Model
2.3. Fluid Structure Interaction Set of Equations
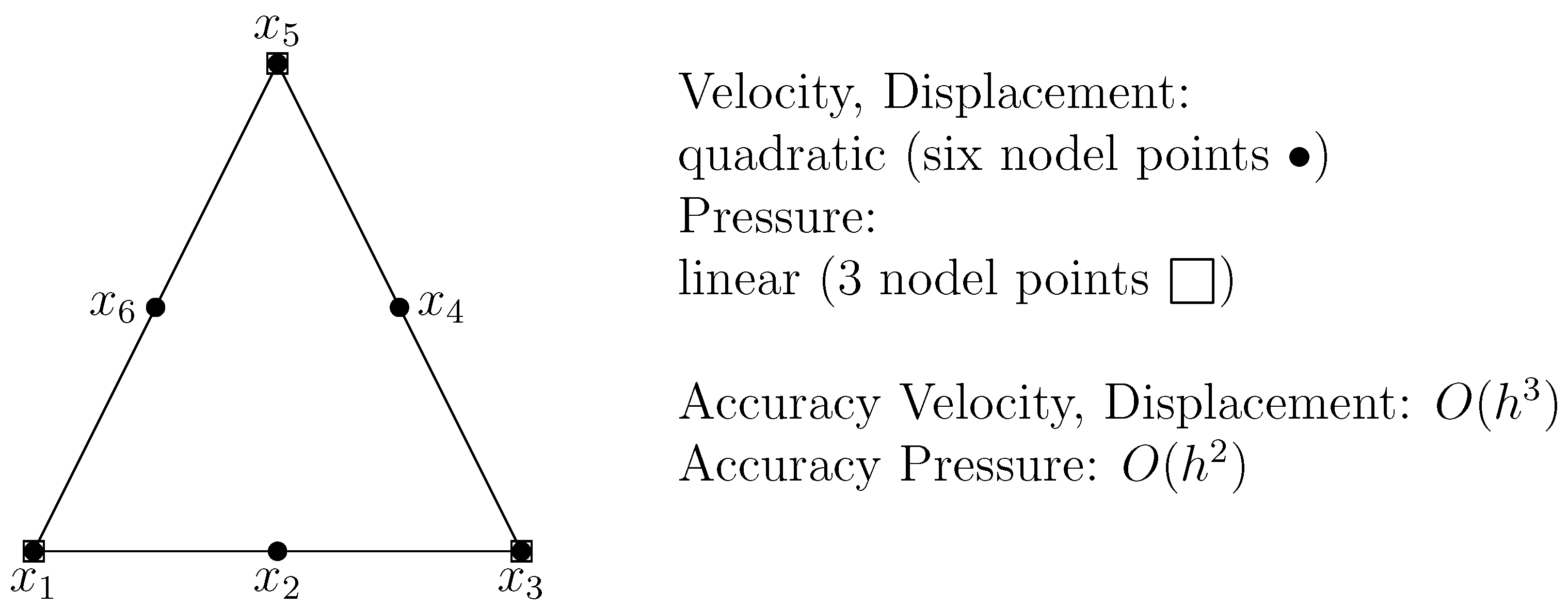
3. The Finite Element Method (FEM) Discretization
4. Solution Algorithm
| Algorithm 1 Newton iteration and line search |
|
