1. Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia encountered in clinical practice and is a major risk factor for ischemic stroke and systemic embolism (SE) [
1,
2]. The majority of these complications are prevented by the use of oral anticoagulant agents (OACs) [
3]. International clinical practice guidelines support and recommend the use of direct OACs (DOACs) in preference to vitamin K antagonists (VKAs) in high-risk patients with nonvalvular AF (NVAF) [
4,
5]. These recommendations were based on randomized clinical trials (RCT) comparing DOACs to VKAs in preventing stroke and SE in patients with NVAF. These benefits of OACs, however, are achieved at the expense of an increased risk of major bleeding (MB) events [
6,
7,
8].
Bleeding events in AF patients using OACs have been extensively studied in many regions across the globe [
9,
10,
11]. In the Middle East, however, there are no published contemporary AF studies that specifically evaluated the incidence of MB and clinically-relevant non-major bleeding (CRNM) events, the demographic profiles of patients who sustained such events, and the predictive factors and prognosis of the bleeding episodes.
The incidence of AF in the Middle East is rising due to the high prevalence of cardiovascular risk factors and the steady increases in life expectancy in this region [
12,
13]. In addition to lacking detailed analysis of bleeding events, the published AF clinical studies and registries in this region suffered considerable limitations including their small size, being single-center with cross-sectional or retrospective design, and being conducted before the wide-spread utilization of DOACs in clinical practice [
14,
15,
16,
17,
18]. The Jordan AF Study is a prospective, multicenter study that enrolled patients with valvular AF (VAF) and nonvalvular AF (NVAF) and followed them for one year. Recently, we have shown that the rate of utilization of OACs, including DOACs in particular, in the study population and adherence rate of the use of these agents according to clinical practice guidelines is high and comparable to rates reported from other regions in the world [
19]. The aim of this study was to determine the one-year incidence of MB and CRNM bleeding events, the clinical and echocardiographic profiles of patients with AF who sustain such events, the factors associated with MB, and the one-year cardiovascular outcomes of patients with bleeding events.
2. Materials and Methods
The methods of the Jordan AF study have been described previously [
19]. In brief, the study involved consecutive adults with AF who were treated at 29 hospitals and ambulatory cardiology clinics in Jordan from May 2019 through October 2020. Diagnosis of AF was based on a 12-lead electrocardiogram (ECG), rhythm strip duration of > 30 seconds, ambulatory ECG monitor showing one episode or more of AF, or a previous diagnosis of AF by a treating physician. Patients with VAF included those with at least moderate rheumatic mitral stenosis or prosthetic mechanical cardiac valve. All other patients were classified as having NVAF. Patients were followed up for one year through out-patient clinic visits, hospital readmissions, or phone calls at 1, 6, and 12 months after enrollment to document the incidence of the prespecified endpoints of MB and CRNM bleeding events, stroke and SE, hospital admission, and all-cause and cardiovascular mortality.
Major bleeding events were defined according to the International Society of Thrombosis and Hemostasis criteria and included fatal bleeding, symptomatic bleeding in a critical area or organ (i.e., intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial) and/or bleeding that causes a fall in hemoglobin level of ≥2 g/dL or requires ≥ 2 units of whole blood or red cells transfusion [
20]. CRNM bleeding events included the events that were not major events but resulted in hospitalization, necessitated medical and/or surgical evaluation or intervention, or required temporary or permanent change in antithrombotic regimen [
20]. Patients who had both MB and CRNM bleeding events were analyzed with the MB group. HAS-BLED and CHA
2DS
2-VASc scores were calculated for each patient according to standard criteria [
21,
22]. Stroke was diagnosed based on neurologist evaluation and standard clinical and imaging criteria. SE was diagnosed based on documented clinical, angiographic, intra-operative, or pathological evidence of embolism to an arterial bed of the extremities or abdominal aorta branches. All-cause mortality included death from non-cardiovascular and cardiovascular causes. Cardiovascular deaths included deaths due to acute coronary syndrome, heart failure, stroke, ventricular tachyarrhythmias, and sudden cardiac death. Death was considered to be related to a major bleeding event in the setting where it could not be attributed to another definite cause. Clinical and echocardiographic baseline profiles and pharmacotherapy in patients with MB were compared to those with no bleeding events.
3. Statistical analysis
Descriptive statistics were performed using means and standard deviation (SD) to describe the continuous variables and percentages were used to describe the categorical variables. Independent t-test was used to compare means and chi-square test was used to compare percentages of the variables in patients with MB and no bleeding events. Multivariable binary logistic regression was conducted to determine factors associated with MB events. The variables in the logistic regression model were selected using the stepwise backward method. A P-value of < 0.05 was considered statistically significant.
