Submitted:
05 March 2023
Posted:
09 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants, design, and ethics of the study
| Participants Characterization | HIIT (n= 21) | SCG (n= 21) | MICT (n=21) | p value |
|---|---|---|---|---|
| Age (Years) | 67.6+8.72 | 68.4+ 7.45 | 69.1+6.73 | >0.05 |
| Weight (Kg) | 76.1+6.08 | 79.2+5.77 | 78.9+7.13 | >0.05 |
| Height (Cm) | 1.68+0.91 | 1.66+0.84 | 1.67+0.93 | >0.05 |
| BMI (Kg/m2) | 27.5+3.73 | 28.5+4.52 | 28.6+5.11 | >0.05 |
| VO2 Max (ml/kg/min) | 32.2+2.64 | 33.4+4.43 | 32.6+7.25 | >0.05 |
Clinical and laboratorial health state of volunteers
Cardiorespiratory fitness assessment
Blood sampling acquisition for immunological analysis
Upper respiratory tract infections assessment
Statistical Analysis
3. Results
The URTI incidence don’t improved in response to the HIIT
Biochemical markers display good health conditions
| Variables | SGC | HIIT | MICT | p value |
|---|---|---|---|---|
| AST (IU/mL) | 39.0±25.6 | 40.5±13.1 | 39.25±20.3 | >0.05 |
| ALT (IU/mL) | 23.67±4.16 | 23.98±2.35 | 24.0±7.2 | >0.05 |
| Creatinine (mg/dL) | 0.75±0.13 | 0.74±0.43 | 0.73±0.1 | >0.05 |
| Urea (mg/dL) | 21.25±2.5 | 26.64±6.1 | 27.25±5.8 | >0.05 |
| CK-MB (IU/mL) | 10.75±2.2 | 10.33±6.7 | 10.25±5.4 | >0.05 |
