1. Introduction
Chemokines are chemotactic cytokines with a small molecular weight (8,000–12,000), and are classified into four categories: CC, CXC, CX3C, and XC subfamilies, according to the number and position of cysteine residues in their N-terminus [
1,
2]. Approximately 50 chemokines, with diverse functions, have been discovered so far. Chemokines were originally thought to function in the leukocyte development, differentiation, and migration, but they are now known to be deeply involved in the pathogenesis of numerous diseases, including inflammatory diseases and cancers [
3,
4]. Chemokines exert their functions by binding to corresponding seven-transmembrane G-protein-coupled receptors (GPCRs). This chemokine-GPCR interaction is important for the physiological functions and pathogenesis of cells [
5].
In 1997, the CC chemokine receptor 6 (CCR6) was discovered as a specific receptor for CC motif chemokine ligand 20 (CCL20) [
6]. CCR6 has been reported to be expressed in tissues, including intestinal mucosa, lung mucosa, and lymph nodes, as well as B and T cells. CCL20, also known as macrophage inflammatory protein-3α (MIP-3α) and liver and activation-regulated chemokine (LARC), is expressed in tissues, such as the liver, lung, and lymph nodes. CCL20 is expressed in immune-related cells, such as dendritic cells, Th17 cells, natural killer cells, and B cells [
4]. CCL20 functions in cooperation with CCR6.
The binding mode of CCL20 and CCR6 has been structurally elucidated [
7]. CCL20 has the shortest N-terminus among chemokines and binds to a shallow surface region of CCR6. CCL20 or/and CCR6 are involved in the progression of numerous pathologies [
8]. CCL20/CCR6-activated M1 macrophages cause adenoid hypertrophy by adenoid epithelium inflammation. An imiquimod-induced psoriasis model reported that skin-homing CCR6+ γδ
Low T cells strongly produce interleukin (IL)-17A and cause inflammation [
9]. Furthermore, a CCR6 antagonist ameliorates inflammatory bowel disease by regulating immune cell chemotaxis [
10]. These reports indicate that CCL20/CCR6 contributes to pathogenesis by controlling immune cells.
Many reports have shown the effects of CCL20/CCR6 in tumors [
11]. The regulatory T (Treg) cells, which is a suppressive factor of cancer immunity, interact with CCL20 in the tumor microenvironment via CCR6 on Treg, and contribute to cancer progression and poor prognoses, such as oral, head and neck squamous carcinomas, and hepatocellular carcinomas [
12,
13,
14]. CCL20, which is secreted by macrophages and stromal fibroblasts, promotes cancer cells or Th17 cell behavior [
15,
16,
17]. CCL20/CCR6 appears to play roles in managing immune cells around tumors. CCR6-expressing chimeric antigen receptor-T cells (CAR-T) have been shown to be effective in lung cancer models [
18]. These findings make the CCL20/CCR6 axis an attractive therapeutic target for various diseases.
Previously, we have developed various monoclonal antibodies (mAbs) against mouse and human chemokine receptors, including an anti-mouse CCR2 mAb [
19], anti-mouse CCR3 mAbs [
20,
21,
22,
23], an anti-mouse CCR4 mAb [
24], anti-mouse CCR6 mAbs [
25], anti-mouse CCR8 mAbs [
26], an anti-mouse CCR9 mAb [
27], an anti-mouse CXCR6 mAb [
28], an anti-human CCR2 mAb [
29], and anti-human CCR9 mAbs [
30,
31]. In this study, we developed anti-human CCR6 (hCCR6) mAbs using the N-terminal peptide immunization method.
2. Materials and Methods
2.1. Cell lines
Human liver carcinoma (HepG2) and differentiated hepatoma (HuH-7) were obtained from the Cell Resource Center for Biomedical Research Institute of Development, Aging and Cancer at Tohoku University. Chinese hamster ovary (CHO)-K1 and P3X63Ag8U.1 (P3U1) cells were obtained from the American Type Culture Collection (Manassas, VA). The expression plasmid of hCCR6 (Accession No.: NM_004367; pCMV6neo-hCCR6-Myc-DDK) was purchased from OriGene Technologies, Inc. (Rockville, MD). A Neon transfection system (Thermo Fisher Scientific Inc., Waltham, MA) was used to transfect the hCCR6 plasmid into CHO-K1 cells. Stable transfectants were established through cell sorting using a cell sorter (SH800; Sony Corp., Tokyo, Japan) and cultivation in a medium containing 0.5 mg/mL of G418 (Nacalai Tesque, Inc., Kyoto, Japan).
CHO-K1, P3U1, hCCR6-overexpressed CHO-K1 (CHO/hCCR6), and HuH-7 cells were cultured in the Roswell Park Memorial Institute (RPMI)-1640 medium (Nacalai Tesque, Inc.). HepG2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Nacalai Tesque, Inc.) RPMI-1640 and DMED were supplemented with 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific Inc.), 100 units/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B (Nacalai Tesque, Inc.). Cells were grown in a humidified incubator at 37°C with an atmosphere of 5% CO2 and 95% air.
2.2. Antibodies
An anti-hCCR6 mAb (clone G034E3) was purchased from BioLegend (San Diego, CA). Secondary Alexa Fluor 488-conjugated anti-mouse IgG was purchased from Cell Signaling Technology, Inc. (Danvers, MA).
