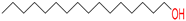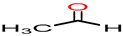1. Introduction
Background. The “emerging pollutants/micro-constituents/trace pharmaceuticals” are substances that enter the water supply from human or agricultural runoff (Hu et al., 2013; Zhang et al. 2013; Ebele et al. 2017). When people dispose their medications in the sink or toilet as well as frequent self-medication pharmaceuticals-waste appear in a significant amount in wastewater, which would make their way into the water system (Plosz et al.,2010). If these pharmaceuticals are detected in drinking water, as well as the soil, even in traces, would, therefore, appear in crops. Crops irrigated with water containing trace pharmaceuticals, present one of the most serious environmental problems, nowadays, that would impact population general health (Rosal et al. 2008; Benotti et al. 2009; Philips et al. 2010).
Problem definition. Per, pharmaceuticals waste and their metabolites are not intended for removal from domestic-waste waters by conventional effluent treatments (Hu et al. 2013; Zhang et al. 2013; Ebele et al. 2017). Therefore, over-time, continuous exposure to low trace pharmaceuticals concentration, from drinking water or in crops irrigated with water containing these traces, would accumulate in tissues causing cellular proliferation inhibition and/or long-term exposure chronic tissue damage (Larson 2007). This presents a serious problem we, as health care professionals, have to face and solve. Therefore, research should be directed to get rid of these “trace pharmaceuticals” whatever their nature, if estrogenic substances, antibiotics or others.
Acetaminophen/APAP/paracetamol® is one of the popular on-the-counter (OTC) analgesic, antipyretic, non-steroidal anti-inflammatory drug (NSAID) (Zhang et al. 2013). APAP is one of the “trace pharmaceuticals” that is present in different environmental domains like sediment, ground, and drinking water (Plosz et al. 2010). Although considered safe at the therapeutic levels (4 g/day or less) (Jaeschke et al., 2012), unmetabolized acetaminophen/paracetamol proved to inhibit ribonucleotide reductase enzyme leading to chromosomal aberration (Cai et al. 2003).
Per, acetaminophen/paracetamol and/or its metabolites are not intended to be removed from home wastewater by conventional effluent treatment methods, therefore, they constitute a serious environmental problem, being present in low traces in water, that will impact health adversely after a while. Therefore, the presence of an effective creative method(s) for degrading acetaminophen/paracetamol-waste remaining’s at the domestic drainage is of great importance (Żur et al. 2012), if we are working hard to achieve “Better Health” as one of the Sustainable Development Goals (SDGs#3).
Currently, sophisticated oxidation techniques and membrane technologies are the examined methodologies for elimination of the primary pharmaceuticals’ contaminants, including acetaminophen/paracetamol (silva et al. 2019). Despite these methods are valid for primary waste, but, still not effective for secondary pollutants and trace acetaminophen/paracetamol bio-degradation products waste (trace pharmaceuticals), making these technologies unfavorable choice(s) (Chen et al. 2010). Moreover, oxidation and membrane techniques high costs, constitutes another constrain for their wide-application.
Thence, the proposed solution should be environment-friendly, green, cost-effective, easy, reliable, and readily available. “Bioremediation” would be attempted to examine, presenting this readily available options (Sattar et al. 2022) that, potentially, fulfils the former mentioned criteria and requirements. The biological process known as “Bioremediation” uses micro-organisms whole cell (either bacteria or fungi or both) and their isolated enzymes to act-upon/convert pollutants or trace pharmaceutical into less harmful (harmless) forms. Several previous studies proposed bacteria can bio-degrade acetaminophen/paracetamol like M. Yunnanensis, Corynebacterium Nuruki, Bacillus Drentensis, Pseudomonas Moorei, M. Yunnanensis, Acinetobacter Bouvetii, producing fewer toxic products (Palma et al. 2021). That is why “bioremediation” via using various bacterial strains, already present (inhabitants) in the near soil or water environment, would present a green, economic, and environment-friendly option to consider for examination. If such attempt was proved potential, would present a step-toward recommending “bacterial bioremediation” use at the domestic drainage, to guard against the long-term exposure to trace pharmaceuticals, that impacts our health adversely.
Research Aim. The aim of the current research is to, first, isolate some potential acetaminophen/paracetamol bio-degrading bacterial stain(s) from marine and terrestrial sources, through screening of different Egyptian habitats such as Wadi El-Natron Lakes, the Red Sea, and the Egyptian Desert. Second, the promising bacterial strain(s) able to bio-degrade acetaminophen/paracetamol will be characterized phenotypically and genotypically and if novel, will be deposited to NCBI database. Third, chemical, biological down-stream targets, and ADME/toxicity predicted characterization of the acetaminophen/paracetamol bio-degradation products in silico/cheminformatics will be done. Finally, safety of these bio-degradation product(s) will be biologically tested for their potential anti-microbial activity (if any) and toxicity experimentally both in vitro and in vivo.
2. Materials
Drugs, Biochemical Reagents, Chemicals, and Solvents
Unless otherwise specified, chemicals, and reagents were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) and were of HPLC grade and some of molecular biology grade. Dimethyl sulfoxide (DMSO) was used as the vehicle for acetaminophen/paracetamol derivatives per their molecular weight. Acetaminophen/paracetamol was a gift from the Advanced Research Center (ARC), Egypt. Trypan blue dye from Sigma-Aldrich, USA and Trypsin from AMRESCo, USA. Other chemicals and solvents such as xylene, paraffin, and alcohol were of the highest grade commercially available.
Bacteria and Fungi
According to
www.ATCC.org the international reference for the biological anti-microbical testing
Escherichia Coli (E. coli) (ATCC 25922) and
Salmonella Typhi (S. typhi) (ATCC 6539) as Gram-negative bacterial strains. Gram-positive strains used are
Staphylococcus Aureus (S. ureus) (ATCC 25923) and
Bacillus Subtilus (B. subtilus) (ATCC 6633), Multidrug-resistant
Staphylococcus Aureus (MRSA) (ATCC 43300), and fungi
Candida albicans (C. albicans) (ATCC 10231) and
Aspergillus niger (A. niger) (ATCC 6275). Stock strains purchased were sub-cultured on nutrient agar plates. Microbial culture suspension standard solution is prepared from these tested bacterial strains.
Experimental Animals
5–7 weeks old mice (weighing 15–20 g, Swiss Albino females) obtained from Nile Co. for Pharmaceutical and Chemical Industries (Egypt). Mice acclimatized for 1 week under standard laboratory conditions in cages in a room with 12 h light/dark cycles, at 25 ± 2°C and 55 ± 5% relative humidity, at the Faculty of Pharmacy, Animal house, Ain Shams University. Mice were fed on standard diet pellets (El Nasr Company for Intermediate Chemicals, Giza, Egypt) containing no less than 20% protein, 3.5% fat, 6.5% ash, 5% fiber, and a vitamin mixture. Animals were allowed free access to drinking water ad libitum.
Biochemical/Molecular Assay Kits
MTT assay kit was purchased from Abcam (Boston, USA), AST, ALT, GGT, total protein (BCA), malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), and total antioxidant capacity (TAC) was all purchased from Biodiagnostics Co. (Cairo, Egypt). Mice interleukin-6 (IL-6) and caspase-9 ELISA kits from IBL America and Elabscience®, respectively. Polymerase chain reaction (PCR) DreamTaq Green PCR Master Mix (2x) (K1081, Thermo Fisher, USA) and E.Z.N.A.®Bacterial DNA Kit (D3350-01, Omega BIO-TEK, USA) for DNA extraction.
3. Methods
Biodiverse Samples Collection, Nature and Type, and Their Processing
Diverse range of 13 samples collected from different locations in Egypt to increase sample biodiversity and to explore different potential novel environmental microbiota, from October to November, 2019. Recording the nature of samples collected, location, and the date of collection, during a period of 14 days, in the morning, from different places. Using a sterile spatula, the samples were taken from a depth of 5 to 25 cm and then transferred into sterile plastic bags numbered/coded. These samples were used to isolate bacterial sp. after being air dried at room temperature for a week.
Fermentation/Isolation of Bacterial Strains from Water, Sediment, and Sand Biodiverse Samples Collected (Hamed et al., 2018)
The air-dried collected samples preserved in falcon tubes, kept in the fridge at 4°C, were suspended in 50 ml sterile water, if sediment or desert sand samples, and left for about 30 min at room temperature with shaking from time to time. Decantation of soil, filteration with a 0.45mm Nalgene filter (Nalgene USA). Sediment, soil filtrate as well as water samples were directly diluted 10 folds with sterile distilled water, plated on 1.5% agar MSM media with 100µl of each dilution uniformly distributed on the plates. Plates were incubated at 30°C (Benchtop laboratory incubator BJPX-H; Biobase, China) for 48 hrs. Then, left at room temp. (no colonies appeared in the pilot study done at higher temp.) for 2 weeks to allow different bacterial colonies, with different morphological characters, to appear on plates. Plates were inspected, frequently, daily for any unwanted Gram-negative bacteria (to be eliminated). Full colony appearance on plates occurred on day 7.
Cultivation and Screening of the Isolated Bacterial Strain’s Ability to Bio-Degrade Acetaminophen/Paracetamol (Rajan and Kannabiran 2014)
Single bacterial colonies were isolated, re-cultured in LB agar plates, and tested for their ability to bio-degrade acetaminophen/paracetamol (500 mg/L) added to the media, as the sole carbon and nitrogen source. Part of the isolated re-cultured potential acetaminophen/paracetamol degrading bacteria was transferred to slant agar and stored at 4℃ till identification/characterization. Another part of these bacterial cultures was stored as frozen stocks in 15% glycerol at -80℃. Stock strains were sub-cultured on nutrient agar plates prior to performing experiments.
The selected bacterial strains isolate that were highly able to bio-degrade acetaminophen/paracetamol, were sub-cultured into 50 ml liquid MSM media containing 500 mg/L acetaminophen/paracetamol in distilled water for terrestrial bacteria or in sea water for marine bacteria. Each bacterial isolate was incubated (Benchtop laboratory incubator BJPX-H; Biobase, China) at 30°C for 7 days in either static (ST) or shaking (SH) conditions. Again, Broth cultures were filtered using 0.45mM Nalgene filters (Nalgene. USA) and the filtrate was extracted repeatedly with an equal volume of ethyl acetate. Organic phases were collected and evaporated giving the minimal volume of “crude extracts” to analyze acetaminophen/paracetamol bio-degraded products within.
Identification of Acetaminophen/Paracetamol Bio-Degradation Products
Chemical analysis of the bio-degradation products by HPLC. Utilizing a reverse-phase HPLC (Agilent Technologies 1200 Series) fitted with a UV detector, the use of acetaminophen/paracetamol was examined (at 240 nm). On an Eclipse XDB-C18 column (5 m, 4.6 mm, 150 mm) the separation was carried out at 30 °C. The ratio of methanol: water CH3OH: H2O in the mobile phase was 15: 85, and the flow rate was 0.8 mL/min. Under these circumstances, acetaminophen/paracetamol retained for 6.492 min. as well as the potential metabolic intermediaries of paracetamol; 4-aminophenol and hydrophinone. This step is attempted to ascertain acetaminophen/paracetamol complete bio-degradation.
Gas chromatography-mass spectrometry GC/EI-MS. The GC/Electron ionization (EI)-MS analysis, was performed using a Thermo Scientific, Trace GC Ultra/ISQ Single Quadrupole MS, TG-5MS fused silica capillary column (30 m, 0.251 mm, 0.1 mm film thickness) and ionization energy of 70 eV was used, Helium gas was used as the carrier gas at a constant flow rate of 1mL/min. The injector and MS transfer line temperature was set at 280 oC. The oven temperature was programmed at an initial temprature 50oC (hold 2 min.), to 150 oC at an increasing rate of 7 oC /min., then to 270 at an increasing rate 5 oC /min (hold 2 min.), then to 310 as a final temperature at an increasing rate of 3.5 oC /min (hold 10 min.).
Quantification, of all the identified intermediate components investigated in the samples done in comparison with structures deposited on the National Institute of Standards and Technology (NIST) Mass Spectrum Interpreter ver. 3.4, released Feb., 2019, which connects mass spectral peaks to their probable chemical structure origin (EI and MS/MS, both nominal and accurate mass)
Liquid Chromatography–Mass Spectrometry (LC-MS)
Structural determination of the bio-degradation products by the promising bacterial strain, isolated, using LC-MS followed by 1H NMR spectra
Electrospray ionization (ESI) MS (Rezk,Basalious&Karim, 2015, Resk et al., 2016a and Resk et al., 2016b) was used to operate the multiple-reaction monitoring mode of the Waters Xevo TQD LC-MS/MS instrument by Waters Corporation, Milford, MA01757 USA. The sample was prepared using ethyl acetate extraction. For ES detection and EI detection, mass spectra were captured using the Schimadzu TripleQuadrupole GC-MS mass Spectrometer and the System mass spectrometer Accuity UPLC BEH C18 (1.7 µm, 2.1 × 50 mm) column, flow rate 0.2 mL/min. The prepared samples were chromatographed by pumping solvent system of gradient (A) water and 0.1% formic acid, (B) methanol, (C) 50%H2O+ 50%MeOH and (D) acetonitrile and 0.1% formic acid (50:50, v/v) in an isocratic mode at a flow rate of 0.3 ml/min. Source voltages 0.14 kV capillary and -1 V cone. Desolution temp. 64°C, source gas flow 3 L/Hr for desolvation, 1 L/hr for cone, analyzer collision energy 2 V. These elemental studies were carried out at Faculty of Pharmacy, Drug Discovery Research Center, Ain Shams University.