5. Mathematical Modeling
5.1. Problem Configuration
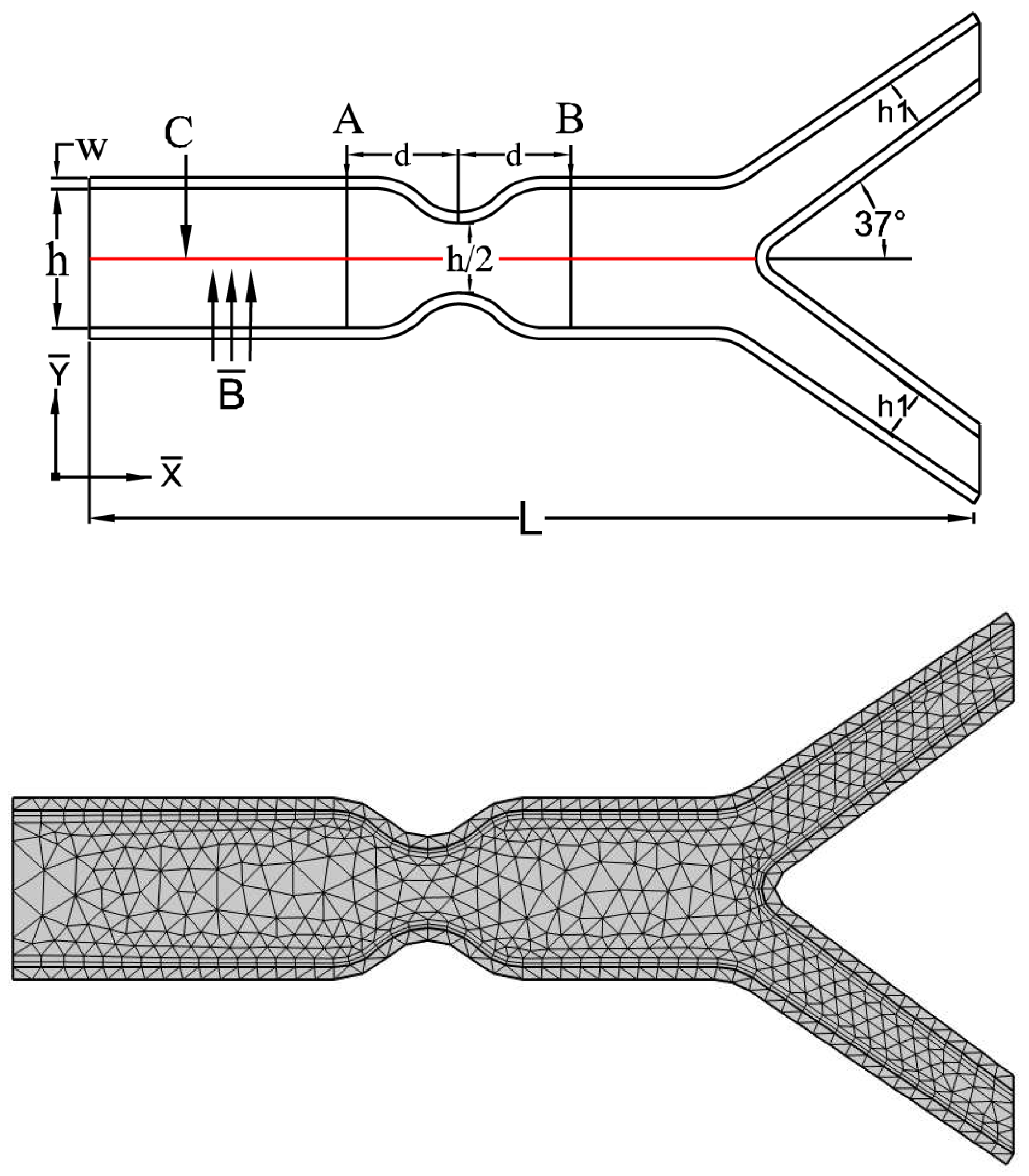
6. Results and Discussion
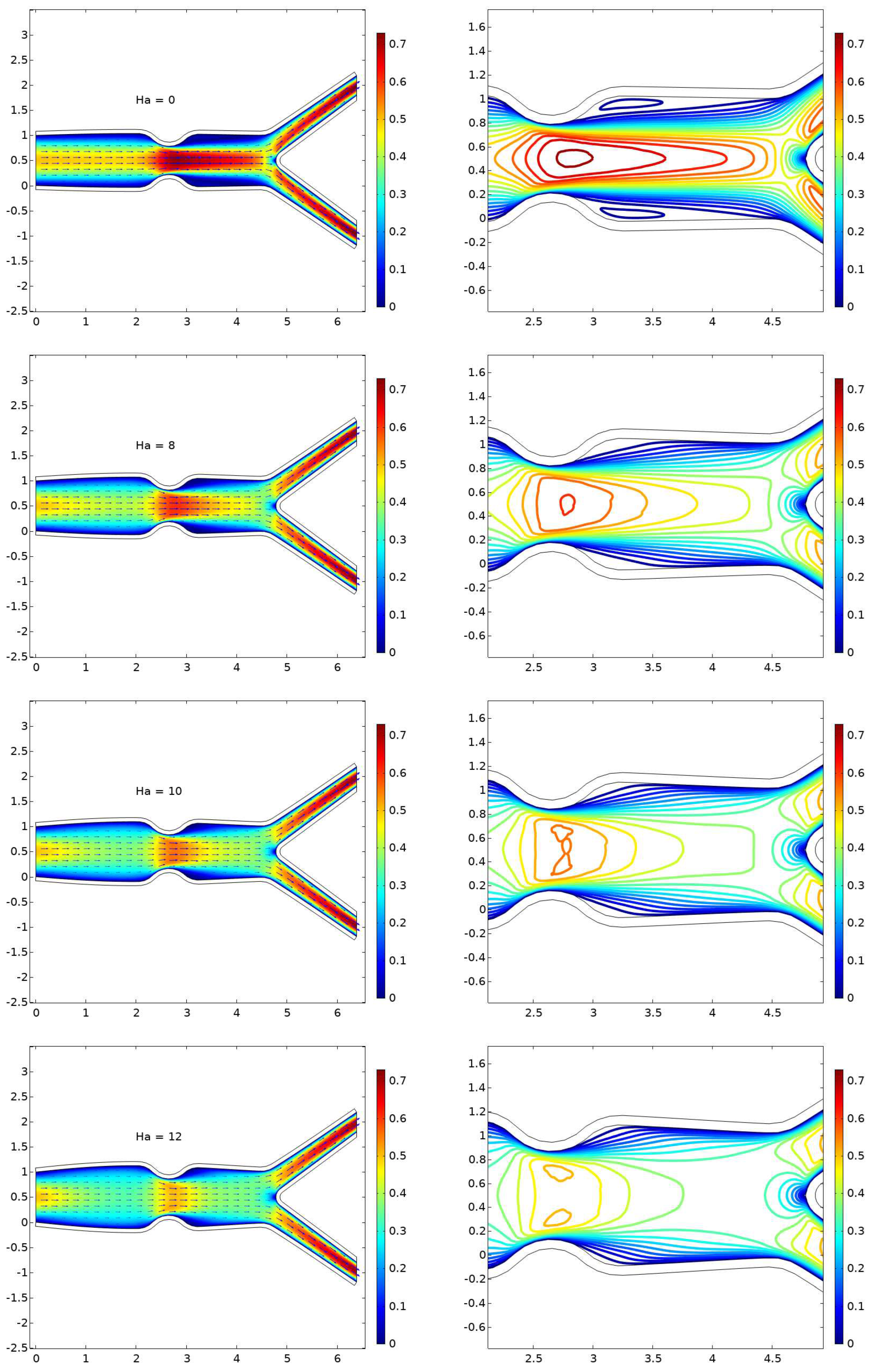
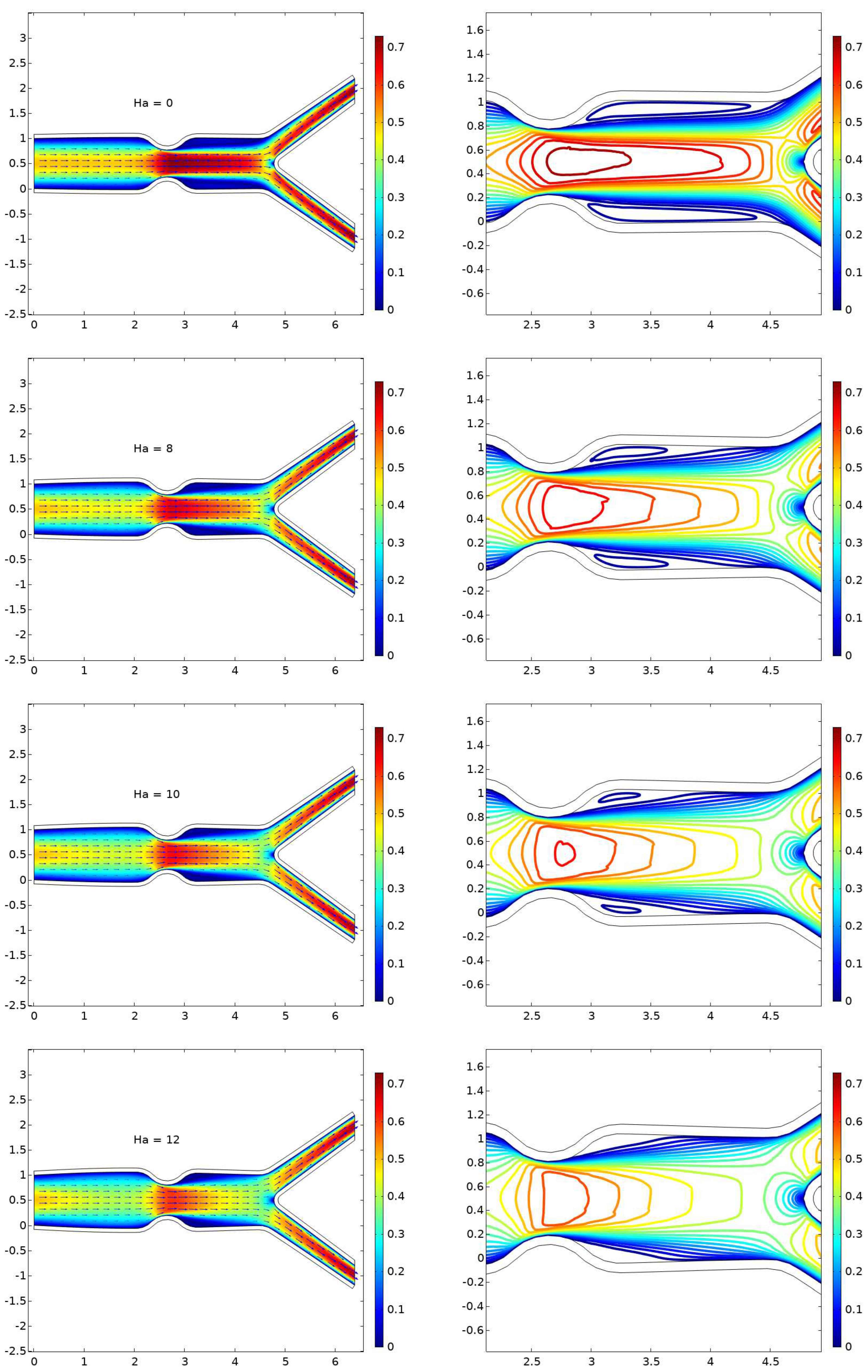
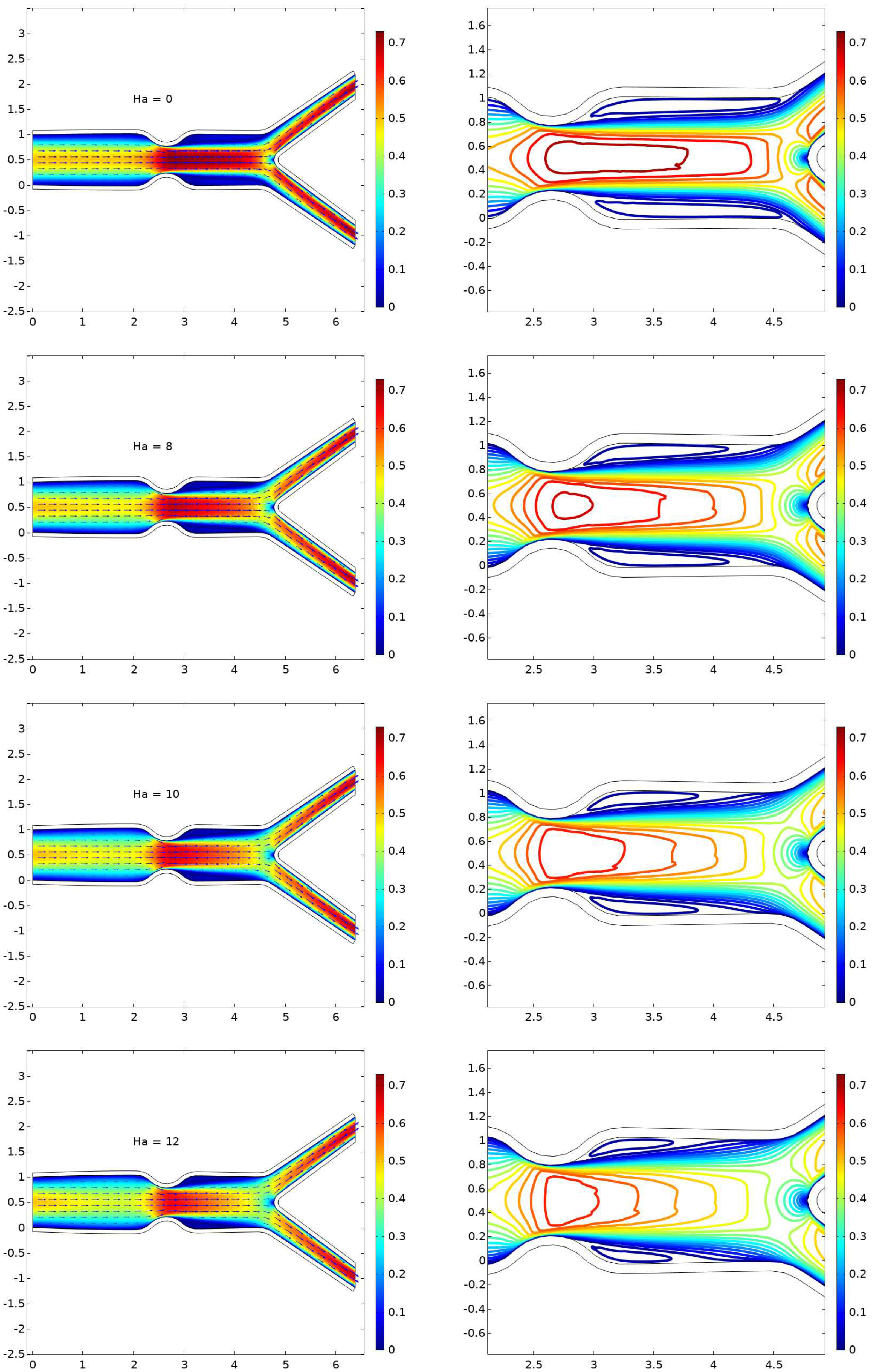
