Submitted:
19 September 2023
Posted:
22 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
“The intricacy of managing balance leads to various issues related to balance, gait, and falling, which require a systematic clinical evaluation for effective intervention. Several clinical tests are designed to assess only one specific “balance system”, but since balance control is a complex process that involves multiple underlying systems, a comprehensive evaluation is necessary.”
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedures
2.4. Postural Exercise Training
2.5. Measurements
2.6. Statistical Analysis
3. Results
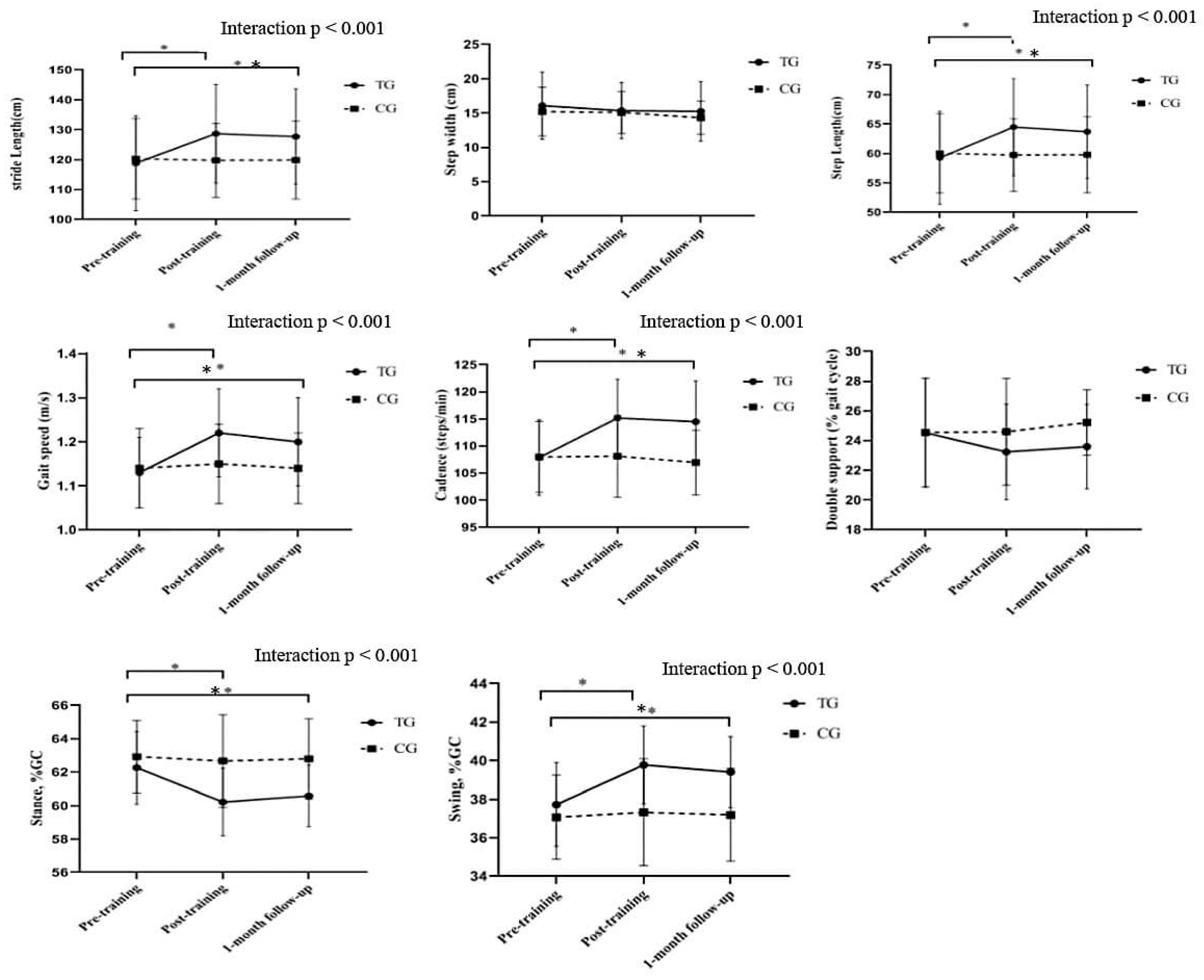
3.1. Gait Parameters
4. Discussion
4.1. Limitations
4.2. Implications and Suggestions for Future Research
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Organization, W.H. , International Classification of Functioning, Disability and Health: ICF; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Oppewal, A.; Hilgenkamp, T.I. The dual task effect on gait in adults with intellectual disabilities: Is it predictive for falls? Disabil. Rehabil. 2019, 41, 26–32. [Google Scholar] [CrossRef]
- Oppewal, A.; Festen, D.A.; Hilgenkamp, T.I. Gait characteristics of adults with intellectual disability. Am. J. Intellect. Dev. Disabil. 2018, 123, 283–299. [Google Scholar] [CrossRef]
- Schalock, R.L.; Luckasson, R.; Tassé, M.J. An overview of intellectual disability: Definition, diagnosis, classification, and systems of supports. Am. J. Intellect. Dev. Disabil. 2021, 126, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Reguera-García, M.M.; Leirós-Rodríguez, R.; Álvarez-Barrio, L. Reliability and Validity of the Six Spot Step Test in People with Intellectual Disability. Brain Sci. 2021, 11, 201. [Google Scholar] [CrossRef] [PubMed]
- Bahiraei, S.; Daneshmandi, H.; Norasteh, A.A.; Yahya, S. Balance stability in intellectual disability: Introductory evidence for the balance evaluation systems test (BESTest). Life Span Disabil. 2019, 22, 7–28. [Google Scholar]
- Bahiraei, S.; Daneshmandi, H.; Sokhangoei, Y. The Study of Biomechanical Gait Cycle and Balance Characteristics in Intellectual Disabilities: A Systematic Review. Phys. Treat. - Specif. Phys. Ther. 2018, 8, 63–76. [Google Scholar] [CrossRef]
- Cleaver, S.; Hunter, D.; Ouellette-Kuntz, H. Physical mobility limitations in adults with intellectual disabilities: A systematic review. J. Intellect. Disabil. Res. 2009, 53, 93–105. [Google Scholar] [CrossRef]
- Galli, M.; Rigoldi, C.; Albertini, G. Postural control in patients with Down syndrome. Disabil. Rehabil. 2008, 30, 1274–1278. [Google Scholar] [CrossRef]
- Giagazoglou, P.; Kokaridas, D.; Neofotistou, K. Effects of a trampoline exercise intervention on motor performance and balance ability of children with intellectual disabilities. Res. Dev. Disabil. 2013, 34, 2701–2707. [Google Scholar] [CrossRef]