Nuclear Magnetic Resonance 1H NMR Spectroscopy
Audit trail, TOPSPIN Version 3.2 Bruker BioSpin GmbH, Germany, used to monitor the acetaminophen/paracetamol intermediate bio-degradation products spots/peaks to identify molecular structures, monitor bio-degradation reactions pathway prediction. Intermediate bio-degradation products functional group chemical shift range was expressed in part per million (ppm).
Acetaminophen/Paracetamol Degradative Pathway Proposed In Silico (Zhang et al., 2012)
Via the Cheminformatics/Bioinformatics tools using the pharmacogenomics knowledge resource PharmGKB
® (Carrillo et al. 2021) registered trademark supported by NIH and managed at Stanford University
https://www.pharmgkb.org/ (Accessed on Nov. 3rd, 2022) identified the small organic molecule acetaminophen/paracetamol-waste (APAP; N-acetyl-para-aminophenol) chemical structure and how affect the biological system. PubChem (Kim et al., 2023)
https://pubchem.ncbi.nlm.nih.gov/ the world’s biggest chemical info interface that is freely accessible, used for acetaminophen/paracetamol bio-degradation products name, IUPAC name, molecular formula, weight, chemical structure, Simplified Molecular-Input Line-Entry System (SMILES) as well as physical properties, and biological activities. The EAWAG Bio-catalysis/Bio-degradation Database (BBD) last modified on July 11, 2017, for information on microbial enzyme-catalyzed biocatalytic reactions and bio-degradation pathways for acetaminophen/paracetamol or its by-products. EAWAG-BBD is provided by NCBI PubChem, last updated 04/06/2020 (USA)
In Silico Prediction of Physicochemical Properties and ADMET Parameters of Acetaminophen/Paracetamol Bio-Degradation Products (Accessed Jan. 26th, 2022)
In Silico Prediction of Physicochemical Properties
SMILES were submitted to the SIB SwissADME (Daina, Michielin & Zoete, 2017) online server
http://www.swissadme.ch/ for calculation and knowledge about structure features; molecular weight (MW g/mol), the logarithm of the partition coefficient (log p), number of hydrogen bond acceptors (HBA), number of hydrogen bond donors (HBD), and the topological polar surface area (TPSA Å
2), pharmacokinetic properties and bioavailability of these biodegradation products. Drug-Likeness Prediction via the Lipinski’r rule by PreADMET
https://preadmet.webservice.bmdrc.org/druglikeness/ to screen drug candidates Lipinski’s rule (Lipinski et al., 2001)
https://preadmet.webservice.bmdrc.org/druglikeness-2/
“Rule of Five” in Comparison to Compounds from World Drug Index (WDI) Database;
If the No. of hydrogen bond donors ≤ 5 (the sum of OHs and NHs) and the No. of hydrogen bond acceptor ≤ 10 (the sum of Os and Ns) and the compound molecular weight is ≤ 500 g/mol, a lipophilicity consensus Log Po/w CLogP ≤ 5 (MlogP ≤ 4.5), then the compound is having better solubility and permeability (if around this figure will be moderately soluble).
SwissTargetPrediction (Daina, Michielin & Zoete, 2019)
http://www.swisstargetprediction.ch/index.php from Swiss Institute of Bioinformatics (SIB) (Lausanne / Switzerland) to find out and identify the possible macromolecular downstream target(s) of the acetaminophen/paracetamol bio-degradation products, assumed-to-be bioactive.
The Most Potent Bacterial Strains Characterization
Phenotypic Characterization. Samples imaged using the whole-mount transmission electron microscopy (TEM) JEOL JEM-1010
https://www.jeol.com/ At Faculty of Pharmacy, Cairo University, sample drop was placed on carbon-coated copper grids and left to dry at room temperature. Electron micrographs of chemically fixed and dried specimens were obtained using an accelerating voltages 100 kV (corresponding to wavelengths of 0.0037nm).
Phylogenetic Characterization. Total fungal genomic DNA was extracted from the two promising bacterial strains A1, through E.Z.N.A.®Bacterial DNA Kit (D3350-01, Omega BIO-TEK, USA) according to manufacturer protocol.
Polymerase Chain Reaction (PCR) Amplification of 16S ribosomal RNA (rRNA) gene of the 2 bacterial strains was done by using Dream Taq Green PCR Master Mix (2X) (K1081, Thermo Fisher, USA) according to manufacturer protocol through Creacon (Holland, Inc).
PCR System Cycler Using Oligonucleotide Universal Actinomycetes Fungal Strain Primer (Mabrouk and Saleh, 2014)
(27F 5′-AGA GTT TGA TCM TGG CTC AG-3′) and (1492R 5′-TAC GGY TAC CTT GTT ACG ACT T-3′) with target fragment length 1500 bp. Agarose gel electrophoresis was used for the detection of the amplification products in the presence of DNA ladder
Actinomycetes (peqGOLD 1 kb DNA-Ladder, Peqlab, VWR) according to the manufacturer protocol. Resultant PCR products were purified with E.Z.N.A.®Gel Extraction Kit, (D2500-01, Omega BIO-TEK, USA) for sequence analysis. Sequence analysis was performed using ABI PRISM® 3100 Genetic Analyzer (Micron-Corp., Korea). Data analysis of the purified PCR products were sequenced using the previous forward and reverse primers. Forward and reverse sequences were aligned together to generate a consensus sequence using DNA Baser Sequence Assembler v. 4.36
https://www.dnabaser.com/ (Accessed on Jan., 2022). The obtained aligned sequences were further identified by NIH/NLM Basic Local Alignment Search Tool (BLAST)
https://blast.ncbi.nlm.nih.gov/blast/Blast.cgi?CMD=Web&PAGE_TYPE=BlastHome
Gel documentation system (Geldoc-it, UVP, England) was applied for data analysis using the image analysis software TotalLab
www.totallab.com V.1.0.1. Genetic distances and multiple alignment of nucleic acid and protein sequences were computed by Pairwise Distance Method (PDM) using Clustal W2 v.2.1(Larkin et al, 2007). Clustal W/X Clustal W/Clustal X Multiple alignment of nucleic acid and protein sequences
http://www.clustal.org/clustal2/ Nucleotide sequences were also compared with
Aspergillus isolates sequences available in the NIH genetic sequence database GenBank
® (Benson et al., 2013)
https://www.ncbi.nlm.nih.gov/genbank/ (Accessed on Feb., 2022).
Finally, phylogenetic tree construction for the 2 micro-organisms based on 16S rRNA gene region sequence was done using Molecular Evolutionary Genetics Analysis MEGA11 program
https://www.megasoftware.net/ downloaded for windows (Accessed on Feb., 2022).
In Silico Prediction of the Isolated Bacterial Strains Enzymatic Activity
BacDive (Reimer et al., 2022) database to explore Bacterial Diversity View for Taxonomy This view contains BacDive IDs (by Leibniz-Institut DSMZ-Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Germany)
https://bacdive.dsmz.de/ via BRENDA (Chang et al., 2021)
https://www.brenda-enzymes.org/index.php structured view of Streptomyces sp. enzymes (release 1, 2023 online including 68 new and 479 updated enzyme classes) by Elixir, USA (Accessed Feb. 17th, 2023).
Biological activity of isolated acetaminophen/paracetamol bio-degradation products
In vitro Anti-microbial Activity of isolated acetaminophen/paracetamol bio-degradation products of the selected potential bacterial strain isolates
According to the Broth microdilution method (Balouiri, M., Sadiki, M., & Ibnsouda., 2016) for in vitro anti-microbial activity testing, Gram-negative bacterial strains E. coli and S. typhiand, Gram-positive strains S. aureus and B. subtilus, MRSA, and fungi C. albicans and A. niger, as well as the fresh microbial cultures of the currently tested selected strains were prepared and the standard was suspension of all these bacteria and fungi without any sample treatment, and were all serially diluted. Microbial counts were adjusted at 107 colony-forming unit/ml (cfu/ml). In 96-well flat polystyrene plates, 10 µl of acetaminophen/paracetamol bio-degradation products samples was incubated with 80 µl of lysogeny broth media and 10 µl of microbial culture suspension standard solution of the tested microbial strains at 37°C (Benchtop laboratory incubator BJPX-H; Biobase, China) overnight (after 20 hr). Bacterial growth was measured as absorbance at OD 600 nm using a Spectro Star Nano Microplate Reader (BMG LABTECH GmbH, Allmendgrun, Germany). The positive anti-microbial effect of the tested acetaminophen/paracetamol bio-degradation products samples monitored as “Clearance zone in the wells” measured in mm = % inhibition zone
In silico PreADMET ver 2.0 by Yonsei University, Yeonsu-gu, Incheon, Republic of Korea, by
www.Cheminformatics.org first released 2004 (Accessed Jan. 26th, 2022). To predict pharmacokinetics and bioavailability of absorption, the volume of distribution, metabolism, and excretion (ADME) parameters predicting small-molecule pharmacokinetic properties using graph-based signatures (
pkCSM) (Pires, Blundell & Ascher, 2015)
https://biosig.lab.uq.edu.au/pkcsm/ for Plasma Protein Binding, Blood Brain Barrier (BBB) Penetration, skin penetration, predict % human intestinal absorption (%HIA). Caco-2 cell model and MDCK cell model as reliable in vitro models for prediction of oral drug absorption.
In Silico Rodent Oral Toxicity Prediction and Indication of Toxicity Targets
Using the PreADMET web server for PreADME/Tox Ames test toxicity prediction
https://preadmet.webservice.bmdrc.org/ Results calculated both with consideration of metabolite (Metabolic activation by rat liver 10% homogenate, as positive) and without consideration of metabolic activation as negative. The actual value of the prediction result is “positive” or “negative”
https://preadmet.webservice.bmdrc.org/toxicity-prediction/ [N.B. PreADMET predicts carcinogenicity on different animals from its model, built from the data of National Toxicology Program (NTP) and US FDA, which are the results of the in vivo
carcinogenicity tests of mice and rats for 2 years]. hERG (human ether-à-go-go-related gene cardiac potassium channel) ion channel inhibition, being an important anti-target in drug discovery, associated with potentially fatal heart conditions.
Furthermore, toxicity could be tested experimentally in vitro using cancer cell lines and in vivo acute single dose oral toxicity examination.
Toxicity Testing
In vitro MTT Cytotoxicity Assay of acetaminophen/paracetamol bio-degradation products produced by the selected potential bacterial strains isolates Extract using two different cancer cell lines.
After bio-degradation of acetaminophen/paracetamol by the selected bacterial strains isolates, identification of these metabolites cytotoxicity in vitro was carried out using 2 different cancer cell lines, of
www.ATCC.org origin, namely, HepG2 and MCF7 cancer cell lines. The half maximal inhibitory concentration (IC50) values were calculated against DMSO non-treated cancer cells (negative control) and acetaminophen/paracetamol-treated cancer cells (positive control). The assay was conducted using 3-(4, 5-di-MethylThiazol-2-yl)-2,5-diphenyl Tetrazolium bromide (MTT) to test cell viability kit, according to the method described by Mosmann,1983 and Denizot, Lang, 1986.
HepG2 and MCF7 cancer cell lines were seeded to 80 % cells confluency on 96 well plate (10x103 cells/well) in RPMI medium with 10% fetal bovine serum (FBS), 1% penicillin, 2 mM glutamine, then incubated at 37°C and 5% CO2 for 24 hr, then cells were treated with either acetaminophen/paracetamol (positive control) or samples of the potential bacterial strain isolates bio-degradation products obtained in the shaking (SH) or the static (ST) states. Serial control or samples concentrations 2.44, 4.88, 9.77, 19.53, 39.06, 78.13, 156.25, 312.50, 625,1250, 2500, and 5000 µg/ml in RPMI serum-free media were used, then incubated for 48 hr at 37°C and 5% CO2. After the incubation period, media was removed carefully, 20 μl MTT stain was added in each well (5 mg/ml MTT solution in 1x PBS) and incubated for 4 hr at 37°C in CO2 incubator till the formation of MTT formazan crystals. MTT solution is now removed and crystals dissolved with 0.05 ml DMSO for 30 min. on a shaker (Staurt, England). Absorbance measured spectrophotometrically at 570 nm with a reference wavelength of 630 nm using ELX800 UV universal microplate reader (BioTek Instruments Inc., VT, Santa Clara, California, USA).
%cell viability = (mean absorbancetreated sample/mean absorbancenegative control sample) × 100
%death rate = 100 – (%cell viability)
The inhibitory concentration of 50% (IC50) was measured from the exponential curve of %cell viability against concentration (dose–response curve) (Kamiloglu et al., 2020) using Master–plex-2010 program. All experiments were done in duplicates.
In Vivo Acute Single Oral Toxicity Study
Sample Size Estimation. The sample size calculation was done to ensure using the least sufficient animal number, to ensure no harm to animals (El-Mesallamy et al., 2014), compiling with the commonly-accepted ‘3Rs rules for animal care; ARRIVE. Sample size was calculated considering the current study is superiority one-sided test as well as depending on a pilot/exploratory study done according to the Resource Approach (Arifin, Zahiruddin, 2017) with an acceptable range of error degrees of freedom (D.F) in an analysis of variance (ANOVA), using design 3 for group comparison, repeated measures, one between and one within factor, repeated-measures ANOVA. Resource equation was used to estimate the sample size needed for the experiment and the number of mice per group, employing group comparison, with D.F within the range of 10 to 20. The study power was set at 0.8 and selecting one-tailed analysis, with an accepted 5% risk of making type I error, so reducing the number of required animals by 14%.