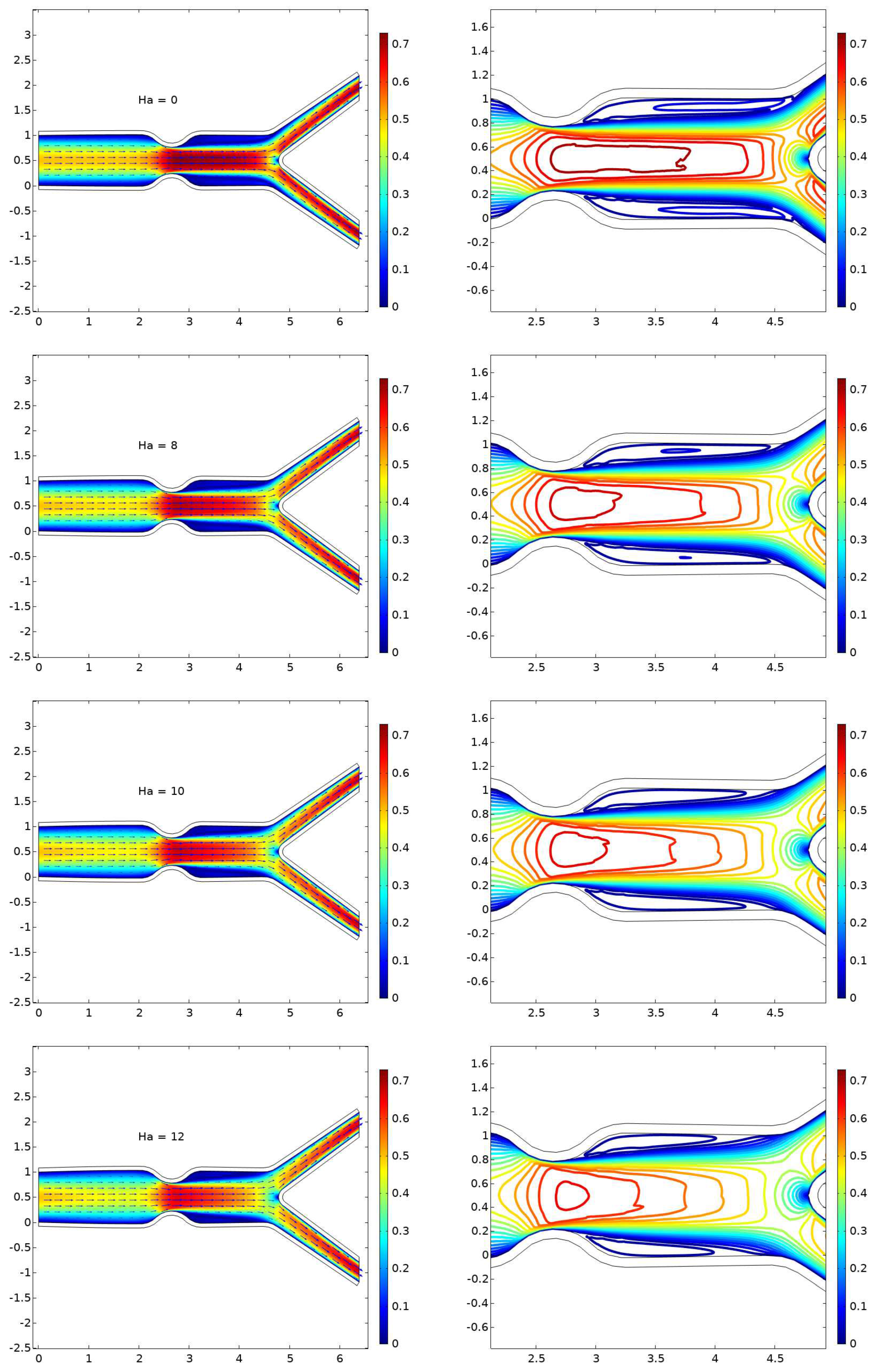
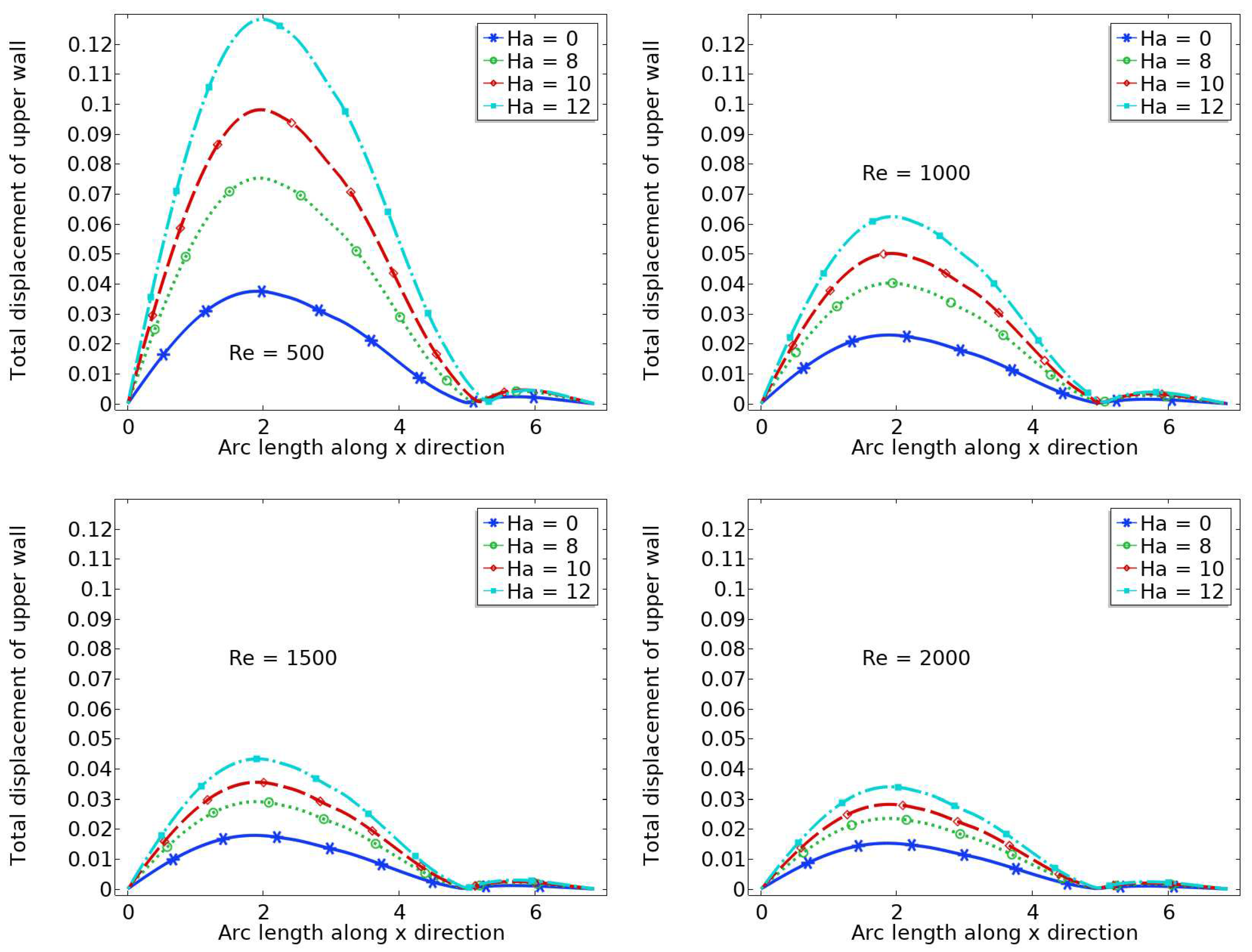
6.1. Displacement
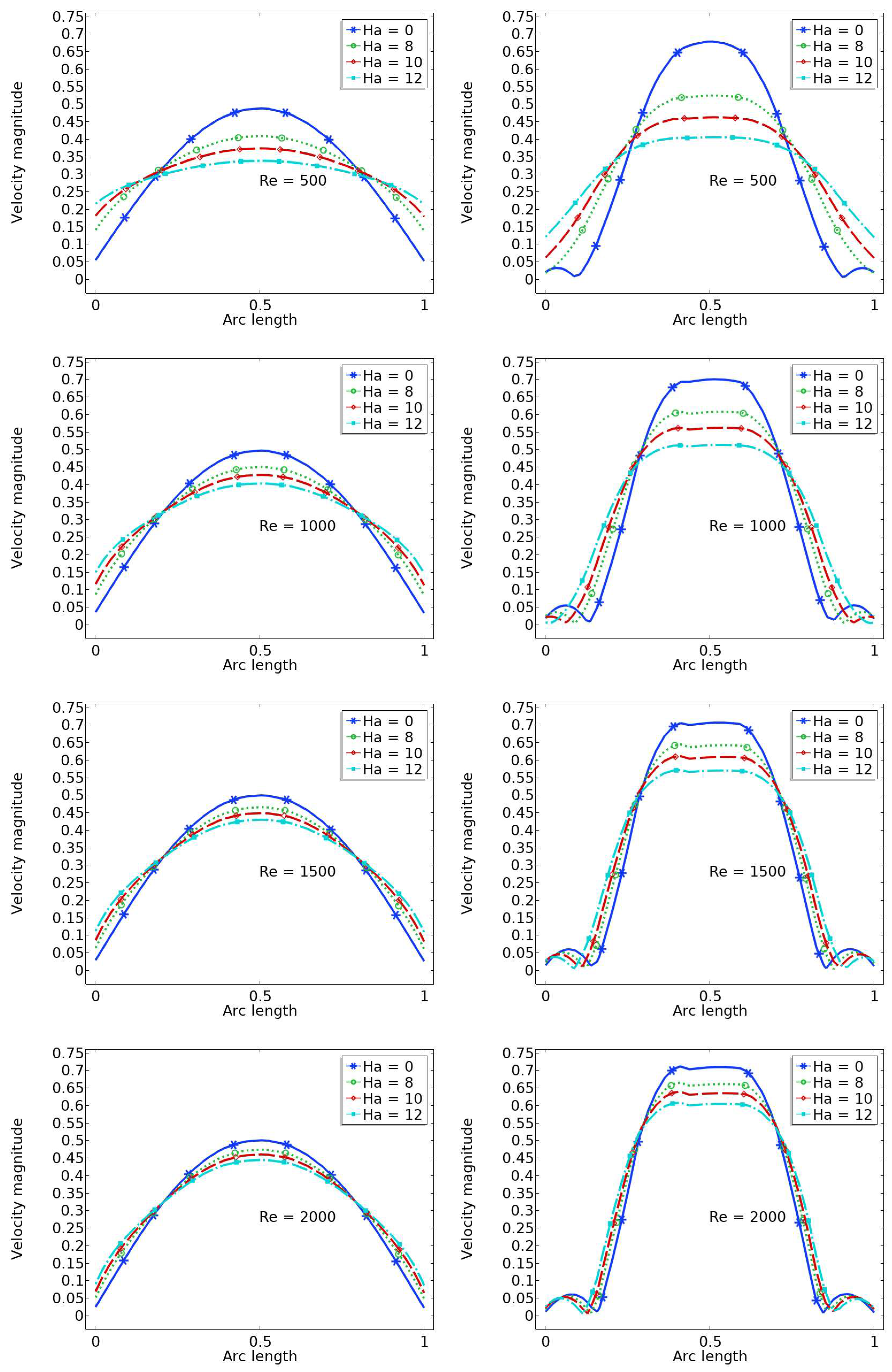
6.2. The Velocity Profile at Position A and B
6.3. Pressure at the centre

