Submitted:
07 March 2023
Posted:
08 March 2023
You are already at the latest version
Abstract
Keywords:
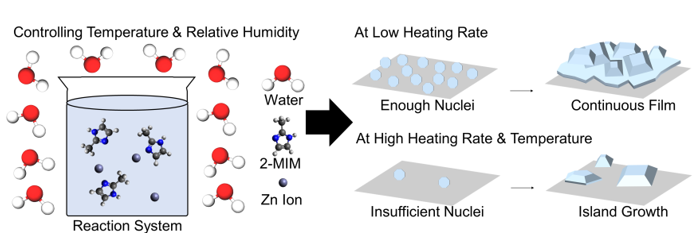
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZIF-8 Layer with Different Humidity and Temperature

2.3. Characterization
3. Results and Discussions
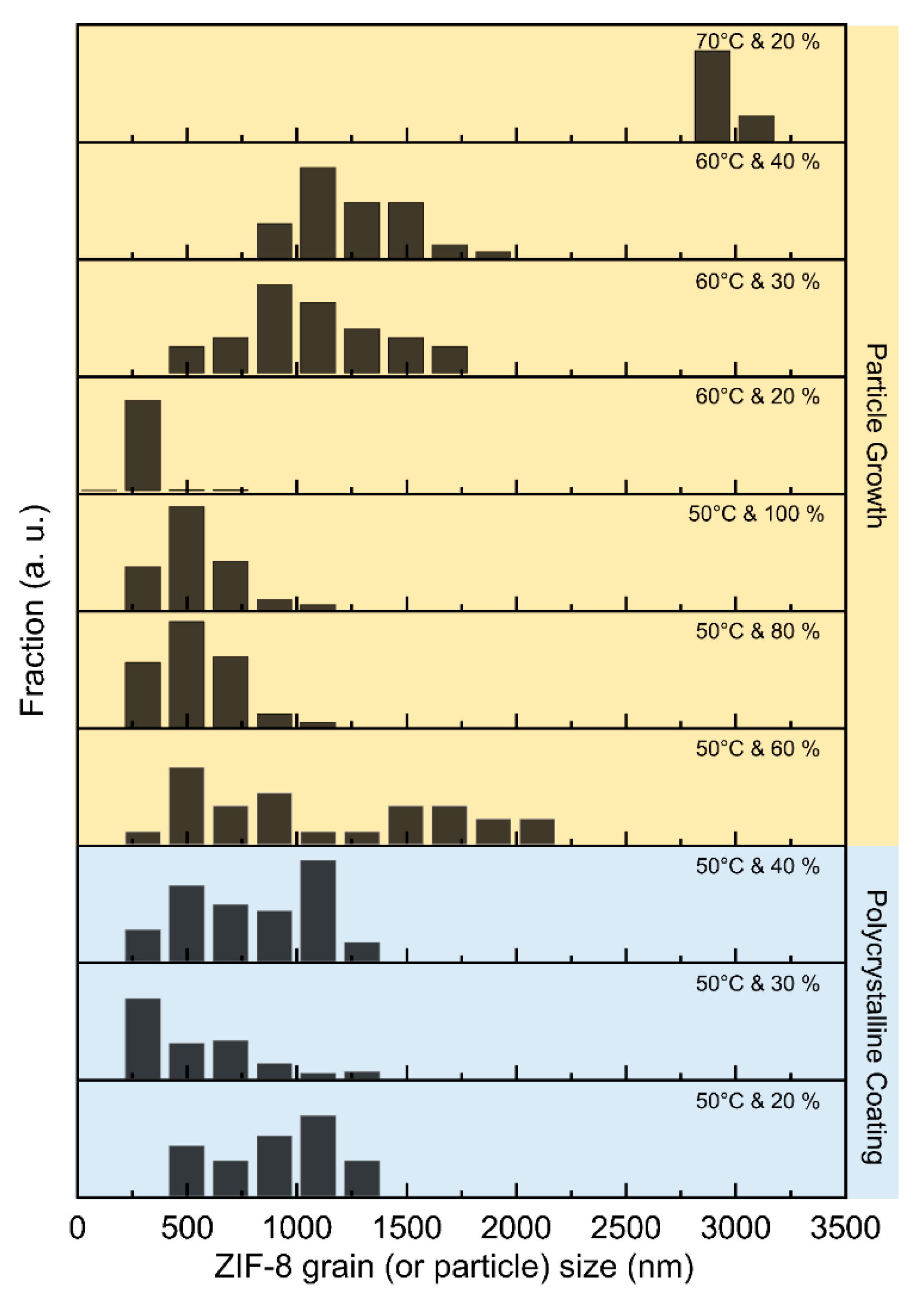
3.1. Morphology of ZIF-8 Crystal Depending on Chamber Temperature and Relative Humidity
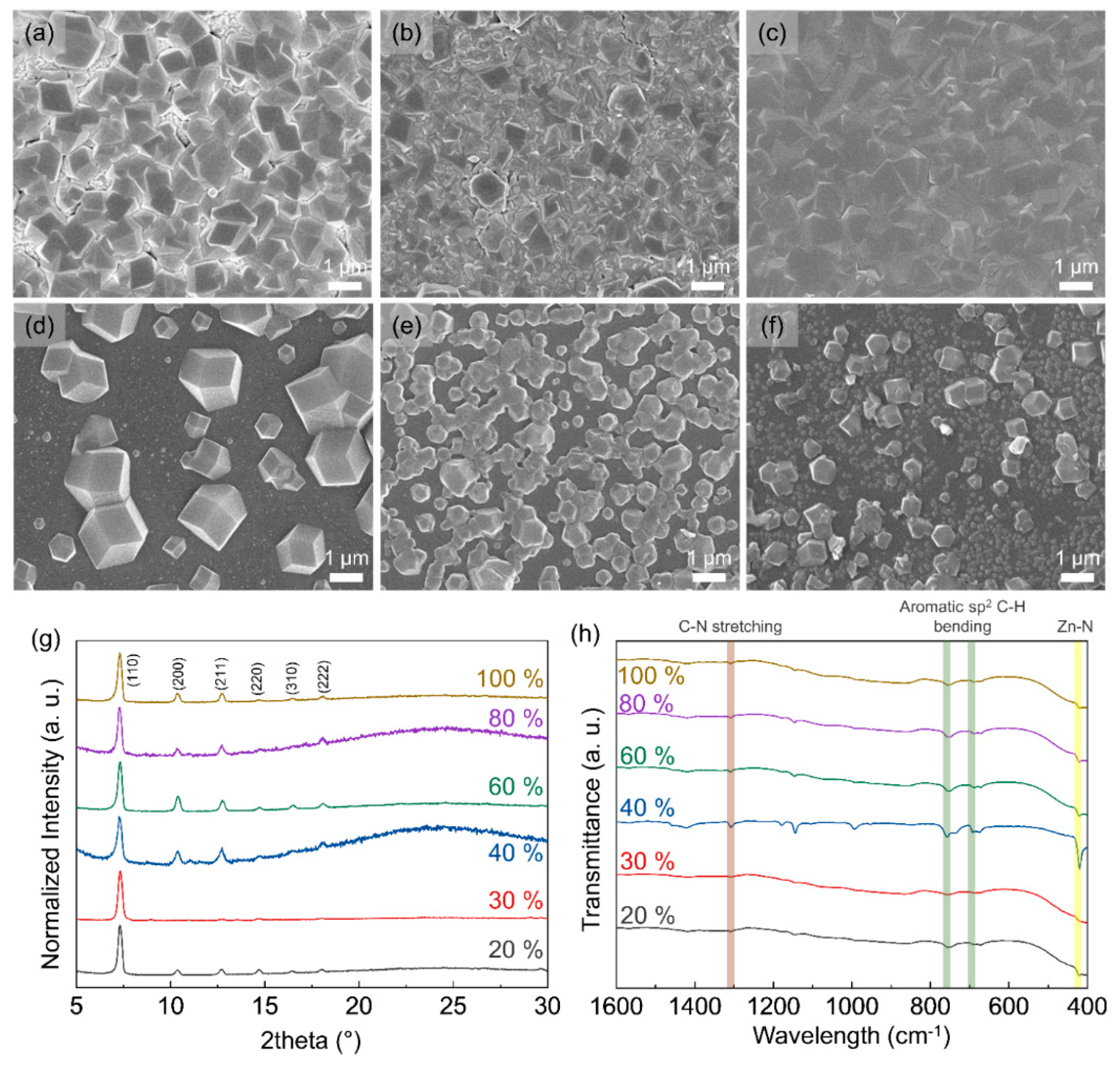
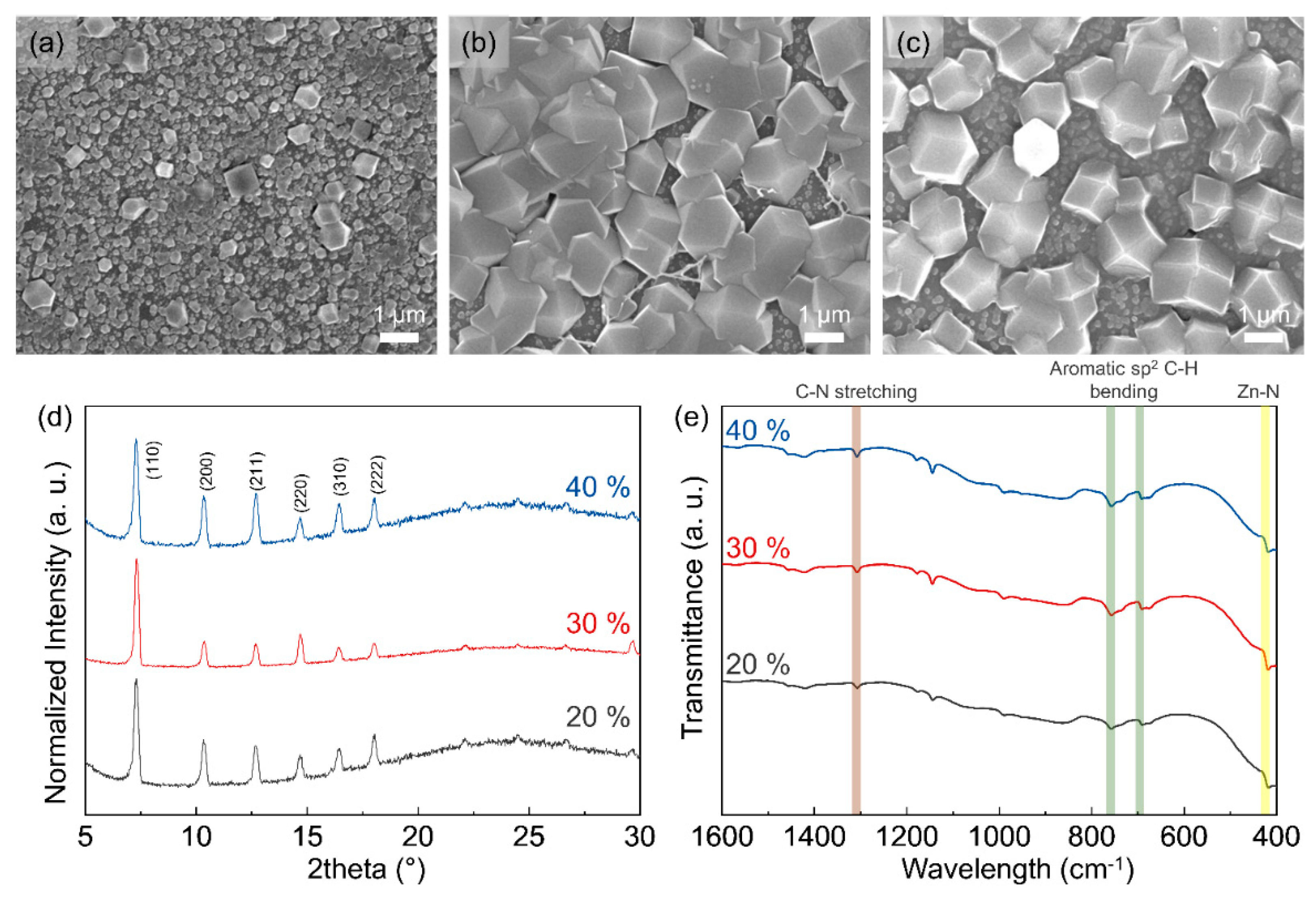
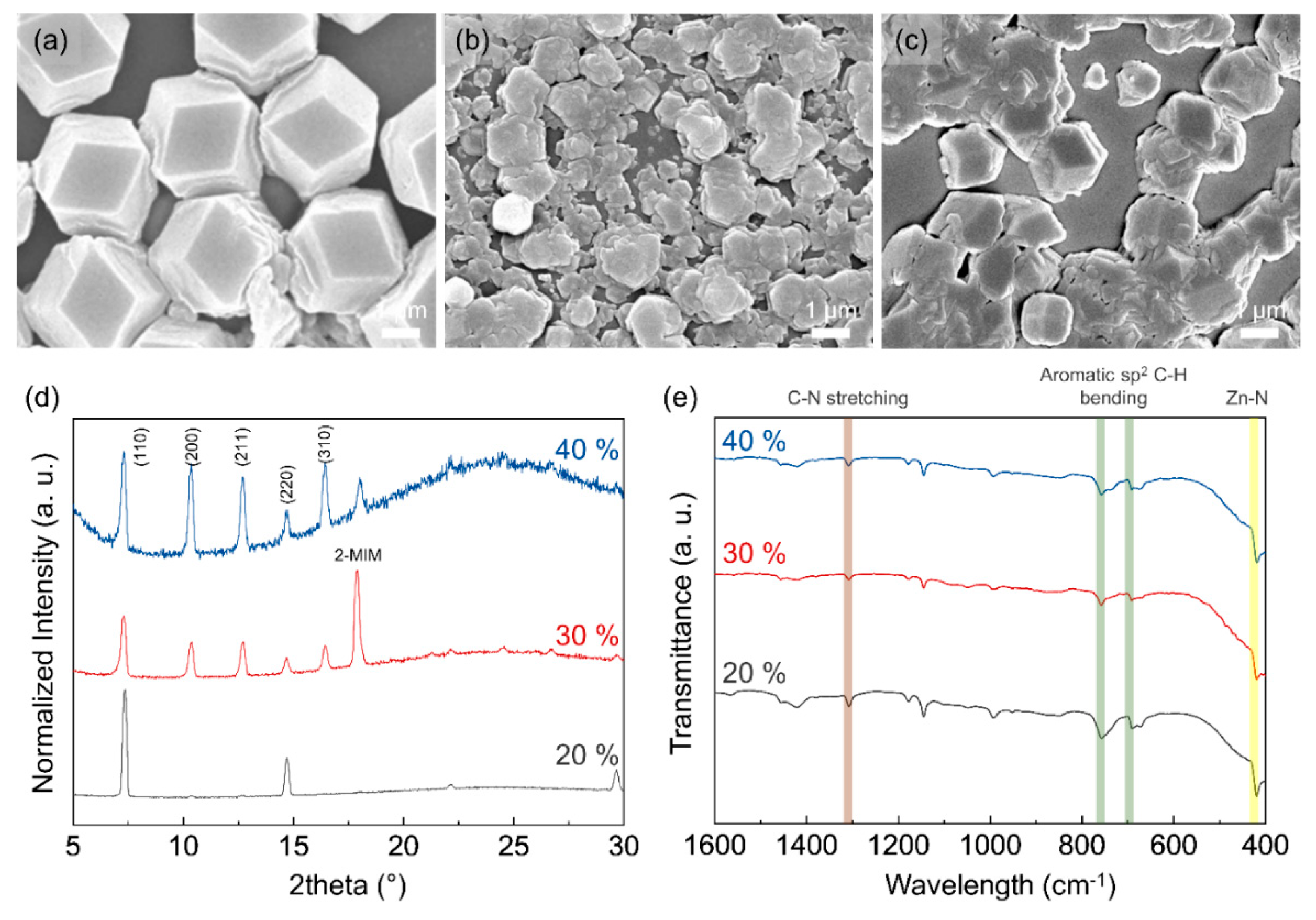
3.2. Particle and Grain Size Distribution

4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Dong, X.; Colombo, V.; Wang, Q.; Liu, Y.; Liu, W.; Wang, X.L.; Huang, X.Y.; Proserpio, D.M.; Sironi, A.; Han, Y.; Li, J. Tailor-Made Microporous Metal–Organic Frameworks for the Full Separation of Propane from Propylene Through Selective Size Exclusion. Adv. Mater. 2018, 30, 1805088. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.G.; Hu, T.L.; Bu, X.H. Metal–Organic Framework Materials for the Separation and Purification of Light Hydrocarbons. Adv. Mater. 2019, 32, 1806445. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; Wang, Q.; Zou, L.; Zhang, Y.; Zhang, L.; Fang, Y.; Li, J.; Zhou, H.-C. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Li, J. Designer Metal–Organic Frameworks for Size-Exclusion-Based Hydrocarbon Separations: Progress and Challenges. Adv. Mater. 