Experimental Design:
Normal control group1: mice received saline orally once,
Negative control group2: mice received vehicle (DMSO) orally once,
Positive control group3: mice given acetaminophen/paracetamol (200 mg/kg BW) once, which is the reported published acute oral dose (Pu et al., 2019),
Group4: received extracted sample 1b obtained from bacterial strain coded M33, while in the static condition, the dose used is the IC50 obtained from the in vitro cancer cell line results,
Group5: received extracted sample 1a obtained from bacterial strain coded M33, while in the in shaking condition, the dose used is the IC50 obtained from the in vitro cancer cell line results.
[N.B.1. No estimation of acute oral toxicity of the bio-degradation products of acetaminophen/paracetamol extracted from the bacterial strain RS2 Extract 2a, 2b in either static or shaking flasks, respectively, per the bio-degradation products were found to be highly similar in structure and to obey the Guidelines No. 407 of the Organization for Economic Cooperation and Development (OECD) Test (OECD, 2008, updated 2017) and the International Academy of Science’s Guide for the Care and Use of Laboratory Animals, as well as the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines
https://arriveguidelines.org/ to use the least number of mice/group for the oral acute single dose toxicity test]. [N.B.2. the objective of the acute oral toxicity study was not LD50 determination].
Experimental Procedure:
Before the experiment, the animal’s body weights (BW) of all mice were recorded. Animals were divided randomly into five groups (5 animals per group) at the Faculty of Pharmacy, Animal House facility, Ain Shams University. All mice were made to fast for 24 hr and treated once (day zero) with the acute single oral dose, using a gastric gavage. Animals were observed for the first 30 min. and 4–5 times at intervals of 48 hr to record any signs of abnormality, then carefully monitored for 14 days (end-point). The animals’ BWs were recorded at the end of the 14-days of observation.
Biochemical Analysis was done at the Faculty of Pharmacy, Biochemistry Dept. Research Lab., Ain Shams University. Blood samples were collected from the retro-orbital plexus and allowed to clot. Sera were prepared by centrifugation at 4000 rpm for 15 min. and then kept frozen at − 80°C for liver function tests (AST, ALT, and GGT calorimetrically). Thereafter, mice were deprived of food overnight, euthanized, and sacrificed by cervical dislocation. Liver tissues were collected, washed with ice-cold saline, and weighed.
One liver tissue part was homogenized and kept frozen at − 80°C for latter analysis of mice IL-6 and mice caspase-9 ELISA in liver tissue homogenate per mgm tissue protein (after total protein estimation in mice liver tissue homogenate calorimetrically by BCA) by sandwich enzyme-linked immune-sorbent assay technology using commercial mice IL-6 ELISA kits (iBL-America; ImmunoBiological Laboratories, Inc., Minneapolis, USA) and mice caspase-9 ELISA (Elabscience; CiteAb, USA).
Super oxide dismutase (SOD) and catalase (CAT) enzymes activities were estimated in liver tissue homogenate as an index of lipid peroxidation by colorimetric methods using Biodiagnostic kits, in accordance with previously established procedures. Levels of total antioxidant capacity (TAC) and the marker of lipid peroxidation malondialdehyde (MDA), were measured spectrophotometrically. MDA measured as thiobarbituric acid (TBA) in an acidic medium at temperature of 95°C for 30 min. to form colored thiobarbituric acid reactive (TBAR) product, the absorbance of which was measured at 534 nm. The MDA kit was purchased from Biodiagnostics, Cairo, Egypt.
Histopathological Examination. Another liver part was excised, weighed, fixed in 10% neutral buffered formaldehyde overnight and then embedded in paraffin, deparaffinized in serial grades of alcohols, cleared in xylene, and were subjected to ultramicrotomy, where 4 μm thick tissue sections were cut by rotatory microtome. Tissue sections were stained with hematoxylin and eosin (H and E) according to Culling, 2013, for histological examination using full HD light microscopic imaging system (Leica Microsystems GmbH, Wetzlar, Germany) (Suvarna et al., 2018).
Statistical Analysis. Data are presented as the mean ± SEM. All experiments were performed in triplicate and repeated twice. Multiple comparisons were done using a one-way analysis of variance (ANOVA) test followed by Duncan as a post hoc test. The statistical significance criterion used is the 0.05 level of probability p. Statistical analyses were carried out using IBM statistical package for social studies (SPSS) v.25 software (ISI software, San Diego, California, USA). Graphs done using GraphPad prism 8 San Diego, California.
4. Results
Biodiverse collected samples nature and type and Fermentation/Isolation of Bacterial Strains and their ability to bio-degrade acetaminophen (
Table 1).
Thirteen samples were collected from different locations. Samples were divided into marine samples (water and sediment samples) and desert samples (sand). Five sediment samples and five water samples were collected from Wadi El Natrun Valley lakes (Naba’ El-Hamra, El-Hamra, EL Samaa, and El khadra) and from Faiyum City Qarun lake. Two sand samples were collected from Wadi El Natrun Valley and Al Giza Desert. One soil sample was collected from the First Industrial District, October City, Giza. Two distinct bacterial strains were able to highly bio-degrade acetaminophen/paracetamol added to the media (coded as RS2 and M33) (
Table 1). Ability to bio-degrade acetaminophen/paracetamol measured as the zone of bacterial growth, on the expense of st. acetaminophen/paracetamol added in the bacterial culture media.
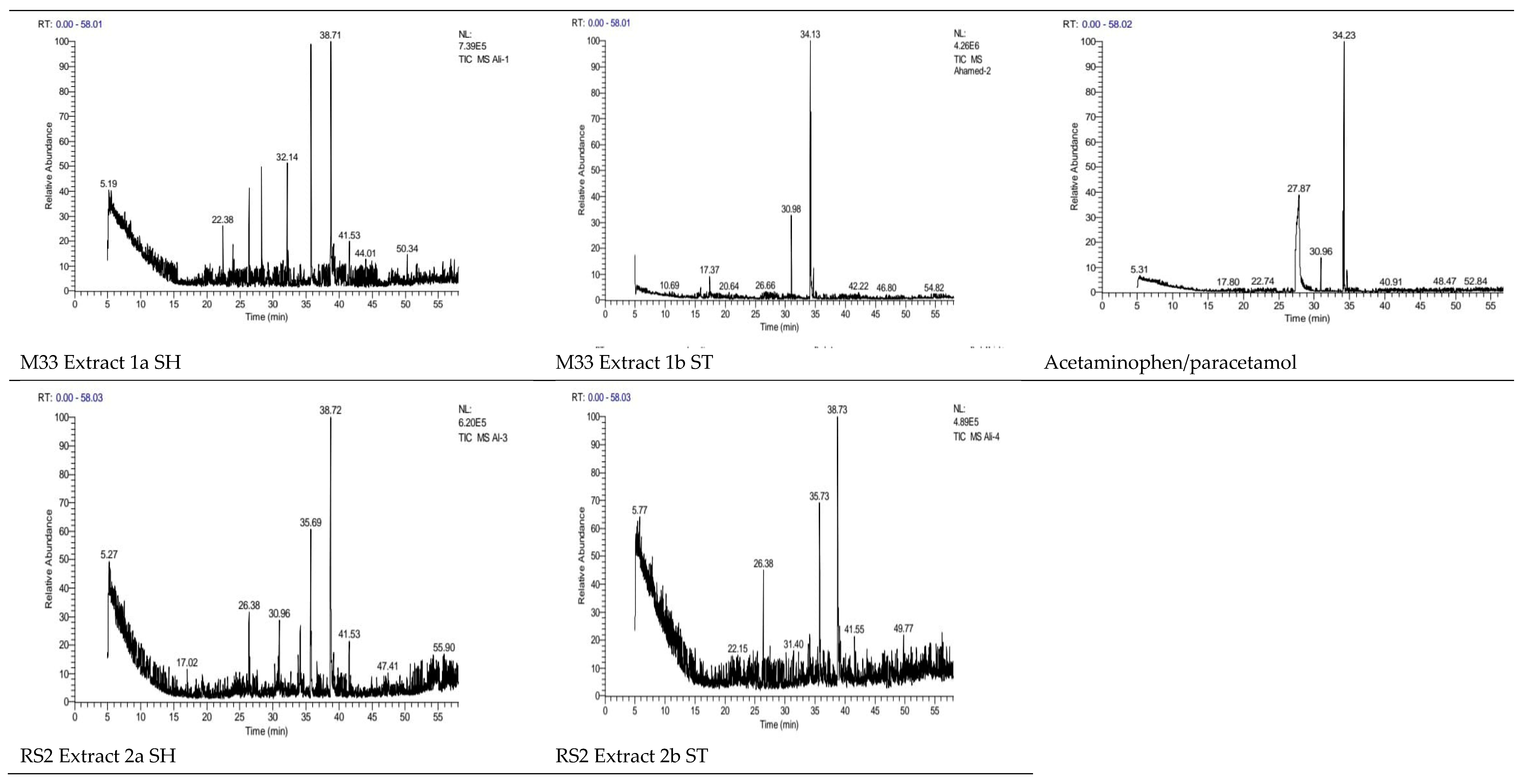
Chemical identification of acetaminophen/paracetamol bio-degradation products using GC/MS (
Table 2 and
Figure 1) after cultivation and screening of the isolated bacterial strain’s ability to degrade acetaminophen/paracetamol. Quantification, of all the identified components investigated in 5 samples done using a % relative peak area (RPA) in comparison of their relative retention time (RRT) and mass spectra with those of the National Institute of Standards and Technology (NIST) Mass Spectrum Interpreter ver. 3.4, released Feb., 2019, which connects mass spectral peaks to their probable chemical structure origin (EI and MS/MS, both nominal and accurate mass)
https://www.nist.gov/mml/biomolecular-measurement/mass-spectrometry-data-center https://chemdata.nist.gov/dokuwiki/doku.php?id=chemdata:start&s[]=gc&s[]=ei&s[]=ms. Acetaminophen/paracetamol (APAP; N-acetyl-para-aminophenol) and its bio-degradation products identity, chemical structure, molecular formula and weight in the filtrates using GC/MS system analysis. Furthermore, SMILES were identified and validation of the obtained chemical structure, mol. Wt, and others, per each compound, via the
in-silico database PharmGKB
® pharmacogenomics
https://www.pharmgkb.org/chemical/PA448015/link and
https://www.pharmgkb.org/chemical/PA448015 (Accessed on Nov. 3rd, 2022). Intermediate metabolites produced by the bio-degradation of acetaminophen/paracetamol, then examined in a hexane extract by GC-MS. Using GC-MS and IC acetaminophen/paracetamol intermediates bio-degradation products were identified. Peaks seen on the gas chromatogram denotes a distinct metabolite eluted closely together (retention times of 17.709 to 51.510). Gas chromatogram peaks are the library that mass spectroscopy produces. The batch culture did not contain any acetaminophen/paracetamol residue, according to the GC-MS data. This suggests that the Streptomyces strain can both use acetaminophen/paracetamol as a source of energy and remove it from waste water. Based on the sample’s peak and retention time (RT), the metabolites of the acetaminophen/paracetamol catabolic pathway would raise several possibilities of acetaminophen/paracetamol bop-degradation pathways involving metabolites appearing in
Table 2. acetaminophen/paracetamol bio-degradation products in
Table 2 are related compounds with annotation
https://pubchem.ncbi.nlm.nih.gov/compound/7689#section=Related-Records.