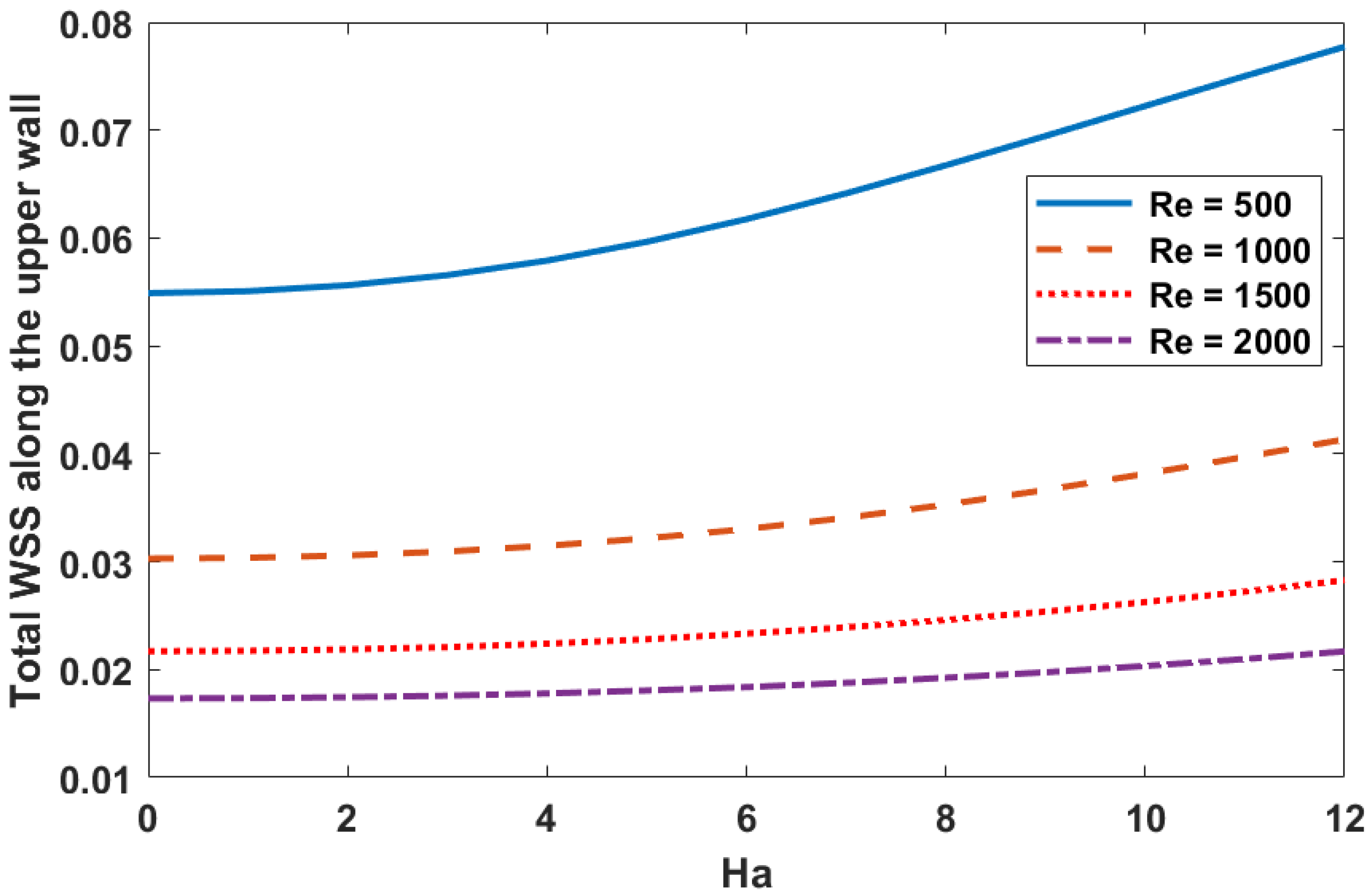
6.4. Wall Shear Stress
7. Conclusions
- The presence of a magnetic field parameter, , in increasing order reduces the size of the cavity adjacent to the stenosis.
- A higher value of the magnetic field parameter decreases the velocity gain after the stenosis compared to the purely hydrodynamic case (). For example, when , the velocity gain is 41
- The pressure increases with higher values of Hartmann number ().
- A flow with a small Reynolds number () and a higher magnetic field parameter value () results in a significant increase in wall shear stress.
- The wall displacement is larger when and , and the maximum wall displacement is observed just before the stenosis.