4. Results
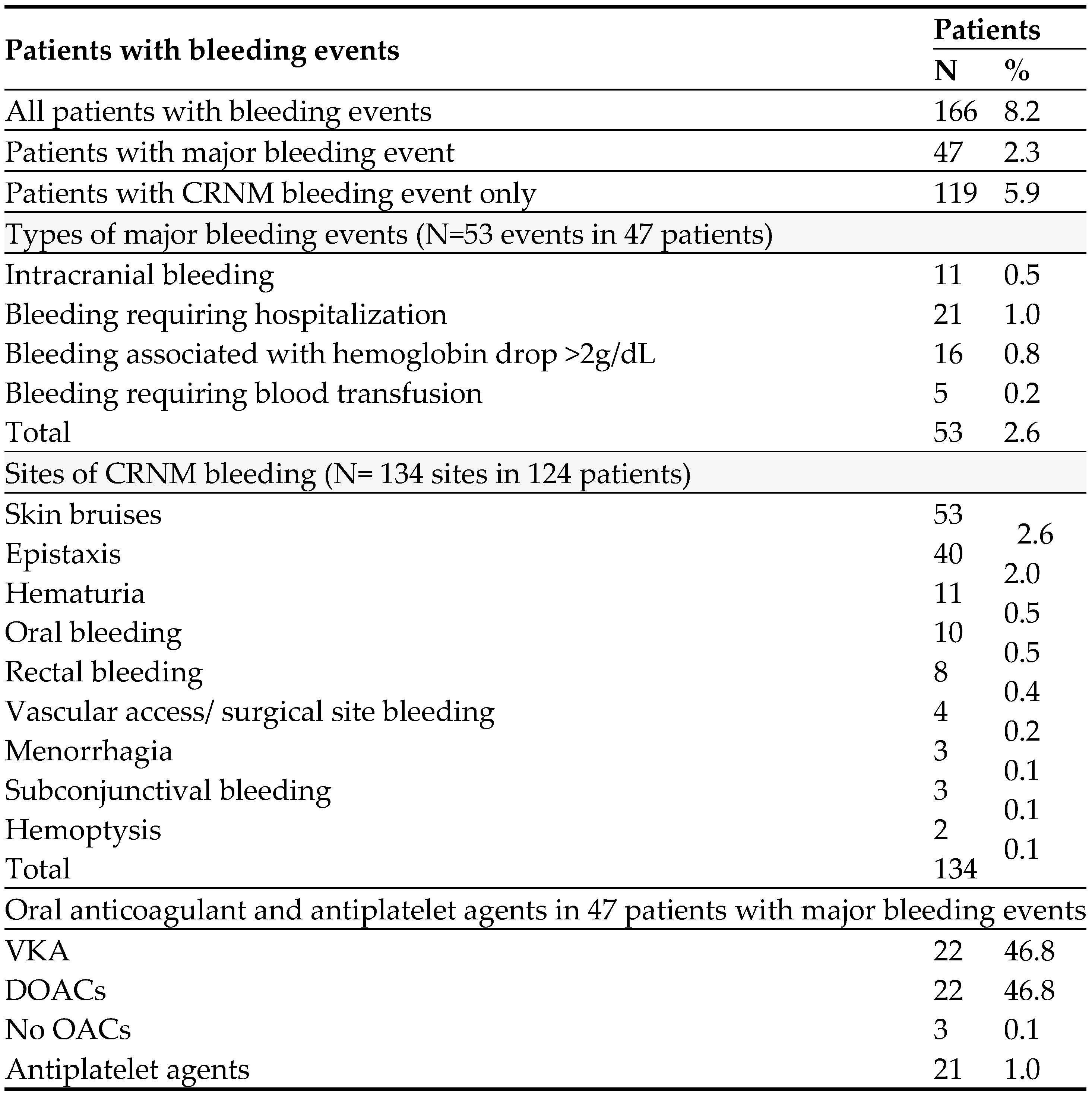
The study enrolled 2020 patients (mean (±SD) age of 67.7±13.0 years and 1096 (54.3%) were women). This analysis included 2018 patients with completed data on all bleeding events. NVAF and VAF were diagnosed in 1847 (91.5%) and 171 (8.5%) patients; respectively. At one year of follow-up, 166 patients had a bleeding event (8.2 events per 100 patient-years). MB occurred in 47 patients (2.3 events per 100 patient-years), and 119 patients had CRNM bleeding (5.9 events per 100 patient-years).
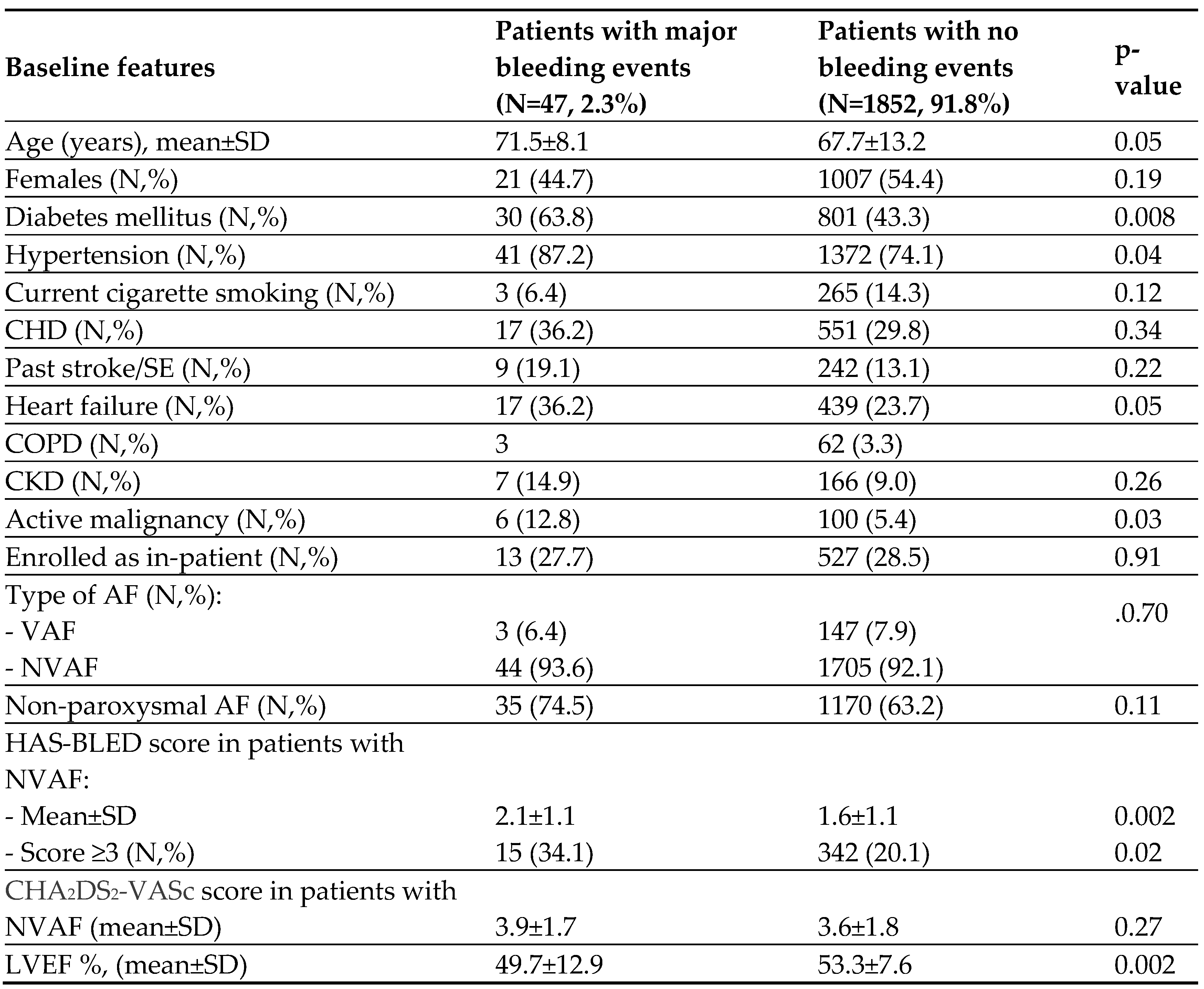
Table 1 depicts the baseline clinical and echocardiographic features in patients with major bleeding, and patients with no bleeding events. Patients with MB events were older, and more likely to have diabetes mellitus, hypertension, heart failure, lower mean left ventricular ejection fraction, chronic lung disease, and active malignancy compared to those who had no bleeding events. They also had higher mean HAS-BLED score and were more likely to have a score of ≥3. However, patients with CRNM bleeding events did not have significant differences in those features compared with patients who had no bleeding events.