2020, 32, 2002603. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bhatt, P.M.; Li, J.; Eddaoudi, M.; Liu, Y. Recent Progress on Microfine Design of Metal–Organic Frameworks: Structure Regulation and Gas Sorption and Separation. Adv. Mater. 2020, 32, 2002563. [Google Scholar] [CrossRef] [PubMed]
- Krokidas, P.; Moncho, S.; Brothers, E.N.; Castier, M.; Economou, I.G. Tailoring the gas separation efficiency of metal organic framework ZIF-8 through metal substitution: A computational study. Phys. Chem. Chem. Phys. 2018, 20, 4879–4892. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Hong, S.J.; Kim, Y.J.; Choi, S.E.; Choi, Y.; Kim, J.H.; Kang, J.; Kwon, O.; Eum, K.; Han, B.; Kim, D.W. Pore Tuning of Metal-Organic Framework Membrane Anchored on Graphene-Oxide Nanoribbon. Adv. Funct. Mater. 2021, 31, 2011146. [Google Scholar] [CrossRef]
- Choi, E.; Choi, J.I.; Kim, Y.J.; Kim, Y.J.; Eum, K.; Choi, Y.; Kwon, O.; Kim, M.; Choi, W.; Ji, H.; Jang, S.S.; Kim, D.W. Graphene Nanoribbon Hybridization of Zeolitic Imidazolate Framework Membranes for Intrinsic Molecular Separation. Angew. Chem. Int. Ed. 2022, 61, e202214269. [Google Scholar] [CrossRef]
- Fan, W.; Ying, Y.; Peh, S.B.; Yuan, H.; Yang, Z.; Yuan, Y.D.; Shi, D.; Yu, X.; Kang, C.; Zhao, D. Multivariate Polycrystalline Metal–Organic Framework Membranes for CO2/CH4 Separation. J. Am. Chem. Soc. 2021, 143, 17716–17723. [Google Scholar] [CrossRef]