| CK-Total (IU/mL) | 91.0±12.6 | 91.3±23.5 | 91.5±38.5 | >0.05 |
| Cholesterol (mg/dL) | 192.5±38.5 | 179.4±77.5 | 164.7±19.7 | >0.05 |
| Triglycerides (mg/dL) | 184.5±38.7 | 176.5±38.7 | 163.7±19.7 | >0.05 |
| LDH (mg/dL) | 358.7±124.2 | 358.7±124.2 | 331.5±70.2 | >0.05 |
| HDL(mg/dL) | 41.5±3.7 | 46.4±1.9 | 46.5±2.97 | >0.05 |
| PCR (mg/dL) | 3.75±4.7 | 3.11±1.9 | 2.75±3.6 | >0.05 |
| Glucose (mg/dL) | 89.5±7.1 | 89.5±7.1 | 92.5±8.2 | >0.05 |
| Haematocrit (%) | 43.4±0.2 | 43.7±0.1 | 44.2±0.2 | >0.05 |
| Haemoglobin (g/dL) | 12.96±0.7 | 12.86±0.9 | 12.81±0.7 | >0.05 |
| MCV (fl) | 86.1±1.3 | 87.9±1.1 | 88.2±1.2 | >0.05 |
| MCH (pg) | 28.8±0.7 | 29.7±0.4 | 29.8±0.6 | >0.05 |
| Platelet levels (uL) | 282650±22951.8 | 283760±22951.8 | 283830±15325 | >0.05 |
The white blood cells display little stress in response to HIIT
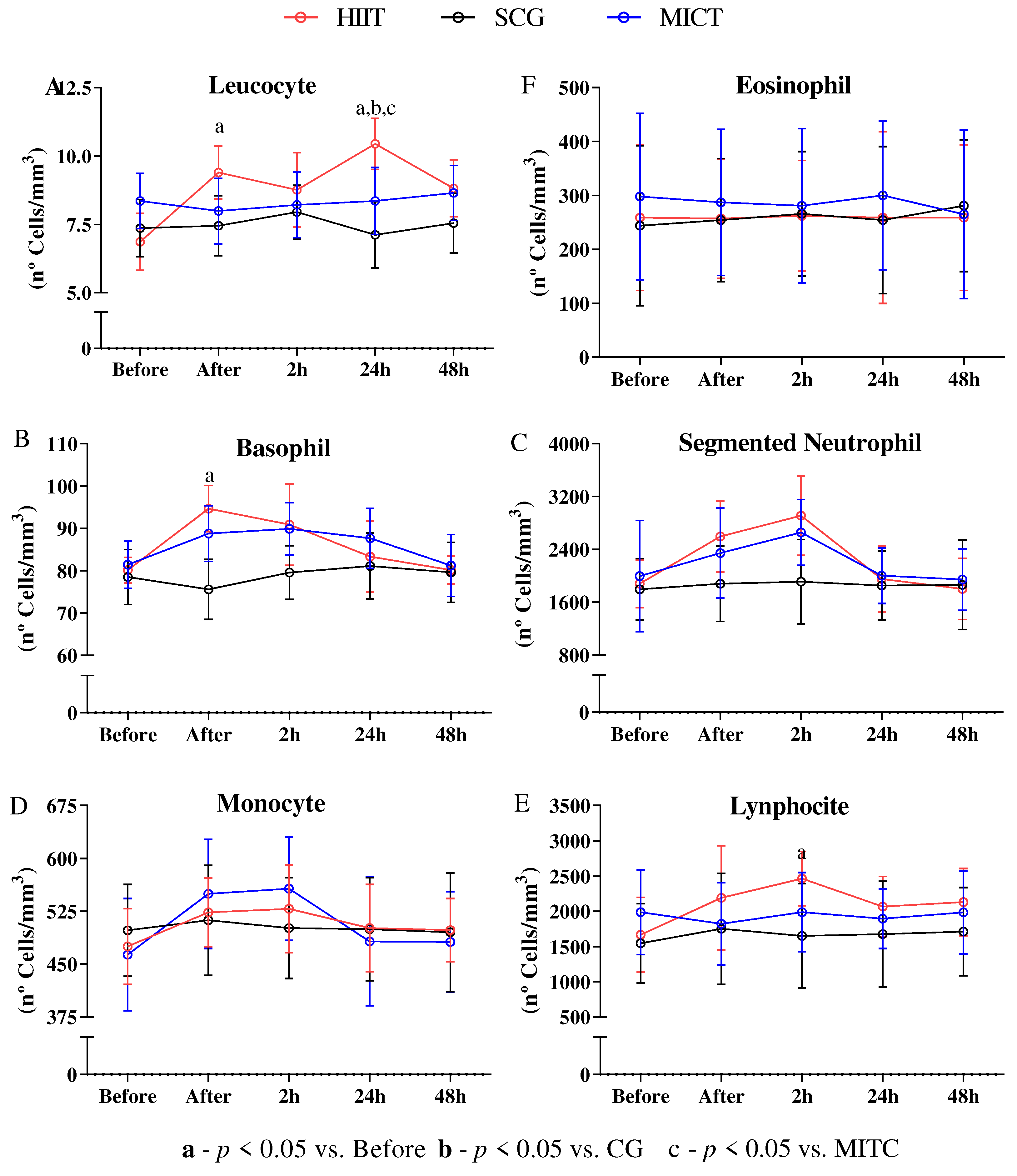
The immunologic markers display little stress in response to HIIT
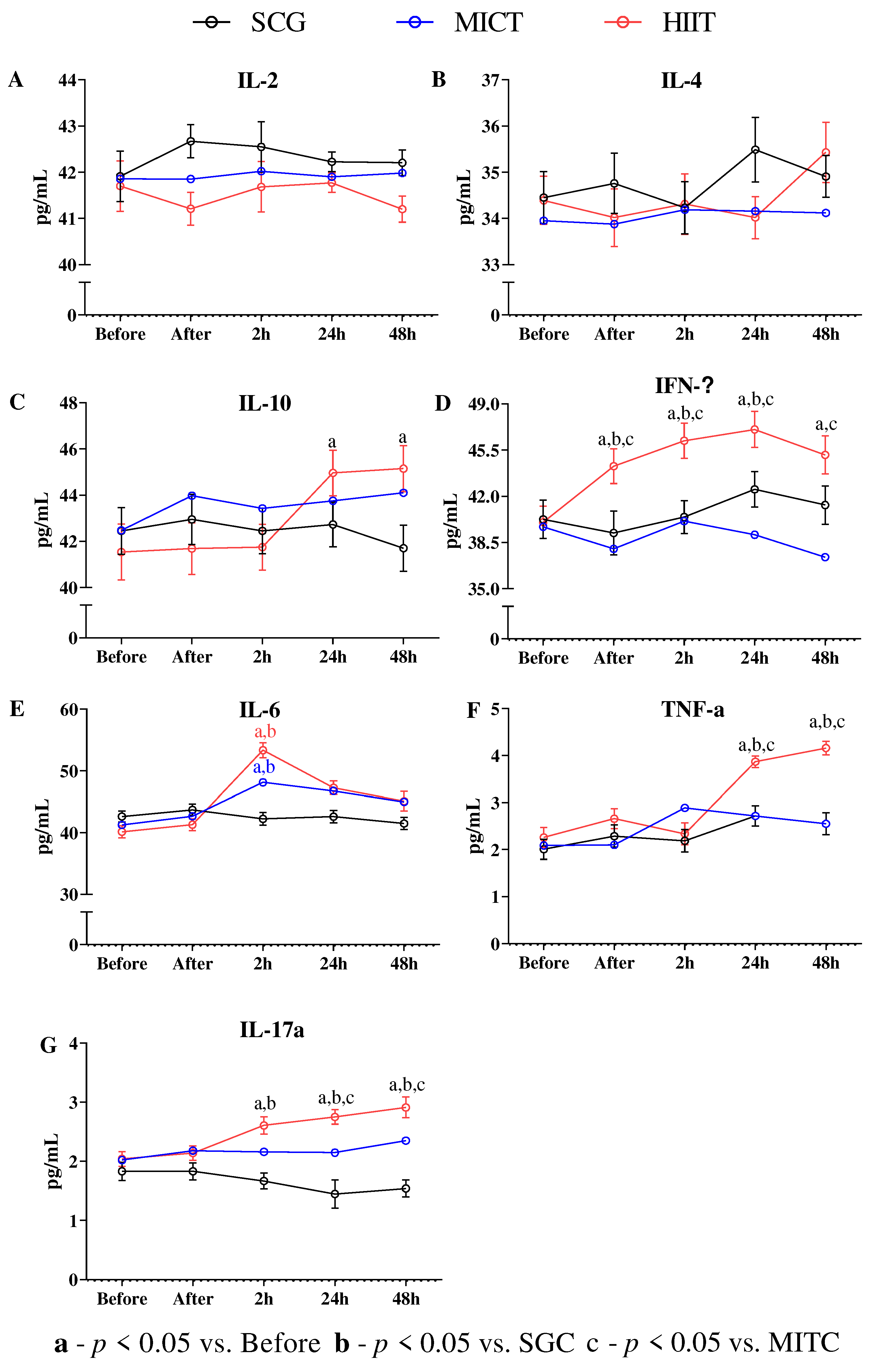
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- American Collegue of Sports Medicine. ACSM’s Guidelines For Exercise Testing And Prescription (10th edition). Sport & Exercise Scientist. 2016. [CrossRef]