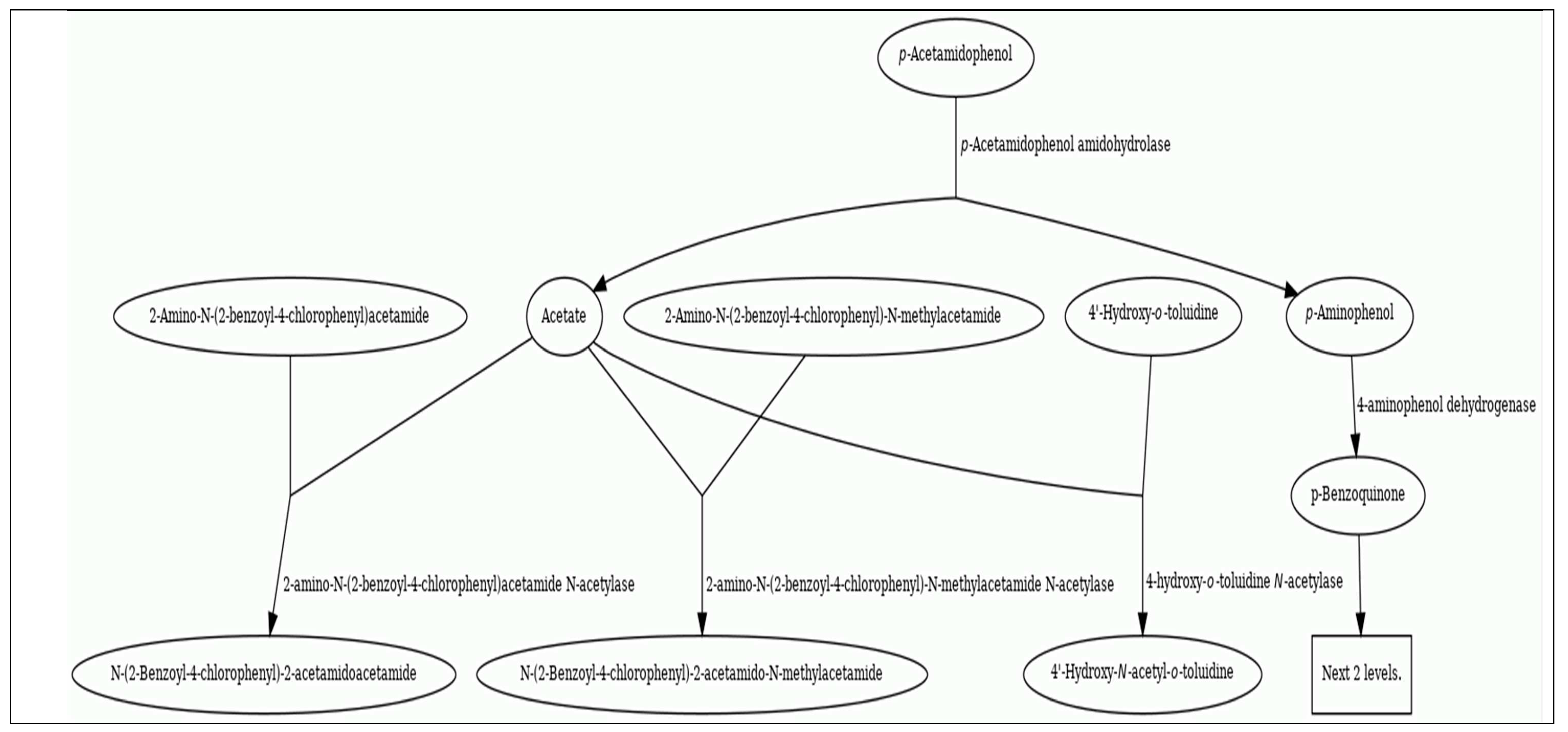
The EAWAG Bio-catalysis/Bio-degradation Database (BBD) for prediction of the pathway(s) and related-enzymes involved in acetaminophen/paracetamol or its bio-degradation products: EAWAG-BBD pathway map, accessed Feb. 16th., 2023 (
Figure 2) proposed pathway may be composed of reactions observed in multiple organisms under different aerobic and/or anaerobic environmental conditions. The first level of reactions begins with 4-acetamidophenol via p-acetamidophenol amidohydrolase enzyme (amidase) (EAWAG-BBD enzyme, enzymeID# e1067) to give acetate and 4-aminophenol. Then during level 2 N-methyl acetamide N-acetylase enzyme (EAWAG-BBD reaction, reacID# r1762) is involved in the bio-degradation of acetate and 4-aminophenol, mainly; p-Acetamidophenol -----> p-Aminophenol + Acetate (reacID# r1629)
. However, some of the bio-degradation products pathways/reactions the EAWAG-PPS does not predict
http://eawag-bbd.ethz.ch/predict/notbepredicted.html#Reactions this would be attributed to being environmental-degradation reactions that are not predicted, also, some reactions are too difficult to predict as detoxification reactions involving conjugation or azo compounds formed from primary amide (-NH
2) groups (compounds 2, 7, 13
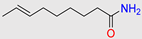
Table 2). Moreover, acetylation of primary amines (compounds 5, 6, 13), formation of intramolecular ring (compounds 7, 8, 14). Finally, from the options that makes products not predicted and are not present on any prediction data bases including the current EAWAG-BBD PPS (accessed on Feb. 16th, 2023) is hydroxylation of aliphatic carbon atoms though environmental non-specific monooxygenases. Formation of EAWAG-BBD PPS termination compounds during bioremediation (bio-degradation) as those containing acetylene or cyanide (compound #1), acetate (compound #4), deconoate (compound #5), proponoate (compound #11) (
Table 2) (accessed on Feb. 16th, 2023)
In Silico Prediction of Physicochemical Properties (Table 3A) (Accessed Nov., 2022)
Calculation and knowledge about structure features physicochemical properties; molecular weight (Mol wt. g/mol), number of hydrogen bond acceptors (HBA), number of hydrogen bond donors (HBD), and the topological polar surface area (TPSA Å2). Pharmacokinetic properties of these bio-degradation products (
Table 3) presented as bioavailability RADAR charts for six important physicochemical properties. The pink area represents the optimal range for each property: lipophilicity (LIPO) XLOGP3 between −0.7 and +5.0; size (SIZE) MW between 150 and 500 g/mol; polarity (POLAR) TPSA between 20 and 130 Å2; sol-ubility (INSOLU) log S not higher than 6; saturation (INSATU) fraction of carbons in the sp 3 hybridiza-tion not less than 0.25; and flexibility (FLEX) no more than nine rotatable bonds. Bioavailability Radar charts for physicochemical properties determined from SwissADME cheminformatics platform
http://www.swissadme.ch/
Drug-Likeness Prediction by PreADMET cheminformatics database (
Table 3A) (Accessed Nov., 2022).
Bioavailability RADAR charts for six important physicochemical properties determined from SwissADME cheminformatics platform
http://www.swissadme.ch/ Lipinski’s rule
https://preadmet.webservice.bmdrc.org/druglikeness-2/ “Rule of Five” in comparison to compounds from WDI database, for solubility and permeability determination (Accessed Nov., 2022). [HBA; hydrogen bond acceptors, HBD; number of hydrogen bond donors, TPSA; topological polar surface area, LIPO; lipophilicity XLOGP3, SIZE; size MW g/mol; POLAR; polarity TPSA Å
2; INSOUL; solubility log S; INSATU; saturation fraction of carbons, FLEX; flexibility]. Assumed-bioactive, the acetaminophen/paracetamol bio-degradation products downstream targets were predicted chemo-informatically using PreADMET database that identified these target(s) enzymes, receptors or cytosolic and membrane proteins
http://www.swisstargetprediction.ch/index.php (Accessed Nov., 2022) (
Table 3B) Taking into consideration that the bio-degradation product compounds with less than 5 heavy atoms cannot be submitted for prediction.
Table 3.
(A) Acetaminophen/paracetamol bio-degradation products physicochemical properties (hydrogen bonnds numbers and TPSA), bioavailability radar charts, lipophilicity, and Lipinski rule (solubility/permeability drug-likeness) in silico prediction using either SwissADME cheminformatics platform or WDI database.
Table 3.
(A) Acetaminophen/paracetamol bio-degradation products physicochemical properties (hydrogen bonnds numbers and TPSA), bioavailability radar charts, lipophilicity, and Lipinski rule (solubility/permeability drug-likeness) in silico prediction using either SwissADME cheminformatics platform or WDI database.
| (A) |
| Bio-degradation product |
|
# |
Bioavailability |
Druglikeness; |
| # |
IUPAC name |
TPSA Ų
|
HBA |
HBD |
Radar |
Lipinski /
Solubility |
| Bacterial strain1 (faint red color on agar); Strain M33 (under shaking condition): Extract 1a |
| 1 |
Cyanoacetylene /
prop-2-ynenitrile |
24 |
1 |
0 |
|
Yes;
0 Violation /
Very soluble |
| 2 |
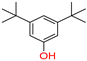
Phenol 3,5-bis(1,1-dimethylethyl)/
3,5-ditert-butylphenol |
20 |
1 |
0 |
|
Yes;
0 Violation / Moderately soluble |
| 3 |
1-Hexadecanol/
hexadecan-1-ol |
20 |
1 |
1 |
|
Yes; 1 violation: MLOGP>4.15/ Poorly soluble
|
| 4 |
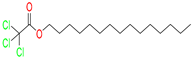
pentadecyl ester Trichloroacetic acid/
pentadecyl 2,2,2-trichloroacetate |
26 |
2 |
0 |
|
Yes;
0 Violation / Moderately soluble |
| 5 |
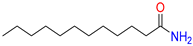
Dodecanamide/
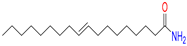
Dodecanamide |
43 |
1 |
1 |
|
Yes;
0 Violation / Moderately soluble |
| 6 |
9-Octadecenamide/
octadec-9-enamide |
43 |
1 |
1 |
|
Yes;
1 violation: MLOGP>4.15/ Poorly soluble |
| 7 |
δ-9-tetrahydrocannbinol/
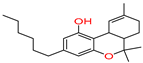
6aR,10aR)-1-methoxy-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydrobenzo[c] chromene |
29 |
2 |
1 |
|
Yes;
1 violation: MLOGP>4.15/ Poorly soluble |
| Bacterial strain1, Strain M33 (under static condition): Extract 1b |
|
| 8 |
N-[4-Bromo-N-Butyl]-2-Piperidinone/
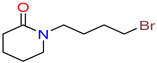
1-(4-bromobutyl)
piperidin-2-one |
20 |
1 |
0 |
|
Yes;
0 Violation / Soluble |
| 6 |
9-Octadecenamide |
- |
- |
- |
- |
- |
| Bacterial strain2, Strain RS2 (under shaking condition): Extract 2a |
|
| 9 |
Acetaldehyde/
Acetaldehyde |
17 |
1 |
0 |
|
Yes;
0 Violation / Highly soluble |
| 10 |
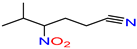
5-Methyl-4-nitrohexane-nitrile |
- |
- |
- |
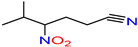 |
Not identified/- |
| 11 |
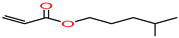
Isohexyl-acrylate/
4-methylpentyl prop-2-enoate |
26 |
2 |
0 |
|
Yes;
0 Violation / Soluble |
| 12 |
10-Undecenoic acid methyl ester/
methyl undec-10-enoate |
26 |
2 |
0 |
|
Yes;
0 Violation / Moderately soluble |
| 5 |
Dodecanamide |
- |
- |
- |
- |
- |
| 6 |
9-Octadecenamide |
- |
- |
- |
- |
- |
| 13 |
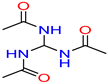
N-(diacetamidomethyl)
acetamide/ N-(diacetamidomethyl)
acetamide |
87 |
3 |
3 |
|
Yes;
0 Violation / Highly soluble |
| Bacterial strain2, Strain RS2 (under static condition): Extract 2b |
|
| 14 |
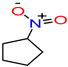
Nitro-cyclopentane/
nitrocyclopentane |
46 |
2 |
0 |
|
Yes;
0 Violation/ Very soluble
|
| 5 |
Dodecanamide |
- |
- |
- |
- |
- |
| 15 |
7-Nonenamide/
(Z)-non-7-enamide |
43 |
1 |
1 |
|
Yes;
0 Violation / Very soluble |
| 7 |
δ-9-tetrahydrocannbinol |
- |
- |
- |
- |
- |
| 16 |
Acetaminophen/
N-(4-hydroxyphenyl)-Acetamide |
49 |
2 |
2 |
|
Yes;
0 violation / Very soluble |
| (B) |
| # |
Bio-degradation product
/IUPAC name |
Target |
| 1 |
Cyanoacetylene /prop-2-ynenitrile |
NA |
| 2 |
Phenol 3,5-bis(1,1-dimethylethyl)/
3,5-ditert-butylphenol |
Family A G protein-coupled receptor; serotonin 2b (5-HT2b) receptor,
Ligand-gated ion channel; GABA-A receptor, alpha-1/beta-2/gamma-2,
Nuclear receptor; Estrogen receptor beta, kinases; serine/threonine-protein kinase AKT |
| 3 |
1-Hexadecanol/hexadecan-1-ol |
Family A G protein-coupled receptor; cannabinoid receptor 1,2,
G-protein coupled bile acid receptor1, Phosphatase; Cdc25A, B, Enzyme; Acyl-CoA desaturase |
| 4 |
pentadecylester Tri-chloroacetic acid/pentadecyl 2,2,2-tri chloroacetate |
Lyase; carbonic anhydrase VII, III, V1, XII, IV,
Family A G protein-coupled receptor; serotonin 5a (5-HT5a) receptor, 1a, 1d |
