1. Introduction to Beer, Hops, Processing and Brewing:
Humulus lupulus, hops, are a foundational beer ingredient that have been used in brewing for over 1000 years. Even then, early brewers understood the important contribution of hops to beer quality and stability. The addition of hops also meant a second vessel was required for the boiling of hops, whereas previously all beer was typically brewed with one vessel.
In the Pacific Northwest growing region alone, hop production increased 11 percent with a total of 52.6 million kilograms harvested in 2021. Similar trends were recorded worldwide (USDA, 2021). American beer production and consumption rose above 27.5 million hectoliters in 2020 (Brewers Association, 2021). As these industries grow exponentially, understanding hops and hop-related issues is of more interest than ever before.
One such pivotal issue that has recently gained industry attention is hop creep, a process where starch-degrading enzymes that persist in or on hop material cleave residual dextrins in the beer creating more simple sugar. A study in 2018 verified alpha amylase, beta amylase, limit dextrinase, and amyloglucosidase were present in hop samples (Kirkpatrik & Shellhammer, 2018). In 2021, another study postulated that the starch-degrading enzymes responsible for hop creep could originate from microbes rather than from the hop plant itself (Teraoka, Kanauchi & Bamforth, 2021). This challenges the body of previous studies on the enzymes being hop derived and raises a question about which microbes commonly exist on hops and which of those populations are able to persist beyond hop processing and into the brewery.
Hop creep has existed for centuries but has only recently transformed into a defining issue for the brewing industry. The popularity of the New England IPA (NEIPA) in recent years has generated two notable developments in hop production and processing that have unintentionally contributed to hop creep’s prevalence. The first is a vast increase in the quantities of hops being added to beer; the second is a deliberate lowering of kiln temperatures during hop processing, in order to preserve the volatile oils responsible for final aromas in beer. This decrease in temperature could unintentionally result in more microbes persisting on the plant, as well as lower denaturation of starch degrading enzymes. Hops have been historically considered to possess antimicrobial mechanisms that protect beer from spoilage (Behre, 1999). Accordingly, commensal microbes on hop cones are relatively unexplored and any microbes that can inhabit hops would be of exceptional interest.
2. Hops, Processing, and Brewing
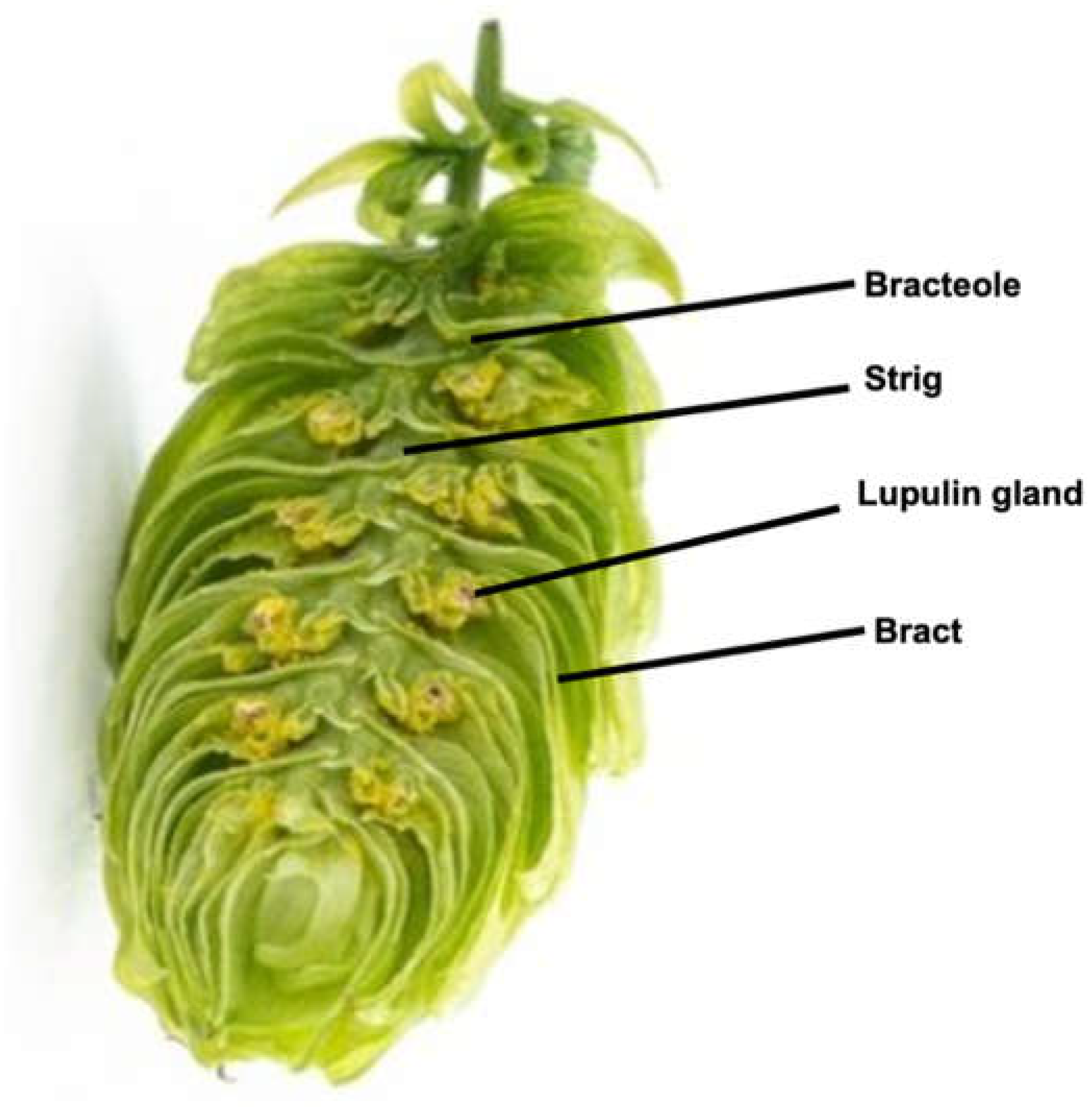
Humulus lupulus is a perennial climbing plant that grows in long vines, called bines, on trellises. Its flowers, strobiles, are better known as hop cones and are harvested for use in the brewing process. Hop cones have four main parts: the bract, bracteole, strig, and lupulin glands
(Figure 1).
