Submitted:
06 March 2023
Posted:
13 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Effects of copper on the growth of Penicillium sp.
| CTR mg/L | 400,0 mg/Lns |
500 mg/L* |
600 mg/L*** |
800 mg/L*** |
ANOVA | ||||||||
| Penicillium sp. |
D (mm) | D (mm) | IC (%) | D (mm) | IC (%) | D (mm) | IC (%) | D (mm) | IC (%) | F | |||
| 90a | 73 ± 1.67a | 19 | 51.50b ± 0.50 | 43 | 40.33c ± 1.89 | 55 | 22.17d ± 1.75 | 75 | 8.57*** | ||||
2.2. Growth in pH 4.0, 7.0 and 9.0
2.3. Toxicological prediction
| Toxicological Prediction | |
| Toxicological Class | 2 |
| LC50 | 25 mg.kg-1 |
| Molecular weight | 187.56 |
| Number of Hydrogen Acceptors | 6 |
| Number of atoms | 9 |
| Number of connections | 6 |
| Molecular polar surface area (PSA) | 137.76 |
2.4. Culture of Penicillium sp. in liquid medium
| Treatment | Cu(NO3)2 | Biomass (µg) | Inhibition (%) | pH |
| 1 | Pkg (control group) | 578 ± 2.75 | 7.00 | |
| 2 | Cu 2+ 400 mg | 257 ± 3.00 | 56% | 4.33 ± 0.032 |
| 3 | Cu 2+ 500 mg | 156.67 ± 2.52 | 73% | 4.19 ± 0.025 |
| 4 | Cu 2+ 600 mg | 55.33 ± 0.58 | 90% | 4.08 ± 0.003 |
| 5 | Cu 2+ 800 mg | 41.67 ± 2.08 | 93% | 3.37 ± 0.078 |
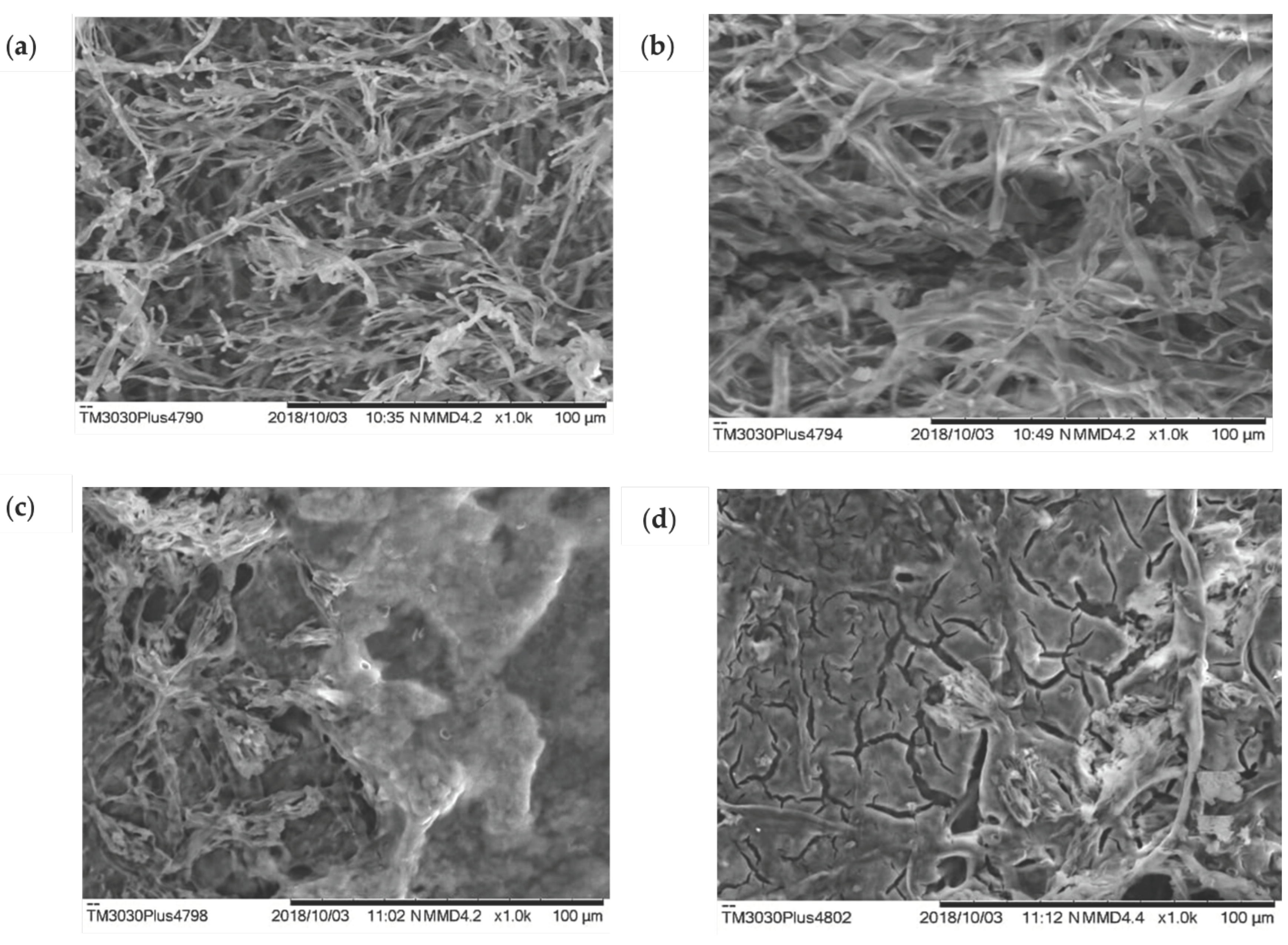
2.5. Biomass analysis of Penicillium sp. isolated from kefir grains by scanning electron microscopy (SEM)
3. Discussion
4. Materials and Methods
4.1. Isolation Methodology of Penicillium sp. of kefir
4.2. Culture Medium Preparation and Radial Growth Measurement
4.3. Toxicological Forecast
4.4. Biomass production of Penicillium sp. and Determination of Minimum Inhibitory Concentration (MIC)
4.5. Preparation of the culture medium
4.6. Cultivation of Penicillium sp. in solid medium and Minimum Inhibitory Concentration (MIC)
4.7. Penicillium sp. biomass and quantification of copper
4.8. Estimation of Residual Metals in the Culture Medium
4.9. Scanning Electron Microscopy (SEM) Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choińska-Pulit, A.; Sobolczyk-Bednarek, J.; Łaba, W. Optimization of Copper, Lead and Cadmium Biosorption onto Newly Isolated Bacterium Using a Box-Behnken Design. Ecotoxicol Environ Saf 2018, 149, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Widmer, J.; Norgrove, L. Identifying Candidates for the Phytoremediation of Copper in Viticultural Soils: A Systematic Review. Environ Res 2023, 216, 114518. [Google Scholar] [CrossRef] [PubMed]