| 5 |
Dodecanamide/Dodecanamide |
Eraser; HDAC1, 3, 6, 5, Lyase; CA2, 1,
Cytochrome P450; thromboxane-A synthase, cytochrome P450 17A1 |
| 6 |
9-Octadecenamide/
octadec-9-enamide |
Enzyme; acyl coenzyme A: cholesterol acyltransferase, Kinase; tyrosine-protein kinase TIE-2, Family A G protein-coupled receptor; melatonin receptor 1A, 1B |
| 7 |
δ-9-tetrahydrocannbinol/6aR,10aR)-1-methoxy-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydrobenzo [c] chromene |
Family A G protein-coupled receptor; n-arachidonyl glycine receptor, G-protein coupled receptor 55, calcium sensing receptor, Kinase; vascular endothelial growth factor receptor 2, Cytosolic protein; 5-lipoxygenase activating protein |
| 8 |
N-[4-Bromo-N-Butyl]-2-Piperidinone/1-(4-bromobutyl) piperidin-2-one |
Family C G protein-coupled receptor; dopamine D4 receptor, serotonin receptor, Enzyme; 11-beta-hydroxysteroid dehydrogenase 1, myeloperoxidase, 3-keto-steroid reductase, Oxidoreductase; steroid 5-alpha-reductase 1, 2 |
| 9 |
Acetaldehyde/Acetaldehyde |
NA |
| 10 |
5-Methyl-4-nitrohexane-nitrile |
Incomplete |
| 11 |
Isohexyl-acrylate/4-methylpentyl prop-2-enoate |
Family A G protein-coupled receptor; hydroxycarboxylic acid receptor 2,
Protease; leukocyte elastase, urokinase-type plasminogen activator,
Kinase; epidermal growth factor receptor erbB1 |
| 12 |
10-Undecenoic acid methyl ester/
methyl undec-10-enoate |
Enzyme; 11-beta-hydroxysteroid dehydrogenase 1, prostaglandin E synthase, hormone sensitive lipase, Cytochrome P450; cytochrome P450 11B1 |
| 13 |
N-(diacetamidomethyl) acetamide / N-(diacetamidomethyl) acetamide |
Protease; leukocyte elastase, epoxide hydrolase 1, cathepsin G,
Enzyme: Poly [ADP-ribose] polymerase 10, 1 |
| 14 |
Nitro-cyclopentane/nitrocyclopentane |
Family A G protein-coupled receptor; alpha-1d adrenergic R, Protease; carboxypeptidase A1 |
| 15 |
7-Nonenamide/
(Z)-non-7-enamide |
Oxidoreductase; steroid 5-alpha-reductase 1, Kinase; inhibitor of nuclear factor kappa B kinase beta subunit, protein tyrosine kinase 2 beta, tyrosine-protein kinase JAK1, Cytosolic proteins; induced myeloid leukemia cell differentiation protein Mcl-1 |
Themost potent bacterial strains characterization
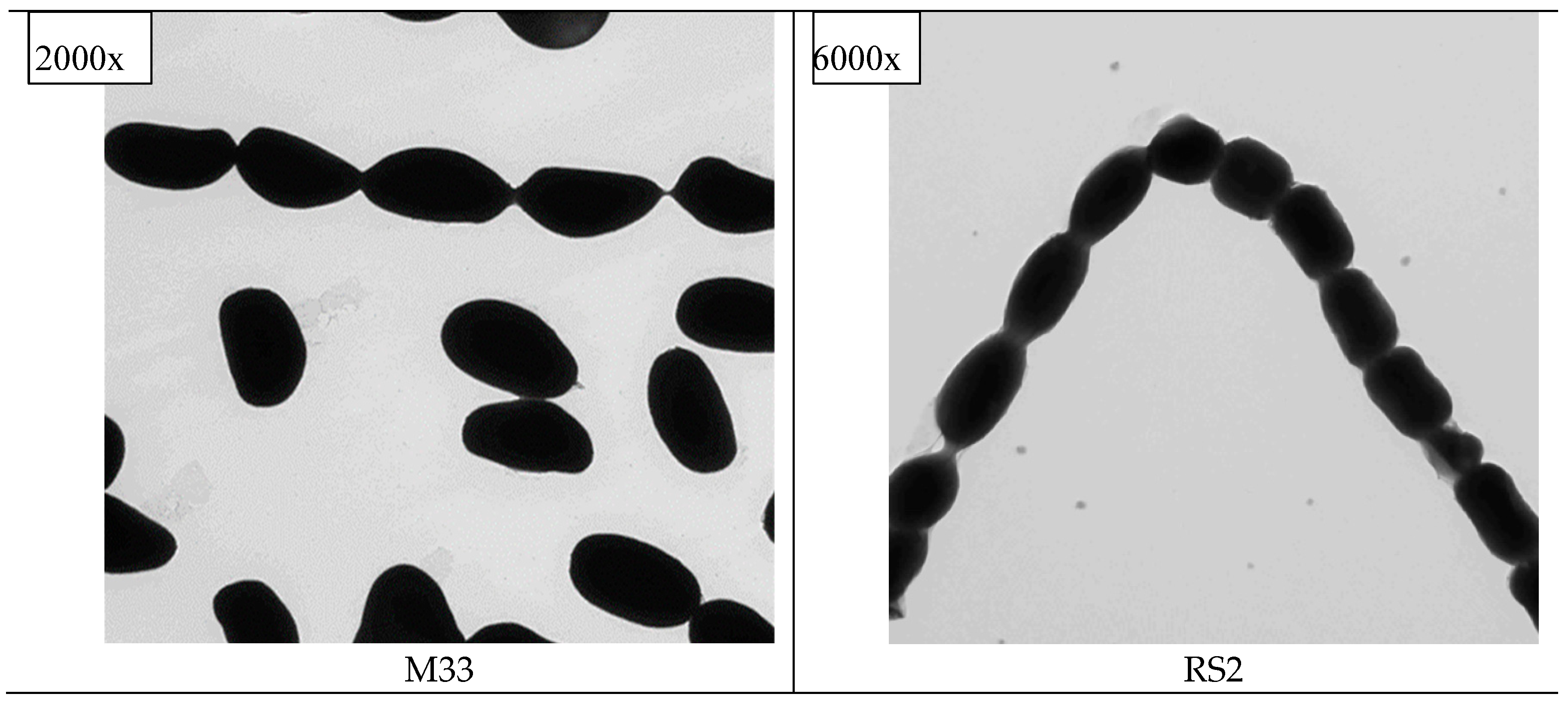
Phenotypic characterization (
Figure 3). Observed cells contracted without tearing the underlying substrate, a feature of the whole mount method was done using TEM, therefore, cellular artitechture is apparent. Smooth surfaced long-chain spores of globose shape is confirmatory of bacteria, as in
Figure 3, being characteristic of
Streptomyces sp.
Phylogenetic Characterization (Figure 4)
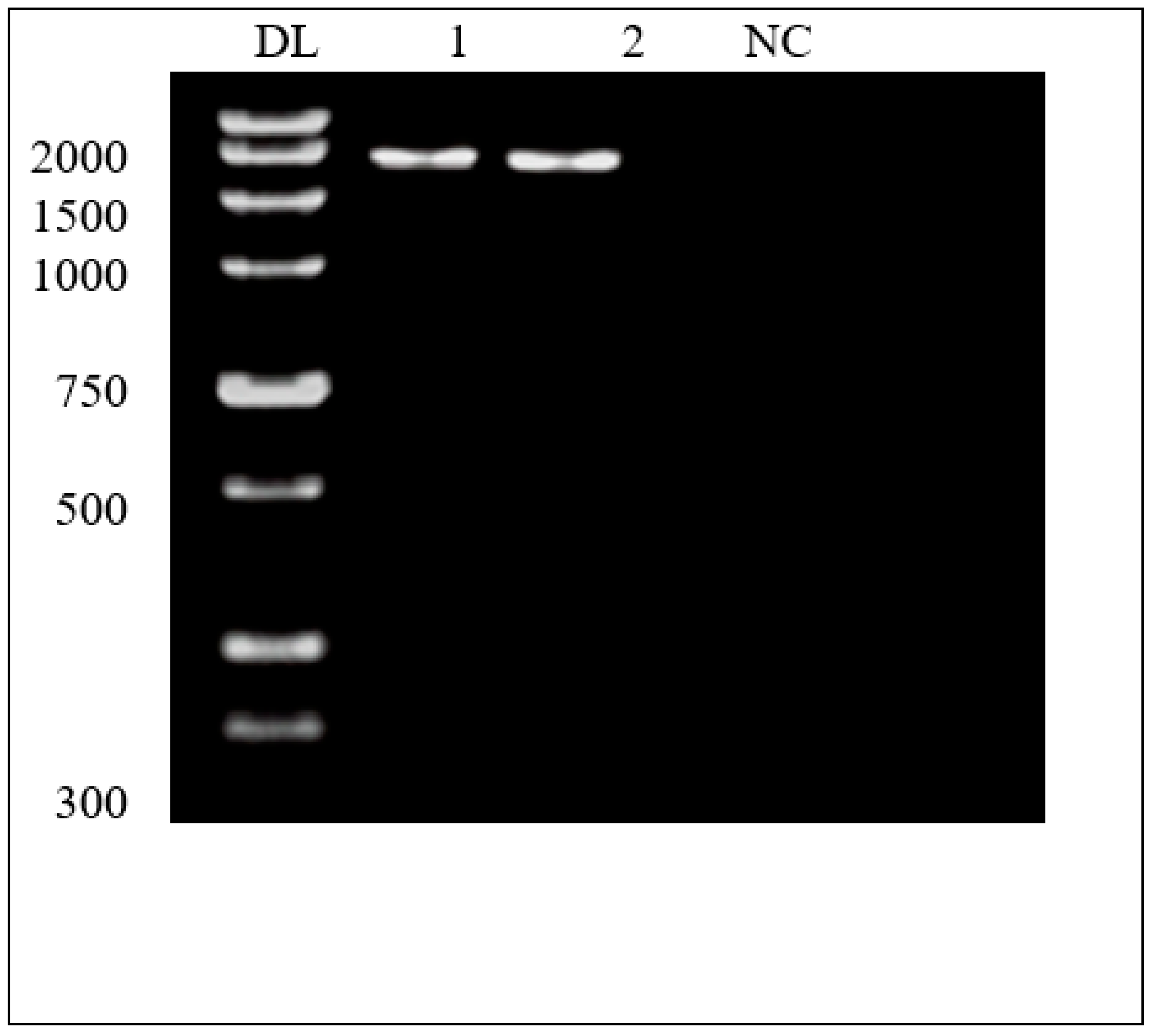
Phylogenetic Sequence analysis after molecular weight detection of the amplified products of 16S ribosomal RNA gene region for two samples with approx. 1500 bp, sample 1 was 1414.49 bp and sample 2 was 1442.62 bp, in comparison to a ladder actinomycetes DNA and the negative control.
Figure 4.
Molecular weight detection of specific amplified products of 16S ribosomal RNA gene region for two samples with approx. 1500 bp [Sample 1 is 1414.49 bp and sample 2 is 1442.62 bp. [DL; DNA ladder; actinomycetes, NC; negative control.].
Figure 4.
Molecular weight detection of specific amplified products of 16S ribosomal RNA gene region for two samples with approx. 1500 bp [Sample 1 is 1414.49 bp and sample 2 is 1442.62 bp. [DL; DNA ladder; actinomycetes, NC; negative control.].
Identified 16S ribosomal RNA gene sequences were aligned, analyzed for identity and genetic distances using NCBI BLAST and multiple alignments sequences were computed by pairwise distance method using Clustal W2
http://www.clustal.org/clustal2/ The identified, aligned nucleotide sequences were compared with Aspergillus isolates sequences available at the NIH genetic sequence database GenBank
® https://www.ncbi.nlm.nih.gov/genbank/ (Accessed on Jan, 2022).
Table 4 addresses 16S rRNA gene, partial sequence, and highest similarity % among the 5 samples for M33 and RS2 bacterial strains. For both the Organism: Bacteria;
Actinomycetota; Streptomycetales; Streptomycetaceae; Streptomyces Spore producing, antibiotic producing organisms, isolated from the soil.
Phylogenetic analysis showed one main clade in which isolate M33 was amongst other
Streptomyces strains in the same clade. Similar 16S rRNA gene sequence belonging to
Streptomyces flavofuscus strain NRRL B-8036 is under the same category.
Figure 5 presents the phylogenetic tree for the 2
Streptomyces strains, based on the previous 16S rRNA gene region sequence from the isolated samples RS2 and M33, using MEGA11 program
https://www. megasoftware.net/
In Silico Prediction of the Isolated Bacterial Strains Enzymatic Activity
Bacterial enzyme activity was retrieved from BcDive database to explore Bacterial Diversity (GmbH, Germany)
https://bacdive.dsmz.de/strain/15148 (Accessed on Feb. 12th, 2023). Enzyme activities include acid and alkaline phosphatases, alpha-chymotrypsin & glucosidase, naphthol-AS-BI-Phosphohydrolase via BRENDA structured view of enzymes (release 1, 2023 online including 68 new and 479 updated enzyme classes) by Elixir, USA (Accessed Feb. 17th, 2023). Streptomyces sp. enzymes are Streptomyces aminopeptidase, Streptomyces dinuclear aminopeptidase, Thermophilic Streptomyces serine proteinase, Proteinase
https://www.brenda-enzymes.org/search_result.php?quicksearch=1&noOfResults=10&a=9&W [2]=Streptomyces+&T [2]=2
In Vitro Anti-Microbial Activity (Table 5)
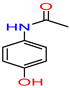
The positive anti-microbial effect of the tested acetaminophen/paracetamol bio-degradation products samples observed as clearance in the wells/inhibition zone %, while compounds that didn’t have an anti-microbial effect, then the growth media appeared opaque with viable bacteria surviving after applying acetaminophen/paracetamol bio-degradation products test extract samples, against the standard mix of microbes used. The positive anti-microbial effect of the tested acetaminophen/paracetamol bio-degradation products (arising from M33) was observed in the shaked sample products, 1a against MRSA and A. Niger. For the static sample products 1b ST an anti-microbial effect of 71.5%, 82%, and 94.5% growth inhibition against S. Arues, E. Coli, and A. Niger, respectively.
Table 5.
Anti-microbial activity monitored as growth inhibition %, of isolated acetaminophen/paracetamol bio-degradation products produced from the static or shaking states for M33 bacterial strain isolate.
Table 5.
Anti-microbial activity monitored as growth inhibition %, of isolated acetaminophen/paracetamol bio-degradation products produced from the static or shaking states for M33 bacterial strain isolate.
| |
M33 tested samples |
| Microorganisms in culture suspension |
1a SH |
1b ST |
| Gram negative |
S. Arues |
-ve |
71.48 |
| E. Coli |
low |
82.18 |
| Gram positive |
MRSA |
81.89 |
low |
| B. Subtills |
-ve |
-ve |
| S. Typhi |
-ve |
-ve |
| Fungi |
C. Albicans |
-ve |
-ve |
| A. Niger |
70.13 |
94.66 |
Values are the average of triplicate experiments. [E. coli; Escherichia coli, S. typhi; Salmonella typhi, S. aureus; Staphylococcus aureus, MRSA; Multidrug-resistant Staphylococcus aureus, B. subtilus; Bacillus subtilus, and C. albicans; Candida albicans, A. niger; Aspergillus niger, 1a; M33 degradation products in shaking flask, 1b: M33 degradation products in static flask.] -ve; No inhibition zone appeared or less than 10% opaque media, if less than 50% inhibition = low activity, inhibition % = positive antimicrobial effect presented as clearance in the wells.
In Silico Prediction of ADMET Parameters for Acetaminophen/Paracetamol Bio-Degradation Products (Accessed Jan. 26th, 2022) (Table 6)
In silico Rodent Oral Toxicity Prediction and Indication of Toxicity Targets
Moreover, to examine interaction with other body proteins and to ensure that these bio-degradation products are safe, with no carcinogenic effect, toxicity prediction is measured as the Ames mutagenicity test. If compounds were anticipated in silico to be non-mutagenic according to Ames test, furthermore, carcinogenicity in animals in vivo (mice) are expected to be negative with a modest risk according to the human ether-à-go-go-related gene cardiac potassium channel (hERG) ion channel inhibition, which is an important antitarget in drug discovery, associated with potentially fatal heart conditions.