Studying individual components is complicated, as the hop compositions differ not only between varieties of hops, but across years and terroirs. Hops contain a variety of compounds, but the lupulin produced by the lupulin glands (depicted in
Figure 1) is the most significant structure with respect to hops’ antimicrobial activity. A large part of the lupulin are resins and oils responsible for bitterness and aroma attributes in the finished beer, respectively. The resin component can be further divided into soft (soluble) and hard (insoluble) resins with other sub-classifications that have not been entirely characterized (Almaguer, Schönberger, Gastl, Arendt & Becker, 2014). These resin fractions are types of complex acids that are classified by their solubility and polarity attributes; they become the bittering compounds linked to beer’s antimicrobial properties. As far back as 800 AD, German monasteries came to understand hops’ inherent stabilizing abilities. Brewing monks noticed and recorded that the addition of hops preserved their beers longer for travel (Behre, 1999).
Now we understand why. Pure-form alpha acids found within the hop plant are antimicrobial; alpha acids become the primary bittering agent within the resins, which ultimately provide microbial stability within the beer (Buggey, Price & Stapely, 2001). The pre-isomerized alpha acids can be used directly, or the antimicrobial traits can become even more effective as hops are isomerized during the boil process in brewing. These iso-alpha acids are more hydrophobic and have better antimicrobial capabilities against Gram-positive bacteria. (Simpson, 1993).
Classic west coast India pale ale (IPA) recipes used approximately one or two pounds of hops per barrel of beer. Comparatively, the newer NEIPA style uses far more hops, generally in the range of four to five pounds per barrel, often translating to over 136.1 kg of hops in a single 35.8 hectoliter (30 barrel) tank. This increase in hop concentration, which can essentially be considered enzyme concentration, can create conditions that exacerbate hop creep to an unprecedented degree. Hop creep generates multiple negative impacts for brewers that can happen in the fermentation vessel, or worse, in the package. The first issue is a resurgence of diacetyl, which is a beer off-flavor characterized by an intense butter aroma. Diacetyl is a natural biproduct of the fermentation process which yeast generally metabolize at the end of fermentation. The second consequence is a drier, further attenuated beer with higher alcohol per volume than intended. The third is extended tank timelines due to longer fermentations. The final impact is increased CO2, which is a serious safety issue in the package, as it can surpass the pressure limit and explode.
Microbes that have the potential to cause hop creep must also endure hop processing. For processing, hops are picked during a very short harvest window of about 30 days. They are quickly removed from the bines and separated from their leaves and stems. While some breweries opt for whole-cone hops as shown in
Figure 1, most use a pelletized product called T-90. The cones are dried in a kiln to reduce the moisture content from about 80% to between 7-12%, then baled and stored in a cold room until pelletization. Pelletization entails breaking up the bale, grinding the hop matter, and then forcing the ground hops through a die. This moment creates high pressures and high temperatures up to 5
5°C (Barth Haas, 2020). Finally, the pellets are cooled, bagged, and stored in an inert gas environment without oxygen. Decreasing moisture content, high temperature and pressure of pelletization, and the specialized storage conditions all aid in preventing and eliminating microbes on hop pellets.
To achieve the isomerization of alpha acids and proper bittering, hops must be added to the boiling stage of wort production on the brewhouse. Alpha acids typically isomerize into iso-alpha acids after at least 60 minutes in the boil. Hops added after this point do not add appreciable amounts of bitterness and therefore contribute less antimicrobial iso alpha acids. Dry hopping, the process of adding hops into the fermentation vessel either during or after fermentation has completed, is primarily used to achieve intense aromas in the beer.
Placing pelletized raw plant material into the fermentation vessel could seem like a cause for concern, but studies have shown that beer is not a favorable environment for most microorganisms. Guinard et al. found that microorganisms, primarily bacteria, and some wild yeast, still exist on both whole cone and pelletized hops (Guinard, Woodmansee, Billovitis, Hanson, Gutierrez, Snider & Lewis, 1990). However, the same study also concluded that dry hopping is not a high-risk process, because none of those microbes can persist in beer for more than three days after dry hopping. Beer is commonly seen as a highly unfavorable environment that can even be stressful for brewer’s yeast, let alone other microorganisms. It contains low levels of carbon sources, high ethanol (5-15%ABV), high CO2(4-5g/L), low pH (3.8-4.8) and a large population of Saccharomyces that outcompetes other organisms (Guinard et. al., 1990).
Despite both the intense processing that hops undergo and the inhospitable beer environment, spoilage can still happen. Infection of a fermentation vessel can occur from improper sanitation processes, scratches in stainless steel that can harbor microorganisms, or from the addition of a non-sterile ingredient, such as fruit.
2. Microbial Communities and Hop Disease
Bacteria, fungi, viruses, and viroids are all found to inhabit the hop plant; some of these microbes cause disease of the plant. The inhabitable region of a plant for microbes is broken into three regions: phyllosphere, rhizosphere, and the endosphere. The cones are within the phyllosphere which is the aerial component of the plant and will therefore be the focus. The phytomicrobiome on hops contains bacteria, fungi, and viruses; not all are necessarily harmful or disease-causing. This review centers on bacteria and fungi, as they have the ability to use multiple carbon sources and not only inhabit plants epiphytically, but also endophytically. Viruses and viroids do not have the ability to produce their own enzymes and therefore cannot contribute to hop creep.