- Cornu, J.-Y.; Huguenot, D.; Jézéquel, K.; Lollier, M.; Lebeau, T. Bioremediation of Copper-Contaminated Soils by Bacteria. World J Microbiol Biotechnol 2017, 33, 26. [Google Scholar] [CrossRef] [PubMed]
- Mohamadhasani, F.; Rahimi, M. Growth Response and Mycoremediation of Heavy Metals by Fungus Pleurotus Sp. Sci Rep 2022, 12, 19947. [Google Scholar] [CrossRef]
- Deniz, F.; Ersanli, E.T. A Natural Macroalgae Consortium for Biosorption of Copper from Aqueous Solution: Optimization, Modeling and Design Studies. Int J Phytoremediation 2018, 20, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Naz, F.; Hamayun, M.; Rauf, M.; Arif, M.; Afzal Khan, S.; Ud-Din, J.; Gul, H.; Hussain, A.; Iqbal, A.; Kim, H.-Y.; et al. Molecular Mechanism of Cu Metal and Drought Stress Resistance Triggered by Porostereum Spadiceum AGH786 in Solanum Lycopersicum L. Front Plant Sci 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Fagnano, M.; Agrelli, D.; Pascale, A.; Adamo, P.; Fiorentino, N.; Rocco, C.; Pepe, O.; Ventorino, V. Copper Accumulation in Agricultural Soils: Risks for the Food Chain and Soil Microbial Populations. Science of The Total Environment 2020, 734, 139434. [Google Scholar] [CrossRef] [PubMed]
- Palanivel, T.M.; Pracejus, B.; Novo, L.A.B. Bioremediation of Copper Using Indigenous Fungi Aspergillus Species Isolated from an Abandoned Copper Mine Soil. Chemosphere 2023, 314, 137688. [Google Scholar] [CrossRef] [PubMed]
- Albert, Q.; Leleyter, L.; Lemoine, M.; Heutte, N.; Rioult, J.-P.; Sage, L.; Baraud, F.; Garon, D. Comparison of Tolerance and Biosorption of Three Trace Metals (Cd, Cu, Pb) by the Soil Fungus Absidia Cylindrospora. Chemosphere 2018, 196, 386–392. [Google Scholar] [CrossRef]
- Oliveira, A.F. de; Santos, C.B.R. dos; Ferreira, A.M.; Bezerra, R.M.; Zamora, R.R.M.; Cruz, R.A.S.; Amado, J.R.R.; Carvalho, J.C.T. A Viability Study for the Production of Biofilms and In Silico Predictions of Major Compounds in Kefir. J Comput Theor Nanosci 2017, 14, 2915–2926. [Google Scholar] [CrossRef]
- Oliveira, A.; Maciel, A.; Florentino, A.; Fernandes, C.; Bezerra, R.; Góes, M.; Salcedo, M.; Zamora, R.; Carvalho, J.C. Study of Kefir Biofilm Associated with Hydroethanolic Extract of Euterpe Oleracea Mart. (Aai). Afr J Microbiol Res 2017, 11, 1474–1483. [Google Scholar] [CrossRef]
- Ortúzar, M.; Trujillo, M.E.; Román-Ponce, B.; Carro, L. Micromonospora Metallophores: A Plant Growth Promotion Trait Useful for Bacterial-Assisted Phytoremediation? Science of The Total Environment 2020, 739, 139850. [Google Scholar] [CrossRef] [PubMed]