Table 2 shows a detailed description of all bleeding events including 53 MB events that occurred in the 47 patients who had MB, and 134 non-major bleeding sites in the 119 patients in patients with CRNM bleeding. Of the 47 patients with 53 MB events; 6 patients had 2 events each, and 10 of them had concomitant minor bleeding events. NVAF was present in 44 (93.6%) and VAF in 3 (6.4%) patients. Overall, there were 11 (0.05%) intracranial hemorrhage (ICH) and 5 (0.2%) gastrointestinal (GI) bleeding events. Nearly one-fourth
(24.5%) of the MB events occurred during the first month, 43.4% occurred between the first and 6th month, and 32.1% occurred between the 6th and 12th months. Of the 9 sites of CRNM bleeding sites, the most common 2 were skin bruises and epistaxis.
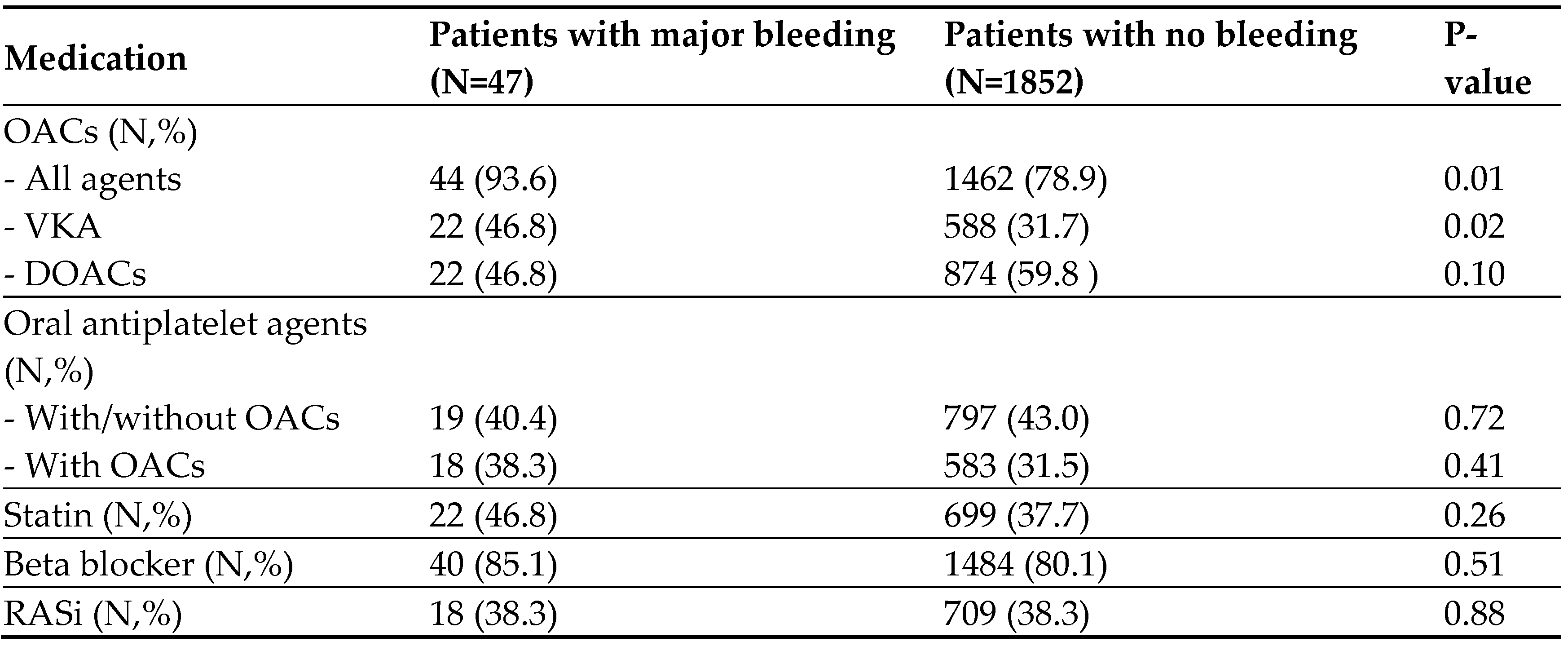
Pharmacotherapy in patients with MB and patients with no bleeding is shown in
Table 3. A significantly higher number of patients with MB than patients with no bleeding events were using OACs. Of the 21.1% of patients with no bleeding who were not using OACs, 51% had a low-risk CHA
2DS
2-VASc score or a contraindication to the use of these medications including thrombocytopenia, bleeding tendency, frailty, and frequent falls.