2.3. Peptides
A partial sequence of the N-terminal extracellular region of hCCR6 (1-MNFSDVFDSSEDYFVSVNT-19) plus C-terminal cysteine (hCCR6p1-19C) was synthesized by Eurofins Genomics KK (Tokyo, Japan). Subsequently, the keyhole limpet hemocyanin (KLH) was conjugated at the C-terminus of the peptide (hCCR6p1-19C-KLH).
2.4. Production of Hybridomas
The two 5-week-old BALB/c mice were purchased from CLEA Japan (Tokyo, Japan) and were housed under specific pathogen-free conditions. All animal experiments were conducted following relevant guidelines and regulations to minimize animal suffering and distress in the laboratory. The Animal Care and Use Committee of Tohoku University (Permit number: 2019NiA-001) approved the animal experiments. The mice were monitored daily for their health during the full 4-week experiment duration. A humane endpoint was defined as a reduction of >25% of total body weight. The mice were euthanized through cervical dislocation, and death was verified through respiratory and cardiac arrest.
The mice were intraperitoneally immunized using 100 µg of the hCCR6p1-19C-KLH peptide with Imject Alum (Thermo Fisher Scientific Inc.) to develop mAbs against hCCR6. The procedure included three additional immunizations, followed by a final booster intraperitoneal injection, 2 days before harvesting the spleen cells. PEG1500 (Roche Diagnostics, Indianapolis, IN) was used to subsequently fuse harvested spleen cells with P3U1 cells. Afterward, hybridomas were grown in the RPMI-1640 medium, supplemented with 10% heat-inactivated FBS, 100 units/mL of penicillin, 100 μg/mL of streptomycin, 0.25 μg/mL of amphotericin B, and hypoxanthine/ aminopterin/ thymidine (HAT) for the selection (Thermo Fisher Scientific Inc.).
Enzyme-linked immunosorbent assay (ELISA) with the hCCR6p1-19C peptide was used to subsequently screen supernatants, followed by flow cytometry, using CHO/hCCR6, CHO-K1, HepG2, and HuH-7 cells.
2.5. ELISA
The hCCR6p1-19C peptide was immobilized on Nunc Maxisorp 96-well immunoplates (Thermo Fisher Scientific Inc.) at a concentration of 1 µg/mL for 30 min at 37°C. After washing with phosphate-buffered saline (PBS) containing 0.05% Tween20 (PBST; Nacalai Tesque, Inc.), wells were blocked with 1% bovine serum albumin (BSA)-containing PBST for 30 min at 37°C. The plates were incubated with hybridoma supernatants, followed by a peroxidase-conjugated anti-mouse immunoglobulins (1:2000 diluted; Agilent Technologies Inc., Santa Clara, CA). Enzymatic reactions were performed using the ELISA POD Substrate TMB Kit (Nacalai Tesque, Inc.). Optical density was measured at 655 nm using an iMark microplate reader (Bio-Rad Laboratories, Inc., Berkeley, CA).
2.6. Flow Cytometric Analysis
Cells were harvested after a brief exposure to 0.25% trypsin and 1 mM of ethylenediaminetetraacetic acid (EDTA; Nacalai Tesque, Inc.). They were washed with 0.1% BSA in PBS and treated with 1, 0.1, 0.01, or 0.001 μg/mL of anti-hCCR6 mAbs for 30 min at 4°C. Then, the cells were treated with Alexa Fluor 488-conjugated anti-mouse IgG (1:2000). Fluorescence data were obtained using SA3800 Cell Analyzer (Sony Corp.).
2.7. Determination of KD by Flow Cytometry
The CHO/hCCR6, HepG2, and HuH-7 cells were suspended in 100 μL of serially diluted anti-hCCR6 mAbs and then 50 μL of Alexa Fluor 488-conjugated anti-mouse IgG (1:200) was added. The BD FACSLyric (BD Biosciences, Franklin Lakes) was used to obtain fluorescence data. The KD was calculated by fitting saturation binding curves to built-in, one-site binding models in GraphPad Prism 6 (GraphPad Software, Inc., La Jolla).
2.8. Immunohistochemical Analysis Using C6Mab-19 against Non-Hodgkin Lymphoma Tissue
A formalin-fixed paraffin-embedded (FFPE) section of lymph nodes from non-Hodgkin lymphoma patients (Catalog No. T2235161-1; BioChain institute Inc., Newark, CA) were autoclaved in citrate buffer (pH6.0; Nichirei Biosciences, Inc., Tokyo, Japan) for 20 min. Then, the section was then incubated with 0.3 µg/mL of C6Mab-19 for 1 h at room temperature followed by treatment of an Envision+ kit (Agilent Technologies Inc.) for 30 min at room temperature. The color was developed using 3,3’-diaminobenzidine tetrahydrochloride (DAB; Agilent Technologies Inc.) for 1 min at room temperature, and the section was then counterstained with hematoxylin (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan).
3. Results
3.1. Establishment of Anti-hCCR6 mAbs Using the Peptide Immunization Method
In this study, we employed the peptide immunization method to develop anti-hCCR6 mAbs (
Figure 1). Two mice were immunized with hCCR6p1-19C-KLH (
Figure 1A). The spleens of the mice were excised, and splenocytes were subsequently fused with myeloma cells (P3U1) using PEG1500 (
Figure 1B). The produced hybridomas were seeded into 96-well plates and cultivated in HAT-contained medium for 7 days.