Table 6.
ADMET Parameters or the PreADME/Tox Prediction for the acetaminophen/paracetamol bio-degradation products using SwissADME cheminformatics platform and the pkCSM Web tool.
Table 6.
ADMET Parameters or the PreADME/Tox Prediction for the acetaminophen/paracetamol bio-degradation products using SwissADME cheminformatics platform and the pkCSM Web tool.
| |
Absorption A |
Distribution D |
Metabolism M |
Excretion E |
Toxicity T |
| Bio-degradation product |
GI % intestinal human abs. |
(log BB) BBB/ CNS permeation |
CYP2D6 inhibitor |
Renal OCT2 substrate |
AMES/ skin sensitization |
Hepatotoxicity/ hERG I inhibitor |
| Cyanoacetylene /prop-2-ynenitrile |
100/high |
-0.039/No |
No |
No |
No/No |
No/No |
Phenol 3,5-bis(1,1-dimethylethyl)/
2,4-ditert-butylphenol |
100/high |
-0.039/No |
No |
No |
No/No |
No/No |
| 1-Hexadecanol/hexadecan-1-ol |
91.63/high |
0.408/Yes |
Yes |
No |
No/Yes |
No/No |
pentadecyl ester Trichloroacetic acid/
pentadecyl 2,2,2-trichloroacetate |
89.803/high |
0.798/Yes |
No |
No |
No/Yes |
No/No |
| Dodecanamide/Dodecanamide |
93.04/high |
0.362/Yes |
No |
No |
Yes/Yes |
No/No |
| 9-Octadecenamide/octadec-9-enamide |
91.726/high |
-0.172/Yes |
No |
No |
No/Yes |
No/No |
δ-9-tetrahydrocannbinol/
6aR,10aR)-1-methoxy-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydrobenzo[c] chromene |
90.218/high |
-0.389/Yes |
No |
No |
No/Yes |
No/No |
N-[4-Bromo-N-Butyl]-2-Piperidinone/
1-(4-bromobutyl)piperidin-2-one |
93.091/high |
0.448/Yes |
No |
No |
No/No |
No/No |
| Acetaldehyde/Acetaldehyde |
93.15/high |
0.58/Yes |
No |
No |
Yes/Yes |
No/No |
| 5-Methyl-4-nitrohexane-nitrile |
100/low |
-0.023/No |
No |
No |
No/No |
No/No |
| Isohexyl-acrylate/4-methylpentyl prop-2-enoate |
Not identified |
NA |
NA |
NA |
NA |
NA |
10-Undecenoic acid methyl ester/
methyl undec-10-enoate |
95.396/high |
0.464/Yes |
No |
No |
No/Yes |
No/No |
| N-(diacetamidomethyl)acetamide |
95.07/high |
0.669/Yes |
No |
Yes |
No/Yes |
No/No |
| Nitrocyclopentane/nitrocyclopentane |
100/high |
-0.278/Yes |
No |
No |
Yes/Yes |
No/No |
| 7-Nonenamide/(Z)-non-7-enamide |
93.31/high |
-0.011/Yes |
No |
No |
No/Yes |
No/No |
| Acetaminophen/N-(4-hydroxyphenyl)-Acetamide |
91.94/high |
-0.219/Yes |
No |
No |
No/No |
No/No |
Toxicity Testing
In vitro MTT cytotoxicity assay of acetaminophen/paracetamol bio-degradation products produced by M33 and RS2 bacterial strain Extracts using two different cancer cell lines (
Table 7)
When HepG2 and MCF7 cancer cells lines were treated with acetaminophen/paracetamol or Extract 1a, Extract 1b, Extract 2a, and Extract 2b with different concentration range. MTT results showed that cancer cell viability decrease by increasing concentration for all bio-degradation products samples. IC50 µg/ml of extracts 1a,1b, 2a, 2b were higher than acetaminophen/paracetamol IC50 µg/ml (safer). Average M33 isolates samples (1a and 1b) IC50 is 200 µg/ml.
In vivo Acute Single Oral Toxicity Test (
Figure 6) [N.B. No estimation of acute oral toxicity of the acetaminophen/paracetamol bio-degradation products extracted from the bacterial strain RS2 Extract 2a in either static or shaking flasks, per the bio-degradation products were highly similar in structure and the anti-microbial effect and the ADMET and SwissTox predicted they are not toxic.]
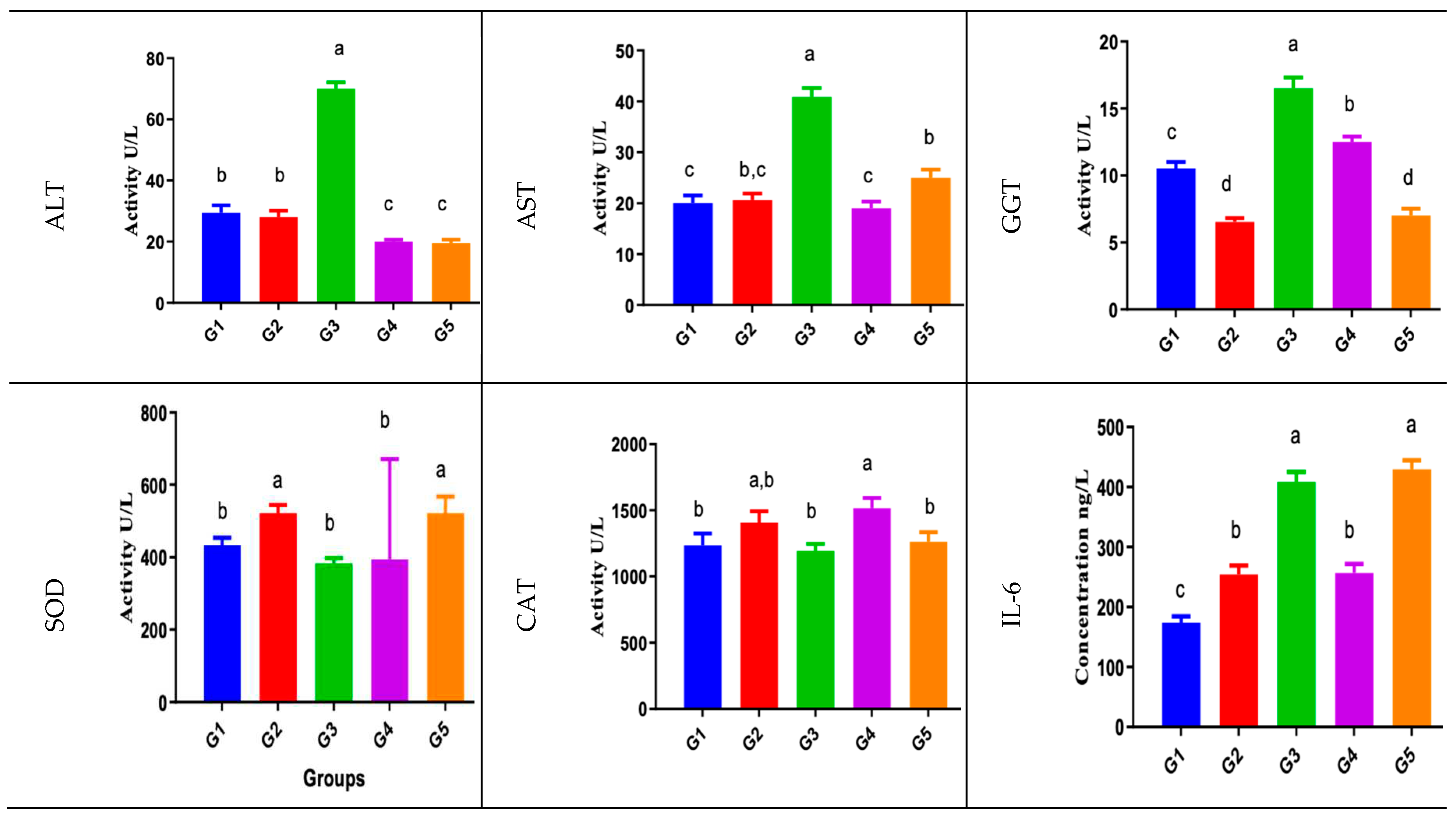
Biochemical Analysis
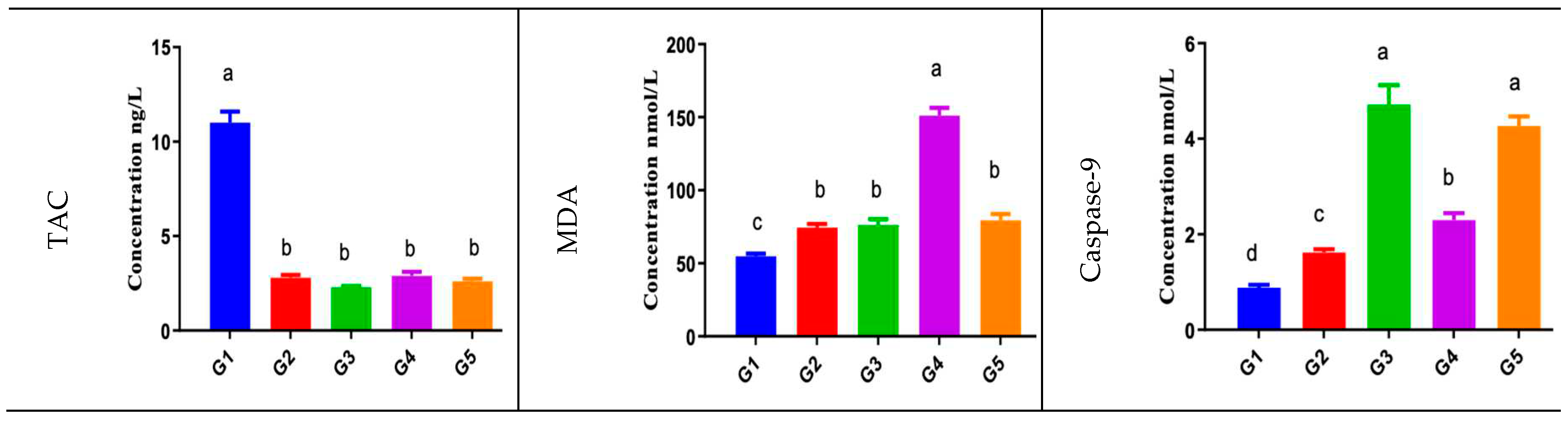
Animals blood liver function tests (ALT, AST, GGT) showed significant increase in acetaminophen/paracetamol group (group3) compared to normal control group (group1) and the negative control group (DMSO) group2. Bio-degradation products groups (group4 and group5) showed relatively normal liver function test values compared to normal control group (group 1). Liver tissue lipid peroxidation level was expressed as MDA showing significant increase in group 4 compared to normal control group (group1) and relative increase in acetaminophen/paracetamol group (group3) and group 5 compared to normal control group (group1).
Data are expressed as mean ± SEM in sera or per mgm tissue protein in the tissue homogenate/total protein conc. (ng/ml). Experiments were performed in triplicate and repeated twice. Significance criterion is set to 0.05 level of probability p. a>b>c>d significance obtained by groups mean comparison difference by ANOVA followed by Duncan post hoc test [Group 1; normal control, group 2; DMSO negative control, group 3; acetaminophen/paracetamol positive control, group 4; M33 Extract 1b, group 5; M33 Extract 1a.]
Liver tissue CAT antioxidant enzyme activity was depressed in the acetaminophen/paracetamol group (group3). However, group4 CAT activity were relatively similar to the normal control group activity, while group 4 showed relative increased CAT activity compared to the normal control group.
Liver Tissue Total Antioxidant Capacity (TAC) Showed Similar Results to CAT
Liver tissue inflammatory mediator marker, IL-6 and the initiator apoptosis enzyme caspase-9 showed significant increase in acetaminophen/paracetamol group (group3), group5, and group4 in comparison to the normal control group (group1) or the negative control group (group2).
The liver tissue oxidative stress enzyme, SOD activity was significantly decreased in the acetaminophen/paracetamol group results (group 3) compared to the normal control group (group1), while group 4 activity was relatively similar to the normal control group and group 5 SOD showed relative increased activity compared to the normal control group.