The rate of use of VKA, but not DOACs, was significantly higher among patients with MB than those with no bleeding. Of the 22 patients with MB on VKA, 12 (54.5%) did drug dose monitoring by the international normalized ratio (INR), and 8 of them had a ratio >3. Of the 11 patients who had ICH, 6 were prescribed VKA (2 of them were taking concomitant antiplatelet agent), 3 received DOAC (one of them1 received concomitant antiplatelet agent), and 2 received antiplatelet agent only. During the follow-up period of the patients who had MB, two were switched from VKA to DOACs, and one was switched from DOAC to VKA. The use of oral antiplatelet agents was not different between the two groups. Patients with CRNM bleeding had a similar rate of use of VKA, but a lower rate of use of DOACs compared with patients who had no bleeding.
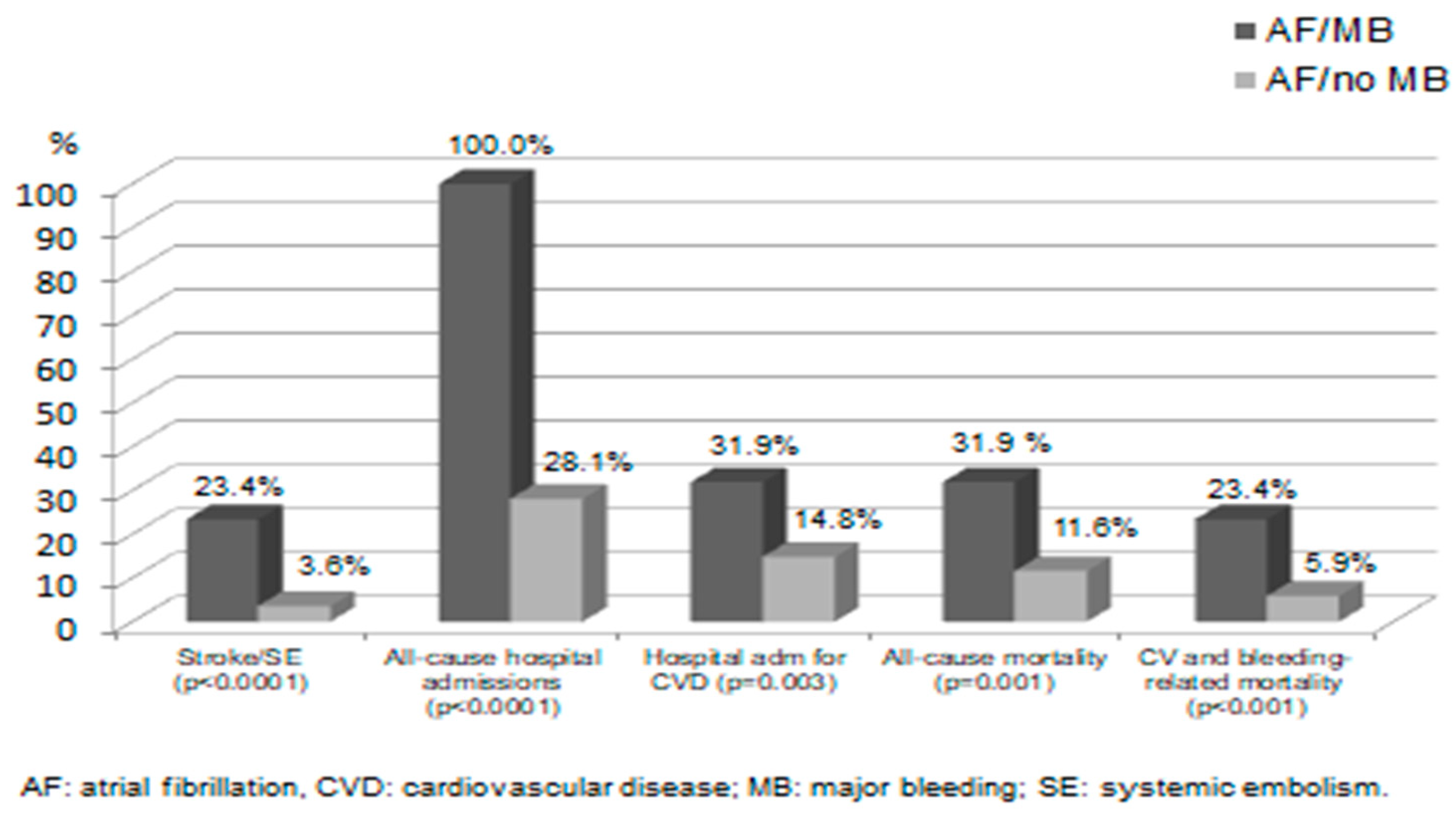
Major cardiovascular events at one year in AF patients with or without MB events are shown in
Figure 1. Patients who had MB had significantly a higher incidence of all of the adverse events reported, including all-cause mortality, all-cause hospitalization, and stroke/SE. Five of the 11 (45.5%) patients who had ICH, and 9 of the 36 (25.0%) patients with other types of major bleeding were dead at one year. Of the 11 cases of stroke/SE reported in the patients with MB, 8 cases occurred before MB and only 3 cases occurred after holding OACs.