- Sevcikova, M.; Modra, H.; Slaninova, A.; Svobodova, Z. Metals as a Cause of Oxidative Stress in Fish: A Review; 2011; Vol. 56;
- Subramaniyam, V.; Subashchandrabose, S.R.; Thavamani, P.; Chen, Z.; Krishnamurti, G.S.R.; Naidu, R.; Megharaj, M. Toxicity and Bioaccumulation of Iron in Soil Microalgae. J Appl Phycol 2016, 28, 2767–2776. [Google Scholar] [CrossRef]
- Torres, E.M.; Hess, D.; McNeil, B.T.; Guy, T.; Quinn, J.C. Impact of Inorganic Contaminants on Microalgae Productivity and Bioremediation Potential. Ecotoxicol Environ Saf 2017, 139, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Schümann, K.; Classen, H.; Dieter, H.; König, J.; Multhaup, G.; Rükgauer, M.; Summer, K.; Bernhardt, J.; Biesalski, H. Hohenheim Consensus Workshop: Copper. Eur J Clin Nutr 2002, 56, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Nishimuta, M.; Masui, K.; Yamamoto, T.; Ikarashi, Y.; Tokushige, K.; Hashimoto, E.; Nagashima, Y.; Shibata, N. Copper Deposition in Oligodendroglial Cells in an Autopsied Case of Hepatolenticular Degeneration. Neuropathology 2018, 38, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.M.; Castro, P.M.L.; Malcata, F.X. Metal Uptake by Microalgae: Underlying Mechanisms and Practical Applications. Biotechnol Prog 2012, 28, 299–311. [Google Scholar] [CrossRef]
- Pereira, A.; Freitas, D.A. USO DE MICRO-ORGANISMOS PARA A BIORREMEDIAÇÃO DE AMBIENTES IMPACTADOS. Revista Eletrônica em Gestão, Educação e Tecnologia Ambiental 2012, 6. [CrossRef]
- Sahu, O. Reduction of Organic and Inorganic Pollutant from Waste Water by Algae. International Letters of Natural Sciences 2014, 8, 1–8. [Google Scholar] [CrossRef]
- Nascimento, R.; Lima, A.; Vidal, B.; Melo, D.; Raulino, G. Adsorção: Aspectos Teóricos e Aplicações Ambientais; 1st ed.; Impressão Universitária: Fortaleza, 20220; Volume 1. [Google Scholar]
- Worms, I.; Simon, D.F.; Hassler, C.S.; Wilkinson, K.J. Bioavailability of Trace Metals to Aquatic Microorganisms: Importance of Chemical, Biological and Physical Processes on Biouptake. Biochimie 2006, 88, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Dusengemungu, L.; Kasali, G.; Gwanama, C.; Ouma, K. O. Recent Advances in Biosorption of Copper and Cobalt by Filamentous Fungi, Front Microbiol. v. 11, p. 582016, 2020.
- Lotlikar, N.; Damare, S.; Meena, R. M.; Jayachandran, S. Variable protein expression in marine-derived filamentous fungus Penicillium chrysogenum in response to varying copper concentrations and salinity. Metallomics, v. 12, n. 7, p.