Hybridoma culture supernatants, which were positive for the hCCR6p1-19C peptide, were screened using ELISA (
Figure 1C). The first screening approach identified signals from hCCR6p1-19C in 134 out of 958 wells (14.0%). We selected the top 86 positive wells for the second screening. The second screening procedure using flow cytometry identified signals against CHO/hCCR6 cells in 49 out of the 86 hybridoma supernatants (57.0%). They did not react with parental CHO-K1 cells. An anti-hCCR6 mAb, C
6Mab-19 (mouse IgG
1, kappa), was finally established after the single-cell cloning of hybridoma by limiting dilution and several additional flow cytometric screenings (
Figure 1D).
3.2. Flow Cytometric Analysis of C6Mab-19 to hCCR6-Expressing Cells
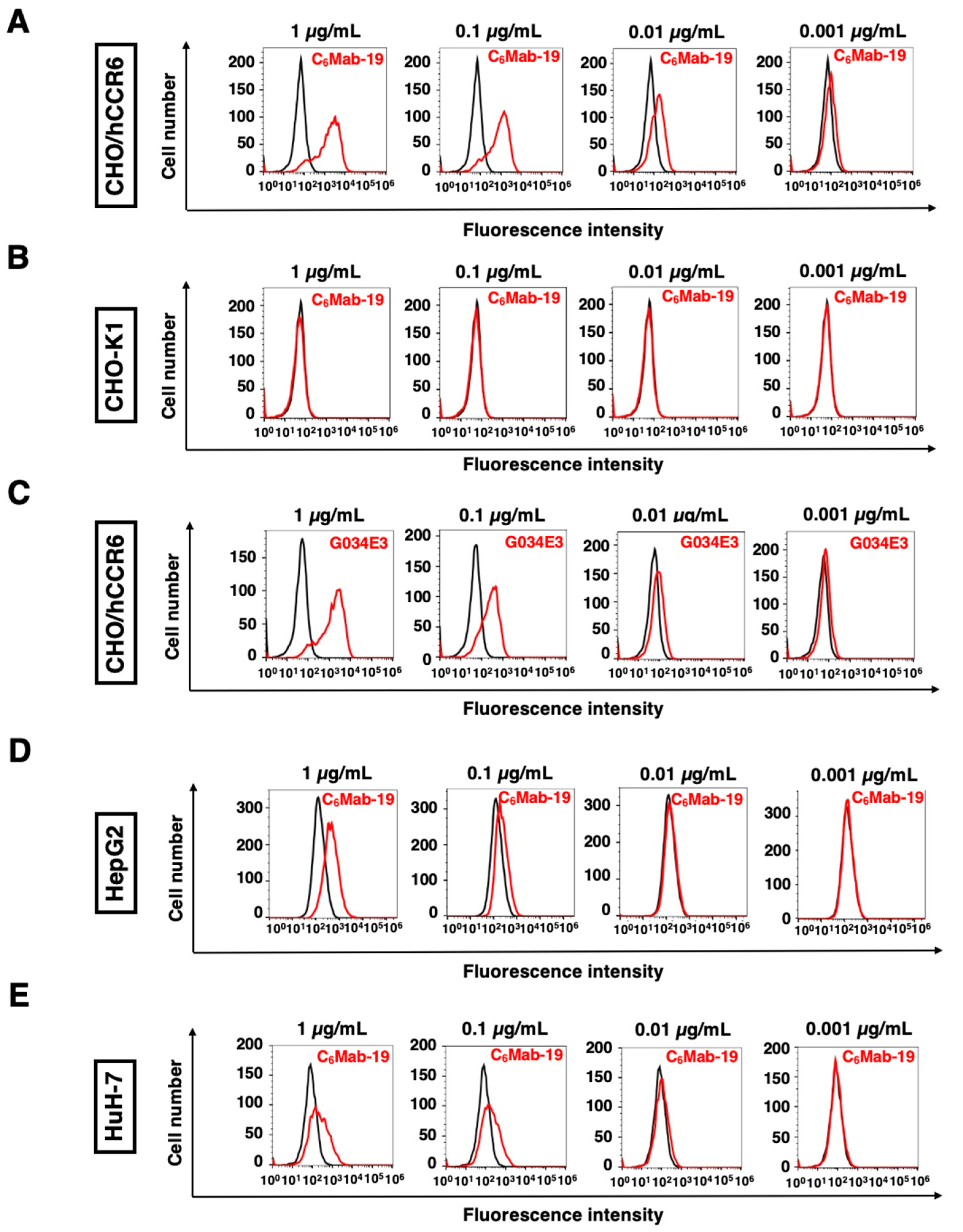
We conducted flow cytometry using C
6Mab-19 and a commercially available anti-hCCR6 mAb (clone G034E3) against CHO/hCCR6, CHO-K1, HepG2, and HuH-7 cells. We selected HepG2 and HuH-7 cells for endogenously hCCR6-expressing cells because CCR6 expression was elevated in the human hepatocellular carcinoma cells in previous studies [
32]. C
6Mab-19 dose-dependently recognized CHO/hCCR6 cells at 1, 0.1, 0.01, and 0.001 μg/mL (
Figure 2A), while C
6Mab-19 did not react to CHO-K1 parental cells even at 1 μg/mL (
Figure 2B). Additionally, C
6Mab-19 possesses higher sensitivity than G034E3, especially at 0.1 and 0.01 µg/mL, although a commercially available anti-hCCR6 mAb (G034E3) recognized CHO/hCCR6 in a dose-dependent manner at 1, 0.1, 0.01, and 0.001 μg/mL (
Figure 2A,C). Moreover, C
6Mab-19 also reacted to endogenously hCCR6-expressing cell lines, such as HepG2 and HuH-7 (
Figure 2D,E). C
6Mab-19 reacted to HepG2 at 1 and 0.1 μg/mL (
Figure 2D), and reacted to HuH-7 at a low concentration of 0.01 μg/mL (
Figure 2E). These results suggest that C
6Mab-19 is applicable for detecting exogenously and endogenously expressed hCCR6 using flow cytometry.