Analysis of the high rate of all-cause hospitalizations and mortality revealed a high rate of deaths and hospital admissions related to non-cardiovascular diseases. Of the 109 non-cardiovascular–related deaths in both groups, there were 68 (62.4%) related to cancer and COVID-19 infection and its complications (respiratory failure, sepsis, pulmonary embolism, and surgery). Furthermore, about one-half of hospital admissions in the group with no bleeding events and one-third of those in the group with MB were related to a non-cardiovascular indication. Patients who had CRNM bleeding only did not have a significantly higher incidence of stroke/SE (1 case, 0.8%), admission for cardiovascular cause (14 cases, 11.8%), or all-cause mortality (4 cases, 3.4%) compared with patients who did not have bleeding events (all p-values=NS).
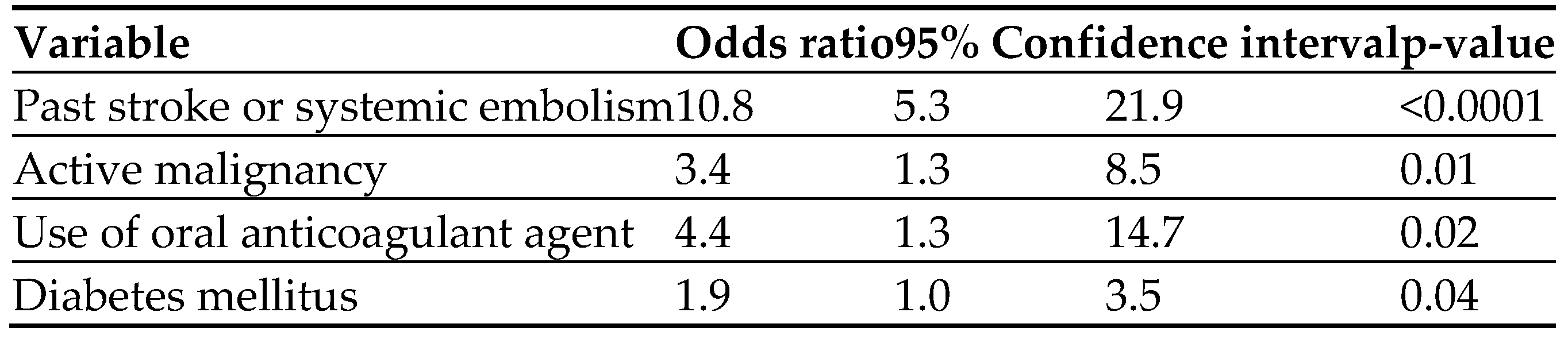
Of 19 variables tested, four were found in the
multivariable analysis to be independent predictors of MB. The strongest factor was past stroke/SE. The other three were the use of OACs, active malignancy, and DM. (
Table 4).
5. Discussion
The present study is the first in the Middle East to evaluate the incident bleeding events in a large cohort of patients with AF. The principal findings were: (i) the incidence rates of one-year major and CRNM bleeding events were 2.3 and 5.9 events per 100 patient-years; respectively, (ii) patients with MB events had worse baseline clinical profiles and significantly higher rates of adverse cardiovascular outcomes compared with patients who did not have bleeding events, and (iii) several independent predictive factors for mortality in patients who sustained an MB event were identified.
Overall, these results are in agreement with findings by studies from other regions which reported similar rates, outcomes, and predictive factors of bleeding events in patients with AF. Three studies from South East Asia, Japan, and Spain reported incidence rates of MB of 4.6%, 4.8%, and 6.9%; respectively [
23,
24,
25]. A fourth study from Norway reported an incidence of MB and CRNM bleeding of 5.9% [
26]. A meta-analysis of eight AF randomized clinical studies found that the rates of MB events ranged between 1.4% and 3.4% among VKA-treated patients [
8]. In the four RCTs of DOACs, the incidence of MB events varied from 3.3% to 5.1% in the DOAC arms and from 4.4% to 5.2% in the VKA arms [
27]. Middle Eastern AF studies reported MB rates ranging from 1.7% to 5.8% [
14,
15,
18,
28]. These ME studies, however, did not report the details of clinical profiles, pharmacological therapy, and outcome in the patients who sustained bleeding events [
12,
13,
14,
15,
16,
17,
18,
29].