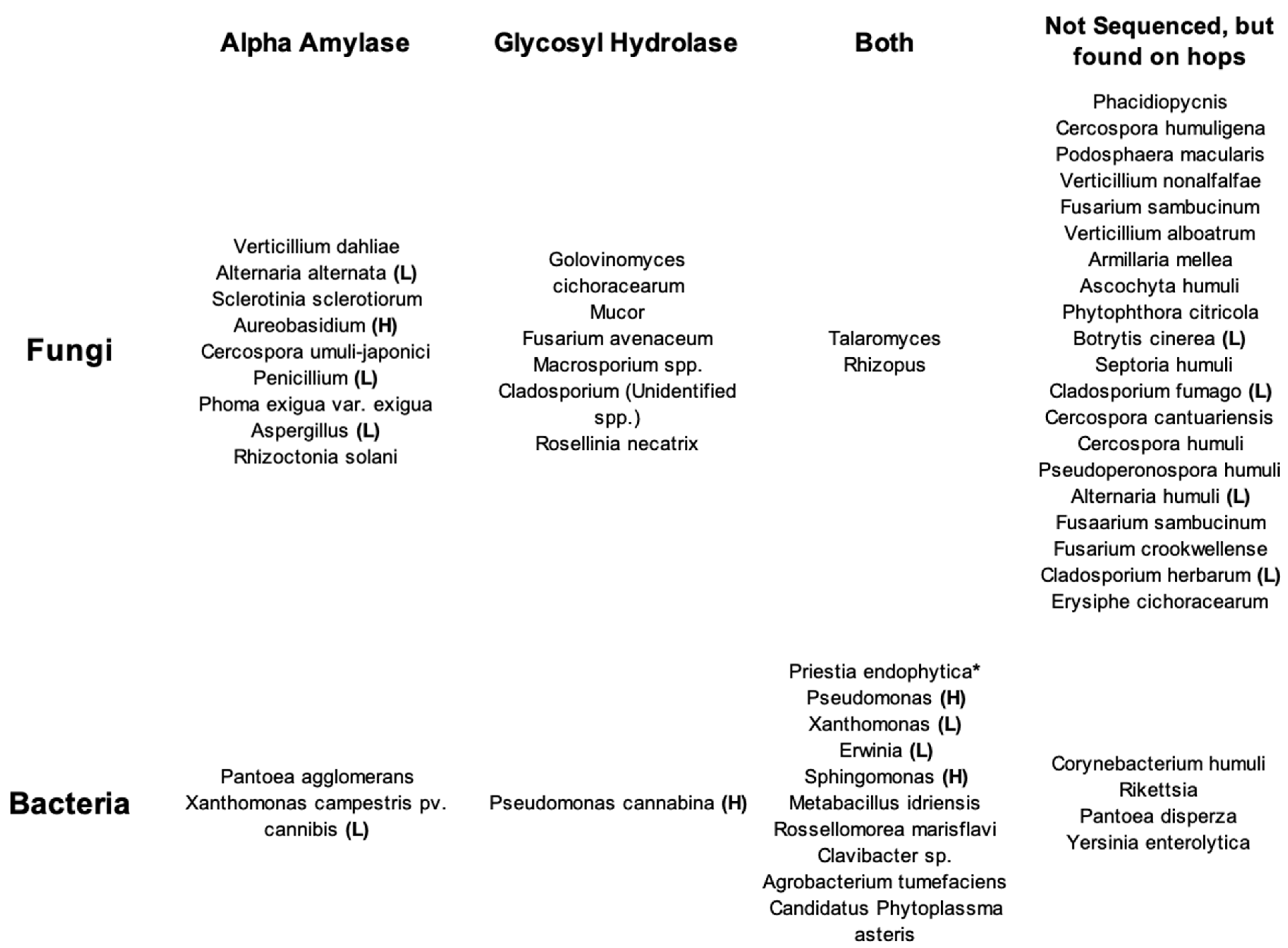
A compendium of known species found on hop plants is below in
Table 1. Most of these microbes do not survive processing and have traditionally been of little concern for brewers as determined previously (Guinard et. al., 1990). Where these organisms do matter is when considering hop agronomics or identifying candidate sources for the starch-degrading enzymes active during hop creep. Although there are common diseases found on several plant species, such as powdery mildew (
Podosphaera macularis), the microbes that infect the hops are specific to
Humulus lupulus as they must possess defenses against the antimicrobial activity associated with the lupulin glands’ alpha acids. A closer look at the microbes able to not only persist through processing and brewing, but also produce dextrin-degrading enzymes can illuminate hop creep perpetrators. Cross-referencing
Table 1 with genome databases revealed whether each microbe’s DNA had been sequenced and if the microbes’ genetic material could possibly express the enzymes responsible.
The microbes listed in
Table 1 are divided based on whether they have been sequenced and found to contain any of the enzymes responsible for hop creep. Interestingly, many do contain both alpha amylase and glycosyl hydrolase family enzymes. The glycosyl hydrolase family can contain limit dextrinase, beta amylase, and amyloglucosidase, thereby requiring further delineation between specific enzymes within the family.
Table 1 includes many hop creep candidates, but a star is placed next to
Priestia endophytica (Bacillus endophytica) because it alone contains alpha amylase, beta amylase, and other glycosyl hydrolases; no other species has attained that level of specificity thus far.
This is a promising start to isolating a single or several species responsible for hop creep, provided the species in question commonly grow on plants. If not, they are unlikely to be the culprit. Hop creep occurs with hop varieties from around the world and with every hop variety so the microbe(s) would need to recur throughout the world’s hop-growing regions. A previous review elucidated many microbes common to flower surfaces and if present, how abundant they are (Kirkpatrik, & Shellhammer, 2018). Microbes that inhabit flower surfaces must have the ability to transfer to other surfaces because of the short-lived nature of flowers. This usually happens via insects but can be done by the wind as well.
Table 1 delineates microbes that have been identified on flowers outside of hops with (H) for high abundance and (L) for low abundance. Thus, the two microbes found on hops at high abundance, and containing both alpha amylase and glycosyl hydrolases, are
Pseudomonas and
Sphingomonas.
An important disease-causing microbe is Podosphaera macularis, the main fungus responsible for powdery mildew disease. The first instance of powdery mildew on hops in the United States was observed in Washington, one of the most important hop growing regions, in June of 1997 (Reeves, 1999). The disease can transmit rapidly, which caused major losses for the hop industry as it spread through the rest of the Pacific Northwest during a single growing year. This fungus is detrimental to hop-growing regions not only because of the disease itself but also how quickly it can spread from farm to farm. Powdery mildew causes the crop to be rejected because of poor cone quality and can lead to complete crop failure (Grenier, Hansen, Harmon, Hirawaki, Horofker, Hughes & Sheer, 2019).
Podosphaera macularis. can grow on all green tissues of hops: cones, leaves, and bines. The visual powdery symptom contains hyphae, conidiophores, and conidia, labeled in
Figure 3. All three structures are significant to growth and reproduction. The fungus grows primarily on the surface of the plant. However, it completely ruins the cones for brewing purposes, with mycelia growing out of the bract as shown on the cone in
Figure 2. The fungus penetrates the epidermal cells to form haustoria, structures which absorb nutrients and water from the host cell (Weldon, Marks, Gevens, D’Arcangelo, Quesada-Ocampo, Parry & Gadoury, 2021).