Histopathological Examination of Liver Tissues Sections (Figure 7)
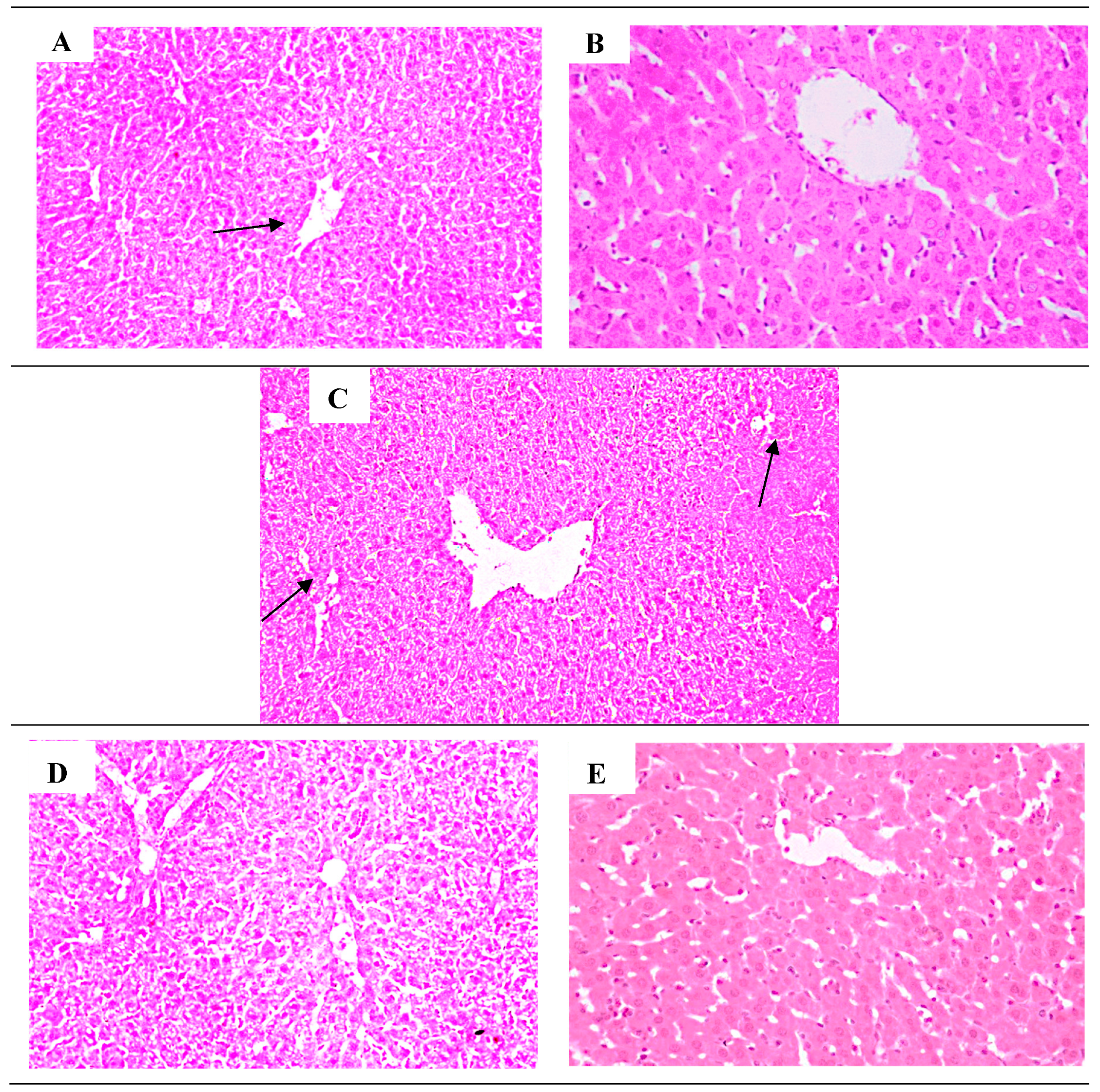
H & E histopathological examination photo-micrograph of hepatic tissue sections following 14-days assigned for the acute single oral toxicity testing of acetaminophen/paracetamol bio-degradation products. Group 1 (normal control group) and group 2 negative control (DMSO) both showed normal liver cells histological structure (A) (B). The positive control group3 administered the acute oral toxic dose of acetaminophen/paracetamol, revealed moderate dilatation of the hepatic central vein and hepatic sinusoids, in addition to, moderate inflammatory changes (grade 2) with lymphocytes and macrophages infiltration and few numbers of neutrophils. Fatty degeneration of the peripheral zone which appeared as intracellular fat droplets. Mid zonal coagulative necrosis of hepatocytes and hyperplasia of Kupffer cells were all seen (grade IV) (C). (D) Group 4 (M33 extract 1b) there is mild vascular change compared to the normal group, but not inflammatory. Group 5 (M33 extract 1a) showed normal histological structure of hepatic lobules with mild dilatated hepatic sinusoids, but, no markers of inflammation (E).
Figure 7.
Photo-micrographs of mice liver sections stained by H&E from A; normal control group1, demonstrated normal histological structure of organized hepatocytes and lobules (black arrow) (100x), B; DMSO negative control group 2, showed hepatocytes parenchymal features comparable to the normal control; A (200x), C; mice treated with acetaminophen/paracetamol as positive control group3, showing mid zonal coagulative necrosis of hepatocytes and hyperplasia of Kupffer cells, leukocytic infiltration with intracellular fat droplets arrow (100x), D; mice treated with M33 extract 1b group4, showed no inflammatory cells infiltrates (100x), E; mice group treated with M33 extract 1a group5, demonstrated normal histological structure of hepatic lobules with mild hepatic sinusoids dilatation (200x). [100 or 200 x magnification.].
Figure 7.
Photo-micrographs of mice liver sections stained by H&E from A; normal control group1, demonstrated normal histological structure of organized hepatocytes and lobules (black arrow) (100x), B; DMSO negative control group 2, showed hepatocytes parenchymal features comparable to the normal control; A (200x), C; mice treated with acetaminophen/paracetamol as positive control group3, showing mid zonal coagulative necrosis of hepatocytes and hyperplasia of Kupffer cells, leukocytic infiltration with intracellular fat droplets arrow (100x), D; mice treated with M33 extract 1b group4, showed no inflammatory cells infiltrates (100x), E; mice group treated with M33 extract 1a group5, demonstrated normal histological structure of hepatic lobules with mild hepatic sinusoids dilatation (200x). [100 or 200 x magnification.].
3. Discussion
Bioremediation is an eco-friendly way to degrade toxic chemicals and trace pharmaceuticals/products that are present in the environment and may have toxic effect on human beings. This study aims to test how to keep the environment clean from acetaminophen/paracetamol, which may be present in waste water or soil or food/crops by using bacterial strains having the ability to degrade acetaminophen/paracetamol and release lower toxic products.
Bacteria via utilizing their own enzymes’ secondary function, is to bio-degrade “trace pharmaceuticals” like acetaminophen/paracetamol, they are exposed to. This is beneficially to be widely used as “bioremediation” method for getting rid of the “trace pharmaceuticals” at their first release, domestically, not after traveling through soil or ground, to irregation water.
By literature retrieval, amidase, deaminase, and dioxygenase(s) are the main enzymes that degrade acetaminophen/paracetamol by Streptomyces bacteria, or any other bioremediation antimicrobial source, to give smaller, simpler metabolites that could be burnt by Kreb’s cycle (Miguel et al., 2022).
In the present study, two bacterial organisms Streptomyces flavofuscus (sample M33) and Streptomyces chrestomyceticus (sample RS2) can utilize acetaminophen/paracetamol, as source of C and N, for growth, in the culture media and moreover, in the environment complex medium such as wastewater or soil.
Identification of the 2 selected strains was done phenotypically using TEM which identified the presence of elongated spores as a strong evidence for Actinomycetes presence. Additionally, molecular identification was then done using 16s rRNA sequencing. The first strain M33 revealed high similarity to Streptomyces flavofuscus strain, about 99.35% identity and the second strain RS2, had high similarity to Streptomyces chrestomyceticus, of 99.57 % identity. The identified, aligned strains nucleotide sequences were compared with Actinomycetes isolates sequences available at the NIH genetic sequence database GenBank® (Accessed on Jan., 2022) and turned to be new strains, so we deposited them to the NCBI/Nucleotide/ Genebank in Feb. 13th., 2022. However, it is important to emphasize on that high similarity is good during relating these organisms to specific species, but, they may contain different characteristics, depending on other environmental factors and/or bacterial mutations. It is noteworthy to mention that the best bacterial isolates were from Wadi El Natrun district in Egypt.
Bio-degradation products of acetaminophen/paracetamol which were produced in the biological system of M33 and RS2 bacterial strains were identified using GC/Mass, which explained the presence of more than one bio-degradation product related to acetaminophen/paracetamol, cyanoacetylene, hexadecanol, dodecenamide, octadecanamide, tetrahydrocannabinol, undecanoic acid, isohexyl acrylate, and acetamide. LC_Mass and 1H NMR were done on the bio-degradation products of M33 bacterial strain to overture the bio-degradation mechanism/reactions pathway, which was predicted computationally. After prediction of bio-degradation products molecular structure, the solubility and absorption, then distribution, metabolism, and excreation (ADME) as well as cellular, metabolic, and functional toxicity prediction (PreADMET) was done.
The rationale for using shaking or static samples, that the bio-degradation/transformation % was increased in the shaken flask runs, with the help of the growth medium formulation’s optimization for C/N ratio and type of nitrogen sources, growth temperature 30 °C/not higher and pH 7.2. This shaking state helped to reduce the harmfull by-product(s) formation as well (Ferraiuolo et al., 2021), confirmed by less toxicity prediction as well as in vivo and in vitro tested toxicity studies.
Taking into consideration that the bio-degradation product compounds could be further studied as new treatment modalities, as assumed-bioactive specially when tested their antimicrobial activity that turned to be positive significantly. The acetaminophen/paracetamol bio-degradation products downstream targets were predicted chemo-informatically using PreADMET database that identified these target(s) enzymes, receptors or cytosolic and membrane proteins (
Table 3B).
Degradation products of M33 sample bacterial stain were tested for the possible antimicrobial activity in the 2 samples during static and shaking conditions, showing high antibacterial activity on S. Arues, during the static phase, high antibacterial activity against E. Coli, during static and shaking phases, high antimicrobial on MRSA if the bio-degradation product sample was produced with shaking.
Via the chemo-informatics/bioinformatics tool(s) using, the pharmacogenomics knowledge resource, PharmGKB® (Carrillo et al. 2021) (accessed on Nov. 3rd, 2022) identified the small organic molecule acetaminophen/paracetamol-waste (APAP; N-acetyl-para-aminophenol) chemical structure and how affect biological system(s), followed by identification of each of the bio-degradation products from various samples.
One of the study obectives is to prove that these bio-degradation products has lower toxic effect(s) when being compared to acetaminophen/paracetamol, ater the majority being predicted as safe computationally. So, we performed the in vitro MTT cytotoxicity assay using M33 and RS2 bio-degradation products on HepG2 and MCF7 cell lines. Significant increased bio-degaradation products IC50 (Kamiloglu et al., 2020) compared to acetaminophen/paracetamol for the 2 cell lines HepG2 and MCF7, which means that acetaminophen/paracetamol has higher toxicity level than all bio-degradation products on both cell lines; bio-degradation products are safer.
Moreover, in vivo acute oral toxicity of M33 sample bio-degradation products were tested on female Swiss albino about 20 gm body weight and 20-weeks age CD1 mice, with histopathological confirmation of hepatic tissue preservation after 14-days of the experiment duration.
Acetaminophen/paracetamol acute single oral dose hepatotoxicity was characterized by OS (Du, Ramachandran, & Jaeschke, 2016) and decreased antioxidant enzymes SOD and CAT and perturbed TAC (Jaeschke, McGill, & Ramachandran., 2012) in cases of toxicity. Caspase-9 and IL-6 levels in liver tissue were done for monitoring apoptosis and inflammation processes, respectively. Finally, findings were confirmed by hepatic tissue H & E histological examination (el deeb et al., 2022).
Limitations. The exact mechanism of the novel bacterial strains biological activity to be characterized and more characterization(s) if they could prove chemotherapeutic effects (El-Mesallamy et al., 2018) based on the bio-degradation products predicted downstream target enzymes or membrane proteins/receptors.
Future Prospective
Identify if these novel bacterial stains can degrade estrogenic waste.
Summary and Conclusions
After isolation of several bacterial strains from some different Egyptian habitats (desert, lakes water, and soil samples) were tested for their ability to degrade standard amount of acetaminophen/paracetamol®, added to the st. culture media, into panel of bio-degradation products, presenting “trace pharmaceuticals” in drinking water. These bio-degradation products panel chemical structure was identified by extensive GC/LC Mass, HPLC and by predictive comparison with literature data, in silico ADME as well as downstream target enzyme(s) and membrane proteins prediction, that would contribute genetic alteration(s) and/or cancer as their adverse impact on population general health if drinking water contains these “trace pharmaceuticals”. Isolation and taxonomic characterization of these potential isolated bacteria was reported on the bases of morphological and genotypic analysis, where 2 Streptomyces strains were found novel, so we deposited them to the NCBI GeneBank Feb.13th, 2022. The biological anti-microbial activity of the acetaminophen/paracetamol bio-degradation products were examined against a panel of ATCC standard test organisms (bacteria and fungi) and some of these bio-degradation compounds proved to be active, having good anti-microbial potential, that mandates further experimental validation.
The most active acetaminophen/paracetamol bio-degradation products were examined for their safety by cheminformatics in silico toxicity prediction and in vitro cytotoxicity against 2 ATCC cancer cell lines (HepG2 and MCF7), where they turned to be safe, with IC50 higher than that of acetaminophen/paracetamol, therefore, we proceeded to perform the in vivo acute single oral toxicity examination to confirm their safety.
Recommendation(s)
The need for proper bio-degradation of “Trace Pharmaceuticals” at its first release domestically.
The need for a “National Task-Force Initiative” for proper environment-friendly disposing unused medication, and terminating with generalized social national awareness opposing being flushed down the toilet, to stay out of our drinking water and crops.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, A.S.A-R., M.M. and N.M.H.; methodology, D.H.E. and A.S.A-R.; software, E.B.B. and N.M.H.; validation, M.M. and N.M.H.; resources, D.H.E; data processing, D.H.E. and N.M.H.; writing— D.H.E.; writing—review and editing, D.H.E., E.B.B. and N.M.H.; visualization, M.M. and N.M.H.; supervision, M.M. and N.M.H.; project administration, M.M. and N.M.H.; funding acquisition, D.H.E. All authors have read and agreed to the published version of the manuscript. All authors have agreed to the authorship of this manuscript. Publication APC funding is supported by authors and the ASRT SNG program.
Funding
ASRT/SNG/BGM/2018-7: Academy of Science, Research and Technology (ASRT)/Scientists for Next Generation (SNG) funding program/ Biochemistry, genetics and Molecular Biology/2018-7 #130; titled: Bioremediation of acetaminophen by Bacterial strains.