- Lee, M.J.; Kwon, H.T.; Jeong, H.K. High-Flux Zeolitic Imidazolate Framework Membranes for Propylene/Propane Separation by Postsynthetic Linker Exchange. Angew. Chem. Int. Ed. 2017, 57, 156–161. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.-F.; Yao, J.; Wang, H. Contra-diffusion synthesis of metal-organic framework separation membranes: A review. Sep. Pur. Technol. 2022, 300, 121837. [Google Scholar] [CrossRef]
- Bux, H.; Liang, F.; Li, Y.; Cravillon, J.; Wiebcke, M.; Caro, J. Zeolitic imidazolate framework membrane with molecular sieving properties by microwave-assisted solvothermal synthesis. J. Am. Chem. Soc. 2009, 131, 16000–16001. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Z.Y.; Zhu, P.W.; Mohamed, A.R.; Chai, S.-P. A well inter-grown ZIF-8 membrane synthesized via two-step hydrothermal synthesis on coarse α-Al2O3 support. Mater. Lett. 2014, 129, 162–165. [Google Scholar] [CrossRef]
- Eum, K.; Rownaghi, A.; Choi, D.; Bhave, R.R.; Jones, C.W.; Nair, S. Fluidic Processing of High-Performance ZIF-8 Membranes on Polymeric Hollow Fibers: Mechanistic Insights and Microstructure Control. Adv. Funct. Mater. 2016, 26, 5011–5018. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, Y.; Lyu, L.; Hou, Q.; Caro, J.; Wang, H. Flexible Polypropylene-Supported ZIF-8 Membranes for Highly Efficient Propene/Propane Separation. J. Am. Chem. Soc. 2020, 142, 20915–20919. [Google Scholar] [CrossRef] [PubMed]