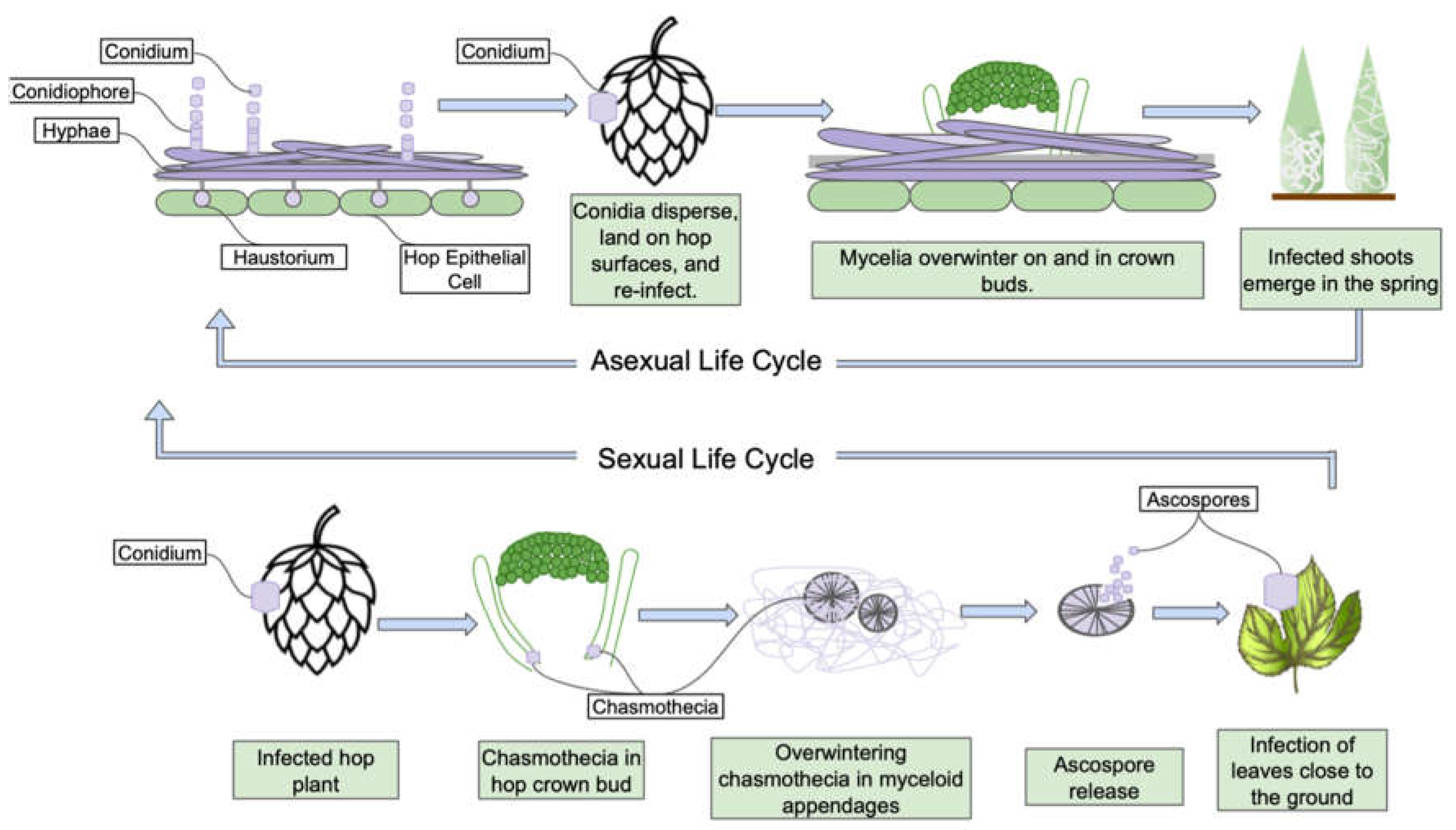
Interestingly, the haustorium is home to several proteins and enzymes made from the fungus and excreted into the plant cell to absorb the plant nutrients. A recent study using genomics and transcriptomics shows other strains of powdery mildew producing cell adhesion proteins, cell wall modification proteins, sugar uptake transport proteins, virulence factors that affect the plant cell membranes, and several other compounds only within this haustorium (Polonio, Seoane, Claros & Perez-Garcia, 2019). It is still unclear if enzymes produced within the haustorium are the same as those responsible for hop creep. In addition to the haustorium’s remarkable properties, powdery mildew can overwinter in the hop plants and spread very quickly throughout a given region the following season.
This fungus reproduces and spreads via two paths: asexually with conidiophores or sexually with chasmothecias. Asexual conidiophores can overwinter as mycelia in the hop bud just below the soil. In the spring, they emerge with the primary root shoots. Sexually, chasmothecias protect ascospores during the overwintering period until the spring when they are released. This lifecycle is depicted in
Figure 3.
3. Beer Spoilage Organisms
With over 175 officially beer styles recorded, understanding and differentiating between them can require an exceedingly nuanced approach (GABF, 2022). Across such a wide variety, hop inputs can range from negligible to nearly six pounds per barrel. Such high concentrations are unprecedented and amount to larger concentrations of enzymes and/or microbes with the enzymes. Moreover, these different styles each encompass slightly different environments in which microbes could persist. The main protection factors for beer include high ethanol, moderately low pH, high dissolved CO
2, low nutrient concentrations, high yeast populations, and high hop bitter acids. Therefore, beer spoilage organisms must be able to live in a low-to-no oxygen environment, endure low pH and high ethanol as well as the hop acids. Beer spoilage is distinguishable by “off” aromas and flavors that can include vinegar, butter, sulfur compounds, and cooked vegetables, along with visible turbidity and viscosity. Unfortunately, the only microbes investigated post-dry hopping are those that cause a negative organoleptic character in the final beer.