- Kachouri, H.; Laatar, R.; Sahli, S. Using a dual-task paradigm to investigate motor and cognitive performance in children with intellectual disability. J. Appl. Res. Intellect. Disabil. 2020, 33, 172–179. [Google Scholar] [CrossRef]
- Lopes Pedralli, M.; Schelle, G.H. Gait evaluation in individuals with Down syndrome. Braz. J. Biomotricity 2013, 7. [Google Scholar]
- Maas, S.; Festen, D.; Hilgenkamp, T.I.; Oppewal, A. The association between medication use and gait in adults with intellectual disabilities. J. Intellect. Disabil. Res. 2020, 64, 793–803. [Google Scholar] [CrossRef]
- Lin, L.-P.; Hsia, Y.-C.; Hsu, S.-W.; Loh, C.-H.; Wu, C.-L.; Lin, J.-D. Caregivers’ reported functional limitations in activities of daily living among middle-aged adults with intellectual disabilities. Res. Dev. Disabil. 2013, 34, 4559–4564. [Google Scholar] [CrossRef]
- Almuhtaseb, S.; Oppewal, A.; Hilgenkamp, T.I. Gait characteristics in individuals with intellectual disabilities: A literature review. Res. Dev. Disabil. 2014, 35, 2858–2883. [Google Scholar] [CrossRef]
- Cioni, M.; Cocilovo, A.; Rossi, F.; Paci, D.; Valle, M.S. Analysis of ankle kinetics during walking in individuals with Down syndrome. Am. J. Ment. Retard. 2001, 106, 470–478. [Google Scholar] [CrossRef]
- Enkelaar, L.; Smulders, E.; van Schrojenstein Lantman-de Valk, H.; Geurts, A.C.; Weerdesteyn, V. A review of balance and gait capacities in relation to falls in persons with intellectual disability. Res. Dev. Disabil. 2012, 33, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Pal, J.; Hale, L.; Mirfin-Veitch, B.; Claydon, L. Injuries and falls among adults with intellectual disability: A prospective New Zealand cohort study. J. Intellect. Dev. Disabil. 2014, 39, 35–44. [Google Scholar] [CrossRef]
- Elshemy, S.A. Comparative study: Parameters of gait in Down syndrome versus matched obese and healthy children. Egypt. J. Med. Hum. Genet. 2013, 14, 285–291. [Google Scholar] [CrossRef]
- Smith, B.A.; Ashton-Miller, J.A.; Ulrich, B.D. Gait adaptations in response to perturbations in adults with Down syndrome. Gait Posture 2010, 32, 149–154. [Google Scholar] [CrossRef]
- Smith, B.A.; Ulrich, B.D. Early onset of stabilizing strategies for gait and obstacles: Older adults with Down syndrome. Gait Posture 2008, 28, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Wrisley, D.M.; Frank, J. The balance evaluation systems test (BESTest) to differentiate balance deficits. Phys. Ther. 2009, 89, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Sibley, K.M.; Beauchamp, M.K.; Van Ooteghem, K.; Straus, S.E.; Jaglal, S.B. Using the systems framework for postural control to analyze the components of balance evaluated in standardized balance measures: A scoping review. Arch. Phys. Med. Rehabil. 2015, 96, 122–132. e29. Arch. Phys. Med. Rehabil. 2015, 96, 122–132.e29. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Lee, M.M.; DShin, C.; Shin, S.H.; Song, C.H. The effects of a balance exercise program for enhancement of gait function on temporal and spatial gait parameters in young people with intellectual disabilities. J. Phys. Ther. Sci. 2014, 26, 513–516. [Google Scholar] [CrossRef]