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Health Estimates 2016: Death by Cause, Age, Sex, by Country and by Region, 2000–2016; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Xu, S.; Jaroslav, P.; Zheng, G.J. Atherosclerosis is an epigenetic disease. Trends in Endocrinology & Metabolism 2018, 29, 739–742. [Google Scholar]
- Oberoi, S.; Schoepf, U.J.; Meyer, M.; Henzler, T.; Rowe, G.W.; Costello, P.; Nance, J.W. Progression of arterial stiffness and coronary atherosclerosis: longitudinal evaluation by cardiac CT. American journal of roentgenology 2013, 200, 798–804. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. Journal of the American College of Cardiology 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Palombo, C.; Kozakova, M. Arterial stiffness, atherosclerosis and cardiovascular risk: pathophysiologic mechanisms and emerging clinical indications. Vascular pharmacology 2016, 77, 1–7. [Google Scholar] [CrossRef]
- Song, P.; Xia, W.; Zhu, Y.; Wang, M.; Chang, X.; Jin, S.; Wang, J., An. Prevalence of carotid atherosclerosis and carotid plaque in Chinese adults: A systematic review and meta-regression analysis. Atherosclerosis 2018, 276, 67–73. [Google Scholar] [CrossRef]
- Gharahi, H.; Zambrano, B.A.; Zhu, D.C.; DeMarco, J.K.; Baek, S. Computational fluid dynamic simulation of human carotid artery bifurcation based on anatomy and volumetric blood flow rate measured with magnetic resonance imaging. International journal of advances in engineering sciences and applied mathematics 2016, 8, 46–60. [Google Scholar] [CrossRef]
- Caro, C.G. Discovery of the role of wall shear in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology 2009, 29, 158–161. [Google Scholar] [CrossRef]
- Zhang, B.; Gu, J.; Qian, M.; Niu, L.; Ghista, D. Correlation between quantitative analysis of wall shear stress and intima-media thickness in atherosclerosis development in carotid arteries. Biomedical engineering online 2017, 16, 137. [Google Scholar] [CrossRef]
- Siasos, G.; Sara, J.D.; Zaromytidou, M.; Park, K.H.; Coskun, A.U.; Lerman, L.O.; Oikonomou, E.; Maynard, C.C.; Fotiadis, D.; Stefanou, K.; et al. Local low shear stress and endothelial dysfunction in patients with nonobstructive coronary atherosclerosis. Journal of the American College of Cardiology 2018, 71, 2092–2102. [Google Scholar] [CrossRef]
- Chiastra, C.; Gallo, D.; Tasso, P.; Iannaccone, F.; Migliavacca, F.; Wentzel, J.J.; Morbiducci, U. Healthy and diseased coronary bifurcation geometries influence near-wall and intravascular flow: a computational exploration of the hemodynamic risk. Journal of biomechanics 2017, 58, 79–88. [Google Scholar] [CrossRef]
- Luo, K.; Jiang, W.; Yu, C.; Zhou, Z.; Ding, Y. Fluid–Solid Interaction Analysis on Iliac Bifurcation Artery: A Numerical Study. International Journal of Computational Methods 2019, 16, 18501. [Google Scholar] [CrossRef]
- Li, Z.Y.; Howarth, S.; Trivedi, R.A.; U-King-Im, J.M.; Brown, A.; Wang, L.; Gillard, J.H. Stress analysis of carotid plaque rupture based on in vivo high resolution MRI. Journal of biomechanics 2006, 39, 2611–2611. [Google Scholar] [CrossRef]
- Tang, D.; Yang, C.; Zheng, J.; Woodard, P.K.; Saffitz, J.E.; Sicard, G.A.; Pilgram, T.K.; Yuan, C. Quantifying effects of plaque structure and material properties on stress distributions in human atherosclerotic plaques using 3D FSI models. J. Biomech Eng. 2005, 127, 1185–1194. [Google Scholar] [CrossRef]
- Saho, T.; Onishi, H. Quantitative analysis of effects of hemodynamic stress on temporal variations of cardiac phases in models of human carotid bulbs. Radiological physics and technology 2017, 10, 475–482. [Google Scholar] [CrossRef]
- Bijari, P.B.; Wasserman, B.A.; Steinman, D.A. Carotid bifurcation geometry is an independent predictor of early wall thickening at the carotid bulb. Stroke 2014, 45, 473–478. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, U.; Katiyar, V.K. Magnetic field effect on flow parameters of blood along with magnetic particles in a cylindrical tube. Journal of Magnetism and Magnetic Materials 2015, 377, 395–401. [Google Scholar] [CrossRef]
- Tashtoush, B.; Magableh, A. Magnetic field effect on heat transfer and fluid flow characteristics of blood flow in multi-stenosis arteries. Heat and Mass transfer 2008, 44, 297–304. [Google Scholar] [CrossRef]
- Abdolmaleki, F.; Hayat SM, G.; Bianconi, V.; Johnston, T.P.; Sahebkar, A. Atherosclerosis and immunity: a perspective. Trends in cardiovascular medicine 2019, 29, 363–371. [Google Scholar] [CrossRef]
- Dutra, R.F.; Zinani, F.S.; Rocha, L.A.; Biserni, C. Effect of non-Newtonian fluid rheology on an arterial bypass graft: A numerical investigation guided by constructal design. Computer Methods and Programs in Biomedicine 2021, 201, 105944. [Google Scholar] [CrossRef]
- De Nisco, G.; Hoogendoorn, A.; Chiastra, C.; Gallo, D.; Kok, A.M.; Morbiducci, U.; Wentzel, J.J. The impact of helical flow on coronary atherosclerotic plaque development. Atherosclerosis 2020, 300, 39–46. [Google Scholar] [CrossRef]
- Hoogendoorn, A.; Kok, A.M.; Hartman, E.M.; de Nisco, G.; Casadonte, L.; Chiastra, C.; Coenen, A.; Korteland, S.-A.; Van der Heiden, K.; Gijsen, F.J.H.; et al. Multidirectional wall shear stress promotes advanced coronary plaque development: comparing five shear stress metrics. Cardiovascular Research 2020, 116, 1136–1146. [Google Scholar] [CrossRef]
- Pinto, S.I.S.; Campos, J. Numerical study of wall shear stress-based descriptors in the human left coronary artery. Computer methods in biomechanics and biomedical engineering 2016, 19, 1443–1455. [Google Scholar] [CrossRef]