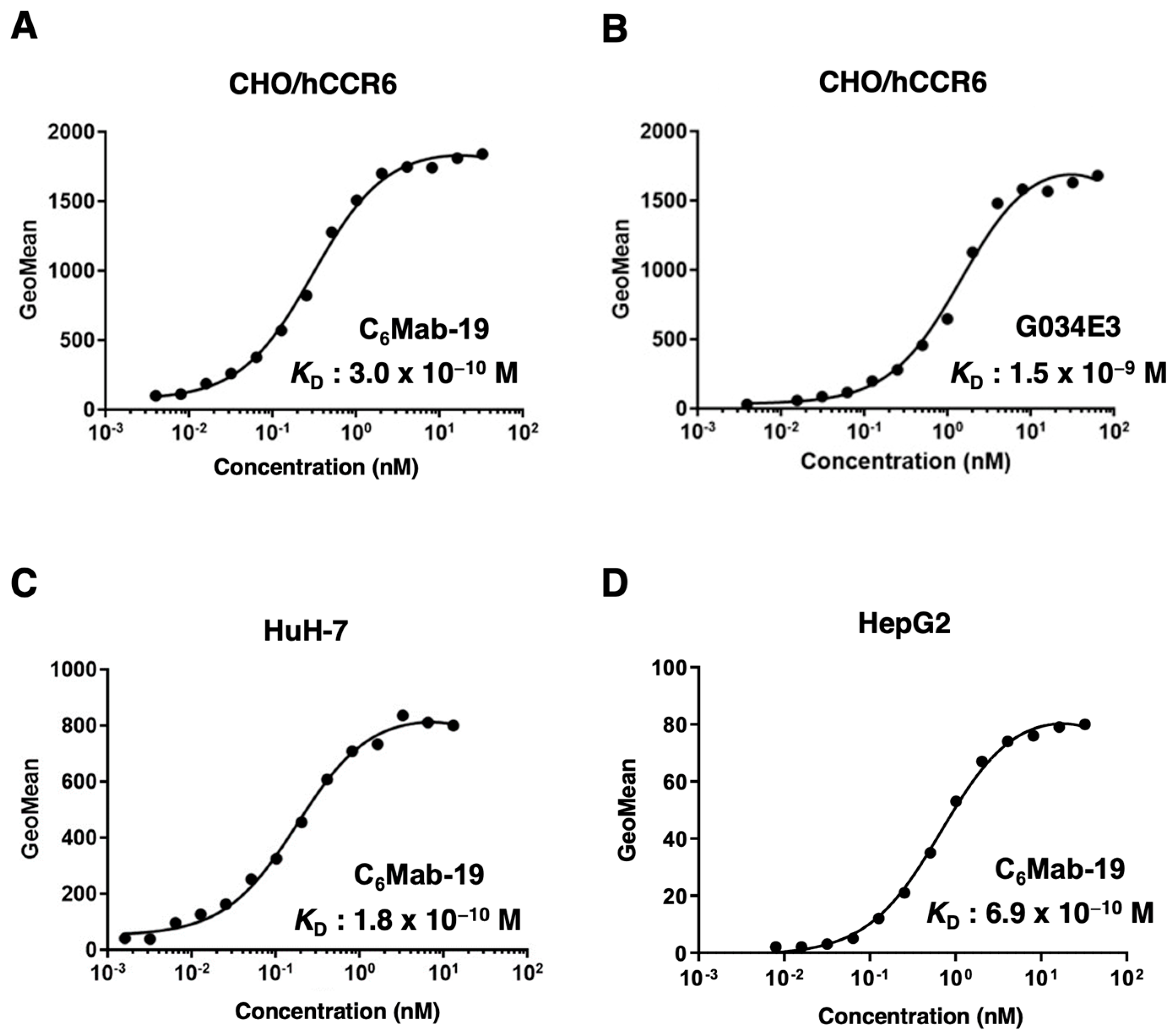
3.3. Determination of the KD for Anti-hCCR6 mAbs against hCCR6-Expressing Cells by Flow Cytometry
We performed the kinetic analysis using flow cytometry to assess the
KD of anti-hCCR6 mAbs, such as C
6Mab-19 and G034E3, for hCCR6-expressing cells. The
KD of C
6Mab-19 for CHO/hCCR6 cells was 3.0 × 10
−10 M, as shown in
Figure 3A. In contrast, the
KD of G034E3 for CHO/hCCR6 cells was 1.5 × 10
-9 M (
Figure 3B), indicating a much higher binding affinity of C
6Mab-19 than that of G034E3.