- Neelakanda, P.; Barankova, E.; Peinemann, K.-V. Polymer supported ZIF-8 membranes by conversion of sputtered zinc oxide layers. Microporous and Mesoporous Mater. 2016, 220, 215–219. [Google Scholar] [CrossRef]
- Valadez Sánchez, E.P.; Gliemann, H.; Haas-Santo, K.; Wöll, C.; Dittmeyer, R. ZIF-8 SURMOF Membranes Synthesized by Au-Assisted Liquid Phase Epitaxy for Application in Gas Separation. Chem. Ing. Tech. 2016, 88, 1798–1805. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.-K. In Situ Synthesis of Thin Zeolitic–Imidazolate Framework ZIF-8 Membranes Exhibiting Exceptionally High Propylene/Propane Separation. J. Am. Chem. Soc. 2013, 135, 10763–10768. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Barkova, M.I.; Kustov, L.M.; Syrtsova, D.A.; Efimova, E.A.; Teplyakov, V.V. In situ synthesis of novel ZIF-8 membranes on polymeric and inorganic supports. J. Mater. Chem. A 2015, 3, 7469–7476. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, N.; Pan, J.H.; Steinbach, F.; Caro, J. In situ synthesis of MOF membranes on ZnAl-CO3 LDH buffer layer-modified substrates. J. Am. Chem. Soc. 2014, 136, 14353–14356. [Google Scholar] [CrossRef]
- Shah, M.; Kwon, H.T.; Tran, V.; Sachdeva, S.; Jeong, H.-K. One step in situ synthesis of supported zeolitic imidazolate framework ZIF-8 membranes: Role of sodium formate. Microporous and Mesoporous Mater. 2013, 165, 63–69. [Google Scholar] [CrossRef]
