1. Introduction
The taxon protozoa is attributed to
Georg August Goldfuss, who proposed the term in 1818 to embrace ‘
infusoria’, some bryozoans, and various other small animal-like creatures; but it was not until the mid-19th century that the term was first used to refer exclusively to single-celled organisms. All free-living protozoa are essentially aquatic, as they live in benthic and planktonic communities of freshwater, brackish and marine environments. Many of them live in semi-terrestrial habitats, in damp moss in the water films around soil particles [
1].
Many protozoa are microaerobic -they seek out habitats with low O
2 tension. These protozoa can be facultative anaerobes [
2]. They live mainly in freshwater and marine sediment; there are many species, but none is ever abundant [
3]. Among the protozoa, now called protists, we can mention the free-living amoebae or naked amoeba. The three free-living amoeba species recognized for causing brain lesions are
Naegleria fowleri,
Acanthamoeba sp (various genotypes, most outstanding T-4) and
Balamuthia mandrillaris [
4,
5,
6]. Primary amebic meningoencephalitis (PAM) is a rare brain infection that is usually fatal and caused by the free-living amoeba
Naegleria fowleri. This ameba lives in soil and warm freshwater around the world. It grows best at high temperatures, up to 115°F (46°C), and can survive for short periods at higher temperatures.
Acanthamoeba is a microscopic, free-living ameba that can cause rare, but severe infections of the eye, skin, and central nervous system. Granulomatous Amebic Encephalitis (GAE) is a serious infection of the brain and spinal cord that typically occurs in people with a compromised immune system.
Balamuthia mandrillaris is a free-living ameba, naturally found in the environment, which cause rare, but severe infections of the skin, and the central nervous system. However, there are many other species of amoeba such as
Sappinea pedata, Vermamoeba vermiformes,
Vanellas sp,
Vahlkampfia sp. and, more recently found,
Paravahlkampfia francinae, which is less virulent and aggressive. This entire amoeba must infect to human and animals Free-living amoebas are eukaryotic microorganisms that have a cosmopolitan distribution worldwide [
5,
6,
7]
In recent years, we have found more species of free-living amoebae or free-living protists implicated in human cases.
It should not be hard to think that there are more potentially pathogenic species in the environment. We can take the number of mycobacterium species that existed in the 1980s and the number of mycobacterium species described today as an example and compare them with the potentially pathogenic and strictly pathogenic species. Undoubtedly, there is a great difference in the number of species over time. However, this does not happen with some protists. Only one pathogenic ciliate, capable of infecting humans, is known: Balantidium coli.
This ciliate is capable of producing dysentery, but not encephalitis. We believe that there should be a greater number of potentially pathogenic microorganisms that affect humans (amoeba and ciliate). The search for microorganisms is related to the finding in the clinical laboratory or pathology laboratory, but recently the diagnosis using molecular tools is one of the best approaches for diagnosis, but it lacks morphological data of the microorganisms.
Here, we describe, for the first time, the morphology of some protists found in cerebrospinal fluid (CSF) of pediatric patients. These microorganisms have not been characterized or categorized yet.
2. Data collection methods and information analysis
For approximately 2 years, (2016-2018), CSF samples have been collected. We chose only 4 samples from four patients, the ones we believed were more significant. Because there is a difference in the age range between patients and survival of them. In addition, the morphological differences of these protists are very striking.
Experienced clinicians took CSF samples. All samples were taken in 15 mL sterile screw cap conical tubes. They were immediately analyzed through wet mount microscopy at 400x and 1000-x magnification.
2.1. Light microscopy
Observations of amoebae in cultures, along with measurements of cell dimensions were made with the use of an inverted microscope normal optic and Primus microscope equipped with phase contrast optics.
Photographs were taken and videos of the samples were recorded under the microscope. To do this, a mobile adapter, which was fixed to the microscope eyepiece, was used. The mobile device used was Sony Xperia lt22i.
All samples were seeded in non-nutritive agar and axenic medium (Proteose peptone 20%, yeast extract 2%, and glucose 18%). The morphology of each protist is described by Page 1988, 1991.
2.2. DNA extraction, PCR amplification and sequencing of SSU ribosomal genes
For the diagnosis and differentiation of free-living amoebas (Balamuthia and Acanthamoeba) associated to encephalitis, we used the primers: 5´Balspec16S (5´-CGCATGTATGAAGAAGACCA-3´ and 3´ Balspec 16S (5´-TTACCTATATAATTGTCGATACCA-3´), which amplifies a 1,075-bp portion of the mitochondrial 16S rRNA gene from
B. mandrillaris. The PCR conditions were modified: for 16S amplification, an initial denaturation at 94°C for 5 min, followed by 35 cycles at 94°C for 30 s, 60°C for 1m and 72°C for 2.20m and an extension at 72°C for 10m [
8].
And for 18S amplicons by using eukaryotic primers Euk1A (5′- AACCTGGTTGATCCTGCCAGT-3′), (5′- TTGATCCTTCTGCAGGTTCACCTAC- 3´). The PCR conditions were 95 °C for 5min, the PCR cycles were: 30 cycles at 94 °C for 30 s, 56 °C for 30 s, 72 °C for 30 s, and an extension at 72 °C [
9].