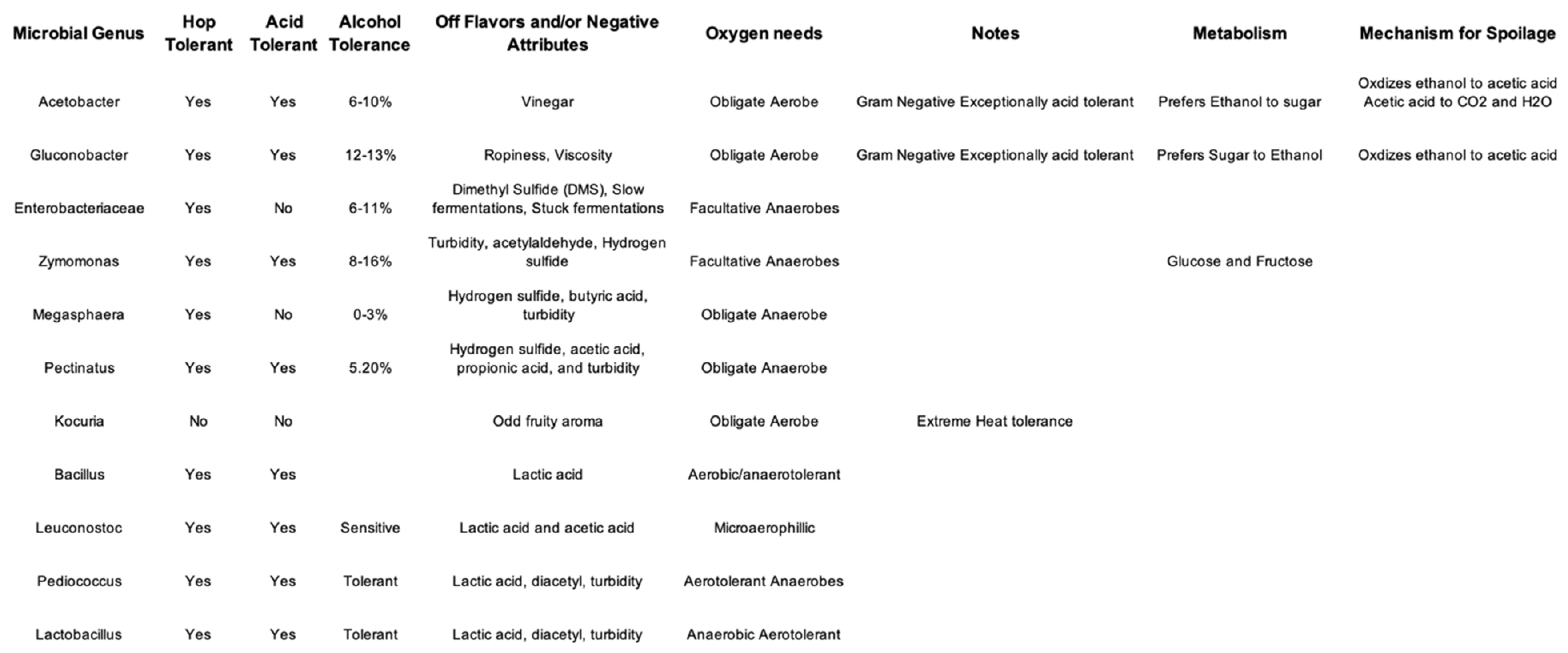
Table 2 outlines the microorganisms known to spoil beer. The most common culprits are lactic acid bacteria, primarily
Lactobacillus and
Pediococcus. The crossover of spoilage organisms and organisms that inhabit hops could also provide clarity on the topic at hand by adding to the list of all possible microbes with potential to be the cause of hop creep. Of additional note,
Bacillus species can be resistant to hop compounds and
Priestia endophytica is (was)
Bacillus.
4. Resistance Mechanisms to Hop Bitter Acids
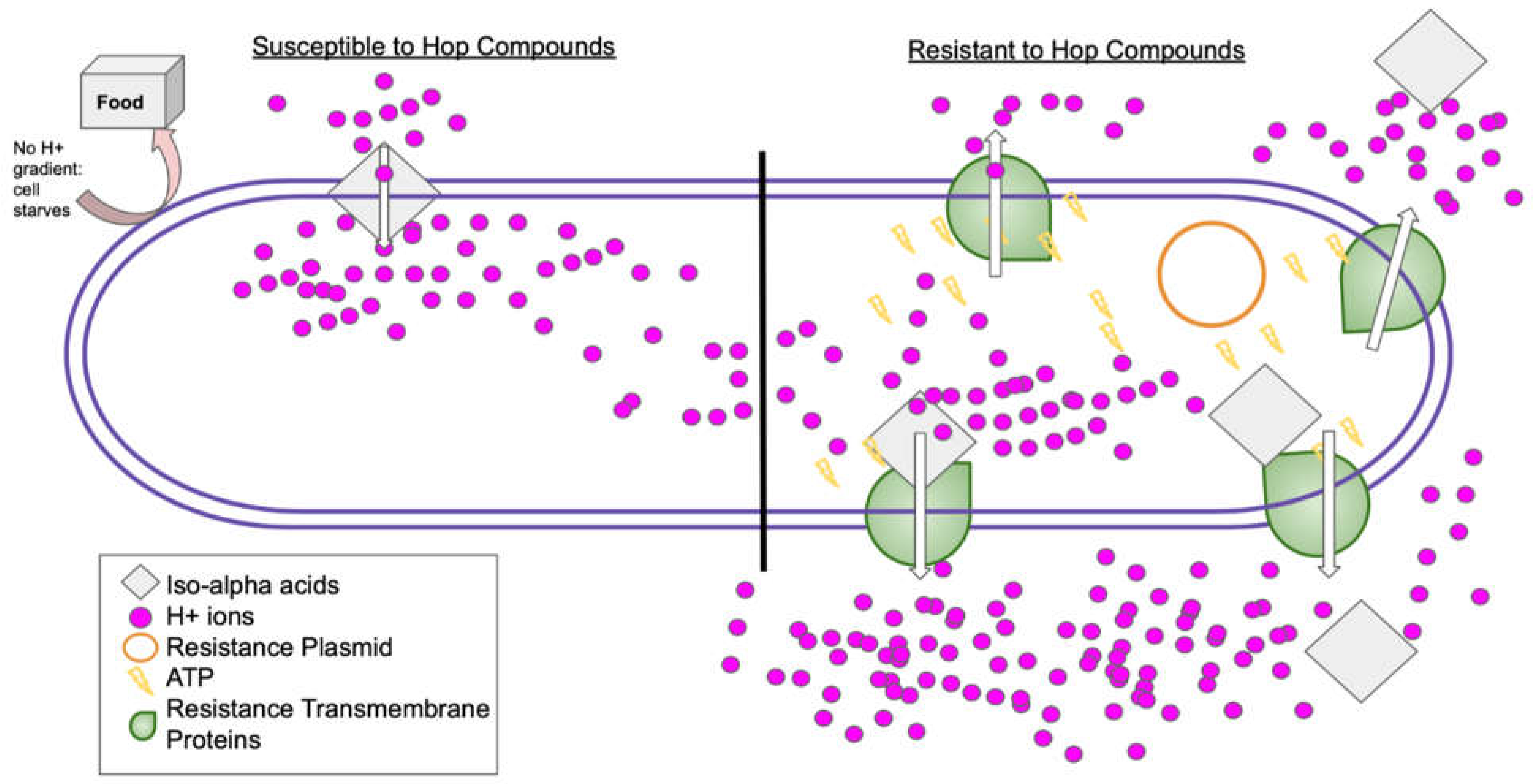
Some of the above spoilage organisms can compete with yeast for sugars or even by utilizing ethanol, but others have evolved a form of resistance involving horizontal gene transfer of a plasmid to overcome the hop iso-alpha acids (Matoulková, Kubizniaková & Sigler, 2021). In
Table 2, the two genera highlighted in yellow have characterized hop resistance from this plasmodial transfer. The exact mechanism responsible for the resistance has not been entirely identified. Fully explaining this resistance requires an additional understanding of bacterial inhibition from the hop iso-alpha acids. Under normal non-beer conditions, the bacteria expel H+ ions to create a proton gradient (Delia, Delvigne, Mabon, Jimboreaan, Forgaarasi, Mihai & Thonart, 2019). Bacteria employ this gradient to subsequently harvest nutrients. The iso-alpha acids (from the lupulin glands) act as ionophores that allow hydrogen ions and the acids themselves to cross the cell membrane, preventing normal nutrient uptake. Additionally, the hop acids enable cations (e.g., Mn
2+) to exit the cell. Interestingly, iso-alpha acids’ antimicrobial properties increase in lower pH environments. The dissociated forms of iso-alpha acids have greater antimicrobial activity, and different pH levels seen in wort fermentation have variable effects on the bactericidal or bacteriostatic activity of those iso-alpha acids. This mostly depends on hop concentration and duration within the wort (Delia et. al., 2019).