- Jayendiran, R.; Nour, B.; Ruimi, A. A fluid–structure interaction analysis of anisotropic Dacron fabric used for aortic replacement. Journal of Fluids and Structures 2020, 97, 103108. [Google Scholar] [CrossRef]
- Jayendiran, R.; Nour, B.; Ruimi, A. Computational fluid–structure interaction analysis of blood flow on patient-specific reconstructed aortic anatomy and aneurysm treatment with Dacron graft. Journal of Fluids and Structures 2018, 81, 693–711. [Google Scholar] [CrossRef]
- Varshney, G.; Katiyar, V.; Kumar, S. Effect of magnetic field on the blood flow in artery having multiple stenosis: a numerical study. International Journal of Engineering, Science and Technology 2010, 2, 967–982. [Google Scholar] [CrossRef]
- Varshney, G.; Katiyar, V.; Kumar, S. Effect of magnetic field on the blood flow in artery having multiple stenosis: a numerical study. International Journal of Engineering, Science and Technology 2010, 2, 967–982. [Google Scholar] [CrossRef]
- Tashtoush, B.; Magableh, A. Magnetic field effect on heat transfer and fluid flow characteristics of blood flow in multi-stenosis arteries. Heat and Mass transfer 2008, 44, 297–304. [Google Scholar] [CrossRef]
- Javadzadegan, A.; Moshfegh, A.; Afrouzi, H.H.; Omidi, M. Magnetohydrodynamic blood flow in patients with coronary artery disease. Computer Methods and Programs in Biomedicine 2018, 163, 111–122. [Google Scholar] [CrossRef]
- Sharifzadeh, B.; Kalbasi, R.; Jahangiri, M.; Toghraie, D.; Karimipour, A. Computer modeling of pulsatile blood flow in elastic artery using a software program for application in biomedical engineering. Computer methods and programs in Biomedicine 2020, 192, 105442. [Google Scholar] [CrossRef]
- Xenos, M.A.; Tzirtzilakis, E.E. MHD effects on blood flow in a stenosis. Advances in Dynamical Systems and Applications 2013, 8, 427–437. [Google Scholar]
- Ali, N.; Zaman, A.; Sajid, M.; Nieto, J.J.; Torres, A. Unsteady non-Newtonian blood flow through a tapered overlapping stenosed catheterized vessel. Mathematical biosciences 2015, 269, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Zaman, A.; Ali, N.; Bég, O.A. Unsteady magnetohydrodynamic blood flow in a porous-saturated overlapping stenotic artery—numerical modeling. Journal of Mechanics in Medicine and Biology 2016, 16, 1650049. [Google Scholar] [CrossRef]
- Zaman, A.; Ali, N. Effects of peripheral layer thickness on pulsatile flow of Herschel–Bulkley fluid through a stenotic artery. Canadian Journal of Physics 2016, 94, 920–928. [Google Scholar] [CrossRef]
- Fetecau, C.; Shah, N.A.; Vieru, D. General solutions for hydromagnetic free convection flow over an infinite plate with Newtonian heating, mass diffusion and chemical reaction. Communications in Theoretical Physics 2017, 68, 768. [Google Scholar] [CrossRef]
- Berger, S.A.; Jou, L.D. Flows in stenotic vessels. Ann. Rev. Fluid. Mech. 2000, 32, 347–382. [Google Scholar] [CrossRef]
- Pedley, T.J. The fluid mechanics of large blood vessels; Cambridge University Press: Cambridge, UK; p. 1980.
- Turek, S.; Hron, J.; Razzaq, M.; Wobker, H.; Schäfer, M. Numerical benchmarking of fluid-structure interaction: A comparison of different discretization and solution approaches. In Fluid Structure Interaction II; Springer: Berlin/Heidelberg, Germany, 2011; pp. 413–424. [Google Scholar]
- Razzaq, M.; Turek, S.; Hron, J.; Acker, J.F.; Weichert, F.; Wagner, M.; Grunwald, I.Q.; Roth, C.; Romeike, B.F. Numerical simulation of fluid-structure interaction with application to aneurysm hemodynamics. Technical University, Fakultat fur Mathematik 2009, 283–294. [Google Scholar]
- Razzaq, M. Numerical techniques for solving fluid-structure interaction problems with applications to bio-engineering. Ph.D. Thesis, TU Dortmund, to appear, 2009. [Google Scholar]
- Ervin, V.J.; Jenkins, E.W. The LBB condition for the Taylor-Hood P2-P1 and Scott-Vogelius P2-discP1 element pairs in 2-D. Technical Report TR2011 04 EJ, Clemson University. 2011. [Google Scholar]
- Kelly, C.T. Iterative methods for optimization. Society for Industrial and Applied Mathematics 1999. [Google Scholar]
- Amaya, R.; Pierides, A.; Tarbell, J.M. The interaction between fluid wall shear stress and solid circumferential strain affects endothelial gene expression. PloS ONE 2015, 10, e0129952. [Google Scholar] [CrossRef]
- Turek, S.; Hron, J.; Razzaq, M.; Wobker, H.; Schäfer, M. Numerical benchmarking of fluid-structure interaction: A comparison of different discretization and solution approaches. In Fluid Structure Interaction II; Springer: Berlin/Heidelberg, Germany, 2011; pp. 413–424. [Google Scholar]
| Mesh Level | Mesh Elements | Wall Shear Stresses | Abs. error |
|---|---|---|---|
| 0 | 1478 | 0.072341 | - |
| 1 | 1922 | 0.072244 | 0.000097 |
| 2 | 2432 | 0.072347 | 0.000103 |
| 3 | 7528 | 0.072557 | 0.00021 |
| 4 | 22153 | 0.072601 | 0.00004 |
| 5 | 24322 | 0.072600 | 0.000001 |
| Ha | A | B | gain % | A | B | gain% | A | B | gain % | A | B | gain% |
| 0 | 0.49 | 0.69 | 41 | 0.50 | 0.70 | 40 | 0.50 | 0.71 | 42 | 0.51 | 0.72 | 39 |
| 8 | 0.42 | 0.52 | 24 | 0.45 | 0.61 | 36 | 0.46 | 0.65 | 41 | 0.47 | 0.66 | 40 |
| 10 | 0.37 | 0.46 | 23 | 0.42 | 0.56 | 33 | 0.45 | 0.61 | 36 | 0.46 | 0.64 | 39 |
| 12 | 0.34 | 0.40 | 18 | 0.37 | 0.51 | 38 | 0.42 | 0.57 | 36 | 0.43 | 0.60 | 40 |
| Ha | ||||
|---|---|---|---|---|
| 0 | 0.05476 | 0.03018 | 0.02161 | 0.01716 |
| 8 | 0.06673 | 0.03522 | 0.02457 | 0.01918 |
| 10 | 0.07223 | 0.03807 | 0.02623 | 0.02030 |
| 12 | 0.07776 | 0.04128 | 0.02824 | 0.02165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