3. Results
In 2016, we received a case of meningitis in the Pathogenic Protozoa and Endosymbiont Laboratory in Lima, Peru. At first, it was assumed that it could be another classic free-living amoeba.
A series of CSF samples arrived, mainly from pediatric patients between four months old and sixteen years of age over a two-year period (2016-2018). The clinical presentation was meningitis or encephalitis of unknown origin. Viral and bacterial infections were discarded and no pathogen was found; therefore, an infection caused by free-living amoebas was suspected.
We observed 7/8 pediatric patients with similar clinical characteristics in 2016; 6/12 patients in 2017; and 11/14 patients until March 2018.
All samples were CSF and were analyzed through microscopy based on morphology. We used non-nutritive agar plates, but the amoeba did not grow. No cysts were observed. The PCR to Balamuthia mandrillaris and others amoeba was negative in all samples.
The EUK A/B primers used amplified human DNA. They were not in one case, they were in all cases. There is no clear explanation. The most plausible thing is that the primers are not specific enough, the variable regions do not amplify, and the parasite load is too low to be detected by these primers.
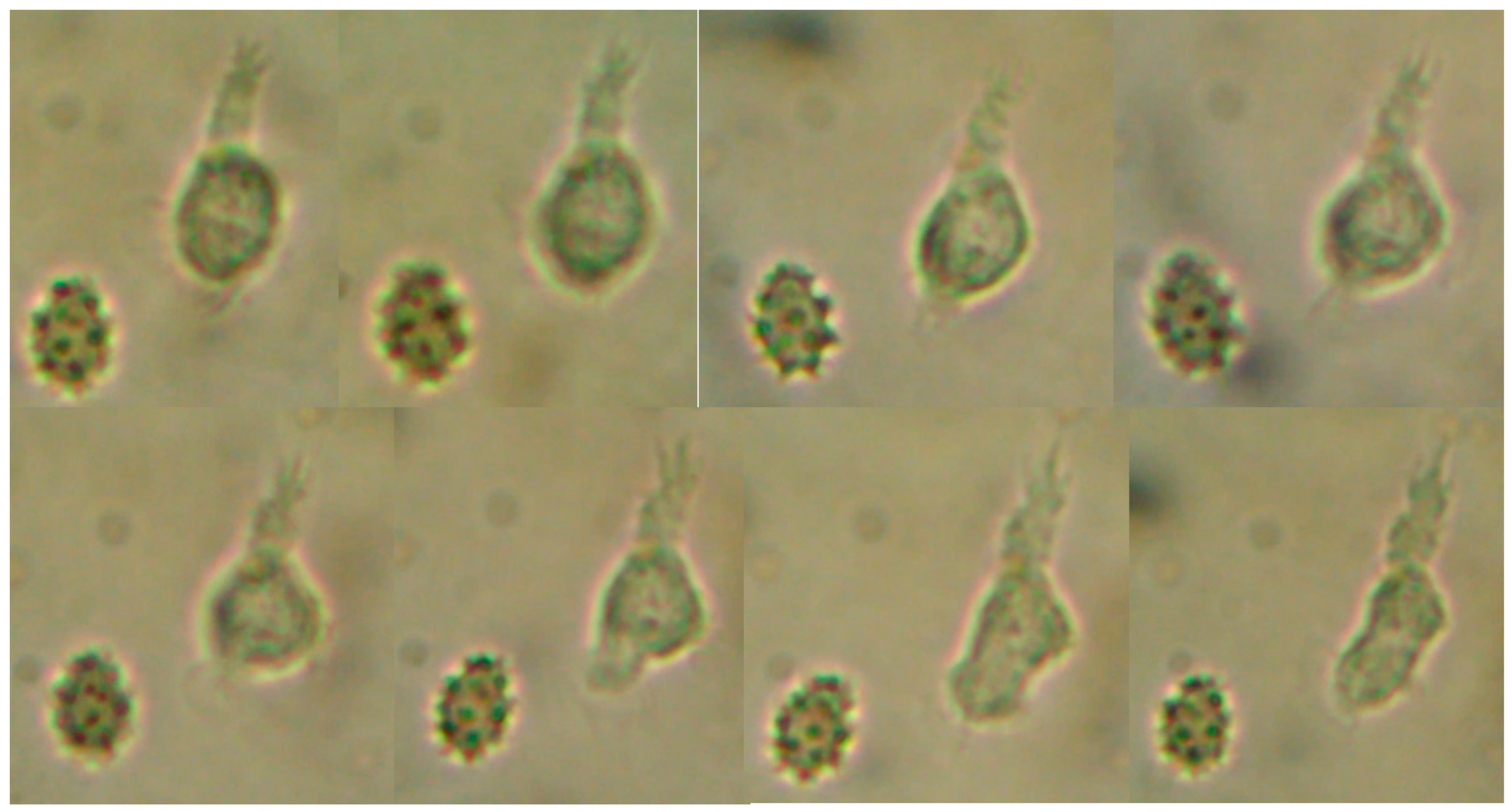
Protist case 1:
The sample was CFS from an 8-year-old boy, found of swimming. He swam in a pool in the south of Lima. His symptoms started with an intense headache and vomits, and was preliminarily diagnosed in a Pediatric Hospital in Lima.