Some resistance genes associated with the resistance plasmid are called
horA, horC, and
hitA. horA and
horC genes encode for transmembrane protein pumps that use ATP to expel the undissociated hop compounds and the buildup of protons.
HitA encodes for a different membrane transport protein responsible for up-taking the cations lost (Matoulková, Kubizniaková & Sigler, 2021). Cells with this plasmid are usually characterized by the buildup of ATP in their cytoplasm, as the transmembrane proteins are consistently using it to perform these processes
(Figure 4). These proteins recreate the gradient between the cytoplasm and the extracellular environment, allowing nutrient uptake to occur and the cells to survive. It was further observed that this mechanism is more complex than a few genes simply encoding resistance apparatus. It is highly strain-dependent and the capacity to survive in a beer environment itself depends on many complex factors in addition to hop acid resistance. Bacterial resistance needs several crucial preconditions, including tolerance for ethanol stress, low oxygen, low nutrients, high carbon dioxide, and sulfur as well as the addition of iso-alpha acids.
5. Conclusions and Opportunities for Future Research:
Hop creep continues to present a persistent and problematic issue within the brewing industry, diminishing beer quality and causing safety concerns. The phytomicrobiome, both commensal and disease causing, remains a possible source for an organism behind hop creep-causing enzymes. This review has clarified the species of microbes that hops can host at all stages of their life cycle: from the plant at the farm to the final beer, including hop processing parameters and resistance mechanisms. The next research phase will explore whether the microbes in
Table 1 with high prevalence and both sets of enzymes survive hop processing and make it into the wort. Additionally, research will need to address the possibility of a combination of plant + microbe interactions providing all four enzymes found to be responsible for hop creep.
The microbes able to survive hop processing conditions and the environment within the finished beer, as this paper described, are a topic that also requires additional exploration. Ultimately, the microbes on
Table 1 -
Pseudomonas and
Sphingomonas - provide a starter set of organisms to further investigate as possible biotic causes of hop creep.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
Deepest appreciation and thanks to Dr. Maria Marco, Melanie Hanlon, and Emily Weintraut.
References
- 2020 Craft Brewing Industry Production Report | Brewers Association. (2021). Retrieved April 27, 2022, from https://www.brewersassociation.org/press-releases/2020-craft-brewing-industry-production-report/.
- Agius, G., Funnell, D., & Matteotti, T. (2010). A Systematic Approach to Investigating Microbiological Infection Sources in the Brewing Process. Technical Quarterly, 47(1). [CrossRef]
- Allen, M. E., Piefer, A. J., Cole, S. N., Werner, J. J., Benziger, P. T., Grieneisen, L., & Britton, S. J. (2019). Characterization of Microbial Communities Populating the Inflorescences of Humulus lupulus L. Journal of the American Society of Brewing Chemists, 77(4), 243–250. [CrossRef]
- Almaguer, C., Schönberger, C., Gastl, M., Arendt, E. K., & Becker, T. (2014). Humulus lupulus - A Story that Begs to be Told. Journal of the Institute of Brewing. [CrossRef]
- Asiegbu, F. O., & Kovalchuk, A. (2021). An Introduction to Forest Biome and Associated Microorganisms. In Forest Microbiology (pp. 3–16). Elsevier. [CrossRef]
- Barney, M., & Kot, E. (1992). A Comparison of Rapid Microbiological Methods for Detecting Beer Spoilage Microorganisms. Master Brewers TQ, 29.
- Barth Haas. (2020). Hop Pellets (Type 90 Pellets). Retrieved March 1, 2022, from https://www.johnihaas.com/wp-content/uploads/2020/06/Hop-Pellets-Type-90-Pellets.pdf.
- Behre, K.-E. (1999). The history of beer additives in Europe — A review. Vegetation History and Archaeobotany, 8(1–2), 35–48. [CrossRef]
- Berg, G., & Smalla, K. (2009). Plant Species and Soil Type Cooperatively Shape the Structure and Function of Microbial Communities in the Rhizosphere. FEMS Microbiology Ecology, 68(1), 1–13. [CrossRef]
- Biendl, M. (2009). Hops and Health. Technical Quarterly, 46(2). [CrossRef]
- Brenner, M. W., & Iffland, H. (1966). Economics of Microbial Stabalization of Beer. Master Brewers TQ, 3(3).
- Buggey, L., Price, A., & Stapely, S. J. (2001). The Antibacterial Activity of Hop Compounds. Poster presented at the Brewing Research International.
- Butu, A., Rodino, S., & Butu, M. (2016). The Antimicrobial Activity of Humulus lupulus Depending on Storage Conditions. Biologie Vegetale, 62(2).