- Gill, S.K.; Teixeira, A.; Rama, L.; Prestes, J.; Rosado, F.; Hankey, J.; Scheer, V.; Hemmings, K.; Ansley-Robson, P.; Costa, R.J.S. Circulatory endotoxin concentration and cytokine profile in response to exertional-heat stress during a multi-stage ultra-marathon competition. Exerc Immunol Rev 2015, 21, 114–28. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25830597. [PubMed]
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Riebe, D.; Franklin, B.A.; Thompson, P.D.; Garber, C.E.; Whitfield, G.P.; Magal, M.; Pescatello, L.S. Updating ACSM’s Recommendations for Exercise Preparticipation Health Screening. Med. Sci. Sports Exerc. 2015, 47, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Smith, L.L.; Utter, A.C.; Vinci, D.M.; Davis, J.M.; Kaminsky, D.E.; Shute, M. Cytokine changes after a marathon race. J. Appl. Physiol. 2001, 91, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, D.T.; Caram, K.; Nogueira, F.R.D.; Libardi, C.A.; Prestes, J.; Cavaglieri, C.R. Immune responses to an upper body tri-set resistance training session. Clin. Physiol. Funct. Imaging 2014, 34, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Zhao G, Zhou S, Davie A, Su Q. Effects of moderate and high intensity exercise on T1/T2 balance. Exerc Immunol Rev. 2012;18: 98–114.