In the CSF, a microorganism of approximately 10-12µm in length with fast but erratic movements and capable of moving through very short pseudopods was observed. These pseudo-pseudopods, were short pseudopods, at some point in the movement, dichotomous pseudopods were observed with slightly granular cytoplasm and extremely light hyaline layer. It was not possible to visualize the nucleus or to grow it in a conventional medium for free-living amoebas. At that time, we did not have standardized molecular methods; that is why it was not possible to establish and confirm the real identity of this protist for molecular biology. We believe that the child acquired it in the pool, since there is no other event that is directly related to the patient’s history. Finally, based on morphology characteristics, we believe this protist is a species related to the Valhkamphiae family (
Naegleria sp.) [
4].
Figure 1.
Trophozoite of freshly extracted CSF observed under the microscope to 40x. It is possible to see in the sequence of images the morphological change of the protist.
Figure 1.
Trophozoite of freshly extracted CSF observed under the microscope to 40x. It is possible to see in the sequence of images the morphological change of the protist.
We could observe erratic movement, although it was sometimes dichotomous with two pseudopods and, at times, an erratic amoeboid movement was seen. Locomotion of the protist and changes in shape can be observed. Through a more detailed analysis of the image of the protist, we could noticed that it produced very well defined short pseudopods and the hyaline layer was not clearly observed. Uroid is not observed.
Host type: Human LCR
Locality: Lima, Peru.
Host collection: Hospital of Lima
Description: 11 μm (9–12 μm, n=30) in length, and 5-6μm (5-8μm) in width. Ovoid cell body shape, with pseudopodos shorts and eruptive.
There was not enough sample to perform the molecular test.
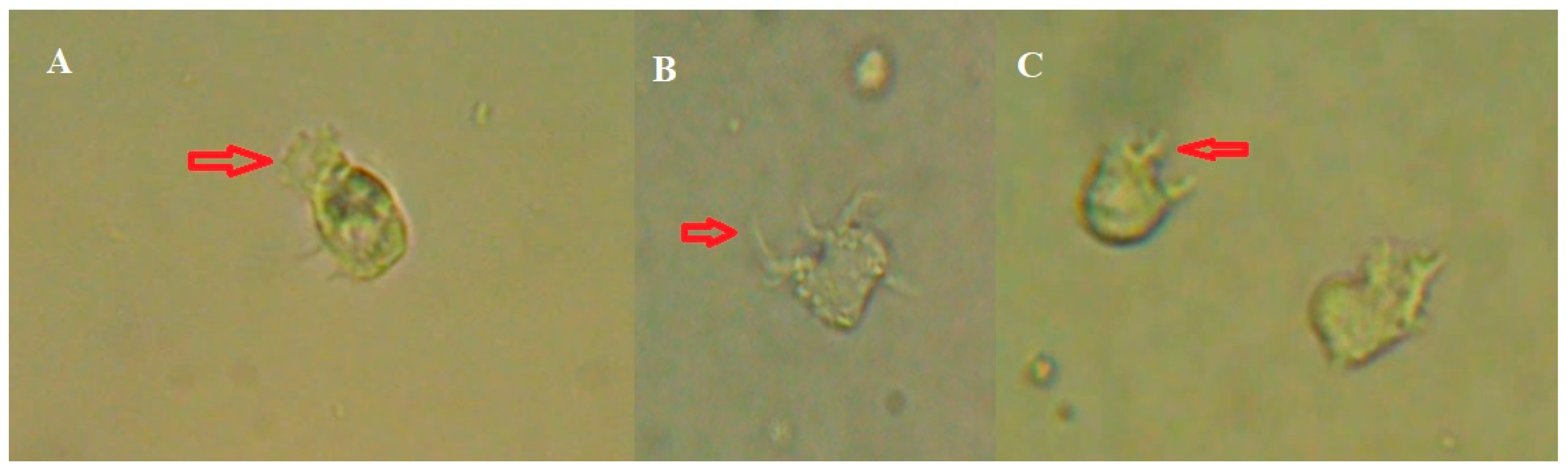
Protist 2
A healthy 12-year-old girl without important risk factors, swimmer of the Peruvian Swimming Federation, had a clinical picture of meningitis. In the CSF, two morphotypes of amoebas were observed: a) Large amoeba of around 20 -25µm with thick and long pseudopods and b) Long amoeba of 25-30 microns with a filamentous uroid which is somewhat longer than other species described. In both, the locomotion was fast and changed in form. The nucleus was not observed in amoeba [amoeba-1], but in amoeba [amoeba-2]. We do not know if they are the same or if they were really two types of amoebas. However, they did not grow in any culture medium. These protists were kept active for several days in axenic medium at 37°C. Then they disappeared. None of these forms were observed in the pool sample.