Next, we analyzed the
KD of C
6Mab-19 against endogenously hCCR6-expressing HuH-7 and HepG2 cells. The
KD of C
6Mab-19 for HuH-7 and HepG2 was determined to be 1.8 × 10
−10 M and 6.9 × 10
−10 M, respectively (
Figure 3C,D). Therefore, C
6Mab-19 possesses a high affinity for exogenously and endogenously hCCR6-expressing cells.
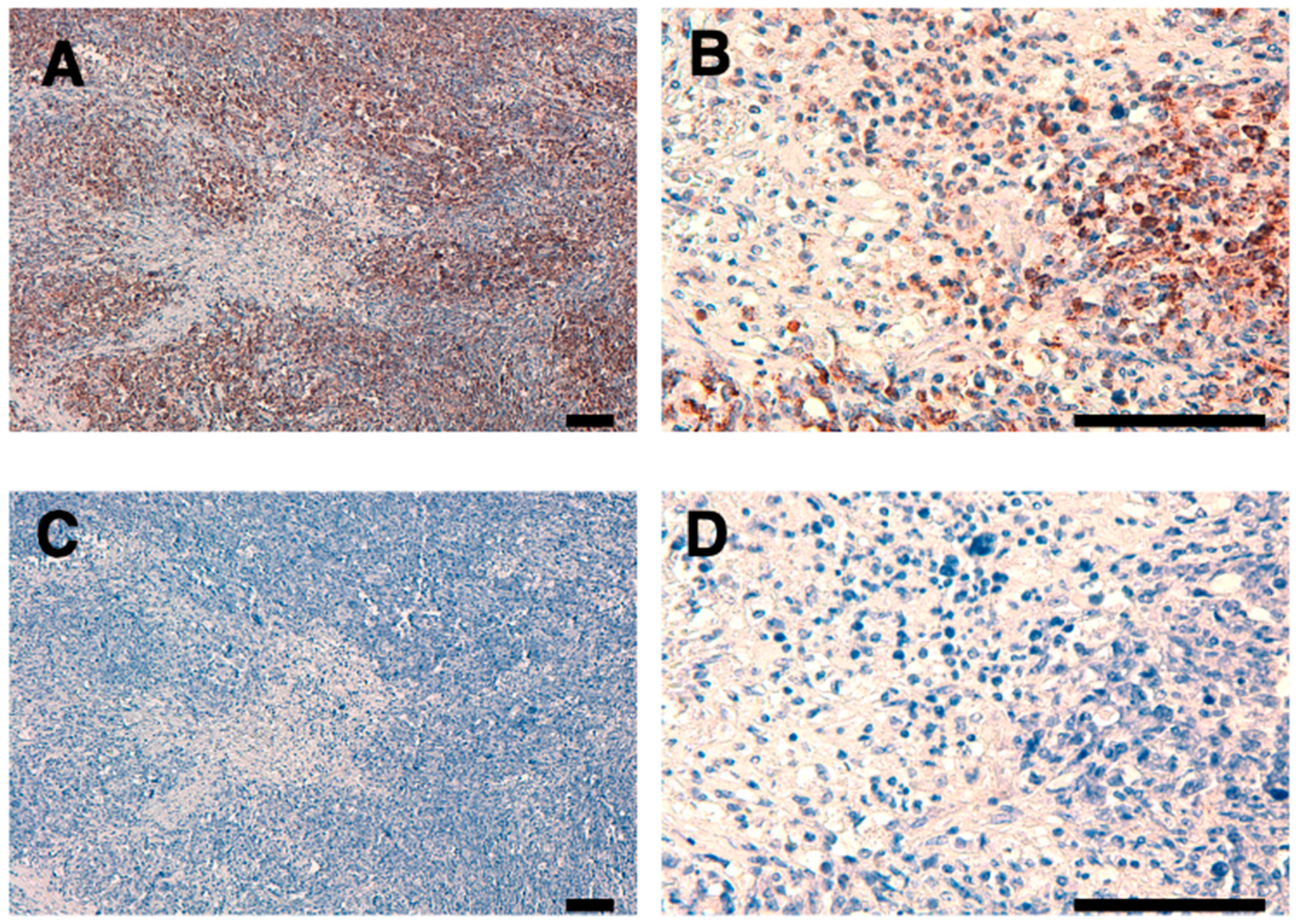
3.4. Immunohistochemical Analysis Using C6Mab-19 against Non-Hodgkin Lymphoma Tissues
We stained a FFPE lymph node tissue from patients with non-Hodgkin lymphoma using C
6Mab-19 to investigate the use of C
6Mab-19 for immunohistochemical analysis. C
6Mab-19 strongly stained non-Hodgkin lymphoma cells, as shown in
Figure 4. In contrast, no staining was observed without primary mAb. Therefore, C
6Mab-19 is useful for immunohistochemical staining of paraffin-embedded sections.
4. Discussion
We have previously established various mAbs against membrane proteins using the Cell-Based Immunization and Screening (CBIS) method. The CBIS method is a valid technique to develop mAbs against complex structured antigens, including GPCRs. Using the CBIS method, we successfully developed an anti-human CCR9 mAb (C
9Mab-1) and confirmed that its epitope is located at the N-terminus of human CCR9 [
30,
33]. By identifying the epitope of C
9Mab-1, we found that the N-terminal region of chemokine receptors is highly antigenic and have successfully developed mAbs against various mouse or human chemokine receptors, including CCR2 [
19,
29], CCR3 [
20,
23], CCR4 [
24], CCR6 [
25], CCR9 [
27,
31], and CXCR6 [
28] by using peptides in the N-terminal region as immunogens. Likewise, we successfully established a novel mAb against hCCR6 (clone C
6Mab-19), which is able to recognize exogenous and endogenous hCCR6 by flow cytometry and immunohistochemistry.