- Lee, K.; Lee, M.; Song, C. Balance training improves postural balance, gait, and functional strength in adolescents with intellectual disabilities: Single-blinded, randomized clinical trial. Disabil. Health J. 2016, 9, 416–422. [Google Scholar] [CrossRef]
- Ahmadi, N.; Peyk, F.; Hovanloo, F.; Garekani, S.H. Effect of functional strength training on gait kinematics, muscle strength and static balance of young adults with Down syndrome. Int. J. Mot. Control Learn. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Huri, M.; Huri, E.; Kayihan, H.; Altuntas, O. Effects of occupational therapy on quality of life of patients with metastatic prostate cancer: A randomized controlled study. Saudi Med. J. 2015, 36, 954. [Google Scholar] [CrossRef]
- Souza, A.P.S.d.; Silva, L.C.D.; Fayh, A.P.T. Nutritional Intervention Contributes to the Improvement of Symptoms Related to Quality of Life in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy: A Randomized Clinical Trial. Nutrients 2021, 13, 589. [Google Scholar] [CrossRef]
- Blomqvist, S.; Wester, A.; Rehn, B. Test–retest reliability, smallest real difference and concurrent validity of six different balance tests on young people with mild to moderate intellectual disability. Physiotherapy 2012, 98, 313–319. [Google Scholar] [CrossRef]
- Rigoldi, C.; Galli, M.; Cimolin, V.; Camerota, F.; Celletti, C.; Tenore, N.; Albertini, G. Gait strategy in patients with Ehlers-Danlos syndrome hypermobility type and Down syndrome. Res. Dev. Disabil. 2012, 33, 1437–1442. [Google Scholar] [CrossRef]
- Rosenthal, J.A. Qualitative descriptors of strength of association and effect size. J. Soc. Serv. Res. 1996, 21, 37–59. [Google Scholar] [CrossRef]
- Rodenbusch, T.L.; Ribeiro, T.S.; Simão, C.R.; Britto, H.M.; Tudella, E.; Lindquist, A.R. Effects of treadmill inclination on the gait of children with Down syndrome. Res. Dev. Disabil. 2013, 34, 2185–2190. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, I.N.; Cámara, M.B.; Gadella, J.B. Gait analysis and Bobath physiotherapy in adults with Down syndrome. Int. Med. Rev. Down Syndr. 2016, 20, 8–14. [Google Scholar] [CrossRef]
- Ulrich, D.A.; Ulrich, B.D.; Angulo-Kinzler, R.M.; Yun, J. Treadmill training of infants with Down syndrome: Evidence-based developmental outcomes. Pediatrics 2001, 108, e84–e84. [Google Scholar] [CrossRef] [PubMed]
- Carmeli, E.; Kessel, S.; Coleman, R.; Ayalon, M. Effects of a treadmill walking program on muscle strength and balance in elderly people with Down syndrome. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M106–M110. [Google Scholar] [CrossRef]
