1. Introduction
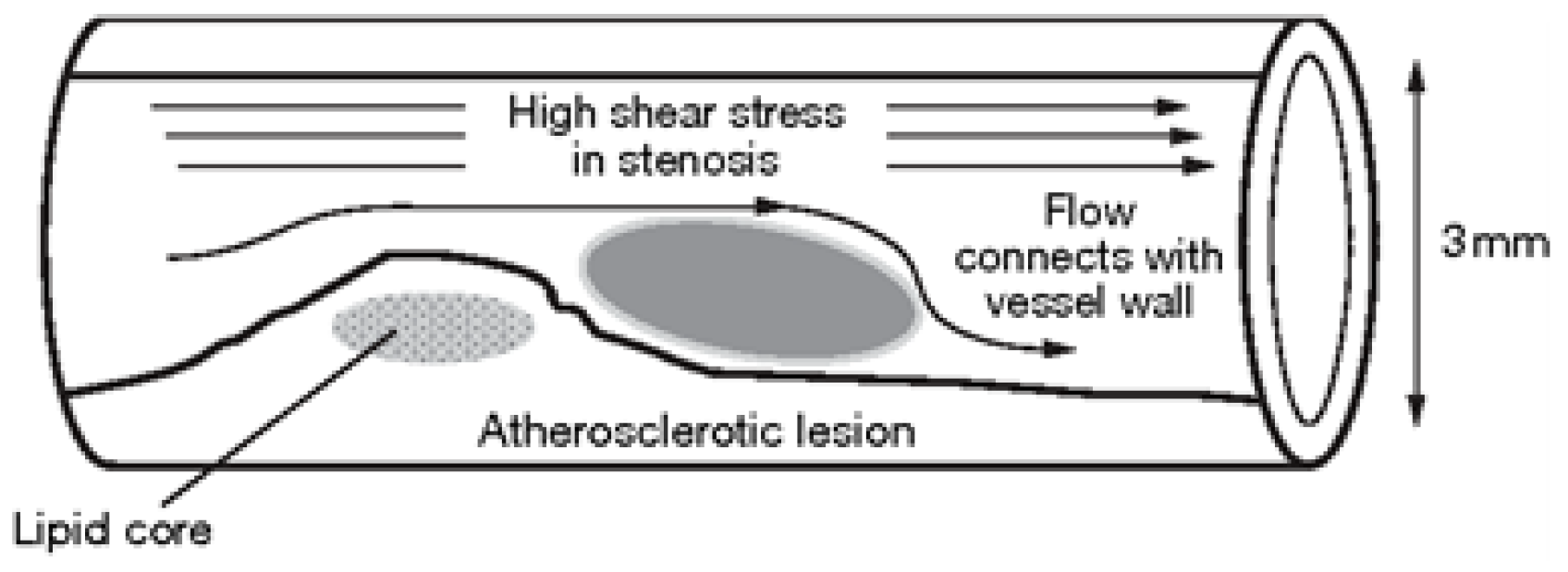
Atherosclerosis is a disease that can be caused by various risk factors, such as high blood pressure, diabetes, smoking, hypercholesterolemia, infections, and modified lipoproteins. Researchers have been investigating atherosclerosis for over a century, with a specific focus on the role of cholesterol in its development, which was first reported by Anitschkow and Chalatov. Although it was initially believed to be a simple lipid storage disease, recent advances in cellular and molecular research have provided new insights into its pathogenesis. The subendothelial space of large and medium-sized arteries contains a monolayer of cells and matrix proteins, with smooth muscular cells occasionally present. As the diseased blood cell thickens over time, focal lesions of lipid accumulation known as fatty streaks develop in the intima. These fatty streaks are believed to be the precursor to advanced atherosclerotic plaques or atheroma, which can lead to clinical symptoms. Advanced lesions occupy a widespread intima region and are characterized by a lipid core with no growth in fibrous tissue at this stage of the disease. Atherosclerotic plaques can be classified as either ’vulnerable’ or ’stable,’ with vulnerable plaques having a significant lipid core and a thin bottom cap that separates tissue factor thrombogenic macrophages from the blood.
According to [
1], the stenosis geometry of some cardiovascular patients cannot be represented by a vertically symmetric function across the stenosis. Consequently, they investigated blood flow through vertically asymmetric stenosis and compared flow volumes in asymmetric and vertically symmetric stenoses. However, a vertically symmetric function, such as an exponential function in a bell shape or a cosine function in a cosine shape, can be used to explain vertically symmetric stenosis.
Zuhaila et al. [
2] conducted a study on the dynamic response of blood flow heat transmission in stenotic conditions through a bifurcated artery with a stiff wall (2D) and Newtonian, incompressible, laminar, and constant blood flow. Their findings revealed that minor changes in stenosis and Reynolds number significantly affected velocity, temperature distribution, and reverse flow recirculation. Diviya et al. [
3] explored the hemodynamics of variable liquid characteristics with heat and mass transmission in the Jeffrey fluid MHD peristaltic mechanism. They observed the effects of an increased variable viscosity parameter on fluid flow and size, as well as on heat transfer to variable thermal conductivity. Li et al. [
4] investigated the impact of various computational models on hydrodynamic factors when an incompressible fluid interacts with two symmetric elastic or poroelastic structures. They performed numerical experiments on blood flow, using the Carreau-Yasuda model to simulate viscosity and examine the influence of non-Newtonian blood rheology and poroelasticity on a benchmark vessel. They also presented a two-dimensional simulation of blood flow in an axisymmetric stenosis artery that considered both non-Newtonian fluid properties and fluid-structure interaction. The results indicated that blood flow exhibits non-Newtonian behavior in small vessels and in settings with complicated geometry.