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- de Souza DC, Matos VAF, dos Santos VOA, Medeiros IF, Marinho CSR, Nascimento PRP, et al. Effects of high-intensity interval and moderate-intensity continuous exercise on inflammatory, leptin, IgA, and lipid peroxidation responses in obese males. Front Physiol. 2018;9: 1–9. [CrossRef] [PubMed]
- Peradini L, Gualano B. Inflammatory cytokine kinetics to single bouts of acute moderate and intense aerobic exercise in women with active and inactive systemic lupus erythematosus. Exerc Immunol Rev. 2015;21: 174–185.
- Suzuki, K. Chronic Inflammation as an Immunological Abnormality and Effectiveness of Exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef]
- Benatti, F.B.; Pedersen, B.K. Exercise as an anti-inflammatory therapy for rheumatic diseases—myokine regulation. Nat. Rev. Rheumatol. 2015, 11, 86–97. [Google Scholar] [CrossRef]
- Furtado-da-Silva V, Braga JC, Santos KM dos, Oliveira GL de, Olivira TAP de, Teixeira AM, et al. PAIN, INFLAMMATION AND PERFORMANCE CAN PREDICT THE IDEAL MOMENT TO APPLY NEW OVERLOAD. Sport Sci. 2021;14: 32–41.
- Fallon, K. Exercise in the time of COVID-19. Aust. J. Gen. Pract. 2020, 49. [Google Scholar] [CrossRef] [PubMed]
- Narici, M.; De Vito, G.; Franchi, M.; Paoli, A.; Moro, T.; Marcolin, G.; Grassi, B.; Baldassarre, G.; Zuccarelli, L.; Biolo, G.; et al. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: Physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. Eur. J. Sport Sci. 2020, 21, 614–635. [Google Scholar] [CrossRef]
- da Silva, V.F.; Silva, D.A.S.; Martins, P.C.; Calomeni, M.R.; Freire, I.d.A.; Militão, A.G.; Borges, C.J.; Junior, G.d.B.V.; de Moraes, M.A.; Silva, A.J.R.M.; et al. Effect of physical exercise and noninvasive brain stimulation on cognition and dementia of elderly people with frailty: A randomized study. Int. J. Imaging Syst. Technol. 2022, 32, 1941–1952. [Google Scholar] [CrossRef]
- Simpson RJ, Campbell JP, Gleeson M, Krüger K, Nieman DC, Pyne DB, et al. Can exercise affect immune function to increase susceptibility to infection? Exercise immunology review. 2020.
- Song, Y.; Ren, F.; Sun, D.; Wang, M.; Baker, J.S.; István, B.; Gu, Y. Benefits of exercise on influenza or pneumonia in older adults: A systematic review. International Journal of Environmental Research and Public Health. 2020, 17, 2655. [Google Scholar] [CrossRef]
- Nogueira TRB, Oliveira GL de, Oliveira TAP de, Pagani MM, Valentim-Silva JR. Efeito do método Pilates nas adaptações neuromusculares e na composição corporal de adultos jovens. Rev Bras Prescrição e Fisiol do Exerc. 2014;8: 296–303. Available: file:///C:/Users/profe/Documents/Documents/Documents/Artigos/tatiane roberta buratti pilates.pdf.
- Silva, J.R.V.; Marques, A.A.; Nogueira, T.R.B.; da Silva, V.F.; de Oliveira, T.A.P.; de Oliveira, G.L.; Dantas, E.H.M.; Gonçalves, P.S.d.P.; Filho, J.F. Pilates plus Cardiovascular Training in Body Composition: Effects of Adding Continuous Cardiovascular Training to the Pilates Method on Adult Body Composition. MOJ Sports Med. 2018, 2, 1–5. [Google Scholar] [CrossRef]
- Lameira-de Oliveira G, Gonçalves PS de P, Perini-de Oliveira TA, Valentim-Silva JR, Roquetti-Fernandes P, Fernandes-Filho J. Body composition and somatotype of athletes of Brazilian w5-a-side football team: Paralympic team rio 2016. Rev Fac Med. 2018;66: 25–29. [CrossRef]
- de Oliveira GL, de Oliveira TAP, de Pinho Gonçalves PS, Silva JRV, Fernandes PR, Filho JF. Body image and eating disorders in female athletes of different sports. J Exerc Physiol. 2017;20: 44–54.