In this sample, we tried to elucidate the identity of this amoeba by performing the PCR using the FLA primers, but we only managed to amplify human DNA.
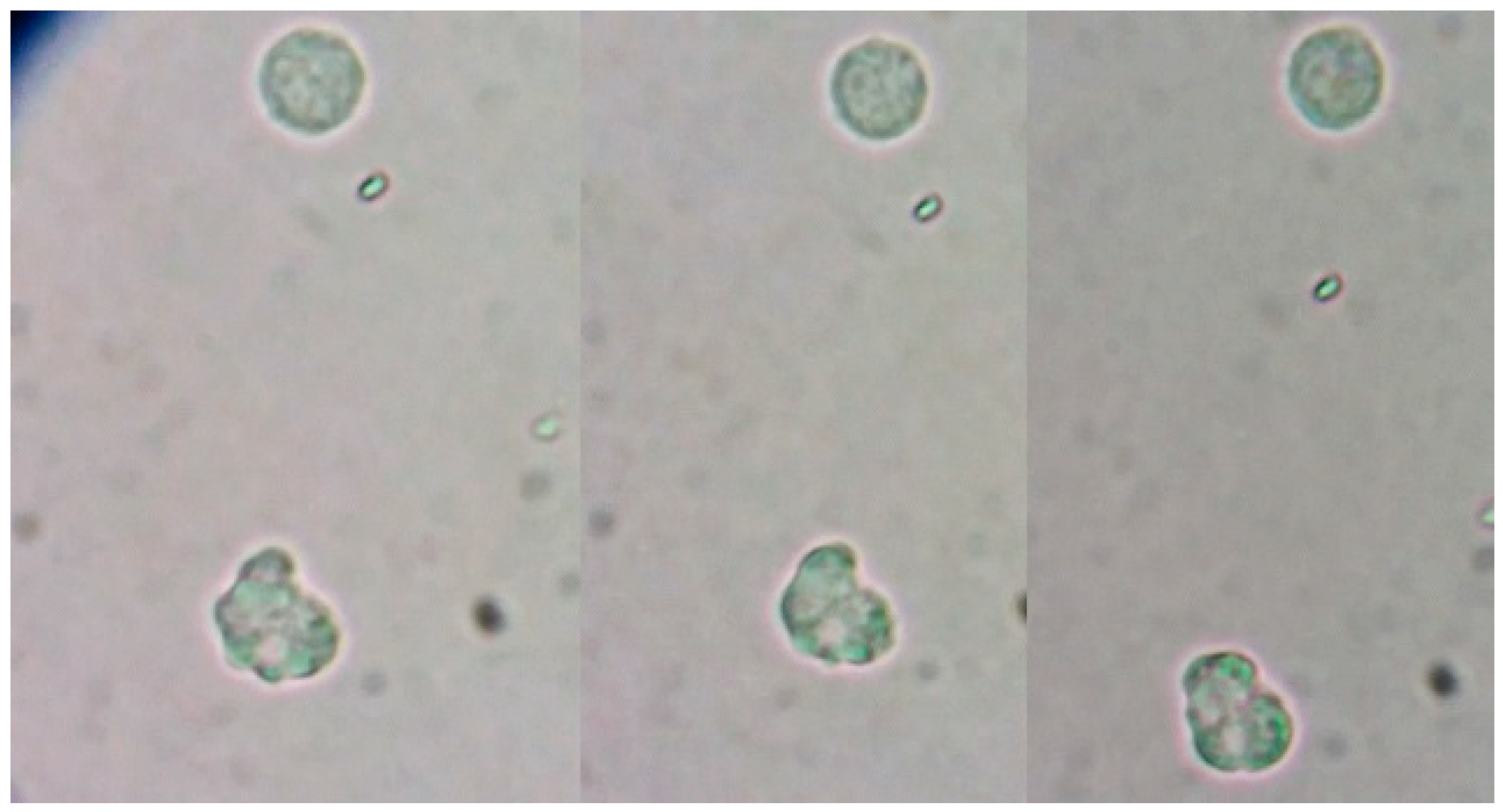
Figure 2.
Amoeba-1: a, b) Pseudopods observed in the patient's CSF. Amoeba-2: c, d, e, f, g) movement sequence of the amoeba observed in the CSF.
Figure 2.
Amoeba-1: a, b) Pseudopods observed in the patient's CSF. Amoeba-2: c, d, e, f, g) movement sequence of the amoeba observed in the CSF.