OACs are highly effective in reducing stroke and SE in patients with VAF and NVAF, and according to the evidence-based clinical practice guidelines, DOACs are considered the anticoagulant agents of choice for eligible high-risk patients with NVAF [
4,
5,
30]. Despite an overall positive benefit-harm ratio of OACs, the increasing utilization of these agents in clinical practice has been closely related to an increased risk of MB and CRNM bleeding events [
3,
4,
5,
6,
7,
31]. This increased risk of bleeding is not entirely related to the use of OACs as patients with AF have a higher intrinsic risk of bleeding in addition to the coexistence of concomitant and comorbid diseases such as hypertension, chronic renal disease, hematological disorders, and malignancies that contribute to the increased bleeding risk [
32].
The most feared and detrimental complication of OACs continues to be ICH, despite the clear evidence from RCTs that DOACs are associated with a reduced risk for this complication compared with VKA [
4,
5,
6,
7,
33]. This event occurred in 0.05% of our patients and accounted for ≈ 20% of all of the MB events. The one-year mortality rate in these patients was close to 50%, a rate double that of patients with other types of MB. Other investigators reported similar results, where the incidence of ICH in AF patients varied between 0.06% and 1.0% with an associated mortality rate ranging between 45% to 55% [
8,
34].
Concomitant use of oral antiplatelet agents is independently associated with an increased risk of ICH [
33]. Of the patients with ICH, 5 were using antiplatelet agents, including 3 patients who used DOAC and antiplatelet agents concomitantly. RCTs and registries have suggested that the risk of MB events, including ICH with OACs use, including VKA and DOACs, was higher for Asians than Caucasians [
23,
35,
36]. The overwhelming majority of patients in the Middle East are Caucasians and do not share this high risk of MB with Asians, as our study has shown.
The other categories of MB events (those associated with significant hemoglobin drop, necessitating hospital admission, or requiring blood products transfusion) are mainly consistent with GI bleeding and accounted for nearly 80% of the MB events in the current study. Several systematic reviews comparing DOACs with VKA demonstrated a 20% higher GI bleeding rate in patients on DOACs probably due to the presence of the active DOAC drug in the GI tract, thus facilitating bleeding from vulnerable lesions [
27,
34,
37]. The current study reinforces the importance of calculating the bleeding risk in patients with NVAF using the HAS-BLED score, which is the most commonly used risk score in clinical practice in AF patients. Patients with a score of 3 or more are considered at high risk of MB, and when prescribed OACs should have a close follow-up and vigilance for the occurrence of bleeding events. The 6.5-fold increased risk of stroke and SE in patients who sustained MB in the current study compared with those who did not have bleeding events is consistent with results from other studies [
38]. This association is related to several factors including the increased thromboembolic risk due to hypotension, hemo-concentration, holding or discontinuing OACs, administration of blood products or antidotes, and undergoing surgical and endoscopic procedures [
38].
MB events were associated with significantly higher rates of all-cause and cardiovascular deaths compared with patients who did not have bleeding events. It is noteworthy that more than half of the study time-frame was conducted in 2020 and 2021 when the COVID-19 pandemic swept through the globe and led to an unprecedented increase in the number of hospital admissions and deaths [
39]. In fact, half (61 of 121) of deaths in the whole cohort were related to COVID-19 infection and its complications, a finding in agreement with other investigators who found that most deaths in AF patients were non-cardiovascular [
40].
Our study shares with many other studies the four independent predictive factors for MB we identified in this study (prior stroke/SE, use of DOACs, DM, and active malignancy). However, the list of bleeding predictive factors is long and includes old age, male sex, thrombocytopenia, anemia, heart failure, kidney and liver disease, peptic ulcer disease, hypertension, concomitant antiplatelet agents, labile INR [
40,
41,
42]. Presence of one or more of these factors in patients with AF should alert the treating physician to the increased risk of MB and hence prescribing OACs (in recommended doses) rather than VKA, offering advice to patients to avoid trauma and falls, and practicing close surveillance of early symptoms and signs of bleeding events.
A few limitations of this study are worth discussing. Non-interventional, observational studies inherently have a potential bias of residual confounding and data collection. This bias was minimized by stressing the importance of recruiting consecutive patients from the participating centers. Patients’ or relatives’ recall of cardiovascular events during the one-year follow-up can also be a source of underreporting of the study endpoints. However, the fact that the studied endpoints were serious events, such as death, stroke, SE, major bleeding, and hospital admission make it very unlikely for these events to be subjected to recall issues. Finally, the high rate of utilization of OACs and the close follow-up of the study population make it difficult to generalize our findings to the whole country or region.
However, enrolling >2000 patients in 5 cities from different sectors of the local health care system might give a credible degree generalizability to the study. Despite these limitations, this study represents an important contribution to the contemporary knowledge of the incidence and impact of MB events in a large Middle Eastern cohort of patients with AF.
6. Conclusions
This Middle Eastern study showed that ≈8% of patients with AF had a bleeding event at one year of follow-up, including ≈2% of patients who had MB events. The incidence of ICH was very low at a rate of 0.05%. Patients who sustained an MB event had worse one year cardiovascular outcomes compared with those who had no bleeding. AF patients with certain clinical predictive factors should have guidance, close observation, and vigilance for incident bleeding events to avoid potentially life-threatening complications.
Clinical studies registration: the study is registered on clinicaltrials.gov (unique identifier number NCT03917992).
Short title: Short title: Bleeding in patients with atrial fibrillation.
Consent for publication
All authors consent to publishing this manuscript. All authors providing consent have been shown the article contents to be published. Authors are prepared to provide copies of signed consent forms to the journal editorial office if requested.
Data availability
Data supporting the results reported in the manuscript can be requested from the corresponding author (rkibdah@just.edu.jo). The additional unpublished data from the study, include excel sheets of enrolled patients and list of all site investigators.