- Da Lage, J.-L., Danchin, E. G. J., & Casane, D. (2007). Where do animal alpha-amylases come from? An interkingdom trip. FEBS Letters, 581(21), 3927–3935. [CrossRef]
- Delia, M., Delvigne, F., Mabon, N., Jimborean, M., Fogarasi, M., Mihai, M., … Thonart, P. (2019). View of Inhibitory Effects of Iso-α and β Hop Acids Against Pediococcus pentosaceus. Notulae Botanicae Horti Agrobotanici Cluj-Napoca.
- GABF. (n.d.). COMPETITION STYLE LIST, DESCRIPTIONS AND SPECIFICATIONS. Retrieved February 15, 2022, from Great American Beer Festival website: https://cdn.greatamericanbeerfestival.com/wp-content/uploads/21_GABF_Beer_Style_Guidelines_Final.pdf.
- Geißler, A. (2016). Lifestyle of Beer Spoiling Lactic Acid Bacteria (Doctoral dissertation). TECHNISCHE UNIVERSITÄT MÜNCHEN.
- Gent, D. H., Claassen, B. J., Gadoury, D. M., Grünwald, N. J., Knaus, B. J., Radišek, S., … Wolfenbarger, S. N. (2020). Population Diversity and Structure of Podosphaera macularis in the Pacific Northwestern United States and Other Populations. Phytopathology, 110(5), 1105–1116. [CrossRef]
- Gent, D. H., Nelson, M. E., George, A. E., Grove, G. G., Mahaffee, W. F., Ocamb, C. M., … Turechek, W. W. (2008). A Decade of Hop Powdery Mildew in the Pacific Northwest. Plant Health Progress, 9(1). [CrossRef]
- Gent, D. H., Woods, J. L., & Putnam, M. L. (2012). New Outbreaks of Verticillium Wilt on Hop in Oregon Caused by Nonlethal Verticillium albo-atrum. Plant Health Progress, 13(1). [CrossRef]
- Grenier, S., Hansen, M., Harmon, J., Hirawaki, Y., Horofker, M., Hughes, M., … Scheer, F. (2019). Hop Selection and Elimination of Hop Diseases: Case Study from the 2018 Master Brewers Brewing and Malting Science Course. Technical Quarterly, 55(4), 93–96. [CrossRef]
- Guinard, J. X., Woodmansee, R. D., Billovits, M. J., Hanson, L. G., Gutierrez, M. J., Snider, M. L., ... & Lewis, M. J. (1990). The microbiology of dry-hopping. Technical quarterly-Master Brewers Association of the Americas (USA).
- Haunold, A., Nickerson, G. B., Gampert, U., Whitney, P. A., & Hampton, R. O. (1993). Agronomic and Quality Characteristics of Native North American Hops. Journal of the American Society of Brewing Chemists, 51(3), 133–137. [CrossRef]
- Jorgenson, E. (2017). An Overview of Bacteria Found in Brewing Ecosystems. Technical Quarterly, 54(2). [CrossRef]
- Karabín, M., Hudcová, T., Jelínek, L., & Dostálek, P. (2016). Biologically Active Compounds from Hops and Prospects for Their Use. Comprehensive Reviews in Food Science and Food Safety, 15(3), 542–567. [CrossRef]
- Kiehne, M., Grönewald, C., & Chevalier, F. (2005). Detection and Identification of Beer-Spoilage Bacteria Using Real-Time Polymerase Chain Reaction. Master Brewers Technical Quarterly, 42(3).
- Kirkpatrick, K. R., & Shellhammer, T. H. (2018). Evidence of Dextrin Hydrolyzing Enzymes in Cascade Hops ( Humulus lupulus). Journal of Agricultural and Food Chemistry, 66(34), 9121–9126. [CrossRef]
- Kolek, J., Patakova, P., Junkova, P., Krofta, K., Hynek, R., & Dostalek, P. (2021). Isolation and Identification of Pantoea agglomerans From the Inflated Bag with Dried Hop Pellets Stored Under a Modified Atmosphere. Journal of Applied Microbiology, 131(1), 281–287. [CrossRef]
- Mahaffee, W. F., Pethybridge, S. J., & Gent, D. H. (2009). Compendium of Hop Diseases and Pests.
- Matoulková, D., Kubizniaková, P., & Sigler, K. (2012). Beer-spoiling Ability of Lactic Acid Bacteria and its Relation with Genes horA, horC and hitA. Kvasny Prumysl, 58(11–12), 336–342. [CrossRef]
- McCaig, R., & Weaver, R. (1983). Physiological Studies on Pediococcus. Master Brewers TQ, 20(1).
- Ocamb, C. M., Klein, R., Barbour, J., Griesbach, J., & Mahaffee, W. (1999). First Report of Hop Powdery Mildew in the Pacific Northwest. Plant Disease, 83(11), 1072. [CrossRef]
- Pethybridge, S. J., Hay, F. S., Barbara, D. J., Eastwell, K. C., & Wilson, C. R. (2008). Viruses and Viroids Infecting Hop: Significance, Epidemiology, and Management. Plant Disease, 92(3), 324–338. [CrossRef]
- Polonio, Á., Seoane, P., Claros, M. G., & Pérez-García, A. (2019). The Haustorial Transcriptome of the Cucurbit Pathogen Podosphaera xanthii Reveals New Insights into the Biotrophy and Pathogenesis of Powdery Mildew Fungi. BMC Genomics, 20(1), 543. [CrossRef]
- Probasco, G., & Murphey, J. M. (1996). The Effects of Hop Viruses on Brewing and Agronomic Characteristics in the Hop Variety Chinook. Master Brewers Technical Quaterly, 33(3).
- Purayannur, S., Gent, D. H., Miles, T. D., Radišek, S., & Quesada-Ocampo, L. M. (2021). The Hop Downy Mildew Pathogen Pseudoperonospora humuli. Molecular Plant Pathology, 22(7), 755–768. [CrossRef]
- Reeves, J. (1999). Assessment of Powdery Mildew Control Measures During the 1998 Hop Growing Season. Master Brewers Technical Quaterly, 36(1).