- Diep, P.; Mahadevan, R.; Yakunin, A.F. Heavy Metal Removal by Bioaccumulation Using Genetically Engineered Microorganisms. Front Bioeng Biotechnol 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Dorr, F.; Guaratini, T.; Cardoso, K.; Pavanelli, D.; Colepicolo, P.; Pinto, E. Toxicologia Ambiental. In Fundamentos de Toxicologia; Oga, S., Camargo, M., Batistuzzo, J., Eds.; Atheneu: São Paulo, 2014; Volume 1. [Google Scholar]
- Zagatto, P.; Bertoletti, E. Ecotoxicologia Aquática : Princípios e Aplicações.; 2nd ed.; Rima: São Carlos, 2014; Volume 1. [Google Scholar]
- Purchase, D.; Scholes, L.N.L.; Revitt, D.M.; Shutes, R.B.E. Effects of Temperature on Metal Tolerance and the Accumulation of Zn and Pb by Metal-Tolerant Fungi Isolated from Urban Runoff Treatment Wetlands. J Appl Microbiol 2009, 106, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, V.; Grujić, S.; Simić, Z.; Ostojić, A.; Radojević, I. Finding the Best Combination of Autochthonous Microorganisms with the Most Effective Biosorption Ability for Heavy Metals Removal from Wastewater. Front Microbiol 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Inès, M.; Mekki, S.; Ghribi, D. Treatment of Heavy Metals Contaminated Water: Use of B. Mojavensis BI2 Derived Lipopeptide and Palm Waste Flour. Water Science and Technology 2022, 86, 1083–1094. [Google Scholar] [CrossRef]
- Kugler, A.; Brigmon, R.L.; Friedman, A.; Coutelot, F.M.; Polson, S.W.; Seaman, J.C.; Simpson, W. Bioremediation of Copper in Sediments from a Constructed Wetland Ex Situ with the Novel Bacterium Cupriavidus Basilensis SRS. Sci Rep 2022, 12, 17615. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, E. C. M.; Baltazar, M. P, G; Dos Reis, T. A.; Do Nascimento, C. A. O.; Côrrea, B.; Gimenes, L. J. Copper biosorption from an aqueous solution by the dead biomass of Penicillium ochrochloron. Environ Monit Assess, v. 2019, 191, 4–247. [Google Scholar] [CrossRef] [PubMed]
- Brinza, L.; Dring, M.J.; Gavrilescu, M. Marine Micro and Macro Algal Species as Biosorbents for Heavy Metals. Environ Eng Manag J 2007, 6, 237–251. [Google Scholar] [CrossRef]
- Kumar, S.; VijayM, A.; KumarKP, S. Biosorption of Lead(II) and Chromium(VI) by Immobilized Cells of Microalga Isochrysis Galbana. Research Article J. Algal Biomass Utln 2013, 1, 42–50. [Google Scholar]