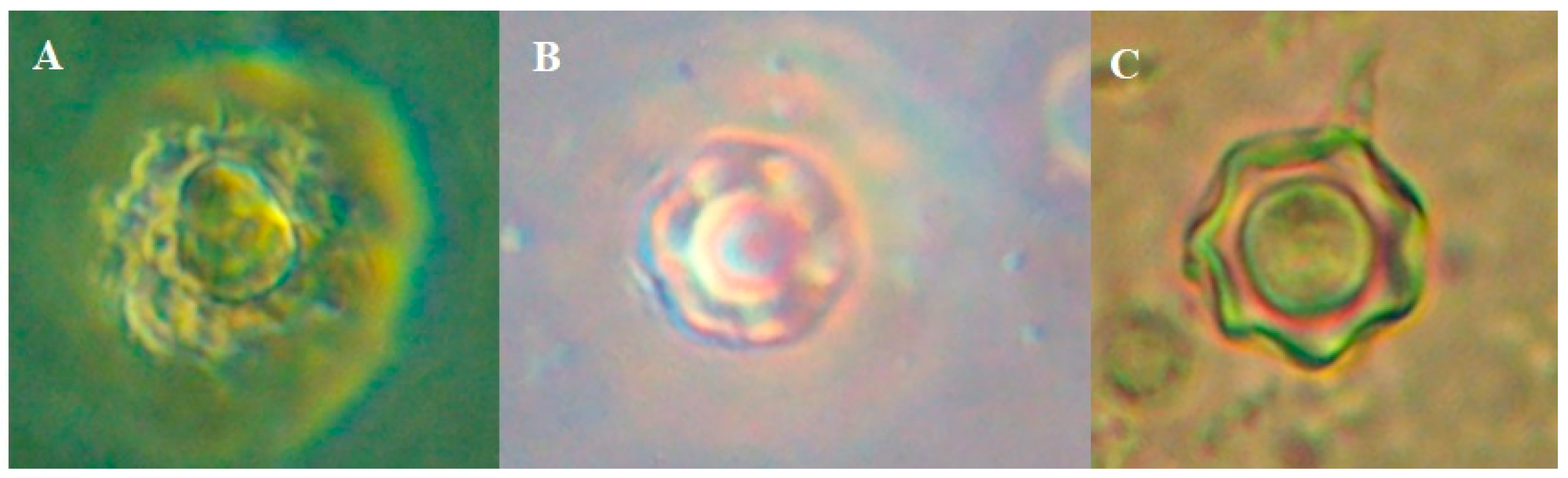
In the image below, we can see one trophozoite with a giant vacuole in axenic culture. The cysts that we see belong to axenic cultivation and the cultivation of the soil with which the girl played in her house. Figure 3
Figure 3.
A; trophozoite with a giant vacuole in axenic culture, B; Cyst in axenic from CSF (face contrast 60x) and C; Cyst in monoxenic culture (soil from home of the girl).
Figure 3.
A; trophozoite with a giant vacuole in axenic culture, B; Cyst in axenic from CSF (face contrast 60x) and C; Cyst in monoxenic culture (soil from home of the girl).
We believe that the cyst and the trophozoite observed in the images belong to the same microorganism. However, we do not have molecular evidence.
Host type: Human CSF
Locality: Lima, Peru.
Host collection: Hospital of Lima
Description: Amoeba -1 size: 50 μm in length (40–60 μm, n=10) and 50 μm (30-40 μm) in width. Ovoid cell body shape, with pseudopods long, branched, and cap hyaline.
The EUK A/B primers used amplified human DNA.
Protist 3
A 4-month-old girl with congenital hydrocephalus, with no clear history of being in contact with any water source, was preliminarily diagnosed in a pediatric hospital in Lima. CFS culture was positive and klebsiella sp was isolated. Subsequently, the presence of an amoeba in the CSF was confirmed. The amoeba did not grow in any culture medium. This protist showed several outstanding features: it did not form a cyst, did not adhere to the slide, but lysed after 2 hours, disappearing from the CSF. We believe lysis occurs because there are anaerobes microorganisms. The movement is active in a floating state, and also could change shape while floating. Cytoplasmic extensions compatible with short pseudopods in the form of plant roots are seen. With all these characteristics, we named it with the candidate name of Rhadiculamoeba anaerobica.