The N-terminus of GPCRs, including CCR6, CCR9, and CXCR6, is identified as the ligand-binding domain [
34,
35,
36,
37]. An anti-hCCR6 mAb (clone 1C6) can block the CCL20-CCR6 interaction and inhibit hCCR6-expressing cells chemotaxis [
36]. The epitope of 1C6 was determined as the N-terminus of hCCR6 [
36]. CCL20 binds to all three extracellular domains of CCR6 and N-terminal residues from Tyr27 to Leu38 [
7]. Conversely, N-terminal residues before Tyr27 in CCR6 may not contribute to the interaction with CCL20 [
7]. The second extracellular domain and N-terminal residues from Tyr27 to Leu38 in CCR6 are the central sites of interaction in CCR6. The epitope of C
6Mab-19 is presented in the 1
st to 19
th amino acids of hCCR6; therefore, C
6Mab-19 might not possess the neutralizing activity for CCL20. We try to establish the mAbs, which have the neutralizing activity for CCL20 by applying the peptide immunization method, in the future studies.
CCL20/CCR6 has been reported to be involved in many inflammatory and autoimmune diseases, and the CCL20/CCR6 axis has been a therapeutic target [
4]. Small molecules, such as compound 1b and MR120, that inhibit CCL20/CCR6 are expected to ameliorate symptoms of chronic colitis in preclinical models [
10,
38]. Interestingly, a recent report revealed that the high prevalence of CCR6 in patients with acute myocardial infarction patients makes it an independent biomarker and correlates with the severity [
39]. Lipid-lowering drugs ameliorate psoriasis by suppressing inflammatory responses that also involve CCL20/CCR6 [
40]. Furthermore, oral squamous cell carcinoma patients with drinking and smoking habits significantly increased the percentage of CD4+ T cells, which express CD45RO and CCR6 in the blood and tumor microenvironment compared with the patients who do not drink or smoke [
41]. Hence, CCR6 is a potent immunoregulatory factor in the formation of pathologies. Therefore, CCR6 is an attractive target to help patients with various inflammatory diseases. C
6Mab-19 can be used in flow cytometry (
Figure 2) and immunohistochemistry (
Figure 4); thus, it may contribute to the diagnosis of such diseases. Additionally, C
6Mab-19 may treat such inflammatory diseases, although further validation is needed.
CCL20, which is secreted into tumor tissues, recruits immunosuppressive CCR6-expressing cells, including Treg cells [
13]. CCL20/CCR6-guided circulating Tregs infiltrate into tumors, resulting in tumor progression and poor prognosis in patients with hepatocellular carcinoma [
14]. Hence, cancer treatment strategies using CCR6-expressing CAR-T cells were designed [
18,
42]. We could detect CCR6-expressing CAR-T cells in blood or tissues using flow cytometry or immunohistochemistry because C
6Mab-19 possesses extremely high binding affinity against hCCR6.
Author Contributions
T.T., K.K., and H.S. performed the experiments. M.K.K. and Y.K designed the experiments. H.S. and Y.K analyzed the data. T.T., H.S. and Y.Kato wrote the manuscript. All authors have read and agreed to the manuscript.
Funding
This research was supported in part by Japan Agency for Medical Research and Development (AMED) under Grant Numbers: JP22ama121008 (to Y.K.), JP22am0401013 (to Y.K.), JP22bm1004001 (to Y.K.), JP22ck0106730 (to Y.K.), and JP21am0101078 (to Y.K.), and by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) grant nos. 21K20789 (to T.T.), 22K06995 (to H.S.), 21K07168 (to M.K.K.), and 22K07224 (to Y.K.).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of Tohoku University (Permit number: 2019NiA-001) for studies involving animals.