- Indhumathi, P.; Syed Shabudeen, P.; Shoba, U.; Saraswathy, C. The Removal of Chromium from Aqueous Solution by Using Green Micro Algae. J Chem Pharm Res 2014, 6, 799–808. [Google Scholar]
- Lau, P.S.; Lee, H.Y.; Tsang, C.C.K.; Tam, N.F.Y.; Wong, Y.S. Effect of Metal Interference, PH and Temperature on Cu and Ni Biosorption by Chlorella Vulgaris and Chlorella Miniata. Environ Technol 1999, 20, 953–961. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Marques, A.P.G.C.; Castro, P.M.L.; Xavier Malcata, F. Characterization of Desmodesmus Pleiomorphus Isolated from a Heavy Metal-Contaminated Site: Biosorption of Zinc. Biodegradation 2009, 20, 629–641. [Google Scholar] [CrossRef]
- Bishnoi, N.R.; Kumar, R.; Kumar, S.; Rani, S. Biosorption of Cr(III) from Aqueous Solution Using Algal Biomass Spirogyra Spp. J Hazard Mater 2007, 145, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Mtengai, K.; Ramasamy, S.; Msimuko, P.; Mzula, A.; Mwega, E.D. Existence of a Novel Heavy Metal–Tolerant Pseudomonas Aeruginosa Strain Zambia SZK-17 Kabwe 1: The Potential Bioremediation Agent in the Heavy Metal–Contaminated Area. Environ Monit Assess 2022, 194, 887. [Google Scholar] [CrossRef]
- Martinelli, P.; Santos, J. Scanning Electron Microscopy of Nematophagous Fungi Associated Tylenchulus semipenetrans and Pratylenchus jaehni. Biosci J 2010, 26, 809–816. [Google Scholar]
- Juříková, T.; Luptáková, D.; Kofroňová, O.; Škríba, A.; Novák, J.; Marešová, H.; Palyzová, A.; Petřík, M.; Havlíček, V.; Benada, O. Bringing SEM and MSI Closer Than Ever Before: Visualizing Aspergillus and Pseudomonas Infection in the Rat Lungs. Journal of Fungi 2020, 6, 257. [Google Scholar] [CrossRef] [PubMed]
- Gams, W.; Bisset, T. Morphology and Identification of Trichoderma. In Trichoderma and Gliocladium: Basic Biology Taxonomy and Genetics Harman GE and CP Kubicek; Harman, G., Kubicek, C., Eds.; Taylor and Francis: London, 1998; Volume 1, pp. 3–34. [Google Scholar]
| 48 h | IC% | 72 h | IC% | 96 h | IC% | 120 h | IC% | F | |
| Pkg -CTR | 20,00 ± 1.26 | 45,00 ± 6.33 | 18,00 ± 1.28 | 90,00 | 932.85** | ||||
| Pkg –Cu pH 4.0 |
07.25 ± 1.18 |
6 | 12,00 ± 0.58 | 6 | 14,00 ± 1.76 |
5 | 24.40 ± 0.5 | 73 | |
| Pkg -Cu pH 7.0 |
6.00 ± 1.06 |
28 | 13,00 ± 1.0 | 3 | 19,00 ± 1.83 |
2 | 22.50 ± 1.9 | 75 | |
| Pkg -Cu pH 9.0 |
5,00 ± 0.65 |
24 | 14,00 ± 20.10 |
19 | 15,00 ± 1.16 | 18 | 20.66 ± 0.76 |
77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