Sohail et al. [
5] conducted a study on Bingham fluid peristaltic flows with inclined magnetic fields or canals to analyze their impact on heat and mass transfer. Bourhan et al. [
6] developed a mathematical model of multi-stenosis to investigate the effects of heat and blood flow in multi-stenosis arteries. They employed a finite difference scheme to solve the governing equations related to the vorticity-stream function and examined the effects of magnetic fields and stenosis on wall shearing stress and nutrient quantities. The study found that magnetic fields can alter flow patterns and enhance heat transfer, and the severity of stenosis affects wall shear stress. Hayat et al. [
7] presented a model of tangent hyperbolic liquid peristaltic, considering chemical processes, magnetohydrodynamics, and thermophoretic deposition analysis in a curved channel and imposing restrictions on speed, temperature, and concentration for slips. Using a magnetohydrodynamics-based mathematical model, Xenos et al. [
8] investigated blood flow in a stenosis channel under a permanent magnetic field and predicted a decrease in blood velocity, stenosis-based circulation, and a decline in blood pressure with increasing magnetic field intensity. Finally, Ogulu and Abbey [
9] simulated heat transfer in a porous artery with oscillatory blood flow and explored the effects of parameters like porosity, amplitude, and frequency of oscillation on heat transfer mechanisms.
Sen and Chakravarty [
10] proposed a mathematical model to study the transport of heat and mass in blood flows under stenotic conditions across bifurcated arteries. Obdulia Ley et al. [
11] explored the impact of arterial geometry and inflammatory cell distribution on hot spots and blood flow in the plaque region, calculating the averaged temperature distribution of atherosclerotic plaques during the inflammatory process. Golam et al. [
12] numerically investigated the effects of non-Newtonian models on unsteady periodic flows in a 2D pipe with two idealized stenoses, comparing various non-Newtonian blood constitutive equations with the Newtonian viscosity model. Wang [
13] proposed a mathematical model for heat-to-blood flow conversion in a narrow tube, while Liu et al. [
14] studied the modeling of blood flow in pulmonary arterial fluid-structure interactions. They developed a general framework for patient-specific simulations for blood flow fluid-structure interaction, including medical image mesh production, low-order VMS finite element, and boundary conditions. Additionally, they identified five crucial parameters that affect blood concentrations: plasma viscosity, hematocrit, red blood cell adhesion, accumulation of red blood cells, and temperature. The physiological parameters hematocrit and erythrocytes are influenced by changes in blood composition and are significant for non-Newtonian shearing and dilatancy. Khaled and Vafaei [
15] developed a biological heat model for tissue heat transmission based on porous media theory, utilizing thermal transport theory and examining the equation of thermal biology in the transport theory of living tissue. Arterial stiffness has emerged as an important tissue biomarker for cardiovascular risk stratification and biological age, with non-invasive methods available for assessing large artery rigidity [
16]. Understanding the distortion of temperature distribution as a function of vessel diameter is crucial for creating adequate bioheat transport models [
17]. Blood flow can create localized areas of cooling in heated hyperthermic tissues, which is important to note [
18,
19]. High temperatures can lead to irreversible harmful effects on blood proteins and cell death [
20]. Intraoperative monitoring is essential for safe and successful open surgery. Numerical simulations are used by some researchers to track heat transfer and the flux of atherosclerotic plaque in a real physiological system, with or without an atherosclerotic plaque, due to their blood viscosity dependence. Xuelan Zhan et al. [
21] conducted research to investigate the effects of hematocrit (H) and operational ambient temperatures on viscosity, velocity, temperature, and wall shear stress (WSS). Their findings indicate that a plaque in the carotid artery increases blood flow, lowers pressure, WSS, and oscillation, and decreases viscosity and temperature variations. A decrease in ambient temperature lowers the WSS region and increases the risk of atherosclerosis and hypothermia. Foong et al. [
22] conducted a continuous heat flow study and used Newton and other non-Newtonian biomedical approaches to analyze the numerical sequence of blood flow within an artery. The recharging of fluids and electrolytes in bodily capillaries can transform blood flow from non-Newtonian to Newtonian, increase heat transmission into the bloodstream, and lower blood flow. The maximum temperature for non-Newtonian fluid flow is 310,007 K, while the Newtonian fluid flow maximum is 310,0045 Kin for this investigation. These findings illustrate the thermal behavior within the body vessels of the Newtonian and non-Newtonian liquid models. As any change in body temperature from the norm can have adverse health effects, medical science centers, vaccination, and serum institutions must carefully monitor any mechanical design of blood fluid medications.