Data Availability Statement
The data presented in this study are available in the article and supplementary material.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Zlotnik, A.; Yoshie, O. The chemokine superfamily revisited. Immunity 2012, 36, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. Febs j 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol 2017, 17, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, R.; Eri, R. Modulation of the CCR6-CCL20 Axis: A Potential Therapeutic Target in Inflammation and Cancer. Medicina (Kaunas) 2018, 54. [Google Scholar] [CrossRef] [PubMed]
- Schöneberg, T.; Liebscher, I. Mutations in G Protein-Coupled Receptors: Mechanisms, Pathophysiology and Potential Therapeutic Approaches. Pharmacol Rev 2021, 73, 89–119. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Imai, T.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Hieshima, K.; Nomiyama, H.; Yoshie, O. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J Biol Chem 1997, 272, 14893–14898. [Google Scholar] [CrossRef]
- Wasilko, D.J.; Johnson, Z.L.; Ammirati, M.; Che, Y.; Griffor, M.C.; Han, S.; Wu, H. Structural basis for chemokine receptor CCR6 activation by the endogenous protein ligand CCL20. Nat Commun 2020, 11, 3031. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Guo, X.; Wu, J.; Wang, M.; Ding, H.; Ren, X. CCL20/CCR6 Mediated Macrophage Activation and Polarization Can Promote Adenoid Epithelial Inflammation in Adenoid Hypertrophy. J Inflamm Res 2022, 15, 6843–6855. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Ichimura, Y.; Kubota, N.; Konishi, R.; Nakamura, Y.; Mizuno, S.; Takahashi, S.; Fujimoto, M.; Nomura, T.; Okiyama, N. The Role of PD-L1 on Langerhans Cells in the Regulation of Psoriasis. J Invest Dermatol 2022, 142, 3167–3174. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.G.; Giorgio, C.; Allodi, M.; Palese, S.; Barocelli, E.; Ballabeni, V.; Szpakowska, M.; Chevigné, A.; Piet van Hamburg, J.; Davelaar, N.; et al. Discovery of small-molecules targeting the CCL20/CCR6 axis as first-in-class inhibitors for inflammatory bowel diseases. Eur J Med Chem 2022, 243, 114703. [Google Scholar] [CrossRef] [PubMed]
- Kadomoto, S.; Izumi, K.; Mizokami, A. The CCL20-CCR6 Axis in Cancer Progression. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Kao, K.C.; Chiu, Y.L.; Jung, C.J.; Liu, C.J.; Cheng, S.J.; Chang, Y.L.; Ko, J.Y.; Chia, J.S. Enrichment of Human CCR6(+) Regulatory T Cells with Superior Suppressive Activity in Oral Cancer. J Immunol 2017, 199, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Rutihinda, C.; Haroun, R.; Saidi, N.E.; Ordoñez, J.P.; Naasri, S.; Lévesque, D.; Boisvert, F.M.; Fortier, P.H.; Belzile, M.; Fradet, L.; et al. Inhibition of the CCR6-CCL20 axis prevents regulatory T cell recruitment and sensitizes head and neck squamous cell carcinoma to radiation therapy. Cancer Immunol Immunother 2022. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.J.; Lin, S.Z.; Zhou, L.; Xie, H.Y.; Zhou, W.H.; Taki-Eldin, A.; Zheng, S.S. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One 2011, 6, e24671. [Google Scholar] [CrossRef] [PubMed]
- Walch-Rückheim, B.; Mavrova, R.; Henning, M.; Vicinus, B.; Kim, Y.J.; Bohle, R.M.; Juhasz-Böss, I.; Solomayer, E.F.; Smola, S. Stromal Fibroblasts Induce CCL20 through IL6/C/EBPβ to Support the Recruitment of Th17 Cells during Cervical Cancer Progression. Cancer Res 2015, 75, 5248–5259. [Google Scholar] [CrossRef] [PubMed]
- Kadomoto, S.; Izumi, K.; Hiratsuka, K.; Nakano, T.; Naito, R.; Makino, T.; Iwamoto, H.; Yaegashi, H.; Shigehara, K.; Kadono, Y.; et al. Tumor-Associated Macrophages Induce Migration of Renal Cell Carcinoma Cells via Activation of the CCL20-CCR6 Axis. Cancers (Basel) 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, W.; Wang, X.; Xu, C.; Zhang, N.; Di, W. Cisplatin-stimulated macrophages promote ovarian cancer migration via the CCL20-CCR6 axis. Cancer Lett 2020, 472, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Cao, L.; Zhu, Y.; Cao, J.; Li, X.; Zhou, J.; Liu, B.; Zhao, T. Enhance anti-lung tumor efficacy of chimeric antigen receptor-T cells by ectopic expression of C-C motif chemokine receptor 6. Sci Bull (Beijing) 2021, 66, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Li, G.; Asano, T.; Saito, M.; Kaneko, M.K.; Suzuki, H.; Kato, Y. Development of a Novel Anti-Mouse CCR2 Monoclonal Antibody (C(2)Mab-6) by N-Terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022, 41, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Nanamiya, R.; Takei, J.; Nakamura, T.; Yanaka, M.; Hosono, H.; Tanaka, T.; Sano, M.; Kaneko, M.K.; Kato, Y. Development of Anti-Mouse CC Chemokine Receptor 3 Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021, 40, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Harigae, Y.; Li, G.; Asano, T.; Tanaka, T.; Suzuki, H.; Kaneko, M.K.; Kato, Y. C(3)Mab-2: An Anti-Mouse CCR3 Monoclonal Antibody for Immunocytochemistry. Monoclon Antib Immunodiagn Immunother 2022, 41, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Suzuki, H.; Tanaka, T.; Saito, M.; Li, G.; Goto, N.; Nanamiya, R.; Kaneko, M.K.; Kato, Y. C(3)Mab-3: A Monoclonal Antibody for Mouse CC Chemokine Receptor 3 for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2022, 41, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Suzuki, H.; Goto, N.