Abbreviations
AF: atrial fibrillation, CI: confidence interval; CRNM: clinically relevant non major (bleeding), DM: diabetes mellitus, DOACs: direct oral anticoagulant agents, ECG: electrocardiogram, GI: gastrointestinal, ICH: intracranial hemorrhage, INR: international normalized ratio, MB: major bleeding, ME: Middle East, NVAF: nonvalvular atrial fibrillation, OACs: oral anticoagulant agents, OR: odds ratio; RCT: randomized clinical trials, SD: standard deviation, SE: systemic embolization, VAF: valvular atrial fibrillation, VKA: vitamin K antagonists.
References
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke. 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, X.; Liang, Y.; et al. Global, regional, and national burden of disease study of atrial fibrillation/flutter, 1990-2019: Results from a global burden of disease study, 2019. BMC Public Health. 2022, 22, 2015. [Google Scholar] [CrossRef] [PubMed]
- Souverein, P.C.; van den Ham, H.A.; Huerta, C.; et al. Comparing risk of major bleeding between users of different oral anticoagulants in patients with nonvalvular atrial fibrillation. Br J Clin Pharmacol. 2021, 87, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; et al.; ESC Scientific Document Group 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019, 74, 104–132. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; et al.; ARISTOTLE Committees and Investigators Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; et al.; RE-LY Steering Committee and Investigators Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.W.; Harrison, S.; Gupta, D.; Lip, G.Y.H.; Lane, D.A. Stroke and Bleeding Risk Assessments in Patients With Atrial Fibrillation: Concepts and Controversies. Sec. Fam. Med. Prim. Care Front. Med 2020. [CrossRef] [PubMed]
- Bassand, J.P.; Accetta, G.; Al Mahmeed, W.; GARFIELD-AF Investigators. Risk factors for death, stroke, and bleeding in 28,628 patients from the GARFIELD-AF registry: Rationale for comprehensive management of atrial fibrillation. PLoS ONE. 2018, 13, e0191592. [Google Scholar] [CrossRef]
- Boriani, G.; Proietti, M.; Laroche, C. , et al; EORP-AF Long-Term General Registry Investigators. Association between antithrombotic treatment and outcomes at 1-year follow-up in patients with atrial fibrillation: The EORP-AF General Long-Term Registry. Europace. 2019, 21, 1013–1022. [Google Scholar] [CrossRef]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro Heart Survey on atrial fibrillation. Chest 2010, 137, 263–372. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.M.; Salim, I.; Kaddoura, R.; Singh, R.; et al. Hypertension in Middle Eastern Arab and South Asian patients with atrial fibrillation: From a 20-year hospital registry in Qatar (1990-2010). Heart Views 2021, 22, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Bhagavathula, S.A.; Shehab, A.; Ullah, A.; Rahmani, J. The burden of cardiovascular disease risk factors in the Middle East: A systematic review and meta-analysis focusing on primary prevention. Curr. Vasc. Pharmacol. 2021, 19. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.M. Atrial Fibrillation in Middle Eastern Arabs and South Asians: Summary of Published Articles in the Arabian Gulf. Heart Views. 2019, 20, 158–165. [Google Scholar] [CrossRef]
- Zubaid, M.; Rashed, W.A.; Alsheikh-Ali, A.A.; et al.; Gulf Survey of Atrial Fibrillation Events (Gulf SAFE) Investigators Management and 1-year outcomes of patients with atrial fibrillation in the Middle East: Gulf survey of atrial fibrillation events. Angiology. 2015, 66, 464–471. [Google Scholar] [CrossRef]
- El Kadri, M.; Ghorab, A.; Joury, J.; et al. Patient characteristics, adherence, and costs of oral anticoagulation therapy in non-valvular atrial fibrillation using the Dubai Real-World Claims Database. Avicenna J Med. 2021, 11, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Hersi, A.; Abdul-Moneim, M.; Almous'ad, A.; Al-Samadi, F.; AlFagih, A.; Sweidan, R. Saudi Atrial Fibrillation Survey: National, observational, cross-sectional survey evaluating atrial fibrillation management and the cardiovascular risk profile of patients with atrial fibrillation. Angiology. 2015, 66, 244–248. [Google Scholar] [CrossRef]
- Azar, R.R.; Ragy, H.I.; Kozan, O.; et al. Antithrombotic treatment pattern in newly diagnosed atrial fibrillation patients and 2-year follow-up results for dabigatran-treated patients in the Africa/Middle-East Region: Phase II results from the GLORIA-AF registry program. Int J Cardiol Heart Vasc. 2021, 34, 100763. [Google Scholar] [CrossRef] [PubMed]
- Hammoudeh, A.J.; Khader, Y.; Kadri, N.; et al. Adherence to the 2019 AHA/ACC/HRS Focused update of the 2014 AHA/ACC/HRS guideline on the use of oral anticoagulant agents in Middle Eastern patients with atrial fibrillation: The Jordan atrial fibrillation (JoFib) study. Int. J. Vasc. Med. 2021. [CrossRef]
- Schulman, S.; Angeras, U.; Bergqvist, D.; Eriksson, B.; Lassen, M.R.; Fisher, W. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis. Defin. Major Bleeding Clin. Investig. Antihemostatic Med. Prod. Surg. Patients. J Thromb Hemost. 2010, 8, 202–204. [Google Scholar] [CrossRef]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro Heart Survey on atrial fibrillation. Chest 2010, 137, 263–372. [Google Scholar] [CrossRef] [PubMed]