- Kubilay, N.S.; Yildirim, Y.; Harutoglu-Akdur, H. Effect of balance training and posture exercises on functional level in mental retardation. Fiz. Rehabil. 2011, 22, 55–64. [Google Scholar]
- Hartman, E.; Houwen, S.; Visscher, C. On the relationship between motor performance and executive functioning in children with intellectual disabilities. J. Intellect. Disabil. Res. 2010, 54, 468–477. [Google Scholar] [CrossRef]
- TS, H. The effects of run/walk exercise on physical fitness and sport skills on individuals with mental retardation. NCYU Phys Educ Health Recreat J. 2008, 7, 44–58. [Google Scholar]
- Nam, H.-C.; Cha, H.-G.; Kim, M.-K. The effects of exercising on an unstable surface on the gait and balance ability of normal adults. J. Phys. Ther. Sci. 2016, 28, 2102–2104. [Google Scholar] [CrossRef]
- Shashidhara, M. The effect of eight-week yoga exercise on balance and gait in girls with intellectual disability. Int. J. Yoga Allied Sci. 2018, 7, 31–35. [Google Scholar]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health= Rev. Can. De Sante Publique 1991, 39, 7–11. [Google Scholar]
- Lipfert, S.W.; Günther, M.; Renjewski, D.; Seyfarth, A. Impulsive ankle push-off powers leg swing in human walking. J. Exp. Biol. 2014, 217, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Rosen, S. Kinesiology and sensorimotor function. Found. Orientat. Mobil. 1997, 170–199. [Google Scholar]
- Angulo-Barroso, R.M.; Wu, J.; Ulrich, D.A. Long-term effect of different treadmill interventions on gait development in new walkers with Down syndrome. Gait Posture 2008, 27, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Holtzer, R.; Lipton, R.B.; Wang, C. Quantitative gait markers and incident fall risk in older adults. J. Gerontol. Ser. A 2009, 64, 896–901. [Google Scholar] [CrossRef]
- Behm, D.G.; Anderson, K.; Curnew, R.S. Muscle force and activation under stable and unstable conditions. J. Strength Cond. Res. 2002, 16, 416–422. [Google Scholar]
- Ardestani, M.M.; Ferrigno, C.; Moazen, M.; Wimmer, M.A. From normal to fast walking: Impact of cadence and stride length on lower extremity joint moments. Gait Posture 2016, 46, 118–125. [Google Scholar] [CrossRef]
- Galli, M.; Rigoldi, C.; Brunner, R.; Virji-Babul, N.; Giorgio, A. Joint stiffness and gait pattern evaluation in children with Down syndrome. Gait Posture 2008, 28, 502–506. [Google Scholar] [CrossRef]
- Long, J.; Feng, Y.; Liao, H.; Zhou, Q.; Urbin, M. Motor sequence learning is associated with hippocampal subfield volume in humans with medial temporal lobe epilepsy. Front. Hum. Neurosci. 2018, 12, 367. [Google Scholar] [CrossRef]
- Llorens-Martin, M.; Rueda, N.; Tejeda, G.S.; Flórez, J.; Trejo, J.L.; Martínez-Cué, C. Effects of voluntary physical exercise on adult hippocampal neurogenesis and behavior of Ts65Dn mice, a model of Down syndrome. Neuroscience 2010, 171, 1228–1240. [Google Scholar] [CrossRef]