- Silva VF da, Junior G de BV, Filho GO, Vieira MAM, Coelho EC da S, Araújo TS de, et al. ACUTE EFFECT OF RESISTANCE VS AEROBIC TRAINING ON EXECUTIVE FUNCTIONS OF OLDER ADULTS. Sport Sci. 2020;13: 122–128.
- Louzada-Júnior, A.; Da-Silva, J.M.; Da-Silva, V.F.; Castro, A.C.M.; De-Freitas, R.E.; Cavalcante, J.B.; Dos-Santos, K.M.; Albuquerque, A.P.A.; Brandão, P.P.; Bello, M.d.N.D.; et al. Multimodal HIIT is More Efficient Than Moderate Continuous Training for Management of Body Composition, Lipid Profile and Glucose Metabolism in the Diabetic Elderly. Int. J. Morphol. 2020, 38, 392–399. [Google Scholar] [CrossRef]
- Braga, J.C.; de Freitas, R.E.; dos Santos, K.M.; da Silva, R.P.; da Silva, J.M.; Junior, A.L.; de Oliveira, G.L.; Oliveir, T.A.P.; Pernambuco, C.S.; da Silva, V.F.; et al. Twelve Weeks of High-Intense Interval Training Enhance the Neuromuscular and Cardiorespiratory Performance of Elderly. Open Sports Sci. J. 2020, 13, 42–48. [Google Scholar] [CrossRef]
- Fischer, C.P. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006, 12, 6–33. [Google Scholar]
- Islam, H.; Neudorf, H.; Mui, A.L.; Little, J.P. Interpreting ‘anti-inflammatory’ cytokine responses to exercise: focus on interleukin-10. J. Physiol. 2021, 599, 5163–5177. [Google Scholar] [CrossRef] [PubMed]
- Silva VF, Marinho DA, Dantas EHM, Santos SS, Teixeira AM, Silva AJRM, et al. Short Exhaustive Intermittent Exercise Results in Acute Inflammatory and Rheumatic Markers Release. J Exerc Physiol Online. 2019.
- Siasos, G.; Athanasiou, D.; Terzis, G.; Stasinaki, A.; Oikonomou, E.; Tsitkanou, S.; Dimitropoulos, S.; Kolokytha, T.; Tzirogiannis, K.; Giannaki, A.; et al. The Acute Impact of Different Types of Aerobic Exercise on Arterial Wave. Cardiology 2016, 135, 81–86. [Google Scholar] [CrossRef]
- Durmus, P.T.; Vardar, M.E.; Kaya, O.; Tayfur, P.; Sut, N.; Vardar, S.A. Evaluation of the Effects of High Intensity Interval Training on Cytokine Levels and Clinical Course in Treatment of Opioid Use Disorder. Turk. J. Psychiatry 2020, 31, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Minuzzi, L.G.; Lira, F.S.; de Poli, R.A.B.; Lopes, V.H.F.; Zagatto, A.M.; Suzuki, K.; Antunes, B.M. High-intensity intermittent exercise induces a potential anti-inflammatory response in healthy women across the menstrual cycle. Cytokine 2022, 154, 155872. [Google Scholar] [CrossRef]
- Pyne, D.B.; Gleeson, M. Effects of Intensive Exercise Training on Immunity in Athletes. Int. J. Sports Med. 1998, 19, S183–S194. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Ringseis R, Eder K, Mooren FC, Krüger K. Metabolic signals and innate immune activation in obesity and exercise. Exerc Immunol Rev. 2015;21: 58–68. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25825956.
- Idorn, M.; Hojman, P. Exercise-Dependent Regulation of NK Cells in Cancer Protection. Trends Mol. Med. 2016, 22, 565–577. [Google Scholar] [CrossRef]
- Wardyn, G.G.; Rennard, S.I.; Brusnahan, S.K.; McGuire, T.R.; Carlson, M.L.; Smith, L.M.; McGranaghan, S.; Sharp, J.G. Effects of exercise on hematological parameters, circulating side population cells, and cytokines. Exp. Hematol. 2008, 36, 216–223. [Google Scholar] [CrossRef]
- Freidenreich DJ, Volek JS. Immune responses to resistance exercise. Exerc Immunol Rev. 2012;18: 8–41.