- Sevigny, J. L., Lloyd, B., McComish, C., Ramsey, A., & Koziol, L. (2019). Whole-Genome Sequences of Pantoea agglomerans BL3, Pseudomonas fluorescens BL, and Pseudomonas stutzeri CM14, Isolated from Hops (Humulus lupulus). Microbiology Resource Announcements, 8(30). [CrossRef]
- Simpson, W. J. (1993). Studies on the Sensitivity of Lactic Acid Bacteria to Hop Bitter Acids. Journal of the Institute of Brewing, 99(5), 405–411. [CrossRef]
- Small, E. (1978). A Numerical and Nomenclatural Analysis of Morpho-Geographic Taxa of Humulus. 3(1), 37. [CrossRef]
- Teraoka, R., Kanauchi, M., & Bamforth, C. W. (2021). Do Starch Degrading Enzymes in Hop Samples Originate in Microorganisms. Technical Quarterly, 58(3). [CrossRef]
- USDA. (2021). National Hop Report. USDA, NASS.
- Vannette, R. L. (2020). The Floral Microbiome: Plant, Pollinator, and Microbial Perspectives. Annual Review of Ecology, Evolution, and Systematics, 51(1). [CrossRef]
- Weldon, W. A., Marks, M. E., Gevens, A. J., D’Arcangelo, K. N., Quesada-Ocampo, L. M., Parry, S., … Gadoury, D. M. (2021). A Comprehensive Characterization of Ecological and Epidemiological Factors Driving Perennation of Podosphaera macularis Chasmothecia on Hop (Humulus lupulus). Phytopathology, 111(11), 1972–1982. [CrossRef]
- Zhang, X., Zhang, E., & Lang, D. (2011). Autotoxic Compounds from Rhizosphere Soil of Humulus lupulus L. Extracts: Identification and Biological Activity. Agronomy Journal, 103(3), 695–701. [CrossRef]
Figure 1.
Humulus lupulus Cone: Parts of the hop cone. Note the lupulin glands which are responsible for the antimicrobial properties.
Figure 1.
Humulus lupulus Cone: Parts of the hop cone. Note the lupulin glands which are responsible for the antimicrobial properties.
Figure 2.
Hyphae from Podosphaera macularis: The hyphae are shown growing through all parts of the hop cone (Mahaffee, Pethybridge, & Gent, 2009).
Figure 2.
Hyphae from Podosphaera macularis: The hyphae are shown growing through all parts of the hop cone (Mahaffee, Pethybridge, & Gent, 2009).
Figure 3.
Podosphaera macularis life cycle. Special focus on the haustorium in the top left of the figure. This specialized structure injects into the plant cell and releases enzymes and metabolites. Adapted from V. Brewster (Mahaffee et. al., 2009).
Figure 3.
Podosphaera macularis life cycle. Special focus on the haustorium in the top left of the figure. This specialized structure injects into the plant cell and releases enzymes and metabolites. Adapted from V. Brewster (Mahaffee et. al., 2009).
Figure 4.
Bacterial cell showing two pathways for interaction with iso-alpha acids. Bacterial cell split to show two possible outcomes upon contact with hop iso-alpha acids. The left side shows the hop compounds acting as an ionophore, allowing hydrogen ions to leak into the cell. This eliminates the gradient that the cell uses to import food and in this scenario the cell will starve. The right sideshows the resistance plasmid that would encode for one of the transmembrane proteins with the ability to export the hop compounds as well as expel the built-up hydrogen ions. These are identified by their notable buildup of ATP (Jorgenson, 2017).
Figure 4.
Bacterial cell showing two pathways for interaction with iso-alpha acids. Bacterial cell split to show two possible outcomes upon contact with hop iso-alpha acids. The left side shows the hop compounds acting as an ionophore, allowing hydrogen ions to leak into the cell. This eliminates the gradient that the cell uses to import food and in this scenario the cell will starve. The right sideshows the resistance plasmid that would encode for one of the transmembrane proteins with the ability to export the hop compounds as well as expel the built-up hydrogen ions. These are identified by their notable buildup of ATP (Jorgenson, 2017).
Table 1.
Bacteria and fungi commonly found on hops. Microbes are divided by sequencing status and whether they have genes that encode for alpha amylase, glycosyl hydrolases, or both. (H) and (L) mark high abundance or low abundance, respectively. (Allen, Piefer, Cole, Werner, Benziger, Grieneisen & Britton, 2019; Asiegbu & Kovaalchuk, 2021; Berg & Smalla, 2009; Gent, Classen, Gadoury, Grünwald, Knaus, Radišek & Wolfenbarger, 2020; Kirkpatrick & Shellhammer, 2018; Kolek, Patakova, Junkova, Krofta, Hynek & Dostalek, 2021; Mahaffee et. al., 2009; NCBI; Protein Data Bank; Pethybridge, Hay, Barbara, Eastwell & Wilson, 2008; Sevigny, Lloyd, McComish, Ramsey & Koziol, 2019; Vannette, 2020).
Table 1.
Bacteria and fungi commonly found on hops. Microbes are divided by sequencing status and whether they have genes that encode for alpha amylase, glycosyl hydrolases, or both. (H) and (L) mark high abundance or low abundance, respectively. (Allen, Piefer, Cole, Werner, Benziger, Grieneisen & Britton, 2019; Asiegbu & Kovaalchuk, 2021; Berg & Smalla, 2009; Gent, Classen, Gadoury, Grünwald, Knaus, Radišek & Wolfenbarger, 2020; Kirkpatrick & Shellhammer, 2018; Kolek, Patakova, Junkova, Krofta, Hynek & Dostalek, 2021; Mahaffee et. al., 2009; NCBI; Protein Data Bank; Pethybridge, Hay, Barbara, Eastwell & Wilson, 2008; Sevigny, Lloyd, McComish, Ramsey & Koziol, 2019; Vannette, 2020).
Table 2.
Organisms responsible for beer spoilage. The final two rows are important due to their increased resistance to hop iso-alpha acids from hit and hor genes. (Agius, Funnell & Matteotti, 2010; Barney & Kot, 1992; Brenner & Iffland, 1996; Geißler, 2016; Jorgenson, 2017; Kiehne, Grönewald & Chevalier, 2005; McCaig & Weaver, 1983; Zhang, Zhang, Lang, 2011).
Table 2.
Organisms responsible for beer spoilage. The final two rows are important due to their increased resistance to hop iso-alpha acids from hit and hor genes. (Agius, Funnell & Matteotti, 2010; Barney & Kot, 1992; Brenner & Iffland, 1996; Geißler, 2016; Jorgenson, 2017; Kiehne, Grönewald & Chevalier, 2005; McCaig & Weaver, 1983; Zhang, Zhang, Lang, 2011).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).