- Novak, I.; Morgan, C. High-risk follow-up: Early intervention and rehabilitation. Handb. Clin. Neurol. 2019, 162, 483–510. [Google Scholar]
- Tong, L.; Shen, H.; Perreau, V.M.; Balazs, R.; Cotman, C.W. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol. Dis. 2001, 8, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- JudgeRoy, J.O.; Davis, B., III; Õunpuu, S. Step length reductions in advanced age: The role of ankle and hip kinetics. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1996, 51, M303–M312. [Google Scholar] [CrossRef] [PubMed]
| Activities | Duration | Activities performed as part of the program | Goal |
|---|---|---|---|
| Warm-up activities | 10 min | Slow and fast walk/ run /stretching |
|
| Postural Exercises Based on BESTest | 40 min | 1- Double leg stance with eyes closed; 2- standing on one leg with eyes open ; 3- Sitting on the Swiss ball; 4- Performing the task of putting on and taking off footwear while sitting on a Swiss ball and lifting one’s lower extremity; 5- Sitting on a Swiss ball and assuming a standing position, participants were instructed to reach for a shelf with their upper extremity, remove a toy, and then return to a seated position ; 6- Walking and increasing step length; 7- Emily post relay walking; 8- Walking posture; 9- Dual-task walking; 10- Performing a lateral movement with the ball while standing on the floor and holding it overhead ; 11- Jumping jacks on the mini-trampoline; 12- Receiving and throwing a ball while jumping on a mini-trampoline ; 13-Standing on two air cushions and catching a ball thrown by a therapist ; 14- Autonomic strategic exercise of the ankle and hip; 15- Automatic strategic exercise (front, back and side) using resistance bands. |
|
| Cool-down activities | 10 min | light stretching/flexibility training |
|
| Outcomes | TG (n = 19) | CG (n = 15) | ||||
|---|---|---|---|---|---|---|
| Pre-training | Post-training | 1-month follow-up | Pre-training | Post-training | 1-month follow-up | |
| Step length (cm) | 59.27±7.90 | 64.19±8.22a,b | 63.70±7.94c,a | 60.01±6.72 | 59.74±6.16 | 59.79±6.47 |
| stride length (cm) | 118.83±15.85 | 128.69±16.48a,b | 127.71±15.92c,a | 120.31±13.47 | 119.78±12.35 | 119.88±12.97 |
| Cadence (steps/min) | 107.88±6.98 | 115.17±7.14a,b | 114.51±7.50c,a | 107.98±6.54 | 108.13±7.56 | 106.96±5.95 |
| Step width (cm) | 16.08±4.90 | 15.37±4.08 | 15.26±4.32 | 15.23±3.56 | 15.10±3.06 | 14.33±2.4 |
| Gait speed (m/s) | 1.13±0.08 | 1.22±0.10a,b | 1.20±0.10c,a | 1.14±0.09 | 1.15±0.09 | 1.14±0.08 |
| Double support %GC | 24.53±3.67 | 23.24±3.22 | 23.60±2.84 | 24.55±3.65 | 24.59±3.59 | 25.22±2.20 |
| Stance, %GC | 62.27±2.17 | 60.22±2.01a,b | 60.59±1.84c,a | 62.93±2.17 | 62.67±2.77 | 62.80±2.4 |
| Swing, %GC | 37.73±2.17 | 39.78±2.01a,b | 39.41±1.84c,a | 37.07±2.17 | 37.33±2.77 | 37.20±2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