- Wee, X.T.; Ho, L.M.; Ho, H.K.; et al. Incidence of thromboembolic and bleeding events in patients with newly diagnosed nonvalvular atrial fibrillation: An Asian multicenter retrospective cohort study in Singapore. Clin Cardiol. 2017, 40, 1218–1226. [Google Scholar] [CrossRef]
- Ogawa, H.; An, Y.; Ishigami, K.; et al.; Fushimi AF Registry investigators Long-term clinical outcomes after major bleeding in patients with atrial fibrillation: The Fushimi AF registry. Eur Heart J Qual Care Clin Outcomes. 2021, 7, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.C.; Cayuelas, J.M.A.; Blanco, P.J.F.; Valdes, M.; Lorenzo, J.L.B.; Fernandez, S.M. Comparison of Bleeding Risk Scores in Patients With Nonvalvular Atrial Fibrillation Starting Direct Oral Anticoagulants. REC: CardioClinics. [CrossRef]
- Rutherford, O.W.; Jonasson, C.; Ghanima, W.; Holst, R.; Halvorsen, S. New score for assessing bleeding risk in patients with atrial fibrillation treated with NOACs. Open Heart. 2018, 5, e000931. [Google Scholar] [CrossRef]
- Undas, A.; Drabik, L. Potpara, Bleeding in anticoagulated patients with atrial fibrillation: Practical considerations. Pol. Arch. Intern. Med. 2020, 130. [Google Scholar] [CrossRef] [PubMed]
- Ouali, S.; Ben Halima, A.; Chabrak, S.; et al. Epidemiological characteristics, management, and outcomes of atrial fibrillation in TUNISIA: Results from the National Tunisian Registry of Atrial Fibrillation (NATURE-AF). Clin Cardiol. 2021, 44, 501–510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shehab, A.; Zubaid, M.; Bhagavathula, A.S. ; on behalf of the Gulf Survey of Atrial Fibrillation Events (Gulf SAFE) investigators. Sex differences in management and outcomes of patients with atrial fibrillation in the Middle East: Gulf survey of atrial fibrillation events (Gulf SAFE). Published: , 2017. 17 May. [CrossRef]
- Hellenbart, E.L.; Faulkenberg, K.D.; Finks, S.W. Evaluation of bleeding in patients receiving direct oral anticoagulants. Vasc Health Risk Manag. 2017, 13, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Hylek, E.M.; Held, C.; Alexander, J.H.; et al. Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin: The ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation): Predictors, Characteristics, and Clinical Outcomes. J Am Coll Cardiol. 2014, 63, 2141–2147. [Google Scholar] [CrossRef]
- Esteve-Pastor, M.A.; Rivera-Caravaca, J.M.; Lip, G.E.H. Hypertension and atrial fibrillation: Balancing stroke and bleeding risks. Am J Hypertension 2017, 30, 1063–1065. [Google Scholar] [CrossRef]
- Lopes, R.D.; Guimaraes, P.O.; Kolls, B.J.; et al. Intracranial hemorrhage in patients with atrial fibrillation receiving anticoagulation therapy. Blood 2017, 129, 2980–2987. [Google Scholar] [CrossRef]
- Archontakis-Barakakis, P.; Li, W.; Kalaitzoglou, D.; Tzelves, L.; et al. Effectiveness and safety of intracranial events associated with the use of direct oral anticoagulants for atrial fibrillation: A systematic review and meta-analysis of 92 studies. Br J Clin Pharmacol. 2022, 88, 4663–4675. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.Y.; Yao, J.F.; Brar, S.S.; Jorgensen, M.B.; Chen, W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol 2007, 50, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Zhu, J.; Liu, L.; et al. Efficacy and safety of apixaban compared with warfarin for stroke prevention in patients with atrial fibrillation from East Asia: A sub-analysis of the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) Trial. Am Heart J 2014, 168, 303–309. [Google Scholar] [PubMed]
- Lau, W.C.Y.; Torre, C.O.; Man, K.K.C.; et al. Comparative effectiveness and safety between Apixaban, Dabigatran, Edoxaban, and Rivaroxaban among patients with atrial fibrillation : A Multinational population-based cohort study. Ann Intern Med 2022, 3, 28–36. [Google Scholar] [CrossRef]
- Harskamp, R.E.; Lucassen, W.A.M.; Lopes, R.D.; Himmelreich, J.C.L.; Parati, G.; van Weert, H.C. Risk of stroke and bleeding in relation to hypertension in anticoagulated patients with atrial fibrillation: A meta-analysis of randomized controlled trials. [CrossRef]
- Farshidfar, F.; Koleini, N.; Ardehali, H. Cardiovascular complications of COVID-19. JCI Insight 2021, 6, e148980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nadarajah, R.; Gale, C.P. Outcomes following major bleeding in atrial fibrillation. Eur Heart J Qual Care Clin Outcomes. 2021, 7, 119–120. [Google Scholar] [CrossRef]
- Tamayo, S.; Peacock, F.W.; Patel, M.; Sicignano, N.; et al. Characterizing major bleeding in patients with nonvalvular atrial fibrillation: A pharmacovigilance study of 27 467 patients taking rivaroxaban. Clin Cardiol. 2015, 38, 63–68. [Google Scholar] [CrossRef]
- Gupta, K.; Trocio, J.; Keshishian, A.; et al. Effectiveness and safety of direct oral anticoagulants compared to warfarin in treatment naive non-valvular atrial fibrillation patients in the US Department of defense population. BMC Cardiovasc Disord. 2019, 19, 142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).