2. Mathematical Modeling
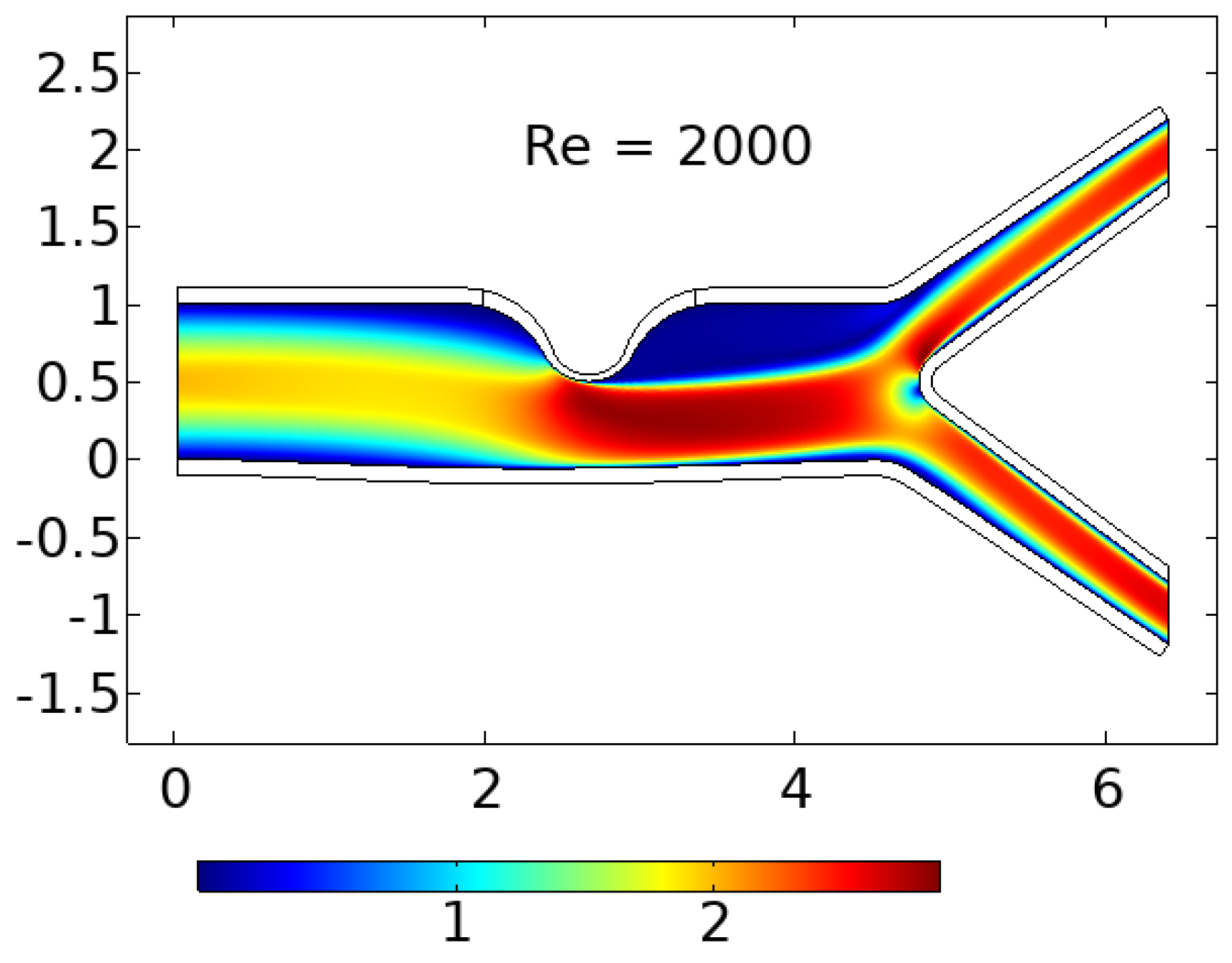
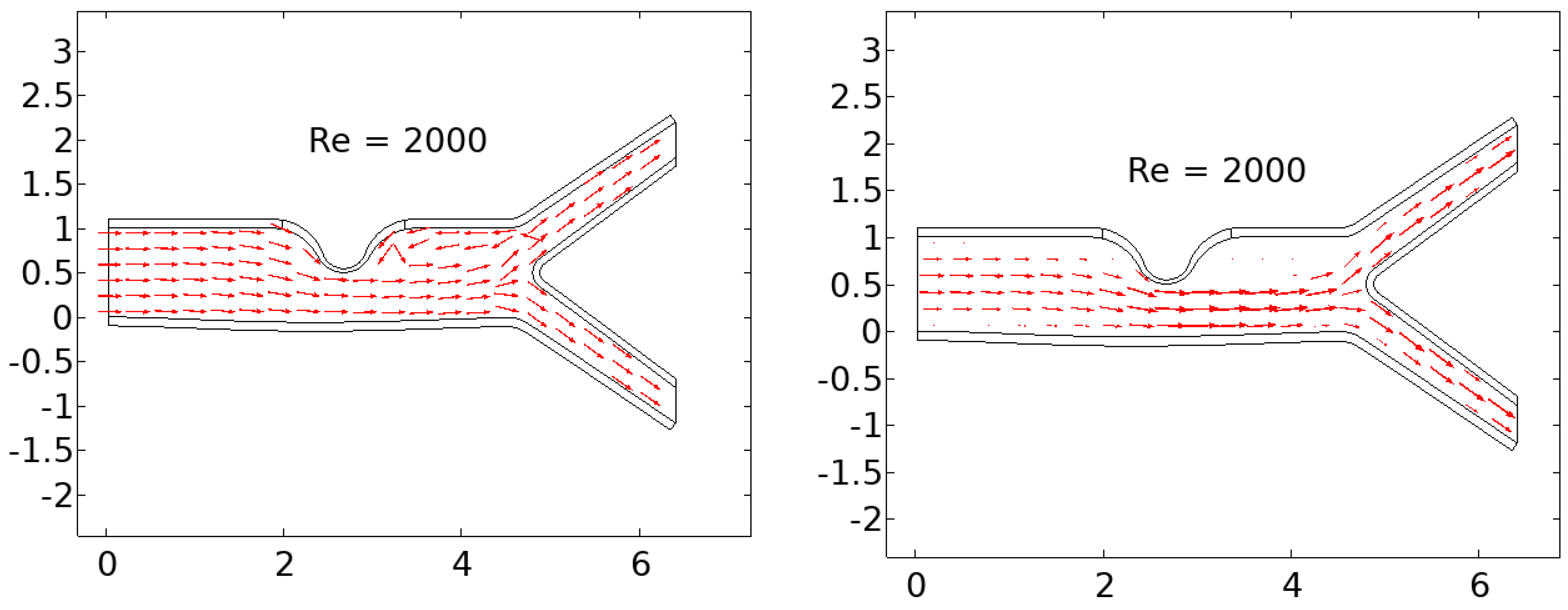
In this study, we are examining the two-dimensional laminar flow of an incompressible viscous fluid in a bifurcated artery with stenosis. To account for the non-Newtonian effect in our analysis, we are utilizing the Casson-Papanastasiou model, which includes an exponential term proposed by Papanastasiou [
27] to remove the requirement for a stress threshold. The regularized Casson model can be expressed as follows:
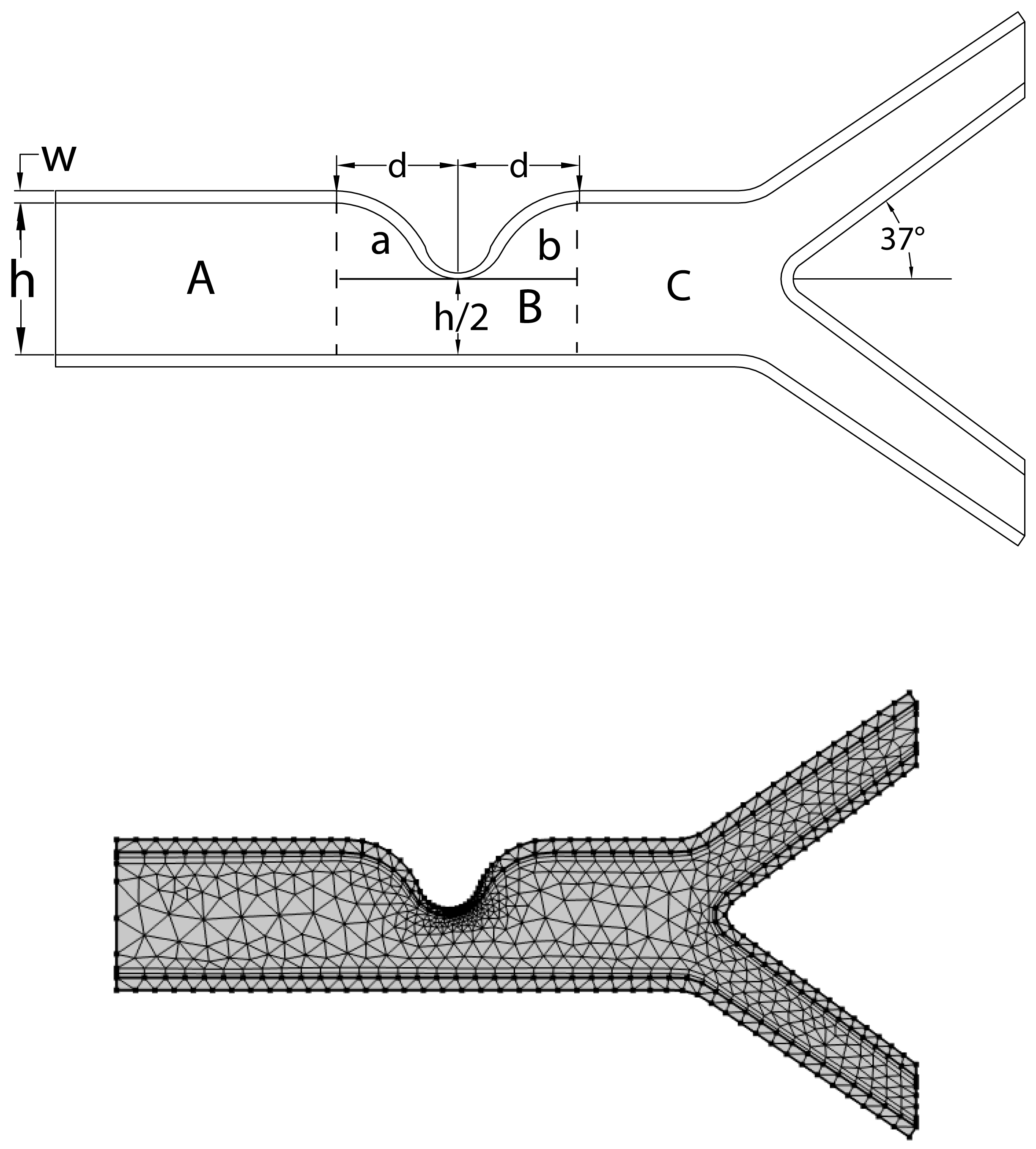
The linearly elastic wall of the arteries is assumed in this study, and the problem geometry and coarse mesh used are depicted in
Figure 1. A parabolic flow inlet is considered, and the pressure at the exit is set to zero. Under these assumptions, the governing equations for flow are expressed in Arbitrary Lagrangian-Eulerian (ALE) formulation, and the conservation of mass is the first equation. Further details on this topic can be found in [
24,
28].