Institutional Review Board Statement
Animal care and all experimental protocols were approved and conducted in accordance with the ethical guidelines approved by the Institutional Review Board of the Faculty of Pharmacy, Ain Shams University, Abassia, Cairo, Egypt (ACUC-FP-ASU RHDIRB2020110301 REC#49).
Informed Consent Statement
Not applicable.
Data Availability Statement
All the source code and data are available within the manuscript. Any further inquiries to be sent to the corresponding author.
Acknowledgments
The authors are thankful to ASRT/SNG program as well as the partial support by the Advanced Biochemistry Research Lab. (ABRL) at the Biochemistry Dept., Faculty of Pharmacy, Ain Shams University (#2021-2022/3NMH6/7).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
List of Abbreviations:
| A. niger: Aspergillus niger |
LC/MS: Liquid chromatography/mass spectrometry |
| ADMET: absorption, distribution, metabolism, excretion - toxicity |
LIPO: lipophilicity |
| ALT: Alanine aminotransferase |
log p: the logarithm of the partition coefficient |
| ANOVA: analysis of variance |
MCF7: human breast cancer cell line |
| APAP: Acetaminophen |
MDA: malondialdehyde |
| AST: Aspartate aminotransferase |
MRSA: Multidrug-resistant Staphylococcus Aureus
|
| B. subtilus: Bacillus Subtilus |
MSM: mineral salt medium |
| BBD: Bio-catalysis/Bio-degradation Database |
MTT: 3-(4, 5-di-MethylThiazol-2-yl)-2,5-diphenyl Tetrazolium bromide |
| BLAST: basic local alignment search tool |
MW: Molecular weight |
| BW: body weight |
NCBI: National Center for Biotechnology Information. |
| C. albicans: Candida albicans |
NIH: National institute of health |
| CAT: Catalase |
NIST: National institute of standards and technology |
| CNS: central Nervous system |
NMR: Nuclear Magnetic resonance |
| D.F: degrees of freedom |
OTC: Over the counter |
| DMSO: Dimethyl sulfoxide |
PCR: polymerase chain reaction |
| DNA: Deoxyribonucleic acid |
PDM: Pairwise Distance Method |
| E-coli: Escherichia Coli
|
PharmGKB: pharmacogenomics knowledge resource |
| EAWAG: Swiss Federal Institute for Environmental Science and Technology |
Ppm: part per million |
| ELISA: enzyme-linked immunosorbent assay |
RPA: relative peak area |
| ESI: Electrospray ionization |
RPMI medium: Roswell Park Memorial Institute Medium |
| FBS: fetal bovine serum |
rRNA: ribosomal ribonucleic acid. |
| FLEX: flexibility |
RRT: relative retention time |
| GC/EI-MS: Gas chromatography/electron ionization/mass spectrometry |
RT: Retention time |
| GC/MS: gas chromatography-mass spectrometry. |
S. typhi: Salmonella Typhi |
| Geldoc-it: Gel documentation system |
S. ureus: Staphylococcus Aureus |
| GGT: gamma glutamyl transferase |
SDG: Sustainable Development Goals |
| HBA: number of hydrogen bond acceptors. |
SH: shaking condition (shaking flask) |
| HBD: number of hydrogen bond donors. |
SIB: Swiss Institute of Bioinformatics |
| HepG2: human liver cancer cell line |
SMILES: Simplified Molecular-Input Line-Entry System |
| hERG: human ether-à-go-go-related gene cardiac potassium channel |
SOD: superoxide dismutase |
| HPLC: High performance liquid chromatography |
SPSS: statistical package for social studies |
| IC50: The half maximal inhibitory concentration. |
ST: static condition (static flask) |
| IL6: interleukin-6 |
TAC: total antioxidant capacity |
| INSATU: saturation |
TBAR: thio barbituric acid reactive |
| INSOLU: solubility |
TEM: Transmission electron microscope |
| IUPAC: International Union of Pure and Applied Chemistry |
TPSA: topological polar surface area |
| LB: Lysogeny broth |
- |
References
- Arifin, W.N.; Zahiruddin, W.M. Sample Size Calculation in Animal Studies Using Resource Equation Approach. The Malaysian journal of medical sciences: MJMS 2017, 24, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. Journal of pharmaceutical analysis 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Barbuto Ferraiuolo, S.; Cammarota, M.; Schiraldi, C.; et al. Streptomycetes as platform for biotechnological production processes of drugs. Appl Microbiol Biotechnol 2021, 105, 551–568. [Google Scholar] [CrossRef]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic acids research 2013, 41, D36–D42. [Google Scholar] [CrossRef]
- Bhatt, P.; Gangola, S.; Bhandari, G.; Zhang, W.; Maithani, D.; Mishra, S.; Chen, S. New insights into the degradation of synthetic pollutants in contaminated environments. Chemosphere 2021, 268, 128827. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Dmitrieva, N.I.; Michea, L.F.; Rocha, G.; Ferguson, D.; Burg, M.B. Toxicity of acetaminophen, salicylic acid, and caffeine for first-passage rat renal inner medullary collecting duct cells. The Journal of pharmacology and experimental therapeutics 2003, 306, 35–42. [Google Scholar] [CrossRef]
- Clarridge, J.E. , 3rd. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clinical microbiology reviews 2004, 17, 840–862. [Google Scholar] [CrossRef]
- Culling, C.F.A. Handbook of Histopathological and Histochemical Techniques: Including Museum Techniques; Butterworth-Heinemann: Cambridge, UK, 2013; ISBN 1483164799. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Scientific reports 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic acids research 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. Journal of immunological methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Du, K.; Ramachandran, A.; Jaeschke, H. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox biology 2016, 10, 148–156. [Google Scholar] [CrossRef]
- Eldeeb, M.; Sanad, E.F.; Ragab, A.; Ammar, Y.A.; Mahmoud, K.; Ali, M.M.; Hamdy, N.M. nticancer Effects with Molecular Docking Confirmation of Newly Synthesized Isatin Sulfonamide Molecular Hybrid Derivatives against Hepatic Cancer Cell Lines. Biomedicines 2022, 10, 722. [Google Scholar] [CrossRef]
- El-Mesallamy, H.O.; Diab, M.R.; et al. Cell-Based Regenerative Strategies for Treatment of Diabetic Skin Wounds, a Comparative Study between Human Umbilical Cord Blood-Mononuclear Cells and Calves’ Blood Haemodialysate. PLoS ONE 2014, 9, e89853. [Google Scholar] [CrossRef]
- El-Mesallamy, H.O.; El Magdoub, H.M.; Chapman, J.M.; et al. Biomolecular study of human thymidylate synthase conformer-selective inhibitors: New chemotherapeutic approach. PLoS ONE 2018, 13, e0193810. [Google Scholar] [CrossRef]
- Hamed, A.; Abdel-Razek, A.; Frese, M.; Stammler, H.; El-Haddad, A.; Ibrahim, T.; Sewald, N.; et al. Terretonin N: A New Meroterpenoid from Nocardiopsis sp. Molecules 2018, 23(2), 299. [Google Scholar] [CrossRef]
- Jaeschke, H.; Ramachandran, A. Oxidant Stress and Lipid Peroxidation in Acetaminophen Hepatotoxicity. Reactive oxygen species (Apex, N.C.) 2018, 5, 145–158. [Google Scholar] [CrossRef]
- Jaeschke, H.; McGill, M.R.; Ramachandran, A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug metabolism reviews 2012, 44, 88–106. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Kim, J.W.; Ryu, S.H.; Kim, S.; Lee, H.W.; Lim, M.S.; Seong, S.J.; Kim, S.; Yoon, Y.R.; Kim, K.B. Pattern recognition analysis for hepatotoxicity induced by acetaminophen using plasma and urinary 1H NMR-based metabolomics in humans. Analytical chemistry 2013, 85, 11326–11334. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; Zaslavsky, L.; Zhang, J.; Bolton, E.E. PubChem 2023 update. Nucleic acids research 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Clustal W and Clustal X version 2. 0. Bioinformatics (Oxford, England) 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Larson, A.M.; Polson, J.; Fontana, R.J.; Davern, T.J.; Lalani, E.; Hynan, L.S.; Reisch, J.S.; Schiødt, F.V.; Ostapowicz, G.; Shakil, A.O.; Lee, W.M.; Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology (Baltimore, Md.) 2005, 42, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced drug delivery reviews 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk and Saleh. Molecular Identification and Characterization of Antimicrobial Active Actinomycetes Strains from Some Egyptian Soils. American-Eurasian J. Agric. & Environ. Sci. 2014, 14, 954–963. [Google Scholar]
- McGill, M.R.; Williams, C.D.; Xie, Y.; Ramachandran, A.; Jaeschke, H. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicology and applied pharmacology 2012, 264, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of immunological methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Oecd, T.N. 425: Acute Oral Toxicity: Up-and-Down Procedure. OECD Guidel. Test. Chem. Sect. 2008, 4, 1–27. [Google Scholar]
- Palma, T.L.; Magno, G.; Costa, M.C. Biodegradation of Paracetamol by Some Gram-Positive Bacterial Isolates. Current microbiology 2021, 78, 2774–2786. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. Journal of medicinal chemistry 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Plósz, B.G.; Benedetti, L.; Daigger, G.T.; Langford, K.H.; Larsen, H.F.; Monteith, H.; Ort, C.; Seth, R.; Steyer, J.P.; Vanrolleghem, P.A. Modelling micro-pollutant fate in wastewater collection and treatment systems: status and challenges. Water science and technology : a journal of the International Association on Water Pollution Research 2013, 67, 1–15. [Google Scholar] [CrossRef]
- Pu, S.; Liu, Q.; Li, Y.; Li, R.; Wu, T.; Zhang, Z.; Huang, C.; Yang, X.; He, J. Montelukast Prevents Mice Against Acetaminophen-Induced Liver Injury. Frontiers in pharmacology 2019, 10, 1070. [Google Scholar] [CrossRef]
- Qin, M.; Qian, Y.; Huang, L.; Zhong, C.; Li, M.; Yu, J.; Chen, H. Extractive electrospray ionization mass spectrometry for analytical evaluation and synthetic preparation of pharmaceutical chemicals. Frontiers in pharmacology 2023, 14, 1110900. [Google Scholar] [CrossRef] [PubMed]
- Rajan, B.M.; Kannabiran, K. Extraction and Identification of Antibacterial Secondary Metabolites from Marine Streptomyces sp. VITBRK2. International journal of molecular and cellular medicine 2014, 3, 130–137. [Google Scholar] [PubMed]
- Ramachandran, A.; Jaeschke, H. Oxidant Stress and Acetaminophen Hepatotoxicity: Mechanism-Based Drug Development. Antioxidants & redox signaling 2021, 35, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Rezk, M.R.; Bendas, E.R.; Basalious, E.B.; et al. Development and validation of sensitive and rapid UPLC-MS/MS method for quantitative determination of daclatasvir in human plasma: Application to a bioequivalence study. J. of pharmaceutical and biomedical analysis 2016, 1028, 61. [Google Scholar] [CrossRef]
- Rezk, M.R.; Basalious, E.B.; Amin, M.E. Novel and sensitive UPLC-MS/MS method for quantification of sofosbuvir in human plasma: application to a bioequivalence study. Biomedical chromatography BMC 2016, 30, 1354–62. [Google Scholar] [CrossRef] [PubMed]
- Rezk, M.R.; Basalious, E.B.; Karim, I.A. Development of a sensitive UPLC-ESI-MS/MS method for quantification of sofosbuvir and its metabolite, GS-331007, in human plasma: Application to a bioequivalence study. Journal of pharmaceutical and biomedical analysis 2015, 114, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Rios-Miguel, A.B.; Smith, G.J.; Cremers, G.; van Alen, T.; Jetten MS, M.; Op den Camp HJ, M.; Welte, C.U. Microbial paracetamol degradation involves a high diversity of novel amidase enzyme candidates. Water research X 2022, 16, 100152. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. Journal of hepatology 2016, 64, 1403–1415. [Google Scholar] [CrossRef]
- Silva, A.; Delerue-Matos, C.; Figueiredo, S.; Freitas, O. The Use of Algae and Fungi for Removal of Pharmaceuticals by Bioremediation and Biosorption Processes: A Review. Water 2019, 11, 1555. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s theory and practice of histological techniques E-Book; Elsevier Health Sciences, 2018; ISBN 0702068861.
- Whirl-Carrillo, M.; Huddart, R.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Whaley, R.; Klein, T.E. An Evidence-Based Framework for Evaluating Pharmacogenomics Knowledge for Personalized Medicine. Clinical pharmacology and therapeutics 2021, 110, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, J.; Zhu, R.; Zhou, Q.; Chen, J. Degradation of paracetamol by pure bacterial cultures and their microbial consortium. Applied microbiology and biotechnology 2013, 97, 3687–3698. [Google Scholar] [CrossRef] [PubMed]
- Żur, J.; Piński, A.; Marchlewicz, A.; Hupert-Kocurek, K.; Wojcieszyńska, D.; Guzik, U. Organic micropollutants paracetamol and ibuprofen-toxicity, biodegradation, and genetic background of their utilization by bacteria. Environmental science and pollution research international 2018, 25, 21498–21524. [Google Scholar] [CrossRef] [PubMed]
- Żur, J.; Wojcieszyńska, D.; Hupert-Kocurek, K.; Marchlewicz, A.; Guzik, U. Paracetamol - toxicity and microbial utilization. Pseudomonas moorei KB4 as a case study for exploring degradation pathway. Chemosphere 2018, 206, 192–202. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).