- He, M.; Yao, J.; Li, L.; Zhong, Z.; Chen, F.; Wang, H. Aqueous solution synthesis of ZIF-8 films on a porous nylon substrate by contra-diffusion method. Microporous and Mesoporous Mater. 2013, 179, 10–16. [Google Scholar] [CrossRef]
- Ramu, G.; Lee, M.; Jeong, H.-K. Effects of zinc salts on the microstructure and performance of zeolitic-imidazolate framework ZIF-8 membranes for propylene/propane separation. Microporous and Mesoporous Mater. 2018, 259, 155–162. [Google Scholar] [CrossRef]
- Watanabe, S.; Ohsaki, S.; Hanafusa, T.; Takada, K.; Tanaka, H.; Mae, K.; Miyahara, M.T. Synthesis of zeolitic imidazolate framework-8 particles of controlled sizes, shapes, and gate adsorption characteristics using a central collision-type microreactor. Chem. Eng. J. 2017, 313, 724–733. [Google Scholar] [CrossRef]
- Yao, J.; Li, L.; Benjamin Wong, W.H.; Tan, C.; Dong, D.; Wang, H. Formation of ZIF-8 membranes and crystals in a diluted aqueous solution. Mater. Chem. Phys. 2013, 139, 1003–1008. [Google Scholar] [CrossRef]
- Bustamante, E.L.; Fernández, J.L.; Zamaro, J.M. Influence of the solvent in the synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals at room temperature. J. Colloid Interface Sci. 2014, 424, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Hashimoto, H. FT-IR-ATR study of depth profile of SiO2 ultra-thin films. Applied surface science 2001, 172, 307–311. [Google Scholar] [CrossRef]