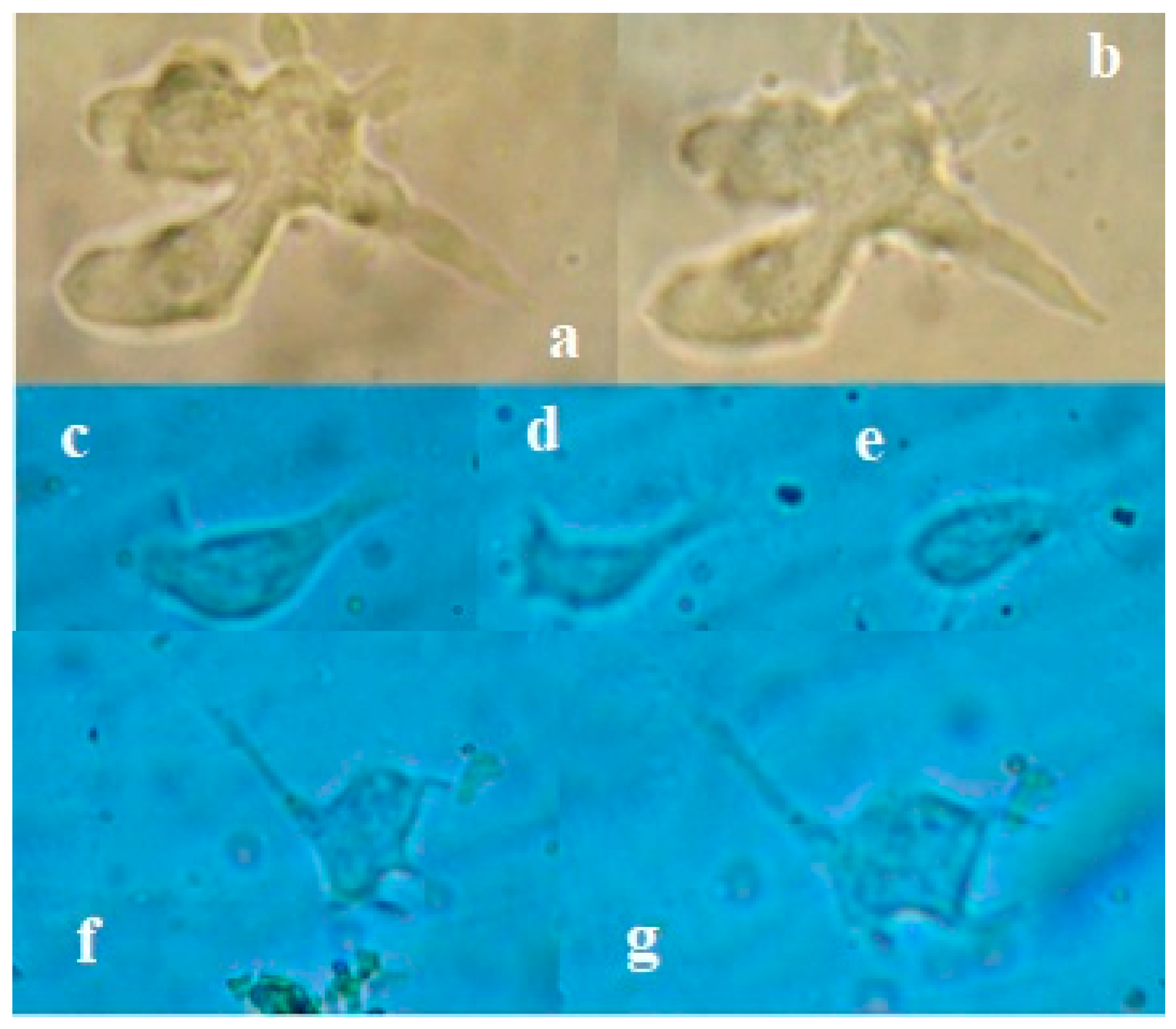
Figure 4.
The images have been taken under a Primus microscope at 40 X. Trophozoites are observed in a floating state; they do not form cysts nor adhere to the coverslip. A) The extension of small pseudopods and a faint hyaline layer can be seen. B) Pseudopods extension, C) Pseudopods with mini lateral extension.
Figure 4.
The images have been taken under a Primus microscope at 40 X. Trophozoites are observed in a floating state; they do not form cysts nor adhere to the coverslip. A) The extension of small pseudopods and a faint hyaline layer can be seen. B) Pseudopods extension, C) Pseudopods with mini lateral extension.
Host type: Human CSF
Locality: Lima, Peru.
Host collection: Hospital of Lima
Description: 30 μm in lenght (25–35 μm, n=30) and 20 μm in width (15–20 μm). Ovoid cell body shape. With pseudopodos long and others short and branched.
Etymology: This species is named Rhadiculamoeba anaerobica due to its morphology and its physiological condition. The protist lysed on contact with oxygen, hence, we assume that it is anaerobic. Rhadicula = Root; Anaeobica = Anaerobic
Name: Candidate Rhadiculamoeba anaerobica
The EUK A/B primers used amplified human DNA
Protist 4
A girl aged 20 months, with no history of contact with water sources, presented with fever, motor deficit, and spoke incoherently. She was taken to a pediatric hospital in Lima. To physicians, their first presumptive diagnosis was tuberculosis. However, acid fast bacilli were not observed in the CSF although an amoeba sp. could be observed. One month later, CSF cultured was positive for Mycobacterium tuberculosis in the MODS and PCR test. The amoeba looked like a leukocyte at first; after four minutes it changed its shape and increased its length. What we see under the microscope is a microorganism that changes shape. We believe that there may be two parts, although we are not very sure: the anterior part and the posterior part. In the anterior part, the structure is circular or oval. In the posterior part, an extension of the cytoplasm is observed that seems to be moving on its axis, implying that it twists forming a kind of screw with terminal filaments. It changes shape on its axis and in the same place, lengthening the posterior part and elongating the spherical part corresponding to the anterior part of the protist's structure. This anterior part seems to have a kind of cilia or cover and, apparently, direction is modified as the anterior part changes from a spherical to an oval.
Figure 5 shows the protist morphological change, before (left) and after (right) in a few minutes and a crenated erythrocyte.