- Communications S, Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002. [CrossRef] [PubMed]
- Peake, J.M.; Della Gatta, P.; Suzuki, K.; Nieman, D.C. Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 2015, 21, 8–25. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25826432. [PubMed]
- Valentim-Silva, J.R.; Macedo, S.R.A.; de Barros, N.B.; Ferreira, A.d.S.; da Silva, J.H.M.; Nicolete, L.D.d.F.; Nicolete, R. Antileishmanial drugs activate inflammatory signaling pathways via toll-like receptors (docking approach) from Leishmania amazonensis-infected macrophages. Int. Immunopharmacol. 2020, 85, 106640. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Gontijo, R.; Peruhype-Magalhães, V.; Costa-Silva, M.F.; Martins-Filho, O.A.; Quaresma, P.F.; Freire, J.d.M.; Moreno, E.d.C.; Teixeira-Carvalho, A.; Gontijo, C.M.F. Protective Profile Involving CD23/IgE-mediated NO Release is a Hallmark of Cutaneous Leishmaniasis Patients from the Xakriabá Indigenous Community in Minas Gerais, Brazil. Scand. J. Immunol. 2015, 81, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Gantt, K.R.; Goldman, T.L.; McCormick, M.L.; Miller, M.A.; Jeronimo, S.M.B.; Nascimento, E.T.; Britigan, B.E.; Wilson, M.E. Oxidative Responses of Human and Murine Macrophages During Phagocytosis ofLeishmania chagasi. J. Immunol. 2001, 167, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Xu, L.; Xie, X.; Yan, H.; Xie, B.; Xu, W.; Liu, X.; Kang, G.; Jiang, W.; Yuan, J. Pulmonary pathology of early-phase COVID-19 pneumonia in a patient with a benign lung lesion. Histopathology 2020, 77, 823–831. [Google Scholar] [CrossRef] [PubMed]
- de Alencar, J.C.G.; Marchini, J.F.M.; Marino, L.O.; Ribeiro, S.C.D.C.; Bueno, C.G.; da Cunha, V.P.; Neto, F.L.; Neto, R.A.B.; Souza, H.P.; COVID U. S., P. Registry Team. Lung ultrasound score predicts outcomes in COVID-19 patients admitted to the emergency department. Ann. Intensiv. Care 2021, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; González-Bernal, J.J.; Sánchez-Serrano, N.; Navascués, L.J.; Ascaso-Del-Río, A.; Mielgo-Ayuso, J. Physical Exercise as a Multimodal Tool for COVID-19: Could It Be Used as a Preventive Strategy? Int. J. Environ. Res. Public Heal. 2020, 17, 8496. [Google Scholar] [CrossRef] [PubMed]
- Barker-Davies, R.M.; O'Sullivan, O.; Senaratne, K.P.P.; Baker, P.; Cranley, M.; Dharm-Datta, S.; Ellis, H.; Goodall, D.; Gough, M.; Lewis, S.; et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br. J. Sports Med. 2020, 54, 949–959. [Google Scholar] [CrossRef]
- Humphreys, H.; Kilby, L.; Kudiersky, N.; Copeland, R. Long COVID and the role of physical activity: a qualitative study. BMJ Open 2021, 11, e047632. [Google Scholar] [CrossRef]
- Shobeiri, P.; Seyedmirzaei, H.; Karimi, N.; Rashidi, F.; Teixeira, A.L.; Brand, S.; Sadeghi-Bahmani, D.; Rezaei, N. IL-6 and TNF-α responses to acute and regular exercise in adult individuals with multiple sclerosis (MS): a systematic review and meta-analysis. Eur. J. Med Res. 2022, 27, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.T.; Shin, D.B.; Winthrop, K.L.; Gelfand, J.M. The risk of respiratory tract infections and symptoms in psoriasis patients treated with interleukin 17 pathway–inhibiting biologics: A meta-estimate of pivotal trials relevant to decision making during the COVID-19 pandemic. J. Am. Acad. Dermatol. 2020, 83, 677–679. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