- Tanaka, S.; Tanaka, Y. A Simple Step toward Enhancing Hydrothermal Stability of ZIF-8. ACS Omega 2019, 4, 19905–19912. [Google Scholar] [CrossRef] [PubMed]
- Saliba, D.; Ammar, M.; Rammal, M.; Al-Ghoul, M.; Hmadeh, M. Crystal Growth of ZIF-8, ZIF-67, and Their Mixed-Metal Derivatives. J. Am. Chem. Soc. 2018, 140, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Fan, T.; Chen, J.; Li, Y. Synthetic Factors Affecting the Scalable Production of Zeolitic Imidazolate Frameworks. ACS Sustain. Chem. Eng. 2019, 7, 3632–3646. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, Q.; Luan, C. The Thermodynamic Relations between the Melting Point and the Size of Crystals. J. Colloid Interface Sci. 2001, 243, 388–390. [Google Scholar] [CrossRef]
- Choi, E.; Lee, J.; Kim, Y.-J.; Kim, H.; Kim, M.; Hong, J.; Kang, Y.C.; Koo, C.M.; Kim, D.W.; Kim, S.J. Enhanced Stability of Ti3C2Tx MXene Enabled by Continuous ZIF-8 Coating. Carbon 2022, 191, 593–599. [Google Scholar] [CrossRef]
- Kwon, O.; Kim, M.; Choi, E.; Bae, J.H.; Yoo, S.; Won, J.C.; Kim, Y.H.; Shin, J.H.; Lee, J.S.; Kim, D.W. High-aspect Ratio Zeolite Framework (ZIF) Nanoplates for Hydrocarbon Separation Membranes. Sci. Adv. 2022, 8, 1, eabl6841. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