Figure 5.
video sequence, where the trophozoites is seen transforming together with the erythrocyte.
Figure 5.
video sequence, where the trophozoites is seen transforming together with the erythrocyte.
Unfortunately, the identity of these protists has not been elucidated yet because they do not grow in conventional culture media for free-living protists. Cell culture was attempted only once. It is worth mentioning that they keep moving for a longer time but exponential growth was not observed.
Monoxenic medium (with an overlay of Escherichia coli on the agar) and axenic medium (liquid medium based on Proteose-peptone, glucose and yeast extract) do not seem to be optimal for the growth of these amoebic forms or they may grow under conditions devoid of oxygen.
The most commonly found morphotypes are Monotactic, Palmado, and Acanthopodia. The Vahlkampfia family appears to be a morphologically dominant group in certain areas. However, the forms observed escape the 19 morphotypes described and do not fit in any of them [
10,
11,
12]
In this document, the clinical cases are not described and, therefore, we will not delve into the treatment; however we can mention that the use of anti-amoebic and antibiotics agents usually gave good results.
We emphasize the morphological description of these protists, since they possibly belong to other classes or kingdoms and, therefore, the primers did not allow DNA amplification.
Host type: Human CSF
Locality: Lima, Peru.
Host collection: Hospital of Lima
Description: 16 μm in length (15–24 μm, n=10) and 10 μm in width (8–12 μm). Ovoid cell elongated body. With filamentous structures on its back or cytoplasmic extension.
Etymology: nameless
The EUK A/B primers used amplified human DNA
4. Discussion
The Protozoa are considered to be a subkingdom of the kingdom Protista, although they were placed in the kingdom Animalia in the classical system. More than 50,000 species have been described, most of which are free-living organisms; protist are found in almost every possible habitat. The free-living amoebas are protozoan parasites that exist in the environment, mainly freshwater, lakes, and rivers without the need for a definitive host.
There are four main genera of amoebas than can cause diseases in human, namely Naegleria fowleri, Acanthamoeba species, Sappinia pedata and Balamuthia mandrillaris.
The very idea that there are only four species of free-living amoebae that can cause brain damage is ludicrous. The number of microorganisms in the environment (soil or water) is very high and believing that, of the 400 species, only four free living amoeba can cause brain damage is simply implausible.
For example, other amoebas may invade and cause a mild brain disease.
Paravahlkampfia francinae was identified in CSF, but it is a non-pathogenic species. It was shown that it invades the brain, causes meningitis but does not destroy it [
12].
Other free-living amoeba has been documented, such as the case of pathogenesis involving
Sappinia species. This resulted in granulomatous amoebic encephalitis in a non-immunocompromised 38-year-old male from Texas in 1998 [
13].
The patient is thought to have inhaled the
Sappinia species while cleaning up animal dung. As described by Gelman et al. (2003) [
14], the patient “presented with a history of loss of consciousness for 45 min and emesis, followed by bifrontal headache, photophobia, and blurry vision for 2 to 3 days”. Qvarnstrom et al. (2009) [
15] found that the sample tested negative for
S. diploidea but had
S. pedata-specific priming sites, which then allowed them to conclude that the causative agent of GAE in this case was
S. pedata [
15].
In our cases, the microorganisms observed in the CSF of pediatric patients make it clear that there is a great diversity of microorganisms with the capacity to injure the human brain and possibly under certain conditions.
The identification of multiple protists using the EUK A/B primers has been described in the literature, all samples are cultures or environmental samples. In our case, the sample was CSF, where eukaryotic cells, amoebas and human cells abound. I have managed to amplify only human DNA. There is no information on the use of these primers in human samples. This is the first time that human DNA amplification using primers for environmental eukaryotes has been reported. I believe that the most plausible is that the primers do not amplify the target of these protists. The variable region is not complementary to the primers. It is necessary to use many more primers to establish the identity of these protists or use Next Generation sequencing (NGS).
Microscopy continues to be the key first step in diagnosis, but the amoeba can be confused with macrophages or other infectious agents if an expert in infectious disease pathology or clinical microbiology is not consulted.
Inevitably, microscopic observation is critical and with it the treatment begins; subsequently, it should be confirmed with molecular tools. However, it is possible that new microorganisms are not identified in the first instance and, hence, more sophisticated tools such as cloning of a cell and the analysis of the complete genome of a single cell are necessary, tools such as next generation sequences (NGS). Studies at the genomic level will be carried out in the future.
Finally, it is necessary to mention that the treatment requires a combination of antibiotics and antifungals and, even with prompt diagnosis and treatment, mortality in regard to neurological diseases is extremely high. In our case, fortunately, the patients did not die, they all recovered [
4,
12].
5. Conclusions
We do not know the origin of these protists. The morphology of these pathogenic protists does not necessarily conform to typical free-living amoebae. They are able to remain in the CSF for a long period around 3-5 weeks or 3 months that is, somewhat prolonged.
It is possible that some of these protists are anaerobic and uncultivable or difficult to grow.