The conservation of momentum is represented by the second equation in the ALE formulation as follows:
The velocity components in dimensional form are represented by
and
, and the dimensional mesh coordinate velocity is represented by
. The volumetric thermal expansion coefficient is denoted by
. The equations that govern the solid component in dimensional form can be expressed as follows:
The stress tensor in its dimensional form is denoted by
, and any external body force acting on the solid is represented by
. The equations governing the blood vessel wall interface are subject to no-slip boundary conditions, which can be mathematically expressed as follows:
the normal component of the stress tensor at the wall interface is represented by
, while
denotes the pressure,
is the dynamic viscosity of the fluid, and
indicates the gradient of the velocity components with respect to the spatial coordinates. Considering a linearly elastic wall, the strain tensor can be expressed as:
The determinant of F, denoted by J, can be defined as . The strain tensor, denoted by , is related to the second Piola-Kirchhoff stress tensor S through , where C is the elasticity tensor. The strain tensor is expressed as follows:
’where
F is the deformation gradient tensor,
is the displacement vector, and
is the Kronecker delta symbol.
The non-dimensional variables can be obtained from their dimensional counterparts by scaling them with appropriate characteristic quantities as follows:
the maximum velocity of blood at the inlet is represented by
, while the diameter of the artery is denoted as
h. The Reynolds number, denoted by
, is a dimensionless parameter used to describe the flow of a fluid. Using the dimensionless parameters given in equation (
9), we can express equation (
5) as:
In the context of linearly elastic walls, the strain tensor, denoted by
, can be expressed as:
the determinant value of
F is represented by
J, where
F is defined as
. The second Piola-Kirchhoff stress tensor
S can be related to the strain
using the equation
, where
, with
E representing Young’s modulus and
representing the Poisson ratio of the solid wall. The strain tensor
is defined as:
Equations (1) to (3) can be expressed using dimensionless parameters as follows:
The boundary condition for the inlet velocity profile is given by equation (
17), where
The outlet boundary conditions are prescribed based on pressure, where the pressure is set to zero at the outlet. The solid domain has a fixed point constraint at the start and end, meaning that those points do not move due to flow, while other parts of the domain are free to move. Initially, the velocity and displacement are set to zero.
2.1. Geometrical Configuration
The computational domain considers a prototype geometric model with stenosis and a bifurcation that has a symmetrical arrangement, as illustrated in
Figure 1. The walls are assumed to be composed of linear elastic and isotropic materials that are characterized by Young’s modulus and Poisson’s ratio. The Lame coefficients