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Establishment of Novel Anti-Mouse CCR3 Monoclonal Antibodies (C(3)Mab-6 and C(3)Mab-7) by N-terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022, 41, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Takei, J.; Suzuki, H.; Asano, T.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Anti-Mouse CCR4 Monoclonal Antibody (C(4)Mab-1) by N-Terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022, 41, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Tanaka, T.; Suzuki, H.; Li, G.; Nanamiya, R.; Tateyama, N.; Isoda, Y.; Okada, Y.; Kobayashi, H.; Yoshikawa, T.; et al. Development of a Novel Anti-Mouse CCR6 Monoclonal Antibody (C(6)Mab-13) by N-Terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022, 41, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nanamiya, R.; Takei, J.; Nakamura, T.; Yanaka, M.; Hosono, H.; Sano, M.; Asano, T.; Kaneko, M.K.; Kato, Y. Development of Anti-Mouse CC Chemokine Receptor 8 Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021, 40, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Asano, T.; Suzuki, H.; Tanaka, T.; Yoshikawa, T.; Kaneko, M.K.; Kato, Y. Establishment of a Sensitive Monoclonal Antibody Against Mouse CCR9 (C(9)Mab-24) for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2022. [Google Scholar] [CrossRef]
- Kitamura, K.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Cx(6)Mab-1: A Novel Anti-Mouse CXCR6 Monoclonal Antibody Established by N-Terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022, 41, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Li, G.; Saito, M.; Suzuki, H.; Asano, T.; Kaneko, M.K.; Kato, Y. Development of an Anti-human CCR2 Monoclonal Antibody (C(2)Mab-9) by N-Terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022, 41, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Nanamiya, R.; Takei, J.; Asano, T.; Tanaka, T.; Sano, M.; Nakamura, T.; Yanaka, M.; Hosono, H.; Kaneko, M.K.; Kato, Y. Development of Anti-Human CC Chemokine Receptor 9 Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021, 40, 101–106. [Google Scholar] [CrossRef]
- Tanaka, T.; Suzuki, H.; Isoda, Y.; Asano, T.; Nakamura, T.; Yanaka, M.; Handa, S.; Takahashi, N.; Okuno, S.; Yoshikawa, T.; et al. Development of a Sensitive Anti-Human CCR9 Monoclonal Antibody (C(9)Mab-11) by N-Terminal Peptide Immunization. Monoclon Antib Immunodiagn Immunother 2022, 41, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Itoh, Y.; Yamaguchi, K.; Yamauchi, N.; Harano, Y.; Nakajima, T.; Minami, M.; Okanoue, T. Chemokine CCL20 enhances the growth of HuH7 cells via phosphorylation of p44/42 MAPK in vitro. Biochem Biophys Res Commun 2004, 322, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Takei, J.; Asano, T.; Li, G.; Saito, M.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Epitope Mapping of an Anti-Human CCR9 Monoclonal Antibody (C(9)Mab-1) Using Enzyme-Linked Immunosorbent Assay. Monoclon Antib Immunodiagn Immunother 2021, 40, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, S.; Vela, M.; Franco-Villanueva, A.; Carramolino, L.; Gutiérrez, J.; Gómez, L.; Lozano, M.; Salvador, B.; García-Gallo, M.; Martínez, A.C.; et al. Antitumor effects of a monoclonal antibody to human CCR9 in leukemia cell xenografts. MAbs 2014, 6, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Chain, B.; Arnold, J.; Akthar, S.; Brandt, M.; Davis, D.; Noursadeghi, M.; Lapp, T.; Ji, C.; Sankuratri, S.; Zhang, Y.; et al. A Linear Epitope in the N-Terminal Domain of CCR5 and Its Interaction with Antibody. PLoS One 2015, 10, e0128381. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Melero, S.; García-Maceira, F.I.; García-Maceira, T.; Luna-Guerrero, V.; Montero-Peñalvo, G.; Túnez-Fiñana, I.; Paz-Rojas, E. Amino terminal recognition by a CCR6 chemokine receptor antibody blocks CCL20 signaling and IL-17 expression via β-arrestin. BMC Biotechnol 2021, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Durán, G.; Romo-Mancillas, A. Behavior of Chemokine Receptor 6 (CXCR6) in Complex with CXCL16 Soluble form Chemokine by Molecular Dynamic Simulations: General Protein‒Ligand Interaction Model and 3D-QSAR Studies of Synthetic Antagonists. Life (Basel) 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Allodi, M.; Giorgio, C.; Incerti, M.; Corradi, D.; Flammini, L.; Ballabeni, V.; Barocelli, E.; Radi, M.; Bertoni, S. Probing the effects of MR120 in preclinical chronic colitis: A first-in-class anti-IBD agent targeting the CCL20/CCR6 axis. Eur J Pharmacol 2023, 175613. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, A.M.N.; Mohamed, A.E.; Bendary, A.M.; Sedki, A.A.; El-Shimi, O.S. Assessment Of Circulating CCR6 Level In Acute Myocardial Infarction And Its Association With Disease Severity. Cardiovasc Hematol Agents Med Chem 2023. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Xing, M.; Hong, S.; Liu, L.; Ding, X.J.; Sun, X.Y.; Luo, Y.; Wang, C.X.; Zhang, M.; et al. Current evidence on the role of lipid lowering drugs in the treatment of psoriasis. Front Med (Lausanne) 2022, 9, 900916. [Google Scholar] [CrossRef] [PubMed]
- Rochefort, J.; Karagiannidis, I.; Baillou, C.; Belin, L.; Guillot-Delost, M.; Macedo, R.; Le Moignic, A.; Mateo, V.; Soussan, P.; Brocheriou, I.; et al. Defining biomarkers in oral cancer according to smoking and drinking status. Front Oncol 2022, 12, 1068979. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Chen, J. CAR-T cell engineering with CCR6 exhibits superior anti-solid tumor efficacy. Sci Bull (Beijing) 2021, 66, 755–756. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).