We believe that it is important to take the greatest care in performing smears to avoid errors in cell identification. For this reason, CSF samples should be stained with Wheatley's trichrome for protists. In some cases, they can reveal morphological differences and help distinguish between human cells and protists.
We have not been able to amplify sequences using universal primers (EUK, to protist) for 18S rRNA. Therefore, we propose the usefulness of others primers and next generation sequencing, which will allow us to establish the identity of these protists.
This finding could open the path to future investigations of the new protist species that provoke neurological diseases.
Finally, we believe in the possibility that these protists could be part of another related phylogenetic group kingdom Protist or definitely, other more elaborate primers are needed to amplify the 18S regions, at least.
Author Contributions
Alfonso Martin Cabello-Vílchez PhD, Conceptualization; Methodology; Validation; Formal Analysis; Investigation; Resources; Data Curation; Writing – Original Draft Preparation; Writing – Review & Editing; Visualization; Supervision; Project Administration; Funding Acquisition; Photos, video and Picture.
Funding
This research received no external funding
Data Availability Statement
The study did not report any data
Acknowledgments
My thanks to my beloved daughter Paula Belén Illary for being the joy of my life. A special thanks to Sheril I. Ramos-Alcántara for all the support provided. The words are insufficient to express my appreciation and my gratitude.
Conflicts of Interest
The author declare no conflict of interest
References
- Finlay, B. J. y Esteban, G. F. Freshwater protozoa: biodiversity and ecological function. Biodiversity and Conservation. 1998; 7(9), 1163-1186. [CrossRef]
- Bernard, C. y Fenchel, T. Some microaerobic ciliates are facultative anaerobes. European Journal of Protistology.1996; 32(3), 293–297. [CrossRef]
- Fenchel, T. and Finlay, B.J. Ecology and evolution in anoxic worlds. Reviews Online.1996;33(05), 2713—33. [CrossRef]
- Martínez DY, Bravo-Cossio F, Valdivia-Tapia MDC, Carreazo NY, Cabello-Vílchez AM. Successful Treatment of Primary Amoebic Meningoencephalitis Using a Novel Therapeutic Regimen Including Miltefosine and Voriconazole. Acta Parasitol. 2022 Sep;67(3):1421-1424.. [CrossRef]
- Trabelsi H, Dendana F, Sellami A, Sellami H, Cheikhrouhou F, Neji S, Makni F, Ayadi A. Pathogenic free-living amoebae: epidemiology and clinical review. Pathol Biol (Paris). 2012 Dec;60(6):399-405.. [CrossRef]
- Visvesvara GS , Moura H , Schuster FL ,. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 2007; 50: 1–26.
- Cabello-Vílchez AM, Chura-Araujo MA, Anicama Lima WE, Vela C, Asencio AY, García H, Del Carmen Garaycochea M, Náquira C, Rojas E, Martínez DY. Fatal granulomatous amoebic encephalitis due to free-living amoebae in two boys in two different hospitals in Lima, Perú. Neuropathology. 2020;40(2):180-184. [CrossRef]
- Ahmad AF, Andrew PW, Kilvington S. Development of a nested PCR for environmental detection of the pathogenic free-living amoeba Balamuthia mandrillaris. J Eukaryot Microbiol. 2011 May-Jun;58(3):269-71.
- Medlin L, Elwood HJ, Stickel S, Sogin ML. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene.1988; 71: 491–499.
- Schuster, F. L. y Ramirez-Avila, L. Current World Status of Balantidium coli. Clinical Microbiology Reviews. 2008; 21(4), 626–638. [CrossRef]
- Smirnov A. V. and S. Brown. Guide to the methods of study and identification of soil Gymnamoebae. Protistology. 2004; 3 (3), 148-190 https://www.zin.ru/journals/protistology/num3_3/smirnov.pdf.
- Visvesvara, G. S., Sriram, R., Qvarnstrom, Y., Bandyopadhyay, K., Da Silva, A. J., Pieniazek, N. J. y Cabral, G. A. Paravahlkampfia francinaen. sp. Masquerading as an Agent of Primary Amoebic Meningoencephalitis. Journal of Eukaryotic Microbiology. 2009;56(4), 357–366. 1. [CrossRef]
- Gelman, B. B. Amoebic Encephalitis Due to Sappinia diploidea. JAMA. 2001; 285(19), 2450. [CrossRef]
- 14. Gelman, B. B., Popov, V., Chaljub, G., Nader, R., Rauf, S. J., Nauta, H. W. y Visvesvara, G. S. Neuropathological and Ultrastructural Features of Amebic Encephalitis Caused by Sappinia diploidea. Journal of Neuropathology & Experimental Neurology. 2003; 62(10), 990 998. [CrossRef]
- Qvarnstrom, Y., da Silva, A. J., Schuster, F. L., Gelman, B. B. y Visvesvara, G. S. Molecular Confirmation of Sappinia pedata as a Causative Agent of Amoebic Encephalitis. The Journal of Infectious Diseases. 2009; 199(8), 1139– 1142. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).