and
, as well as the shear modulus
, are related through the following equations:
The value of Poisson’s ratio
determines the compressibility of a structure, with
representing incompressible structures, while
representing compressible structures [
29]. Unless otherwise stated, the Young’s modulus
E is set to
and the Poisson’s ratio
is set to
. The diameter of the artery is 1 mm and it shrinks to
at the region of stenosis, indicating that stenosis restricts the artery about
of the time. The elastic wall’s width is estimated to be
cm. The bifurcation artery has an inclination of
. The central line along which pressure is recorded is denoted by
C, while points A and B are chosen to anticipate the behavior of the velocity profile prior to and following stenosis. Additionally, a coarse mesh consisting of triangular components, as shown in
Figure 1, is used to represent the geometry.
4. Summary and Conclusion
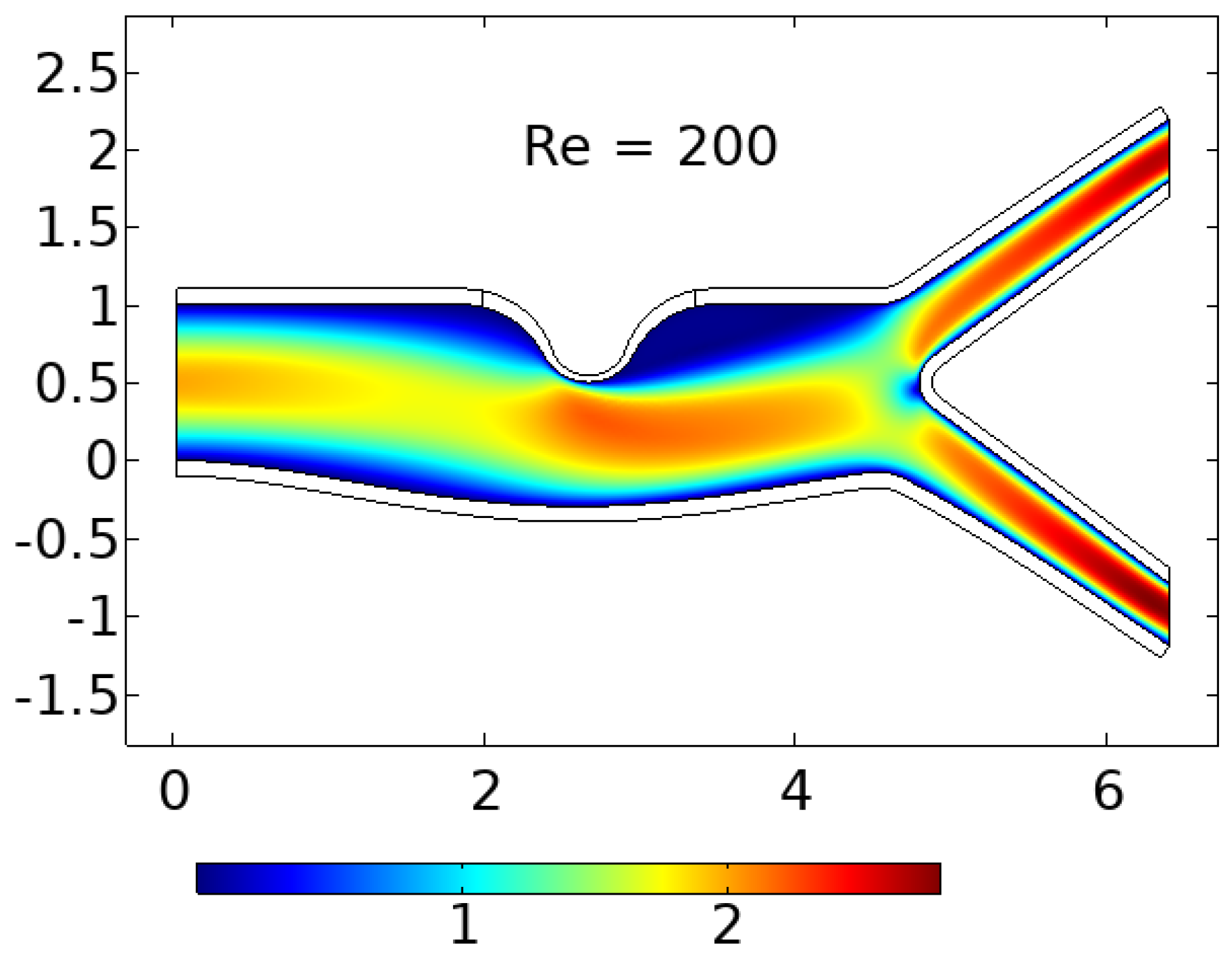
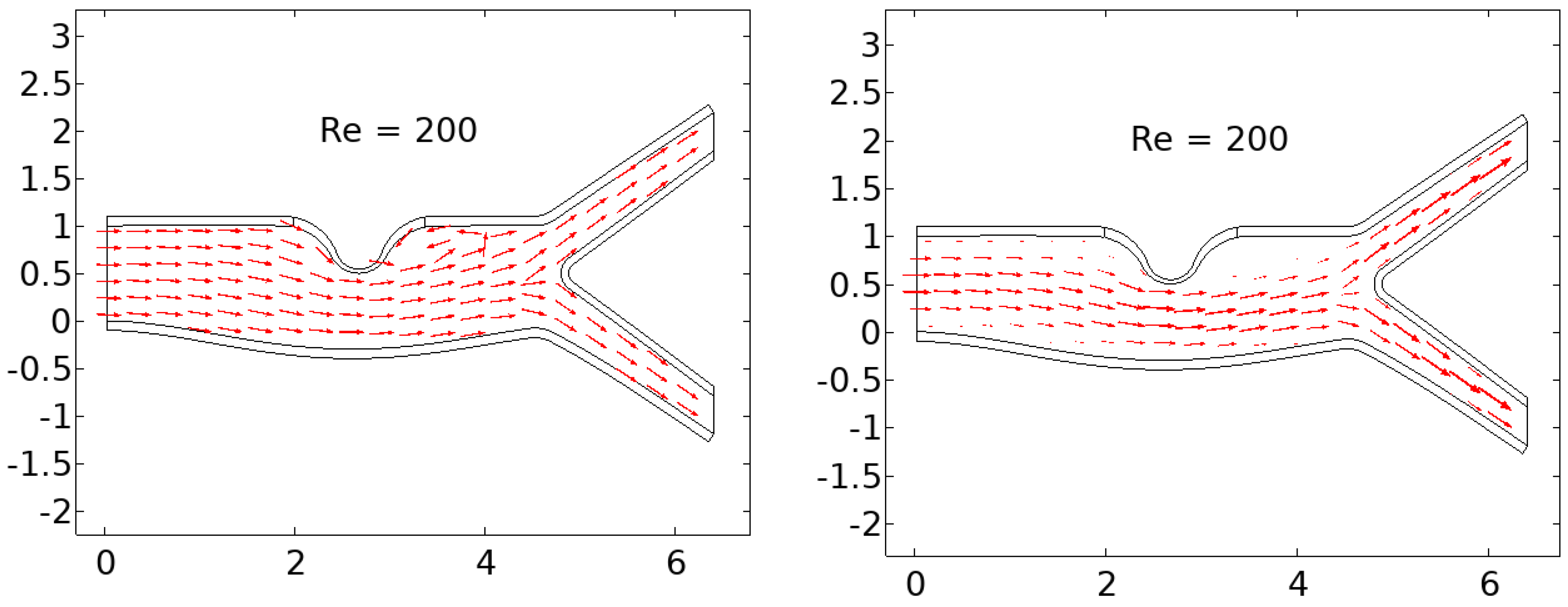
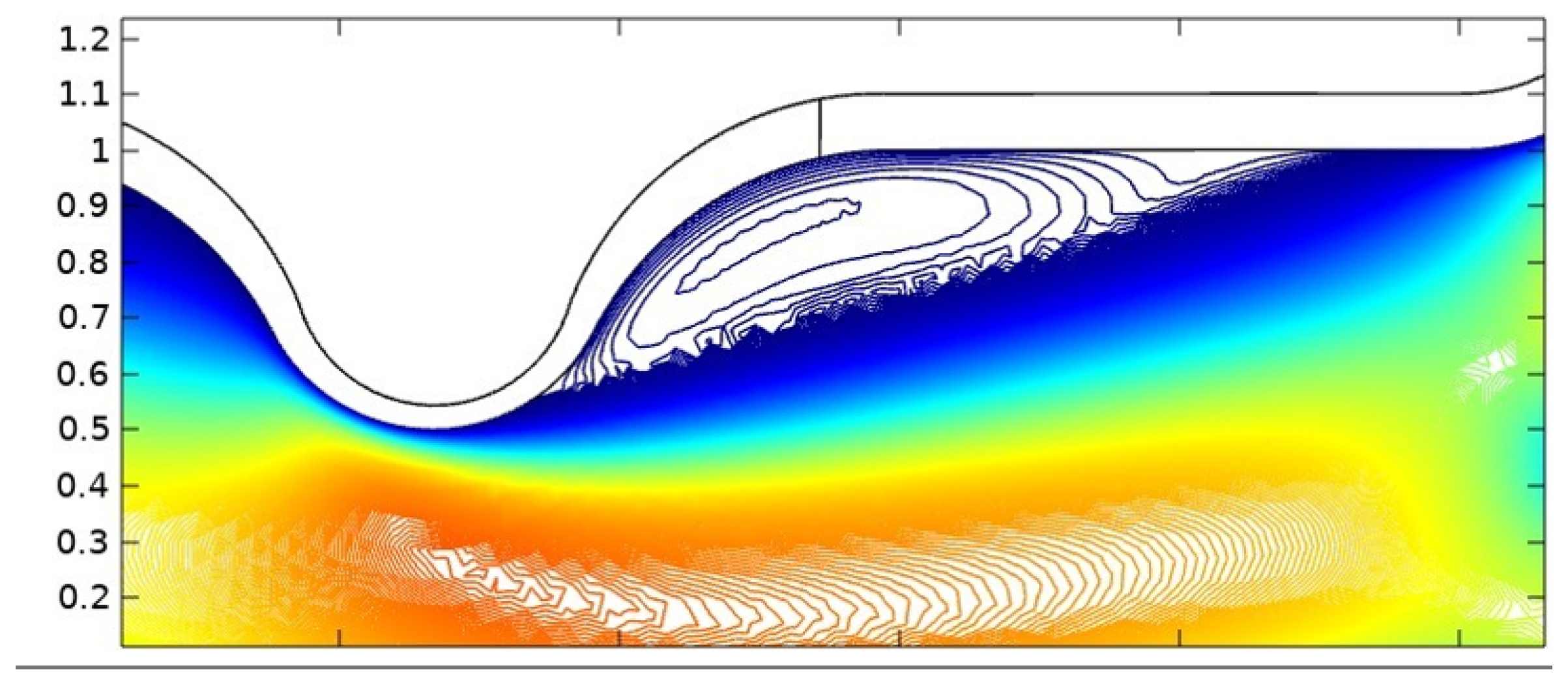
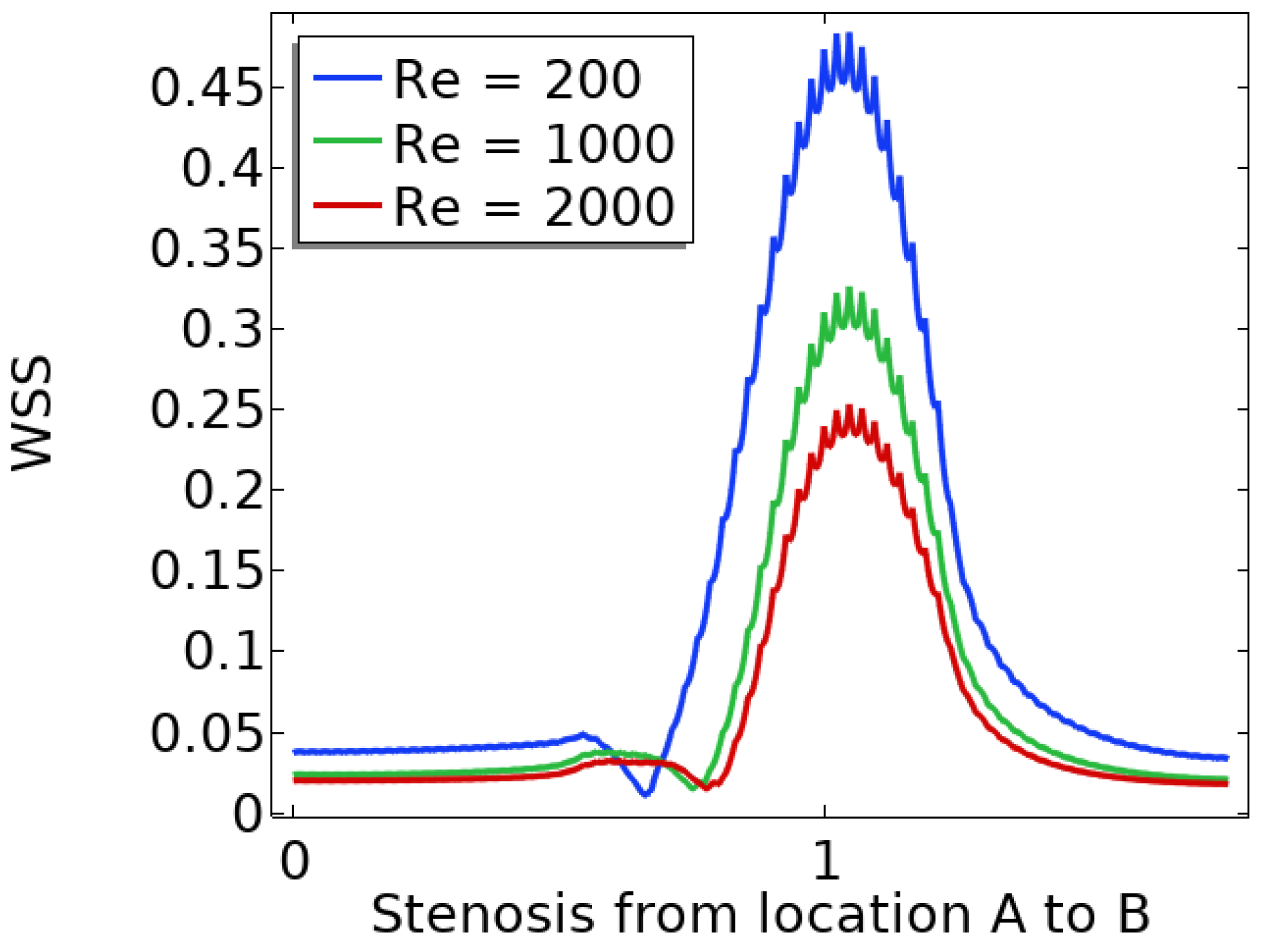
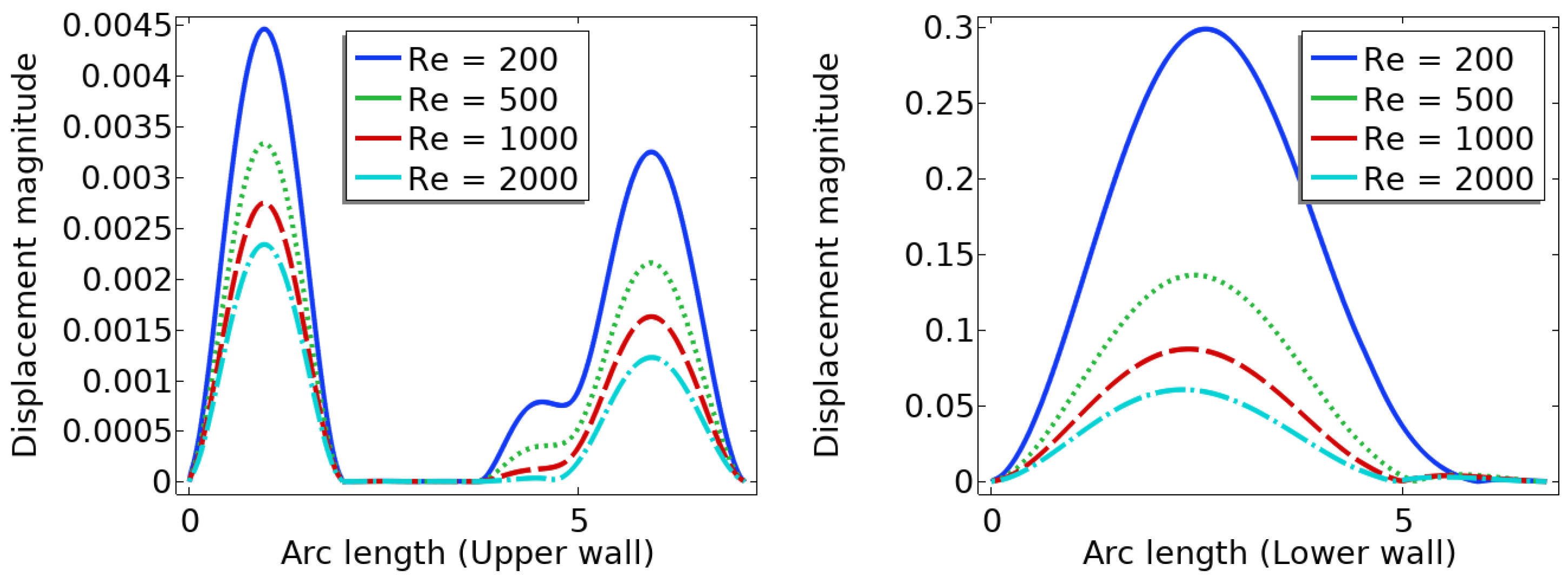
In summary, the blood flow in a stenosed artery can significantly impact the development and progression of atherosclerosis. Key factors to observe when analyzing blood flow behavior are high shear stress and wall displacement. Shear stress caused by the high blood flow velocity in the narrowest region or cap of the plaque can cause the cap to fracture, leading to thrombosis. Wall displacement or deformation caused by different elastic modulus at the stenosis and other wall can decrease as the Reynolds number increases. Wall shear stress can also help reduce the recirculation area in a stenosed artery. As the Reynolds number decreases, the magnitude of WSS increases, reducing the chance of atherosclerosis.
The computer model and simulation results demonstrated significant changes in blood flow dynamics as it moves through the stenotic region of the artery. These changes include the expansion of the arterial wall, modifications in the velocity profiles, and the presence of blood stagnation and backflow. These changes may lead to the formation of highly dynamic energized hot spots (DEHS), leading to the development of a larger plaque, further narrowing the artery lumen. Additionally, the simulation results revealed that high shear stress at the narrowest part of the artery, located at the cap of the plaque, may cause it to rupture.
In conclusion, understanding the behavior of blood flow in a stenosed artery can aid in the prevention and treatment of atherosclerosis. Factors such as shear stress and wall displacement should be considered when analyzing the risk of atherosclerosis in a patient. Future research could focus on developing more accurate and comprehensive models of blood flow behavior in a stenosed artery, as well as investigating new methods for preventing and treating atherosclerosis.